Abstract
Ovarian cancer is the deadliest gynecologic cancer due to lack of early effective diagnosis and development of resistance to platinum-based chemotherapy. Several studies reported that adenosine concentrations are higher in tumor microenvironment than in non-tumor tissue. This finding inspired us to study the role of adenosine in ovarian cancer cells and to investigate if adenosine pathways offer new treatment options urgently needed to prevent or overcome chemoresistance. The ovarian cancer cell lines HEY, A2780, and its cisplatin-resistant subline A2780CisR were used in this study. Expression and functional activity of adenosine receptors were investigated by RT-PCR, Western blotting, and cAMP assay. A1 and A2B adenosine receptors were expressed and functionally active in all three cell lines. Adenosine showed moderate cytotoxicity (MTT-IC50 values were between 700 and 900 μM) and induced apoptosis in a concentration-dependent manner by increasing levels of sub-G1 and cleaved PARP. Apoptosis was diminished by QVD-OPh, confirming caspase-dependent induction of apoptosis. Forty-eight hours pre-incubation of adenosine prior to cisplatin significantly enhanced cisplatin-induced cytotoxicity in a synergistic manner and increased apoptosis. SLV320 or PSB603, selective A1 and A2B antagonists, was not able to inhibit adenosine-induced increase in cisplatin cytotoxicity or apoptosis whereas dipyridamole, a nucleoside transporter inhibitor, completely abrogated both effects. Mechanistically, adenosine increased pAMPK and reduced pS6K which was prevented by dipyridamole. In conclusion, application of adenosine prior to cisplatin could be a new therapeutic option to increase the potency of cisplatin in a synergistic manner and thus overcome platinum resistance in ovarian cancer.
Electronic supplementary material
The online version of this article (10.1007/s11302-018-9622-7) contains supplementary material, which is available to authorized users.
Keywords: Adenosine, Ovarian cancer, Cisplatin, Adenosine receptors, Nucleoside transporter, Dipyridamole
Introduction
Ovarian cancer is the most lethal gynecologic cancer and the fifth common cause of cancer-related death in women [1]. Worldwide, 225,000 new cases were detected each year, and 140,000 people annually die from the disease [2]. Platinum-based chemotherapy is the recommended first-line chemotherapy for ovarian cancer treatment [3]. Resistance development to platinum-based chemotherapy is one of the crucial problems in ovarian cancer treatment. An estimated 85% of patients with epithelial ovarian cancer achieving a full remission following first-line therapy will develop a recurrence of cancer [4]. Patients experiencing relapses later than 6 months following a response to platinum-based therapy are characterized as having platinum-sensitive disease whereas patients who experience recurrences within 6 months following an initial response to platinum-based therapy are defined as having platinum-resistant ovarian cancers [5]. Many mechanisms are responsible for the development of resistance to platinum-based chemotherapy including reduction of drug uptake, increasing drug inactivation, and increase in DNA repair. These mechanisms decrease signal transduction pathways that activate apoptosis [6, 7]. There are second-line therapeutic options including PARP inhibition, recently approved by the US FDA for patients with advanced ovarian cancer carrying hereditary BRCA mutations and previously receiving three or more chemotherapy regimens [8, 9]. However, PARP inhibitors are limited to BRCA-mutated ovarian cancers only [8]. Further, there are several targeted therapies in progression of extensive studies, including agents targeting folate metabolism, cell adhesion molecules, hormonal therapy, agents associated with DNA repair pathways, and immunotherapy. However, encouraging results for these targeted therapies are limited [5]. Further investigations to offer new strategies for treatment of ovarian cancers remain an unmet medical need.
It is well-known that adenosine concentrations are higher in the tumor microenvironment than in the normal tissue [10, 11]. An earlier study reported that concentrations of extracellular adenosine in solid carcinoma may rise to 100 μM or higher which is 10- to 20-fold above the concentration in normal tissue [12]. Adenosine is a purine nucleoside originating from the metabolism of ATP, ADP, and AMP by the ecto-nucleotidase CD39 and the 5′-ectonucleotidase CD73 [11]. Extracellular adenosine transduces signals via four different G protein-coupled receptors. Adenosine relaxes vascular smooth muscles by activation of Gs-coupled A2A receptors leading to adenylyl cyclase activation and reduction of intracellular calcium concentrations in smooth muscle cells [13]. Clinically, adenosine has negative chronotropic and inotropic effects on the heart by slowing the conduction time through the AV node and interrupting AV nodal reentry pathways. The US FDA approved adenosine as intravenous injection solution (3 mg/ml) (Adenocard®) for the conversion to sinus rhythm of paroxysmal supraventricular tachycardia. Methylxanthines such as caffeine and theophylline are competitive antagonists of adenosine while dipyridamole inhibits adenosine uptake by inhibition of nucleoside transporters [13].
Extracellular adenosine mediates effects via G protein-coupled P1 (adenosine) receptors divided into four subtypes A1, A2A, A2B, and A3. They are distributed throughout the body and involved in many cell signaling pathways [14]. A1 receptors interact with pertussis toxin-sensitive G proteins (Gi and Go), inhibiting adenylyl cyclase (AC) and modulating calcium and potassium channels and phospholipase C (PLC). A2A receptors are coupled to Gs/Golf proteins, stimulating AC and thus increasing intracellular cyclic adenosine monophosphate (cAMP) concentration. A2B receptors are coupled to Gs/Gq proteins, increasing AC activity and stimulating PLC. Finally, A3 receptors couple to Gi and Gq proteins leading to inhibition of AC and stimulating PLC [14]. However, adenosine also has receptor-independent intracellular effects after nucleoside transporter-mediated intracellular uptake [11, 15]. One of the major intracellular effects of adenosine is (after conversion of adenosine to AMP) the activation of AMP-activated protein kinase (AMPK) and downstream pathways [15].
AMPK is an evolutionary conserved cellular energy sensor and plays a major role in the regulation of cellular metabolism. AMPK is essential for embryonic growth and development. It also has impact in adult tissues under stress conditions [16]. AMPK is now further recognized to own antineoplastic efficacy and as a target of chemotherapy. Under low energy (high AMP/ATP ratio), AMPK is activated in an LKB1-dependent manner. AMPK directly phosphorylates Raptor at S792 which is required for inhibition of mTOR1 and growth arrest under energy stress [17, 18]. Metformin-induced activation of AMPK was shown to inhibit cell proliferation and induce apoptosis in triple-negative breast cancer cell lines [19]. Moreover, the combination treatment of metformin and chemotherapeutic agents carboplatin, paclitaxel, or doxorubicin showed a synergistic inhibition of G1 phase of cell cycle [19]. A study in non-small cell lung cancer (NSCLC) patients showed that high levels of phosphorylated AMPK were associated with increased overall survival and recurrence-free survival [20]. These findings indicate a beneficial role of AMPK activation in cancer treatment.
While the LKB1/AMPK pathway can act as tumor suppressor through its ability to inhibit tumor cell growth, it can also behave as tumor promoter allowing tumor cells to acquire resistance to metabolic stress, so-called “metabolic adaptation” [21, 22]. Noteworthy, it was reported that active AMPK can provide stress resistance in human cancers [23] eventually leading to chemoresistance. These oncogenic functions of AMPK are in agreement with a study reporting that AMPK is rapidly activated upon cisplatin treatment in colon cancer cells and an inhibition of AMPK resulted in a remarkable increase in cisplatin-induced apoptosis [24]. The controversial role of AMPK in cancer treatment needs to be clarified before AMPK activators or inhibitors can be added to particular cancer treatments.
Even though effects of adenosine and its molecular mechanisms have been studied in many kinds of cancer, knowledge about adenosine effects in ovarian cancer is limited and remains unclear. Therefore, the purpose of this study was to examine effects and underlying mechanisms of adenosine on ovarian cancer cells as well as its effects on sensitivity of ovarian cancer cells towards cisplatin. These finding may open new treatment options to prevent or overcome chemoresistance against platinum-based chemotherapy in ovarian cancer.
