Abstract
Polycystic kidney diseases (PKDs) are a group of inherited nephropathies marked by formation of fluid-filled cysts along the nephron. Growing evidence suggests that in the kidney formation of cysts and alteration of cystic electrolyte transport are associated with purinergic signaling. PCK/CrljCrl-Pkhd1pck/CRL (PCK) rat, an established model of autosomal recessive polycystic kidney disease (ARPKD), was used here to test this hypothesis. Cystic fluid of PCK rats and their cortical tissues exhibited significantly higher levels of ATP compared to Sprague Dawley rat kidney cortical interstitium as assessed by highly sensitive ATP enzymatic biosensors. Confocal calcium imaging of the freshly isolated cystic monolayers revealed a stronger response to ATP in a higher range of concentrations (above 100 μM). The removal of extracellular calcium results in the profound reduction of the ATP evoked transient, which suggests calcium entry into the cyst-lining cells is occurring via the extracellular (ionotropic) P2X channels. Further use of pharmacological agents (α,β-methylene-ATP, 5-BDBD, NF449, isoPPADS, AZ10606120) and immunofluorescent labeling of isolated cystic epithelia allowed us to narrow down potential candidate receptors. In conclusion, our ex vivo study provides direct evidence that the profile of P2 receptors is shifted in ARPKD cystic epithelia in an age-related manner towards prevalence of P2X4 and/or P2X7 receptors, which opens new avenues for the treatment of this disease.
Keywords: ATP, PCK rat, Intracellular calcium flux, P2X receptors, Kidney, Polycystic kidney disease, ARPKD, P2rx7, P2rx4, P2X7, P2X4, Purinergic receptor
Autosomal dominant and recessive polycystic kidney diseases (ADPKD and ARPKD, respectively) are genetic disorders characterized by the development of renal cysts from tubular epithelial cells. ADPKD is caused by mutations in the PKD1 or PKD2 genes, which encode polycystins 1 and 2, membrane proteins involved in the regulation of cell proliferation that form a Ca2+-permeable cation channel [1]. ARPKD is a result of mutations in PKHD1, a gene encoding fibrocystin, which is a protein capable of interacting with the polycystin-1/2 complex [2]. Development of PKDs is a complex process which depends on inherited and acquired factors of cystogenesis, i.e., the balance between mutant protein expression and reactivity of the epithelia with surrounding tissues determines severity of disease complications [3].
In recent years, P2 (purinergic) receptors have been proposed to be potential targets for the treatment of various cardiorenal disorders with the use of P2 subtype-selective pharmacological tools [4–6]. A panel of studies report that P2X signaling is an important pharmacological target in hypertension; polymorphisms in P2X receptors reduce the risk of cardiovascular effects in humans [7, 8]. However, little is known about purinergic signaling in renal cyst epithelium, which is characterized by loss of polarity, dedifferentiation, and other abnormalities which can lead to purinergic signaling remodeling [9, 10]. This phenomenon has been the focus of recent comprehensive reviews [11, 12] due in no small part to reported high ATP concentrations in cystic fluid [13, 14] compared to normal nephron ATP concentrations [15].
Under normal conditions, ATP is a powerful regulator of epithelial transport and renal hemodynamics; it acts through metabotropic P2Y and ionotropic P2X receptor families [16–21]. G protein-coupled P2Y receptors were shown to mediate ATP-induced release of Ca2+ from intracellular stores [22–24]. P2X family receptors are non-selective ion channels, permeable for Ca2+ ions, and characterized by high affinity to extracellular ATP, which also makes them critical for controlling calcium-dependent intracellular mechanisms [4].
There is evidence that both P2Y and P2X receptors are potentially involved in different models of cystogenesis [11, 12]. P2X7, P2Y2, and P2Y6 receptors were highly expressed in a Han:SPRD cy/+ rat model of ADPKD [25]. Additionally, P2X7 agonists have been shown to be able to modulate the development of renal cysts in an in vitro model of cyst formation derived from the cpk/cpk mouse [26]. Finally, it was shown that inhibition of P2X7 receptors with antagonist A438079 or through morpholino-mediated knockdown of P2RX7 strongly regulates cyst development in the pkd2 knockdown zebrafish model of PKD [27]. We and others also recently reported the abnormal effects of ATP in cystic tubules isolated from PCK rats compared to normal tubules from Sprague-Dawley (SD) rats [28, 29].
The goal of this study was to profile P2 receptors in freshly isolated cysts from PCK rats using available pharmacological tools. Here, we characterized P2 signaling in ARPKD renal cysts to determine potential targets for pharmacological treatment of PKD. PCK rats, derived from the SD strain, carry a mutation in the Pck gene, the orthologous gene for human PKHD1, and serve as a well-established model of ARPKD [30, 31]. Pharmacological screening, calcium imaging, and immunostaining in the ex vivo preparation (freshly isolated ARPKD cystic epithelia) were employed here to test P2 receptor profiles using ATP-induced calcium response. Our data revealed the that highly expressed P2X receptors, P2X7 and P2X4, play a crucial role in ATP signaling in cystic epithelia. The downstream signaling cascade mediated by the corresponding purinergic channels may play an essential role in the development of cysts and progression of ARPKD.
Materials and methods
Animals
Male Sprague-Dawley and PCK rats (PCK/CrljCrl-Pkhd1pck/Crl) were purchased from Charles River. Animal use and welfare adhered to the NIH Guide for the Care and Use of Laboratory Animals following a protocol reviewed and approved by the IACUC at the Medical College of Wisconsin. Animals were maintained in a standard 12/12 dark/light cycle with water and food (no. 5L0D, LabDiet, St. Louis, MO) provided ad libitum.
Tissue harvesting and processing
Kidneys were flushed with PBS to remove blood as described previously [32]. Cystic monolayers were isolated from large developed cysts by microdissection under a dissection microscope and immediately used for experiments.
ATP level measurements in the kidney
In this study, microenzymatic ATP-sensitive biosensors (Sarissa Biomedical Limited, Coventry, UK) were used for the detection of ATP concentrations. Real-time extracellular ATP concentrations from kidneys of 10–12 weeks old SD and PCK rats were measured using amperometry detection in biosensors inserted into the kidney as described earlier [32, 33]. Microelectrode biosensors were inserted in the cortical tissue of freshly isolated kidneys or immersed in collected cystic fluid. For the collection of cystic fluid, full-size kidneys were snap-frozen in plastic vials, which were put into liquid nitrogen immediately after the tissue was harvested. Next, cortical cystic fluid was collected from kidney slices under binoculars using fine forceps (kidney slices were cut with a fine sharp blade and kept on dry ice to prevent collected frozen fluid from thawing). Frozen cystic fluid was immediately put into pre-chilled vials and frozen at − 80 °C until further analysis.
Calcium imaging in isolated cysts
At the age of 16 weeks, rats were euthanized and their kidneys were flushed, harvested and cut to 1–1.5-mm-thick slices. The internal monolayer of the cyst-lining cells was manually microdissected and attached to a cover glass as described earlier [29]. Samples were incubated with 3.5 μM Fluo-8 dye (no. 21091 AAT Bioquest, Sunnyvale, CA) in presence of 0.05% pluronic acid (no. F-68, Sigma, St. Louis, MO), then rinsed and imaged via confocal laser-scanning fluorescence microscopy with a Leica TCS SP5 system (IRAPO 25x, NA 0.95 objective lens). The bath solution contained (in mM) 145 NaCl, 4.5 KCl, 2 MgCl2, 2 CaCl2 (except for calcium-free experiments), and 10 HEPES (pH 7.35). Treatment with pharmacological agents targeting P2 receptors was performed as described in Table 2. The basal calcium level in the cystic epithelium was evaluated by the application of 10 μM ionomycin followed by 1 mM MnCl2. ATP was obtained from Sigma (St. Louis, MO; no. A2383). P2 receptor modulating compounds were obtained from Tocris/Bio-Techne (Minneapolis, MN): α,β-meATP (no. 3209), 5-BDBD (no. 3579), NF 449 (no. 1391), AZ10606120 (no. 3323), and isoPPADS (no. 0683) and diluted according to manufacturer’s guidelines.
Table 2.
