Abstract
Background
Primary malignant or aggressive benign bone tumors rarely occur in distal tibia, and limb salvage remains the mainstay of surgical options. However, reconstruction methods for large bone defect after wide tumor resection in this location are debatable. The purpose of this systematical review is to critically evaluate each reconstruction method regarding the postoperative complications and functional outcome.
Methods
A systematic review of the 33 studies including 337 cases with tumors affecting distal tibia was performed after searching the PubMed and EMBASE databases. Pooled descriptive statistics with separate analyses for postoperative complications and functional outcome of different reconstruction options were performed.
Results
290 (86.1%) patients received limb salvage procedures. Reconstruction strategies including biological reconstruction, such as autograft, allograft, distraction osteogenesis and non-biological prosthetic replacement. The patients received limb salvage procedures tended to have a higher MSTS score (77.1% vs 70.9%, P = .055) and a higher incidence of local relapse (28/290 vs 0/47, P = .052) than those amputated. Biological reconstruction methods provided better functional outcome (78.4% vs 72.2%, P = .017) compared with non-biological prosthetic reconstruction, although similarity of incidence of major complications (51/253 vs 12/37, P = .091). With respect to the comparison between autograft and allograft reconstruction, the autograft seemed to have less major postoperative complications occurrence (27/165 vs 22/78, P = .032), and consequently better functional outcome (MSTS score, 80.2% vs 74.3%, P = .025) than allograft reconstruction.
Conclusions
Limb salvage results in better functional outcome compared with amputation. Biological reconstruction is more advocated than prosthetics replacement, and furthermore, autograft might be suggested to be the optimal reconstructive method with regard to better postoperative functional outcome and less major complications.
Keywords: Distal tibia, Bone tumor, Complications, Function, Systematic review
1. Introduction
Primitive bone malignancies affecting the distal tibia are rare, and there are no large series covering this issue [1], [2], [3]. In the past, the standard treatment for this issue was below-knee amputation [4]. However, following the development of more effective chemotherapy drugs and therapy schemes and the availability of modern surgical techniques, limb salvage has become the mainstay of treatment for this issue [4], [5], [6]. Multiple local recurrences of aggressive giant cell tumor, the osteolytic area may expand rapidly with no residual bone left and the tumor mass protrude into soft tissue. In this circumstance, re-curettage procedure is not feasible, marginal- or wide- resection of tumor become necessary.
In recent decades, variety of reconstruction methods for bone defect of distal tibia after tumor resection have been explored, including non-biological reconstruction, i.e. prosthetic replacement [7], [8], [9], [10], and biological methods, i.e. autograft (vascularized or non-vascularized fibula [2], [4], [6], [11], [12], [13], [14], [15], recycled tumor-bearing bone [15], [16], [17]), allograft [3], [6], [15], [18], [19] and distraction osteogenesis [4], [20], [21]. However, the outcomes of each method are not well known as most studies with a small sample size due to its rarity. Therefore, controversies regarding the best reconstructive method persist. To our knowledge, there is no meta-analyses or systematical review of the literature to illustrate this issue.
Studies concerning complications data and functional outcome of each surgical option and reconstruction method was systematically reviewed, aiming to make recommendations based on evidence for the management of bone tumors affecting distal tibia. We also evaluated patient demographics, diagnosis, and survival in this study.
2. Methods
2.1. Search strategy and inclusion criteria
A literature review was performed using the PubMed and EMBASE databases. The search string employed was the following: (((((lower extremity) OR lower limb) OR distal tibia)) AND (((tumor) OR sarcoma) OR neoplasm)) AND ((((limb salvage) OR limb sparing) OR amputation)).
Criteria for possible inclusion were as follows: articles published between January 1, 1970 and June 30, 2018; all articles written in English or with an English translation; articles describing the surgical methods for primary malignant or aggressive benign tumors of distal tibia; articles reporting postoperative complications and function outcome. Both retrospective studies and case reports were considered. Reviews, letters, comments, conference abstracts, editorials, and non-English publications were excluded.
2.2. Study selection and assessment of quality
To identify potentially relevant studies, two authors (ZZQ and WW) independently evaluated the titles and then the abstracts on the basis of the eligibility criteria. Then full-text articles were screened to test the eligibility. References of obtained articles were screened manually to identify additional references not captured by the original search. The quality of these studies was assessed using Grading Quality of Evidence and Strength of Recommendations (GRADE) [22] by two authors independently. Disagreement was solved through discussion.