Materials and methods
Materials
Adenosine, dipyridamole, and cisplatin were purchased from Sigma-Aldrich (Germany), and SCH442416, SLV320, and PSB603 were purchased from Tocris Bioscience (Germany). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) was purchased from Serva (Germany) and was dissolved in PBS 1× at a concentration of 5 mg/ml. Roswell Park Memorial Institute (RPMI) 1640, Dulbecco’s modified eagle medium (DMEM), 0.05% trypsin/0.02% EDTA in PBS, penicillin/streptomycin (10,000 IU/ml, 10 mg/ml), and fetal bovine serum (FBS) were supplied by PAN Biotech (Germany). my-Budget RNA Mini Kit and PolyFect transfection reagent were obtained from Qiagen (Germany). High-Capacity cDNA Reverse Transcription Kit was purchased from Applied Biosystems (USA). Oligo(dT)23 anchored primer was supplied by Sigma-Aldrich (Germany). 10,000× GelRed™ in water was purchased from Biotium (USA). GeneRuler 50 bp was obtained from Thermo Fisher Scientific (USA). Adenosine primer sequences were produced by Eurofins Genomics (Germany). Agarose gel was obtained from Carl Roth GmbH & Co. KG (Germany). PathDetect cis pCRE-luc reporter plasmid was purchased from Stratagene (Germany). ATP was obtained from Sigma-Aldrich (Germany). Co-enzyme A and D-luciferin sodium salt were purchased from AppliChem (Germany). Oregon Green™ 488 BAPTA-1 AM was obtained from Thermo Fisher Scientific (USA). Propidium iodide (PI) was purchased from Santa Cruz Biotechnology (Germany). Rabbit anti-A1, A2A, A2B, and A3 adenosine receptor antibodies were obtained from Alomone labs (Israel). Mouse anti-β-actin, rabbit anti-pAMPK (Thr172), and Western blotting luminol reagent were purchased from Santa Cruz Biotechnology (Germany). Mouse anti-pS6K (Thr389) was obtained from Cell signaling (USA). Goat anti-PARP antibody, anti-rabbit, mouse, and goat horseradish peroxidase (HRP)-coupled secondary antibodies were purchased from R&D Systems (Germany). Protease/phosphatase inhibitor mini tablets were purchased from Thermo Fisher Scientific (USA).
Cell lines and cell culture
Human ovarian cancer cell lines A2780 and HEY cells were obtained from the European Collection of Cell Cultures (ECACC, UK). The cisplatin-resistant subline A2780CisR was generated by intermittent treatment of A2780 cells with cisplatin for 24 weekly cycles according to methods previously published [25]. Human embryonic kidney cell line (HEK293) was obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Germany). The A2780, A2780CisR, and HEY cell lines were grown in Roswell Park Memorial Institute 1640 (RPMI1640), while HEK293 cell line was cultivated in Dulbecco’s modified eagle medium (DMEM). The RPMI1640 and DMEM were supplemented with 10% fetal bovine serum (FBS), 120 IU/ml penicillin, and 120 μg/ml of streptomycin. Cell lines were grown at 37 °C under humidified atmosphere containing 5% CO2 until they reached 80 to 90% confluence before used for assays.
MTT cell viability assay
The rate of cell survival under the action of substances was evaluated by an improved MTT assay as previously described [25, 26]. Briefly, ovarian cancer cell lines were seeded at a density of 1 × 104 cells per well while HEK293 cells were seeded at a density of 8 × 103 cells per well in 96-well plates (Corning, Germany). After 24 h, cells were exposed to increasing concentrations of test compounds. Incubation was ended after 48 h, and cell survival was determined by addition of MTT solution (5 mg/ml in phosphate-buffered saline). The formazan precipitate was dissolved in DMSO (VWR, Germany). Absorbance was measured at 544 and 690 nm at a FLUOstar microplate reader (BMG LabTech, Offenburg, Germany).
Combination treatment
For the investigation of the adenosine effect on cisplatin-induced cytotoxicity, adenosine was pre-incubated 48 h prior to cisplatin administration. After 72 h, the cytotoxic effect was determined with MTT cell viability assay. CompuSyn software version 1.0 (ComboSyn, USA) was used to calculate the combination index (CI) as a quantitative measurement of the degree of drug interactions.
RT-PCR
Cell lines were grown in T25 flasks until they reached 80 to 90% confluence. RNA was extracted using my-Budget RNA Mini Kit (Qiagen, Germany) following the protocol for eukaryotic cells. A total of 1 μg of purified RNA was taken to prepare cDNA using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, California, USA) and oligo(dT)23 anchored primers (Sigma-Aldrich, USA). Thermocycler program setting was initiated for 10 min at 25 °C then followed by 120 min at 37 °C. A total of 20 μl of cDNA were diluted in 100 μl of TE buffer 1× (1 mM of Tris-Cl and 0.1 mM EDTA in distilled water) pH 7.5 and stored at − 20 °C for further PCR. Specific primers for adenosine receptors were designed using primer design tool from the website www.ncbi.nlm.nih.gov/tools/primer-blast/ [27]. The primer sequences of all adenosine receptors are shown in Table S1 (Electronic Supplementary Material). The PCR program consisted of an initial denaturation step at 95 °C for 120 s for 1 cycle then continued with 94 °C for 20 s followed by 57 °C for 30 s and 72 °C for 60 s for a total of 40 cycles. The PCR products were separated using 2% agarose gel in TAE buffer 1× (TAE 10× consisted of 24.2 g Tris base, 5.7 ml acetic acid, and 1.85 g EDTA disodium salt in 500 ml distilled water). DNA bands were stained using GelRed™ and detected under Intas Gel iX Imager UV system. GeneRuler 50 bp was used as DNA ladder, and β-actin served as constitutive gene.
cAMP reporter gene assay
Cell lines were transiently transfected with CRE-luc reporter plasmid (Stratagene, Germany) using PolyFect transfection reagent (Qiagen, Germany), following the transient transfection protocol. Briefly, cells were seeded at a density of 4 × 105 cells per well in 6-well plates (Sarstedt, Germany). Cell lines were incubated for 24 h in an incubator. The formation of transfection complex containing CRE-luc reporter plasmid and PolyFect transfection reagent in RPMI1640 without FBS-supplemented medium was performed at room temperature for 10 min. During the time of transfection complex formation, the cells were washed with PBS 1× once, and new complete RPMI1640 was added to the cells. The transfection complex was diluted in complete RPMI1640 medium and transferred to the cells in each well, and the cells were incubated for 24 h to allow reporter gene expression. Then, the cells were harvested and seeded at a density of 6 × 104 cells per well in white, clear-bottom 96-well plates (Greiner, Germany). After 48 h, the cells were exposed to various concentrations of adenosine for 3 h (agonist activity study). In case of antagonist activity study, selective antagonists were incubated 30 min prior to adenosine administration. After 3 h of adenosine incubation, cells were lysed using lysis reagent (8 mM Tricine, 1 mM DTT, 2 mM EDTA, and 5% w/v Triton X-100) for 10 to 20 min at 4 to 8 °C. Luminescence was measured after addition of luciferase assay reagent (30 mM Tricine, 10 mM MgSO4, 0.5 mM EDTA, 10 mM DTT, 0.5 mM ATP, 0.5 mM coenzyme A, and 0.05 mM D-luciferin) by a LUMIstar Galaxy microplate reader (BMG Labtech, Germany).
Measurement of apoptotic cells
The cells were seeded at a density of 5 × 104 cells per well in 24-well plates (Sarstedt, Germany). They were treated with various concentrations of adenosine alone or in combination with cisplatin for indicated time points. The supernatant was removed after centrifugation step, and the cells were lysed in 500 μl of hypotonic PI-staining buffer (0.1% sodium citrate, 0.1% Triton X-100, and 100 μg/ml propidium iodide solution in filtered distilled water) at 4 to 8 °C in the dark for at least 6 h. The percentage of apoptotic nuclei with DNA content in sub-G1 was analyzed by flow cytometry (CyFlow, Partec, Germany) or by fluorescence imaging (Thermo Fisher Array Scan XTI, Schwerte, Germany).
Immunoblotting
The cells were treated with various concentrations of adenosine alone or in combination with cisplatin for indicated time points. Protein samples were prepared from cell lysate in a reducing condition using lysis buffer 6 (Bio-Tech, Germany) or RIPA lysis buffer (50 mM Tris HCl, 2 mM EDTA, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, and 0.5% sodium deoxycholate) plus protease/phosphatase inhibitor. Equal amounts of total protein (25 to 35 μg) were resolved by SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes. Blots were incubated with primary antibodies against β-actin, PARP, pAMPK, and pS6K. After washing, the blots were incubated with HRP-coupled secondary antibodies. After additional washing, the proteins were visualized by luminol reagent under Intas imager (Intas, Germany). Densitometric analysis was performed on scanned images using ImageJ software (National Institutes of Health) [28].
Statistical analysis
EC50 and IC50 values were estimated after fitting the pooled data from at least three independent experiments to the four-parameter logistic equation using GraphPad Prism version 4.00 for Windows (GraphPad, USA). Data were presented as mean ± standard error of the mean (mean ± SEM). Statistical comparison was analyzed using Student’s t test. (*), (**), and (***) indicate P value < 0.05, < 0.01, and < 0.001, respectively.