P2 receptor pharmacology used
| Target receptor | IC50/EC50 | Applied concentrations | |
|---|---|---|---|
| P2 Receptors Agonists | |||
| αβmeATP | Most P2X (P2X4 in high concentrations) | > 100 μM for P2X4; 0.5 μM for other P2X | 50–400 μM |
| P2 Receptors Antagonists | |||
| 5-BDBD | P2X4 | 0.50 μM | 10 μM |
| NF 449 | P2X1/P2X1/5/P2X2/3/P2X3/P2X2/P2X4 | 0.28 nM/0.69 nM/120 nM/1820 nM/47 μM/300 μM | 40 μM |
| AZ10606120 | P2X7 | 20 nM | 10 μM |
| isoPPADS | non-selective P2X antagonist, affects P2Y (>IC50 ~ 0.9 mM) | 1–2.6 μM | 20 μM |
Western blotting
Kidney cortical lysates were prepared as previously described [34]. The PCK rat kidneys were excised and cut into 1–2 mm slices under a dissecting microscope with × 6 magnification. The approximate apical kidney cortex sections were carved and then diced into small pieces with a razor blade. Samples were pulse sonicated in Laemmli buffer with a protease inhibitor cocktail (Roche) for 10 s and spin cleared at 10,000×g for 10 min. The resulting supernatant was subjected to PAGE, transferred onto nitrocellulose membrane (Millipore) for probing with P2X4 and P2X7 antibodies (1:1000; Alomone, APR-002 and APR-008, correspondingly), and subsequently visualized by enhanced chemiluminescence (ECL; Amersham Biosciences).
Histochemistry
Formalin-fixed kidney sections were cut into 4 μm slices, dried and deparaffinized for subsequent streptavidin-biotin immunohistochemistry. After deparaffinization, the slides were treated with a citrate buffer (pH 6) for a total of 35 min. The slides were blocked with a peroxidase block (DAKO, Carpinteria, CA, USA), Avidin Block (Vector Labs, Burlingame, CA, USA), Biotin Block (Vector Labs), and serum-free Protein Block (DAKO). For P2Y2 and P2X7 staining, kidney sections were embedded in one paraffin block for tissue microarrays and slices were incubated 1:200 overnight with antibodies (P2Y2: Alomone APR-010, P2X7: Alomone APR-008).
Immunofluorescence and imagining
Freshly isolated cysts were stripped of adventitia and allowed to adhere to microscopy slides double-coated with poly-l-lysine. Sectioned tissue was fixed with chilled 4% paraformaldehyde in PBS for 20 min, then washed 5× with ice-cold PBS. Next, cells were permeabilized with a 2% bovine serum albumin + 0.1% Triton-X100 PBS solution for 30 min and washed 5× with chilled PBS. Cyst sections were probed with primary antibody (1:100) overnight in a 2% BSA-PBS solution at 4 °C (P2X4:Alomone APR-002, P2X7: Alomone APR-008, AQP2: Santa Cruz sc-9882). The following day, tissue sections were washed 5× with cold PBS and incubated with appropriate Alexa fluorophore-labeled secondary antibodies (1:500, ThermoFisher, Pittsburgh, PA) in a 2% BSA-PBS solution at room temperature in the dark. Following 5 PBS washes, cysts were incubated with 1 μg/mL Hoescht nuclear stain in PBS for 10 min at room temperature in the dark. After a final 5 washes with PBS, tissue was preserved and coverslip-mounted with Vectashield (Vector Laboratories, Burlingame, CA). Images were captured on a confocal Nikon A1R inverted microscope using a Plan Apo 60× Oil DIC objective with 1.4 numerical aperture controlled by Nikon Elements AR software (Nikon, Tokyo, Japan).
RNA isolation and PCR analysis
Sections of whole kidney were flash frozen in liquid nitrogen. Total RNA was extracted using TRIzol Reagent (ThermoFisher) according to the manufacturer’s protocol. Total RNA quantity was determined by Nanodrop 2000 (ThermoFisher), and cDNA from 1 μg of RNA was synthesized using the RevertAid First-Strand cDNA Synthesis Kit (ThermoFisher). SYBR Green real-time PCR reactions were carried out on an ABI Prism 7900HT (ABI, Applied Biosytems, Foster City, CA) using Bullseye EvaGreen qPCR Master Mix (MedSci, Valley Park, MO) according to the manufacturer’s directions in 10 μL final volume with samples run in triplicate. Exon spanning primers were designed from the rat sequences of P2rx4, P2rx7, and 18S (Sigma-Aldrich) (Table 1) and assessed for specificity by sequencing the PCR product. Negative controls for the reverse transcription reactions were included to confirm the absence of genomic DNA. Final Ct values were determined using SDS software, version 2.3, and quantification of P2rx4 and P2rx7 mRNA copy number was determined by normalizing to 18S.
Table 1.
qPCR primer sequences
| Gene | Forward | Reverse |
|---|---|---|
| P2xr4 | GACGTGGCGGACTATGTGAT | TCTTCCAGTCGCAACTCCAC |
| P2xr7 | CAAAGGTCAAGAGGTCCCGAGAC | GCCCTGCGGTTCTCTGGTAG |
| 18S | CGGCTACCACATCCAAGGAA | CCTGTATTGTTATTTTTCGTCACTACCT |
Statistical methods
Data are presented as mean ± SEM. Following normality and equal variance tests, data were compared using the t test or one-way ANOVA with Tukey comparison of means. P < 0.05 was considered significant.
Results
ATP level in rat cortical tissues and cystic fluid
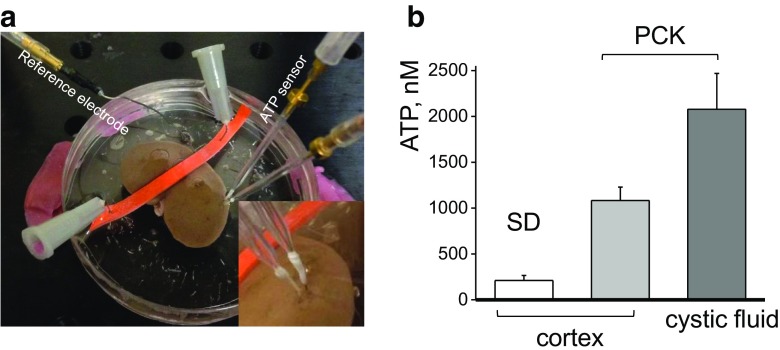
It was previously proposed that the purinergic signaling axis is disturbed in ARPKD, although no exact mechanism or any direct proof of the involvement of this type of signaling in the development of the disease has been discovered so far. High micromolar concentrations of ATP were detected in the fluid from microdissected human ADPKD cysts, and enhanced ATP exocytosis from an ARPKD epithelial cell model has been shown [14]. Here, we compared the levels of ATP in the cortex of the freshly isolated SD and PCK rat kidneys using an enzymatic biosensors approach, which allows detecting a wide range of ATP concentrations [35]. Microenzymatic biosensors were inserted either in the cortex of the whole freshly isolated kidney or immersed into the cystic fluid. The detected concentrations of ATP were more than five times higher in PCK rat cortex (211 ± 55 vs 1082 ± 147 nM for SD and PCK cortex tissue correspondingly), and up to ten times for isolated cystic fluid (2078 ± 391 nM), compared to normal SD cortex (Fig. 1).
Fig. 1.
ATP levels in PCK cystic fluid and cortical tissues of the isolated kidneys from PCK and Sprague-Dawley (SD) rats. a Enzymatic biosensor setup for detection of ATP in the isolated kidney tissues. ATP and Null (to subtract noise and non-selective interferences) biosensors were inserted into the kidney cortical layer and connected to a dual channel potentiostat for real-time amperometry recordings. Inset shows a close-up image with sensors’ tips inserted into a cortex of the freshly isolated kidney from PCK rat. b ATP concentrations were detected using enzymatic biosensors in the cortical tissues of the freshly extracted kidneys from PCK and SD rats and isolated cystic fluid from PCK rats (N = 4 rats for each group)
Calcium transients evoked by purinergic agonists in isolated ARPKD cysts
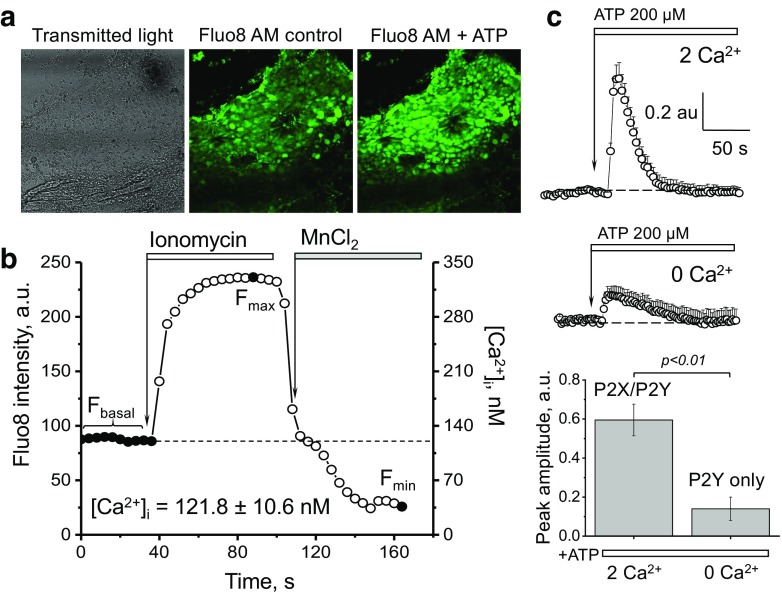
To test calcium handling and response to ATP [36] in the ARPKD cystic epithelia, we manually isolated cystic epithelial monolayers from the PCK rat kidney cortex, loaded them with a fluorescent calcium dye (Fluo-8) (see Fig. 2a for a representative image of the Fluo-8 loaded monolayer of cystic cells), and performed confocal calcium imaging. First, we assessed the basal calcium level in the cystic epithelium using a standard ionophore/MnCl2 approach [36] (Fig. 2b); these experiments revealed that basal intracellular calcium ([Ca2+]i) level in the cystic epithelial cells was 121.8 ± 10.6 nM.
