Abstract
Knowing the extent to which a clinical trial's findings translate into clinical practice can be challenging. One practical approach to estimating a trial's influence on clinical practice can be achieved by assessing how the trial informed relevant clinical practice guidelines (CPGs). Accordingly, the objectives of this study were to provide an overview of all the clinical trials involving the Department of Veterans Affairs (VA) Cooperative Studies Program (CSP) that aimed at informing or resulted in informing the management of high blood pressure and to identify and describe the extent to which these trials informed CPGs for the management of high blood pressure. A total of 26 clinical trials involving the VA CSP were identified. Using bibliographic information, 21 CPGs for the management of hypertension representing over 40 years of treatment recommendations from eight collectives were evaluated to determine how they were informed by trials involving the VA CSP. From 1977 to 2018, 13 of the 26 trials (50.0%) were found to have informed 19 of the 21 CPGs (90.5%) a total of 54 times (mean = 2.6 trial citations per CPG, SD ± 1.8). Clinical trials involving the VA CSP have informed a sizeable proportion of CPGs for the management of high blood pressure over the past 40 years. Because of this impact on the CPGs, these trials are also likely to have had at least moderate influence on clinical practice.
Keywords: Clinical trials, Clinical impact, Implementation, Clinical practice guidelines, Hypertension
1. Introduction
Many clinical trials are conducted with the intent of influencing clinical practice; however, knowing the extent to which a clinical trial has achieved this intent is difficult. Given the varying and complex ways that research findings can influence clinical practice, there is no consensus regarding the best indicator or approach to demonstrate this type of impact [1,2]. Furthermore, clinical trial stakeholders may greatly desire to know if a clinical trial has appropriately influenced clinical practice as this knowledge can be helpful in demonstrating accountability and value from research, justifying the investment in research, identifying next steps, and executing future research that has a greater likelihood of influencing clinical practice. One practical approach to estimating influence on clinical practice involves evaluating bibliometric data to determine the extent to which a study informed the synthesized literature base, such as clinical practice guidelines (CPGs) [2,3]. Although this approach is limited to evaluating an intermediate outcome for influence on clinical practice instead of directly evaluating the full clinical impact realized from the clinical trial, it can complement other means used to assess impact. Specifically, this approach serves to document a key step along the translational research timeline and contributes to the body of evidence supporting that a clinical trial has likely achieved the intent of influencing clinical practice.
The US Department of Veterans Affairs (VA) Cooperative Studies Program (CSP) is a research infrastructure dedicated to fulfilling the VA's health care mission by generating definitive answers to vital clinical questions through the conduct of multicenter clinical trials [4]. The VA CSP has investigated treatments for a variety of conditions, including psychiatric disorders, neurologic disorders, infectious diseases, and cardiovascular disease [5]. While the VA CSP has sponsored and supported many trials to improve the management of high blood pressure, the extent to which this body of research has influenced the clinical management of hypertension has not been assessed. Since CPGs for the management of hypertension have a long history and since there is some evidence of their successful influence on clinical practice [6,7], it is suitable to assess the extent to which these clinical trials have informed the CPGs and to use this assessment to estimate their impact on clinical practice. The objectives of this study were to provide an overview of all the clinical trials involving the VA CSP that aimed at informing or resulted in informing hypertension management and to identify and describe the extent to which these trials informed CPGs for the management of hypertension.
2. Methods
A comprehensive list of hypertension management CPGs was identified in order to represent CPGs from different periods of time and from the most influential guideline-producing bodies. All the guidelines from the Joint National Committee (JNC) on Detection, Evaluation, and Treatment of High Blood Pressure were included to provide a representation spanning the entire era of hypertension guidelines. To provide representation of guidelines from other frequently utilized sources in more recent time, up to two of the most recent guidelines published in the last two decades were selected from the following collectives: the American Diabetes Association (ADA), the American Heart Association (AHA)/American College of Cardiology (ACC), the American Society of Hypertension (ASH)/International Society of Hypertension (ISH), the British Hypertension Society (BHS)/the National Institute for Health and Clinical Excellence (NICE), the European Society of Cardiology (ESC)/European Society of Hypertension (ESH), the Kidney Disease Improving Global Outcomes (KDIGO)/Kidney Disease Outcomes Quality Initiative (KDOQI), and the Department of Veterans Affairs (VA)/Department of Defense (DoD). In addition to including the two most recent position statements regarding diabetes and hypertension from the ADA, the most recent edition of the Standards of Medical Care in Diabetes was also included.
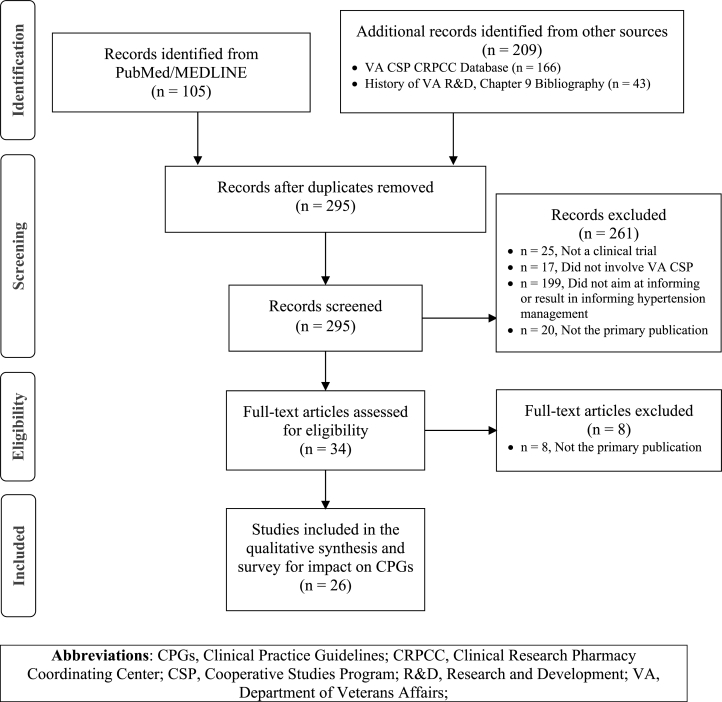
Eligible studies were identified from the following sources: (1) an internal database maintained by the VA CSP Clinical Research Pharmacy Coordinating Center containing bibliographic information for all the clinical trials involving the VA CSP since 1975; (2) the bibliography for a text describing the early history of hypertension research conducted by the VA's Research & Development [8]; and (3) search results from the PubMed/MEDLINE database using the terms “(Veterans[All Fields] AND Cooperative[All Fields] AND (“Blood Pressure”[All Fields] OR Hypertension[All Fields] OR Antihypertensive[All Fields] OR Vasodilator[All Fields])) AND Clinical Trial[ptyp]“. The data from these three sources were combined, and duplicate references were removed. Abstracts, and when needed full-text publications, were screened and reviewed to identify eligible clinical trials (Fig. 1 [9]).
Fig. 1.
PRISMA flow diagram [9] for the process of identifying the clinical trials involving the department of veterans affairs cooperative studies program that aimed at informing or resulted in informing high blood pressure management.
A study was eligible for inclusion by meeting the following conditions: (1) it was a randomized clinical trial; (2) it involved the VA CSP as indicated by VA CSP sponsorship (or it was a “Cooperative Study” performed by the VA before the formal reorganization of the VA CSP in 1972) or by VA CSP collaboration to provide funding, distribution of study interventions, and/or coordination of study sites; and (3) it was aimed at informing or resulted in informing high blood pressure management evidenced by using an intervention or primary outcome that addressed hypertension or by being cited by a CPG when making a recommendation for hypertension management. Finally, studies meeting the inclusion criteria were then excluded if they were not the main publication for the study. A publication was considered to be the main publication if it reported the final results for the trial's primary outcome. The main characteristics for each trial found eligible for this review were summarized. Additionally, support of a trial by a pharmaceutical company was ascertained by identifying any acknowledgement listed in the main publication describing material support, funding, or grants received from a for-profit corporation.
For the purposes of this study, a clinical trial was considered to have informed a CPG when it was referenced by a CPG while making or describing a recommendation for the management of hypertension. The bibliographies from each CPG were searched for reference to the titles of the included trials. When a reference to a trial was found within a CPG, the usage of the reference in the CPG was reviewed to confirm that its purpose was to support or describe a recommendation. Each trial that was found to be referenced by a CPG was summarized in text, and the nature of its use in that guideline was described. The total number of instances that these trials were found to have informed the CPGs was summed. This count was then divided by the total number of CPGs and by the total number of trials that informed CPGs to estimate the average number of trial citations per CPG and the average number of CPG citations per influential trial, respectively.
