Abstract
The pathogenesis of endometriosis (EMS) is complicated, and treatment results are unsatisfactory. It has become the focus of gynecological research. Analysis targeting the pathogenesis of EMS is the key to providing more effective treatments. In recent years, the superiority of traditional Chinese medicine in treating EMS has been highlighted, so we investigated the impact of a Chinese medicinal formula (Xiao Liu Fang) on the “3A” ability, in situ, of ectopic endometrial stromal cells in patients with EMS. Primary endometrial stromal cells were isolated using a modified net filtration method, cultured, and identified in different groups. Endometrial cell attachment was measured using the methyl thiazolyl tetrazolium (MTT) colorimetric assay, cell aggression was detected by the transwell cell-invasion assay, and angiogenesis was defined by evaluating the mRNA concentrations of intercellular cell adhesion molecule 1 (ICAM-1), cyclooxygenase 2 (COX-2), matrix metallo peptidase 9 (MMP-9), and vascular endothelial growth factor (VEGF). Attachment, aggression, and angiogenesis (AAA) plays an important role in EMS, and a high dose of the Xiao Liu Fang extract can be used for the treatment of EMS owing to its inhibition of the AAA of endometrial stromal cells. Therefore, in-depth studies targeting the effective mechanisms and targets of traditional Chinese medicine (TCM) are of great significance for the prevention and treatment of EMS.
Keywords: endometriosis, EMS, endometrial stromal cells, invigorating the kidney and promoting blood circulation, attachment, aggression, and angiogenesis, AAA
Introduction
Endometriosis (EMS) is a common gynecological disease that affects 10%–15% of women; 80% of patients have symptoms such as pelvic pain, and 30%–50% experience infertility.1 The recurrence rate of EMS is 40% over 5 years, regardless of the treatment administered,2 which can seriously affect the quality of life. An investigation into the mechanism of EMS revealed that attachment, aggression, angiogenesis (AAA) plays an important role.3 Previous studies have demonstrated that the endometrium can develop in parts of the body other than the uterus, and its development involves AAA. Blocking any one of the three factors can affect endometrium formation and development.
The intercellular cell adhesion molecule 1 (ICAM-1), cyclooxygenase 2 (COX-2), matrix metallopeptidase 9 (MMP-9), and vascular endothelial growth factor (VEGF), the levels of which are increased in EMS, have been shown to promote AAA in different phases of EMS.4, 5 Traditional Chinese medicines used for the treatment of EMS6, 7 are based on the assumption that EMS is a result of kidney deficiency and blood stasis. The effects of Xiao Liu Fang were investigated by Q.C.,8 who previously confirmed its activity based on the decrease in the recurrence rates of EMS9 and the induction of apoptosis in ectopic endometrial cells. In combination with modern medicines for EMS and the ectopic cell theory proposed in a most recent report,10 this study focused on understanding whether the mechanism of EMS is related to AAA.
Results
Primary Endometrial Stromal Cells
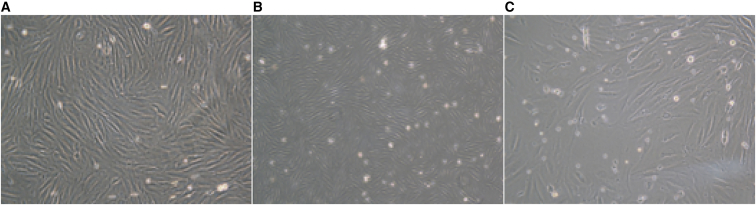
The primary endometrial stromal cells began to adhere to the wall approximately 2 hr after inoculation, had a fusi form or polygonal fibroblast morphology, a full cytoplasm, and a round and centrally located nucleus. The cells were flat, with clear boundaries, and arranged in parallel (Figure 2).
Figure 2.
Primary Cultured Endometrial Stromal Cells
Primary cultured endometrial stromal cells (×100): normal menstruation (A), EMS cyst (B), and EMS uterus (C).
Immunohistochemical Staining
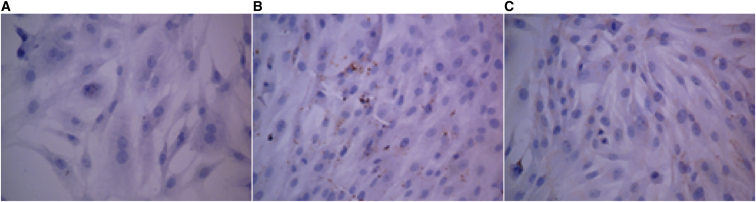
A specific marker of stromal cells is the cytoskeletal protein vimentin, which was stained brownish yellow in the cytoplasm of the primary endometrial stromal cells (Figure 3).
