Abstract
Plasma microparticles (MP) bear functional active ectonucleotidases of the CD39 family with implications in vascular inflammation. MP appear to be able to fuse with cells and transfer genetic information. Here, we tested whether levels of different immunomodulatory microRNAs (miRs) in plasma MP are modulated by CD39 after experimental hepatectomy. We further investigated whether horizontal transfer of miR-142-3p between mononuclear (MNC) and endothelial cells via MP is regulated by purinergic signaling. Partial hepatectomy was performed in C57BL/6 wild type and Cd39 null mice. MP were collected via ultracentrifugation. MNC were stimulated with nucleotides and nucleosides, in vitro, and tested for miR-142-3p levels. Fusion of MNC-derived MP and endothelial cells with subsequent transfer of miR-142-3p was imaged by flow cytometry and confocal microscopy. Endothelial inflammation and apoptosis were quantified after transfection with miR-142-3p. Significantly lower miR-142-3p levels were observed in plasma MP of Cd39 null mice after partial hepatectomy, when compared to C57BL/6 wild types (p < 0.05). In contrast to extracellular nucleotides, anti-inflammatory adenosine significantly increased miR-142-3p levels in MNC-derived MP, in vitro (p < 0.05). MNC-derived MP are able to transfer miR-142-3p to endothelial cells by fusion. Transfection of endothelial cells with miR-142-3p decreased TNF-α levels (p < 0.05) and endothelial apoptosis (p < 0.05). MiR-142-3p levels in MNC-derived MP are modulated by nucleoside signaling and might reflect compensatory responses in vascular inflammation. Our data suggest the transfer of genetic information via shed MP as a putative mechanism of intercellular communication—with implications in organ regeneration.
Electronic supplementary material
The online version of this article (10.1007/s11302-018-9624-5) contains supplementary material, which is available to authorized users.
Keywords: Partial hepatectomy, Liver regeneration, Vascular inflammation, Purinergic signaling, Microvesicles, MicroRNA
Introduction
Liver regeneration after partial hepatectomy is characterized by complex interactions of intra- and extra-hepatic responses. Increasing evidence suggests that regenerative processes might be modulated by immunomodulatory and regenerative responses from the bone marrow [1–5]. We and others have shown that hematopoietic stem cells (HSC) are mobilized at high levels from the bone marrow in response to surgical and pharmacological liver injury in rodents and patients [4, 6]. Administration of HSC has been confirmed in several publications to boost liver regeneration in experimental models and first clinical settings, at least in parts, by modulating vascular inflammation [7–9].
Own observations indicate that HSC might not only be mobilized from the bone marrow, but also rapidly shed plasma microparticles (MP) in response to acute liver injury [10]. MP are small submicron membrane vesicles (≤ 1 μm), characterized by surface profiles and contents according to their cells of origin. Shedding of MP in response to cellular stress is believed to be due to a loss of membrane asymmetry, increased intracellular calcium levels, and subsequently reorganization of the cytoskeleton [11, 12].
We have previously shown that HSC-derived MP can be divided in functionally distinct subsets according to their expression of CD39, also known as ectonucleoside triphosphate diphosphohydrolase 1 (E-NTPDase 1) [6, 10], the dominant immune and hematopoietic ectonucleotidase. CD39 bearing cells and MP are able to phosphohydrolyse extracellular pro-inflammatory adenosine triphosphate (ATP) to anti-inflammatory adenosine, with important implications in HSC mobilization and modulation of vascular inflammation in liver regeneration [6, 13–16]. We have shown that CD39high HSC subsets are preferentially mobilized from the bone marrow by purinergic signaling via adenosine-type P1 receptors (A2A) [6].
Besides the potential role as potent biomarkers in liver diseases, shed MP might modulate the microenvironment at sites of inflammation. Shedding of MP might represent an alternative way of communication between hematopoietic bone marrow cells and primary liver cells, e.g., endothelial cells, during liver regeneration [17]. MP have been shown to fuse with different cell types and transfer genetic information such as messenger RNA (mRNA) and microRNA (miR, miRNA) [18–24], which in turn are involved in the regulation of sterile inflammation and liver regeneration [25, 26]. However, mechanisms of MP shedding and transfer of information by MP, e.g., immunomodulatory miRNA, are poorly understood.
We here suggest the horizontal transfer of genetic information via shed monocyte-derived MP as a putative mechanism of intercellular communication in settings of sterile endothelial inflammation. Among other immunomodulatory miRNA tested, e.g., miR-21, our results suggest an important role of the hematopoietic-lineage restricted miR-142-3p, which is known to be highly expressed in all stages of HSC lineage during development and both necessary for immunomodulatory and regulation functions of endothelial progenitor cells. MiR-142-3p has already been described to modulate different inflammatory responses [27–31] and serves as a diagnostic marker, e.g., after renal transplantation or during systemic sclerosis [32, 33].
Materials and methods
Animals
Wild type C57BL/6 and Cd39 null mice (derived as previously described [2, 34]) were used in accordance with the guidelines from the American Association for Laboratory Animal Care and the Federation of European Laboratory Animal Science Associations (FELASA), respectively. Animal research protocols were approved by the Beth Israel Deaconess Medical Centre Institutional Animal Care and Use Committees, Boston, USA and the federal state government of Saxony, Germany, respectively.
Liver injury model
Partial hepatectomy (70%) and sham operation were performed, as previously described [2].
Isolation of mononuclear cells and plasma-derived MP
Bone marrow was obtained from hind legs of wild type mice and was pestled using wash buffer (phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA; w/v) and 0.6% citrate phosphate dextrose (CPD; v/v)). Murine bone marrow mononuclear cell (BM-MNC) suspension was filtered through a 70 μM cell strainer. Red blood cells were lysed for 10 min at room temperature (RT) using lysis buffer (1.5 M NH4Cl, 100 mM NaHCO3, 1 mM EDTA, pH 7.4; dilution 1:4). Cells were washed (10 min, 300g, 12 °C) and lysis was repeated. Isolation of human peripheral blood mononuclear cells (PB-MNC) was performed similarly to the isolation of murine BM-MNC. Human PB-MNC were obtained from fresh EDTA-blood of healthy volunteers via blood lysis. Blood samples were diluted 1:4 with lysis buffer for 10 min at RT and washed (PBS containing 0.5% HSA (human serum albumin) w/v and 0.6% CPD v/v; 10 min, 300g, 12 °C). Lysis was repeated three times. After the second lysis, cell suspension was filtered through a 70 μM cell strainer. MP were collected from the plasma and cell culture supernatants by two-step ultracentrifugation (10.000g for 30 min and 100.000g for 90 min), as described previously [18]. Analysis of blood samples from healthy humans for mechanistic studies was approved by the ethics committee of the University of Leipzig, Germany.