3. Results
3.1. Summary
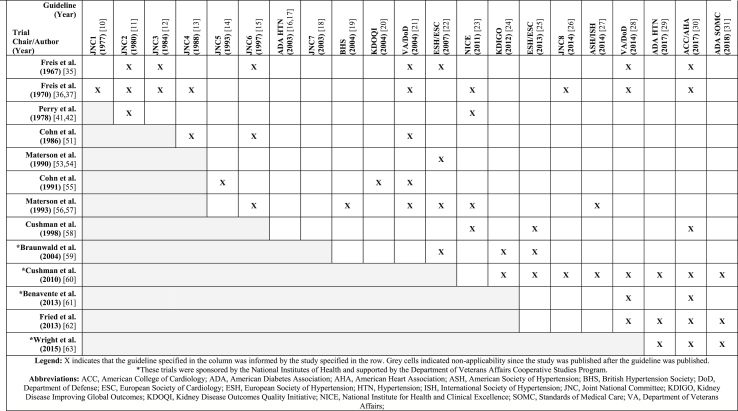
The 21 hypertension CPGs that were evaluated in this study are listed in Table 1 [[10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]]. These guidelines span a period of over 40 years and represent recommendations from at least eight distinct collectives. Since the early 1960s, there have been 26 clinical trials involving the VA CSP which aimed at informing or resulted in informing hypertension management (Fig. 1 [9]). The main characteristics of these trials are summarized in Table 2 [[32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]]. A total of 22 trials were sponsored by the VA CSP while four trials were sponsored by the National Institutes of Health (NIH) and utilized VA CSP collaboration. There were eleven trials that received support, funding, or grants from a pharmaceutical company. Of the 26 trials, 13 (50.0%) were found to have informed 19 (90.5%) of the 21 CPGs included in this review. The number of instances these trials informed the CPGs is portrayed in Table 3 and is described further for each of these trials in section 3.2 [[10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31],[35], [36], [37],41,42,51,[53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]]. From 1977 to 2018, these 13 clinical trials were cited by 19 CPGs a total of 54 times, resulting in an average of 2.6 trial citations per CPG (SD ± 1.8) or an average of 4.2 CPG citations per influential trial (SD ± 2.5). Nine of these 13 trials were sponsored by the VA CSP while the remaining four were sponsored by the NIH and supported by the VA CSP. Finally, six of the 13 trials that informed a CPG received support from a pharmaceutical company (46.2%), and five of the 13 trials that did not inform a CPG received support from a pharmaceutical company (38.5%).
Table 1.
Clinical practice guidelines for the management of high blood pressure, ordered chronologically.
| Year | Guideline Title and Source | Abbreviation |
|---|---|---|
| 1977 | Report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure [10]. | JNC1 |
| 1980 | The 1980 report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure [11]. | JNC2 |
| 1984 | The 1984 Report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure [12]. | JNC3 |
| 1988 | The 1988 report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure [13]. | JNC4 |
| 1993 | The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V) [14]. | JNC5 |
| 1997 | The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure [15]. | JNC6 |
| 2003 | Treatment of hypertension in adults with diabetes. The American Diabetes Association [16,17]. | ADA 2003 |
| 2003 | The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report [18]. | JNC7 |
| 2004 | Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004-BHS IV [19]. | BHS |
| 2004 | K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease [20]. | KDOQI |
| 2004 | VA/DoD Clinical Practice Guideline For Diagnosis and Management of Hypertension in the Primary Care Setting, Version 2.0b- 2004 [21]. | VA/DoD 2004 |
| 2007 | 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) [22]. | ESH/ESC 2007 |
| 2011 | Hypertension: The Clinical Management of Primary Hypertension in Adults: Update of Clinical Guidelines 18 and 34. The National Institute for Health and Clinical Excellence [23]. | NICE |
| 2012 | KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease [24]. | KDIGO |
| 2013 | 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) [25]. | ESH/ESC 2013 |
| 2014 | 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) [26]. | JNC8 |
| 2014 | Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension [27]. | ASH/ISH |
| 2014 | VA/DoD Clinical Practice Guideline For Diagnosis and Management of Hypertension in the Primary Care Setting, Version 3.0–2014 [28]. | VA/DoD 2014 |
| 2017 | Diabetes and Hypertension: A Position Statement by the American Diabetes Association [29]. | ADA HTN 2017 |
| 2017 | 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [30]. | ACC/AHA |
| 2018 | Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2018. The American Diabetes Association [31]. | ADA SOMC 2018 |
Table 2.
Main characteristics of the clinical trials involving the department of veterans affairs cooperative studies program that aimed at informing or resulted in informing the management of high blood pressure.
| Chair/Author (Year) | Guideline Reference | Industry Support |
n | Design | Population | Intervention/Comparator | Primary Outcome | Primary Results |
|---|---|---|---|---|---|---|---|---|
| Freis et al. (1962) [32,33] | N | N | 426 | R, DB, PC | DBP > 90 mm Hg | Mild & Moderate: Reserpine ± Hydralazine or Placebo Moderate & Severe: Reserpine + Ganglion Blocking Agent |
Mean change in SBP/DBP from baseline to 12 months | Placebo: ↑ by 3.7/2.0 mm Hg Reserpine: ↓ by 3.0/5.4 mm Hg Reserpine + Hydralazine: ↓ by 4.9/10.5 mm Hg Reserpine + Ganglion Blocking Agent: ↓ equally across all agents by 15.5/13 mm Hg |
| Freis et al. (1962) [34] | N | N | 411 | R, DB, PC | DBP > 90 mm Hg | Mild & Moderate: Chlorothiazide ± Hydralazine ± Reserpine or Placebo Moderate & Severe: Chlorothiazide + Reserpine + (Hydralazine or Cryptenamine or Ganglion Blocking Agent) |
Mean change in SBP/DBP from baseline to 3 months | Placebo: BP unchanged Chlorothiazide: ↓ by 7.9/3.8 mm Hg Chlorothiazide + Hydralazine: ↓ by 10.2/11.8 mm Hg Chlorothiazide + Reserpine: ↓ by 14/11 mm Hg Chlorothiazide + Reserpine + Hydralazine: ↓ by 16/14.3 to 22.7/20.9 mm Hg Chlorothiazide + Reserpine + Cryptenamine: ↓ by 17.9/16.4 mm Hg Chlorothiazide + Reserpine + Ganglion Blocking Agent: ↓ by 29/23 mm Hg |
| Freis et al. (1967) [35] | Y | N | 143 | R, DB, PC, RI | Male, DBP 115–129 mm Hg (untreated) | HCTZ + Reserpine + Hydralazine vs. Placebo | Cardiovascular mortality and morbidity after a mean of 20 months | Significant ↓ in cardiovascular mortality and morbidity among patients receiving antihypertensive agents when compared to placebo |
| Freis et al. (1970) [36,37] | Y | N | 380 | R, DB, PC, RI | Male, DBP 90–114 mm Hg (untreated) | HCTZ + Reserpine + Hydralazine vs. Placebo | Cardiovascular mortality and morbidity after a mean of 3.2 years | Significant ↓ in cardiovascular mortality and morbidity among patients with moderate HTN (DBP 105–114 mm Hg) receiving antihypertensive agents when compared to placebo |
| Freis et al. (1975) [38] | N | N | 86 | R, DB, PC | Male, DBP < 95 mm Hg (treated), on antihypertensive treatment for 2 years | Discontinue prior treatment vs. Continue treatment with HCTZ + Reserpine + Hydralazine | DBP >95 mm Hg or cardiovascular morbidity after 72 weeks | Incidence of DBP >95 mm Hg or cardiovascular mortality was significantly greater after antihypertensive discontinuation (85%) compared to continuing antihypertensive treatment (4%) |
| Ramirez et al. (1977) [39] | N | N | 108 | R, DB, RI | Male, Age < 60 years, DBP 100–124 mm Hg (treated) | Adjunctive Guanethidine vs. Adjunctive Bethanidine | DBP <90 mm Hg after 6 months | Significantly more patients on guanethidine (68.8%) achieved DBP < 90 mm Hg compared to bethanidine (45.5%) |
| Thomas et al. (1977) [40] | N | N | 450 | R, DB, AC, RI | Male, Age < 60 years, DBP 90–114 mm Hg (untreated) |
Propranolol ± HCTZ ± Hydralazine vs. Reserpine + HCTZ | DBP <90 mm Hg after 6 months | Compared to Reserpine + HCTZ (88%): Significantly fewer patients on Propranolol Alone (52%) or Propranolol + Hydralazine (72%) achieved DBP < 90 mm Hg, and no significant difference in the proportion of patients taking Propranolol + Hydralazine + HCTZ (92%) or Propranolol + HCTZ (81%) who achieved DBP < 90 mm Hg |
| Perry et al. (1978) [41,42] | Y | N | 1012 | R, DB, PC, Pilot study | Age 21–50 years, DBP 85–105 mm Hg, no prior cardiovascular disease | Chlorthalidone ± Reserpine vs. Placebo | Rates of progression to significant HTN, cardiovascular morbidity, and side effects | Compared to placebo, treatment appeared to ↓ progression to significant HTN, ↑ cardiovascular morbidity, and ↑ occurrence of side effects (significance not reported) |
