Abstract
Adenosine is a versatile signaling molecule recognized to physiologically influence gut motor functions. Both the duration and magnitude of adenosine signaling in enteric neuromuscular function depend on its availability, which is regulated by the ecto-enzymes ecto-5′-nucleotidase (CD73), alkaline phosphatase (AP), and ecto-adenosine deaminase (ADA) and by dipyridamole-sensitive equilibrative transporters (ENTs). Our purpose was to assess the involvement of CD73, APs, ecto-ADA in the formation of AMP-derived adenosine in primary cultures of ileal myofibroblasts (IMFs). IMFs were isolated from rat ileum longitudinal muscle segments by means of primary explant technique and identified by immunofluorescence staining for vimentin and α-smooth muscle actin. IMFs confluent monolayers were exposed to exogenous 5′-AMP in the presence or absence of CD73, APs, ecto-ADA, or ENTs inhibitors. The formation of adenosine and its metabolites in the IMFs medium was monitored by high-performance liquid chromatography. The distribution of CD73 and ADA in IMFs was detected by confocal immunocytochemistry and qRT-PCR. Exogenous 5′-AMP was rapidly cleared being almost undetectable after 60-min incubation, while adenosine levels significantly increased. Treatment of IMFs with CD73 inhibitors markedly reduced 5′-AMP clearance whereas ADA blockade or inhibition of both ADA and ENTs prevented adenosine catabolism. By contrast, inhibition of APs did not affect 5′-AMP metabolism. Immunofluorescence staining and qRT-PCR analysis confirmed the expression of CD73 and ADA in IMFs. Overall, our data show that in IMFs an extracellular AMP-adenosine pathway is functionally active and among the different enzymatic pathways regulating extracellular adenosine levels, CD73 and ecto-ADA represent the critical catabolic pathway.
Keywords: Intestine, Adenosine, CD73/ecto-5′nucleotidase, Adenosine deaminase, Alkaline phosphatase, Adenosine receptor, Rat, Myofibroblasts
Introduction
Adenosine is considered an important signaling molecule involved in the modulation of gastrointestinal motor, secretory/absorptive, and immune responses. This nucleoside exerts its effects through four G protein-coupled P1 purinergic receptors, namely A1, A2A, A2B, and A3 receptors, located on the surface of diverse enteric cells such as neuronal, glial, epithelial, smooth muscle, and immune cells [1, 2]. Each P1 receptor subtype is characterized by distinct binding affinities for adenosine as well as by diverse expression and functional profile along the gastrointestinal tract in both physiological and pathological conditions [3–6].
Adenosine levels in interstitial fluids are dependent on the combined actions of several processes, including cellular release and re-uptake, extracellular and intracellular metabolism [7–11]. In pathological conditions, such as hypoxia or inflammation, extracellular adenosine levels considerably raise, playing a pivotal role in the regulation of neuromuscular and inflammatory responses [3–5, 12]. The major process responsible for the rapid extracellular adenosine increase following a harmful insult is represented by ATP and ADP dephosphorylation, which are transformed into 5′-AMP (AMP) by the ecto-nucleoside triphosphate diphosphohydrolase 1 (CD39). AMP is successively dephosphorylated to adenosine by the ecto-5′-nucleotidase (CD73) and/or by the alkaline phosphatases (APs) [13–15].
In physiological conditions, a more amendable source of extracellular adenosine is represented by cyclic adenosine monophosphate (cAMP), which, once extruded from activated cells, can be converted into AMP and then to adenosine by ecto-phosphodiesterase and by CD73, respectively [16]. In this regard, considering the findings of Nitahara et al. in isolated longitudinal muscle strips of guinea pig ileum [7], we were the first to reveal CD73 expression in rat ileum muscularis externa and in primary culture of ileal smooth muscle cells and to highlight the critical role of CD73 in the formation of extracellular adenosine at both levels [10]. Intriguingly, in rat ileal longitudinal muscle-myenteric preparations (LMMPs), Duarte-Araùjo et al. [8] and Correia-de-Sà et al. [9] have shown that extracellular AMP-derived adenosine by interacting with pre-junctional A2A facilitatory receptors (but not with A1 inhibitory receptors) evoked an increase in the release of acetylcholine from electrically stimulated myenteric neurons.
The effect of both adenosine and AMP is interrupted by equilibrative nucleoside transporters (ENTs) and/or by ecto-adenosine deaminase (ecto-ADA), a ubiquitous catabolic enzyme localized on cell surfaces, which reduces adenosine extracellular levels in the biophase of its receptors [17]. Of note, ecto-ADA may be found either in a soluble form in interstitial fluids or in a cell-associated form, bound to CD26 or adenosine receptor [9, 15, 17–19].
Although several studies have revealed a critical role of CD73 in the control of extracellular adenosine levels in the gut [4, 13–15, 19], the relevant enzymes involved in regulating adenosine levels at P1 receptors located on gut primary culture of myofibroblasts are still unknown. Thus, the purpose of the present study was to assess the physiological role of the ecto-enzymes CD73 and ecto-ADA in modulating extracellular adenosine levels in primary culture of rat ileal myofibroblasts (IMFs).
Materials and methods
Animals and tissue preparation
Male Wistar rats (250 ± 50 g body weight; Harlan, Italy) were housed at the conventional animal facility of the Department of Pharmaceutical and Pharmacological Sciences of the University of Padova under temperature and humidity-controlled conditions with ad libitum access to standard laboratory chow and tap water, and a regular 12/12-h light/dark cycle. All experimental protocols were approved by the Animal Care and Use Committee of the University of Padova and by the Italian Ministry of Health and were in compliance with the national and EU guidelines for handling and use of experimental animals. Immediately after decapitation, segments of ileum were excised, deprived of the mesentery and related tissues, and placed in ice-cold phosphate-buffered saline (PBS), supplemented with 0.25 μg/mL amphotericin B and 20 μg/mL gentamicine, and aerated with 95% O2/5% CO2 [10].
Cultures of ileal myofibroblasts
IMFs were explanted from the muscularis externa of the rat ileum, as previously reported [10]. Briefly, isolated longitudinal smooth muscle strips were collectively minced and cultured in DMEM (Cambrex, Italy) supplemented with 20% fetal bovine serum (FBS, Celbio, Pero, Italy), 20 μg/mL gentamicin, 2 mM L-glutamine, and 0.1 mM MEM Eagle NEAA and maintained at 37 °C in a humidified 5% CO2 atmosphere. On reaching confluence, explants were dissociated using 0.125% (wt/vol) trypsin and EDTA*4Na (0.38 g/L) in Hanks’ balanced salt solution (HBBS) for 1 min at 37 °C. Isolated cells were then seeded onto 60-mm dishes at a density of 3 × 105 cells/mL and maintained in DMEM containing 10% FBS. All experiments were performed on almost confluent cultures (3 × 105 per well), for no more than two passages [10]. IMFs integrity and viability were determined by light microscopy observations and trypan blue dye exclusion assay. After incubation with or without AMP in absence or presence of the enzyme inhibitors, cells were treated with 0.4% trypsin to obtain cell suspensions that were thoroughly mixed with equal volumes of 0.4% trypan blue solution (Gibco, Grand Island, NY, USA) and incubated for 3 min at 37 °C. Live (non-stained) and dead (stained) cells were estimated using a hemocytometer according to manufacturer’s instructions.
Measurement of surface enzymes activity
IMFs, grown to subconfluence in DMEM supplemented with 10% FBS, were washed twice with PBS and incubated for 24 h with 1 mL of DMEM supplemented with 0.4% FBS. AMP (50 μM) was added to the cells in 1 ml-final volume and incubated for diverse time periods. In different experimental settings, IMFs were treated with 50 μM AMP in the presence or absence of the following: (i) α,β-methyleneadenosine-5′-diphosphate (AOPCP, 0.2 mM) [20]; (ii) concanavalin A (ConA, 0.1 mg/mL) [21]; (iii) levamisole (10 mM) [22]; (iv) erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA, 10 μM) [9, 10]; (v) dipyridamole (0.5 μM) added 15 min before the exogenous nucleotide. After incubation, 1-mL samples of IMFs incubation medium were collected into tubes containing HClO4 (1 M, final concentration) and stored at − 80 °C until HPLC analysis.