MiRNA and gene expression analysis
Total RNA (including miRNA) from MP, cells, and liver tissue was isolated using Qiazol® Lysis Reagent (Qiagen, Hilden, GER) and purified with modifications, as described in the user’s manual (miRNeasy Micro Kit and miRNeasy Serum/Plasma Kit, Qiagen, Hilden, GER). Purified RNA was reverse transcribed and qPCR was performed using manufacturer’s instruction (miScript System II, Qiagen, Hilden, GER; RevertAid First Strand cDNA Synthesis Kit, Life Technologies, Karlsruhe, GER and GoTaq qPCR Master Mix, Promega, Mannheim, GER) and the 7500 Real Time PCR System (Applied Biosystems by Life Technologies, California, USA). Primer sequences used for gene expression analysis (TNFa: (f) ATG TTG TAG CAA ACC CTC AAG C; (r) TGA AGA GGA CCT GGG AGT AGA T; 18rRNA: (f) ACT CAA CAC GGG AAA CCT CAC C; (r) CGC TCC ACC AAC TAA GAA CGG) and miScript Primer Assays (Qiagen) sequences of the mature microRNA (hsa-miR-21: 5′UAG CUU AUC AGA CUG AUG UUG A 3′; hsa-miR-126: 5′UCG UAC CGU GAG UAA UAA UGC G 3′; hsa-miR-142-3p:5′UGU AGU GUU UCC UAC UUU AUG GA 3′; hsa-miR-146a: 5′UGA GAA CUG AAU UCC AUG GGU U 3′; hsa-miR-155: 5′UUA AUG CUA AUC GUG AUA GGG GU 3′). As internal control for the miRNA studies, RNU6 (Hs_RNU6-2-1; Qiagen; Hilden, GER) was used in murine as well as human cells and miR-39 from Caenorhabditis elegans (included in the miRNeasy Serum/Plasma Kit, Qiagen, Hilden, GER) was used as internal spike-in control in MP. Human 18S rRNA was applied as housekeeping gene in target gene expression analysis. MiRNA target prediction was done using prediction algorithms (TargetScan; release 6.2; June 2012; http://www.targetscan.org). The qPCR results were analyzed using the 7500 Software (v2.0.6).
Stimulation of BM-MNC
Murine BM-MNC were stimulated with 100 μM ATP (Sigma Aldrich, Taufkirchen, GER), 100 μM non-degradable ATPγS (Adenosine 5′-O-(3-thio)triphosphate; Sigma Aldrich, Taufkirchen, GER) and 100 μM adenosine (Sigma Aldrich, Taufkirchen, GER) for 4 h, in vitro, respectively. BM-MNC were pre-stimulated with different adenosine receptor antagonists (theophylline: unspecific, 50 μM; xanthine amine congener (XAC): A1, 1 μM; 8-(3-chlorostyryl)caffeine (CSC): A2A 1 μM; alloxazine: A2B, 1 μM; MRS1523: A3, 5 μM; all purchased from Sigma Aldrich, Taufkirchen, GER) for 30 min. During stimulation, BM-MNC were cultured in alphaMEM (Lonza, Köln, GER) with 10% fetal bovine serum (FBS), glutamine, and penicillin/streptavidin.
Transfection studies
Primary human umbilical vein endothelial cells (HUVEC) were purchased from Promocell (Heidelberg, GER). HUVEC were cultured in endothelium media II+supplements (Promocell, Heidelberg, GER) and passaged after > 80% confluence. Cells were cryopreserved in media containing 10% dimethyl sulfoxide (DMSO; VWR, Dresden, GER). HUVEC were transfected with miR-142-3p (Pre-miR™ miRNA Precursors, Life Technologies, Karlsruhe, GER) and BM-MNC were transfected with miR-142-3p or a Cy3-labeled RNA oligonucleotide (Cy3™ Dye-Labeled Pre-miR, Life Technologies, Karlsruhe, GER) via lipofection (reagent: INTERFERin, PEQLAB, Erlangen, GER) for 24 h using manufacturer’s instructions. HUVEC were activated via incubation with ATP for 30 min. After transfection, cells were harvested and miRNA as well as target gene expression analysis were performed.
Co-culture of human PB-MNC-derived MP with HUVEC
Cell numbers were adapted from Aucher et al. [35]. For fluorescence-activated cell sorting (FACS) analysis, MP of 2 × 107 human PB-MNC were isolated and transfected with a Cy3-labeled RNA oligonucleotide. To analyze the fusion of HUVEC and PB-MNC-derived MP, MP were generated via stimulating 4 × 107 PB-MNC with ATP, as described. In both cases, 180.000 HUVEC were co-cultured with MP for 5 h.
Fluorescence-activated cell sorting (FACS)
FACS analysis of HUVEC after co-culture with MP was performed on a FACS Canto II (BD, Becton, Dickinson and Company, Heidelberg, GER). After ultracentrifugation, plasma MP and MP enriched in Cy3-labeled RNA oligonucleotides from cell culture supernatant were resuspended in PBS and then measured. Cells were harvested and washed twice with PBS. To separate nonviable cells, a 7-AAD Viability Staining Solution (eBioscience, Frankfurt a. M., GER) was used according to the user’s manual.
Imaging
For fusion studies of human PB-MNC-derived MP with HUVEC, the PKH67 Green Fluorescent Cell Linker (Sigma Aldrich, Taufkirchen, GER) was used for general membrane staining. After collecting MP, labelling was performed following the manufacturer’s instructions and modified as described previously [36]. After staining, MP were washed through filling up the staining volume with PBS and subsequent ultracentrifugation as described previously. MP were resuspended in endothelium media. After co-culture with HUVEC for 5 h, cells were washed and fixed with 4% paraformaldehyde for 20 min. Then, cells were washed three times with PBS and nuclear staining was performed with DAPI (4′,6-diamidino-2-phenylindole; Carl Roth, Karlsruhe, GER). Fluorescence was detected using the Keyence-BZ 9000 (Keyence, Osaka, JP). Images were taken with 20× and 40× dry objectives (N.A. = 0.5).
Apoptosis assay
Apoptosis was induced in HUVEC by stimulation with camptothecin (CPT) for 24 h (positive control). The commercial PE Annexin V Apoptosis Detection Kit I (BD Bioscience, Heidelberg, GER) was used following the user’s manual. After performing the assay, cells were gently harvested using accutase (VWR, Dresden, GER), washed twice with PBS and analyzed by FACS.