| Freis et al. (1979) [43] | N | Y | 240 | R, DB, AC, RI | Male, age < 65 years, DBP 95–114 mm Hg (untreated) | Ticrynafen vs. HCTZ | Mean change in BP and serum uric acid after 6 weeks | No significant difference in BP reductions between treatments Ticrynafen significantly ↓ serum uric acid compared to HCTZ |
| Thomas et al. (1981) [44] | N | N | 211 | R, DB, AC, RI | Male, age < 65 years, DBP 95–114 mm Hg (untreated) | Propranolol + HCTZ vs. Oxprenolol + HCTZ | DBP <90 mm Hg after 6 months | Significantly more patients on propranolol (50%) achieved DBP < 90 mm Hg compared to oxprenolol (27%) |
| Ramirez et al. (1981) [45] | N | N | 198 | R, DB, RI | Male, age < 75 years, DBP 95–114 mm Hg (untreated) | Prazosin + HCTZ vs. Hydralazine + HCTZ | DBP <90 mm Hg and mean HR after 6 months | No significant difference in patients on prazosin (44.6%) achieving DBP < 90 mm Hg compared to hydralazine (39.6%) No significant difference in mean HR between treatments |
| Freis et al. (1982) [46,47] | N | Y | 683 | R, DB, RI | Male, age ≤ 65 years, DBP 95–114 mm Hg (untreated) | HCTZ vs. Propranolol | Mean change in SBP/DBP and number of discontinuations after a 10-week titration | BP reduction from HCTZ (−18.1/-12.0 mm Hg) was significantly greater than BP reduction from propranolol (−10.4/-10.8 mm Hg), especially in African Americans No significant difference in number of discontinuations |
| Thomas et al. (1982) [48] | N | Y | 311 | R, DB, RI | Male, age < 70 years, DBP 90–114 mm Hg (treated) | Low dose Reserpine + Chlorthalidonevs. Standard dose Reserpine + Chlorthalidone | DBP <90 mm Hg and side effects after12 weeks | No significant difference in percentage of patients with DBP <90 mm Hg or patients experiencing side effects in low dose compared to standard dose reserpine |
| Freis et al. (1983) [49] | N | Y | 308 | R, DB, RI | Male, age < 70 years, DBP 95–114 mm Hg (untreated) |
Nadolol vs. Bendroflumethiazide vs. Nadolol + Bendroflumethiazide | DBP <90 mm Hg after 12 weeks | Significantly more patients on the nadolol + bendroflumethiazide combination (85%) achieved DBP < 90 mm Hg compared to nadolol alone (49%) or bendroflumethiazide alone (46%) |
| Materson et al. (1984) [50] | N | Y | 363 | R, DB, PC, RI | Male, age < 70 years, DBP 92–109 mm Hg (untreated) | Captopril (4 doses) ± HCTZ vs. Placebo ± HCTZ | Mean change in BP after 14 weeks | Significantly greater BP reductions in patients taking the captopril + HCTZ combination compared to either agent alone Higher doses of captopril did not result in significantly different BP reductions compared to lower doses of captopril |
| Cohn et al. (1986) [51] | Y | N | 642 | R, DB, PC | Stable chronic congestive heart failure, male, age ≤ 75 years, treatment with digoxin and diuretics | Hydralazine + Isosorbide Dinitrate vs. Prazosin vs. Placebo |
Overall mortality after a mean follow-up of 2.3 years | Non-significant trend for lower mortality in patients taking hydralazine and isosorbide dinitrate (38.7%) compared to placebo (44.0%) No significant difference in mortality for patients taking prazosin (49.7%) compared to placebo (44.0%) |
| Freis et al. (1989) [52] | N | N | 606 | R, DB, PC, RI | DBP < 115 mm Hg (treated) | Antihypertensive Titration followed by Discontinuation vs. Dose Reduction vs. No Change | DBP <90 mm Hg after 6 months | Significantly fewer patients discontinuing therapy maintained DBP <90 mm Hg compared to patients continuing therapy No significant difference in percent of patients with a dose reduction maintaining DBP <90 mm Hg compared to patients continuing therapy |
| Materson et al. (1990) [53,54] | Y | Y | 672 | R, DB, RI | Male, age > 60 years, DBP 90–114 mm Hg (untreated), DBP < 110 mm Hg (treated) | HCTZ 50 mg vs. HCTZ 100 mg THEN Hydralazine vs. Methyldopa vs. Metoprolol vs. Reserpine |
DBP <90 mm Hg after 6 and 12 months | No significant difference in patients on HCTZ 50 mg/day achieving DBP <90 mm Hg compared to patients on HCTZ 100 mg/day No significant difference in patients achieving DBP <90 mm Hg between 2nd-line treatments |
| Cohn et al. (1991) [55] | Y | N | 804 | R, DB, AC | Stable chronic congestive heart failure, male, age ≤ 75 years, treated with digoxin and diuretics | Enalapril vs. Hydralazine + Isosorbide Dinitrate | Overall mortality after a mean follow-up of 2.5 years | Non-significant trend for lower mortality in patients taking enalapril (32.8%) compared to hydralazine and isosorbide dinitrate (38.2%) |
| Materson et al. (1993) [56,57] | Y | Y | 1292 | R, DB, PC | Male, age > 21 years, DBP 95–109 mm Hg (untreated) | HCTZ vs. Atenolol vs. Clonidine vs. Captopril vs. Prazosin vs. Diltiazem SR vs. Placebo | DBP <95 mm Hg after 1 year | Significantly fewer patients taking Placebo (31%) achieved DBP < 95 mm Hg compared to Diltiazem SR (72%), Clonidine (62%), Atenolol (60%), HCTZ (55%), Prazosin (54%), and Captopril (50%) |
| Cushman et al. (1998) [58] | Y | N | 641 | R, OL | Nondependent moderate-to-heavy alcohol consumption, DBP 80–99 mm Hg | Cognitive-Behavior Program to Reduce Alcohol Consumption Vs. No Intervention |
Mean difference in BP after 6 months | No significant difference in SBP/DBP reductions in patients receiving cognitive-behavior program compared to patients receiving no intervention (−1.2/-0.7 mm Hg; p-values > 0.05). |
| *Braunwald et al. (2004) [59] | Y | Y | 8290 | R, DB, PC, RI | Coronary artery disease, age ≥ 50 years, left ventricular ejection fraction > 40% | Trandolapril vs. Placebo | Rate of nonfatal myocardial infarction, cardiac death, or revascularization after a median follow-up of 4.8 years | No significant difference in the rate of cardiovascular events with trandolapril (21.9%) compared to placebo (22.5%): hazard ratio of 0.96 (95% confidence interval of 0.88–1.06) |
| *Cushman et al. (2010) [60] | Y | Y | 4773 | R, OL | Type 2 diabetes, age 40–79 years, increased risk for cardiovascular events | Intensive-treatment (SBP < 120 mm Hg) vs. Standard-treatment (SBP < 140 mm Hg) | Rate of major cardiovascular events after a mean follow-up of 4.7 years | No significant difference in the rate of major cardiovascular events with intensive-treatment (1.87%/year) compared to standard-treatment (2.09%/year): hazard ratio of 0.88 (95% confidence interval of 0.73–1.06) |
| *Benavente et al. (2013) [61] | Y | N | 3020 | R, OL | Age > 30 years, recent lacunar stroke | Lower-target BP(SBP < 130 mm Hg) vs. Higher-target BP (SBP 130–149 mm Hg) | Rate of all strokes after a mean follow-up of 3.7 years | No significant difference in the rate of all strokes with lower-target BP (2.25%/year) compared to higher-target BP (2.77%/year): hazard ratio of 0.81 (95% confidence interval of 0.64–1.03) |
| Fried et al. (2013) [62] | Y | Y | 1448 | R, DB, PC, RI | Type 2 diabetes, glomerular filtration rate 30–89.9 mL/min/1.73m2, urinary albumin-to-creatinine ratio > 300 | Losartan + Placebo (Monotherapy) vs. Losartan + Lisinopril (Combination-Therapy) | Rate of glomerular filtration rate decline, development of end-stage renal disease, and death after a median follow-up of 2.2 years | No significant difference in rate of primary outcome with monotherapy (10.8 events per 100 person-years) compared to combination therapy (9.5 events per 100 person-years): hazard ratio 0.88 (95% confidence interval of 0.70–1.12) |
| *Wright et al. (2015) [63] | Y | Y | 9361 | R, OL | Age > 50 years, SBP 130–180 mm Hg, increased risk for cardiovascular events | Intensive therapy (SBP < 120 mm Hg) vs. Standard therapy (SBP < 140 mm Hg) | Rate of major cardiovascular events after a median follow-up of 3.26 years | Significantly lower rate of major cardiovascular events with intensive-treatment (1.65%/year) compared to standard-treatment (2.19%/year): hazard ratio of 0.75 (95% confidence interval of 0.64–0.89) |
*These trials were sponsored by the National Institutes of Health and supported by the Department of Veterans Affairs Cooperative Studies Program.