For Eadie–Hofstee graphical analysis, incubation time was 5 min to prevent substrate depletion. Product formation was expressed as μmol/min of adenosine + inosine + hypoxanthine.
HPLC analysis of purines
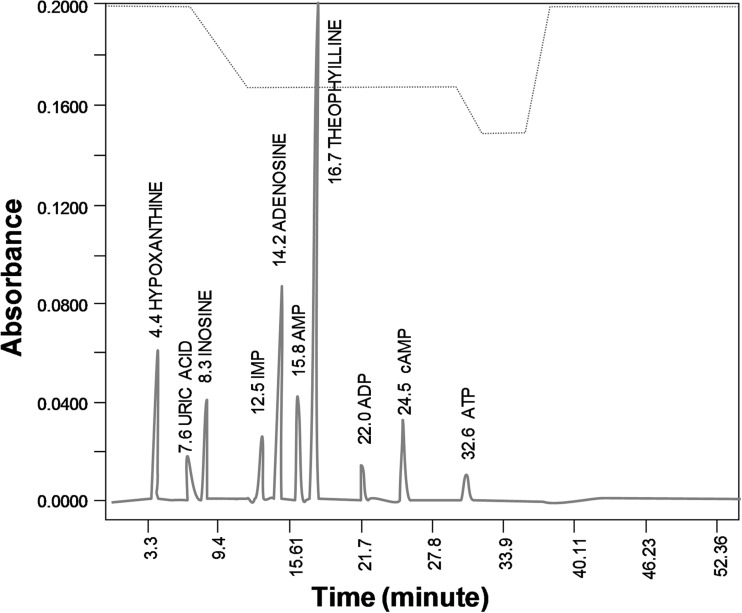
Samples of IMFs incubation medium were brought to about pH 4–5 with 1 N NaOH and then mixed with 50 μM theophylline as internal standard. After centrifugation (3000 g for 5 min), clear supernatants were filtered through a 0.22-μm syringe filter and analyzed by HPLC-UV (Beckman System Solvent Module 125) on a Beckman C18 Analytical Ultrasphere column, using a mobile phase consisting of 73.5 mM NaH2PO4/6 mM tetra-n-butylammonium bromide (pH 5.8; solvent A) and methanol (solvent B), applying a non-linear gradient at a flow rate of 1 mL/min, as previously described [10]. AMP, adenosine, inosine, and hypoxanthine and ATP were detected by UV absorbance (254 nm, Beckman Detector 166) and quantified from absorbance peak area. The concentration of purines was extrapolated from calibration curves. A representative chromatogram of standards mixture of purines (i.e., AMP, adenosine, inosine, and hypoxanthine and ATP) and internal standard (i.e., theophylline) is depicted in Fig. 1.
Fig. 1.
Purines HPLC analysis. A representative chromatogram of standards mixture of purines (i.e., ATP, ADP, cAMP, AMP, adenosine, IMP, inosine, hypoxanthine, and uric acid) and internal standard (i.e., theophylline)
Immunocytochemistry
Desmin, vimentin, alpha smooth muscle actin (α-SMA), CD73 and ADA were detected by indirect immunofluorescence in IMFs plated in glass cover slips. Cells were fixed in 4% paraformaldehyde for 15 min and incubated with 0.05 M NH4Cl. Fixed cells were rinsed with PBS, permeabilized with 0.1% Triton X-100, and incubated with the following primary antibodies: rabbit polyclonal anti-human desmin (1:100, Thermo Fisher Scientific, Milan, Italy), mouse monoclonal anti-human vimentin (1:100, clone V9, Dako, Italy), mouse monoclonal α-SMA FITC-conjugated (1:200; clone 1A4, Sigma Aldrich, Milan, Italy), mouse anti-rat CD73 (1:500; BD Biosciences Pharmigen, Belgium), and biotin-labeled rabbit anti-bovine ADA (1:100; Alpha Diagnostic International Inc., USA), diluted in PBS containing 0.1% BSA and 10% normal goat serum for 1 h at room temperature [10, 23, 24].
Thereafter, IMFs were incubated with goat anti-mouse or streptavidin labeled with Alexa Fluor 488 and with anti-rabbit labeled with Alexa Fluor 555 (1:1000; Thermo Fisher Scientific, Milano, Italy), for 1 h at room temperature. Cell nuclei were stained with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI) (1:1000; Thermo Fisher Scientific, Milan, Italy), added together with the secondary antibodies. To obtain negative controls, IMFs were also incubated with appropriate isotype-matched control antibodies or without primary antibody or pre-incubated with each antibody with the corresponding control peptide (final concentration as indicated by manufacturer’s instructions) [10, 23]. Stained cells were imaged with a Zeiss LSM 800 confocal laser-scanning microscope (Carl Zeiss AG, Germany) and analyzed using NIH Image J software (version 1.50a) [23].
RNA isolation and quantitative RT-PCR
Quantitative reverse transcription PCR (qRT-PCR) analysis was conducted on rat IMFs lysates obtained from three different cell cultures. To conduct each experiment, 1.2 × 106 cells were collected from each cell culture. Total RNA was extracted using a Quick-RNA MicroPrep (ZYR1050, Zymo Res, Euroclone, Milan, Italy) according to the manufacturer’s instructions.
Two microgram of total RNA was reverse transcribed using High Capacity cDNA Reverse Transcription (Applied Biosystems, Milan, Italy). Quantitative RT-PCR was performed on the Abi Prism 7000 real-time thermocyclator (Applied Biosystems, Milan, Italy) with Power Sybr Green Universal PCR Master Mix (Applied Biosystems, Milan, Italy) according to manufacturer’s instructions. Primers were designed using Primer Express software (Applied Biosystems, Milan, Italy) according to the available sequences deposited in open access databases. The primer sequences are listed in Table 1. For qRT-PCR a 500-nM-final concentration for each primer was used. Primers were designed to have a similar amplicon size and similar amplification efficiency as required for the application of the Ct (cycle threshold) method as a relative measure of the concentration of target gene in our samples, where Ct represents the number of cycles required for the fluorescent signal to cross the threshold, thus exceeding background level [25]. β-actin was used as a housekeeping gene. Experiments were carried out two times for each different preparation (n = 3).
Table 1.
Sequence of primers used for the q-RT PCR analysis of ADA and CD73 expression in IMFs and relative length of the amplification products
| Gene | Sequence 5′-3′ | Length |
|---|---|---|
| ADA | Fw CTGGAATCCCAAAACGACGC Rev TCGTCCGAGTTGAGGGAGTA |
75 bp |
| CD73 | Fw TGTTGGGACCAGCAACTCAA Rev TTTGAGGCTCAGTGGTAGCC |
136 bp |
| β-actin | Fw TGACAGGATGCAGAAGGAGA Rev TAGAGCCACCAATCCACACA |
104 bp |
Drugs and chemicals
Amphotericin B, trypsin, EDTA, and gentamicin were purchased from Invitrogen-Gibco (Milano, Italy). Fetal bovine serum (FBS) was from Celbio (Pero, Italy). Formaldehyde (37%) and Triton X-100 were from Applichem (Milano, Italy). Tetra-n-butylammonium bromide (TBA) was from Merck (Darmstadt, Germany). Unless otherwise specified, all other chemicals were obtained from Sigma Aldrich (Milan, Italy) and were of the highest available analytical grade. Drugs were prepared as concentrated stock solutions in sterile H2O mQ grade and diluted into DMEM or Krebs solution just before use.
Statistical analysis
All data were expressed as mean ± SEM; n refers to the number of animal preparations on which observations were made. Statistical analysis of data was performed by paired or unpaired Student’s t tests or by one-way analysis of variance (ANOVA) followed by Neuman-Keuls multicomparison test when appropriate, using Graph Pad Prism 3.03 (San Diego, CA-USA). p values of < 0.05 were considered statistically significant. Linear logistic regression (Pearson’s correlation coefficient) and correlation analysis were performed in selected experiments.