Fig. 2.
Calcium level and response to ATP in the freshly isolated PCK rat cystic epithelium. a A representative confocal image showing a cystic monolayer isolated from a PCK rat kidney (bright field image, and the same cystic monolayer loaded with Fluo8 calcium dye before and after application of ATP). Scale bar is 100 μm. b Experimental protocol to determine intracellular calcium concentrations in the cells of the cystic epithelium. Average basal calcium concentration in the cystic cells is shown on the graph. To measure intracellular calcium concentration, cystic monolayers were loaded with Fluo-8, AM, and fluorescence intensity was recorded in the baseline and after addition of ionomycin and MnCl2. The graph demonstrates the fluorescence signal changes in response to a calcium ionophore (ionomycin, producing the maximum Fluo-8 fluorescence, Fmax) and MnCl2, which quenches the dye and results in the lowest fluorescence intensity (Fmin). Intensity of fluorescence (left axis) for each time point was recalculated into the actual calcium concentration in nanomoles (right axis). The transient shown on the graph reflects fluorescence intensity of a ROI (single cell) selected from a cystic monolayer; images were taken every 8 s. Calcium levels were assessed in 12 cystic monolayers isolated from 6 different rats, and 140 ROIs containing multiple cells were analyzed in this group. c Representative calcium transients recorded in an isolated cystic monolayer. The fluorescent responses to 200 μM ATP application in media containing 2 mM of Ca2+ (upper panel) and the same ATP concentration without extracellular Ca2+ (middle panel). Summary for the changes of the amplitude of intracellular calcium concentration in response to ATP with or without the presence of Ca2+ ions in extracellular solution (N ≥ 6 cyst monolayers for each group)
We then asked whether ATP elevates [Ca2+]i and whether it is ionotropic P2X or metabotropic P2Y receptors that account for the calcium level change in response to ATP. To address this question, we applied a high concentration of ATP (200 μM) to the cystic monolayers in the absence or presence of 2 mM CaCl2 in the bath solution; calcium transients in the calcium-free solution were significantly blunted compared to calcium-containing solution (the maximum response was 0.14 ± 0.06 versus 0.60 ± 0.08 a.u., respectively; Fig. 2c). The area under curve (AUC), which accounts for the total calcium release, was also dramatically decreased in the absence of calcium (11.6 ± 0.8 versus 22.3 ± 1.8 a.u). Therefore, we hypothesized that the major component of the calcium elevation in response to ATP is mediated via ionotropic P2X receptors.
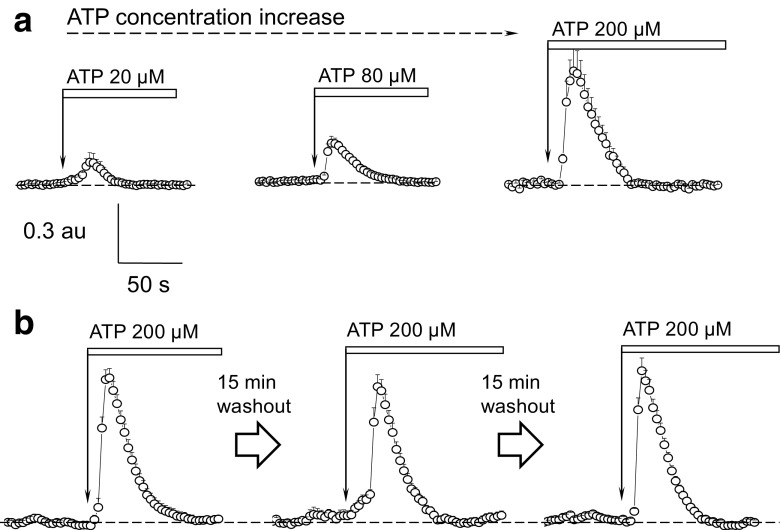
Application of various concentrations of ATP in the calcium-containing solution demonstrated that this effect was dose-dependent and further allowed us to establish a specific concentration for subsequent imaging experiments involving pharmacological modulators of purinoceptors activity. Use of lower concentrations of ATP (20 and 80 μM) resulted in small calcium transients, whereas 200 μM of ATP caused a robust increase in calcium influx (Fig. 3a) (three cystic monolayers were tested in each group from three different rats). According to the published ATP concentration dependence for the rodent P2X receptors [37], P2X4 and P2X7 have the closest ATP dose response characteristics to those described in the current literature, as they respond to higher ATP concentrations. We further conducted control experiments to ensure that the response to ATP is sustained after the washout, and it is feasible to perform pharmacological testing. Figure 3b demonstrates a response of the same cystic monolayer to three consecutive applications of ATP interspersed with 15 min washout periods. As clearly seen from the representative transients, the response remains stable, and no desensitization of the receptors is observed after 15-min washout (maximum amplitude of the transient in response to ATP: 1.01 ± 0.07 during the first application of ATP, 0.9 ± 0.08 and 1.04 ± 0.09 a.u. after the first and second washouts, respectively; 5 cystic monolayers were tested from 3 different rats).
Fig. 3.
Calcium response in the cystic monolayer is triggered by high concentrations of ATP and consecutive ATP applications/washouts protocol do not cause P2 receptor desensitization. a Changes of the cystic cells’ intracellular calcium levels in response to various concentrations of ATP. Shown are representative transients from different cystic monolayers (12÷15 cells/ROIs were analyzed per each transient). b Representative calcium transients in response to three consecutive applications of ATP interleaved with 15-min long washout periods; 15 cells/ROIs were analyzed per each transient
α,β-Methylene adenosine 5′-triphosphate (αβmeATP), a phosphonic analog of ATP and a common agonist of the P2X receptors, was further used to detect specific P2 receptors involved in elevation of [Ca2+]i in cystic cells. αβmeATP has the EC50 ~ 1 μM for P2X1 and P2X3 and ~ 1000-fold less potent for P2X2 and P2X4,5,6,7 receptors [38–40]. Similarly to ATP, high concentrations of αβmeATP (~ 400 μM) were required for the elevation of [Ca2+]i in the cystic monolayer (Fig. 4a) (for three cystic monolayers, 12÷15 cells/ROIs analyzed per each transient) from 3 different animals per group). There are two potential P2X receptors, P2X4 and P2X7, with a low affinity to both αβmeATP and ATP [5, 41, 42]. Figure 4b also shows that the [Ca2+]i transient was reduced in response to αβmeATP compared to ATP, and that amplitude of the response to ATP was reproducible following the washout of αβmeATP (5÷8 cystic monolayers were tested in each group from 4 different rats). Summary data for the high agonist concentrations (200 μM) are shown in Fig. 4c. The amplitude of the response to ATP was significantly higher than response to αβmeATP. However, total Ca2+ production (as represented by AUC) did not reach statistical difference due to the slow transient decay of abMeATP transient.
Fig. 4.