Abbreviations: AC, Active-Controlled; BP, Blood Pressure; ↓, Decrease; DB, Double-Blind; DBP, Diastolic Blood Pressure; HCTZ, Hydrochlorothiazide; Hg, Mercury, HR, Heart Rate; ↑, Increase; Industry Support, Supported, Funded, or Received a Grant from a For-Profit Corporation; m, Meters; min, Minutes; mL, Milliliters; mm, Millimeters; n, Sample Size; N, No; OL, Open-Label; PC, Placebo-Controlled; R, Randomized; RI, Run-in Phase; SBP, Systolic Blood Pressure; SR, Sustained-Release; Y, Yes.
Table 3.
Instances when clinical practice guidelines for the management of high blood pressure were informed by clinical trials involving the department of veterans affairs cooperative studies program, ordered chronologically.
3.2. Clinical trials that informed the CPGs
3.2.1. Freis et al., 1967 [35].
This randomized, double-blind, placebo-controlled trial evaluated the effectiveness of antihypertensive agents in preventing cardiovascular morbidity and mortality. After completing a run-in period evaluating adherence to placebo, 143 males with untreated diastolic blood pressures averaging 115–129 mm mercury (mm Hg) were assigned to receive either placebo or a combination of hydrochlorothiazide, reserpine, and hydralazine. After an average follow-up of 15.7 months for the placebo-treated patients and 20.7 months for the active-treated patients, significantly fewer morbid events and deaths occurred in the active-treated patients (two morbid events and zero deaths) compared to the placebo-treated patients (27 morbid events and four deaths).
The findings of this study informed seven of the CPGs, starting in 1980 and continuing into 2017 [11,12,15,21,22,28,30]. The results of this trial were most frequently used by guidelines when describing the benefits of treating moderate and severe hypertension and when justifying recommendations for pharmacologic treatment of hypertension for patients with a diastolic blood pressure greater than or equal to 90 mm Hg. Notably, the recent 2017 ACC-AHA hypertension guidelines credited this study and the authors’ subsequent trial published in 1970 with ushering in the era of effective treatment for high blood pressure.
3.2.2. Freis et al., 1970 [36,37].
Following their previous trial published three years earlier [35], the authors released the results of a second similarly-designed trial that was conducted using patients with lower diastolic blood pressures. This was a randomized, double-blind, placebo-controlled trial that evaluated the effectiveness of antihypertensive agents in preventing cardiovascular morbidity and mortality. After completing a run-in period evaluating adherence to placebo, 380 males with untreated diastolic blood pressures averaging 90–114 mm Hg were assigned to receive either placebo or a combination of hydrochlorothiazide, reserpine, and hydralazine. After an average follow-up of 3.9 years for the placebo-treated patients and 3.7 years for the active-treated patients, significantly fewer morbid events and deaths occurred in the active-treated patients (nine morbid events and eight deaths) compared to the placebo-treated patients (35 morbid events and 19 deaths). This trend was most profound in patients with baseline diastolic blood pressures greater than 104 mm Hg. Study findings were also analyzed according to age finding that older patients also benefitted from antihypertensive therapy as exhibited by a reduction of morbidity within this sub-group.
The findings of this study informed nine of the CPGs, starting in 1977 and continuing into 2017 [[10], [11], [12], [13],21,23,26,28,30]. The results of this trial were most frequently used by guidelines when describing the benefits of treating moderate and severe hypertension and when justifying recommendations for pharmacologic treatment of hypertension for patients with a diastolic blood pressure greater than or equal to 90 mm Hg. The findings from the study's analysis on the effect of age were also used to support recommendations for treating elderly patients with antihypertensive medications. Notably, this trial was the only study referenced by the first CPG from the JNC in 1977.
3.2.3. Perry et al., 1978 [41,42].
The VA and the National Heart, Lung, and Blood Institute jointly organized and undertook a randomized, double-blind, placebo-controlled, feasibility trial to understand whether a full-scale definitive trial could recruit and retain patients with mild hypertension. After two-years of recruitment, 1012 patients with diastolic blood pressures between 85 and 105 mm Hg were randomly assigned to receive chlorthalidone with or without reserpine or placebo. Although the main objective of the study was to determine the feasibility of performing a full-scale study, the trial also reported findings on the rate of progression to significant hypertension, cardiovascular morbidity, and antihypertensive side effects. In addition to finding that a full-scale study would be difficult to achieve, the trial also reported that patients given active-treatment appeared to experience less progression to significant hypertension, more cardiovascular morbidity, and more medication side effects. However, as this trial was a feasibility study and was not powered to detect differences in these clinical endpoints, the statistical significance for these findings was not reported.
The findings from this study were cited by two of the CPGs in 1980 and 2011 [11,23]. This trial was used by guidelines when advising providers to weigh the benefits of treating mild hypertension against its potential side effects and when deriving pooled estimates showing the benefit of using thiazide-type diuretics for hypertension.
3.2.4. Cohn et al., 1986 [51].
The first Vasodilator-Heart Failure Trial (V-HeFT I) was a randomized, double-blind, placebo-controlled trial that evaluated the effect of various vasodilators on mortality in chronic heart failure. A total of 642 men with stable chronic congestive heart failure being treated with digoxin and diuretics were randomly assigned to receive hydralazine with isosorbide dinitrate, prazosin, or placebo. After a mean of 2.3 years of follow-up, there were no significant differences between the overall mortality rates in patients taking hydralazine with isosorbide dinitrate (38.7%), prazosin (44.0%), or placebo (49.7%). However, cumulative mortality at two years, which was also considered an important end point, was significantly reduced by 34% in patients taking hydralazine with isosorbide dinitrate compared to placebo.
Despite this trial's focus on investigating the effect of vasodilator use in patients with chronic heart failure, three blood pressure guidelines used the trial findings when making recommendations for blood pressure management in patients with comorbid heart failure [11,15,21]. Specifically, these guidelines recommended using hydralazine with isosorbide dinitrate as a first- or second-line option to control hypertension in patients with less severe heart failure.
3.2.5. Materson et al., 1990 [53,54].
This randomized, double-blind clinical trial evaluated the effect of various antihypertensive regimens on blood pressure changes and cognitive function in elderly patients. After completing a run-in period evaluating adherence to a placebo, 672 males age greater than 60 years with an untreated diastolic blood pressure averaging 90–114 mm Hg were assigned to receive either low- or high-dose hydrochlorothiazide and were then potentially randomized again to receive hydralazine, methyldopa, metoprolol or reserpine. After six to twelve months of treatment, the rates of diastolic blood pressure control as well as cognitive function were significantly improved within all groups when compared to baseline; however, blood pressure control rates and cognitive function did not differ between regimens. These results were discussed in one guideline when reviewing the evidence for the therapeutic management of hypertension and how antihypertensives may affect cognitive function [22].
3.2.6. Cohn et al., 1991 [55].
The second Vasodilator-Heart Failure Trial (V-HeFT II) was a randomized, double-blind, active-controlled trial that evaluated the effect of enalapril on mortality in chronic heart failure. A total of 804 men with stable chronic congestive heart failure being treated with digoxin and diuretics were randomly assigned to receive enalapril or hydralazine with isosorbide dinitrate. After a mean of 2.5 years of follow-up, there was no significant difference between the overall mortality rate in patients taking enalapril (32.8%) or hydralazine with isosorbide dinitrate (38.2%). However, cumulative mortality at two years, which was also considered an important end point, was significantly lowered by 28.2% in patients taking enalapril compared to hydralazine with isosorbide dinitrate.
As in V-HeFT I [51], despite this trial's focus on investigating the effect of vasodilator use in patients with chronic heart failure, three of the CPGs used this trial's findings when making recommendations for blood pressure management in patients with comorbid heart failure [14,20,21]. Specifically, this study's findings were used by guidelines when recommending the first-line use of angiotensin-converting enzyme (ACE) inhibitors for the management of blood pressure in patients with heart failure and when recommending hydralazine with isosorbide dinitrate as a second-line option in this population.
3.2.7. Materson et al., 1993 [56,57].
This randomized, double-blind, placebo-controlled trial evaluated the benefit of six antihypertensive agents representing six distinct classes of antihypertensive agents. A total of 1292 men ages greater than 21 years with an untreated diastolic blood pressure of 95–109 mm Hg received hydrochlorothiazide, atenolol, clonidine, captopril, prazosin, diltiazem, or placebo. After one year, significantly fewer patients taking placebo (31%) achieved a target diastolic blood pressure less than 95 mm Hg compared to diltiazem (72%), clonidine (62%), atenolol (60%), hydrochlorothiazide (55%), prazosin (54%), and captopril (50%). Furthermore, when compared to the other antihypertensive options, significantly more patients taking diltiazem achieved a diastolic blood pressure less than 95 mm Hg. This trial also analyzed data according to age and race. In so doing, the trial found that African American males responded best to diltiazem while Caucasian males responded equally well to all active agents. Finally, this trial indicated that younger patients generally appeared to experience a better response to beta-blockers compared to other agents.
From 1997 to 2014, six guidelines referenced this trial when making treatment recommendations [15,19,[21], [22], [23],27]. These recommendations focused on giving preference to calcium channel blockers for African Americans, considering the use of beta-blockers in younger patients, and considering the need for a second antihypertensive agent to obtain blood pressure targets for most patients.