Results
IMFs metabolize exogenous AMP
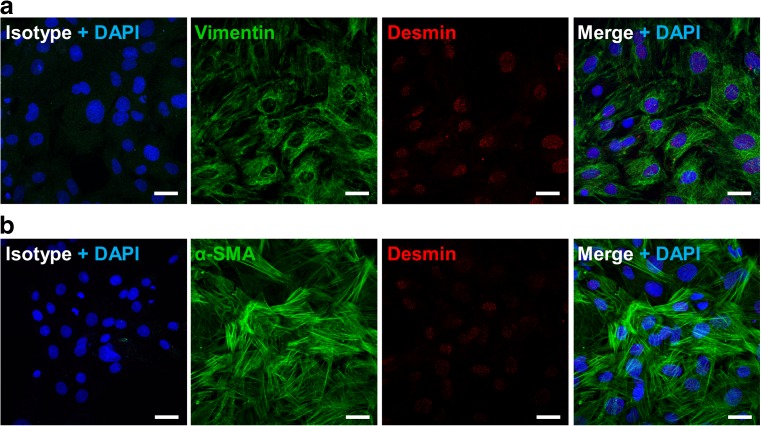
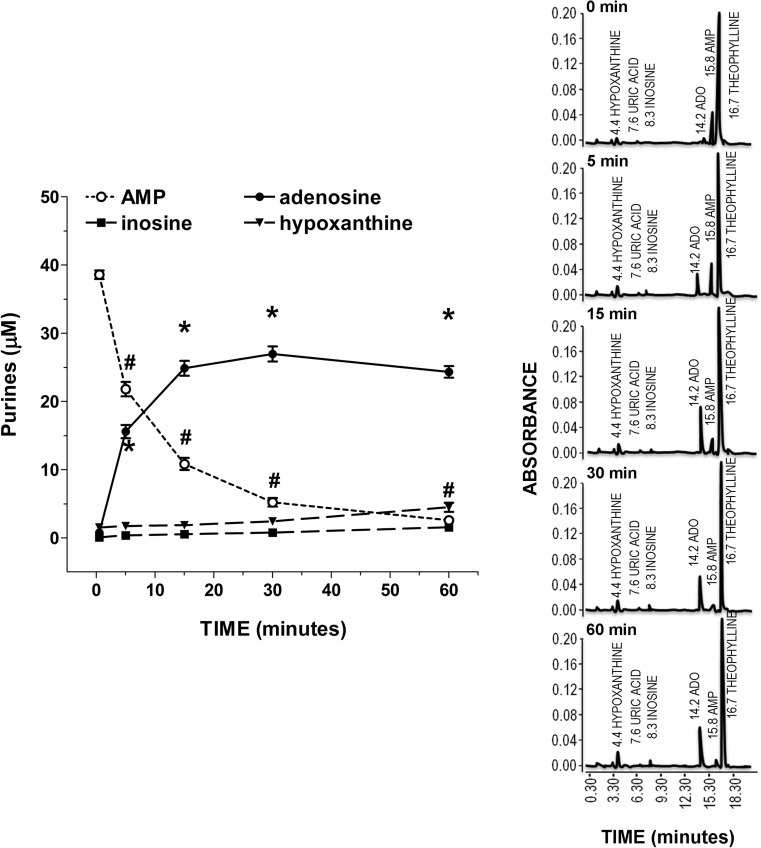
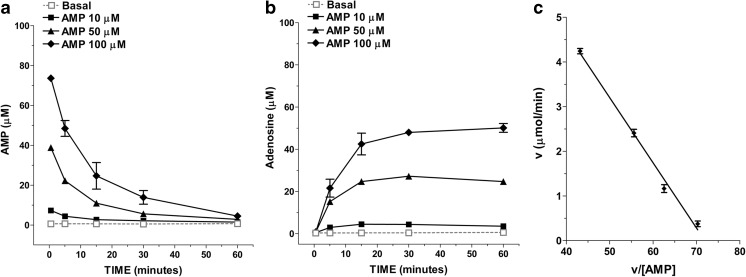
To characterize the enzyme machinery involved in controlling adenosine levels in the enteric smooth muscle layer, a primary culture of rat IMFs from rat small intestine was obtained using the tissue explant technique, without the use of enzymatic tissue dissociation. The lineage identity of IMFs was confirmed by the expression of α-SMA and vimentin and the absence of desmin immunoreactivity (Fig. 2) [24]. After 60-min incubation of IMFs in the absence of AMP the release of 0.54 ± 0.24 μM AMP, 0.45 ± 0.03 μM adenosine, 0.16 ± 0.12 μM inosine, and 1.34 ± 0.29 μM hypoxanthine was determined in the incubation medium. Following the addition of 50 μM AMP to the incubation medium a time-dependent decrease in the nucleotide concentration occurred (Fig. 3). The average half-life of exogenously added AMP was 4.1 ± 0.6 min. The disappearance of AMP from the incubation medium was associated with a time-related increase in the levels of its metabolites over basal values (Fig. 3), suggesting that these metabolites originated from AMP catabolism by IMFs enzymes. During a 60 min-incubation period, the total metabolite pool (i.e., adenosine + inosine + hypoxanthine) reached a maximum concentration in the medium after 15 min and remained steady thereafter (Fig. 3), although the time course describing the enhancement of each metabolite concentration in the medium was different. As shown in Fig. 3, adenosine concentration reached a maximum value of 27.0±1.1 μM at 30 min, while inosine and hypoxanthine highest concentrations were 1.53±0.50 μM and 4.65±0.67 μM, respectively, at 60 min. In addition, adenosine levels were already statistically significant versus basal control levels at 5 min, while a 60-min time period was required for inosine and hypoxanthine to rise above basal levels (Fig. 3). After 60 min, the pool of adenosine + inosine + hypoxanthine accounted for approximately 61% of the amount of AMP cleared from the medium. When increasing concentrations of AMP (10–100 μM) were added to IMFs, the reduction of AMP levels (Fig. 4a) was paralleled by a proportional increase in the levels of adenosine (Fig. 4b) and its metabolites (data not shown) after 60 min-incubation period. The kinetics of AMP catabolism at increasing concentrations after 5-min incubation, evaluated by Eadie–Hofstee graphical technique, gave a linear plot (Fig. 4b) with a Km value of 146 μM. These findings suggest that, in our model, adenosine production is far more dependent on AMP than inosine and hypoxanthine production.
Fig. 2.
Immunohistochemical characterization of rat IMFs. a Representative confocal microphotographs showing the distribution of vimentin (green) and desmin (red) in rat IMFs. b Representative confocal microphotographs showing the distribution of α-SMA (green) and desmin (red) in rat IMFs. Isotype fluorescent images were obtained by labeling with Alexa Fluor 488 and Alexa Fluor 555 conjugated secondary antibodies in presence of nonimmune rabbit or mouse antiserum instead of the primary antibodies. Cell nuclei were stained with DAPI (blue). n = 6. Scale bars = 25 μm
Fig. 3.
IMFs metabolize exogenous AMP. a Residual concentration of exogenous AMP and time-dependent conversion of AMP to extracellular adenosine, inosine and hypoxanthine in the medium of cultured IMFs. IMFs were incubated for the indicated times with exogenous AMP (50 μM, n = 6). *,#p < 0.05 versus time = 0 min. b Representative chromatograms illustrating the changes in AMP, IMP, adenosine, inosine, hypoxanthine, and uric acid levels obtained by injecting IMFs-free supernatants collected after 0.5, 5, 15, 30, and 60 min-incubation with exogenous AMP (50 μM) and processed for separation and quantification by reverse phase HPLC and UV absorbance detection
Fig. 4.