Calcium transient in the cystic monolayer in response to P2X receptor agonist αβmeATP. a Representative changes of the cystic cells’ intracellular calcium levels in response to various concentrations of α,β-methylene-ATP (αβmeATP). Shown are representative transients from different cysts (12÷15 cells/ROIs were analyzed per each transient). b Response to ATP in a cystic monolayer is sustained after application of αβmeATP with 15-min-long washouts between applications of the compounds. N = 4 rats per group, 5÷8 cystic monolayers were tested in each group. c Summary for the amplitude and total [Ca2+]i production (AUC) for ATP and αβmeATP (200 μM each) (N ≥ 3 cyst monolayers for each group at selected concentration of agonist)
Pharmacological screening of the P2 receptor profile in freshly isolated cystic epithelia
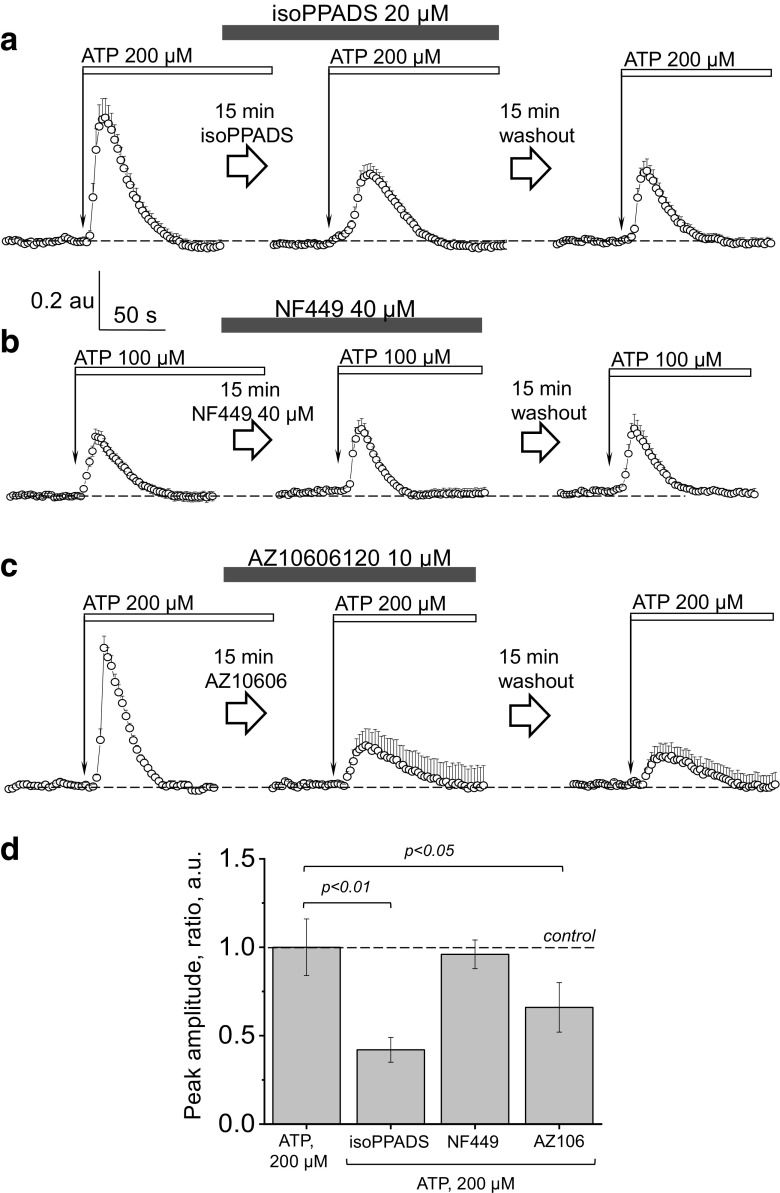
To further study the profile of P2X receptors in cystic cells, we tested [Ca2+]i transients in response to ATP in the presence of various pharmacological modulators of the ionotropic purinoceptors (see Table 2 for the list of used agonists and antagonists). The same experimental design was used in all the conducted experiments: first, an application of ATP in a high micromolar range was performed to establish the control level of the response. Then, during the washout period (15 min), the drug was added to the bath solution, and incubation was performed. Next, the same concentration of ATP was applied to the cystic monolayer in the presence of antagonist. Following a second washout, ATP was applied again to test the reversibility of the response. As shown in control experiments on Fig. 3b, three consecutive applications of ATP are feasible (following the washouts) and produce comparable responses.
In order to confirm our initial observations suggesting P2X purinoceptors are the primary receptors mediating ATP-induced calcium transients, we employed isoPPADS (a non-selective P2X antagonist). As seen in Fig. 5a, isoPPADS resulted in a blunted response to ATP compared to control (the AUC (total calcium release) were 30.1 ± 0.9, 22.2 ± 1.8 and 19.5 ± 3.2 a.u., 3 cystic monolayers; 11÷15 cells/ROIs analyzed per each transient from 3 different rats) in control, after incubation with isoPPADS, and after the second washout, respectively. Notably, from the P2X family, rat P2X4 purinoreceptor is only P2X receptor relatively insensitive to PPADS (although it is able to fully inhibit mouse and human receptors with pIC50 ~ 5) [43].
Fig. 5.
The effects of P2X antagonists isoPPADS, NF449 and AZ10606120 on calcium transients evoked by ATP in freshly isolated cystic epithelium. ATP was applied to establish the control level of the response (left), then, during the washout period, isoPPADS (a), NF449 (b), or AZ10606120 (c) were added to the bath solution, and incubation was performed (15 min); ATP was applied to the cystic monolayer in presence of the antagonist (middle). Following a second washout of all applied drugs (next 15 min), ATP was applied again to test the reversibility of the response (right). Shown are the representative responses from single cystic monolayers (11÷15 cells/ROIs were analyzed per transient). d The summary of the changes in the [Ca2+]i transient amplitude for the applied agonists/antagonists of purinergic signaling to the cystic epithelial cells (N = 3–8 cyst monolayers for each group)
To narrow down the list of candidate P2X receptors, we tested NF449 (a potent purinergic receptor antagonist that displays high selectivity for P2X1) (IC50 values are 0.28, 0.69, 1.20, 1.820, 47.000, and > 300.000 nM for P2X1, P2X1 + 5, P2X2 + 3, P2X3, P2X2 and P2X4 receptors, respectively). The tested concentration of 40 μM did not result in any decrease in the ATP-mediated calcium transient (Fig. 5b); in the tested concentration, NF449 did not exert any effects on P2X4 or P2X7 (4 cystic monolayers, 11÷15 cell/ROIs analyzed per each transient, from 4 different rats); these data suggest that the observed response to ATP is likely mediated via one or both of these two receptors. Next, to specifically assess the role of P2X7 in the ATP-mediated calcium influx, we employed AZ10606120, which is a potent P2X7 receptor antagonist. [44] As seen from Fig. 5c, application of AZ10606120 (10 μM) resulted in a bunted calcium response to ATP (32.4 ± 2.2, 13.5 ± 6.4, and 11.1 ± 3.1 a.u. of total calcium release in control, after incubation with AZ10606120, and after washout, respectively; P < 0.05 ANOVA; for 4 cystic monolayers, 11÷15 cells/ROIs analyzed per each transient, from 4 different rats), which corroborates the hypothesized role of P2X7 in cyst development. The summary of the changes in the [Ca2+]i transient amplitude for the applied agonists/antagonists of purinergic signaling are shown at Fig. 5d (1.0 ± 0.16; 0.42 ± 0.07; 0.96 ± 0.08 and 0.66 ± 0.14 for the ATP, isoPPADS, NF449, and AZ10606120).
In order to reveal the involvement of P2X4 in calcium influx, we tested the effect of 5-BDBD (5-(3-bromophenyl)-1,3-dihydro-2H-benzofuro[3,2-e]-1,4-diazepin-2-one), a well-known potent antagonist of this purinoreceptor. Incubation with 5-BDBD resulted in reduction of the response to both ATP (Fig. 6a) and αβmeATP (Figs. 6b), and the observed block was not reversed by a washout. The maximum amplitude of the response after incubation with 5-BDBD was reduced from 0.71 ± 0.06 to 0.27 ± 0.04 and from 1.1 ± 0.07 to 0.22 ± 0.05 a.u. in response to ATP and αβmeATP, respectively (P < 0.05 ANOVA; for 9 cystic monolayers (9÷13 cells/ROIs analyzed per each transient, from 7 different rats for each drug). The summary of the changes in the [Ca2+]i transient amplitude for the applied purinergic agonists/5-BDBD are shown at Fig. 6c (1.0 ± 0.16, 0.33 ± 0.08, 0.25 ± 0.03 and 0.11 ± 0.05 for the ATP, αβmeATP and ATP or αβmeATP in the presence of 5-BDBD—right panel). These data allowed us to conclude that in addition to P2X7, P2X4 is an ionotropic receptor involved in handling of ATP-mediated calcium influx in cystic epithelium.
