Abstract
Purpose
This study aimed to elucidate the molecular mechanisms of the anti-pancreatic fibrosis effects of matrine in rats.
Materials and Methods
Trinitrobenzene sulfonic acid was administrated to rats to establish a pancreatic fibrosis model. Rats were divided into four groups: Control, Sham, Model, and Matrine (n=8). Hematoxylin-eosin staining, Masson staining, and Azan staining were performed to evaluate pancreatic fibrosis. Expression of transforming growth factor-β1 (TGF-β1), α-smooth muscle actin (α-SMA), and collagen I in pancreatic tissues was evaluated by immunohistochemical staining. mRNA and protein levels of TGF-β receptor 1 (TβR1), TβR2, and Smad2 in pancreatic tissues were determined by RT-PCR and Western blot, respectively.
Results
In the model group, hyperplasia of glandules around the glandular ducts, mitochondrial swelling of acinous cells, and severe fibrosis were found. Interestingly, in the Matrine group, mitochondrial swelling was only found in a small number of acinous cells, and the fundamental structures of pancreatic tissues were intact. Moreover, pancreatic fibrosis was markedly alleviated. Comparing to the Sham group, expression of α-SMA, TGF-β1, and collagen I was sharply elevated in the Model group (p<0.05); however, their expressions were much lower in the Matrine group, compared to the Model group (p<0.05). Compared with the Sham group, mRNA and protein levels of Smad2, TβR1, and TβR2 in the Model group were notably raised (p<0.05). However, their high expression was significantly downregulated in the Matrine group (p<0.05).
Conclusion
Matrine suppressed pancreatic fibrosis by regulating TGF-β/Smad signaling in rats.
Keywords: Matrine, pancreatic fibrosis, signal pathway, Smad2, TGF-β
INTRODUCTION
Pancreatic fibrosis poses a serious threat to human health. It can be found in different pancreatic diseases, such as chronic pancreatitis, type 2 diabetes, severe acute pancreatitis, and pancreatic cancer.1,2,3 The clinical manifestations of pancreatic fibrosis are persistent or recurrent upper abdominal pain, pancreatic calcification, steatorrhea, diabetes, pancreatic pseudocyst, and even insufficient endocrine and exocrine functioning of the pancreas. Fibrosis is not only irreversible in morphology, but also tends to be progressive. Generally, pancreatic fibrosis is characterized by increased extracellular matrix, infiltration of inflammatory cells, damage to and reduction of acinar cells, structural changes in glandular ducts, and stone formation in the glandular ducts. The pathological changes are featured by irreversible destruction of the pancreas, including multiple stenoses of calcified glandular tubes, atrophy and diffuse fibrosis of glands, reduction of pancreatic acini, beaded changes in pancreatic ducts, and cystic dilatation.
Because the pathogenic factors of pancreatic fibrosis are complicated, it is difficult to effectively diagnose in clinics, and treatments are also inefficient. Its exact pathogenesis is not definitely clear at present. Previous studies have shown that pancreatic stellate cells play an important role in the process of pancreatic fibrosis.4 The cells can promote fibrosis by activating, transforming, migrating, and promoting the synthesis of extracellular matrix. Many cytokines can stimulate the activation of pancreatic stellate cells. For example, inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), transforming growth factor-β1 (TGF-β1), and interleukin-6 (IL-6), secreted by the damaged pancreas can change the pancreatic stellate cells from quiescent state to activated state.4,5,6 The activation of pancreatic stellate cells by cytokines through a variety of signal transduction pathways might be key to fibrosis formation. Therefore, in the prevention and treatment of fibrosis, the inhibition of the activation and proliferation of pancreatic stellate cells is critical. TGF-β1/Smad2 signaling pathway plays a major role in the activation and proliferation of pancreatic stellate cells. If we can block the TGF-β1/Smad2 signaling pathway, pancreatic fibrosis may be improved.7
Matrine is an alkaloid extracted from sophora flavescens, sophora alopecuroide, and subprostrate sophora root. Modern studies have shown that matrine exerts anti-inflammation, immunomodulation, antivirus, and anti-tumor effects.8,9 Interestingly, matrine has been shown to be able to inhibit pancreatic fibrosis and to achieve satisfying results in clinical care.9 However, the related mechanisms are still not clear at present. Whether the regulation of TGF-β1/Smad signaling pathway is involved in the prevention and suppression of pancreatic fibrosis by matrine is an interesting research topic.
Therefore, in this study, we investigated the effects of matrine on the expression of signal molecules in the TGF-β/Smad pathway in pancreatic fibrosis. To do so, a Sprague Dawley (SD) rat model with pancreatic fibrosis was first established. The suppression effect of matrine on pancreatic fibrosis was evaluated by hematoxylin-eosin (HE) staining, Masson staining, and Azan staining. Expression of TGF-β1, α-smooth muscle actin (α-SMA), and collagen I in pancreatic tissues was assessed by immunohistochemical staining. mRNA and protein levels of TGF-β receptor 1 (TβR1), TβR2, and Smad2 in pancreatic tissues were determined by realtime (RT) PCR and Western blot, respectively.