2.3. Data extraction
The patient characteristics recorded from each study included number of patients who met inclusion criteria, mean age at the time of diagnosis and type of neoplasm. The postoperative results gathered included length of follow-up, major postoperative complications (fracture, deep infection, nonunion, prosthetic loosening, and talar collapse, etc), and functional scores.
2.4. Statistical analysis
Two review authors (ZZQ and YTQ) analyzed data. Microsoft Excel ® (Microsoft Corp.) was utilized to calculate averages and standard deviations. The SPSS version 22.0 (IBM Corp., Armonk, New York, USA) was used for statistical analysis. Cohort differences were analyzed by Student's t-test for the followup time and functional score, Chi-square test (Pearson's and Fisher's Exact tests, as appropriate) for incidence of postoperative complications and local relapse. Survival analysis was performed using the Kaplan–Meier method. Statistical significance was considered at P < .05.
3. Results
3.1. Search results and characteristics of included studies
The search returned 2657 articles (Fig. 1) from PubMed (n = 1701) and EMBASE (n = 956). Duplicates (n = 650) were removed and the remaining articles (n = 2007) were screened and excluded (n = 1956) if distal tibia tumor was not a focus of the paper. 51 citations were identified for full-text review. Ultimately, 33 studies including 337 patients met the inclusion criteria (Table 1).
Fig. 1.
Flow chart of literature search.
Table 1.
General characteristics of the included studies.
| Author | Year | Source | Design/Evidence Class | Reconstruction | Grade | N |
|---|---|---|---|---|---|---|
| Gebhardt | 1987 | J Bone Joint Surg [Am] | RCS/IV | Allograft | Low | 9 |
| Casadei | 1994 | Foot Ankle Int | RCS/IV | Allograft/Autograft | Low | 12 |
| Abudu | 1999 | Int Orthop | RCS/IV | Prostheses | Low | 4 |
| Lee | 1999 | J Bone Joint Surg [Br] | RCS/IV | Prostheses | Low | 5 |
| Natarajan | 2000 | Int Orthop | RCS/IV | Prostheses | Low | 6 |
| Moore | 2005 | Clin Orthop Relat Res | RCS/IV | Allograft | Low | 8 |
| Laitinen | 2005 | Int Orthop | RCS/IV | Allograft/Autograft/Prostheses/Distraction osteogenesis | Low | 15 |
| Shalaby | 2006 | J Bone Joint Surg [Br] | RCS/IV | Autograft | Low | 6 |
| Balsamo | 2007 | Clin Orthop Relat Res | RCS/IV | Allograft | Low | 12 |
| Ebeid | 2007 | Acta Orthop Belg | RCS/IV | Autograft | Low | 13 |
| Campanacci | 2008 | Foot Ankle Int | RCS/IV | Allograft/Autograft | Low | 8 |
| Niimi | 2008 | J Cancer Res Clin Oncol | RCS/IV | Autograft/Allograft/Prostheses | Low | 10 |
| Saglik | 2008 | Foot Ankle Int | Case report/V | Autograft | Low | 2 |
| Jeon | 2008 | Arch Orthop Trauma Surg | RCS/IV | Recycled autograft | Low | 9 |
| EL-Sherbiny | 2008 | J Egypt | RCS/IV | Autograft | Low | 16 |
| Shekkeris | 2009 | J Bone Joint Surg [Br] | RCS/IV | Prostheses | Low | 6 |
| Ste´phane | 2009 | J Pediatr Orthop | RCS/IV | Autograft | Low | 13 |
| Ozaki | 2009 | Acta Orthop Scand | RCS/IV | Allograft | Low | 3 |
| Hamada | 2011 | The Foot | Case report/V | Prostheses | Low | 1 |
| Mavrogenis | 2012 | Clin Orthop Relat Res | Retrospective comparative study/III | Allograft/Amputation | Low | 42 |
| Kundu | 2013 | J Orthopaed Traumatol | RCS/IV | Autograft | Low | 9 |
| Liu | 2013 | Bull Cancer | RCS/IV | Recycled autograft | Low | 10 |
| Scaglioni | 2013 | Ann Plas Surg | RCS/IV | Autograft | Low | 5 |
| Ajit Singh | 2013 | Asia-Pac J Clin Oncol | Case report/V | Prostheses | Low | 4 |
| Quyang | 2015 | World J Surg Oncol | Case report/V | Distraction osteogenesis | Low | 1 |
| Fan | 2016 | World J Surg Oncol | Case report/V | Autograft | Low | 1 |
| Xu | 2017 | Foot Ankle Surg | RCS/IV | Allograft | Low | 5 |
| Zhang | 2017 | Foot Ankle Surg | RCS/IV | Autograft | Low | 5 |
| Gede | 2017 | J Orthop Surg | Case report/V | Recycled autograft | Low | 4 |
| Han | 2017 | Clin Orthop Relat Res | Retrospective comparative study/III | Recycled autograft/Amputation | Low | 79 |
| Yang | 2017 | J Orthop | RCS/IV | Prostheses | Low | 8 |
| Mizoshiri | 2018 | BMC Cancer | Case report/V | Distraction osteogenesis | Low | 1 |
| Lou | 2018 | Int Orthop | RCS/IV | Distraction osteogenesis | Low | 5 |
RCS = retrospective case series. N = number of patients included for analysis.