Concentration-dependence of exogenous AMP catabolism by rat IMFs. a Purine metabolites (adenosine, inosine and hypoxanthine) levels in IMFs medium after 60-min incubation with AMP (10–100 μM; n = 6). b Eadie–Hofstee plot of v (μmol/min) versus v/[AMP] illustrating CD73 activity after 5-min incubation with 5–100 μM AMP (n = 6)
IMFs express CD73 and adenosine deaminase
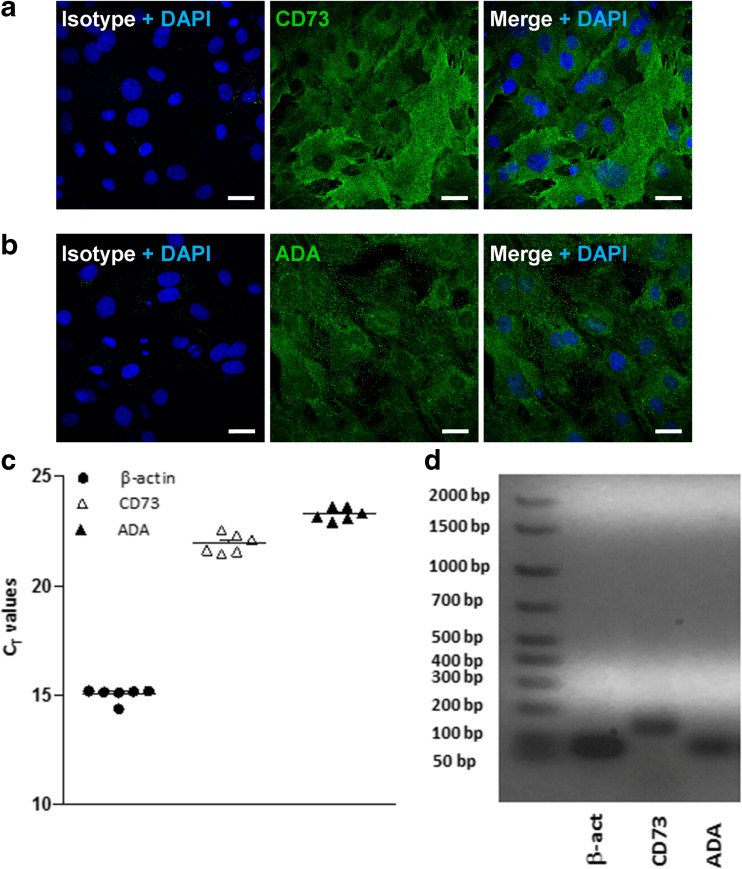
To further characterize the extracellular AMP-adenosine pathway in IMFs primary cultures, we evaluated the expression of the enzymes CD73 and ecto-ADA. IMFs showed positive immunoreactivity for CD73 and ecto-ADA (Fig. 5a, b), suggesting that these cells are potentially capable of metabolizing extracellular adenosine. qRT-PCR analysis showed that mRNAs for both CD73 and ADA are expressed in rat IMFs, yielding Ct values of 21.94 ± 0.18 (n = 3) and 23.22 ± 0.20 (n = 3), respectively (Fig. 5c). Correct length amplification was confirmed by running qRT-PCR products on an agarose gel, which showed the predicted amplification bands at 136 bp and 75 bp for CD73 and ADA, respectively (Fig. 5d).
Fig. 5.
Rat IMFs express CD73 and ADA enzymes. a Representative confocal microphotographs showing the distribution of CD73 (green) or b ADA (green) in rat IMFs. Isotype fluorescent images were obtained by labelling with Alexa Fluor 488 conjugated secondary antibody in presence of nonimmune rabbit or mouse antiserum instead of the primary antibodies. Cell nuclei were stained with DAPI (blue). n = 6. Scale bars = 25 μm. c RT-PCR analysis of CD73, ADA, and of β-actin transcripts in rat IMFs. Values are expressed as number of threshold cycles (Ct) ± SEM, n = 3. d Representative microphotograph showing qRT-PCR products on an agarose gel
CD73 and ADA regulate adenosine levels in IMFs
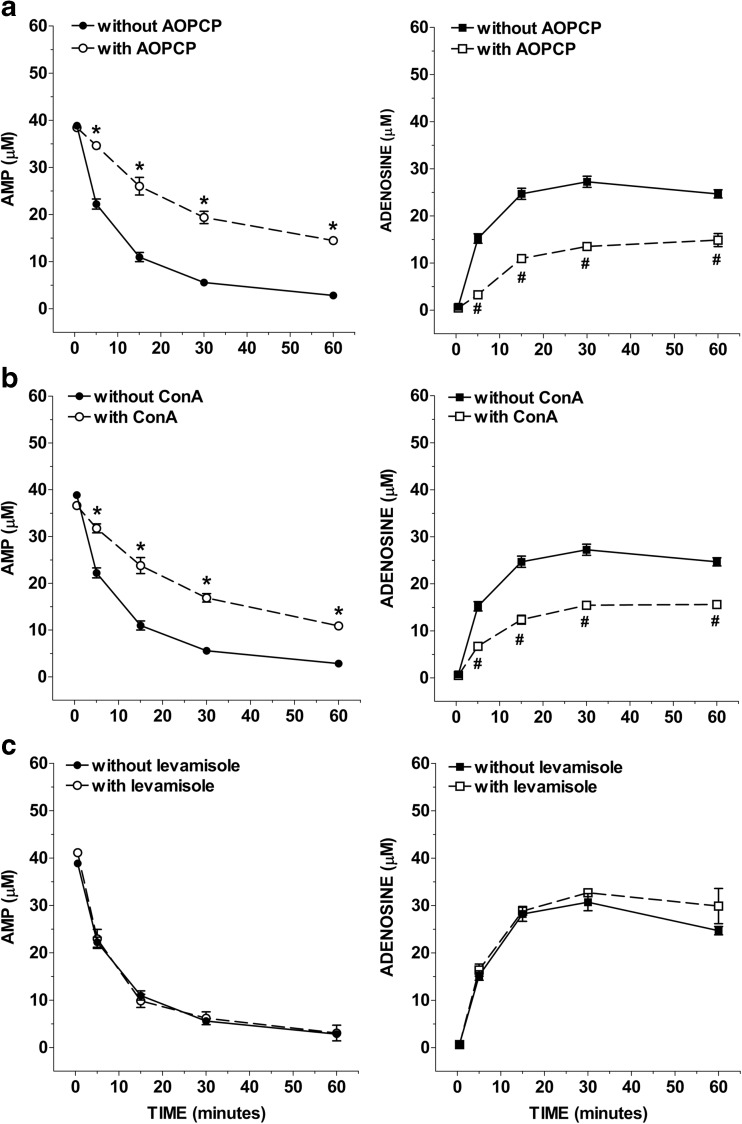
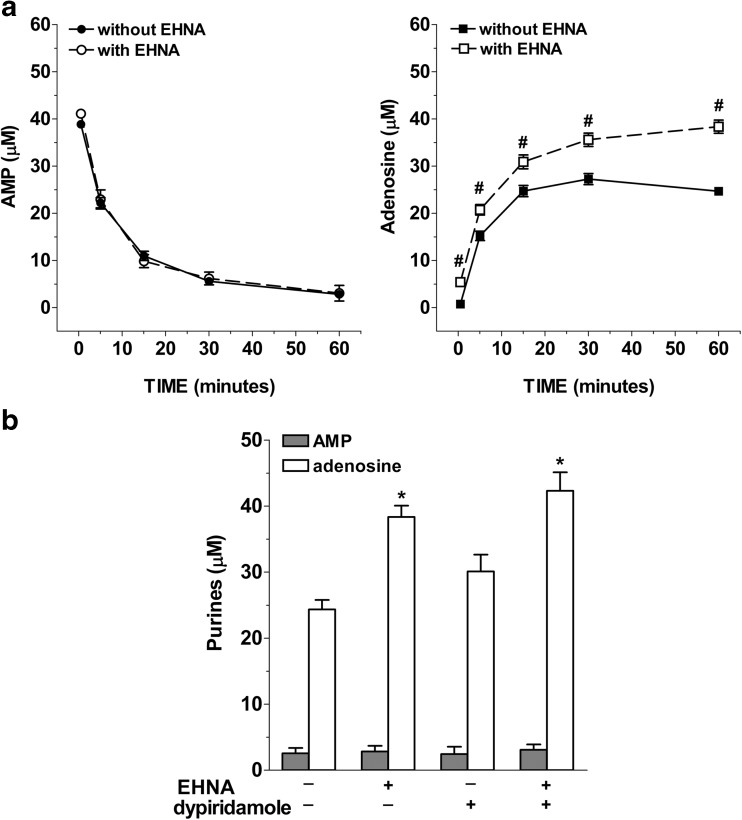
To test if the activation of ecto-ADA and CD73 and APs may underlie adenosine metabolism, IMFs were incubated with 50 μM AMP in the presence of specific inhibitors for these ecto-enzymes. Figure 6 shows the time course of AMP catabolism and adenosine formation in the presence of two chemically distinct CD73 inhibitors, the ADP analogue, AOPCP (0.2 mM; Fig. 6a), and the non-nucleoside lectin, concanavalin A (ConA 0.1 mg/mL; Fig. 6b). Both the two membrane-impermeable inhibitors of CD73 proportionally reduced AMP clearance and adenosine formation by about 60% (Fig. 6a, b). After 60-min incubation with AOPCP (Fig. 6a) or ConA (Fig. 6b), the average half-life of exogenously added AMP increased to 9.9 min and 9.7 min, respectively. Treatment of IMFs with AMP in the presence of 10 mM levamisole, an APs inhibitor [22], did not affect the conversion of AMP to adenosine as demonstrated by the average half-life which remains equal to 4.1 min (Fig. 6c). The ecto-ADA inhibitor EHNA (10 μM) did not influence AMP metabolism (Fig. 7a) but significantly increased adenosine accumulation in AMP-treated IMFs (Fig. 7a) and markedly reduced AMP-induced inosine + hypoxanthine accumulation that resulted comparable to basal levels (0.22 ± 0.08 μM inosine and 1.14 ± 0.19 μM hypoxanthine). Treatment of IMFs with AMP in the presence of dipyridamole, an inhibitor of ENTs, did not affect the conversion of AMP to adenosine after 60 min of incubation (Fig. 7b). However, a significant increase of adenosine levels was found following 60 min-incubation with both dipyridamole + EHNA (Fig. 7b), to suggest that IMFs clear adenosine primarily through adenosine deaminase but also through its uptake via ENTs to prevent long-term increases of extracellular adenosine, as previously observed in rat ileal longitudinal muscle—myenteric plexus preparations [8]. These results support the presence of a very intense extracellular CD73 activity compared to that of APs and ecto-ADA in IMFs, suggesting that CD73 is primarily responsible for rapid AMP dephosphorylation to adenosine in our model.