MATERIALS AND METHODS
Materials and animals
Horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG was obtained from ZSGB-BIO (ZB-2305, Beijing, China). Matrine injections were obtained from Chia Tai Tianqing Pharmaceutical Group Co., Ltd. (1201117204, Jiangsu, China). Pentobarbital sodium (P3761, 5 g) and trinitrobenzene sulfonic acid (P2297, 10 mL) were bought from Sigma-Aldrich (St. Louis, MO, USA). Rabbit anti-collagen I antibody (bs-0578R), α-SMA rabbit polyclonal antibody (bs-10196R), and TGF-β receptor 2 rabbit polyclonal antibody (bs-0117R) were purchased from Biosynthesis Biotechnology Co., Ltd. (Beijing, China). TGF-β1 rabbit polyclonal antibody was purchased from ABclonal Technology (A2124, Woburn, MA, USA). TGF-β receptor 1 rabbit polyclonal antibody (ab31013), Smad2 rabbit monoclonal antibody (ab33875), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) rabbit polyclonal antibody (ab9485) were obtained from Abcam (Cambridge, MA, USA). TRIzol reagent (CW0580), HiFiScript cDNA synthesis kit (CW2569), BCA kit, and UltraSYBR Mixture (CW0957) were purchased from CWBiotech (Beijing, China).
SD rats (male, 8 weeks) were purchased from Changzhou Cavens Lab Animal Co., Ltd. [License No. SCXK (Su) 2016-0010, Jiangsu, China]. All animals were raised with free access to food and drink. The study protocol was reviewed and approved by the Institutional Animal Care and Use Committee, the First Affiliated Hospital of Nanchang University, China (2018 medical research No. 094).
Pancreatic fibrosis model establishment
Rats were weighed and anesthetized by intraperitoneal injection of 1% pentobarbital sodium at a dose of 45 mg/kg. The abdominal fur of the rats was removed with scissors, and operative sites were disinfected with iodine. A median incision was made along the abdominal raphe. The skin was cut first and then the peritoneum, cutting into the abdominal cavity. The biliopancreatic duct near the porta hepatis was found with a cotton swab and clamped with an artery clamp. The duodenum was fixed with left finger pulp, and the opening of the biliopancreatic duct in the duodenum was found. An injection needle type 4.5 was then inserted into the biliopancreatic duct along the bowel wall as much as about 1 cm. Then, 12.5 mL of 2% trinitrobenzene sulfonic acid-ethanol phosphate buffer solution containing 1 mL of 5% trinitrobenzene sulfonic acid and 1.5 mL of 10% ethanol phosphate buffer solution were injected with a micro-injection pump (AJ-5805, Angel Electronic Equipment Co., Ltd., Shanghai, China). The duodenal end was clamped with an artery clamp. Pressure of micro-injection was maintained for 10 min. Finally, the peritoneal cavity was closed and the success of the model establishment was judged by pathologic analysis based on HE staining, Masson staining, and Azan staining.
Animal grouping
Thirty two rats were divided into four groups: Control, Model, Sham, and Matrine (n=8). Rats without any treatments served as the control. Rats in the Model group were ones receiving the above operation of model establishment. Rats suffering the similar operation without injection of trinitrobenzene sulfonic acid-ethanol phosphate buffer solution were divided into the Sham group. In the Matrine group, at three weeks after establishment of the pancreatic fibrosis model, rats were intraperitoneally injected with matrine injection every day at a dose of 100 mg/kg for four weeks.10,11 Finally, rats in all groups were sacrificed for the following experiments.
HE staining
Pancreatic tissues of suitable size were collected and rinsed with phosphate buffer solution. After being fixed in 10% neutral formaldehyde, tissues were embedded with paraffin and cut into slices by using a microtome (BQ-318D, Bona Medical Technology, Hubei, China). The paraffin sections were dewaxed and hydrated. These slices were incubated in hematoxylin solution for 5 min and rinsed with running water. Then the slices were immersed in eosin solution for 3 min and washed again with running water. After dehydration, the slices were mounted with neutral resin and viewed under a light microscope (CX41, Olympus, Tokyo, Japan) for pathologic analysis.
Masson staining
Pancreatic tissues of suitable size were collected and washed with running water for several hours. They were then dehydrated in 70, 80, 90, and 100% ethanol and transparentized in dimethylbenzene. After being embedded in paraffin, they were cut into slices. The slices were routinely dewaxed, hydrated, incubated in Weigert solution for 5–10 min. They were then differentiated in acidic ethanol for 5–15 s, slightly washed with water, and blued in Masson bluing buffer for 3–5 min. After being washed with water, the slices were incubated in ponceau-fuchsin solution for 5–10 min, washed with weak acid solution for 1 min, and washed with phosphomolybdic acid solution for 1–2 min. The slices were subsequently stained in aniline blue solution for 1–2 min. They were then washed with weak acid solution, dehydrated in absolute ethanol, transparentized in dimethylbenzene, mounted with neutral resin, and observed under a microscope (CX41).