3.2.8. Cushman et al., 1998 [58].
The Prevention and Treatment of Hypertension Study (PATHS) was jointly sponsored by the NIH and the VA CSP. This randomized, open-label clinical trial evaluated whether blood pressure was reduced by a non-pharmacologic intervention that lowered alcohol consumption. A total of 641 patients with nondependent moderate-to-heavy alcohol consumption and average diastolic blood pressures between 80 and 99 mm Hg were randomly assigned to receive a cognitive-behavioral program focused on reducing alcohol intake or no intervention. While weekly alcohol consumption was significantly lower in the patients receiving the intervention compared to patients receiving no intervention after 6 months (difference = −124 g/week; p-value < 0.001), systolic and diastolic blood pressures were not significantly lower (systolic/diastolic blood pressure difference = −1.2/-0.7 mm Hg; p-values > 0.05).
This trial was used by three CPGs when making recommendations [23,25,30]. Despite finding non-significant benefit in terms of reducing blood pressure, these CPGs cited this trial when discussing the benefit of limiting alcohol consumption to no more than two and one drinks per day for men and women, respectively, as a lifestyle measure to reduce blood pressure.
3.2.9. Braunwald et al., 2004 [59].
The Prevention of Events with Angiotensin Converting Inhibition (PEACE) trial was sponsored by the National Heart, Lung, and Blood Institute and supported by the VA CSP Clinical Research Pharmacy Coordinating Center. In this trial, 8290 patients with stable coronary artery disease, normal or slightly reduced left ventricular function, and ages greater than 50 years were randomly assigned to receive the ACE inhibitor trandolapril or placebo in a double-blind manner. After a median follow-up of 4.8 years, the primary composite end point of death from cardiovascular causes, myocardial infarction, or coronary revascularization was not significantly different between the patients treated with trandolapril and those that received placebo (hazard ratio [HR] = 0.96; 95% Confidence Interval [CI] = 0.88–1.06).
Although this trial investigated an intervention for coronary artery disease, three of the CPGs for blood pressure management cited this trial's findings when making recommendations [22,24,25]. These guidelines recommended that healthcare providers consider the total level of cardiovascular risk when determining the timing of antihypertensive initiation. These findings were also discussed when noting that the evidence for benefit from ACE inhibitors in this population was inconsistent.
3.2.10. Cushman et al., 2010 [60].
The Action to Control Cardiovascular Risk in Diabetes Blood Pressure (ACCORD BP) trial was sponsored by the National Heart, Lung, and Blood Institute and supported by the VA CSP Clinical Research Pharmacy Coordinating Center. In this trial, 4733 patients with type 2 diabetes were randomly assigned to have systolic blood pressure treated to a target of less than 120 mm Hg (intensive therapy) or to a target of less than 140 mm Hg (standard therapy) in a non-blinded manner. After a mean follow-up of 4.7 years, the primary composite end point consisting of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death was not significantly different between intensive or standard therapy (HR = 0.88; 95% CI = 0.73–1.06). While the risk of stroke was significantly reduced by intensive therapy (HR = 0.59; 95% CI = 0.39–0.89), the incidence of serious adverse events attributable to antihypertensive medications was significantly higher with intensive therapy compared to standard therapy (3.3% and 1.27%, respectively; p-value < 0.001).
The results of ACCORD BP informed eight of the CPGs [[24], [25], [26], [27], [28], [29], [30], [31]]. Depending on the CPG, the findings from ACCORD BP were cited when making recommendations indicating that most patients with diabetes should be treated to obtain a systolic blood pressure less than 140 mm Hg or less than 130 mm Hg. Additionally, guidelines used these findings to make recommendations for the use of a similar blood pressure target when treating other populations without diabetes, including patients with chronic kidney disease.
3.2.11. Benavente et al., 2013 [61].
The Secondary Prevention of Small Subcortical Stroke (SPS3) trial was sponsored by the National Institute of Neurologic Disorders and Stroke and supported by the VA CSP Clinical Research Pharmacy Coordinating Center. A total of 3020 patients with recent lacunar stroke were randomly treated to a systolic blood pressure target of 130–149 mm Hg (higher-target) or to a systolic blood pressure target of less than 130 mm Hg (lower-target) in a non-blinded manner. After a mean of 3.7 years, the rate of all strokes was non-significantly lower in patients in the lower-target group compared to the higher-target group (HR = 0.81; 95% CI = 0.64–1.03), and the rates of serious adverse events attributable to study treatment were comparable between the two groups.
The SPS3 trial informed two of the CPGs [28,30]. In one of the CPGs, the findings from SPS3 were discussed when describing the recommendation to treat all patients to a systolic blood pressure goal of less than 150 mm Hg. The other CPG influenced by SPS3 cited the study when recommending that a systolic blood pressure target of less than 130 mm Hg may be a reasonable measure for secondary prevention of lacunar stroke.
3.2.12. Fried et al., 2013 [62].
The Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) study, a randomized, double-blind, placebo-controlled trial, evaluated the effect of combining an ACE Inhibitor with an Angiotensin Receptor Blocker (ARB) in treating diabetic nephropathy. After completing an initial run-in phase to establish tolerance to losartan, 1448 patients with type 2 diabetes mellitus, estimated glomerular filtration rates of 30–89.9 mL per minute per 1.73 m2 of body-surface area, and urinary albumin-to-creatinine ratios of at least 300 received the addition of either lisinopril or placebo. After a median follow-up of 2.2 years, the trial was stopped early after showing significantly higher rates of hyperkalemia (HR = 2.8; 95% CI = 1.8–4.3), significantly higher rates of acute kidney injury (HR = 1.7; 95% CI = 1.3–2.2), and a non-different rate of combined glomerular filtration rate decline, development of end-stage renal disease, and death (HR = 0.88; 95% CI = 0.70–1.12) in patients taking an ARB and an ACE Inhibitor compared to patients taking an ARB and placebo.
Since its publication and despite being conducted in patients with diabetic nephropathy, four hypertension CPGs have made recommendations based on this trial's findings [[28], [29], [30], [31]]. The recommendations in these guidelines supported by VA NEPHRON-D urge caution when treating diabetic patients with a combination of an ACE Inhibitor and an ARB, especially in patients with volume depletion. Further, these guidelines recommend against the use of the ACE Inhibitor with ARB combination for most patients, even in the absence of diabetic nephropathy.
3.2.13. Wright et al., 2015 [63].
The Systolic Blood Pressure Intervention Trial (SPRINT) was sponsored by the NIH and supported by the VA CSP Clinical Research Pharmacy Coordinating Center. This randomized, open-label, clinical trial assigned 9361 patients with ages greater than 50 years, systolic blood pressures from 130 to 180 mm Hg, and at increased risk for cardiovascular events to a systolic blood pressure target of less than 120 mm Hg (intensive treatment) or to a target of less than 140 mm Hg (standard treatment). After a median of 3.26 years, patients receiving intensive treatment had a significantly lower rate of the composite primary end point of myocardial infarction, acute coronary syndrome, acute decompensated heart failure, or cardiovascular death compared to patients receiving standard treatment (HR = 0.75; 95% CI = 0.64–0.89). The rates of serious adverse events due to syncope, hypotension, electrolyte abnormalities, and acute kidney injury were significantly higher in patients receiving intensive treatment compared to standard treatment.
Three of the CPGs were informed by SPRINT [[29], [30], [31]]. The findings from SPRINT were cited when recommending the use of pharmacologic antihypertensive treatment to maintain systolic and diastolic blood pressures below 130 mm Hg and 80 mm Hg, respectively, in patients being at risk for or having atherosclerotic cardiovascular disease. Due to these findings, guidelines also made recommendations for other aspects of managing blood pressure including performing follow-up and managing blood pressure in the presence of other comorbidities. Finally, the recommendations from the ADA for treating diabetic patients acknowledged the findings from SPRINT but noted that their relevance to diabetic patients was less clear, indicating that a lower blood pressure goal could be appropriate for diabetic patients assuming that the additional treatment would not pose undue burden.
4. Discussion
4.1. Significance
One of most desirable outcomes a clinical trial can achieve is to appropriately influence clinical practice. A trial's influence on clinical practice can be demonstrated in part through its use in the relevant CPGs [2,3]. Although CPGs are not the only force shaping the care a patient ultimately receives, they represent a systematic aid when optimizing patient care, having the potential to improve healthcare quality and safety, reduce inappropriate variations in practice, more efficiently translate research into practice, and establish performance measures for physicians and hospitals [64]. Accordingly, incorporation of a trial's finding into the CPG recommendations is likely to facilitate the trial's implementation in clinical practice.
This study demonstrated that half of the clinical trials involving the VA CSP informed CPGs for the management of hypertension and that approximately 90% of these CPGs were informed by at least one of these clinical trials. While the two trials from Freis et al., in 1967 and 1970 as well as ACCORD BP and SPRINT were previously noted as having great influence on the clinical management of hypertension [5,65], the extent to which the body of clinical trials involving the VA CSP informed hypertension management was heretofore unknown.