All the 337 cases’ demographic and clinical characteristics are summarized in Table 2. There were 75.1% (253/337) osteosarcoma, 7.4% (25/337) Ewing's sarcoma, 6.8% (23/337) giant cell tumor of bone, and most patients (222/310, 71.6%) were under 30 years old. 290 patients (86.1%) underwent limb-sparing operation while 47 patients received below-knee amputation. Autograft was applied to reconstruct the bone defect after tumor resection in 165 patients (56.9%, 165/290), allograft in 79 (26.9%, 78/290), prosthetic replacement in 37 (12.8%, 37/290), and distraction osteogenesis in 10 cases (3.4%, 10/290), respectively. Table 3 summarized the complications and function outcome of different methods.
Table 2.
Demographic and clinical characteristics.
| N | 337 |
|---|---|
| Age (yrs) (no. [%]) | |
| 0–10 | 20 (6.4) |
| 11–20 | 118 (38.1) |
| 21–30 | 84 (27.1) |
| 31–40 | 36 (11.6) |
| >40 | 52 (16.8) |
| Pathologic diagnosis (no. [%]) | |
| Osteosarcoma | 253 (75.1) |
| Ewing sarcoma | 25 (7.4) |
| Giant cell tumor of bone | 23 (6.8) |
| Others | 36 (10.7) |
| Surgery (no. [%]) | |
| Amputation | 47 (13.9) |
| Limb salvage | 290 (86.1) |
| Allograft (alone or combined with autograft) | 78 |
| Autograft | 165 |
| Prostheses | 37 |
| Distraction osteogenesis | 10 |
Table 3.
Pooled analysis of literatures on complications and functional outcome.
| Reconstruction | Allograft | Autograft | p Value | Amputation | Prosthesis | Distraction osteogenesis |
|---|---|---|---|---|---|---|
| Number of literatures | 9 | 16 | 3 | 9 | 4 | |
| Followup duration (mon), mean (range) | 68.5 (8–288) | 67.5 (6–288) | 0.899 | 65.3 (10–268) | 78.4 (12–324) | 52.3 (16–109) |
| MSTS (%), mean (range) | 74.3 (20–97) | 80.2 (56.7–100) | 0.025 | 70.9 (47.0–87.0) | 72.2 (50–90) | 91.0 (83–100) |
| Fracture (%) (cases) | 15.1 (11/73) | 7.0 (11/158) | 0.051 | / | / | 0 (0/10) |
| Nonunion (%) (cases) | 10.3 (8/78) | 5.5 (9/165) | 0.171 | / | / | 20.0 (2/10) |
| Deep infection (%) (cases) | 9.6 (7/73) | 4.4 (7/158) | 0.218 | 0 (0/47) | 16.2 (6/37) | 10.0 (1/10) |
| Loosening (%) (cases) | / | / | / | 13.5 (5/37) | / | |
| Talar collapse (%) (cases) | / | / | / | 5.4 (2/37) | / | |
| Local relapse (%) (cases) | 7.7 (6/78) | 9.7 (16/165) | 0.611 | 0 (0/47) | 13.5 (5/37) | 10.0 (1/10) |
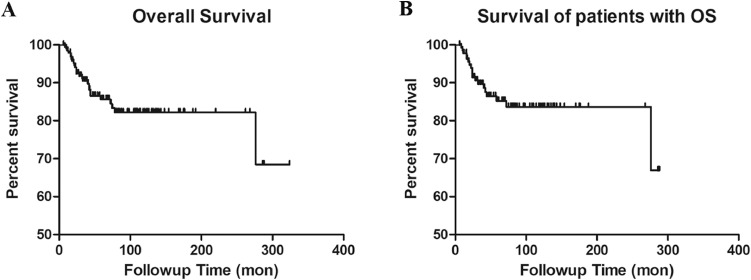
Twenty-three articles [2], [15], [18], [19], [20], [21], [23], [24], [25], [26], [27] including 198 (139 osteosarcoma) patients documented the detailed survival outcome. 27 (19 osteosarcoma) of 198 patients died of disease. The Kaplan Meier 2-year and 5-year overall survival rates for all the 198 patients with malignant or aggressive benign distal tibia tumor were 92.4% and 85.6% respectively, for the 139 patients with distal tibial osteosarcoma were 91.4% and 85.2% respectively (Fig. 2).