Azan staining
Pancreatic tissues of suitable size were collected and washed with running water for several hours. They were dehydrated, transparentized, embedded in paraffin, and cut into slices. The slices were baked for 1.5 h and routinely dewaxed and hydrated. For staining, slices were incubated in azocarmine G solution at 60℃ for 1 h, kept at room temperature for 10 min, and washed twice with water for each 3 min. After the slices were immersed in 1% aniline ethanol for 2 min, they were successively immersed in 95% ethanol, 80% ethanol, 50% ethanol, water and fresh water for each 3 min. They were stained in 4% phosphotungstic acid for 2 h and washed twice with water for each 3 min. They were stained in aniline blue-orange G solution for 2 h and washed twice with water for each 3 min. Subsequently, they were dehydrated in absolute ethanol, transparentized in dimethylbenzene, mounted with neutral resin, and observed under a microscope (CX41).
Immunohistochemical staining
Pancreatic tissues of suitable size were collected and rinsed with phosphate buffer solution. After being fixed in 10% neutral formaldehyde, tissues were embedded with paraffin and cut into slices by using a microtome (BQ-318D, Bona Medical Technology, Hubei, China). After baking in an oven at 65℃ for 1.5 h, the paraffin sections were immersed in xylene for 10 min and fresh xylene for another 10 min. Then, sections were successively put in 100% ethanol, 100% ethanol, 95% ethanol, 80% ethanol, and purified water for 3 min in each. The slices were placed in a repair box, and citrate buffer solution (antigen retrieval buffer) was added. The box was placed in a pressure cooker and heated to automatic deflation. After 2 min, the box was taken out and naturally cooled. Then the antigen retrieval buffer was discarded, and the slices were eluted with phosphate buffer solution. The slices were transferred to a wet box and incubated in fresh 3% hydrogen peroxide at room temperature for 10 min to remove endogenous peroxidase blocking buffer. The slices were eluted three times with phosphate buffer solution for 5 min every time, and absorbent papers were used to absorb residual phosphate buffer solution around the tissues. Bovine serum albumin (BSA, 5%) was dropwise added onto the slices, which were blocked at 37℃ for 30 min. Absorbent papers were used to absorb residual blocking buffer around the tissues. Enough diluted primary antibodies (TGF-β1 antibody, 1:200; Rabbit a collagen I, 1:200; α-SMA antibody, 1:200) were dropwise added onto every slice. After placed in a wet box at 4℃ overnight, the slices were taken out in room temperature for 45 min. Secondary antibody buffer (1:2000) was dropwise added onto every slice. After incubation at 37℃ for 30 min, the slices were sufficiently rinsed with phosphate buffer solution. Diaminobenzidine developing was performed for 5–10 min, and staining degree was monitored under a microscope. After washing with running water for 1 min, the slices were stained in hematoxylin for 1 min. After being differentiated with hydrochloric-alcohol solution, washed with running water for 1 min, dehydrated, and mounted, the slices were subjected to be examined under a microscope (CX41). Results were quantified with software Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD, USA).
RT-PCR
Tissues in various groups were grinded in liquid nitrogen. Total RNA was collected by using TRIzol reagent according to its instruction for use. Reverse transcription from RNA to cDNA by using HiFiScript cDNA synthesis kit according to its instruction for use. Sequences of primers were listed in Table 1. PCR system (25 µL) included RNase free dH2O 9.5 µL, cDNA/DNA 1 µL, forward primer 1 µL, reverse primer 1 µL, ULtra-SYBR Mixture (2×) 12.5 µL. Reaction parameters were shown as follows: pre-denaturation for 10 min at 95℃, denaturation for 10 s at 95℃, annealing for 30 s at 58℃, elongation for 30 s at 72℃, and 40 circles. Dissociation curve was analyzed as follows: 15 s at 95℃, 1 min at 58℃, 15 s at 95℃, 15 s at 58℃, 15 s at 58℃, and measured stepwise from 95℃, every 0.5℃. Finally, it was evaluated on a fluorescent quantitation PCR (CFX Connect™, Bio-Rad, Hercules, CA, USA). GAPDH served as internal control.