Fig. 6.
P2X4 receptor inhibition results in a blunted calcium transient in response to ATP or αβmeATP. The specific P2X4 receptor inhibitor 5-BDBD was applied to the cystic monolayers following an application of ATP (a) or αβmeATP (b) and a washout. After incubation with 5-BDBD was performed, ATP was applied to the cystic monolayer in presence of the antagonist; then following a second washout, ATP was applied to test the reversibility of the response. Representative responses from single cystic monolayers are shown. c Summary of the changes in the [Ca2+]i transient amplitude for the applied agonists/5-BDBD of purinergic signaling to the cystic epithelial cells (N = 3–8 cyst monolayers for each group)
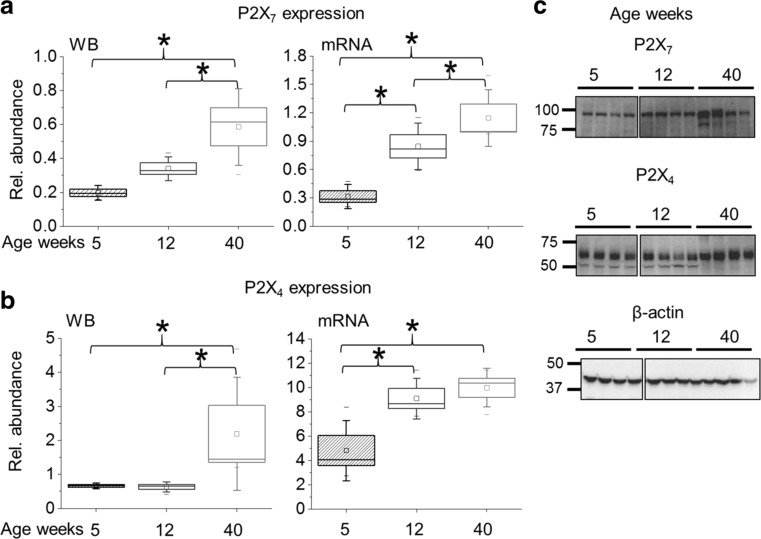
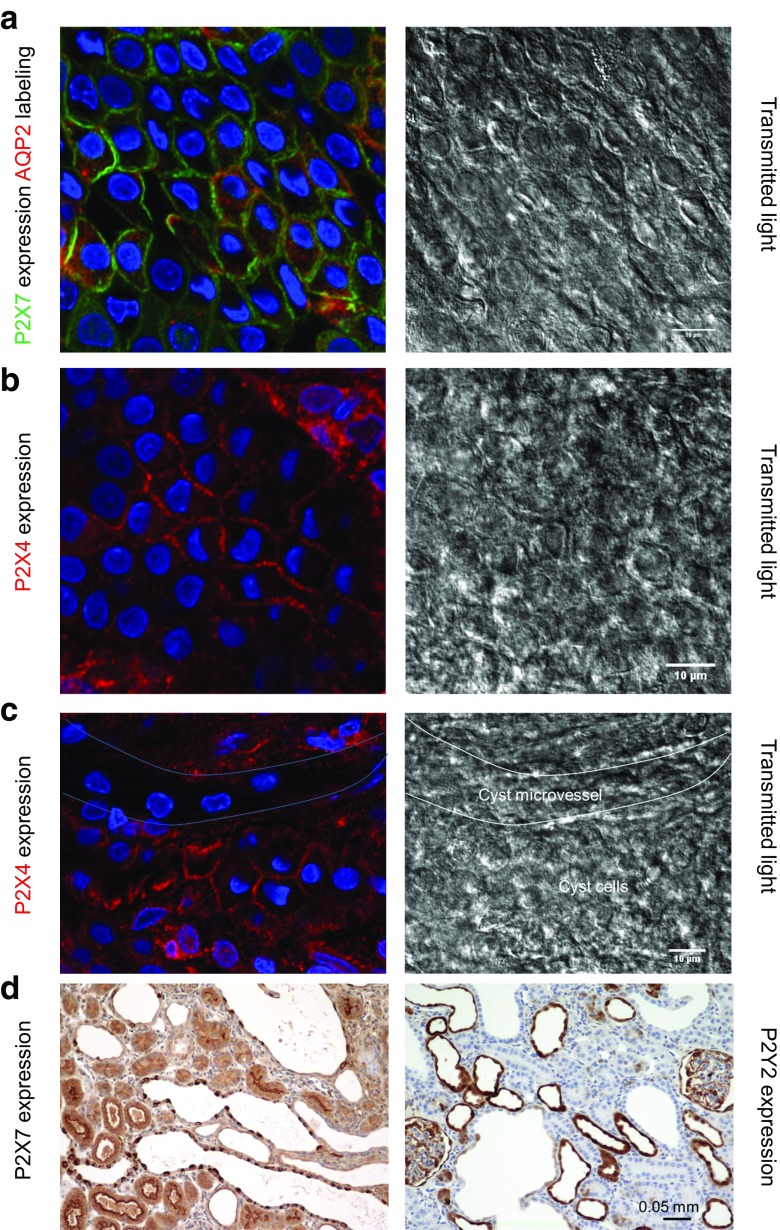
To detect changes in the expression of identified P2X receptors, we performed Western blot and RT-PCR analyses from samples of the kidney cortex of 5, 12, and 40 weeks old PCK rats when cysts are in the initial stage of development or later in the progression of disease. The expression of both P2X7 and P2X4 receptors increased significantly during the progression of ARPKD (Fig. 7). Moreover, immunostaining and fluorescence microscopy of isolated cystic epithelia showed strong localization of both types of receptors at the plasma membrane (Fig. 8a, b). In contrast, these receptors were absent in the cells of cystic microvessels (Fig. 8c). Finally, immunohistochemical staining in the kidney confirmed strong expression of P2X7 receptors in cystic cells, while P2Y2, metabotropic receptors typically expressed in cortical collecting ducts, is observed in small, early cysts and healthy tubules, but not in larger cysts (Fig. 8d).
Fig. 7.
Expression of P2X7 and P2X4 receptors in the cortex of PCK rats during the development of cytogenesis. Average relative density analysis for Western blotting (N ≥ 8) and mRNA levels (N ≥ 6) of P2X7 (a) P2X4 receptors (b), and corresponding for the P2X7 receptor (normalized to loading controls) in the kidney cortex lysates of 5, 12, and 40 weeks old PCK rats. c Examples of the corresponding Western blotting (each line represents 1 rat)
Fig. 8.
Intracellular localizations of P2X7 and P2X4 receptors in PCK cysts. Transmitted light images depict the epithelial cellular structure in cystic monolayers. Due to the thin, squamous nature of the epithelia, nuclear protrusions can occasionally be observed in the transmitted light. Freshly isolated cyst sections were fixed and probed for P2X7 (green) and AQP2 (red) (a). Cells demonstrate distinctive membrane localization in classic Voronoi patterning for both P2X7 and AQP2. b P2X4 channels (red) are also targeted to the plasma membrane of cyst epithelia. c PCK cyst epithelia contain unique microvasculature. In this image, the intermingling microvessel can be observed by lack of P2X4 staining and structural features in the transmitted light. Approximate outlines of the cystic and vessel regions are shown in the transmitted light image. d Polycystic kidneys were embedded in paraffin, sectioned, and probed for P2X7 and P2Y2. Similar to the isolated cyst epithelial staining, P2X7 is observed in cystic cells, while P2Y2 are observed in small, early cysts, but not in larger cysts
Discussion
The therapeutic potential of targeting P2 pathways in polycystic kidney diseases has recently been attracting research interest. P2 signaling is tightly bound to regulation of epithelial ion and water transport, which is recognized as a major factor mediating cystic fluid accumulation and cyst progression. It is well known that the expression of purinergic receptors in different tissues including kidney epithelia is dynamic and depends on age, disease states, and other factors [45]. In normal tubules, ATP downregulates sodium reabsorption [21] via inhibition of epithelial Na+ channel (ENaC) through the purinergic P2Y metabotropic receptors cascade [46]. This could result in an increase of transepithelial fluid secretion, driven by active Cl− transport [47]. The cyst-lining cells exhibit impaired ENaC-mediated Na+ reabsorption [48] and active fluid secretion driven by the Cl− transport [49, 50]. Fluid secretion was also found to depend on extracellular ATP in MDCK cell culture and to act synergistically with purinergic signaling. This was confirmed by either the addition of ATP scavenger apyrase or P2 receptor blocker suramin [51].