Fig. 2.
(A) Kaplan-Meier curve of overall survival probability of all the 198 patients. (B) Kaplan-Meier curve of overall survival probability for 139 patients with osteosarcoma.
3.2. Amputation vs Limb salvage
Three studies [3], [4], [28] of amputation and 33 studies [2–[21], [23]–35] of limb salvage were included for comparative analysis. Pooled analysis showed that the mean functional MSTS score of patients received limb salvage tended to be higher than those amputated (77.1% vs 70.9%, P = .055). Regarding the complications, only few patients had minor wound problems in amputation group (47 patients), such as wound dehiscence or superficial infection in 3 (6.4%) and 1 patient (2%) respectively, while more patients in limb salvage group suffered major postoperative complications, including graft fracture (26/253, 10.3%), deep infection (23/290, 7.9%), nonunion (19/253, 7.5%), prosthetic loosening (5/37, 13.5%), and talar collapse (2/37, 5.4%). With regard to local recurrence after treatment, 28 of 290 patients who had limb salvage experienced local relapse, whereas none of the patients received below-knee amputation have this problem (28/290 vs 0/47, P = .052).
3.3. Biological vs non-biological reconstruction
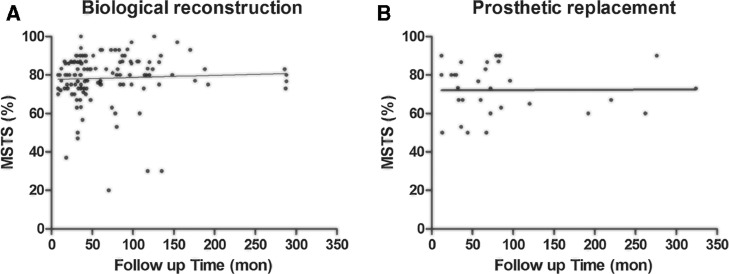
Twenty-six studies [2–[6], [11]–[21], [23], [25], [26], [28], [30]–35] of biological reconstruction and 9 studies [[4], [7]–[10], [15], [24], [27], [29]] of prosthetic replacement were included for comparative analysis. The mean followup durations of biological reconstruction were 67.2 (range, 6–288) and 78.4 (range, 12–324) months for prosthetic replacement, there was no significant difference between these two group (P = .251). The functional score of patients treated by biological reconstruction was higher than those treated by non-biological one (78.4% vs 72.2%, P = .017) (Fig. 3). The major complications (fracture, nonunion, or deep infection) occurred in 51 of 253 patients of biological reconstruction group, and that of prosthetic replacement (loosening, deep infection and talar collapse) developed in 12 of 37 patients (P = .091). Table 4 showed that the main complications of prosthetic replacement were deep infection (6/37, 16.2%) prosthetic loosening (5/37, 13.5%), talar collapse (2/37, 5.4%).
Fig. 3.
Scatterplot graphs showing the MSTS scores and the followup time. (A) Biological reconstruction, (B) Prosthetic reconstruction.
Table 4.
Comparison between biological reconstruction and prosthetic replacement.