Table 1. PCR Primers.
| Gene | Primer (5′-3′) |
|---|---|
| TGF-β receptor 1 (TβR1) | For: TGTCTCCAAATCCAACTCCTC |
| Rev: GACTGGCCCTACCTCACCTAT | |
| TGF-β receptor 2 (TβR2) | For: CAGAGTGAAGCCGTGGTAGGT |
| Rev: CTGTGAGAAGCCGCAGGAAGT | |
| Smad2 | For: AAGCCATCACCACTCAGAATTG |
| Rev: CACTGATCTACCGTATTTGCGT | |
| GAPDH | For: GCAAGTTCAACGGCACAG |
| Rev: CGCCAGTAGACTCCACGAC |
Western blot
Tissues in various groups were grinded in liquid nitrogen and incubated in lysis buffer for 30 min. The lysate was centrifugated at 10000 rpm and 4℃ for 10 min. The supernatant was carefully collected to obtain total protein. Protein concentration was determined with BCA kit. Protein was degenerated and loaded quantitatively to conduct SDS-PAGE for 1–2 h. The gel was immersed in transfer buffer to form transfer sandwich. The membrane was transferred for 30–50 min by a wet method. Subsequently, the membrane was incubated with diluted primary antibodies (TβR1 antibody, 1:1000; TβR2, 1:600; Smad2 antibody, 1:1500; GAPDH antibody, 1:2500) at 4℃ overnight. It was rinsed and incubated in HRP labeled IgG buffer (1:2000) at room temperature for 1–2 h. Finally, enhanced chemiluminescence substrate was dropwise added onto the membrane and then the membrane was exposed on a gel imaging system (ChemiDoc™ XRS+, Bio-Rad). Gray values of protein blots were calculated with a software “Quantity one” (v4.62, Bio-Rad). GAPDH served as the internal control.
Statistical analysis
All data were expressed as mean±standard deviation. Statistical analysis was carried out using one way analysis of variance followed by a Tukey post-hoc test via SPSS version 19.0 software (IBM Corp., Armonk, NY, USA). p<0.05 was judged to indicate a statistically significant difference.
RESULTS
Pancreatic fibrosis model establishment
The establishment of the pancreatic fibrosis model in rats and an image of typical pancreatic tissue evaluated by HE staining are shown in Fig. 1. After the operation for model establishment, the physical states of all rats were fine, and none of the rats died. Pathologic analysis identified hyperplasia of glandules around the glandular ducts and mitochondrial swelling of acinous cells. A small amount of fibers accumulated in the mesenchyme, and cellular infiltration was not found. These results indicated the success of the pancreatic fibrosis model establishment in rats.
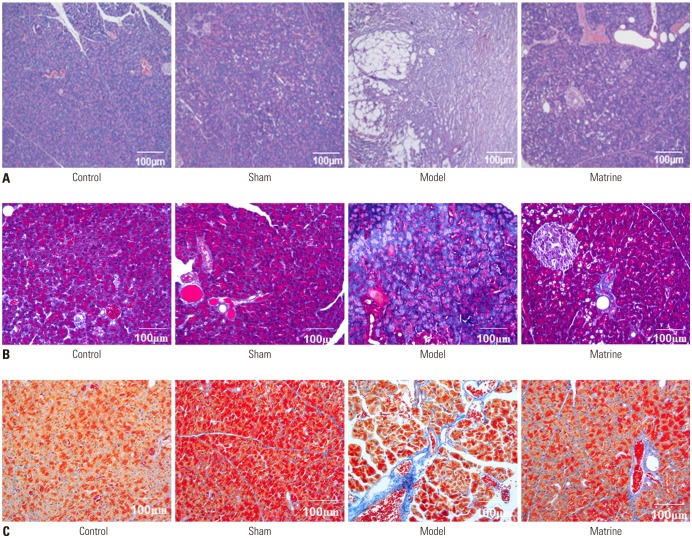
Fig. 1. Establishment of the pancreatic fibrosis model in rats and a typical image of pancreatic tissue evaluated by hematoxylin-eosin staining (×200).
Pathologic analysis evaluated by HE staining, Masson staining, and Azan staining
Pathologic analysis of the pancreatic tissues in various groups evaluated by HE staining is depicted in Fig. 2A. In the Control and Sham groups, there were no obvious fibrosis or progressive changes. In the Model group, we found hyperplasia of glandules around the glandular ducts, mitochondrial swelling of acinous cells, and accumulation of a small amount of fibers in the mesenchyme, as well as no cellular infiltration. However, in the Matrine group, mitochondrial swelling was only found in a small number of acinous cells, and the fundamental structures of pancreatic tissues were intact.
Fig. 2. Pathologic analysis of pancreatic tissues from the study groups evaluated by hematoxylin-eosin staining (×200) (A), Masson staining (×200) (B), and Azan staining (×200) (C).