Previously, it has been proposed that the purinergic signaling axis is disturbed in ARPKD, although there is no exact mechanism known and no specific research has been conducted to confirm of the involvement of this type of signaling in the development of the disease. Micromolar concentrations of ATP were detected in the fluid from microdissected human ADPKD cysts, and enhanced ATP exocytosis from ARPKD epithelial cell model has been shown [13, 14]. Moreover, degradation of ATP released from the cystic epithelia could take hours (with only 50% of the initial ATP concentration being degraded after approximately 4 h) [14]. This mechanism of slow ATP degradation in ADPKD cells could be due to reduced expression of CD39 as was shown previously [52]. Here, we compared the concentration levels of extracellular ATP in the cortex of freshly isolated SD and PCK rat kidneys using a novel enzymatic biosensors approach, which is a real-time measurement capable of detecting the full physiological range of ATP in tissues [32, 33]. These experiments allowed measurement of ATP in freshly isolated preparations of whole rat kidney. Our results demonstrate that concentrations of ATP were higher in PCK rats, compared to normal SD cortex (Fig. 1) proving that ATP concentrations are significantly increased in the ARPKD animals compared to the controls. This finding supports the idea that the P2 signaling axis in cystic cells is altered and warrants further experiments. We used a unique preparation of the cystic epithelium freshly isolated from the PCK rat cysts and employed calcium imaging with a set of pharmacological modulators to determine the presence and functional role of purinergic receptors in the cystic epithelial cells. The involvement of P2Y receptors in calcium-dependent chloride secretion was shown previously in cultured collecting duct cells [53]. However, initial experiments in freshly isolated cystic monolayers revealed sensitivity to high ATP concentrations, low calcium release form intracellular stores, and sustained response to P2X receptor activator αβmeATP, which demonstrates a modest contribution of the P2Y component in the cystic cell response to ATP. Further, the combination of pharmacological tools and calcium imaging allowed us to narrow down the cystic P2 profile to P2X4 and/or P2X7 receptors.
The release of ATP is the key element of the purinergic signaling system. Kidney cells, including renal tubular cells, are capable of releasing physiologically relevant amounts of ATP in response to different stimuli including mechanical (blood pressure) or hormonal (such as vasopressin) changes [33, 54]. It is well established that ATP and its metabolites can stimulate Cl− secretion across epithelia derived from airway, gastrointestinal, and renal tissues [14, 55]. The abnormally potentiated ATP release observed in both ARPKD and ADPKD was shown to mediate stimulation of Cl− secretion via cytosolic Ca2+-dependent signaling, which could be a potential mechanism of cyst growth and expansion [14, 56]. Under normal conditions, P2Y2 receptors stimulate cystic fibrosis transmembrane conductance regulator (CFTR) Cl− secretion and reduce ENaC Na+ reabsorption [45]. As we discussed previously [12], significant increases in mRNA and protein expression levels of P2X7, P2Y2, and P2Y6 receptors throughout disease progression were detected in a Han:SPRD cy/+ rat model of ADPKD [25]. We also found a strong increase in P2X4, in addition to P2X7, which was not tested in the Han:SPRD cy/+ rats. Our calcium data, as well as IHC staining, however, shows the absence of P2Y2 receptors in the developed cystic epithelia. During the development of pathologies such as ARPKD, however, P2X4 and/or P2X7 receptors may become main regulators of secretion in conjunction with purinergic agonists or hormones. Importantly, the P2X receptors are non-specific cation channels that allow movement of Ca2+, Na+, and K+ ions along electrochemical gradient. In contrast, P2Y receptors do not actively transport ions, but rather trigger downstream signaling cascades that affect ion transport indirectly. Both P2X4 and P2X7 receptors can have relatively high calcium permeability, and under prolonged agonist exposure, P2X4 and P2X7 receptors can become permeable to large organic cations such as NMDG [38], which might also contribute to cyst development.
Previous work has shown that the P2X4 receptors (expressed in renal epithelia) are strongly linked to the regulation of epithelial sodium transport and particularly ENaC activity [57]. Interestingly, it was reported that activation of purinergic cascades could lead to either activation or inhibition of ENaC, depending on the localization of the P2X4 receptor (on the basolateral or apical membrane) [4]. We have previously shown that impaired ENaC activity contributes to cytogenesis [48], and thus apical expression and activation of P2X4 could be involved in PKD progression.
Several studies also suggested that P2X7 receptor is a potential target for treatment of PKD. Hillman and colleagues demonstrated a non-apoptotic role for P2X7 in cyst enlargement in cpk/cpk mice [26]. In vitro experiments revealed reduced cystogenesis in cell culture in the presence of bzATP, a non-specific P2X7 agonist [58]. In additional studies, a morpholino-driven knockout of p2rx7 in zebrafish was shown to have a beneficial effect in embryos developing cyst-like formations in pronephros [27]. In non-cystic cortical duct cells, P2X7 receptors have been found to be involved in production of endothelin-1, a powerful tubular transport regulator [59–61]. It is important to note that of all members of the P2X family, P2X7 and P2X4 are the most closely related, with a 49.8% similarity in amino acid sequence in rat [4, 62]. Moreover, they can form functional heteromeric channels [63] or regulate expression of each other in the kidney, where knockout of one of them inhibits the expression of the other [4]. However, it does not exclude the possibility that in pathological conditions blockade or knockout of either P2X7 or P2X4 may result in compensatory overexpression of the other.
In conclusion, our experiments demonstrate the significant involvement of the P2X4 and P2X7 signaling axis in the regulation of cytosolic calcium levels in cystic epithelia. Our ex vivo study provides evidence that the P2 receptor profile is shifted in ARPKD cystic epithelia towards predominance of P2X receptor activity. Remodeling of the purinergic signaling axis in cystic cells (changes from metabotropic P2Y to ionotropic P2X receptors) occurring in ARPKD may lead to altered calcium influx in these cells, and subsequent changes in intracellular signaling cascades. Therefore, targeting P2X signaling in the ARPKD cysts (specifically, P2X4 and/or P2X7) opens new avenues for the treatment of this disease.
Acknowledgements
Christine Duris (Children’s Hospital of Wisconsin) and Elena Sorokina (Department of Pediatrics, Medical College of Wisconsin) are recognized for excellent technical assistance with immunostaining and RNA isolation, respectively.
Funding information
This research was supported by the National Institute of Health grants: R35 HL135749 (to A.S.); R00 HL116603 and P30 DK090868 via Baltimore PKD Center P&F Grant (to TSP); R00 DK105160 and PKD Foundation (221G18a) award (to DVI); T32 HL134643 and CVC A.O. Smith Fellowship (to C.A.K); and American Heart Association grants: 16EIA26720006 (to A.S.) and 17SDG33660149 (to OP); and Department of Veteran Affairs I01 BX004024 (AS).
Conflict of interest
Oleg Palygin declares that he/she has no conflict of interest.
Daria V. Ilatovskaya declares that he/she has no conflict of interest.
Vladislav Levchenko declares that he/she has no conflict of interest.
Christine A. Klemens declares that he/she has no conflict of interest.
Lashodya Dissanayake declares that he/she has no conflict of interest.
Anna Marie Williams declares that he/she has no conflict of interest.
Tengis S. Pavlov declares that he/she has no conflict of interest.
Alexander Staruschenko declares that he/she has no conflict of interest.
Ethical approval
Animal use and welfare adhered to the NIH Guide for the Care and Use of Laboratory Animals following a protocol reviewed and approved by the IACUC at the Medical College of Wisconsin.
Contributor Information
Tengis S. Pavlov, Phone: (313) 916-7198, Email: tpavlov1@hfhs.org
Alexander Staruschenko, Phone: (414) 955-8475, Email: staruschenko@mcw.edu.