| Reconstruction | Biological reconstruction | Prosthetic replacement | p value |
|---|---|---|---|
| Number of literatures | 26 | 9 | |
| Followup duration (mon), mean (range) | 67.2 (6–288) | 78.4 (12–324) | 0.251 |
| MSTS (%), mean (range) | 78.4 (20–100) | 72.2 (50–90) | 0.017 |
| Fracture (%) (cases) | 10.3 (26/253) | / | |
| Deep infection (%) (cases) | 6.7 (17/253) | 16.2 (6/37) | 0.095 |
| Nonunion (%) (cases) | 7.5 (19/253) | / | |
| Loosening (%) (cases) | / | 13.5 (5/37) | |
| Talar collapse (%) (cases) | / | 5.4 (2/37) | |
| Local relapse (%) (cases) | 9.1 (23/253) | 13.5 (5/37) | 0.580 |
3.3. Allograft vs autograft
Ten studies [3–[6], [15], [18], [19], [30], [32], [34]] of allograft reconstruction and 17 studies [[2], [4], [6], [11]–[17], [23], [26], [28], [30], [31], [33], [35]] of autograft reconstruction were included for comparative analysis. Among the 16 studies (165 cases), five [15–[17], [28], [33]] including 82 cases documented the use of recycled tumor-bearing bone. The mean followup durations were 68.5 (range, 8–288) and 67.5 (range, 6–288) months, respectively (P = .899). Pooled analysis indicated that the mean functional score of patients who underwent autograft reconstruction was higher than those allograft reconstruction (80.2% vs 74.3%, P = .025), and less patients in autograft reconstruction group had major complications, the difference reached clinical significance between autograft (16.4%, 27/165) and allograft (28.2%, 22/78) reconstruction (P = .032). As shown in Table 3, the fracture, nonunion, and deep infection occurred in autograft group was 11, 9, and 7 cases respectively, while in allograft group was 11, 8, and 7 cases respectively.
3.4. Distraction osteogenesis
Of the 33 publications selected for this review, four studies [4], [20], [21], [25] including 10 cases investigated the outcome of distraction osteogenesis for large bone defect after tumor resection of distal tibia. The mean followup time was 52.3 (range, 16–109) months. The length of bone transport did not exceed 15 cm in all cases. Pooled analysis indicated the incidence of infection and nonunion was 10% (1/10) and 20% (2/10) respectively, and the average functional score was 91% (range, 83%−100%).
4. Discussion
In this systematic review, we can see that the majority of patients (86.1%) could preserve the lower limb with primary malignant or aggressive benign bone tumor of distal tibia. Pooled analysis indicated that limb salvage can provide a superior function of the limb as compared to amputated limbs (77.1% vs 70.9%, P = .055). Only two studies compared the survival and incidence of local recurrence between those who underwent limb salvage and below-knee amputation [3], [28]. Han [28] reported that 6-year survival of patients in salvage (n = 52) and in amputation (n = 27) group were (80% vs70%, P = .301) and found no difference in local recurrence (6/52 vs 0/27, P = .066). In Mavrogenis’ study, the survival of patients who had limb salvage (n = 23) was similar to that of patients who had amputations (n = 19): 84% vs 74% at 120 and 240 months (P = .599), and three of the 23 patients who had limb salvage experienced local recurrence whereas no patients with amputation experienced local recurrence [3]. Nowadays, amputation still considered for malignancy treatment in distal tibia, especially the tumor with poor response to chemotherapy, involving the crucial neurovascular tissue, or limb salvage failure [3].
Pooled analysis indicated that limb sparing operation can result in acceptable survival rate and disease-free periods. Meanwhile, the survivorship of malignant tumors, including osteosarcoma, in distal tibia location seems to be better than that of proximal ones, in agreement with that of previous literatures reported [1], [36].
Salvaged lower limb offers a better psychological acceptance and an intact body image, and various reconstruction techniques have been attempted in the literature. The application of prosthetic replacement, as a non-biological method, had been tried in a few studies. 37 of 290 (12.8%) limb salvage cases received prosthetic replacement, and the functional outcome was acceptable with a mean of MSTS score 72.2% (range, 50%−90%), the followup period varied from 12 to 324 months with a mean followup of 78.4 month. However, concerns were evident in the literature. Firstly, lack of muscle coverage in this region is anatomical constraints. Secondly, prostheses need a certain amount of residual host bone to allow adequate fixation of the stem, and this may be another limiting factor to their use after resection in tumors involving the tibial diaphysis and distal metaphysis [7], [8], [10]. Thirdly, deep infection (16.2%), and late stage complications such as loosening (13.5%) and talar collapse (5.4%) were major concern after prosthetic replacement, which may deteriorate ankle stability, contributing to the deterioration of the function over time. The study conducted by Abudu has reported that the functional results showed a deterioration in time with an average MSTS score of 81% at one year, decreasing to 65% at last follow-up (median 57 months; range, 33–84) [8]. We suppose that the new developed 3D-printed prosthesis which allows bone ingrown to fuse the ankle joint might decrease the possibility of the late loosening and talar collapse.