Pathologic analysis of pancreatic tissues in the study groups evaluated by Masson staining and Azan staining is demonstrated in Fig. 2B and C. Collagen fibers with positive Masson or Azan staining were blue. In the Sham group, there was only a small amount of blue fibrous tissue in the interstitium of pancreatic tissue. However, compared to the Sham group, the distribution of blue collagen fibers in the pancreatic tissue increased markedly, and fibrosis was aggravated greatly in the Model group. Moreover, infiltration of inflammatory cells was observed in the Model group. Interestingly, the distribution of collagen fibers in the Matrine group was much less than that in the Model group, and the infiltration of inflammatory cells was also alleviated in the Matrine group.
Expression of α-SMA, TGF-β1, and collagen I assessed by immunohistochemical staining
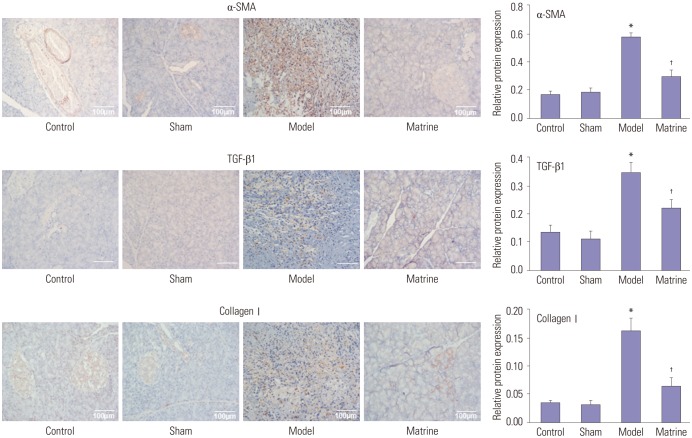
Expression of α-SMA, TGF-β1, and collagen I in pancreatic tissues from the study groups assessed by immunohistochemical staining is depicted in Fig. 3. Expression levels of α-SMA, TGF-β1, and collagen I were low in the Control and Sham group. However, compared to the Control group, their levels were sharply elevated in the Model group (p<0.05). The expression of α-SMA, TGF-β1, and collagen I in the Matrine group was much lower than that in the Model group (p<0.05). This indicated that matrine administration was able to downregulate the expression of α-SMA, TGF-β1, and collagen I in the fibrous pancreatic tissues from rats.
Fig. 3. Expression of α-SMA, TGF-β1, and collagen I in the fibrous pancreatic tissues upon immunohistochemical staining (×200). *p<0.05 vs. control group, †p<0.05 vs. model group. α-SMA, α-smooth muscle actin; TGF-β1, transforming growth factor-β1.
Levels of Smad2, TβR1, and TβR2 determined by RT-PCR and Western blot
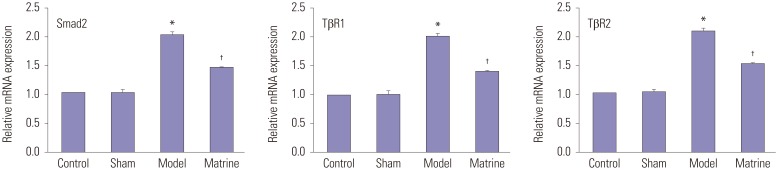
Fig. 4 shows the mRNA levels of Smad2, TβR1, and TβR2 in the fibrous pancreatic tissues, which were determined by RT-PCR. Low mRNA levels of Smad2, TβR1, and TβR2 were similarly found between the Control and Sham groups. Compared with the Control group, mRNA levels of Smad2, TβR1, and TβR2 in the Model group were notably raised (p<0.05). However, the high mRNA expression was significantly downregulated by matrine treatment to a relative low level in the Matrine group (p<0.05).
Fig. 4. mRNA levels of Smad2, TβR1, and TβR2 in the fibrous pancreatic tissues as determined by RT-PCR. *p<0.05 vs. control group, †p<0.05 vs. model group. α-SMA, α-smooth muscle actin; TGF-β1, transforming growth factor-β1.
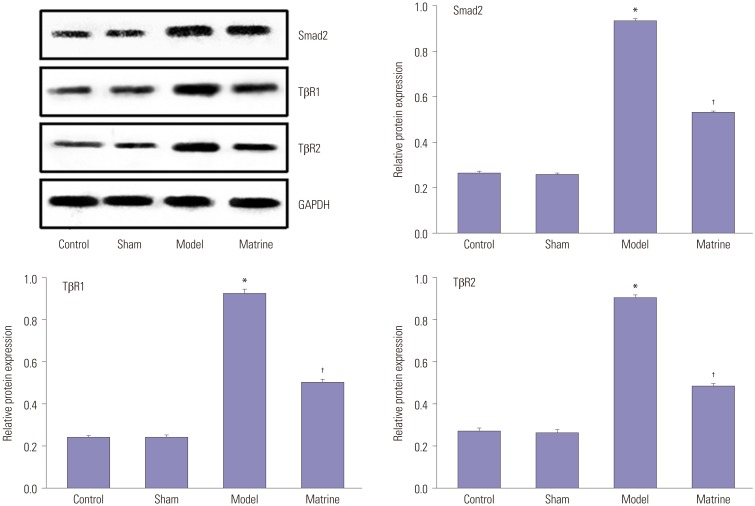
Fig. 5 demonstrates the protein levels of Smad2, TβR1, and TβR2 in the fibrous pancreatic tissues as determined by Western blot. The qualitative and quantitative results of Western blots agreed with those of RT-PCR. Protein expression of Smad2, TβR1, and TβR2 in the fibrous pancreatic tissues was low in the Control and Sham groups. Although protein expression thereof was sharply increased to high levels in the Model group (p<0.05), this was reversed upon matrine treatment (p<0.05).