Statistics
All results are reported as mean ± SEM. Significance were analyzed using Student’s t test and Mann-Whitney rank sum test, where appropriate. Normal distribution was checked via Shapiro Wilk Test. A p value of < 0.05 was considered significant.
Results
MiR-142-3p MP levels are modulated after partial hepatectomy in a CD39-dependent manner
We first investigated plasma MP responses after partial hepatectomy and demonstrated comparable absolute MP counts in wild type and Cd39 null mice by FACS (Supp. Fig. 1). We then isolated plasma MP from wild type and mutant mice 12 h after partial hepatectomy and screened them for levels of different immunomodulatory miRNAs. Thus, for example, miR-155 was not detectable in these MP. MiR-21, miR-146a, and miR-126 were rather different in sham operated wild type and mutant mice, indicating either liver independent mechanisms or no differences could be observed at all (Supp. Fig. 2). MiR-142-3p plasma MP levels in wild type mice after partial hepatectomy were comparable to control mice undergoing sham operation (Fig. 1a). In contrast, a five-fold decrease of miR-142-3p MP levels was observed after partial hepatectomy in Cd39 null mice, when compared to shams (12 h, p < 0.05). Further, we noted a 7.5-fold decrease of miR-142-3p MP levels in Cd39 null mice relative to wild type plasma MP 12 h after partial hepatectomy (p < 0.001).
Fig. 1.
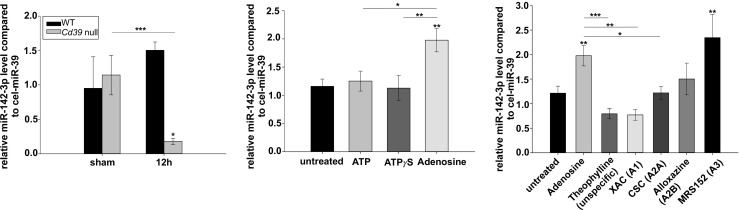
Purinergic modulation of miR-142-3p encapsulation in MP. a MiR-142-3p levels in plasma MP-derived from wild type and Cd39 null mice 12 h after partial hepatectomy (n = 3/group). MiR-142-3p MP levels were compared to sham operated mice. MiR-39 from C. elegans was used as internal spike-in control. b MiR-142-3p levels in MP-derived from wild type BM-MNC after stimulation with ATP, ATPγS or adenosine (all 100 μM), respectively, for 4 h, when compared to MP shed by untreated BM-MNC (n = 3–9, representative for 3–4 independent experiments). c MiR-142-3p level of wild type BM-MNC-derived MP after pre-incubation with specific and unspecific P1 receptor antagonists (theophylline: unspecific, 50 μM; XAC: A1, 1 μM; CSC: A2A, 1 μM; alloxazine: A2B, 1 μM; MRS1523: A3, 5 μM) for 30 min followed by stimulation with adenosine (100 μM) for 4 h (n = 6–9). Error bars represent standard error of mean. *p < 0.05, **p < 0.01, ***p < 0.001. BM-MNC, bone-marrow-derived mononuclear cells; CSC, 8-(3-chlorostyryl)caffeine; miR, microRNA; MP, microparticles; WT, wild type; XAC, xanthine amine congener
Fig. 2.
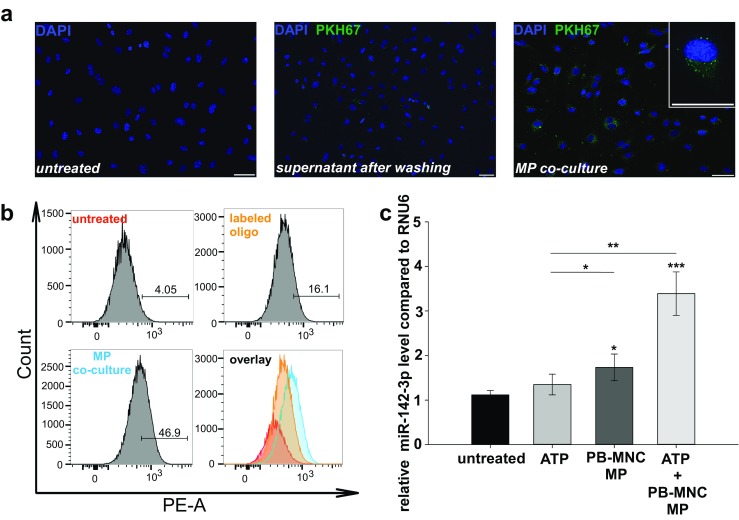
Co-culture of HUVEC with human PB-MNC-derived MP. a Representative fluorescence images of HUVEC after co-culture with PB-MNC-derived MP, compared to untreated cells and cells treated with the supernatant from the washing step after MP staining. PB-MNC were pre-treated with ATP (100 μM) to shed a high number of MP, which were subsequently stained with the PKH67 fluorescence dye, usually used for general membrane staining. Scale bars: 50 μM. b FACS histograms showing HUVEC positive for Cy3 after co-culture with Cy3-labeled PB-MNC-derived MP for 5 h, when compared to untreated cells and labeled oligo alone. Before, human PB-MNC were transfected with Cy3-labeled RNA oligonucleotide (25 nM) for 24 h to shed MP enriched in these RNA oligonucleotides. Numbers represent the amount of Cy3-positive HUVEC in percent. c MiR-142-3p levels in HUVEC after co-culture with PB-MNC-derived MP from 1 × 105 BM-MNC for 5 h with (right bar) and without (middle bar) pre-incubation with ATP (5 mM) for 30 min. The left bar shows the miR-142-3p level in non-co-cultured HUVEC after pre-stimulation with ATP. MiR-142-3p levels were compared to untreated HUVEC (n = 6–8, representative for 1–2 independent experiments). Shortly before co-culture, PB-MNC were stimulated with adenosine (100 μM) for 4 h to shed MP enriched in miR-142-3p. Error bars represent standard error of mean. *p < 0.05, **p < 0.01, ***p < 0.001. Cy3, cyanine 3; FACS, fluorescence-activated cell sorting; HUVEC, human umbilical vein endothelial cells; miR, microRNA; MP, microparticles; PB-MNC, peripheral-blood-derived mononuclear cells; PE, phycoerythrin
Encapsulation of miR-142-3p in murine BM-MNC-derived MP is modulated by purinergic receptor signaling
We next tested whether miR-142-3p levels in BM-MNC-derived MP are linked to purinergic signaling. Therefore, we examined if miR-142-3p levels might be modulated by extracellular nucleot(s)ides. We isolated BM-MNC from bone marrow of untreated wild type mice and incubated them with ATP, non-degradable ATPγS, and adenosine (all 100 μM), respectively, for 4 h, in vitro. Derived MP were collected from the supernatant and analyzed for miR-142-3p MP levels (Fig. 1b). Incubation of BM-MNC with ATP and ATPγS led to slightly higher miR-142-3p levels in derived MP relative to MP shed from untreated cells; however, this did not reach statistical significance. Rather, stimulation of BM-MNC with adenosine lead to a twofold increase in the miR-142-3p MP levels, when compared to MP shed by untreated cells (p < 0.01). Interestingly, no such changes in miR-142-3p levels were evident in BM-MNC themselves (Supp. Fig. 3), suggesting adenosine-mediated modulations in the process of miRNA encapsulation in derived MP.