Figure 3.
Immunohistochemical Results of Endometrial Stromal Cells
Immunohistochemical results of endometrial stromal cells (SP, ×200), normal menstruation (A), EMS cyst (B), and EMS uterus (C).
Immunofluorescence Identification of Endometrial Cells
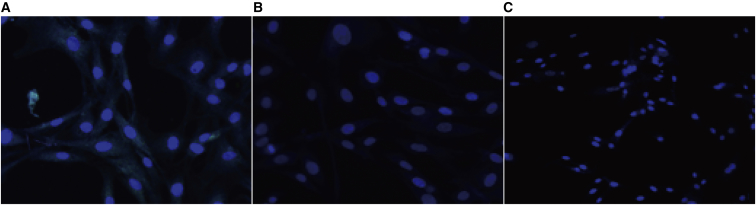
The vimentin staining of cells in different groups was identified by immunofluorescence (Figure 4).
Figure 4.
The Immunofluorescence of Endometrial Cells
The immunofluorescence of endometrial cells (×250), normal menstruation (A), EMS cyst (B), and EMS uterus (C).
Effect of Xiao Liu Fang on Attachment
MTT Colorimetric Assay
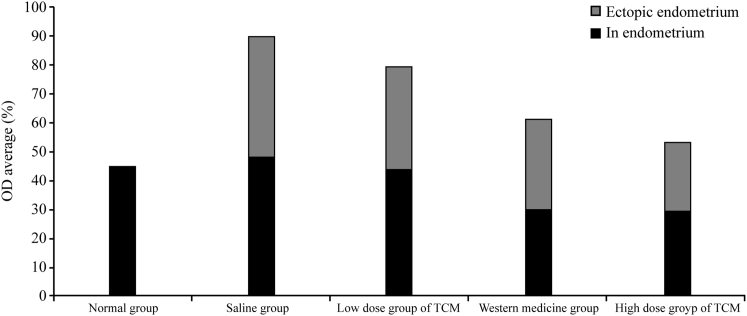
In comparison with the control and saline-treated groups, the high-dose traditional Chinese medicine (TCM) treatment and gestrinone treatment groups exhibited a significant inhibition of endometrial cell attachment (p < 0.01 or 0.05; Table 1; Figure 5).
Table 1.
The Attachment of the Endometrial Cells (n, ± s)
| Group | Group EU (OD) | Group EC (OD) |
|---|---|---|
| N (n = 5) | 0.45 ± 0.04 | – |
| B (n = 5) | 0.47 ± 0.05 | 0.42 ± 0.10 |
| LD-TCM (n = 5) | 0.43 ± 0.07 | 0.36 ± 0.06 |
| G (n = 5) | 0.30 ± 0.14*ΔΔ# | 0.31 ± 0.06Δ# |
| HD-TCM (n = 5) | 0.29 ± 0.09*ΔΔ# | 0.24 ± 0.05ΔΔ# |
Note: compare with group N, *p < 0.05 (p = 0.011); compare with group B, Δp < 0.05 (p = 0.032), ΔΔp < 0.01(p = 0.002); compare with group LD-TCM, #p < 0.05 (p = 0.019).
Figure 5.
Impact of Xiao Liu Fang on Adhesion Ability of Endometrium Cells
Note: compare with group N, *p < 0.05 (p = 0.011); compare with group B, Δp < 0.05 (p = 0.032), ΔΔp < 0.01 (p = 0.002); compare with group LD-TCM, #p < 0.05 (p = 0.019).
Aggression Assay
Transwell Cell Aggression Test
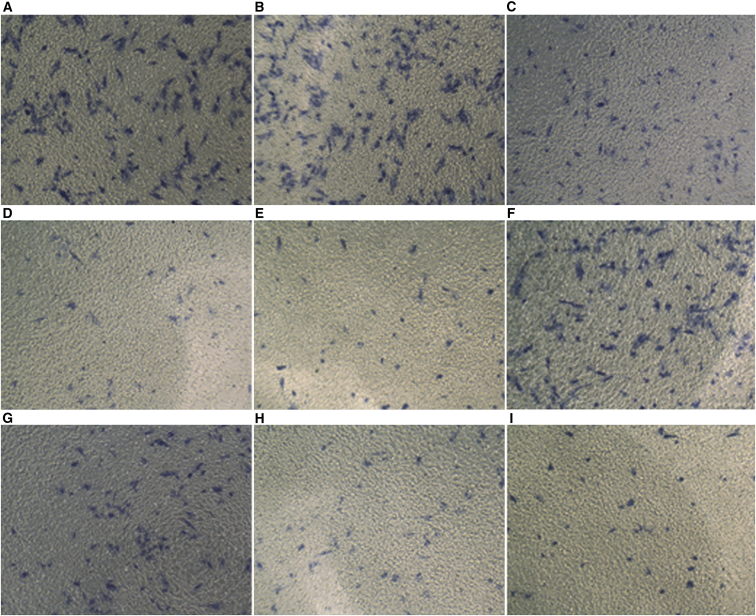
Gestrinone and Xiao Liu Fang reduced the aggression of endometrial cells; the inhibition was more noticeable in the gestrinone and high-dose TCM groups (p < 0.01 or 0.05; Figure 6).