Among biological reconstruction methods, the bone graft was widely used for restoring bone continuity after tumor resection. The bone grafts that may be used for an arthrodesis can be autografts, i.e. taken from other anatomic regions of the same individual, such as the contralateral tibia (n = 4), vascularized fibula (n = 54), non-vascularized fibula (n = 5), tumor-bearing bone graft after devitalization (n = 82), or can be allografts (n = 78). In this systematic review, regarding the followup duration, there was no significant difference between different methods. Pooled analysis indicated that reconstruction with autograft can provide better functional limb than those allograft reconstruction (80.2% vs 74.3%, P = .025). Regarding major postoperative complications, 22 of 78 patients in allograft group while 27 of 165 patients in autograft group experienced major complications (P = .032). However, it did not reach significant differences between allograft and autograft in fracture (P= .051), nonunion (P = .171), and deep infection (P = .218). Several factors may count for this: the allografts in reported studies may be processed by different technique, some were processed by high dose of gamma irradiation which has altered biological potential and weak mechanical strength, while some are fresh-frozen which preserved the biological potential; and the application of chemotherapy. In spite of that a little of studies [2], [12], [13], [18], [19] reported the bone union time of host-graft junctions, all the reported average union time of fibular graft was shorter than allograft, which indicated that this technique, especially using the vascularized fibula graft can accelerate bone healing and prevents the formation of non-union characteristics.
As to the reconstruction of distraction osteogenesis, only four studies with a small number of cases (n = 10) reported the outcomes. It provided good functional outcome (mean MSTS 91%; range, 83%−100%), however, the prolonged duration the external fixator has to be kept to achieve ankle fusion beside distraction osteogenesis which imposed restriction on its use. In Mizoshiri's case report, the bone union time exceeded 30 months [25]. Another study conducted by Lou et al reported five patients with a mean bone defect of 11.8 (range, 8–14) cm and the external fixation index was29.3 (range, 22.8–36.3) days/cm, all achieved solid union of the lengthening site and fusion of the ankle [21]. Other disadvantages include high incidence of nonunion at the docking site (2/10, 20%) and pin- or wire-tract infection (2/10, 20%). Additionally, the length of bone transport did not exceed 15 cm in all cases, it does not prove the feasibility of this method for patients whose bone defect longer than 15 cm.
Is it necessary to perform ankle arthrodesis? In this systematic review, twenty-one studies [2], [3], [4], [5], [6], [11], [13], [14], [15], [16], [17], [19], [20], [21], [23], [25], [26], [30], [33], [34], [35] including 142 cases of ankle arthrodesis and six studies [3], [4], [15], [18], [28], [31], [32], [34] including 111 cases of ankle joint preservation were included for comparative analysis. Most researchers hold the notion that arthrodesis was the best way to provide excellent ankle stability, and the final functional outcome further confirmed this theory. However, pooled analysis of functional outcome indicated similar outcome between ankle arthrodesis and ankle joint preservation (MSTS 78.7% vs 77.2%, P = .573). Reasons may be that in some studies, intercalary allograft reconstruction or osteoarticular allograft reconstructions were performed for patients whose tumors without involving the distal tibiofibular joint and articular surface, therefore, the ankle stability was preserved for these patients. In Mavrogenis’ study, primary ankle arthrodesis was only preferred for adults and for tumors involving the distal tibiofibular joint. Intercalary resection and reconstruction were performed for tumors 3 cm or greater from the distal tibiofibular joint. Intraarticular resection and osteoarticular allograft reconstruction were performed for tumors less than 3 cm from the distal tibiofibular joint without joint involvement [3]. So, if the joint capsule and the ligaments around the ankle joint were could not preserved, ankle arthrodesis is necessary to achieve ankle stability.
Whether vascularized fibular graft does better than non-vascularized one? Allsopp et al performed a systematic review in 2016 to compare the vascularized and non-vascularized bone grafts, concluded that vascularization might increase the risk of complications without increasing union rates or time to union [37]. Moreover, reconstruction by vascularized fibular grafts require microsurgery to anastomose vessels and this will prolong operating time distinctly. From this literature review, only two studies documented the use of non-vascularized fibular grafts. Shalaby [13] has reported three patients treated by non-vascularized fibular grafts and three by vascularized ones. Five patients achieved successful host-graft junctions union in both ends, and the mean union time in patients with vascularized grafts was 10 (range, 8–12) months and 18 (range, 16–20) months in those with non-vascularized grafts. All five patients have similar MSTS score (MSTS 70%) and only one patient had a stress fracture at the proximal end of the non-vascularized fibular graft postoperatively. Another study conducted by Saglik [26] reported two cases with giant cell tumor in the distal tibia treated with fibular autograft and ankle arthrodesis. Both of them achieved bone union and able to walk independently without pain. However, the bone union time was not recorded in his study. In Zhang's study, bone union of vascularized fibula graft was achieved in all five patients at an average time of 7 (range, 5.6–8.5) months[12]. So, in this systematic review, we could not compare the vascularized or non-vascularized graft.