Fig. 6.
Influence of alkaline phosphatase and CD73 inhibitors on AMP levels and adenosine accumulation in IMFs incubation medium. IMFs were incubated with AMP (50 μM) for 60 min in presence or absence of AOPCP (0.2 mM; n = 6; a), or ConA (0.1 mg/ml; n = 6; b), or levamisole (10 mM; n = 6; c). *p < 0.05 versus AMP; #p < 0.05 versus adenosine
Fig. 7.
Effects of ecto-ADA and ENTs inhibition on AMP levels and adenosine accumulation in IMFs incubation medium. IMFs were incubated with AMP (50 μM) for 60 min in presence or absence of EHNA (1 μM; n = 4), dipyridamole (0.5 μM; n = 4), or EHNA + dipyridamole (n = 4). *p < 0.05 versus adenosine in absence of the inhibitors
Discussion
In the gut, adenosine acts as an autocrine and paracrine messenger on most cell types [1, 4, 12]. Until now, most of the research has been devoted to understanding adenosine-signaling in the myenteric plexus; however, data concerning the physiological relevance of AMP and related ecto-enzymes CD73 and ADA in controlling adenosine levels in gut IMFs are still limited [8–10, 15, 19]. Smooth muscle myopathies have been observed in various gastrointestinal diseases, including chronic intestinal pseudo-obstruction (CIP) [26] and inflammatory bowel disease (IBD) [27–29]. IMFs show morphologic and functional features of both fibroblasts and smooth muscle cells and appear to transdifferentiate from these cells or to originate from primitive stem cells. Even if IMFs exact function, content and involvement are still to be defined, they play an important role in intestinal injury, inflammation, fibrosis, and tissue repair [30]. In Crohn’s disease (CD), ileal strictures are associated to increased intestinal obstruction, often resulting in surgical intervention, and usually developed from a chronic transmural inflammation that elicits an exaggerated deposition of extracellular matrix released by activated IMFs. Of note, in CD, a constant process of differentiation and de-differentiation between the three mesenchymal phenotypes (i.e., fibroblasts, smooth muscle cells, and IMFs) has been detected with a prevalent phenotypic switch of fibroblasts into IMFs [30]. During CD, also interstitial cells of Cajal (ICC), another type of myofibroblasts involved in the propagation of electrical events, have been shown to almost completely disappear within both muscle layers or to be destroyed during fibrosis and replaced by fibroblasts [31]. Thus, a detailed characterization of the enzymatic pathways that modulate extracellular adenosine levels may be useful for defining potential therapies [32].
Adenosine production can take place both in the intracellular and extracellular compartments. The intracellular pathway comprises the constitutive transmethylation pathway involving the cleavage of S-adenosyl-L-homocysteine (SAH) to L-homocysteine and adenosine by the SAH hydrolase, and the enzymatic ATP dephosphorylation into adenosine by cytosolic nucleotidase when energy supply fails to meet cellular energy demand [32, 33]. Once produced, adenosine can be extruded through the cell membrane via facilitated diffusion nucleoside transporters to interact with its G protein-coupled receptors. The extracellular ATP-adenosine pathway involves adenine nucleotides released by several cell populations and then metabolized to adenosine by different ecto-enzymes such as ecto-ATPase, ecto-ADPase and CD73 [12]. The postsynaptic localization of CD73 was firstly shown by immunohistochemistry combined with electron microscopy in the guinea pig ileum [7]. Upon release, adenosine can then activate adenosine P1 receptors or can be internalized through equilibrative or concentrative transporters, representing a recognized re-uptake system involved in the control of the nucleoside extracellular levels at the myenteric neuromuscular junction [8, 9]. In rat ileum preparations of LMMPs, Duarte-Araújo et al. [8] and Correia-de-Sá et al. [9] demonstrated that the adenosine, generated from exogenous AMP in the extracellular milieu, interacts with pre-junctional facilitatory A2A adenosine receptors (but not with A1 inhibitory receptors) and determines the release of acetylcholine from electrically activated myenteric neurons. The cAMP-adenosine pathway has also been put forward as a more suitable mechanism underlying the hormonal modulation of adenosine levels at the cell surface, where adenosine receptors are located [16]. In this respect, we have provided substantial evidence for a physiological role played by the extracellular cAMP-adenosine pathway in the small intestine [10].
Our study provides demonstration that IMFs express CD73, which retains a physiological role in modulating adenosine generation in the gut neuromuscular layer [7]. Furthermore, we show that AMP is metabolized to adenosine by CD73 and the nucleoside is subsequently deaminated into inosine by ecto-ADA; both are key enzymes involved in controlling adenosine levels in IMFs. In our experiments, a progressive and rapid decrease of exogenously added AMP was found in IMFs medium, with a t1/2 of about 4 min. This clearance was paralleled by a rapid accumulation of AMP-derived metabolites, namely, adenosine, inosine, and hypoxanthine. In particular, 15 min after AMP addition to the medium, adenosine levels reached a steady state while no changes in inosine levels were detected at end of the 60-min incubation period. CD73 is claimed to be the rate limiting enzyme of adenosine generation from the extracellular catabolism of adenine nucleotides catalyzed by the ectonucleotidase cascade, which, in the myenteric neuromuscular junction of the rat ileum, involves, at least, NTPDase 2 and 3 along with ecto-5′-nucleotidase/CD73 [19]. This finding is functionally relevant considering that AMP precursors, such as both ATP and ADP, feed-forwardly inhibit CD73 contributing to slow down adenosine formation and, thus, to the dissociation of the effects of nucleotide-sensitive P2 receptors from adenosine-operated P1 receptors [8, 9, 19].