Figure 6.
Changes of Invasion Ability of Endometrium Cells in Each Group
(A) Control group; (B) eutopic saline group; (C) eutopic low-dose TCM group; (D) eutopic gestrinone group; (E) eutopic high-dose TCM group; (F) ectopic saline group; (G) ectopic low-dose TCM group; (H) ectopic gestrinone group; (I) ectopic high-dose TCM group. Note: compare with group N, **p < 0.01 (p = 0.004); compare with group B, ΔΔp < 0.01 (p = 0.005); compare with group LD-TCM, #p < 0.05 (p = 0.013).
The Effect of the Treatments on ICAM-1, COX-2, MMP-9, and VEGF mRNA Expression
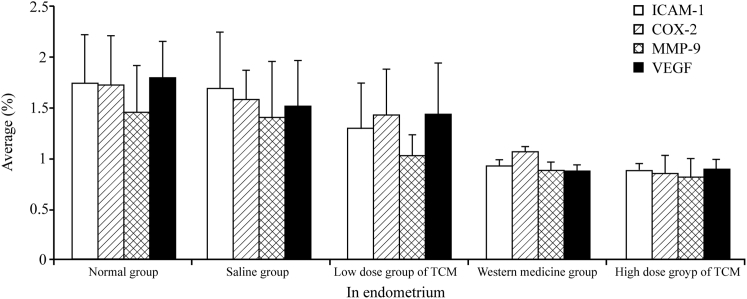
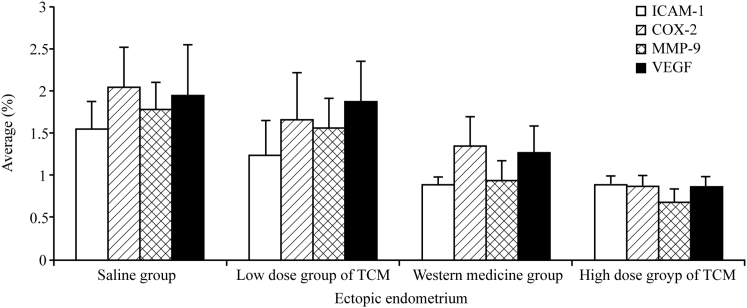
In comparison to the normal and low-dose TCM groups, the high-dose TCM and gestrinone groups exhibited a significant inhibition of the mRNA expression levels of ICAM-1, COX-2, MMP-9, and VEGF, as determined by RT-PCR (p < 0.01 or 0.05; Figures 7 and 8).
Figure 7.
Impact of Xiao Liu Fang on Expression of 3A Factor in Endometrium Cells
Note: compare with group N, *p < 0.05 (p = 0.03), **p < 0.01 (p = 0.002); compare with group B, Δp < 0.05 (p = 0.014), ΔΔp < 0.01 (p = 0.007).
Figure 8.
Impact of Xiao Liu Fang on Expression of 3A Factor in Ectopic Endometrium Cells
Note: compare with group B, Δp < 0.05 (p = 0.012), ΔΔp < 0.01 (p = 0.006).
Discussion
The Xiao Liu Fang formula comprises fried turtle shells, sliced Cornus Cervi, leech, Eupolyphaga seu Steleophaga, Vaccaria segetalis, Rhizoma curcumae, steamed buns, Salvia chinensis, Smilax china, Radix codonopsis, and Rhizoma zingiberis Preparatum). Among these components, sliced Cornus Cervi has a salty flavor and a mild Qi, can enter the blood, softens hardness, and disperses viciousness, thus reinforcing Yang and benefitting vital energy. Fried turtle shells can nourish the Yin energy and suppress Yang, dispelling stasis and dissipating knots, softens hardness, and suppresses disease. These two synergic TCMs can nourish the kidneys and soften knots, thus activating the blood and dispersing disease, and are therefore defined as sovereign drugs. Leech, Eupolyphaga seu Steleophaga, steamed buns, Salvia chinensis, and Rhizoma curcumae can activate the blood circulation and dissipate blood stasis. Vaccaria segetalis and Smilax china can remove dampness, disperse knots, and remove toxins for detumescence and are defined as minister drugs in TCM. Radix Codonopsis can strengthen the spleen and benefit vital energy, namely “raising positive energy so as to eliminate disease automatically.” Rhizoma zingiberis Preparatum is, the envoy drug, that can activate Qi and disperse cold temperatures, thus allowing other drugs to directly access the lesion.