Results
Expression and functional activity of adenosine receptors
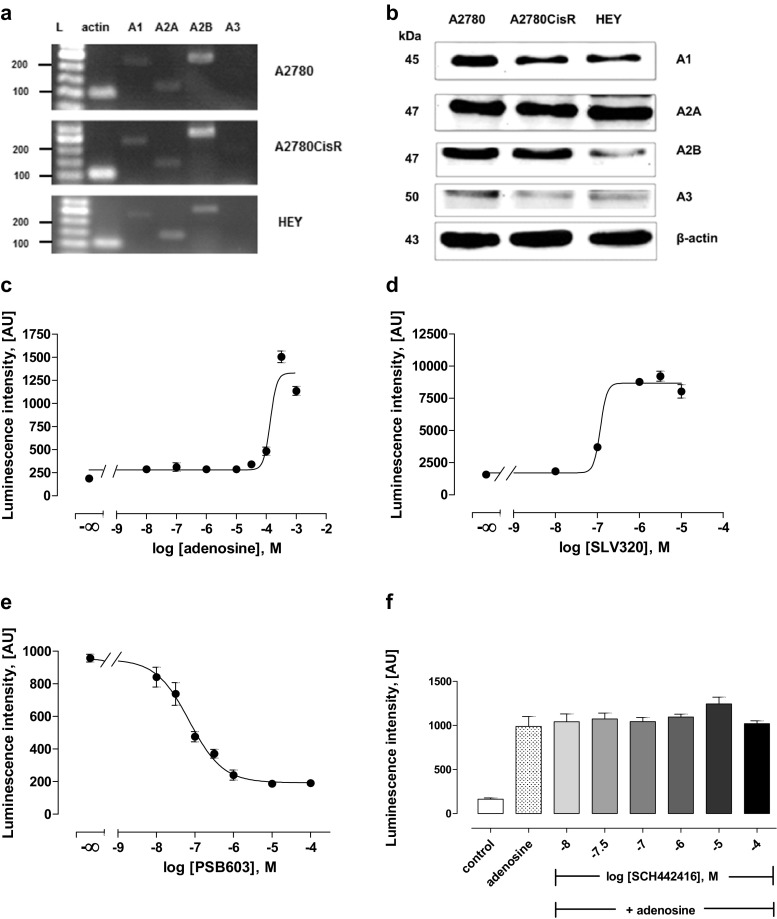
As detected by RT-PCR and Western blotting, adenosine receptors A1, A2A, and A2B were expressed in A2780, A2780CisR, and HEY cell lines, while A3 receptors were not detected by PCR and gave only slight bands in Western blotting (Fig. 1a,b). Functional activity of A1, A2A, and A2B receptors was then analyzed by cAMP reporter gene assay. Adenosine showed a concentration-dependent increase in cAMP levels starting only at 100 μM as shown for A2780 cells in Fig. 1c. This could however be due to parallel stimulation of Gs and Gi-coupled adenosine receptors. Similar results were obtained for A2780CisR and HEY cells. EC50 values and pEC50 ± SEM of adenosine in all three cell lines are displayed in Table 1. Next, selective antagonists of A1, A2A, and A2B receptors were examined. Results for A2780 cells are displayed in Fig. 1d–f. Data for A2780CisR and HEY were similar (not shown). Raising concentrations of the selective A1 receptor antagonist SLV320 led to an increase in adenosine-induced luminescence, resulting in an IC50 value of 0.16 μM (pIC50 6.81 ± 0.14) (Fig. 1d). At concentrations of SLV320 beyond 10 μM (31.6 and 100 μM), adenosine-induced luminescence decreased, likely because SLV320 lost its selectivity for A1 receptors [29]. Increasing concentrations of PSB603, a selective A2B receptor antagonist, led to a decrease in luminescence intensity, giving an IC50 value of 78.5 nM (pIC50 7.10 ± 0.12) (Fig. 1e), whereas SCH 442416, a selective A2A receptor antagonist, did not show any effects up to 100 μM on adenosine-induced luminescence intensity (Fig. 1f). These results indicated that A1 and A2B receptors were functionally active whereas A2A was not functionally active even though expressed as shown in the Western blotting (Fig. 1b).
Fig. 1.
Expression and functional activity of adenosine receptors in A2780, A2780CisR, and HEY cells. a Gene expression of adenosine receptors using RT-PCR. b Protein expression of adenosine receptors using Western blot. c Concentration-dependent effect of adenosine on cAMP-related luminescence in A2780 cells. d–f Concentration-dependent effect of SLV320, PSB603, and SCH 442416 (selective A1, A2B, and A2A antagonists, respectively) on 200 μM adenosine-induced luminescence in A2780 cells
Table 1.
Summary of EC50 and pEC50 ± SEM of adenosine obtained from cAMP reporter gene assay. Data shown are from at least three independent experiments
| Cell lines | EC50 (μM) | pEC50 ± SEM |
|---|---|---|
| A2780 | 166 | 3.78 ± 0.21 |
| A2780CisR | 324 | 3.49 ± 0.34 |
| HEY | 347 | 3.46 ± 0.33 |
Adenosine enhances cisplatin cytotoxicity
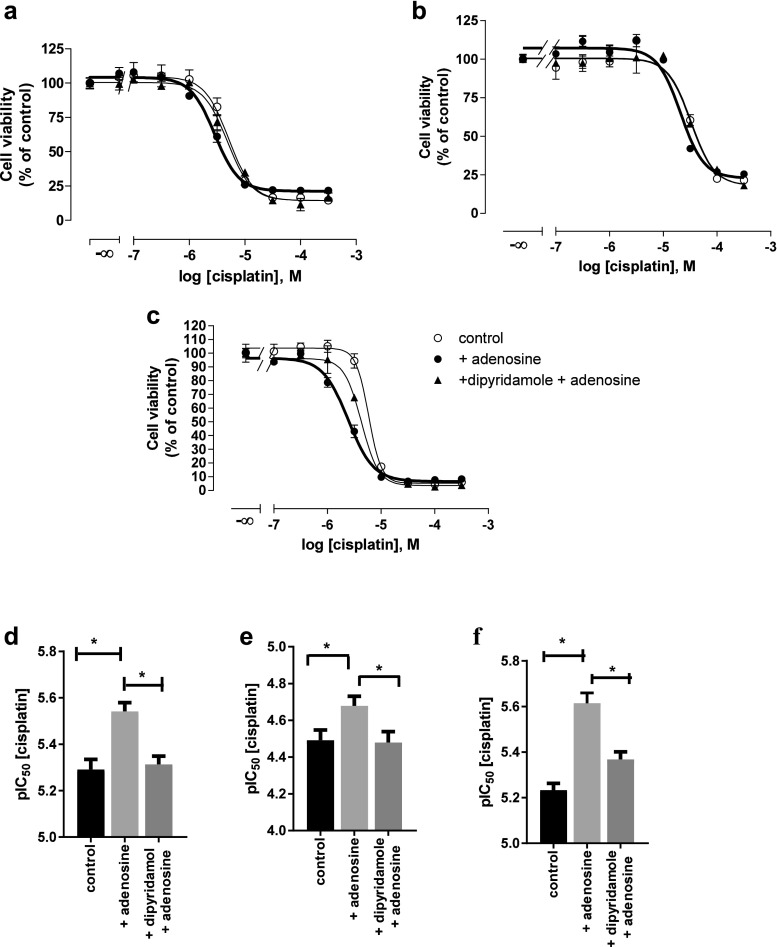
Adenosine cytotoxicity was examined by MTT cell viability assay (Fig. 2a). After 48 h of adenosine treatment, IC50 of adenosine was 700 to 900 μM in all three cell lines. Next, combinations of adenosine and cisplatin were examined by MTT assay. Adenosine in a concentration of 100, 300, and 500 μM was pre-incubated for 48 h prior to 72 h co-incubation of adenosine with cisplatin. The IC50 of cisplatin significantly decreased in the presence of adenosine compared to cisplatin alone (Fig. 2c–e and Table 2). Shift factor is the ratio of IC50 values of cisplatin alone and the combination of adenosine with cisplatin. A total of 500 μM of adenosine significantly shifted the IC50 of cisplatin by a factor of 4.1 in A2780 cells (Fig. 2b). Similarly, significant shift factors were obtained in the presence of 500 μM of adenosine in A2780CisR and HEY cells (Fig. 2b). In addition, combination indices (CI) were calculated according to the method of Chou by CompuSyn software [30] to evaluate the mode of interaction (additive effect or synergism) between adenosine and cisplatin. Table 3 shows the CI values for various concentrations in all three ovarian cancer cell lines. At higher concentrations of cisplatin and adenosine, CI values were below 0.9 indicating synergism between adenosine and cisplatin.