Detection of high adenosine concentrations compared to those of inosine and hypoxanthine confirms the presence of a very intense CD73 activity and a low ecto-ADA activity in IMFs cultures. The ability of IMFs to clear much higher extracellular AMP concentrations than those present in physiological conditions without showing any sign of cell damage as well as the high rate of AMP clearance and its metabolites formation in the medium is indicative for a high metabolic activity of IMFs versus the nucleotide. The kinetics of AMP catabolism in cultured IMFs was found to be significantly different from that shown in isolated LMMPs of the rat ileum [8]. Moreover, in LMMPs, the half-life of exogenously added AMP (i.e., 15.04 ± 2.42 min) was longer than in IMFs primary culture, possibly reflecting a diverse expression and/or activity of CD73 in the different cell subtypes composing the isolated ileal LMMPs. Indeed, the Km value of 146 μM obtained with our primary culture was similar to values found in vascular smooth muscle cell cultures (100–150 μM) and arterial smooth muscle cells (82 μM) [34, 35]. Other in vitro studies, carried out in many different cell types, such as rat Sertoli cells [36], human intestinal epithelial cells [37], microvascular endothelial cells [38], and bronchial epithelial cells [22], showed that extracellular dephosphorylation of AMP into adenosine is primarily mediated by CD73, the so called purine rescue enzyme. This enzyme, also identified as ecto-5′-nucleotidase (ecto-5′-NT, EC 3.1.3.5) is a 70-kD glycosylphosphatidylinositol protein, anchored to the cell surface, and encoded by the NT5E gene. CD73 plays a crucial role in switching purinergic signaling from ATP-dependent to adenosine-dependent signaling cascades [8, 9, 13, 19]. In the last years, the modulation of this protein has been also suggested to represent a critical checkpoint not only in the control of tumor development but also in the severity of a variety of other diseases, including tissue fibrosis, infections, and autoimmune diseases [5, 39, 40].
Alkaline phosphatases (APs) are tissue-nonspecific enzymes, expressed on intestinal, placental, and germ cells, which contribute to the metabolism of AMP to adenosine and share many common features with CD73, such as being glycosylphosphatidylinositol (GPI)-anchored ecto-enzymes, displaying similar molecular weight and forming homomeric dimers [32, 41]. Similarly to CD73, APs can be released as soluble forms and are highly expressed in the intestines and kidneys where they are considered specific markers [41, 42]. In spite of these similarities, the contribution of APs to adenosine generation in the gastrointestinal tract has only been partially investigated until now [43]. We have thus tried to evaluate the differential contribution of CD73 and APs in modulating AMP levels in IMFs by measuring the rate of AMP catabolism and metabolite formation in the absence or presence of specific CD73 and APs inhibitors, such as AOPCP or ConA and levamisole, respectively. AOPCP, a more stable analogue of ADP, is one of the most potent available competitive inhibitors of ecto-5′-NT, and has recently been used as a lead compound for the development of new potent CD73 inhibitors [44]. ConA, a plant lectin that binds specifically to α-d-glucosyl and α-d-mannosyl residues of glycoproteins [21], has been shown to completely block the activity of CD73 without affecting APs whereas levamisole has been shown to inhibit the enzymatic activity of tissue-nonspecific, placental and intestinal APs [22]. The kinetic profiles of AMP clearance and generation of related metabolites in IMFs medium were influenced by both AOPCP or ConA, but not by levamisole addition. These findings suggest that CD73, but not APs, is responsible for AMP-derived adenosine production in IMFs.
The ADA inhibitor EHNA increased adenosine-to-inosine ratio without altering AMP levels, revealing that extracellular inosine accumulation is mostly derived from adenosine metabolism by ecto-ADA, which is extensively expressed in mouse [45] and rat tissues and highly active in the small intestine, as previously shown in full-thickness preparations of rat ileum by our group [10] and in rat colon by Antonioli et al. [46]. The resulting inosine could be then metabolized by purine nucleoside phosphorylase (PNP; EC 2.4.2.1) to hypoxanthine and ribose-1-phosphate [47]. The concomitant inhibition of ADA and ENTs revealed that in IMFs, adenosine deamination is a more effective pathway than the dipyridamole-sensitive equilibrative nucleoside uptake system in regulating adenosine levels in the extracellular milieu, as previously shown in rat ileal preparations of LMMPs [8].
Interestingly, CD73 blockade reduced, but did not abolish AMP clearance. It is conceivable that in the enteric smooth muscle microenvironment, an ensemble of enzymes that includes, but extends beyond, CD73 and ecto-ADA may be responsible for AMP homeostasis. For example, after knocking out CD73 in mouse dorsal root ganglia and spinal neurons, adenosine generation was only partially reduced [48]. Analogously, in neurons genetically deprived of both prostatic acid phosphatase (PAP; EC 3.1.3.2) and CD73, adenosine production was reduced by 69% [48]. A possible alternative player involved in adenosine metabolism is the prostatic acid phosphatase (PAP), a tartrate-sensitive histidine acid phosphatase enzyme with two known isoforms, the secreted (sPAP) and the type-I transmembrane (TMPAP) isoform, widely expressed in human and rodent tissues [49]. We can hypothesize that besides CD73, also PAPs might be involved in regulating extracellular adenosine levels, AMP metabolism, and smooth muscle physiology in IMFs. However, this possibility merits further investigation, especially considering the importance of an efficient metabolism and recycling of extracellular AMP in gastrointestinal pathophysiology.
In line with the critical role of adenosine signaling in the intestine, here, we showed the expression of CD73 and ADA proteins in cultured IMFs. This finding suggests that surface-bound CD73 and ADA convert AMP to adenosine, which, in turn, can potentially activate P1 receptors [50].
However, a significant accumulation of adenosine was found in the incubation medium from IMFs compared to rat ileum preparations [8, 10] suggesting that ecto-ADA activity could be depressed or be less efficient in physiological conditions. These results highlight the involvement of IMFs together with other cells (e.g., interstitial cells of Cajal) located at the tripartite myenteric synapse in controlling extracellular adenosine accumulation for regulating inflammatory response [51–53].
Considering the potent anti-inflammatory action of adenosine and the ability of adenosine receptors to regulate a variety of important physiological processes [50], these data evoke important challenges for the future such as to decipher the extracellular information encoded in AMP-derived adenosine signaling and the pathophysiological implications of the ecto-enzymes responsible for its metabolism in the gut. Our results may also have potential implications for the pharmacological manipulation of endogenous adenosine content by targeting these ecto-enzymes in the enteric neuromuscular layer through the inhibition of ecto-ADA, and/or the administration of AMP-derived prodrugs able to selectively interact with P1 receptor co-localized with CD73 [19]. This is still an unexplored area, which may have beneficial effects during enteric pathogen infection, dysbiosis, motility disturbances, and immune reactivity inherent to inflammatory enteric disorders, or chronic inflammation-induced fibrosis [5, 28–30, 52, 53].
Acknowledgments
We thank Dr. Francesca Patrese and Dr. Ludovico Scenna for veterinary assistance; Mauro Berto, Massimo Rizza, and Andrea Pagetta for technical assistance in animal handling and experimental procedures.
Abbreviations
- ADA
Adenosine deaminase
- AOPCP
α,β-methyleneadenosine 5′-diphosphate sodium salt
- CD73
ecto-5′-nucleotidase
- EHNA
Erythro-9-(2-hydroxy-3-nonyl) adenine
- ENTs
Equilibrative transporters
- HPLC
High-pressure liquid chromatography
- IMFs
Ileal myofibroblasts
- AP
Alkaline phosphatase
- PAP
Prostatic acid phosphatase
Author contributions
Conceived and designed the experiments: MCG, AB, VC. Performed the experiments: AB, VC, IC, GO, MB. Analyzed the data: MCG, AB, VC, IC, GO, SDM, MM, LA, CG, RC, PD. Contributed reagents/materials/analysis tools: MCG, GO, IC, PD, CG. Wrote the manuscript: MCG, AB, VC. All the authors reviewed the manuscript.
Funding information
This work was supported by grants from University of Padova (UNIPD-CPDR155591/15 Assegno di Ricerca 2016, UNIPD-DSF-DOR-2016 and 2017 funds, and UNIPD-DSF-PRID-2017) and from San Camillo Hospital, Treviso (Italy) to MCG. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
All experimental protocols were approved by the Animal Care and Use Committee of the University of Padova and by the Italian Ministry of Health and were in compliance with the national and EU guidelines for handling and use of experimental animals.