References
- 1.Torres VE, Harris PC. Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol. 2006;2(1):40–55. doi: 10.1038/ncpneph0070. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann C, Senderek J, Kupper F, Schneider F, Dornia C, Windelen E, Eggermann T, Rudnik-Schoneborn S, Kirfel J, Furu L, Onuchic LF, Rossetti S, Harris PC, Somlo S, Guay-Woodford L, Germino GG, Moser M, Buttner R, Zerres K. PKHD1 mutations in autosomal recessive polycystic kidney disease (ARPKD) Hum Mutat. 2004;23(5):453–463. doi: 10.1002/humu.20029. [DOI] [PubMed] [Google Scholar]
- 3.Antignac C, Calvet JP, Germino GG, Grantham JJ, Guay-Woodford LM, Harris PC, Hildebrandt F, Peters DJM, Somlo S, Torres VE, Walz G, Zhou J, Yu ASL. The future of polycystic kidney disease research—as seen by the 12 Kaplan awardees. J Am Soc Nephrol. 2015;26(9):2081–2095. doi: 10.1681/ASN.2014121192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craigie E, Birch RE, Unwin RJ, Wildman SS. The relationship between P2X4 and P2X7: a physiologically important interaction? Front Physiol. 2013;4:216. doi: 10.3389/fphys.2013.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy C, Chootip K, Mitchell C, Syed NI, Tengah A. P2X and P2Y nucleotide receptors as targets in cardiovascular disease. Future Med Chem. 2013;5(4):431–449. doi: 10.4155/fmc.13.6. [DOI] [PubMed] [Google Scholar]
- 6.Menzies RI, Tam FW, Unwin RJ, Bailey MA. Purinergic signaling in kidney disease. Kidney Int. 2017;91(2):315–323. doi: 10.1016/j.kint.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 7.Gidlöf O, Smith JG, Melander O, Lövkvist H, Hedblad B, Engström G, Nilsson P, Carlson J, Berglund G, Olsson S, Jood K, Jern C, Norrving B, Lindgren A, Erlinge D. A common missense variant in the ATP receptor P2X7 is associated with reduced risk of cardiovascular events. PLoS One. 2012;7(5):e37491. doi: 10.1371/journal.pone.0037491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palomino-Doza J, Rahman TJ, Avery PJ, Mayosi BM, Farrall M, Watkins H, Edwards CRW, Keavney B. Ambulatory blood pressure is associated with polymorphic variation in P2X receptor genes. Hypertension. 2008;52(5):980–985. doi: 10.1161/HYPERTENSIONAHA.108.113282. [DOI] [PubMed] [Google Scholar]
- 9.Wilson PD. Apico-basal polarity in polycystic kidney disease epithelia. Biochim Biophys Acta. 2011;1812(10):1239–1248. doi: 10.1016/j.bbadis.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Ilatovskaya DV, Palygin O, Levchenko V, Staruschenko A. Pharmacological characterization of the P2 receptors profile in the podocytes of the freshly isolated rat glomeruli. Am J Phys Cell Phys. 2013;305(10):C1050–C1059. doi: 10.1152/ajpcell.00138.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rangan G. Role of extracellular ATP and P2 receptor signaling in regulating renal cyst growth and interstitial inflammation in polycystic kidney disease. Front Physiol. 2013;4:218. doi: 10.3389/fphys.2013.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilatovskaya DV, Palygin O, Staruschenko A. Functional and therapeutic importance of purinergic signaling in polycystic kidney disease. Am J Physiol Ren Physiol. 2016;311(6):F1135–F1139. doi: 10.1152/ajprenal.00406.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson PD, Hovater JS, Casey CC, Fortenberry JA, Schwiebert EM. ATP release mechanisms in primary cultures of epithelia derived from the cysts of polycystic kidneys. J Am Soc Nephrol. 1999;10(2):218–229. doi: 10.1681/ASN.V102218. [DOI] [PubMed] [Google Scholar]
- 14.Schwiebert EM, Wallace DP, Braunstein GM, King SR, Peti-Peterdi J, Hanaoka K, Guggino WB, Guay-Woodford LM, Bell PD, Sullivan LP, Grantham JJ, Taylor AL. Autocrine extracellular purinergic signaling in epithelial cells derived from polycystic kidneys. Am J Physiol Ren Physiol. 2002;282(4):F763–F775. doi: 10.1152/ajprenal.0337.2000. [DOI] [PubMed] [Google Scholar]
- 15.Vekaria RM, Unwin RJ, Shirley DG. Intraluminal ATP concentrations in rat renal tubules. J Am Soc Nephrol. 2006;17(7):1841–1847. doi: 10.1681/ASN.2005111171. [DOI] [PubMed] [Google Scholar]
- 16.de Bruijn PIA, Bleich M, Praetorius HA, Leipziger J. P2X receptors trigger intracellular alkalization in isolated perfused mouse medullary thick ascending limb. Acta Physiol. 2015;213(1):277–284. doi: 10.1111/apha.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan Z, Fellner RC, Van Beusecum J, Inscho EW. P2 receptors in renal autoregulation. Curr Vasc Pharmacol. 2014;12(6):818–828. doi: 10.2174/15701611113116660152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birch RE, Schwiebert EM, Peppiatt-Wildman CM, Wildman SS. Emerging key roles for P2X receptors in the kidney. Front Physiol. 2013;4:262. doi: 10.3389/fphys.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burnstock G. Purinergic signalling: its unpopular beginning, its acceptance and its exciting future. BioEssays. 2012;34(3):218–225. doi: 10.1002/bies.201100130. [DOI] [PubMed] [Google Scholar]
- 20.Vallon V, Rieg T. Regulation of renal NaCl and water transport by the ATP/UTP/P2Y2 receptor system. Am J Physiol Ren Physiol. 2011;301(3):F463–F475. doi: 10.1152/ajprenal.00236.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallon V. P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Ren Physiol. 2008;294(1):F10–F27. doi: 10.1152/ajprenal.00432.2007. [DOI] [PubMed] [Google Scholar]
- 22.Vallon V, Stockand J, Rieg T. P2Y receptors and kidney function. Wiley Interdiscip Rev Membr Transp Signal. 2012;1(6):731–742. doi: 10.1002/wmts.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geyti CS, Odgaard E, Overgaard MT, Jensen MEJ, Leipziger J, Praetorius HA. Slow spontaneous [Ca2+]i oscillations reflect nucleotide release from renal epithelia. Pflugers Arch. 2008;455(6):1105–1117. doi: 10.1007/s00424-007-0366-4. [DOI] [PubMed] [Google Scholar]
- 24.Mori M, Hosomi H, Nishizaki T, Kawahara K, Okada Y. Calcium release from intracellular stores evoked by extracellular ATP in a Xenopus renal epithelial cell line. J Physiol. 1997;502:365–373. doi: 10.1111/j.1469-7793.1997.365bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner CM, Ramesh B, Srai SKS, Burnstock G, Unwin RJ. Altered ATP-sensitive P2 receptor subtype expression in the Han:SPRD cy/+ rat, a model of autosomal dominant polycystic kidney disease. Cells Tissues Organs. 2004;178(3):168–179. doi: 10.1159/000082247. [DOI] [PubMed] [Google Scholar]
- 26.Hillman KA, Johnson TM, Winyard PJ, Burnstock G, Unwin RJ, Woolf AS. P2X(7) receptors are expressed during mouse nephrogenesis and in collecting duct cysts of the cpk/cpk mouse. Exp Nephrol. 2002;10(1):34–42. doi: 10.1159/000049896. [DOI] [PubMed] [Google Scholar]
- 27.Chang M-Y, Lu J-K, Tian Y-C, Chen Y-C, Hung C-C, Huang Y-H, Chen Y-H, Wu M-S, Yang C-W, Cheng Y-C. Inhibition of the P2X7 receptor reduces cystogenesis in PKD. J Am Soc Nephrol. 2011;22(9):1696–1706. doi: 10.1681/ASN.2010070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaika O, Mamenko M, Berrout J, Boukelmoune N, O'Neil RG, Pochynyuk O. TRPV4 dysfunction promotes renal cystogenesis in autosomal recessive polycystic kidney disease. J Am Soc Nephrol. 2013;24(4):604–616. doi: 10.1681/ASN.2012050442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavlov TS, Ilatovskaya DV, Palygin O, Levchenko V, Pochynyuk O, Staruschenko A. Implementing patch clamp and live fluorescence microscopy to monitor functional properties of freshly isolated PKD epithelium. J Vis Exp. 2015;103:e53035. doi: 10.3791/53035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katsuyama M, Masuyama T, Komura I, Hibino T, Takahashi H. Characterization of a novel polycystic kidney rat model with accompanying polycystic liver. Exp Anim. 2000;49(1):51–55. doi: 10.1538/expanim.49.51. [DOI] [PubMed] [Google Scholar]