To test whether those effects are dependent on adenosine binding to P1 receptors, we next pre-incubated wild type BM-MNC with selective adenosine-type P1 receptor antagonists (Fig. 1c). We noted significantly decreased miR-142-3p MP levels after pre-incubation of adenosine-stimulated BM-MNC with the unselective P1-receptor inhibitor theophylline (p < 0.001) and selective antagonists XAC (p < 0.05) and CSC (p < 0.01), when compared to adenosine, indicating effects to be mediated by A1 and A2A receptor signaling.
Adhesion and fusion of human PB-MNC-derived MP with activated endothelial cells
Previous studies have shown that disordered vascular inflammation is associated with exacerbated tissue damage after partial hepatectomy. Thus, we next focused on the modulatory potential of MNC-derived MP on activated endothelial cells with regard to implications in regeneration. We also tested whether human PB-MNC-derived MP are able to fuse with endothelial cells and to transfer genetic information. Liver sinusoidal endothelial cells (LSEC) would be the ideal cells for in vitro experiments, but those are not readily available, difficult to isolate, lose phenotype rapidly after isolation, and are not viable in monoculture [37]. Own in vitro experiments resulted in failed cell culturing. Isolation of LSEC is described in the Supp. File. Thus, for co-culture experiments commercially available HUVEC cells were used. First, MP-derived from PB-MNC were stained by PKH67 and subsequently co-cultured for 5 h with commercially available untreated HUVEC. Fusion of MP with HUVEC could be confirmed by confocal microscopy (Fig. 2a). MP were pictured as green dots attached to the membrane of unstained HUVEC. Diffuse distribution of the green fluorescence dye over the cytoplasm may reveal the uptake of MP into HUVEC.
We next investigated whether human PB-MNC-derived MP might be able to transfer miRNA to endothelial cells by fusion. Successful transfection of Cy3-labeled RNA oligonucleotide to PB-MNC was confirmed by FACS analysis (Supp. Fig. 4). The derived MP were then co-cultured with HUVEC for 5 h (Fig. 2b). Co-culture of HUVEC with Cy3-labeled RNA oligonucleotide bearing PB-MNC-derived MP resulted in 46.9% Cy3 positive HUVEC, when compared 16.1% achieved by the incubation with free Cy3-labeled RNA oligonucleotide (25 nM).
Finally, we tested whether PB-MNC-derived MP are able to transfer immunomodulatory hematopoietic-restricted miR-142-3p. Note that in contrast to mononuclear cells, miR-142-3p is found at negligibly low levels in endothelial cells (HUVEC and LSEC; Supp. Fig. 5). Human PB-MNC were stimulated with adenosine, as described earlier, for miR-142-3p enrichment in shed MP. Subsequently, these PB-MNC-derived MP were co-cultured with HUVEC, which resulted in a 1.7-fold increase of miR-142-3p levels, when compared to untreated HUVEC (Fig. 2c). To simulate the inflammatory micro-environment, HUVEC were activated with ATP before transfection. Importantly, this pre-activation was associated with a 3.5-fold increase in the miR-142-3p level compared to untreated HUVEC (p < 0.001) and a 2.6-fold increase compared to HUVEC treated with ATP (p < 0.01), respectively.
MiR-142-3p decreases tumor necrosis factor (TNF-α) expression in endothelial cells and decreases endothelial apoptosis
We have shown that PB-MNC-derived MP are potent carriers of genetic information, able to fuse and transfer miR-142-3p from hematopoietic to endothelial cells, in vitro. We next investigated the importance of miR-142-3p in modulating endothelial inflammation and apoptosis, characteristic for disordered liver regeneration after partial hepatectomy [2, 6, 38, 39]. To prove successful transfection, Fig. 3a shows HUVEC transfected with miR-142-3p (25 nM) for 24 h via lipofection, which resulted in significant increased miR-142-3p levels, when compared to controls (p < 0.05; Fig. 3a). Pre-experiments in HUVEC regarding TNF-α expression resulted in inhomogeneous and low expression levels of TNF-α. To induce TNF-α, cultured HUVEC were activated with ATP and subsequently transfected with miR-142-3p for 24 h. Transfection with miR-142-3p was associated with a significant decrease in transcriptional levels of TNF-α by ~ 50%, when compared to HUVEC incubated with the transfection reagent and ATP, respectively (p < 0.01; Fig. 3b). Treatment with the transfection reagent and ATP also induces apoptosis (Fig. 3c, d). Interestingly, transfection of HUVEC with miR-142-3p resulted in significant increased levels of cells alive (10–15%) and decreased endothelial apoptosis rate by 10% (p < 0.05), when compared to controls.
Fig. 3.