References
- 1.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 2.Christofi FL, Zhang H, Yu JG, Guzman J, Xue J, Kim M, Wang YZ, Cooke HJ. Differential gene expression of adenosine A1, A2a, A2b, and A3 receptors in the human enteric nervous system. J Comp Neurol. 2001;439:46–64. doi: 10.1002/cne.1334. [DOI] [PubMed] [Google Scholar]
- 3.Kolachala VL, Bajaj R, Chalasani M, Sitaraman SV. Purinergic receptors in gastrointestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2008;294(2):G401–G410. doi: 10.1152/ajpgi.00454.2007. [DOI] [PubMed] [Google Scholar]
- 4.Antonioli L, Colucci R, Pellegrini C, Giustarini G, Tuccori M, Blandizzi C, Fornai M. The role of purinergic pathways in the pathophysiology of gut diseases: pharmacological modulation and potential therapeutic applications. Pharmacol Ther. 2013;139(2):157–188. doi: 10.1016/j.pharmthera.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Zoppellaro C, Bin A, Brun P, Banzato S, Macchi V, Castagliuolo I, Giron MC. Adenosine-mediated enteric neuromuscular function is affected during herpes simplex virus type 1 infection of rat enteric nervous system. PLoS One. 2013;8(8):e72648. doi: 10.1371/journal.pone.0072648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature. 2014;509(7500):310–317. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitahara K, Kittel A, Liang SD, Vizi ES. A1-receptor-mediated effect of adenosine on the release of acetylcholine from the myenteric plexus: role and localization of ecto-ATPase and 5′-nucleotidase. Neuroscience. 1995;67(1):159–168. doi: 10.1016/0306-4522(94)00585-S. [DOI] [PubMed] [Google Scholar]
- 8.Duarte-Araujo M, Nascimento C, Alexandrina Timoteo M, Magalhaes-Cardoso T, Correia-de-Sa P. Dual effects of adenosine on acetylcholine release from myenteric motoneurons are mediated by junctional facilitatory A(2A) and extrajunctional inhibitory A(1) receptors. Br J Pharmacol. 2004;141(6):925–934. doi: 10.1038/sj.bjp.0705697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Correia-de-Sa P, Adaes S, Timoteo MA, Vieira C, Magalhaes-Cardoso T, Nascimento C, Duarte-Araujo M. Fine-tuning modulation of myenteric motoneurons by endogenous adenosine: on the role of secreted adenosine deaminase. Autonomic neuroscience: basic & clinical. 2006;126–127:211–224. doi: 10.1016/j.autneu.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Giron MC, Bin A, Brun P, Etteri S, Bolego C, Florio C, Gaion RM. Cyclic AMP in rat ileum: evidence for the presence of an extracellular cyclic AMP-adenosine pathway. Gastroenterology. 2008;134(4):1116–1126. doi: 10.1053/j.gastro.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 11.Fukuuchi T, Kobayashi M, Yamaoka N, Kaneko K. Evaluation of cellular purine transport and metabolism in the Caco-2 cell using comprehensive high-performance liquid chromatography method for analysis of purines. Nucleosides Nucleotides Nucleic Acids. 2016;35(10–12):663–669. doi: 10.1080/15257770.2016.1205195. [DOI] [PubMed] [Google Scholar]
- 12.Antonioli L, Pellegrini C, Fornai M, Tirotta E, Gentile D, Benvenuti L, Giron MC, Caputi V, Marsilio I, Orso G, Bernardini N, Segnani C, Ippolito C, Csóka B, Németh ZH, Haskó G, Scarpignato C, Blandizzi C, Colucci R. Colonic motor dysfunctions in a mouse model of high-fat diet-induced obesity: an involvement of A(2B) adenosine receptors. Purinergic Signal. 2017;13(4):497–510. doi: 10.1007/s11302-017-9577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duarte-Araujo M, Nascimento C, Timoteo MA, Magalhaes-Cardoso MT, Correia-de-Sa P. Relative contribution of ecto-ATPase and ecto-ATPDase pathways to the biphasic effect of ATP on acetylcholine release from myenteric motoneurons. Br J Pharmacol. 2009;156(3):519–533. doi: 10.1111/j.1476-5381.2008.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19(6):355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardoso AM, Schetinger MR, Correia-de-Sa P, Sevigny J. Impact of ectonucleotidases in autonomic nervous functions. Auton Neurosci. 2015;191:25–38. doi: 10.1016/j.autneu.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Jackson EK, Mi Z, Dubey RK. The extracellular cAMP-adenosine pathway significantly contributes to the in vivo production of adenosine. J Pharmacol Exp Ther. 2007;320(1):117–123. doi: 10.1124/jpet.106.112748. [DOI] [PubMed] [Google Scholar]
- 17.Pacheco R, Martinez-Navio JM, Lejeune M, Climent N, Oliva H, Gatell JM, Gallart T, Mallol J, Lluis C, Franco R. CD26, adenosine deaminase, and adenosine receptors mediate costimulatory signals in the immunological synapse. Proc Natl Acad Sci U S A. 2005;102(27):9583–9588. doi: 10.1073/pnas.0501050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antonioli L, Colucci R, La Motta C, Tuccori M, Awwad O, Da Settimo F, Blandizzi C, Fornai M. Adenosine deaminase in the modulation of immune system and its potential as a novel target for treatment of inflammatory disorders. Curr Drug Targets. 2012;13(6):842–862. doi: 10.2174/138945012800564095. [DOI] [PubMed] [Google Scholar]
- 19.Vieira C, Magalhaes-Cardoso MT, Ferreirinha F, Silva I, Dias AS, Pelletier J, Sevigny J, Correia-de-Sa P. Feed-forward inhibition of CD73 and upregulation of adenosine deaminase contribute to the loss of adenosine neuromodulation in postinflammatory ileitis. Mediat Inflamm. 2014;2014:254640. doi: 10.1155/2014/254640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Investig. 2002;110(7):993–1002. doi: 10.1172/jci15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stefanovic V, Mandel P, Rosenberg A. Concanavalin A inhibition of ecto-5′-nucleotidase of intact cultured C6 glioma cells. J Biol Chem. 1975;250(17):7081–7083. [PubMed] [Google Scholar]
- 22.Picher M, Burch LH, Hirsh AJ, Spychala J, Boucher RC. Ecto 5′-nucleotidase and nonspecific alkaline phosphatase. Two AMP-hydrolyzing ectoenzymes with distinct roles in human airways. J Biol Chem. 2003;278(15):13468–13479. doi: 10.1074/jbc.M300569200. [DOI] [PubMed] [Google Scholar]
- 23.Caputi V, Marsilio I, Filpa V, Cerantola S, Orso G, Bistoletti M, Paccagnella N, De Martin S, Montopoli M, Dall'Acqua S, Crema F, Di Gangi IM, Galuppini F, Lante I, Bogialli S, Rugge M, Debetto P, Giaroni C, Giron MC. Antibiotic-induced dysbiosis of the microbiota impairs gut neuromuscular function in juvenile mice. Br J Pharmacol. 2017;174(20):3623–3639. doi: 10.1111/bph.13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mifflin RC, Pinchuk IV, Saada JI, Powell DW. Intestinal myofibroblasts: targets for stem cell therapy. Am J Physiol Gastrointest Liver Physiol. 2011;300(5):G684–G696. doi: 10.1152/ajpgi.00474.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Achenbach JE, Topliff CL, Vassilev VB, Donis RO, Eskridge KM, Kelling CL. Detection and quantitation of bovine respiratory syncytial virus using real-time quantitative RT-PCR and quantitative competitive RT-PCR assays. J Virol Methods. 2004;121(1):1–6. doi: 10.1016/j.jviromet.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Qin X, Liu S, Lu Q, Zhang M, Jiang X, Hu S, Li J, Zhang C, Gao J, Zhu MS, Feil R, Li H, Chen M, Weinstein LS, Zhang Y, Zhang W. Heterotrimeric G stimulatory protein alpha subunit is required for intestinal smooth muscle contraction in mice. Gastroenterology. 2017;152(5):1114–1125.e1115. doi: 10.1053/j.gastro.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brun P, Giron MC, Zoppellaro C, Bin A, Porzionato A, De Caro R, Barbara G, Stanghellini V, Corinaldesi R, Zaninotto G, Palù G, Gaion RM, Tonini M, De Giorgio R, Castagliuolo I. Herpes simplex virus type 1 infection of the rat enteric nervous system evokes small-bowel neuromuscular abnormalities. Gastroenterology. 2010;138(5):1790–1801. doi: 10.1053/j.gastro.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 28.Chen W, Lu C, Hirota C, Iacucci M, Ghosh S, Gui X. Smooth muscle hyperplasia/hypertrophy is the most prominent histological change in Crohn’s fibrostenosing bowel strictures: a semiquantitative analysis by using a novel histological grading scheme. J Crohn’s Colitis. 2017;11(1):92–104. doi: 10.1093/ecco-jcc/jjw126. [DOI] [PubMed] [Google Scholar]