Fig. 2.
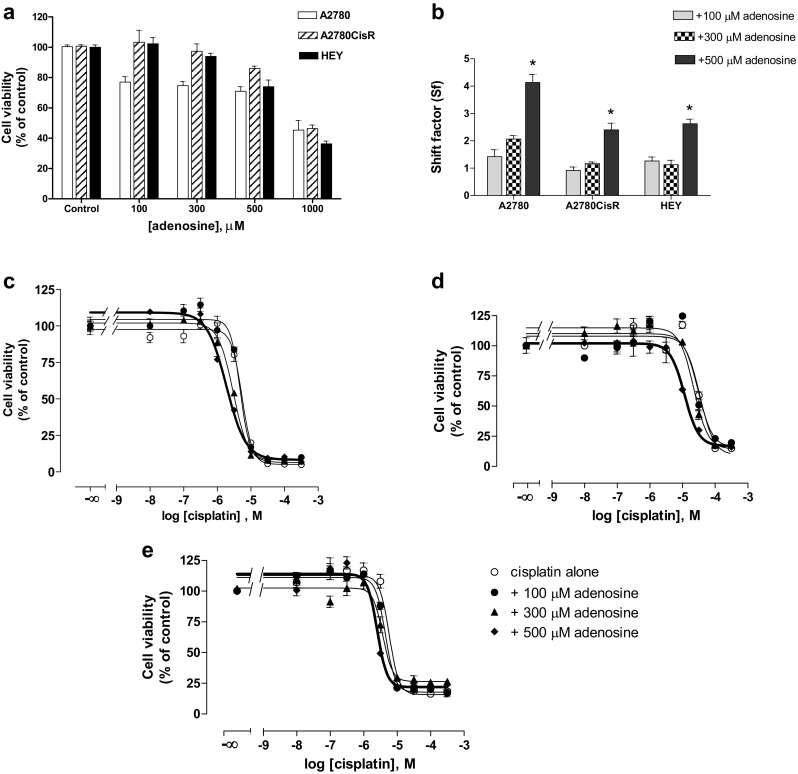
Effect of adenosine alone (a) or in combination with cisplatin (b–e) on the cell viability (MTT assay) of A2780, A2780CisR, and HEY cells. a Percentage of cell viability after treatment with 100 to 1000 μM of adenosine for 48 h compared to untreated control. b Shift factors (Sf) of adenosine effect on cisplatin potency. Data shown are mean ± SEM of at least three independent experiments. (*) indicates statistical significance (P value < 0.05). c–e Concentration–effect curves of cisplatin in the absence or presence of 100, 300, or 500 μM adenosine in A2780, A2780CisR, and HEY cells, respectively. Adenosine was incubated 48 h prior to 72 h co-incubation of increasing concentrations of cisplatin. Data shown are mean ± SEM of at least three independent experiments
Table 2.
Summary of IC50, pIC50 ± SEM values of cisplatin, and shift factors (Sf) derived from combination treatments of adenosine and cisplatin. Adenosine was incubated 48 h prior to 72 h co-incubation with cisplatin. Data shown are representative data from at least three independent experiments. (*) indicates statistical significance (P value < 0.05) in comparison to cisplatin alone (control).
| Condition | IC50 (μM) | pIC50 ± SEM | Shift factor (Sf) |
|---|---|---|---|
| A2780 | |||
| Control | 5.46 | 5.26 ± 0.03 | – |
| + 100 μM adenosine | 4.77 | 5.32 ± 0.03 | 1.14 |
| + 300 μM adenosine | 3.01* | 5.52 ± 0.02 | 1.81 |
| + 500 μM adenosine | 1.26* | 5.90 ± 0.04 | 4.32 |
| A2780CisR | |||
| Control | 34.0 | 4.47 ± 0.07 | – |
| + 100 μM adenosine | 30.1 | 4.52 ± 0.10 | 1.13 |
| + 300 μM adenosine | 22.2 | 4.65 ± 0.06 | 1.53 |
| + 500 μM adenosine | 15.4* | 4.81 ± 0.03 | 2.20 |
| HEY | |||
| Control | 5.81 | 5.24 ± 0.05 | – |
| + 100 μM adenosine | 4.46 | 5.35 ± 0.03 | 1.30 |
| + 300 μM adenosine | 3.68* | 5.43 ± 0.04 | 1.58 |
| + 500 μM adenosine | 2.54* | 5.60 ± 0.05 | 2.29 |
Table 3.
Combination indices (CI) derived from combination treatments of adenosine and cisplatin. Adenosine in a concentration of 100, 300, 500, and 700 μM was pre-incubated 48 h prior to 72 h co-incubation with various concentrations of cisplatin. Then, MTT data of a particular combination treatment were used to calculate CI values according to the method of Chou using CompuSyn. Data shown are mean CI values ± SD from at least three independent experiments
| Cell line | Combination index (CI) ± SD | ||||
|---|---|---|---|---|---|
| Cisplatin (μM) | Plus adenosine (μM) | ||||
| 100 | 300 | 500 | 700 | ||
| A2780 | 2 | 0.52 ± 0.03 | 0.79 ± 0.04 | 0.88 ± 0.02 | 0.55 ± 0.07 |
| 5 | 0.70 ± 0.05 | 0.78 ± 0.02 | 0.78 ± 0.03 | 0.41 ± 0.05 | |
| 10 | 0.49 ± 0.03 | 0.42 ± 0.05 | 0.46 ± 0.05 | 0.25 ± 0.04 | |
| A2780CisR | 25 | 0.95 ± 0.08 | 0.91 ± 0.06 | 0.95 ± 0.02 | 0.82 ± 0.08 |
| 30 | 0.90 ± 0.10 | 0.76 ± 0.11 | 0.87 ± 0.03 | 0.82 ± 0.05 | |
| HEY | 4 | 0.76 ± 0.06 | 0.93 ± 0.03 | 0.80 ± 0.07 | 0.48 ± 0.10 |
| 6 | 0.74 ± 0.07 | 0.74 ± 0.05 | 0.78 ± 0.03 | 0.64 ± 0.04 | |
| 8 | 0.83 ± 0.12 | 0.79 ± 0.04 | 0.83 ± 0.04 | 0.80 ± 0.09 | |
Adenosine uptake plays an essential role in adenosine cytotoxicity and synergistic effect with cisplatin
To examine a possible contribution of adenosine receptors for the observed synergistic cytotoxic effect of adenosine and cisplatin, selective A1 and A2B receptor antagonists SLV320 and PSB603 were incubated 1 h prior to adenosine administration. Neither SLV320 nor PSB603 was able to inhibit adenosine-induced cytotoxicity, whereas dipyridamole, a nucleoside transporter inhibitor, significantly inhibited adenosine-induced cytotoxicity in A2780, A2780CisR, and HEY cells (Fig. 3). Moreover, the presence of dipyridamole completely abrogated adenosine-induced increase in cisplatin cytotoxicity (Fig. 4 and Table 4). These results demonstrated that the uptake of adenosine through nucleoside transporters and not G protein-coupled adenosine receptors are crucial for adenosine cytotoxicity and adenosine-induced increase in cisplatin cytotoxicity.
Fig. 3.
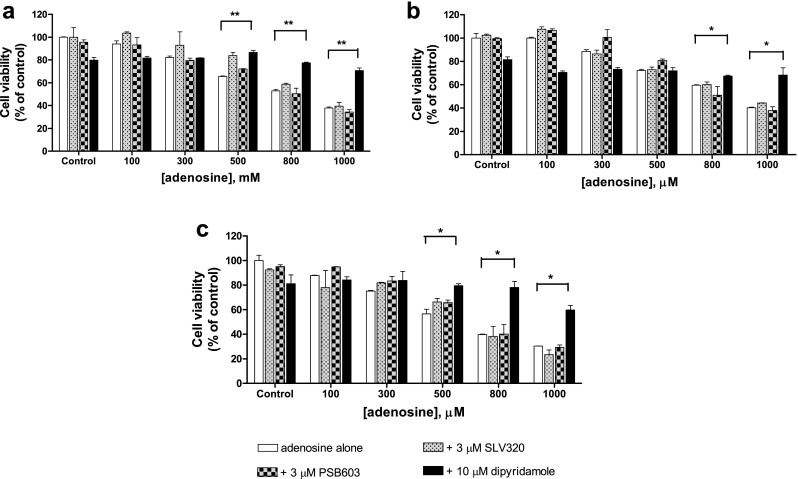
Effect of SLV320, PSB603, and dipyridamole on adenosine cytotoxicity (MTT assay). Viability of A2780 (a), A2780CisR (b), and HEY (c) cells treated with 3 μM of either SLV320 or PSB603 1 h or 10 μM of dipyridamole 30 min prior to increasing concentrations of adenosine for 48 h compared to untreated control cells. Data shown are mean ± SEM from at least three independent experiments. (*) and (**) indicate statistical significance compared to adenosine alone, P value < 0.05 and < 0.01, respectively
Fig. 4.