Previous clinical studies have shown that Xiao Liu Fang has a role in reducing the recurrence rate of EMS,9 and experimental studies have shown that this formula can promote apoptosis of ectopic endometrial cells.8
EMS is the growth of the endometrium in places outside the uterine cavity. The infiltration, proliferation, diffusion, and recurrence of EMS are estrogen dependent and typically affect women within the reproductive age, affecting their physical, mental, and social well-being and causing pain and infertility.11, 12 The treatment of EMS includes operations and administration of hormone drugs, but it is impossible to remove the ectopic endometrium completely, and ovarian functions can be impaired by repeated operations. Additionally, the long-term administration of hormones can result in many side effects, including perimenopausal symptoms, masculinity, and damages to liver and kidney functions.
There are many theories about the pathogenesis of EMS, which include endometrial implantation, lymphatic and venous dissemination, cavity metaplasia, and immunity.13 Recent studies have focused on eutopic endometrium determinism and AAA capability. The AAA of endometrium pieces in menstruation blood is the main indication of EMS,14 and it has been found that the endometrium of patients with EMS was much more active, with stronger AAA capabilities. It is known that the attachment of the endometrium in the pelvic region or other organs is the first step of EMS, aggression is the second step necessary for breaking through the extracellular matrix and invading for implantation, and angiogenesis is the last step, which includes neovascularization and ectopic endometrial hyperplasia.15, 16, 17 There are many factors that play different roles in the development of EMS: ICAM-1 mediates the separation between homotypic cells and the adhesion between different types of cells; COX-2 increases cell aggression and induces angiogenesis; MMP and tissue inhibitor of metalloproteinases (TIMP) are involved in the degradation of the extracellular matrix and mediates cell aggression; and VEGF promotes angiogenesis.18 As the development of EMS is related to all of the above factors, we chose to analyze them in this study.
Recently, the advantages of Chinese medicine in the treatment of EMS have been evaluated and accepted by an increasing number of people.19, 20 Q.C. found that the main pathogenesis of EMS was kidney deficiency and blood stasis and developed the Xiao Liu Fang extract for invigorating the kidneys and promoting blood circulation. The results of this study showed that a high-dose of Xiao Liu Fang extract and gestrinone could inhibit the AAA associated with EMS by reducing the expression levels of ICAM-1, COX-2, MMP-9, and VEGF mRNAs, suggesting that Xiao Liu Fang has an effect similar to that of gestrinone, which is used to treat EMS. The findings of this study support the above theory.
Materials and Methods
Experimental Materials
Reagents and Instruments
DMEM and F12 was supplemented with fetal bovine serum (FBS) (Hyclone, USA), 0.25% type I collagenase, 5% normal goat serum, 0.25% trypsin (Lanzhou Minhai Bioengineering), and the total RNA extract reagent Trizol was used along with the enzymes and reagents necessary for reverse transcription and PCR amplification (Invitrogen, USA). The following NCBI gene sequences were used: ICAM-1, NM_000201.3; COX-2, M90100; MMP-9, NM_004994.2; VEGFE14233.1 (https://www.ncbi.nlm.nih.gov/nuccore).
Endometrial Materials
Five patients with ovarian endometrial cysts were considered for the study. The patients provided written informed consent, and the study was approved by the ethics committee of the hospital. The patients were aged between 25 and 38 years and had normal menstruation cycles and no other complications. The patients had not received hormone therapy within the last 3 months and had no visible pathological changes in the endometrium. Endometrial samples were collected from the uterus and the ectopic cyst. Endometrial tissues collected from healthy women having normal menstrual cycles were used as controls. The specimens were washed with 0.9% sterile NaCl solution, cultured in the DMEM and F12 culture medium (Hyclone) in an ice bath, isolated, and subjected to further analyses (Figure 1).
Figure 1.
Endometrium
Endometrium, normal menstruation (A), EMS cyst (B), and EMS uterus (C).