Fig. 5. Protein levels of Smad2, TβR1, and TβR2 in the fibrous pancreatic tissues as determined by Western blot. *p<0.05 vs. control group, †p<0.05 vs. model group. TβR1, transforming growth factor-β receptor 1; TβR2, transforming growth factor-β receptor 2.
DISCUSSION
Pancreatic fibrosis is a segmental, local, or diffuse chronic progressive inflammation of the pancreas induced by diverse reasons, causing irreversible damage to pancreatic function or pancreatic tissue. In this study, trinitrobenzene sulfonic acid was employed to induce pancreatic fibrosis in rats. While ethanol itself as a solvent can induce mild fibrosis of the pancreas, trinitrobenzene sulfonic acid is able to interact with ascorbic acid, and the free radicals produced have toxic effects on pancreatic duct epithelial cells. Pathologic characterization upon HE staining, Masson staining, and Azan staining confirmed the success of the establishment of the pancreatic fibrosis model in rats and the suppression of pancreatic fibrosis by matrine.
α-SMA is a marker of the activation of pancreatic stellate cells in rats.12 Rat pancreatic stellate cells are surrounded by acinar cells and connected with surrounding acinar cells. Their cytoplasm is polygonal, and there are large amounts of vitamin A lipid droplets in the cytoplasm at stationary state. After activation, lipid droplets in the rat pancreatic stellate cells disappear, and α-SMA is expressed. Meanwhile, the synthesis and secretion of collagen increase. These substances are deposited around the pancreatic lobules and acinar cells, generating fibrosis.12,13,14,15 In this study, α-SMA showed positive expression in both Model and Matrine groups, indicating the activation of pancreatic tissues in both groups. However, the expression of α-SMA in the Matrine group was significantly lower than that in the Model group, suggesting matrine might act as an inhibitor in the early activation stage of pancreatic stellate cells in rats to prevent the formation of pancreatic fibrosis by reducing the activation and proliferation thereof.
TGF-β family is composed of TGF-β subtypes, activator/repressor, Mullerrian inhibiting substance, and bone morphogenetic proteins, etc.16 The binding of TGF-β and receptor can initiate signal transduction. TβR1 and TβR2 are found on the surface of cell membranes, acting as a serine/threonine protein kinase.17 The TGF-β family first binds to and activates TβR2, which then binds to TβR1 to form a complex and to activate the receptors via phosphorylation.18 Smad protein is one type of TβR1 and plays a crucial role in the TGF-β signal pathway.19 TGF-β1 recognizes and binds to TβR2 on the cell membrane. After phosphorylation, TβR2 induces the phosphorylation of the GS domain of TβR1 and subsequently activates TβR1, and it can bind to TGF-β1 at the same time to form a heterotetramer complex.20
Matrine is an alkaloid extracted from sophora flavescens and exerts an anti-fibrosis effect in multiple organs of the body in a variety of ways.21,22 Many studies have shown that matrine can reduce the levels of TGF-β1 and TNF-α in the serum of chronic hepatitis B patients, thereby inhibiting the activation of hepatic stellate cells, decreasing the release of extracellular matrix, and ultimately achieving an anti-hepatic fibrosis effect.23,24,25 In animal experiments of pulmonary fibrosis induced by bleomycin in mice, matrine was capable of reducing the expression of IL-6, TNF-α, and TGF-β1 in lung tissues and played an anti-fibrosis role through TGF-β/Smad signaling.26 In this study, the expression of Smad2, TβR1, and TβR2 were significantly upregulated, demonstrating that trinitrobenzene sulfonic acid induced pancreatic fibrosis in rats through TGF-β/Smad signaling. Interestingly, the expression of Smad2, TβR1, and TβR2 in the Matrine group was significantly lower than that in the Model group. One mechanism of anti-pancreatic fibrosis might be that matrine reduces the expression of Smad2 by downregulating the expression of α-SMA, TGF-β1, and collagen.
In addition, oxymatrine had been found to inhibit the growth of human hypertrophic scar fibroblasts by inhibiting the TGF-β/Smad signal pathway and thereby inhibiting collagen synthesis and the contractile function of cells.27 Matrine was reported to suppress cardiac fibrosis and improve cardiac function by inhibiting the TGF-β/Smad signal pathway.28,29 From these previous reports and the present results, matrine may suppress fibrosis, such as scar fibrosis, cardiac fibrosis and pancreatic fibrosis, by inhibiting the TGF-β/Smad signal pathway.