4.2. Trends in the clinical trials
From this study, it is possible to identify trends within the trials cited by the CPGs in order to gain some understanding as to possible reasons for their inclusion in the CPGs. One interesting trend pertains to the primary outcomes of the clinical trials. Every trial wherein the primary outcome consisted of clinical endpoints such as cardiovascular mortality or morbidity, as opposed to a surrogate marker such as change in blood pressure, informed a CPG. In contrast, only three of the sixteen trials that used a surrogate marker for the primary outcome informed a CPG. The selection for trials with clinical endpoints by CPGs is anticipated given the focus of CPGs to improve patient healthcare in a meaningful way [64].
Another difference between those trials that informed and did not inform the CPGs pertains to sample size. Even after excluding the four large trials sponsored by the NIH, the average sample size of trials that informed CPGs (781.6 persons per trial) was larger than the average sample size of trials that did not inform CPGs (331.3 persons per trial). Given that these sample sizes were likely derived, at least in part, from anticipated effect size, anticipated variance, and desired power, trials with larger sample sizes may have been more frequently included in the CPGs since they often provide more definitive and generalizable answers to vital clinical questions.
It is also interesting to observe that four of the 13 trials that resulted in informing the hypertension CPGs did not aim at informing the management of high blood pressure based on the populations, interventions, or outcomes utilized by these trials. This unintended benefit supports to the notion that there can often be great and unforeseeable value to conducting clinical trials. Also of note is the observation that the proportion of trials that informed CPGs and which were supported by pharmaceutical companies was approximately similar to the proportion of trials that did not inform CPGs and which were supported by pharmaceutical companies. The apparent similarity in these proportions may simply reflect that there can often exist a mutual interest in obtaining answers to vital clinical questions that is shared by all types of clinical trial sponsors. Finally, this study also serves to demonstrate the extent to which clinical trials advance organically along the translational timeline from the publication of findings to the uptake by CPGs and may highlight the need for additional efforts that supplement the conduct of clinical trials in order to facilitate this progression.
4.3. Limitations
While the approach of evaluating how clinical trials informed the CPGs can be more objective and feasible than attempting to directly measure their influence in clinical practice, there are several limitations to this method. The care patients receive in clinical practice is not always consistent with the care recommended by CPGs. As such, to assume that clinical practice has necessarily been influenced once the CPGs have been informed may overestimate the true clinical impact. Although modest evidence exists supporting the general uptake of hypertension guideline recommendations by healthcare providers [7], directly evaluating the uptake of the specific recommendations informed by the trials would have further elucidated the true clinical impact from these trials.
This study was also limited in that it evaluated how the clinical trials informed just one type of synthesized literature, CPGs. It did not consider the extent to which a trial might have informed the unaffiliated systematic reviews and meta-analyses which are occasionally used by CPGs to support recommendations. Although there is a growing number of unaffiliated systematic reviews and meta-analyses upon which CPGs base recommendations, identifying these reviews and then evaluating the impact of the clinical trials on this additional intermediate outcome was beyond the scope of this paper. Instead, this study focused only on more proximal impact on CPGs as this provided for a more compelling demonstration of potential influence on clinical practice. However, by not considering the impact upon these other types of synthesized literature, this study may have underestimated the extent to which the VA CSP clinical trials informed the CPGs.
This study primarily focused on CPGs published after the year 2000. Before the year 2000, only CPGs from the JNC were included. While this approach provided for an assessment that was most relevant to recent clinical practice, it failed to consider how the clinical trials may have informed the CPGs that were published before the year 2000 and produced by other sources. However, given that the JNC was likely the most influential source of CPGs for hypertension management before the year 2000, the findings from this study are likely to serve as an adequate estimation of impact on clinical practice achieved through the CPGs.
Although the characteristics of trials involving VA CSP pertaining to population and interventions may differ from those utilized in trials without VA CSP involvement, it was not possible to evaluate how these differences may have modified the extent to which trials informed CPGs. This study was able to estimate the extent to which trials involving the VA CSP informed CPGs; however, additionally estimating how trials without VA CSP involvement informed CPGs and comparing this to how the trials involving VA CSP informed the CPGs was beyond the intended scope of this study. Finally, this study was focused on estimating impact on clinical practice and did not consider the impact of the clinical trials on the advancement of research, as exhibited by traditional citation indexes and other indicators. However, given that many of the early trials conducted by the VA CSP are not yet indexed by the PubMed/MEDLINE database, evaluating the impact of the VA CSP clinical trials by creating citation indexes or other similar indicators was infeasible.
5. Conclusions
Clinical trials involving the VA CSP have informed a sizeable proportion of hypertension management CPGs over the past 40 years. CPGs primarily relied on trials with larger sample sizes and ones which focused on clinical endpoints. Because of this impact on the CPGs, these trials are also likely to have had at least moderate influence on clinical practice.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the development, authorship, and/or publication of this manuscript.
Funding
Development of this manuscript was supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Cooperative Studies Program using resources and facilities at the VA Cooperative Studies Program Clinical Research Pharmacy Coordinating Center, Raymond G. Murphy VA Medical Center, and the Biomedical Research Institute of New Mexico. The contents do not represent the views of the U.S. Department of Veterans Affairs of the United States Government.
Disclaimer
Contents are expressed by the authors and do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Thonon F., Boulkedid R., Delory T., Rousseau S., Saghatchian M., van Harten W., O'Neill C., Alberti C. Measuring the outcome of biomedical research: a systematic literature review. PloS One. 2015;10 doi: 10.1371/journal.pone.0122239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosas S.R., Schouten J.T., Cope M.T., Kagan J.M. Modeling the dissemination and uptake of clinical trials results. Res. Eval. 2013;22:179–186. doi: 10.1093/reseval/rvt005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant J., Cottrell R., Cluzeau F., Fawcett G. Evaluating “payback” on biomedical research from papers cited in clinical guidelines: applied bibliometric study. BMJ. 2000;320:1107–1111. doi: 10.1136/bmj.320.7242.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang G.D., Ferguson R.E., Peduzzi P.N., O'Leary T.J. Scientific and organizational collaboration in comparative effectiveness research: the VA cooperative studies program model. Am. J. Med. 2010;123:e24–31. doi: 10.1016/j.amjmed.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Saklayen M.G., Deshpande N.V. Timeline of history of hypertension treatment. Front. Cardiovasc. Med. 2016;3:3. doi: 10.3389/fcvm.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotchen T.A. Developing hypertension guidelines: an evolving process. Am. J. Hypertens. 2014;27:765–772. doi: 10.1093/ajh/hpt298. [DOI] [PubMed] [Google Scholar]
- 7.Ardery G., Carter B.L., Milchak J.L., Bergus G.R., Dawson J.D., James P.A., Franciscus C., Kim Y. Explicit and implicit evaluation of physician adherence to hypertension guidelines. J. Clin. Hypertens. 2007;9:113–119. doi: 10.1111/j.1524-6175.2007.06112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hays M. Hist. Look Dep. Veterans Aff. Res. Dev. Program. Department of Veterans Affairs Office of Research & Development; 2010. 9. The hypertension studies; pp. 227–240.https://www.research.va.gov/pubs/docs/ORD-85yrHistory.pdf [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 10.Report of the Joint national committee on detection, evaluation, and treatment of high blood pressure. A cooperative study. J. Am. Med. Assoc. 1977;237:255–261. [PubMed] [Google Scholar]