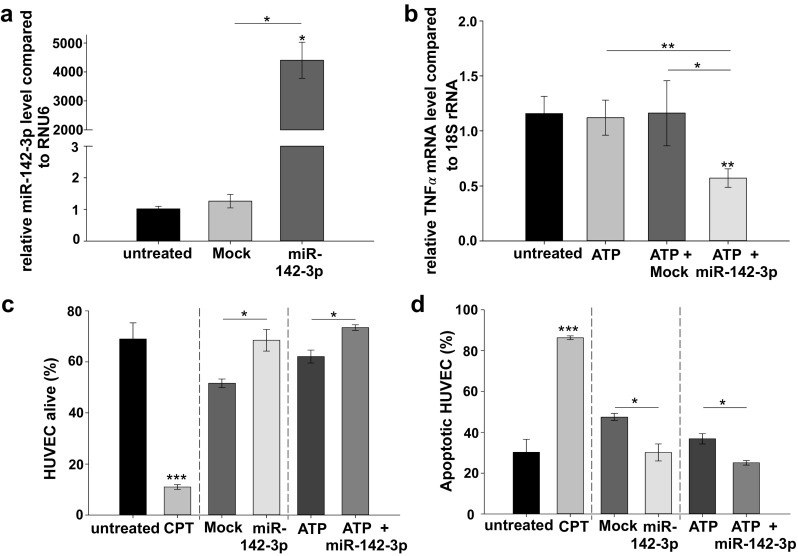
Effect on TNF-α mRNA levels and apoptosis rate after successful transfection of HUVEC with miR-142-3p. a MiR-142-3p levels transferred into HUVEC after transfection of miR-142-3p (25 nM) for 24 h (n = 3), when compared to untreated cells and transfection reagent (Mock). b TNF-α mRNA levels after transfection of miR-142-3p (25 nM) for 24 h, when compared to ATP and the transfection reagent (Mock). Before transfection, HUVEC were cultured for 5 h after treatment with ATP (5 mM) for 30 min (n = 3–9, representative for 2–4 independent experiments). c, d Amount of apoptotic HUVEC and HUVEC alive (in percent) after miR-142-3p (25 nM) transfection. HUVEC were compared to untreated cells and cells treated with CPT (5 μM) for 24 h as positive control. Cells were activated by incubation with ATP (5 mM) for 30 min followed by culture for 5 h. Transfection time 24 h (n = 3). Error bars represent standard error of mean. *p < 0.05, **p < 0.01, ***p < 0.001. CPT, camptothecin; HUVEC; human umbilical vein endothelial cells; miR, microRNA; Mock, transfection reagent alone; TNF-α, tumor necrosis factor
Discussion
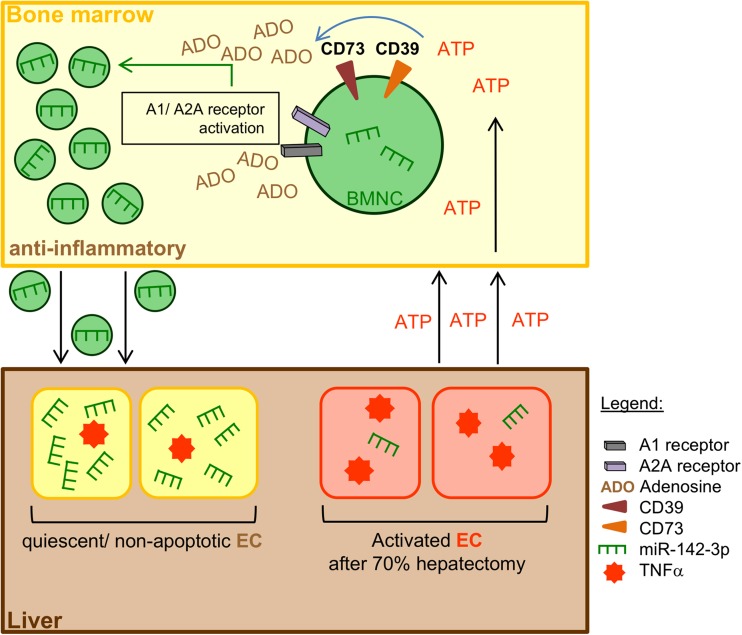
Our results indicate that circulating plasma MP contain hematopoietic-restricted miR-142-3p, with levels being modulated after experimental partial hepatectomy in a CD39-dependent manner. Mechanistic studies, in vitro, revealed that the encapsulation of miR-142-3p in MP-derived from BM-MNC is boosted by adenosine signaling via A1 and A2A receptors. MP-derived from human PB-MNC act as potent carriers of immunomodulatory miR-142-3p and are able to fuse with activated endothelial cells. MiR-142-3p dampens endothelial activation and apoptosis, with possible implications in vascular inflammation and liver regeneration after partial hepatectomy (Fig. 4).
Fig. 4.
Proposed mechanism of intercellular communication between BM-MNC and endothelial cells (EC) during liver regeneration. Release of the immunomodulatory miR-142-39 into BM-MNC-derived MP is modulated by the degradation of extracellular ATP to adenosine. This miRNA encapsulation can be boosted via A1 and A2A receptors. Transfer of miR-142-3p via the fusion of enriched MP with activated/apoptotic EC diminishes TNF-α mRNA levels and limits endothelial apoptosis. Inactivation of EC may also lead to a kind of feedback mechanism to prevent an abundant immune response. BM-MNC, bone-marrow-derived mononuclear cells; EC, endothelial cells
Extracellular pro-inflammatory ATP is released by apoptotic and necrotic cells in response to acute liver injury [16]. CD39 is the dominant hematopoietic and vascular ectonucleotidase and, together with CD73, phosphohydrolyses extracellular ATP to anti-inflammatory adenosine [6, 13–15]. We have shown that mutant mice null for Cd39 were characterized by significantly lower miR-142-3p levels in plasma MP after partial hepatectomy (Fig. 1a). In Cd39 null mice the phosphohydrolysis of extracellular ATP via CD39 is inhibited, resulting in an excess of pro-inflammatory extracellular ATP and shortage of anti-inflammatory adenosine. Additional evidence from mechanistic studies, in vitro, revealed that the encapsulation of miR-142-3p into BM-MNC-derived MP is enhanced by adenosine signaling via A1 and A2 receptors (Fig. 1c). Regulated ATP phosphohydrolysis by CD39 appears to be pivotal in modulating encapsulation of immunomodulatory miRNA in MNC-derived MP during liver regeneration. We noted miR-142-3p levels in BM-MNC-derived MP to be modulated by CD39-dependent phosphohydrolysis of ATP. Interestingly, no such changes were evident on a cellular level in parent BM-MNC, suggesting selective purinergic modulation of mechanisms involved in MP shedding or miRNA packaging. Recent evidence suggests the secretion of RNA via MP as energy dependent [40] or with plasma membrane anchors transferring RNA and proteins into exosomes [41]. MiRNA, RNA interference essential protein human Argonaute 2 (hAGO2) as well as the target mRNA appear to be associated with processing bodies (P-bodies) and stress granules [42–44], whereas ribonucleoproteins seem to be involved in the intracellular traffic and RNA compartmentation [21]. However, downstream pathways involved in the intracellular compartmentation and encapsulation of miR into derived MP remain poorly understood and further research is needed in this regard.
We could show that PB-MNC-derived MP attach to HUVEC after co-culture (Fig. 2a). The ability of fusion and transfer of miRNA was confirmed by the transfer of Cy3-labeled-RNA oligonucleotides from PB-MNC-derived MP to HUVEC, in vitro (Fig. 2b). Together, these results have important implications for the communication between bone marrow cells, e.g., HSC, and primary liver cells in liver regeneration after partial hepatectomy. Fusion and internalization of MP to recipient cells have been suggested in previous studies to be adhesion molecule and/or integrin dependent via, e.g., CD29, CD44, or CD54 [18–20]. Others suggest that fusion occurs through phagocytosis or directly fusing with the plasma membrane of the target cells [41]. Further studies are needed to elucidate the importance of proposed mechanisms in post-hepatectomy liver regeneration.