In conclusion, matrine exerted its anti-pancreatic fibrosis effect by regulating the TGF-β/Smad signal pathway in pancreatic stellate cells of rats. Matrine might inhibit the activation of pancreatic stellate cells via downregulating the expression of TGF-β1 and Smad2, consequently suppressing the development and progression of pancreatic fibrosis. These results might provide a reliable and solid theoretical basis for matrine-related drug development and clinical application of matrine in treatment of pancreatic fibrosis.
ACKNOWLEDGEMENTS
This work was supported by financial support from the Jiangxi Provincial Education Department of China (GJJ10055).
Footnotes
The authors have no potential conflicts of interest to disclose.
References
- 1.Xue J, Sharma V, Hsieh MH, Chawla A, Murali R, Pandol SJ, et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun. 2015;6:7158. doi: 10.1038/ncomms8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka K, Tomita H, Osada S, Watanabe H, Imai H, Sasaki Y, et al. Significance of histopathological evaluation of pancreatic fibrosis to predict postoperative course after pancreatic surgery. Anticancer Res. 2015;35:1749–1756. [PubMed] [Google Scholar]
- 3.Lu X, Chen Y. Pathologic assessment of pancreatic fibrosis in predicting pancreatic fistula and management of prophylactic drain removal after pancreaticoduodenectomy. World J Surg. 2016;40:1520–1521. doi: 10.1007/s00268-015-3301-4. [DOI] [PubMed] [Google Scholar]
- 4.Nagathihalli NS, Castellanos JA, VanSaun MN, Dai X, Ambrose M, Guo Q, et al. Pancreatic stellate cell secreted IL-6 stimulates STAT3 dependent invasiveness of pancreatic intraepithelial neoplasia and cancer cells. Oncotarget. 2016;7:65982–65992. doi: 10.18632/oncotarget.11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tjomsland V, Sandnes D, Pomianowska E, Cizmovic ST, Aasrum M, Brusevold IJ, et al. The TGFβ-SMAD3 pathway inhibits IL-1α induced interactions between human pancreatic stellate cells and pancreatic carcinoma cells and restricts cancer cell migration. J Exp Clin Cancer Res. 2016;35:122. doi: 10.1186/s13046-016-0400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tjomsland V, Pomianowska E, Aasrum M, Sandnes D, Verbeke CS, Gladhaug IP. Profile of MMP and TIMP expression in human pancreatic stellate cells: regulation by IL-1α and TGFβ and implications for migration of pancreatic cancer cells. Neoplasia. 2016;18:447–456. doi: 10.1016/j.neo.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu L, Zheng N, He Q, Li R, Zhang K, Liang T. Puerarin, isolated from Pueraria lobata (Willd.), protects against hepatotoxicity via specific inhibition of the TGF-β1/Smad signaling pathway, thereby leading to anti-fibrotic effect. Phytomedicine. 2013;20:1172–1179. doi: 10.1016/j.phymed.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Zhang YJ, Chan J, Xiang MX, Cheng G, Wang SS. Effect of matrine on fibrosis of hypertrophy myocardium induced by pressure overload. Bull Sci Technol. 2007;23:67–71. [Google Scholar]
- 9.Gao HY, Li GY, Lou MM, Li XY, Wei XY, Wang JH. Hepatoprotective effect of Matrine salvianolic acid B salt on carbon tetrachloride-induced hepatic fibrosis. J Inflamm (Lond) 2012;9:16. doi: 10.1186/1476-9255-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang HW, Jin Y. [Renoprotective effects of matrine on experimental glomerulosclerosis in rats] Zhonghua Er Ke Za Zhi. 2004;42:737–740. [PubMed] [Google Scholar]
- 11.Yuan X, Sun H, Miao R, Zhang Z, Zhao X, Guo Y, et al. Effect of matrine on NF-κB and collagen protein III of rats with pulmonary fibrosis. J Xinxiang Med College. 2009;26:327–330. [Google Scholar]
- 12.Bikádi P, Szabó J, Szabára Á, Jakab C. Internal positive controls of alpha-smooth muscle actin (α-SMA) in bovine tissues Immunohistochemical study. Magyar Allatorvosok Lapja. 2015;137:151–158. [Google Scholar]
- 13.Meng LP, Wang GG, Hu MX, Zhang H, Guan B, Hong YP. The correlation of magnetic resonance perfusion parameters and CD34, a-SMA of liver fibrosis and cirrhosis in rats. Radiol Pract. 2016;31:580–585. [Google Scholar]