Drugs and Extraction
Xiao Liu Fang (composed of fried turtle shells, sliced Cornus Cervi, leech, Eupolyphaga seu Steleophaga, Vaccaria segetalis, Rhizoma curcumae, steamed buns, Salvia chinensis, Smilax china, Radix codonopsis, and Rhizoma zingiberis Preparatum) was provided by the Department of TCM Preparation, affiliated to Shuguang Hospital of Shanghai TCM University. Gestrinone was purchased from Beijing Zizhu Pharm (batch no H20080256). For extracting the formula, the method of water-extract-alcohol-precipitation was used: the components were first soaked in tap water at room temperature for 1 hr, followed by simmering for half an hour and collecting the fluid extract. The dregs were soaked in tap water at room temperature for 1 hr, simmered for half an hour, and the fluid extract was collected. The two liquid extracts thus obtained were combined, filtered, and concentrated by simmering until 2 g of the crude drug/mL was obtained. This was precipitated by successive concentrations of 70% and 85% alcohol. The supernatant was then evenly mixed, sealed, stored at 4°C for 24 hr, and filtered. The supernatant was diluted with 20 mL of distilled water and the ethanol was volatilized by simmering, to achieve a concentrated volume of 18 mL (1 g of crude drug/mL). To the concentrated solution, sodium hydroxide particles were added and mixed thoroughly to adjust the pH to 7.0. Water was added to obtain a solution having 0.5 g of crude drug per mL. The solution was sterilized, labeled, and stored at −80°C. Gestrinone was first dissolved in DMSO and then diluted with the culture medium to achieve the desired concentration.
Specimen Collection
This research study was reviewed and approved by the Ethics Committee of Shuguang Hospital. All patients signed the informed consent form and voluntarily joined the research.
Isolation, Culture, and Identification of Endometrial Stromal Cells
In Vitro Isolation and Culture
The primary endometrial stromal cells were isolated using a modified filtering method.21 In this method, the cells were washed in FBS, and the endometrial tissues were cut into pieces (<1 mm); 0.25% type I collagenase (containing 0.005% DNA enzyme, Lanzhou min Hai Bioengineering, China) was added for digestion. Digestion was carried out in a CO2 incubator supplied with 5% CO2 for 60 min at 37°C, with oscillations at every 15-min interval. DMEM supplemented with 10% FBS was added to terminate digestion, and the cells were filtered through a 100-mesh (150-μm aperture) screen. The endometrial cell suspension was divided into two equal parts and centrifuged (50 × g × 1 min). The supernatant was filtered through a 400-mesh (40 μm aperture) screen and centrifuged to collect the endometrial cells (count number). The cells were cultured in a CO2 incubator supplied with 5% CO2 at 37°C.The culture medium was first changed after 24 hr and subsequently refreshed every 2 days. Cell morphology and growth were observed every day under an inverted microscope until the cells covered the surface of the plate. The third-generation cells were used for the experiments.
Grouping and Intervention
The third-generation endometrial cells were divided into two groups, the eutopic group (EU) and the ectopic group (EC). The two groups were further randomly sub-grouped into the saline control group (B), the gestrinone group (G), the low-dose TCM group (LD-TCM), and the high-dose TCM group (HD-TCM). The concentration of the drugs and doses administered were as follows: group G received gestrinone at a dose of 1.8 mg/mL, the LD-TCM group was administered a dose of 5 mg of extract/mL, the HD-TCM group received a dose of 10 mg of extract/mL, while groups N and B were administered the same amounts of 0.9% NaCl solution. The cells in each group were collected and tested after a 24-hr culture.
Treatments
Gestrinone was supplied by Beijing Zizhu Pharmaceutical. The Chinese medicine H19980020 (Xiao Liu Fang) comprising fried turtle shells, sliced Cornus Cervi, leech, Eupolyphaga sinensis Walker, the seed of cowherb, zedoary, wood, climbing fig receptacle, Salvia chinensis Benth., iron thorn, Codonopsis pilosula, and dry ginger, was supplied by the Department of Traditional Chinese Medicine, Shuguang Hospital, affiliated to Shanghai University of Traditional Chinese Medicine. The Chinese formula was a decoction of a Chinese herbal medicine, which was mixed with water at 25°C for 1 hr and gently heated for 30 min before collecting the juice. The residue was again mixed with water and boiled for 30 min; the juice collected was mixed with the previously collected juice. The liquor was filtered, concentrated to 2 g, dissolved in ethanol at concentrations of 70% and 85%, and stored for 24 hr at 4°C. The ethanolic solution of the formulation was mixed with 20 mL water and warmed until the ethanol was volatilized and the volume was concentrated to 18 mL (1 g/mL). The pH was adjusted to 7.0 by adding sodium hydroxide particles. Water was added to achieve a final concentration of 0.5 g/mL. The solution was then sterilized and stored at −80°C.