Mutant mice null for Cd39 are characterized by increased vascular inflammation, apoptosis, and attenuated regenerative capacities after partial hepatectomy. Our results indicate that, at least in parts, this might be due to attenuated immunomodulatory miR-142-3p bearing MP responses seen in Cd39 null mice. The miR-142-3p sequence is highly conserved in human and mice. Also, targets vary between the two species, the inflammation-associated targets like IRAK1/Irak1, TAB2/Tab2, TGFBR1/Tgfbr1, or BCLAF1/Bclaf1 are relevant for both murine and human miR-142-3p. We and others have shown that miR-142-3p is restricted to hematopoietic cells [45, 46], with no relevant levels found in HUVEC and other endothelial cells. MiR-142-3p directly targets the hematopoietic stem cell marker CD133 [27, 28]. In addition, miR-142-3p influences the activation and function of T cell subsets via modulating the final cAMP levels [29]. Danger et al. describes miR-142-3p as a potential biomarker after renal transplantation with increased miR-142-3p levels in PB-MNC of operationally tolerant patients. In vitro studies suggest a role for this miRNA in modulating inflammatory responses via modulating the transforming growth factor beta 1 (TGFβ) signaling pathway [32]. Addition of TGFβ induces miR-142-3p which is described by Kim et al. associated with the regulation of the vascular smooth muscle cell phenotype [30]. Both studies suggest a negative feedback loop mechanism for anti-inflammatory TGFβ and therefore termination of the immune response.
After co-culturing MP enriched in miR-142-3p with HUVEC in vitro, the cellular level of miR-142-3p is significantly increased (Fig. 2c). We provide evidence that upregulation of miR-142-3p levels in HUVEC leads to decreased transcriptional levels of the pro-inflammatory cytokine TNF-α, in vitro (Fig. 3b). Our results underline results of Fordham et al., who showed that high levels of miR-142-3p in macrophages and dendritic cells lead to a decreased release of TNF-α [31]. Further studies in liver endothelial cells, e.g., liver sinusoidal or portal vein endothelial cells are needed to confirm our results. HUVEC treated with the transfection reagent or with ATP are more apoptotic than untreated cells. This might be of importance after partial hepatectomy, as lower TNF-α levels impede the inflammation process by dampening apoptosis and inactivating endothelial cells. Furthermore, we could show an increased apoptosis resistance in HUVEC after transfection with miR-142-3p (Fig. 3c, d). Overexpression of miR-142-3p ameliorates the rate of viable cells. Reduced apoptosis of endothelial cells and hepatocytes after injury may impact on the reversal of liver fibrosis via less activated hepatic stellate cells [47].
Despite all, this study has some limitations. We showed that the encapsulation of miR-142-3p is ATP-dependent. But there is no evidence for a selective transport of this miRNA. There are no changes in the miR-142-3p level in the cells of origin. Furthermore, we have to assume that our isolated cells from the bone marrow and the peripheral blood and the corresponding microparticles are heterogeneous. Investigations with specific subpopulations require further refinement. It is necessary to examine the observed miRNA effects, using RNase treatment and/or specific inhibition of Dicer, which is involved in RNA interference. Furthermore, exclusion of newly synthesized miR-142-3p via detection of the appropriate pre-/pri-miRNA is needed to confirm the horizontal genetic transfer. Experiments in HUVEC should be repeated in LSEC to confirm implications in liver regeneration. Our co-culture experiments have to be understood as a proof of concept and further in vivo studies are needed to confirm the physiological relevance of proposed mechanisms. Administration of cell-derived MP in mice should be part of these investigations.
In conclusion, we here provide first evidence that miR-142-3p levels in MNC-derived MP are modulated by nucleoside signaling. Our data further suggest the horizontal transfer of genetic information via bone-marrow-derived MP as a putative mechanism of intercellular communication in sterile inflammation with potential implications in liver regeneration. Further studies need to confirm whether proposed mechanism reflects compensatory responses in sterile vascular inflammation in settings of acute liver deterioration.
Electronic supplementary material
(PDF 634 kb)
Acknowledgments
This work was presented in part at The Liver Meeting®, the 65th Annual Meeting of the AASLD in Boston, MA, USA (2014). The abstract “Plasma microparticles modulate vascular inflammation and liver regeneration via ectonucleotidase-dependent levels of miR-142-3p” was selected as a Presidential Poster of Distinction and was in the top 10% off all abstracts accepted for poster presentation.
Abbreviations
- ATP
Adenosine triphosphate
- ATPγS
Adenosine 5′-O-(3-thio)triphosphate
- BM-MNC
Bone-marrow-derived mononuclear cells
- BSA
Bovine serum albumin
- CD
Cluster of differentiation
- cel-miR-39
miR-39-derived from nematode Caenorhabditis elegans
- CPD
Citrate phosphate dextrose
- CSC
8-(3-chlorostyryl) caffeine
- DAPI
4′,6-diamidino-2-phenylindole
- DMSO
Dimethyl sulfoxide
- EDTA
Ethylenediamine tetraacetic acid
- E-NTPDase 1
Ectonucleoside triphosphate diphosphohydrolase 1
- FBS
Fetal bovine serum
- hAGO2
Human argonaute 2
- HSA
Human serum albumin
- HSC
Hematopoietic stem cells;
- HUVEC
Human umbilical vein endothelial cells
- LSEC
Liver sinusoidal endothelial cells
- MP
Microparticles; miR
- miRNA
MicroRNA
- PBS
Phosphate buffered saline
- PB-MNC
Peripheral-blood-derived mononuclear cells
- P-bodies
Processing bodies
- RNU6
U6 small nuclear RNA
- TGFβ
Transforming growth factor beta 1
- TNF-α
Tumor necrosis factor alpha
- XAC
Xanthine amine congener
- 18S rRNA
18S ribosomal RNA
Funding
The project was funded by the German Ministry of Education and Research (BMBF 1315883) and the Deutsche Forschungsgemeinschaft (DFG 2661/3-1).