- 11.The 1980 report of the Joint national committee on detection, evaluation, and treatment of high blood pressure. Arch. Intern. Med. 1980;140:1280–1285. [PubMed] [Google Scholar]
- 12.The 1984 report of the Joint national committee on detection, evaluation, and treatment of high blood pressure. Arch. Intern. Med. 1984;144:1045–1057. [PubMed] [Google Scholar]
- 13.The 1988 report of the Joint national committee on detection, evaluation, and treatment of high blood pressure. Arch. Intern. Med. 1988;148:1023–1038. [PubMed] [Google Scholar]
- 14.The fifth report of the Joint national committee on detection, evaluation, and treatment of high blood pressure (JNC V) Arch. Intern. Med. 1993;153:154–183. [PubMed] [Google Scholar]
- 15.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch. Intern. Med. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 16.Arauz-Pacheco C., Parrott M.A., Raskin P. The treatment of hypertension in adult patients with diabetes. Diabetes Care. 2002;25:134–147. doi: 10.2337/diacare.25.1.134. [DOI] [PubMed] [Google Scholar]
- 17.Arauz-Pacheco C., Parrott M.A., Raskin P. Treatment of hypertension in adults with diabetes. Diabetes Care. 2003;26(Suppl 1):S80–S82. doi: 10.2337/diacare.26.2007.s80. [DOI] [PubMed] [Google Scholar]
- 18.Chobanian A.V., Bakris G.L., Black H.R., Cushman W.C., Green L.A., Izzo J.L.J., Jones D.W., Materson B.J., Oparil S., Wright J.T.J., Roccella E.J. The seventh report of the Joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. J. Am. Med. Assoc. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 19.Williams B., Poulter N.R., Brown M.J., Davis M., McInnes G.T., Potter J.F., Sever P.S., McG Thom S. Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004-BHS IV. J. Hum. Hypertens. 2004;18:139–185. doi: 10.1038/sj.jhh.1001683. [DOI] [PubMed] [Google Scholar]
- 20.K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2004;43:S1–S290. [PubMed] [Google Scholar]
- 21.The Management Of Hypertension In The Primary Care Setting Working Group . Department of Veterans Administration and Department of Defense; 2004. VA/DoD Clinical Practice Guideline for Diagnosis and Management of Hypertension in the Primary Care Setting.https://www.healthquality.va.gov/hypertension/htn04_pdf1.pdf Version 2.0b- 2004. [Google Scholar]
- 22.Mancia G., De Backer G., Dominiczak A., Cifkova R., Fagard R., Germano G., Grassi G., Heagerty A.M., Kjeldsen S.E., Laurent S., Narkiewicz K., Ruilope L., Rynkiewicz A., Schmieder R.E., Boudier H.A.J.S., Zanchetti A., Vahanian A., Camm J., De Caterina R., Dean V., Dickstein K., Filippatos G., Funck-Brentano C., Hellemans I., Kristensen S.D., McGregor K., Sechtem U., Silber S., Tendera M., Widimsky P., Zamorano J.L., Erdine S., Kiowski W., Agabiti-Rosei E., Ambrosioni E., Lindholm L.H., Viigimaa M., Adamopoulos S., Agabiti-Rosei E., Ambrosioni E., Bertomeu V., Clement D., Erdine S., Farsang C., Gaita D., Lip G., Mallion J.-M., Manolis A.J., Nilsson P.M., O'Brien E., Ponikowski P., Redon J., Ruschitzka F., Tamargo J., van Zwieten P., Waeber B., Williams B. 2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the european society of hypertension (ESH) and of the european society of Cardiology (ESC) J. Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 23.Hypertension . Royal College of Physicians (UK); London: 2011. The Clinical Management of Primary Hypertension in Adults: Update of Clinical Guidelines 18 and 34. [PubMed] [Google Scholar]
- 24.Kidney Disease Improving global outcomes (KDIGO) blood pressure work group, KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2012;(Suppl):337–414. [Google Scholar]
- 25.Mancia G., Fagard R., Narkiewicz K., Redon J., Zanchetti A., Bohm M., Christiaens T., Cifkova R., De Backer G., Dominiczak A., Galderisi M., Grobbee D.E., Jaarsma T., Kirchhof P., Kjeldsen S.E., Laurent S., Manolis A.J., Nilsson P.M., Ruilope L.M., Schmieder R.E., Sirnes P.A., Sleight P., Viigimaa M., Waeber B., Zannad F., Redon J., Dominiczak A., Narkiewicz K., Nilsson P.M., Burnier M., Viigimaa M., Ambrosioni E., Caufield M., Coca A., Olsen M.H., Schmieder R.E., Tsioufis C., van de Borne P., Zamorano J.L., Achenbach S., Baumgartner H., Bax J.J., Bueno H., Dean V., Deaton C., Erol C., Fagard R., Ferrari R., Hasdai D., Hoes A.W., Kirchhof P., Knuuti J., Kolh P., Lancellotti P., Linhart A., Nihoyannopoulos P., Piepoli M.F., Ponikowski P., Sirnes P.A., Tamargo J.L., Tendera M., Torbicki A., Wijns W., Windecker S., Clement D.L., Coca A., Gillebert T.C., Tendera M., Rosei E.A., Ambrosioni E., Anker S.D., Bauersachs J., Hitij J.B., Caulfield M., De Buyzere M., De Geest S., Derumeaux G.A., Erdine S., Farsang C., Funck-Brentano C., Gerc V., Germano G., Gielen S., Haller H., Hoes A.W., Jordan J., Kahan T., Komajda M., Lovic D., Mahrholdt H., Olsen M.H., Ostergren J., Parati G., Perk J., Polonia J., Popescu B.A., Reiner Z., Ryden L., Sirenko Y., Stanton A., Struijker-Boudier H., Tsioufis C., van de Borne P., Vlachopoulos C., Volpe M., Wood D.A. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the european society of hypertension (ESH) and of the european society of Cardiology (ESC) Eur. Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 26.James P.A., Oparil S., Carter B.L., Cushman W.C., Dennison-Himmelfarb C., Handler J., Lackland D.T., LeFevre M.L., MacKenzie T.D., Ogedegbe O., Smith S.C.J., Svetkey L.P., Taler S.J., Townsend R.R., Wright J.T.J., Narva A.S., Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) J. Am. Med. Assoc. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 27.Weber M.A., Schiffrin E.L., White W.B., Mann S., Lindholm L.H., Kenerson J.G., Flack J.M., Carter B.L., Materson B.J., Ram C.V.S., Cohen D.L., Cadet J.-C., Jean-Charles R.R., Taler S., Kountz D., Townsend R.R., Chalmers J., Ramirez A.J., Bakris G.L., Wang J., Schutte A.E., Bisognano J.D., Touyz R.M., Sica D., Harrap S.B. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J. Clin. Hypertens. 2014;16:14–26. doi: 10.1111/jch.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Diagnosis and Management of Hypertension Working Group . Department of Veterans Affairs and Department of Defense; 2014. VA/DoD Clinical Practice Guideline for Diagnosis and Management of Hypertension in the Primary Care Setting.https://www.healthquality.va.gov/guidelines/CD/htn/VADoDCPGHTN2014.pdf Version 3.0- 2014. [Google Scholar]
- 29.de Boer I.H., Bangalore S., Benetos A., Davis A.M., Michos E.D., Muntner P., Rossing P., Zoungas S., Bakris G. Diabetes and hypertension: a position statement by the American diabetes association. Diabetes Care. 2017;40:1273–1284. doi: 10.2337/dci17-0026. [DOI] [PubMed] [Google Scholar]
- 30.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E.J., Collins K.J., Dennison Himmelfarb C., DePalma S.M., Gidding S., Jamerson K.A., Jones D.W., MacLaughlin E.J., Muntner P., Ovbiagele B., Smith S.C.J., Spencer C.C., Stafford R.S., Taler S.J., Thomas R.J., Williams K.A.S., Williamson J.D., Wright J.T.J. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2017 [Google Scholar]
- 31.9. Cardiovascular disease and risk management: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S86–S104. doi: 10.2337/dc18-S009. [DOI] [PubMed] [Google Scholar]
- 32.Veterans Administration Cooperative Study Group on Antihypertensive Agents A double blind control study of antihypertensive agents. II. Further report on the comparative effectiveness of reserpine, reserpine and hydralazine, and three ganglion blocking agents, chlorisondamine, mecamylamine, and pentolinium tartrate. Arch. Intern. Med. 1962;110:230–236. [PubMed] [Google Scholar]
- 33.Veterans Administration Cooperative Study Group on Antihypertensive Agents A double blind control study of antihypertensive agents. I. Comparative effectiveness of reserpine, reserpine and hydralazine, and three ganglionic blocking agents, chlorisondamine, mecamyamine, and pentolinium tartrate. Arch. Intern. Med. 1960;106:81–96. [PubMed] [Google Scholar]
- 34.Veterans Administration Cooperative Study Group on Antihypertensive Agents A double blind control study of antihypertensive agents. III. Chlorothiazide Alone and in Combination with Other Agents; Preliminary Results. Arch. Intern. Med. 1962;110:230–236. [Google Scholar]
- 35.Veterans Administration Cooperative Study Group on Antihypertensive Agents Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. J. Am. Med. Assoc. 1967;202:1028–1034. [PubMed] [Google Scholar]
- 36.Veterans Administration Cooperative Study Group on Antihypertensive Agents Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. J. Am. Med. Assoc. 1970;213:1143–1152. [PubMed] [Google Scholar]