Following transfer into 6-well plates for normal culture, the cells occupied 80% of the well on the following day, at which time the corresponding intervention drugs were added for a 24-hr intervention. Serum-free DMEM (50 μL) and Matrigel (ratio 1:4) were added into the individual wells of 96-well culture plates and gently shaken so as to evenly tile the Matrigel. The plates were then incubated at 37°C for 1 hr. After removing from the incubator, the cells were rinsed three times using sterile PBS buffer; 50 μL of 10 g ⋅ L−1 BSA was then added into each well for another 30 min of incubation. The cells in all the groups were then digested with 0.25% trypsin and the cell density was adjusted to 5 × 104 ⋅ mL−1. 100 μL of this cell suspension was inoculated into the Matrigel-filled wells in the 96-well culture plates. For each concentration, three parallel samples were prepared. The cells were cultured in DMEM supplemented with 10% FBS for 2 hr. After carefully discarding the culture medium, the cells were rinsed three times using PBS to wash away the free cells. To each well, 200 μL of 2% FBS-supplemented DMEM and 20 μL of 5 mg ⋅ mL−1 methyl thiazolyl tetrazolium (MTT) were added and incubated for 4 hr. After discarding the medium, 150 μL of DMSO was added into each well for a 10-min vibration. The absorbance (OD) of each well was measured at 490 nm, using the all-in-one microplate reader.
The cells were divided into five groups and the following were administered to each group: the control (normal saline group) received 0.9% saline (1 mL/day), the gestrinone group received gestrinone at a dose of 1.8 mg/mL/day), the low-dose Xiao Liu Fang group received 5 mg/mL/day of the Xiao Liu Fang extract, and the high-dose Xiao Liu Fang group received 10 mg/mL/day of the Xiao Liu Fang extract. All the treatments were administered once daily for 7 days, and the cell samples were collected 2 hr after administration.
Following transfer into 6-well plates for normal culture, the cells occupied 80% of the well on the following day, at which time the corresponding intervention drugs were added for a 24-hr intervention. All procedures were performed aseptically. Matrigel, stored at −20°C, was thawed overnight on ice at temperatures ranging between 2°C and 8°C. Then, 100 μL of Matrigel was added to 300 μL of pre-cooled serum-free medium and thoroughly mixed. The diluted Matrigel (25 μL) thus prepared was added to the upper chamber of the transwell plate and covered entirely with a polycarbonate ester film for 30 min at 37°C to enable Matrigel polymerization. The cells in each group were rinsed three times with PBS, and a single cell suspension was prepared using serum-free medium by conventional methods to achieve a cell density of 5 × 105 cells/mL. The placenta staining test requires a cell viability of over 95%. Then 100 μL of the cell suspension (5 × 104 cells) was added into the upper chamber of the transwell plate along with 200 μL of serum-free medium. After this, 500 μL of 10% FBS-supplemented DMEM was added into the lower chamber of the transwell plate, and the plate was subsequently incubated for 5 hr at 37°C at a CO2 concentration of 48%. After gently wiping the Matrigel and the cells on the upper surface of the polycarbonate film with a damp swab, a defined amount of ice-cold methanol was added to achieve a 30-min fixation. The film was stained with crystal violet (0.1%) for 30 min at room temperature, followed by rinsing with PBS two times. Five fields of view were then randomly selected for photography and counting.
The total RNA was extracted using Trizol reagent (Invitrogen, USA) and reverse-transcribed into cDNA. The 20-mL reaction system included 1 μL of 10 mol/L deoxy-ribonucleoside triphosphate (dNTP), 1 μL of random primer, and 2 μg of sample RNA, which were mixed thoroughly at 65°C for 5 min and then quickly introduced into ice water for centrifugation. Using 4 μL of 5× buffer, 2 μL DTT (100 mmol/L), 1 μL of RNAsin (40 U/mL), and 1 μL of Moloney murine leukemia virus reverse transcriptase (M-MLVRT; 200 U/mL), 37°C for 50 mi, 70°C for 15 min, and stored at −20°C. For PCR amplification, 10 μL of Sybr green, 2 μL of the upstream primer (5 mmol/L), 2 μL of 5 mmol/L of the downstream primer, and 2 μL of reverse transcription products were used. The amplification was performed for 40 cycles under the following conditions: 95°C for 10 s, 95°C for 5 s, 60°C for 20 s.