How were these complications further treated? From the identified studies, we can see that the reported postoperative complication rates ranged from 0% to 50%. Topping that ranking in biological reconstruction were fracture, nonunion, and deep infection; and in non-biological reconstruction were deep infection, prosthetic loosening, and talar collapse. Wound problem would be solved by wound debridement and local care (or and oral antibiotics) [3], [8], [28], [30]. Patients who had fracture or nonunion were treated with autologous bone grafting at the site of the fracture and re-osteosynthesis, those experienced deep infection were treated with debridement (and revision of the osteosynthesis), and administration of systemic antibiotics [2], [3], [4], [5], [6], [13], [18]. Those who underwent intercalary reconstruction experienced fracture would be treated with arthrodesis [3], [18]. Below-knee amputation would be considered when persistent chronic infection or nonunion developed, or with local recurrence [4], [13], [18], [27].
As this study has sought to provide a comprehensive review of the surgical options for the treatment of distal tibia tumors, note that the ability to draw comprehensive conclusions from the results provided in there viewed studies was limited given the extensive variation in the scales. We included 26 retrospective case series studies and seven case reports, it inevitably limited the comparability due to the retrospective design. The sources of allograft or autograft maybe differed in each study, so the incidence of complication maybe inaccuracy. Additionally, many articles did not record the bone union time or the data was ambiguous. So, we did not analyze the bone union time between different method. Even in light of these shortcomings, the systematic review described here may serve as a framework which to help the surgeon to determine the optimal reconstruction strategy for larger bone defect in distal tibia.
5. Conclusion
In conclusion, limb salvage in distal tibia results in acceptable survival rates and provides better functional outcome compared with those received amputation. Each reconstructive procedure has advantages and disadvantages and each reconstructive technique indeed, caused many complications. However, biological reconstruction is suggested to serve as the first choice with regard to better postoperative functional outcome and less major complications, moreover, autograft might be more advocated.
Conflict of interest statement
Authors have no conflicts of interest or financial support to declare.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2018.100209.
Appendix. Supplementary materials
References
- 1.Bielack S.S., Kempf-Bielack B., Delling G., Exner G.U., Flege S., Helmke K., Kotz R., Salzer-Kuntschik M., Werner M., Winkelmann W., Zoubek A., Jurgens H., Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002;20(3):776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 2.Ebeid W., Amin S., Abdelmegid A., Refaat Y., Ghoneimy A. Reconstruction of distal tibial defects following resection of malignant tumours by pedicled vascularised fibular grafts. Acta orthopaedica Belgica. 2007;73(3):354–359. [PubMed] [Google Scholar]
- 3.Mavrogenis A.F., Abati C.N., Romagnoli C., Ruggieri P. Similar survival but better function for patients after limb salvage versus amputation for distal tibia osteosarcoma. Clinical Orthopaedics Related Res. 2012;470(6):1735–1748. doi: 10.1007/s11999-011-2238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laitinen M., Hardes J., Ahrens H., Gebert C., Leidinger B., Langer M., Winkelmann W., Gosheger G. Treatment of primary malignant bone tumours of the distal tibia. Int. Orthopaedics. 2005;29(4):255–259. doi: 10.1007/s00264-005-0656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore D.R., Halpern J.L., Schwartz H.S. Allograft ankle arthrodesis: a limb salvage technique for distal tibial tumors. Clinical Orthopaedics Related Res. 2005;440:213–221. doi: 10.1097/01.blo.0000176449.77149.81. [DOI] [PubMed] [Google Scholar]