Effect of dipyridamole on the combination treatment of adenosine and cisplatin. Concentration–effect curves of cisplatin at A2780 (a), A2780CisR (b), and HEY (c) cells, pre-incubated with 500 μM adenosine for 48 h prior to 72 h co-incubation with cisplatin in the presence or absence of 10 μM dipyridamole. Comparison of pIC50 ± SEM of cisplatin alone or in combination with adenosine in the presence or absence of dipyridamole at A2780 (d), A2780CisR (e), and HEY (f) cells. Data shown are representative data from at least three independent experiments. (*) indicates statistical significance at P value < 0.05
Table 4.
IC50 and pIC50 ± SEM values of cisplatin achieved from combination treatment of adenosine and cisplatin in the presence or absence of 10 μM dipyridamole. Adenosine at 500 μM concentration was pre-incubated for 48 h prior to 72 h co-incubation with increasing concentrations of cisplatin in the presence or absence of 10 μM dipyridamole. Data shown are representative data from at least three independent experiments. (*) and (#) indicate statistical significance compared to cisplatin alone (control) and cisplatin plus adenosine, respectively (P value < 0.05)
| Cell lines | IC50 (μM) | ||
|---|---|---|---|
| (pIC50 ± SEM) | |||
| Control | + Adenosine | + Dipyridamole | |
| + Adenosine | |||
| A2780 | 5.11 | 2.88* | 4.86# |
| (5.29 ± 0.04) | (5.54 ± 0.04) | (5.31 ± 0.04) | |
| A2780CisR | 32.4 | 20.8* | 33.1# |
| (4.49 ± 0.06) | (4.68 ± 0.05) | (4.48 ± 0.06) | |
| HEY | 5.89 | 2.45* | 4.26# |
| (5.23 ± 0.03) | (5.61 ± 0.04) | (5.37 ± 0.03) | |
Adenosine induces apoptosis via a caspase-dependent pathway
A 48 h incubation with adenosine induced apoptosis in a concentration-dependent manner (Fig. 5a). Adenosine- or cisplatin-induced apoptosis was inhibited by 20 μM of the caspase inhibitor QVD-OPh applied 1 h prior to adenosine or cisplatin application, thus assuming that adenosine-induced apoptosis is mediated by caspase activation (Fig. 5b). Further confirmation of caspase-mediated apoptosis came from Western blots displaying a concentration-dependent increase in PARP cleavage for adenosine (Fig. 5c,d). The presence of dipyridamole significantly reduced the percentage of apoptotic nuclei (subG1 phase) of both cells treated with adenosine alone and cells treated with adenosine in combination with cisplatin, while SLV320 and PSB603 did not show any inhibition of apoptosis (Fig. 6a,b). These results indicate that adenosine uptake but not G protein-coupled adenosine receptors play an important role for adenosine-induced apoptosis. In addition, combination treatment of adenosine and cisplatin increased PARP cleavage even more than the treatment with either compound alone, again confirming a beneficial effect of the dual combination (Fig. 6c,d).
Fig. 5.
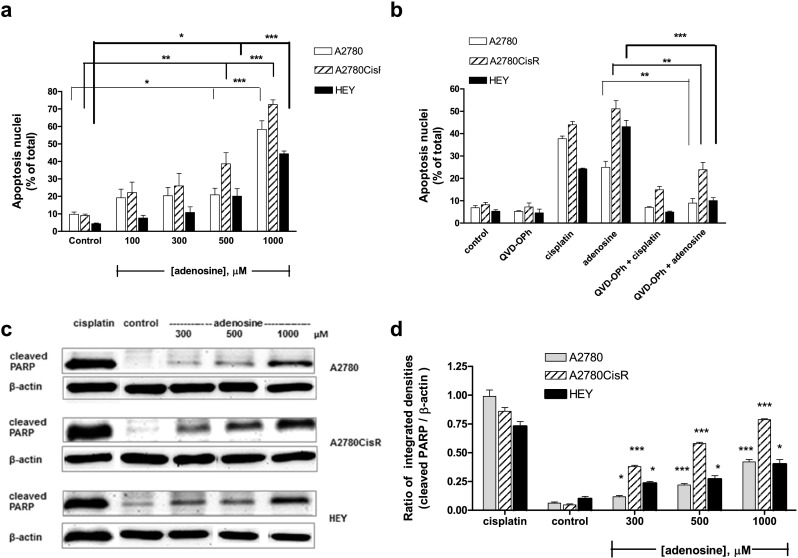
Induction of apoptosis by adenosine. a Concentration-dependent induction of apoptosis by adenosine treatment for 48 h. b 20 μM QVD-OPh inhibited apoptosis induction by 1000 μM adenosine (48 h) and 5-fold IC50 of cisplatin (24 h). QVD-OPh was incubated 1 h prior to adenosine or cisplatin administration. c 48 h Adenosine treatment increased PARP cleavage (24 kDa) concentration-dependent in A2780, A2780CisR, and HEY cells. d Quantification of immunoblots by densitometric analysis and ratio calculation of protein bands of PARP cleavage and β-actin after adenosine treatment. Data shown are mean ± SEM of the integrated density ratio of protein bands analyzed by ImageJ software from at least three independent experiments. Statistical significance (*) and (***) compared to untreated control P value < 0.05 and < 0.001, respectively
Fig. 6.
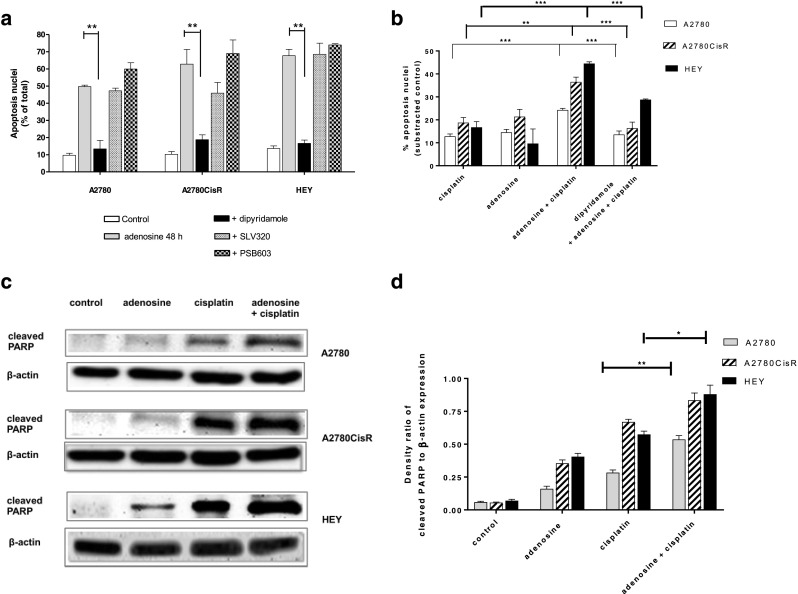
Effect of SLV320, PSB603, and dipyridamole on the induction of apoptosis by adenosine. a Effect of 10 μM dipyridamole, 3 μM PSB603, and 3 μM SLV320 on the ability of 1000 μM adenosine to induce apoptosis over 48 h. b Increased effect of apoptosis induction by combination of adenosine (500 μM) and cisplatin (3-fold IC50). Adenosine was incubated for 48 h prior to addition of cisplatin for 24 h. Dipyridamole significantly decreased the effect of adenosine. c The combination of 500 μM adenosine 48 h prior to 3-fold IC50 of cisplatin for 24 h increased cleaved PARP (24 kDa). d Quantification of immunoblots by densitometric analysis and ratio calculation of protein bands of PARP cleavage and β-actin was performed. Data shown are mean ± SEM of the integrated density ratio of protein bands analyzed by ImageJ software from at least three independent experiments. Statistical significance (*) and (**) was compared to cisplatin alone treatment P value < 0.05 and < 0.01, respectively
Adenosine activates AMPK leading to mTOR inhibition
Next, we performed studies on the mechanism of adenosine-induced increase in apoptosis in the three ovarian cancer cell lines. First, the effect of adenosine on AMP-activated kinase, AMPK, was studied. Treatment of the respective cell lines for 48 h with adenosine increased the phosphorylation of AMPK at Thr172 in all three cell lines similarly to metformin which was used as positive control (Fig. 7a,c). pAMPK (activated AMPK) is known to mediate inhibition of the mTOR1 complex by phosphorylation of Raptor [31]. We thus studied if activation of AMPK by adenosine treatment in ovarian cancer cells would lead to inhibition of mTORC1 by analyzing the phosphorylation status of downstream S6K. Indeed, pS6K was decreased under high-dose adenosine (1000 μM) treatment compared to control (Fig. 7b,d). Adenosine-induced decrease of pS6K could partially be reversed by dipyridamole. Interestingly, whereas cisplatin had no effect on pS6K (approximately same intensity as untreated control), the combination of adenosine and cisplatin decreased pS6K. Thus, adenosine treatment in ovarian cancer cells led to activation of AMPK and subsequent inhibition of mTOR as estimated by decreased pS6K (Fig. 7b,d). Noteworthy, treatment-induced changes in pS6K were strongest in A2780 whereas slight but still significant differences occurred in HEY and A2780CisR cells (Fig. 7b,d).