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
References
- 1.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 2.Beldi G, Wu Y, Sun X, Imai M, Enjyoyi K, Csizmadia E, Candinas D, Erb L, Robson SC. Regulated catalysis of extracellular nucleotides by vascular CD39/ENTPD1 is required for liver regeneration. Gastroenterology. 2008;135:1751–1760. doi: 10.1053/j.gastro.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gehling UM, Willems M, Dandri M, Petersen J, Berna M, Thill M, Wulf T, Müller L, Pollok JM, Schlagner K, Faltz C, Hossfeld DK, Rogiers X. Partial hepatectomy induces mobilization of a unique population of haematopoietic progenitor cells in human healthy liver donors. J Hepatol. 2005;43:845–883. doi: 10.1016/j.jhep.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Harb R, Xie G, Lutzko C, Guo Y, Wang X, Hill CK, Kanel GC, DeLeve LD. Bone marrow progenitor cells repair rat hepatic sinusoidal endothelial cells after liver injury. Gastroenterology. 2009;137:704–712. doi: 10.1053/j.gastro.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine P, McDaniel K, Francis H, Kennedy L, Alpini G, Meng F. Molecular mechanisms of stem cell therapy in alcoholic liver disease. Dig Liver Dis. 2014;46:391–397. doi: 10.1016/j.dld.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Schmelzle M, Duhme C, Junger W, Salhanick SD, Chen Y, Wu Y, Toxavidis V, Csizmadia E, Han L, Bian S, Fürst G, Nowak M, Karp SJ, Knoefel WT, Schulte am Esch J, Robson SC. CD39 modulates hematopoietic stem cell recruitment and promotes liver regeneration in mice and humans after partial hepatectomy. Ann Surg. 2012;257:693–701. doi: 10.1097/SLA.0b013e31826c3ec2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulte am Esch J, Knoefel WT, Klein M, Ghodsizad A, Fuerst G, Poll LW, Piechaczek C, Burchardt ER, Feifel N, Stoldt V, Stockschläder M, Stoecklein N, Tustas RY, Eisenberger CF, Peiper M, Häussinger D, Hosch SB. Portal application of autologous CD133+ bone marrow derived stem cells to the liver: a novel concept to support hepatic regeneration. Stem Cells. 2005;2:463–470. doi: 10.1634/stemcells.2004-0283. [DOI] [PubMed] [Google Scholar]
- 8.Fürst G, Schulte am Esch J, Poll LW, Hosch SB, Fritz B, Klein M, Godehardt E, Krieg A, Wecker B, Stoldt V, Stockschläder M, Eisenberger CF, Mödder U, Knoefel WT. Portal vein embolization and autologous CD133+ bone marrow stem cells for liver regeneration: initial experience. Radiology. 2007;243:171–179. doi: 10.1148/radiol.2431060625. [DOI] [PubMed] [Google Scholar]
- 9.Schulte am Esch J, Schmelzle M, Fürst G, Robson SC, Krieg A, Duhme C, Tustas RY, Alexander A, Klein HM, Topp SA, Bode JG, Häussinger D, Eisenberger CF, Knoefel WT. Infusion of CD133+ bone marrow-derived stem cells after selective portal vein embolization enhances hepatic reserves after extended right hepatectomy. Ann Surg. 2012;255:79–85. doi: 10.1097/SLA.0b013e31823d7d08. [DOI] [PubMed] [Google Scholar]
- 10.Schmelzle M, Splith K, Andersen LW, Kornek M, Schuppan D, Jones-Bamman C, Nowak M, Toxavidis V, Salhanick SD, Han L, Schulte am Esch J, Jonas S, Donnino MW, Robson SC. Increased plasma levels of microparticles expressing CD39 and CD133 in acute liver injury. Transplantation. 2013;95:63–69. doi: 10.1097/TP.0b013e318278d3cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burger D, Schock S, Thompson CS, Montezano AC, Hakim AM, Touyz RM. Microparticles: biomarkers and beyond. Clin Sci. 2013;124:423–441. doi: 10.1042/CS20120309. [DOI] [PubMed] [Google Scholar]
- 12.Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM. Membrane microparticles: two sides of the coin. Physiology. 2005;20:22–27. doi: 10.1152/physiol.00029.2004. [DOI] [PubMed] [Google Scholar]
- 13.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crumm S, Cofan M, Juskeviciute E, Hoek JB. Adenine nucleotide changes in the remnant liver: an early signal for regeneration after partial hepatectomy. Hepatology. 2008;48:898–908. doi: 10.1002/hep.22421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzales E, Julien B, Serrière-Lanneau V, Nicou A, Doignon I, Lagoudakis L, Garcin I, Azoulay D, Duclos-Vallée JC, Castaing D, Samuel D, Hernandez-Garcia A, Awad SS, Combettes L, Thevananther S, Tordjmann T. ATP release after partial hepatectomy regulates liver regeneration in the rat. J Hepatol. 2010;52:54–62. doi: 10.1016/j.jhep.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banz Y, Beldi G, Wu Y, Atkinson B, Usheva A, Robson SC. CD39 is incorporated into plasma microparticles where it maintains functional properties and impacts endothelial activation. Br J Haematol. 2008;142:627–637. doi: 10.1111/j.1365-2141.2008.07230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornek M, Popov Y, Libermann TA, Afdhal NH, Schuppan D. Human T cell microparticles circulate in blood of hepatitis patients and induce fibrolytic activation of hepatic stellate cells. Hepatology. 2011;53:230–242. doi: 10.1002/hep.23999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fonsato V, Collino F, Herrera MB, Cavallari C, Deregibus MC, Cisterna B, Bruno S, Romagnoli R, Salizzoni M, Tetta C, Camussi G. Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells. 2012;30:1985–1998. doi: 10.1002/stem.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, Calogero R, Bussolati B, Tetta C, Camussi G. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med. 2010;14:1605–1618. doi: 10.1111/j.1582-4934.2009.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, Tetta C, Camussi G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5:e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camus SM, De Moraes JA, Bonnin P, Abbyad P, Le Jeune S, Lionnet F, Loufrani L, Grimaud L, Lambry JC, Charue D, Kiger L, Renard JM, Larroque C, Le Clésiau H, Tedgui A, Bruneval P, Barja-Fidalgo C, Alexandrou A, Tharaux PL, Boulanger CM, Blanc-Brude OP. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasco-occlusions in sickle cell disease. Blood. 2015;125:3805–3814. doi: 10.1182/blood-2014-07-589283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das S, Halushka MK. Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovasc Pathol. 2015;24:199–206. doi: 10.1016/j.carpath.