- 37.Veterans Administration Cooperative Study Group on Antihypertensive Agents Effects of treatment on morbidity in hypertension. III. Influence of age, diastolic pressure, and prior cardiovascular disease; further analysis of side effects. Circulation. 1972;45:991–1004. doi: 10.1161/01.cir.45.5.991. [DOI] [PubMed] [Google Scholar]
- 38.Veterans Administration Cooperative Study Group on Antihypertensive Agents Return of elevated blood pressure after withdrawal of antihypertensive drugs. Circulation. 1975;51:1107–1113. doi: 10.1161/01.cir.51.6.1107. [DOI] [PubMed] [Google Scholar]
- 39.Ramirez E.A., Elson L., Gear A.S., Oster J.R., Talmers F.N., Thomas J.R. Multiclinic controlled trial of bethanidine and guanethidine in severe hypertension. Circulation. 1977;55:519–525. doi: 10.1161/01.cir.55.3.519. [DOI] [PubMed] [Google Scholar]
- 40.Thomas J.R., Elson L., Freis E.D., Gear A.S., Oster J.R., Ramirez E.A., Talmers F.N. Propranolol in the treatment of essential hypertension. J. Am. Med. Assoc. 1977;237:2303–2310. [Google Scholar]
- 41.Perry H.M., Goldman A.I., Lavin M.A., Schnaper H.W., Fitz A.E., Frohlich E.D., Steele B., Richman H.G. Evaluation of drug treatment in mild hypertension: VA-NHLBI feasibility trial. Plan and preliminary results of a two-year feasibility trial for a multicenter intervention study to evaluate the benefits versus the disadvantages of treating mild hypertension. Prepared for the Veterans Administration-National Heart, Lung, and Blood Institute Study Group for Evaluating Treatment in Mild Hypertension. Ann. N. Y. Acad. Sci. 1978;304:267–292. doi: 10.1111/j.1749-6632.1978.tb25604.x. [DOI] [PubMed] [Google Scholar]
- 42.Perry H.M.J. Treatment of mild hypertension. Preliminary results of a two-year feasibility trial. Circ. Res. 1977;40:I180–I187. [PubMed] [Google Scholar]
- 43.Veterans administration cooperative study group on antihypertensive agents, comparative effects of ticrynafen and hydrochlorothiazide in the treatment of hypertension. N. Engl. J. Med. 1979;301:293–297. doi: 10.1056/NEJM197908093010602. [DOI] [PubMed] [Google Scholar]
- 44.Veterans Administration Cooperative Study Group Oxprenolol vs propranolol: a randomized, double-blind, multiclinic trial in hypertensive patients taking hydrochlorothiazide. Veterans Administration Cooperative Study Group. Hypertension. 1981;3:250–256. [PubMed] [Google Scholar]
- 45.Veterans Administration Cooperative Study Group on Antihypertensive Agents Comparison of prazosin with hydralazine in patients receiving hydrochlorothiazide. A randomized, double-blind clinical trial. Circulation. 1981;64:772–779. doi: 10.1161/01.cir.64.4.772. [DOI] [PubMed] [Google Scholar]
- 46.Veterans Administration Cooperative Study Group on Antihypertensive Agents Comparison of propranolol and hydrochlorothiazide for the initial treatment of hypertension. I. Results of short-term titration with emphasis on racial differences in response. Veterans Administration Cooperative Study Group on Antihypertensive agents. J. Am. Med. Assoc. 1982;248:1996–2003. [PubMed] [Google Scholar]
- 47.Veterans Administration Cooperative Study Group on Antihypertensive Agents Comparison of propranolol and hydrochlorothiazide for the initial treatment of hypertension. II. Results of long-term therapy. Veterans Administration Cooperative Study Group on Antihypertensive Agents. J. Am. Med. Assoc. 1982;248:2004–2011. [PubMed] [Google Scholar]
- 48.Veterans Administration Cooperative Study Group on Antihypertensive Agents, Low doses v standard dose of reserpine. A randomized, double-blind, multiclinic trial in patients taking chlorthalidone. J. Am. Med. Assoc. 1982;248:2471–2477. [PubMed] [Google Scholar]
- 49.Veterans Administration Cooperative Study Group on Antihypertensive Agents Efficacy of nadolol alone and combined with bendroflumethiazide and hydralazine for systemic hypertension. Am. J. Cardiol. 1983;52:1230–1237. doi: 10.1016/0002-9149(83)90579-9. [DOI] [PubMed] [Google Scholar]
- 50.Materson B.J., Freis F.D., Anderson S., Taguchi J.T. Low-dose captopril for the treatment of mild to moderate hypertension. I. Results of a 14-week trial. Veterans administration cooperative study group on antihypertensive agents. Arch. Intern. Med. 1984;144:1947–1953. doi: 10.1001/archinte.144.10.1947. [DOI] [PubMed] [Google Scholar]
- 51.Cohn J.N., Archibald D.G., Ziesche S., Franciosa J.A., Harston W.E., Tristani F.E., Dunkman W.B., Jacobs W., Francis G.S., Flohr K.H. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N. Engl. J. Med. 1986;314:1547–1552. doi: 10.1056/NEJM198606123142404. [DOI] [PubMed] [Google Scholar]
- 52.Freis E.D., Thomas J.R., Fisher S.G., Hamburger R., Borreson R.E., Mezey K.C., Mukherji B., Neal W.W., Perry H.M., Taguchi J.T. Effects of reduction in drugs or dosage after long-term control of systemic hypertension. Am. J. Cardiol. 1989;63:702–708. doi: 10.1016/0002-9149(89)90255-5. [DOI] [PubMed] [Google Scholar]
- 53.Materson B.J., Cushman W.C., Goldstein G., Reda D.J., Freis E.D., Ramirez E.A., Talmers F.N., White T.J., Nunn S., Chapman R.H. Treatment of hypertension in the elderly: I. Blood pressure and clinical changes. Results of a department of veterans Affairs cooperative study. Hypertension. 1990;15:348–360. doi: 10.1161/01.hyp.15.4.348. [DOI] [PubMed] [Google Scholar]
- 54.Goldstein G., Materson B.J., Cushman W.C., Reda D.J., Freis E.D., Ramirez E.A., Talmers F.N., White T.J., Nunn S., Chapman R.H. Treatment of hypertension in the elderly: II. Cognitive and behavioral function. Results of a department of veterans Affairs cooperative study. Hypertension. 1990;15:361–369. doi: 10.1161/01.hyp.15.4.361. [DOI] [PubMed] [Google Scholar]
- 55.Cohn J.N., Johnson G., Ziesche S., Cobb F., Francis G., Tristani F., Smith R., Dunkman W.B., Loeb H., Wong M. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N. Engl. J. Med. 1991;325:303–310. doi: 10.1056/NEJM199108013250502. [DOI] [PubMed] [Google Scholar]
- 56.Materson B.J., Reda D.J., Cushman W.C., Massie B.M., Freis E.D., Kochar M.S., Hamburger R.J., Fye C., Lakshman R., Gottdiener J. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The department of veterans Affairs cooperative study group on antihypertensive agents. N. Engl. J. Med. 1993;328:914–921. doi: 10.1056/NEJM199304013281303. [DOI] [PubMed] [Google Scholar]
- 57.Materson B.J., Reda D.J., Cushman W.C. Department of veterans Affairs single-drug therapy of hypertension study. Revised figures and new data. Department of Veterans Affairs cooperative study group on antihypertensive agents. Am. J. Hypertens. 1995;8:189–192. doi: 10.1016/0895-7061(94)00196-i. [DOI] [PubMed] [Google Scholar]
- 58.Cushman W.C., Cutler J.A., Hanna E., Bingham S.F., Follmann D., Harford T., Dubbert P., Allender P.S., Dufour M., Collins J.F., Walsh S.M., Kirk G.F., Burg M., Felicetta J.V., Hamilton B.P., Katz L.A., Perry H.M.J., Willenbring M.L., Lakshman R., Hamburger R.J. Prevention and Treatment of Hypertension Study (PATHS): effects of an alcohol treatment program on blood pressure. Arch. Intern. Med. 1998;158:1197–1207. doi: 10.1001/archinte.158.11.1197. [DOI] [PubMed] [Google Scholar]
- 59.Braunwald E., Domanski M.J., Fowler S.E., Geller N.L., Gersh B.J., Hsia J., Pfeffer M.A., Rice M.M., Rosenberg Y.D., Rouleau J.L. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N. Engl. J. Med. 2004;351:2058–2068. doi: 10.1056/NEJMoa042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cushman W.C., Evans G.W., Byington R.P., Goff D.C.J., Grimm R.H.J., Cutler J.A., Simons-Morton D.G., Basile J.N., Corson M.A., Probstfield J.L., Katz L., Peterson K.A., Friedewald W.T., Buse J.B., Bigger J.T., Gerstein H.C., Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N. Engl. J. Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benavente O.R., Coffey C.S., Conwit R., Hart R.G., McClure L.A., Pearce L.A., Pergola P.E., Szychowski J.M. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507–515. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fried L.F., Emanuele N., Zhang J.H., Brophy M., Conner T.A., Duckworth W., Leehey D.J., McCullough P.A., O'Connor T., Palevsky P.M., Reilly R.F., Seliger S.L., Warren S.R., Watnick S., Peduzzi P., Guarino P. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N. Engl. J. Med. 2013;369:1892–1903. doi: 10.1056/NEJMoa1303154. [DOI] [PubMed] [Google Scholar]
- 63.Wright J.T.J., Williamson J.D., Whelton P.K., Snyder J.K., Sink K.M., Rocco M.V., Reboussin D.M., Rahman M., Oparil S., Lewis C.E., Kimmel P.L., Johnson K.C., Goff D.C.J., Fine L.J., Cutler J.A., Cushman W.C., Cheung A.K., Ambrosius W.T. A randomized trial of intensive versus standard blood-pressure control. N. Engl. J. Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Institute of Medicine (US) National Academies Press (US); Washington (DC): 2011. Committee on Standards for Developing Trustworthy Clinical Practice Guidelines, Clinical Practice Guidelines We Can Trust.http://www.ncbi.nlm.nih.gov/books/NBK209539/ [PubMed] [Google Scholar]
- 65.Moser M. Historical perspectives on the management of hypertension. J. Clin. Hypertens. 2006;8:15–20. doi: 10.1111/j.1524-6175.2006.05836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]