Fig. 7.
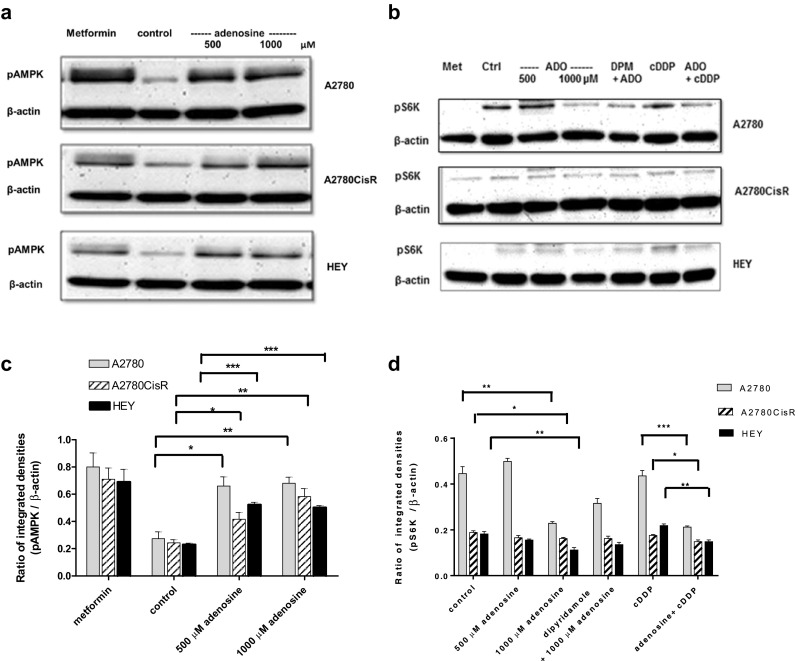
Activation of AMPK and inhibition of mTOR by adenosine treatment. a Phosphorylation of AMPK at Thr172 (60 kDa) was detected by Western blotting after treatment for 48 h with 500 or 1000 μM of adenosine. A total of 30 mM metformin (Met) for 24 h served as positive control. b Phosphorylation of S6K at Thr389 (70 kDa) was detected by Western blotting after treatment for 48 h with 500 or 1000 μM adenosine (ADO) in the absence or presence of 20 μM of dipyridamole (DPM) 30 min prior to 1000 μM of adenosine for 48 h or treatment with a 3-fold IC50 of cisplatin (cDDP) for 24 h or 500 μM of adenosine for 48 h followed by 3-fold IC50 of cisplatin for another 24 h. Metformin-treated cells served as positive control. Control indicates untreated cells. c,d Quantification of immunoblots in panels a and c. Densitometric analysis and calculation of the ratios of pAMPK or pS6K bands, respectively, and β-actin were performed after treatment with adenosine alone or a combination of adenosine and cisplatin. Data shown are mean ± SEM ratios of protein band-integrated densities analyzed by ImageJ software from at least three independent experiments. (*), (**), and (***) indicate statistical significance at P value < 0.05, < 0.01, and < 0.001, respectively
Discussion
Several studies address the association between adenosine and its signaling on growth, proliferation, or death of different kinds of cancer cells including ovarian cancer cells [32–39]. These studies report on effects of adenosine only and do not examine combination treatments. Besides adenosine effects mediated by G protein-coupled P1 receptors, adenosine uptake was identified as effector of the cytotoxic effect of ATP after ATP degradation to adenosine by ectonucleotidases [35, 36]. Moreover, adenosine signaling plays a major role for tumor immune escape in the tumor microenvironment. Thus, adenosine receptor antagonists, mainly A2A antagonists, may be beneficial for immune cell-mediated cancer cell removal [40, 41]. Summarizing these studies, the role of adenosine in cancer treatment is controversial and needs a differentiated evaluation.
In the present study, we wanted to clarify if and how adenosine exerts cytotoxic effects on three ovarian cancer cell lines of different sensitivity against cisplatin (or carboplatin), a cornerstone drug in the chemotherapy of ovarian cancer patients. Moreover, in addition to the effects of adenosine alone, we were interested in possible effects of adenosine on the chemosensitivity of cisplatin. A2780 cells are derived from low-grade serous ovarian carcinoma (LGSOC), and HEY cells are moderately differentiated [42]. LGSOC can largely be treated (and often cured) by surgery. However, despite good prognosis for LGSOC, a substantial number of patients show relapse [43]. We thus decided for this study to choose cell lines (A2780, HEY, A2780CisR) according to their sensitivity to cis- or carboplatin, first-line drugs in ovarian cancer treatment, rather than to differentiate the grade of the cell lines. A2780 cells are cisplatin-sensitive, HEY cells show mediocre sensitivity, and A2780CisR, previously established in our laboratory [25], are chemoresistant against cisplatin as defined by their IC50 values (Table 4). Mutations in various signaling pathway genes such as BRAF (mutated in A2780 and HEY) or KRAS (wt in A2780, mutated in HEY) may play an additional role in the effect of cisplatin and adenosine and warrant studies with targeted therapies such as MEK inhibitors [44], but they were not the focus of this study.
All three cell lines expressed A1, A2A, and A2B receptors as detected by PCR and Western blotting (Fig. 1). Functional activity of these adenosine receptors was confirmed for A1 and A2B as detected by changes in intracellular cAMP concentrations upon selective A1 and A2B receptor inhibition by SLV320 and PSB603, respectively (Fig. 1d,e). The selective A2A antagonist SCH442416 had no effect in cAMP levels (Fig. 1f), suggesting lack of a role of A2A in adenosine signaling under the experimental conditions.
In A2780, A2780CisR, and HEY cells, adenosine displayed concentration-dependent cytotoxicity and induction of caspase-mediated apoptosis shown by an increase in apoptotic nuclei (sub G1), by PARP cleavage, and by using the caspase inhibitor QVD-OPh (Figs. 2 and 5). These results are in accordance with data from Shirali et al. [32] confirming cytotoxicity of adenosine and its ability to induce apoptosis via caspase-dependent pathways in ovarian cancer cells and support the idea to use adenosine signaling for inhibition of cancer cell growth and proliferation. Several studies found the mechanism of adenosine-induced growth arrest and apoptosis induction by activation of A2B receptors [34, 37] or A1 receptors [38], both adenosine receptors activated in our ovarian cancer cell lines (Fig. 1). In contrast to these studies [34, 37, 38], adenosine-induced growth arrest and apoptosis induction in our ovarian cancer cell lines were however not mediated by G protein-coupled adenosine receptors but by intracellular uptake of adenosine by nucleoside transporters as shown by the effect of dipyridamole and a lack of effects of adenosine receptor antagonists (Figs. 3, 4 and 6). These findings are supported by studies in other cancer cell lines. In cervical cancer cells, dipyridamole, an adenosine uptake inhibitor prevented apoptosis and PARP cleavage mediated after extracellular ATP degradation to adenosine [35]. Virtanen et al. found that treatment with adenosine, but not other nucleosides or other adenosine receptor agonists, inhibited cell invasion and migration in breast and prostate cancer cells. These inhibitory effects occurred only after adenosine uptake despite abundant expression of A2B receptors [45]. Studies in hepatocellular carcinoma cell lines demonstrated that adenosine induces apoptosis, and this effect can be inhibited by dipyridamole [46, 47]. Adenosine-induced apoptosis in gastric cancer cell lines was also inhibited by adenosine transporter inhibitors while it was not affected by adenosine receptor antagonists [48, 49]. In line with these studies, our data on ovarian cancer cell lines using the nucleoside uptake transporter inhibitor dipyridamole support an important role of intracellular adenosine uptake for growth inhibitory effects and apoptosis induction after adenosine treatment. Similar to literature reports in other cell lines [49–51], we found that adenosine (after intracellular uptake) leads to activation of AMPK (phosphorylated at Thr172) in all three ovarian cancer cell lines (Fig. 7a,c). This is in accordance with well-known knowledge that incubation of cells or tissues with adenosine increases the energy charge by activation of AMPK [52, 53]. Adenosine is intracellularly converted into AMP by adenosine kinase mimicking energy depression, i.e. low energy charge. High AMP levels in turn are known to induce phosphorylation and activation of AMPK by the upstream kinase LKB1. Therefore, adenosine treatment leads to activation of AMPK and subsequently influences downstream signaling. One of the pleiotropic effects of activated AMPK is inhibition of mTOR. Indeed, we could indirectly demonstrate mTOR inhibition as a result of adenosine treatment by decreased phosphorylation of ribosomal protein S6 kinase (S6K) at Thr389 (Fig. 7b,d). S6K phosphorylation (pS6K) at Thr389 is a hallmark of mTOR activation and results in an increase in protein synthesis and cell proliferation. Vice versa, inhibition of mTOR (e.g. by activation of AMPK after adenosine treatment) reduces pS6K. Reduced pS6K can then lead to inhibition of cell growth and proliferation and, moreover, to the induction of apoptosis [54] which was observed in our adenosine-treated cell lines.