- 31.Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet. 2002;30(3):259–269. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- 32.Palygin O, Levchenko V, Ilatovskaya DV, Pavlov TS, Ryan RP, Cowley AW, Jr, Staruschenko A. Real-time electrochemical detection of ATP and H2O2 release in freshly isolated kidneys. Am J Physiol Ren Physiol. 2013;305(1):F134–F141. doi: 10.1152/ajprenal.00129.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palygin O, Evans LC, Cowley AW, Jr, Staruschenko A. Acute in vivo analysis of ATP release in rat kidneys in response to changes of renal perfusion pressure. J Am Heart Assoc. 2017;6(9):e006658. doi: 10.1161/JAHA.117.006658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavlov TS, Levchenko V, O'Connor PM, Ilatovskaya DV, Palygin O, Mori T, Mattson DL, Sorokin A, Lombard JH, Cowley AW, Jr, Staruschenko A. Deficiency of renal cortical EGF increases ENaC activity and contributes to salt-sensitive hypertension. J Am Soc Nephrol. 2013;24(7):1053–1062. doi: 10.1681/ASN.2012080839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palygin O, Levchenko V, Evans LC, Blass G, Cowley AW, Staruschenko A. Use of enzymatic biosensors to quantify endogenous ATP or H2O2 in the kidney. J Vis Exp. 2015;104:53059. doi: 10.3791/53059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ilatovskaya DV, Palygin O, Levchenko V, Staruschenko A. Single-channel analysis and calcium imaging in the podocytes of the freshly isolated glomeruli. J Vis Exp. 2015;100:e52850. doi: 10.3791/52850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xing S, Grol MW, Grutter PH, Dixon SJ, Komarova SV. Modeling interactions among individual P2 receptors to explain complex response patterns over a wide range of ATP concentrations. Front Physiol. 2016;7:294. doi: 10.3389/fphys.2016.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82(4):1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 39.Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011;63(3):641–683. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helms N, Kowalski M, Illes P, Riedel T. Agonist antagonist interactions at the rapidly desensitizing P2X3 receptor. PLoS One. 2013;8(11):e79213. doi: 10.1371/journal.pone.0079213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PP. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53(1):107–118. [PubMed] [Google Scholar]
- 42.Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP. Pharmacology of P2X channels. Pflugers Arch. 2006;452(5):513–537. doi: 10.1007/s00424-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 43.Jones CA, Chessell IP, Simon J, Barnard EA, Miller KJ, Michel AD, Humphrey PP. Functional characterization of the P2X(4) receptor orthologues. Br J Pharmacol. 2000;129(2):388–394. doi: 10.1038/sj.bjp.0703059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allsopp RC, Dayl S, Schmid R, Evans RJ. Unique residues in the ATP gated human P2X7 receptor define a novel allosteric binding pocket for the selective antagonist AZ10606120. Sci Rep. 2017;7(1):725. doi: 10.1038/s41598-017-00732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novak I. Purinergic signalling in epithelial ion transport: regulation of secretion and absorption. Acta Physiol. 2011;202(3):501–522. doi: 10.1111/j.1748-1716.2010.02225.x. [DOI] [PubMed] [Google Scholar]
- 46.Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J. 2007;21(13):3717–3726. doi: 10.1096/fj.07-8807com. [DOI] [PubMed] [Google Scholar]
- 47.Nanami M, Pech V, Lazo-Fernandez Y, Weinstein AM, Wall SM. ENaC inhibition stimulates HCl secretion in the mouse cortical collecting duct. II. Bafilomycin-sensitive H+ secretion. Am J Physiol Ren Physiol. 2015;309(3):F259–F268. doi: 10.1152/ajprenal.00120.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pavlov TS, Levchenko V, Ilatovskaya DV, Palygin O, Staruschenko A. Impaired epithelial Na+ channel activity contributes to cystogenesis and development of autosomal recessive polycystic kidney disease in PCK rats. Pediatr Res. 2015;77(1–1):64–69. doi: 10.1038/pr.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hooper KM, Unwin RJ, Sutters M. The isolated C-terminus of polycystin-1 promotes increased ATP-stimulated chloride secretion in a collecting duct cell line. Clin Sci. 2003;104(3):217–221. doi: 10.1042/CS20020239. [DOI] [PubMed] [Google Scholar]
- 50.Wildman SS, Hooper KM, Turner CM, Sham JS, Lakatta EG, King BF, Unwin RJ, Sutters M. The isolated polycystin-1 cytoplasmic COOH terminus prolongs ATP-stimulated Cl- conductance through increased Ca2+ entry. Am J Physiol Ren Physiol. 2003;285(6):F1168–F1178. doi: 10.1152/ajprenal.00171.2003. [DOI] [PubMed] [Google Scholar]
- 51.Buchholz B, Teschemacher B, Schley G, Schillers H, Eckardt KU. Formation of cysts by principal-like MDCK cells depends on the synergy of cAMP- and ATP-mediated fluid secretion. J Mol Med. 2011;89(3):251–261. doi: 10.1007/s00109-010-0715-1. [DOI] [PubMed] [Google Scholar]
- 52.Xu C, Shmukler BE, Nishimura K, Kaczmarek E, Rossetti S, Harris PC, Wandinger-Ness A, Bacallao RL, Alper SL. Attenuated, flow-induced ATP release contributes to absence of flow-sensitive, purinergic Cai2+ signaling in human ADPKD cyst epithelial cells. Am J Physiol Ren Physiol. 2009;296(6):F1464–F1476. doi: 10.1152/ajprenal.90542.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kraus A, Grampp S, Goppelt-Struebe M, Schreiber R, Kunzelmann K, Peters DJ, Leipziger J, Schley G, Schodel J, Eckardt KU, Buchholz B. P2Y2R is a direct target of HIF-1alpha and mediates secretion-dependent cyst growth of renal cyst-forming epithelial cells. Purinergic Signal. 2016;12(4):687–695. doi: 10.1007/s11302-016-9532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Odgaard E, Praetorius HA, Leipziger J. AVP-stimulated nucleotide secretion in perfused mouse medullary thick ascending limb and cortical collecting duct. Am J Physiol Ren Physiol. 2009;297(2):F341–F349. doi: 10.1152/ajprenal.00190.2009. [DOI] [PubMed] [Google Scholar]
- 55.Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V, Stockand JD. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem. 2008;283(52):36599–36607. doi: 10.1074/jbc.M807129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zsembery A, Boyce AT, Liang L, Peti-Peterdi J, Bell PD, Schwiebert EM. Sustained calcium entry through P2X nucleotide receptor channels in human airway epithelial cells. J Biol Chem. 2003;278(15):13398–13408. doi: 10.1074/jbc.M212277200. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Sanchez D, Gorelik J, Klenerman D, Lab M, Edwards C, Korchev Y. Basolateral P2X4-like receptors regulate the extracellular ATP-stimulated epithelial Na+ channel activity in renal epithelia. Am J Physiol Ren Physiol. 2007;292(6):F1734–F1740. doi: 10.1152/ajprenal.00382.2006. [DOI] [PubMed] [Google Scholar]
- 58.Hillman KA, Woolf AS, Johnson TM, Wade A, Unwin RJ, Winyard PJD. The P2X7 ATP receptor modulates renal cyst development in vitro. Biochem Biophys Res Commun. 2004;322(2):434–439. doi: 10.1016/j.bbrc.2004.07.148. [DOI] [PubMed] [Google Scholar]
- 59.Pandit MM, Inscho EW, Zhang S, Seki T, Rohatgi R, Gusella L, Kishore B, Kohan DE. Flow regulation of endothelin-1 production in the inner medullary collecting duct. Am J Physiol Ren Physiol. 2015;308(6):F541–F552. doi: 10.1152/ajprenal.00456.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kohan DE, Inscho EW, Wesson D, Pollock DM. Physiology of endothelin and the kidney. Compreh Physiol. 2011;2:883–919. doi: 10.1002/cphy.c100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pavlov TS, Chahdi A, Ilatovskaya DV, Levchenko V, Vandewalle A, Pochynyuk O, Sorokin A, Staruschenko A. Endothelin-1 inhibits the epithelial Na+ channel through betaPix/14-3-3/Nedd4-2. J Am Soc Nephrol. 2010;21(5):833–843. doi: 10.1681/ASN.2009080885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim MJ, Turner CM, Hewitt R, Smith J, Bhangal G, Pusey CD, Unwin RJ, Tam FW. Exaggerated renal fibrosis in P2X4 receptor-deficient mice following unilateral ureteric obstruction. Nephrol Dial Transplant. 2014;29(7):1350–1361. doi: 10.1093/ndt/gfu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD. Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol. 2007;72(6):1447–1456. doi: 10.1124/mol.107.035980. [DOI] [PubMed] [Google Scholar]