- 29.Longhi MS, Moss A, Jiang ZG, Robson SC. Purinergic signaling during intestinal inflammation. J Mol Med (Berlin, Germany) 2017;95(9):915–925. doi: 10.1007/s00109-017-1545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Severi C, Sferra R, Scirocco A, Vetuschi A, Pallotta N, Pronio A, Caronna R, Di Rocco G, Gaudio E, Corazziari E, Onori P. Contribution of intestinal smooth muscle to Crohn’s disease fibrogenesis. Eur J Histochem. 2014;58(4):2457. doi: 10.4081/ejh.2014.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porcher C, Baldo M, Henry M, Orsoni P, Jule Y, Ward SM. Deficiency of interstitial cells of Cajal in the small intestine of patients with Crohn’s disease. Am J Gastroenterol. 2002;97(1):118–125. doi: 10.1111/j.1572-0241.2002.05430.x. [DOI] [PubMed] [Google Scholar]
- 32.Yegutkin GG. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: functional implications and measurement of activities. Crit Rev Biochem Mol Biol. 2014;49(6):473–497. doi: 10.3109/10409238.2014.953627. [DOI] [PubMed] [Google Scholar]
- 33.Antonioli L, Colucci R, Pellegrini C, Giustarini G, Sacco D, Tirotta E, Caputi V, Marsilio I, Giron MC, Németh ZH, Blandizzi C, Fornai M. The AMPK enzyme-complex: from the regulation of cellular energy homeostasis to a possible new molecular target in the management of chronic inflammatory disorders. Expert Opin Ther Targets. 2016;20(2):179–191. doi: 10.1517/14728222.2016.1086752. [DOI] [PubMed] [Google Scholar]
- 34.Pearson JD, Carleton JS, Gordon JL. Metabolism of adenine nucleotides by ectoenzymes of vascular endothelial and smooth-muscle cells in culture. The Biochemical journal. 1980;190(2):421–429. doi: 10.1042/bj1900421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon EL, Pearson JD, Dickinson ES, Moreau D, Slakey LL. The hydrolysis of extracellular adenine nucleotides by arterial smooth muscle cells. Regulation of adenosine production at the cell surface. J Biol Chem. 1989;264(32):18986–18995. [PubMed] [Google Scholar]
- 36.Casali EA, da Silva TR, Gelain DP, Kaiser GR, Battastini AM, Sarkis JJ, Bernard EA. Ectonucleotidase activities in Sertoli cells from immature rats. Braz J Med Biol Res. 2001;34(10):1247–1256. doi: 10.1590/S0100-879X2001001000003. [DOI] [PubMed] [Google Scholar]
- 37.Strohmeier GR, Lencer WI, Patapoff TW, Thompson LF, Carlson SL, Moe SJ, Carnes DK, Mrsny RJ, Madara JL. Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. J Clin Invest. 1997;99(11):2588–2601. doi: 10.1172/jci119447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narravula S, Lennon PF, Mueller BU, Colgan SP. Regulation of endothelial CD73 by adenosine: paracrine pathway for enhanced endothelial barrier function. J Immunol (Baltimore, Md: 1950) 2000;165(9):5262–5268. doi: 10.4049/jimmunol.165.9.5262. [DOI] [PubMed] [Google Scholar]
- 39.Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev. 2017;276(1):121–144. doi: 10.1111/imr.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrari D, Gambari R, Idzko M, Muller T, Albanesi C, Pastore S, La Manna G, Robson SC, Cronstein B. Purinergic signaling in scarring. FASEB J. 2016;30(1):3–12. doi: 10.1096/fj.15-274563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson EK, Cheng D, Verrier JD, Janesko-Feldman K, Kochanek PM. Interactive roles of CD73 and tissue nonspecific alkaline phosphatase in the renal vascular metabolism of 5′-AMP. Am J Physiol Ren Physiol. 2014;307(6):F680–F685. doi: 10.1152/ajprenal.00312.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmermann H, Zebisch M, Strater N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8(3):437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bilski J, Mazur-Bialy A, Wojcik D, Zahradnik-Bilska J, Brzozowski B, Magierowski M, Mach T, Magierowska K, Brzozowski T. The role of intestinal alkaline phosphatase in inflammatory disorders of gastrointestinal tract. Mediat Inflamm. 2017;2017:9074601. doi: 10.1155/2017/9074601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhattarai S, Freundlieb M, Pippel J, Meyer A, Abdelrahman A, Fiene A, Lee SY, Zimmermann H, Yegutkin GG, Strater N, El-Tayeb A, Muller CE. alpha, beta-Methylene-ADP (AOPCP) derivatives and analogues: development of potent and selective ecto-5′-nucleotidase (CD73) inhibitors. J Med Chem. 2015;58(15):6248–6263. doi: 10.1021/acs.jmedchem.5b00802. [DOI] [PubMed] [Google Scholar]
- 45.Xu PA, Kellems RE. Function of murine adenosine deaminase in the gastrointestinal tract. Biochem Biophys Res Commun. 2000;269(3):749–757. doi: 10.1006/bbrc.2000.2357. [DOI] [PubMed] [Google Scholar]
- 46.Antonioli L, Fornai M, Awwad O, Giustarini G, Pellegrini C, Tuccori M, Caputi V, Qesari M, Castagliuolo I, Brun P, Giron MC, Scarpignato C, Blandizzi C, Colucci R. Role of the A(2B) receptor-adenosine deaminase complex in colonic dysmotility associated with bowel inflammation in rats. Br J Pharmacol. 2014;171(5):1314–1329. doi: 10.1111/bph.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zamzow CR, Xiong W, Parkinson FE. Adenosine produced by neurons is metabolized to hypoxanthine by astrocytes. J Neurosci Res. 2008;86(15):3447–3455. doi: 10.1002/jnr.21789. [DOI] [PubMed] [Google Scholar]
- 48.Street SE, Kramer NJ, Walsh PL, Taylor-Blake B, Yadav MC, King IF, Vihko P, Wightman RM, Millan JL, Zylka MJ. Tissue-nonspecific alkaline phosphatase acts redundantly with PAP and NT5E to generate adenosine in the dorsal spinal cord. J Neurosci. 2013;33(27):11314–11322. doi: 10.1523/jneurosci.0133-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Araujo CL, Quintero IB, Kipar A, Herrala AM, Pulkka AE, Saarinen L, Hautaniemi S, Vihko P. Prostatic acid phosphatase is the main acid phosphatase with 5′-ectonucleotidase activity in the male mouse saliva and regulates salivation. Am J Physiol Cell Physiol. 2014;306(11):C1017–C1027. doi: 10.1152/ajpcell.00062.2014. [DOI] [PubMed] [Google Scholar]
- 50.Vieira C, Ferreirinha F, Silva I, Duarte-Araujo M, Correia-de-Sa P. Localization and function of adenosine receptor subtypes at the longitudinal muscle--myenteric plexus of the rat ileum. Neurochem Int. 2011;59(7):1043–1055. doi: 10.1016/j.neuint.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 51.Caputi V, Marsilio I, Cerantola S, Roozfarakh M, Lante I, Galuppini F, Rugge M, Napoli E, Giulivi C, Orso G, Giron MC. Toll-like receptor 4 modulates small intestine neuromuscular function through nitrergic and purinergic pathways. Front Pharmacol. 2017;8:350. doi: 10.3389/fphar.2017.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brun P, Gobbo S, Caputi V, Spagnol L, Schirato G, Pasqualin M, Levorato E, Palu G, Giron MC, Castagliuolo I. Toll like receptor-2 regulates production of glial-derived neurotrophic factors in murine intestinal smooth muscle cells. Mol Cell Neurosci. 2015;68:24–35. doi: 10.1016/j.mcn.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 53.Vieira C, Ferreirinha F, Magalhaes-Cardoso MT, Silva I, Marques P, Correia-de-Sa P. Post-inflammatory ileitis induces non-neuronal purinergic signaling adjustments of cholinergic neurotransmission in the myenteric plexus. Front Pharmacol. 2017;8:811. doi: 10.3389/fphar.2017.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]