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Duchez AC, Boudreau LH, Naika GS, Bollinger J, Belleannée C, Cloutier N, Laffont B, Mendoza-Villarroel RE, Lévesque T, Rollet-Labelle E, Rousseau M, Allaeys I, Tremblay JJ, Poubelle PE, Lambeau G, Pouliot M, Provost P, Soulet D, Gelb MH, Boilard E. Platelet microparticles are internalized in neutrophils via the concerted activity of 12-lipoxygenase and secreted phospholipase A2-IIA. Proc Natl Acad Sci U S A. 2015;112:E3564–E3573. doi: 10.1073/pnas.1507905112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-Messina L, Gutierrez-Vazquez C, Rivas-Garcia E, Sanchez-Madrid F, de la Fuente H. Immunomodulatory role of microRNAs transferred by extracellular vesicles. Biol Cell. 2015;107:61–77. doi: 10.1111/boc.201400081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beninson LA, Fleshner M. Exosomes: an emerging factor in stress-induced immunomodulation. Semin Immunol. 2014;26:394–401. doi: 10.1016/j.smim.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Bissels U, Wild S, Tomiuk S, Hafner M, Scheel H, Mihailovic A, Choi YH, Tuschl T, Bosio A. Combined characterization of microRNA and mRNA profiles delineates early differentiation pathways of CD133+ hematopoietic stem and progenitor cells. Stem Cells. 2011;29:847–857. doi: 10.1002/stem.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chai S, Tong M, Ng KY, Kwan PS, Chan YP, Fung TM, Lee TKW, Wong N, Xie D, Yuan YF, Guan X-Y, Ma S. Regulatory role of miR-142-3p on the functional hepatic cancer stem cell marker CD133. Oncotarget. 2014;5:5725–5735. doi: 10.18632/oncotarget.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang B, Zhao J, Lei Z, Shen S, Li D, Shen GX, Zhang GM, Feng ZH. MiR-142-3p restricts cAMP production in CD4+CD25- T cells and CD4+CD25+ TREG cells by targeting AC9 mRNA. EMBO Rep. 2009;10:180–185. doi: 10.1038/embor.2008.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim K, Yang DK, Kim S, Kang H. MiR-142-3p is a regulator of the TGFβ-mediated vascular smooth muscle cell phenotype. J Cell Biochem. 2015;116(10):2325–2333. doi: 10.1002/jcb.25183. [DOI] [PubMed] [Google Scholar]
- 31.Fordham JB, Naqvi AR, Nares S. Regulation of miR-24, miR-30b, and miR-142-3p during macrophage and dendritic cell differentiation potentiates innate immunity. J Leukoc Biol. 2015;98(2):195–207. doi: 10.1189/jlb.1A1014-519RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danger R, Pallier A, Giral M, Martínez-Llordella M, Lozano JJ, Degauque N, Sanchez-Fueyo A, Soulillou JP, Brouard S. Upregulation of miR-142-3p in peripheral blood mononuclear cells of operationally tolerant patients with renal transplant. J Am Soc Nephrol. 2012;23:597–606. doi: 10.1681/ASN.2011060543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makino K, Jinnin M, Kajihara I, Honda N, Sakai K, Masuguchi S, Fukushima S, Ihn H. Circulating miR-142-3p levels in patients with systemic sclerosis. Clin Exp Dermatol. 2011;37:34–39. doi: 10.1111/j.1365-2230.2011.04158.x. [DOI] [PubMed] [Google Scholar]
- 34.Enjyoji K, Sévigny J, Lin Y, Frenette PS, Christie PD, Schulte am Esch J, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–101.7. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 35.Aucher A, Rudnicka D, Davis DM. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J Immunol. 2013;191:6250–6260. doi: 10.4049/jimmunol.1301728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Vlist EJ, Nolte-‘t Hoen ENM, Stoorvogel W, Arkesteijn GJA, Wauben MHM. Fluorescent labeling of nano-sized vesicles released by cells and subsequent quantitative and qualitative analysis by high-resolution flow cytometry. Nat Protoc. 2012;7:1311–1326. doi: 10.1038/nprot.2012.065. [DOI] [PubMed] [Google Scholar]
- 37.Bale SS, Golberg I, Jindal R, McCarty WJ, Luitje M, Hedge M, Bhushan A, Usta OB, Yarmush ML. Long-term coculture strategies for primary hepatocytes and liver sinusoidal endothelial cells. Tissue Eng Part C Methods. 2014;21:413–422. doi: 10.1089/ten.tec.2014.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greene AK, Wiener S, Puder M, Yoshida A, Shi B, Perez-Atayde AR, Efstathiou JA, Holmgren L, Adamis AP, Rupnick M, Folkman J, O'Reilly MS. Endothelial-directed hepatic regeneration after partial hepatectomy. Ann Surg. 2003;237:530–535. doi: 10.1097/01.SLA.0000059986.96051.EA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun X, Han L, Seth P, Bian S, Li L, Csizmadia E, Junger WG, Schmelzle M, Usheva A, Tapper EB, Baffy G, Sukhatme VP, Wu Y, Robson SC. Disordered purinergic signaling and abnormal cellular metabolism are associated with development of liver cancer in Cd39/Entpd1 null mice. Hepatology. 2003;57:205–216. doi: 10.1002/hep.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopatina T, Deregibus MC, Cantaluppi V, Camussi G. Stem cell-derived microvesicles: a cell free therapy approach to the regenerative medicine. Curr Biotechnol. 2012;1:11–22. doi: 10.2174/2211550111201010011. [DOI] [Google Scholar]
- 41.Lee Y, Andaloussi SE, Wood MJA. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21:R125–R134. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- 42.Leung AKL, Sharp PA. Function and localization of microRNAs in mammalian cells. Cold Spring Harb Symp Quant Biol. 2006;71:29–38. doi: 10.1101/sqb.2006.71.049. [DOI] [PubMed] [Google Scholar]
- 43.Detzer A, Engel C, Wünsche W, Sczakiel G. Cell stress is related to re-localization of Argonaute 2 and to decreased RNA interference in human cells. Nucleic Acids Res. 2011;39:2727–2741. doi: 10.1093/nar/gkq1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asirvatham AJ, Magner WJ, Tomasi TB. MiRNA regulation of cytokine genes. Cytokine. 2009;45:58–69. doi: 10.1016/j.cyto.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 46.Xu S, Wei J, Wang F, Kong LY, Ling XY, Nduom E, Gabrusiewicz K, Doucette T, Yang Y, Yaghi NK, Fajt V, Levine JM, Qiao W, Li XG, Lang FF, Rao G, Fuller GN, Calin GA, Heimberger AB. Effect of miR-142-3p on the macrophage and therapeutic efficacy against murine glioblastoma. J Natl Cancer Inst. 2014;106(8):dju162. doi: 10.1093/jnci/dju162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chakraborty JB, Oakley F, Walsh MJ (2012) Mechanisms and biomarkers of apoptosis in liver disease and fibrosis. Int J Hepatol 2012:648915 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 634 kb)