The most important finding of our study is, however, that adenosine treatment of ovarian cancer cells acts in a synergistic manner with cisplatin, pharmacodynamically equivalent to the clinically more widely used carboplatin (Figs. 2, 4 and 5, Tables 2 and 3). Presence of adenosine significantly enhanced cisplatin-induced cytotoxicity by decreasing the IC50 of cisplatin by a factor of up to 4 (Table 2). This was confirmed in MTT assay, PARP cleavage, apoptosis induction, and CI analysis according to Chou [30]. Remarkably, dipyridamole completely abrogates adenosine-enhanced cisplatin cytotoxicity and induction of apoptosis whereas no significant inhibitory effect was observed by selective adenosine receptor antagonists (Fig. 4). Noteworthy, treatment with dipyridamole in combination with cisplatin had no significant effect on the IC50 of cisplatin compared to cisplatin treatment alone (data not shown). These results confirm that adenosine uptake via nucleoside transporters but not GPCRs is crucial for the observed effects in A2780, HEY, and A2780CisR ovarian cancer cells. In addition, mTOR inhibition as observed by a decrease in pS6K did not occur upon cisplatin treatment alone but upon the combination of adenosine and cisplatin (Fig. 7b,d). These findings may obtain importance since adenosine acts as a dual-edged sword: whereas adenosine can inhibit cancer cell growth and induces apoptosis, adenosine may also suppress anti-cancer immune response via A2A receptor stimulation in immune cells. If thus adenosine mediates cell growth inhibition and apoptosis induction after intracellular uptake and not via G protein-coupled adenosine receptors, this may open a dual strategy using A2A antagonists for immune cell-mediated cancer cell removal [40, 41] and adenosine treatment to inhibit cancer cell growth and to induce apoptosis.
Interestingly, the synergistic effect between adenosine and cisplatin was more pronounced in A2780 cells, showing the highest sensitivity against cisplatin, than in HEY or A2780CisR cells (Fig. 2b). This finding could indicate that a co-treatment of cisplatin with adenosine should start early to maintain a high sensitivity of cisplatin and possibly prevent the development of cisplatin resistance in ovarian cancer cells. However, as discussed previously, the histotype (low- versus high-grade serous ovarian cancer cells) may play an additional role. Noteworthy, the synergistic effects of adenosine and cisplatin did not occur in the non-cancer cell line HEK293, indicating some selectivity of adenosine effects on cisplatin sensitization for cancer over non-cancer cells (Fig. S1 and Fig. S2).
In conclusion, our study reveals that adenosine acts synergistically with cisplatin in ovarian cancer cell lines and improves the chemosensitivity to cisplatin—adenosine receptor independent—by increased adenosine uptake with subsequent AMPK activation and mTOR inhibition. These results may open a novel strategy to prevent/delay the development of platinum resistance and overall improve the treatment of ovarian cancer but need confirmation in further ovarian cancer histotypes, such as high-grade serous ovarian carcinoma.
Electronic supplementary material
(DOCX 35 kb)
Funding
This study was supported by the Bundesministerium für Forschung (BMBF, Germany, Grant: BMBF-16GW0108). We acknowledge funding by the DFG for the Thermofisher Arrayscan XTI (Grant: INST 208/690-1 FUGG).
Conflicts of interest
All the authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with participants or animals performed by any of the authors.
References
- 1.Coward JI, Middleton K, Murphy F. New perspectives on targeted therapy in ovarian cancer. Int J Womens Health. 2016;7:189–203. doi: 10.2147/IJWH.S52379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C (2013;24 Suppl 6) Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol:vi24–vi32 [DOI] [PubMed]
- 4.Foley OW, Rauh-Hain JA, Carmen MG. Recurrent epithelial ovarian cancer: an update on treatment. Oncology (Williston Park) 2013;27(4):288–294. [PubMed] [Google Scholar]
- 5.Mantia-Smaldone GM, Edwards RP, Vlad AM. Targeted treatment of recurrent platinum-resistant ovarian cancer: current and emerging therapies. Cancer Manag Res. 2011;3:25–38. doi: 10.2147/CMR.S8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 7.Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 8.Hijaz M, Chhina J, Mert I, Taylor M, Dar S, Al-Wahab Z, et al. Preclinical evaluation of olaparib and metformin combination in BRCA1 wild type ovarian cancer. Gynecol Oncol. 2016;142(2):323–331. doi: 10.1016/j.ygyno.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Kim G, Ison G, McKee AE, Zhang H, Tang S, Gwise T, et al. FDA approval summary: olaparib monotherapy in patients with deleterious germline BRCA-mutated advanced ovarian cancer treated with three or more lines of chemotherapy. Clin Cancer Res. 2015;21(19):4257–4261. doi: 10.1158/1078-0432.CCR-15-0887. [DOI] [PubMed] [Google Scholar]
- 10.Di Virgilio F, Adinolfi E. Extracellular purines, purinergic receptors and tumor growth. Oncogene. 2017;36(3):293–303. doi: 10.1038/onc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar V. Adenosine as an endogenous immunoregulator in cancer pathogenesis: where to go? Purinergic Signal. 2013;9(2):145–165. doi: 10.1007/s11302-012-9349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blay J, White TD, Hoskin DW. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res. 1997;57(13):2602–2605. [PubMed] [Google Scholar]
- 13.US Food & Drug Administration (USFDA). Adenosine. https://www.accessdata.fda.gov. Accessed 27 July 2017
- 14.Gessi S, Merighi S, Sacchetto V, Simioni C, Borea PA. Adenosine receptors and cancer. Biochim Biophys Acta. 2011;1808(5):1400–1412. doi: 10.1016/j.bbamem.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Antonioli L, Blandizzi C, Pacher P, Hasko G. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer. 2013;13(12):842–857. doi: 10.1038/nrc3613. [DOI] [PubMed] [Google Scholar]
- 16.Dasgupta B, Chhipa RR. Evolving lessons on the complex role of AMPK in normal physiology and cancer. Trends Pharmacol Sci. 2016;37(3):192–206. doi: 10.1016/j.tips.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10(5):307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe R, Wei L, Huang J. mTOR signaling, function, novel inhibitors, and therapeutic targets. J Nucl Med. 2011;52(4):497–500. doi: 10.2967/jnumed.111.089623. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Scholz C, Zang C, Schefe JH, Habbel P, Regierer AC, Schulz CO, Possinger K, Eucker J. Metformin and the mTOR inhibitor everolimus (RAD001) sensitize breast cancer cells to the cytotoxic effect of chemotherapeutic drugs in vitro. Anticancer Res. 2012;32(5):1627–1637. [PubMed] [Google Scholar]
- 20.William WN, Kim JS, Liu DD, Solis L, Behrens C, Lee JJ, Lippman SM, Kim ES, Hong WK, Wistuba II, Lee HY. The impact of phosphorylated AMP-activated protein kinase expression on lung cancer survival. Ann Oncol. 2012;23(1):78–85. doi: 10.1093/annonc/mdr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zadra G, Batista JL, Loda M. Dissecting the dual role of AMPK in cancer: from experimental to human studies. Mol Cancer Res. 2015;13(7):1059–1072. doi: 10.1158/1541-7786.MCR-15-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang J, Mills GB. AMPK: a contextual oncogene or tumor suppressor? Cancer Res. 2013;73(10):2929–2935. doi: 10.1158/0008-5472.CAN-12-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koumenis C, Hammond E, Giaccia A. Tumor microenvironment and cellular stress: signaling, metabolism, imaging, and therapeutic targets. Preface. Adv Exp Med Biol. 2014;772:v–viii. doi: 10.1007/978-1-4614-5915-6. [DOI] [PubMed] [Google Scholar]