Data analyses were performed with the Prism 7500 SDS Software Analysis (ABI) program, and the expression levels of ICAM-1, COX-2,MMP-9, and VEGF mRNAs were calculated based on their relative expression levels against glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Immunocytochemical Staining
Cell Migration onto the Slide
The cell suspension (106/mL) was cultured in 6-well plates in a cell culture chamber for 24 hr. The cells migrated onto the slide when cell coverage was over 90% of the total plated area. The cells were fixed in 4% paraformaldehyde and permeabilized by adding 0.1%–0.2% polyglycol-octyl-phenyl ether. The cells were then incubated overnight at 4°C with 5% normal goat serum (Lanzhou min Hai Bioengineering, China) for 30 min along with an antibody at an antibody-to-vimentin ratio of 1:50 (Cedarlane, USA). PBS was added as a control for the antibody. The cells were incubated with a secondary antibody at 37°C for 30 min and stained by streptavidin-perosidase (SP).22
Immunofluorescence
After the cells had migrated onto the slide and had conjugated with the primary antibody, they were incubated with the secondary fluorescein isothiocyanate (FITC)-labeled goat anti-mouse antibody and protected from light at 37°C for 1 hr, and the nuclei were stained with DAPI. A seal (50%–90% glycerol) was applied, and the samples were observed under a light microscope.23
MTT assay showed that in comparison to groups N, B, and LD-TCM, the cells in groups HD-TCM and G were significantly inhibited with respect to their eutopic and ectopic adhesion ability (p < 0.01 or 0.05; Table 1; Figure 5).
Attachment
The attachment of endometrial cells to the extracellular matrix was measured by the MTT colorimetric assay.
The transwell cell-invasion assay showed that compared to that observed for groups N and B, the invasive ability of the eutopic and ectopic cells was significantly inhibited in the other intervention groups and was particularly significant in groups HD-TCM and G (Figure 6).
Aggression
The transwell invasion assay was used to calculate the number of aggressive cells. The mRNA expression levels of ICAM-1, COX-2, MMP-9, and VEGF were detected by RT-PCR.
The results of RT-PCR showed that compared to that observed for groups N and B, the expression levels of ICAM-1, COX-2, MMP-9, and VEGF mRNAs in groups HD-TCM and G were significantly reduced (p < 0.01 or 0.05; Figures 7 and 8).
Statistical Analyses
Statistical analyses were carried out using the SPSS22.0 software. All data were expressed as ± s and were analyzed by performing a variance analysis. p < 0.05 indicated statistical significance.
Author Contributions
H.Z. conducted the molecular studies and drafted the manuscript. Q.Z. participated in the laboratory measurement and data analysis. C.Q. conceived the study and contributed to the study design and coordination. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81574012), the Xinglin star plan of Shanghai, and the Inheriting talent training project of Shanghai school of traditional Chinese medicine (LPRC2017032).
References
- 1.Dunselman G.A., Vermeulen N., Becker C., Calhaz-Jorge C., D’Hooghe T., De Bie B., Heikinheimo O., Horne A.W., Kiesel L., Nap A., European Society of Human Reproduction and Embryology ESHRE guideline: management of women with endometriosis. Hum. Reprod. 2014;29:400–412. doi: 10.1093/humrep/det457. [DOI] [PubMed] [Google Scholar]
- 2.Yang X.H., Ji F., AiLi A., TuerXun H., He Y., Ding Y. Effects of laparoscopic ovarian endometriosis cystectomy combined with postoperative GnRH-a therapy on ovarian reserve, pregnancy, and outcome recurrence. Clin. Exp. Obstet. Gynecol. 2014;41:272–275. [PubMed] [Google Scholar]
- 3.Zhao Y., Gong P., Chen Y., Nwachukwu J.C., Srinivasan S., Ko C., Bagchi M.K., Taylor R.N., Korach K.S., Nettles K.W. Dual suppression of estrogenic and inflammatory activities for targeting of endometriosis. Sci. Transl. Med. 2015;7:271ra9. doi: 10.1126/scitranslmed.3010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szubert M., Suzin J., Duechler M., Szuławska A., Czyż M., Kowalczyk-Amico K. Evaluation of selected angiogenic and inflammatory markers in endometriosis before and after danazol treatment. Reprod. Fertil. Dev. 2014;26:414–420. doi: 10.1071/RD12258. [DOI] [PubMed] [Google Scholar]