- 14.Hu GX, Wan ZY, Shao JL, Zhang Y, Zhang LL, Gong ZJ. [Effects of hydroxycamptothecin on TGFb1, a-SMA and collagen I expression in rat hepatic satellite cells] Zhonghua Gan Zang Bing Za Zhi. 2012;20:453–457. doi: 10.3760/cma.j.issn.1007-3418.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 15.De Jesus Araújo L, Yamamoto De Almeida L, Santos Lima J, Martelli-Júnior H, Ferreti Bonan PR. Evaluation of MMP-1, MMP-10, TIMP-1, a-SMA and TGF-b1 in angiofibromas of tuberous sclerosis. Minerva Stomatol. 2011;60:25–33. [PubMed] [Google Scholar]
- 16.David CJ, Huang YH, Chen M, Su J, Zou Y, Bardeesy N, et al. TGF-β tumor suppression through a lethal EMT. Cell. 2016;164:1015–1030. doi: 10.1016/j.cell.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Kozono DE, O'Connor KW, Vidal-Cardenas S, Rousseau A, Hamilton A, et al. TGF-β inhibition rescues hematopoietic stem cell defects and bone marrow failure in Fanconi anemia. Cell Stem Cell. 2016;18:668–681. doi: 10.1016/j.stem.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan X, Liao H, Cheng M, Shi X, Lin X, Feng XH, et al. Smad7 protein interacts with receptor-regulated Smads (R-Smads) to inhibit transforming growth factor-β (TGF-β)/Smad signaling. J Biol Chem. 2016;291:382–392. doi: 10.1074/jbc.M115.694281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Da C, Liu Y, Zhan Y, Liu K, Wang R. Nobiletin inhibits epithelialmesenchymal transition of human non-small cell lung cancer cells by antagonizing the TGF-β1/Smad3 signaling pathway. Oncol Rep. 2016;35:2767–2774. doi: 10.3892/or.2016.4661. [DOI] [PubMed] [Google Scholar]
- 20.Ma M, He M, Jiang Q, Yan Y, Guan S, Zhang J, et al. MiR-487a promotes TGF-β1-induced EMT, the migration and invasion of breast cancer cells by directly targeting MAGI2. Int J Biol Sci. 2016;12:397–408. doi: 10.7150/ijbs.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang YB, Zhan LQ, Li GQ, Wang F, Wang Y, Li YL, et al. Dimeric matrine-type alkaloids from the roots of Sophora flavescens and their anti-epatitis B virus activities. J Org Chem. 2016;81:6273–6280. doi: 10.1021/acs.joc.6b00804. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Wu Y, Deng L, Chen L, Zhao D, Lv L, et al. The alkaloid matrine of the root of Sophora flavescens prevents arrhythmogenic effect of ouabain. Phytomedicine. 2014;21:931–935. doi: 10.1016/j.phymed.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Sun N, Sun P, Lv H, Sun Y, Guo J, Wang Z, et al. Matrine displayed antiviral activity in porcine alveolar macrophages co-infected by porcine reproductive and respiratory syndrome virus and porcine circovirus type 2. Sci Rep. 2016;6:24401. doi: 10.1038/srep24401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pu J, Fang FF, Li XQ, Shu ZH, Jiang YP, Han T, et al. Matrine exerts a strong anti-arthritic effect on type II collagen-induced arthritis in rats by inhibiting inflammatory responses. Int J Mol Sci. 2016;17:1410. doi: 10.3390/ijms17091410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Yang Y, Xu J, Jiang X, Wang J. GW27-e1128 Matrine inhibits cardiac fibrosis by inhibiting TGFβ1/smad signaling pathway. J Am Coll Cardiol. 2016;68(16 Suppl):C40. [Google Scholar]
- 26.Mihailova S, Ivanova-Genova E, Lukanov T, Stoyanova V, Milanova V, Naumova E. A study of TNF-α, TGF-β, IL-10, IL-6, and IFN-γ gene polymorphisms in patients with depression. J Neuroimmunol. 2016;293:123–128. doi: 10.1016/j.jneuroim.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Yang P, Wu Z, Huang J, Wang A, Xu S, You P, et al. Regulation of fibroblasts proliferation and function of human hypertrophic scar by Oxymatrine through TGF-β signaling pathway. Chin J Aesth Plast Surg. 2010;21:557–559. [Google Scholar]
- 28.Zhang Y, Cui L, Guan G, Wang J, Qiu C, Yang T, et al. Matrine suppresses cardiac fibrosis by inhibiting the TGF-β/Smad pathway in experimental diabetic cardiomyopathy. Mol Med Rep. 2018;17:1775–1781. doi: 10.3892/mmr.2017.8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao N, Pan S, Zhang Y, Liu X, Guan G, Wang J, et al. TGF-β/ Smads signal pathway is involved in the anti-fibrotic effect of matrine and improvement of the cardiac function in the diabetic cardiomyopathy rats. J Shanxi Med Univ. 2015;13:312–327. [Google Scholar]