- 24.Kim H, Hwang J, Yun H, et al. Inhibition of AMPK-activated protein kinase sensitizes cancer cells to cisplatin-induced apoptosis via hyper-induction of p53. J Biol Chem. 2008;283:3731–3742. doi: 10.1074/jbc.M704432200. [DOI] [PubMed] [Google Scholar]
- 25.Engelke LH, Hamacher A, Proksch P, Kassack MU. Ellagic acid and resveratrol prevent the development of cisplatin resistance in the epithelial ovarian cancer cell line A2780. J Cancer. 2016;7(4):353–363. doi: 10.7150/jca.13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stenzel K, Hamacher A, Hansen FK, Gertzen CGW, Senger J, Marquardt V, Marek L, Marek M, Romier C, Remke M, Jung M, Gohlke H, Kassack MU, Kurz T. Alkoxyurean-based histone deacetylase inhibitors increase cisplatin potency in chemoresistant cancer cell lines. J Med Chem. 2017;60(13):5334–5348. doi: 10.1021/acs.jmedchem.6b01538. [DOI] [PubMed] [Google Scholar]
- 27.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden T. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalk P, Eggert B, Relle K, Godes M, Heiden S, Sharkovska Y, Fischer Y, Ziegler D, Bielenberg GW, Hocher B. The adenosine A1 receptor antagonist SLV320 reduces myocardial fibrosis in rats with 5/6 nephrectomy without affecting blood pressure. Br J Pharmacol. 2007;151(7):1025–1032. doi: 10.1038/sj.bjp.0707319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 31.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirali S, Aghaei M, Shabani M, Fathi M, Sohrabi M, Moeinifard M. Adenosine induces cell cycle arrest and apoptosis via cyclinD1/Cdk4 and Bcl-2/Bax pathways in human ovarian cancer cell line OVCAR-3. Tumour Biol. 2013;34(2):1085–1095. doi: 10.1007/s13277-013-0650-1. [DOI] [PubMed] [Google Scholar]
- 33.Hajiahmadi S, Panjehpour M, Aghaei M, Mousavi S. Molecular expression of adenosine receptors in OVCAR-3, Caov-4 and SKOV-3 human ovarian cancer cell lines. Res Pharm Sci. 2015;10(1):43–51. [PMC free article] [PubMed] [Google Scholar]
- 34.Hajiahmadi S, Panjehpour M, Aghaei M, Shabani M. Activation of A2b adenosine receptor regulates ovarian cancer cell growth: involvement of Bax/Bcl-2 and caspase-3. Biochem Cell Biol. 2015;93(4):321–329. doi: 10.1139/bcb-2014-0117. [DOI] [PubMed] [Google Scholar]
- 35.Mello Pde A, Filippi-Chiela EC, Nascimento J, Beckenkamp A, Santana DB, Kipper F, et al. Adenosine uptake is the major effector of extracellular ATP toxicity in human cervical cancer cells. Mol Biol Cell. 2014;25(19):2905–2918. doi: 10.1091/mbc.e14-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mello Pde A, Coutinho-Silva R, Savio LEB (2017) Multifaceted effects of extracellular adenosine triphosphate and adenosine in the tumor–host interaction and therapeutic perspectives. Frontier Immunol 8 article 1526 [DOI] [PMC free article] [PubMed]
- 37.Wei Q, Costanzi S, Balasubramanian R, Gao ZG, Jacobson KA. A2B adenosine receptor blockade inhibits growth of prostate cancer cells. Purinergic Signal. 2013;9(2):271–280. doi: 10.1007/s11302-012-9350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mirza A, Basso A, Black S, Malkowski M, Kwee L, Pachter JA, et al. RNA interference targeting of A1 receptor-overexpressing breast carcinoma cells leads to diminished rates of cell proliferation and induction of apoptosis. Cancer Biol Ther. 2005;4(12):1355–1360. doi: 10.4161/cbt.4.12.2196. [DOI] [PubMed] [Google Scholar]
- 39.Ahmad A, Ahmad S, Glover L, Miller SM, Shannon JM, Guo X, Franklin WA, Bridges JP, Schaack JB, Colgan SP, White CW. Adenosine A2A receptor is a unique angiogenic target of HIF-2alpha in pulmonary endothelial cells. Proc Natl Acad Sci. 2009;106(26):10684–10689. doi: 10.1073/pnas.0901326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci. 2006;103(35):13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montalban Del Barrio I, Penski C, Schlahsa L, Stein RG, Diessner J, Wockel A, et al. Adenosine-generating ovarian cancer cells attract myeloid cells which differentiate into adenosine-generating tumor associated macrophages—a self-amplifying, CD39- and CD73-dependent mechanism for tumor immune escape. J Immunother Cancer. 2016;4:49. doi: 10.1186/s40425-016-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gershenson DM. Low-grade serous carcinoma of the ovary or peritoneum. Ann Oncol. 2016;27(suppl 1):i45–i49. doi: 10.1093/annonc/mdw085. [DOI] [PubMed] [Google Scholar]
- 44.Fernández ML, DiMattia GE, Dawson A, Bamford S, Anderson S, Hennessy BT, Anglesio MS, Shepherd TG, Salamanca C, Hoenisch J, Tinker A, Huntsman DG, Carey MS. Differences in MEK inhibitor efficacy in molecularly characterized low-grade serous ovarian cancer cell lines. Am J Cancer Res. 2016;6(10):2235–2251. [PMC free article] [PubMed] [Google Scholar]
- 45.Virtanen SS, Kukkonen-Macchi A, Vainio M, Elima K, Harkonen PL, Jalkanen S, Yegutkin GG. Adenosine inhibits tumor cell invasion via receptor-independent mechanisms. Mol Cancer Res. 2014;12(12):1863–1874. doi: 10.1158/1541-7786.MCR-14-0302-T. [DOI] [PubMed] [Google Scholar]
- 46.Yang D, Yaguchi T, Nakano T, Nishizaki T. Adenosine activates AMPK to phosphorylate Bcl-XL responsible for mitochondrial damage and DIABLO release in HuH-7 cells. Cell Physiol Biochem. 2011;27(1):71–78. doi: 10.1159/000325207. [DOI] [PubMed] [Google Scholar]
- 47.Wu LF, Li GP, Feng JL, Pu ZJ. Molecular mechanisms of adenosine-induced apoptosis in human HepG2 cells. Acta Pharmacol Sin. 2006;27(4):477–484. doi: 10.1111/j.1745-7254.2006.00302.x. [DOI] [PubMed] [Google Scholar]
- 48.Saitoh M, Nagai K, Nakagawa K, Yamamura T, Yamamoto S, Nishizaki T. Adenosine induces apoptosis in the human gastric cancer cells via an intrinsic pathway relevant to activation of AMP-activated protein kinase. Biochem Pharmacol. 2004;67(10):2005–2011. doi: 10.1016/j.bcp.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 49.Tsuchiya A, Nishizaki T. Anticancer effect of adenosine on gastric cancer via diverse signaling pathways. World J Gastroenterol. 2015;21(39):10931–10935. doi: 10.3748/wjg.v21.i39.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alers S, Loffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32(1):2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mihaylova MM, Shaw RJ. The AMPK signaling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13(9):1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chagoya de Sánchez V, Brunner A, Pina E. In vivo modification of the energy charge in the liver cell. Biochem Biophys Res Commun. 1972;46(3):1441–1445. doi: 10.1016/S0006-291X(72)80138-4. [DOI] [PubMed] [Google Scholar]
- 53.Oakhill JS, Steel R, Chen ZP, Scott JW, Ling N, Tam S, Kemp BE. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332(6036):1433–1435. doi: 10.1126/science.1200094. [DOI] [PubMed] [Google Scholar]
- 54.Tandon P, Gallo CA, Khatri S, Barger JF, Yepiskoposyan H, Plas DR. Requirement for ribosomal protein S6 kinase 1 to mediate glycolysis and apoptosis resistance induced by Pten deficiency. Proc Natl Acad Sci U S A. 2011;108(6):2361–2365. doi: 10.1073/pnas.1013629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 35 kb)