- 5.Pluchino N., Taylor H.S. Endometriosis and Stem Cell Trafficking. Reprod. Sci. 2016;23:1616–1619. doi: 10.1177/1933719116671219. [DOI] [PubMed] [Google Scholar]
- 6.Han Y.F., Hou L.H., Zhou Y.J., Wu X.K. A survey of TCM treatment for endometriosis. J. Tradit. Chin. Med. 2009;29:64–70. doi: 10.1016/s0254-6272(09)60034-0. [DOI] [PubMed] [Google Scholar]
- 7.Jingwei C., Huilan D., Ruixiao T., Hua Y., Huirong M. Effect of Bushenwenyanghuayu decoction on nerve growth factor and bradykinin/bradykinin B1 receptor in a endometriosis dysmenorrhea mouse model. J. Tradit. Chin. Med. 2015;35:184–191. doi: 10.1016/s0254-6272(15)30026-1. [DOI] [PubMed] [Google Scholar]
- 8.Zhou H., Yin X.Q., Ma Q.L., Li J.H., Qi C. Eliminate tumor side effects on human endometrial stem cells apoptosis. Shanghai J. Traditional. Chin. Med. 2014;31:76–79. [Google Scholar]
- 9.Zhou H., Song L.N. Ovarian chocolate cyst surgery plus adjuvant treatment of Chinese and western medicine clinical observation on 120 cases. Chinese J. Traditional. Chin. Med. 2013;31:43–45. [Google Scholar]
- 10.Shimizu Y., Mita S., Takeuchi T., Notsu T., Mizuguchi K., Kyo S. Dienogest, a synthetic progestin, inhibits prostaglandin E2 production and aromatase expression by human endometrial epithelial cells in a spheroid culture system. Steroids. 2011;76:60–67. doi: 10.1016/j.steroids.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Benagiano G., Brosens I., Lippi D. The history of endometriosis. Gynecol. Obstet. Invest. 2014;78:1–9. doi: 10.1159/000358919. [DOI] [PubMed] [Google Scholar]
- 12.Hickey M., Ballard K., Farquhar C. Endometriosis. BMJ. 2014;348:g1752. doi: 10.1136/bmj.g1752. [DOI] [PubMed] [Google Scholar]
- 13.Sasson I.E., Taylor H.S. Stem cells and the pathogenesis of endometriosis. Ann. N Y Acad. Sci. 2008;1127:106–115. doi: 10.1196/annals.1434.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers P.A., Adamson G.D., Al-Jefout M., Becker C.M., D’Hooghe T.M., Dunselman G.A., Fazleabas A., Giudice L.C., Horne A.W., Hull M.L., WES/WERF Consortium for Research Priorities in Endometriosis Research Priorities for Endometriosis. Reprod. Sci. 2017;24:202–226. doi: 10.1177/1933719116654991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yotova I.Y., Quan P., Leditznig N., Beer U., Wenzl R., Tschugguel W. Abnormal activation of Ras/Raf/MAPK and RhoA/ROCKII signalling pathways in eutopic endometrial stromal cells of patients with endometriosis. Hum. Reprod. 2011;26:885–897. doi: 10.1093/humrep/der010. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Q.Y., Wu R.J. Growth mechanisms of endometriotic cells in implanted places: a review. Gynecol. Endocrinol. 2012;28:562–567. doi: 10.3109/09513590.2011.650662. [DOI] [PubMed] [Google Scholar]
- 17.Agic A., Djalali S., Diedrich K., Hornung D. Apoptosis in endometriosis. Gynecol. Obstet. Invest. 2009;68:217–223. doi: 10.1159/000235871. [DOI] [PubMed] [Google Scholar]
- 18.Kang S., Zhao J., Liu Q., Zhou R., Wang N., Li Y. Vascular endothelial growth factor gene polymorphisms are associated with the risk of developing adenomyosis. Environ. Mol. Mutagen. 2009;50:361–366. doi: 10.1002/em.20455. [DOI] [PubMed] [Google Scholar]
- 19.Xia J.F., Inagaki Y., Zhang J.F., Wang L., Song P.P. Chinese medicine as complementary therapy for female infertility. Chin. J. Integr. Med. 2017;23:245–252. doi: 10.1007/s11655-016-2510-5. [DOI] [PubMed] [Google Scholar]
- 20.Huang C., Song K., Ma W., Ding J., Chen Z., Zhang M. Immunomodulatory mechanism of Bushen Huoxue Recipe alleviates cyclophosphamide-induced diminished ovarian reserve in mouse model. J. Ethnopharmacol. 2017;208:44–56. doi: 10.1016/j.jep.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Chen S., Jin W., Huang J., Jiang H., Yao S. A modified filter method to isolate and culture human endometrial cell. Progress in Obstetrics & Gynecology. 2012;21:280–285. [Google Scholar]
- 22.Zhang L., Guo W., Chen Q., Fan X., Zhang Y., Duan E. Adam12 plays a role during uterine decidualization in mice. Cell Tissue Res. 2009;338:413–421. doi: 10.1007/s00441-009-0884-9. [DOI] [PubMed] [Google Scholar]
- 23.Wang L.N., Wang Y., Lu Y., Yin Z.F., Zhang Y.H., Aslanidi G.V., Srivastava A., Ling C.Q., Ling C. Pristimerin enhances recombinant adeno-associated virus vector-mediated transgene expression in human cell lines in vitro and murine hepatocytes in vivo. J. Integr. Med. 2014;12:20–34. doi: 10.1016/S2095-4964(14)60003-0. [DOI] [PubMed] [Google Scholar]