- 6.Campanacci D.A., Scoccianti G., Beltrami G., Mugnaini M., Capanna R. Ankle arthrodesis with bone graft after distal tibia resection for bone tumors. Foot Ankle Int. 2008;29(10):1031–1037. doi: 10.3113/FAI.2008.1031. [DOI] [PubMed] [Google Scholar]
- 7.Shekkeris A.S., Hanna S.A., Sewell M.D., Spiegelberg B.G., Aston W.J., Blunn G.W., Cannon S.R., Briggs T.W. Endoprosthetic reconstruction of the distal tibia and ankle joint after resection of primary bone tumours. J. Bone Joint Surg. British. 2009;91(10):1378–1382. doi: 10.1302/0301-620X.91B10.22643. [DOI] [PubMed] [Google Scholar]
- 8.Abudu A., Grimer R.J., Tillman R.M., Carter S.R. Endoprosthetic replacement of the distal tibia and ankle joint for aggressive bone tumours. Int. Orthopaed. 1999;23(5):291–294. doi: 10.1007/s002640050374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Natarajan M.V., Annamalai K., Williams S., Selvaraj R., Rajagopal T.S. Limb salvage in distal tibial osteosarcoma using a custom mega prosthesis. Int. Orthopaed. 2000;24(5):282–284. doi: 10.1007/s002640000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S.H., Kim H.S., Park Y.B., Rhie T.Y., Lee H.K. Prosthetic reconstruction for tumours of the distal tibia and fibula. J. Bone Joint Surg. 1999;81(5):803–807. doi: 10.1302/0301-620x.81b5.9588. [DOI] [PubMed] [Google Scholar]
- 11.Kundu Z.S., Gogna P., Gupta V., Singla R., Sangwan S.S., Mohindra M., Singh A. Ankle fusion with centralisation of the fibula after distal tibia bone tumour resection. J. Orthopaed. Traumatol. Offic. J. Ital. Soc. Orthopaed. Traumatol. 2014;15(2):95–101. doi: 10.1007/s10195-013-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C., Zeng B., Zhu K., Zhang L., Hu J. Limb salvage for malignant bone tumours of distal tibia with dual ipsilateral vascularized autogenous fibular graft in a trapezoid-shaped array with ankle arthrodesis and preserving subtalar joint. Foot Ankle Surg. 2017 doi: 10.1016/j.fas.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Shalaby S., Shalaby H., Bassiony A. Limb salvage for osteosarcoma of the distal tibia with resection arthrodesis, autogenous fibular graft and Ilizarov external fixator. J. Bone Joint Surg. 2006;88(12):1642–1646. doi: 10.1302/0301-620X.88B12.17879. [DOI] [PubMed] [Google Scholar]
- 14.S. Stephane, M. Eric, W. Philippe, D.J. Felix, S. Raphael, Resection arthrodesis of the ankle for aggressive tumors of the distal tibia in children, J. Pediatr. Orthop. 29(7) (2009) 811–6. [DOI] [PubMed]
- 15.Niimi R., Matsumine A., Kusuzaki K., Kuratsu S., Araki N., Aoki Y., Ueda T., Kudawara I., Myoui A., Ieguchi M., Hashimoto N., Yoshikawa H., Uchida A. Usefulness of limb salvage surgery for bone and soft tissue sarcomas of the distal lower leg. J. Cancer Res. Clin. Oncol. 2008;134(10):1087–1095. doi: 10.1007/s00432-008-0386-2. [DOI] [PubMed] [Google Scholar]
- 16.Jeon D.G., Kim M.S., Cho W.H., Song W.S., Lee S.Y. Reconstruction with pasteurized autograft for distal tibial tumor. Arch. Orthopaed. Trauma Surg. 2008;128(2):159–165. doi: 10.1007/s00402-007-0445-1. [DOI] [PubMed] [Google Scholar]
- 17.Liu T., Guo X., Zhang X., Li Z., Zhang Q. Reconstruction with pasteurized autograft for primary malignant bone tumor of distal tibia. Bull Cancer. 2012;99(9):87–91. doi: 10.1684/bdc.2012.1626. [DOI] [PubMed] [Google Scholar]
- 18.Balsamo L.H., Malinin T.I., Temple H.T. Distal tibial osteoarticular allografts. Clin. Orthop. Related Res. 2007;459:92–95. doi: 10.1097/BLO.0b013e3180514c20. [DOI] [PubMed] [Google Scholar]
- 19.Xu L., Zhou J., Wang Z., Xiong J., Qiu Y., Wang S. Reconstruction of bone defect with allograft and retrograde intramedullary nail for distal tibia osteosarcoma. Foot Ankle Surg. 2018;24(2):149–153. doi: 10.1016/j.fas.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Ouyang Z., Xu X., Li L., Luo Y., Liu J., Wang X., Yao X., Huang G., Li X. Distraction osteogenesis and arthrodesis as a new surgical option for chondrosarcoma in the distal tibia. World J. Surg. Oncol. 2015;13:187. doi: 10.1186/s12957-015-0604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lou T.F., Li H., Chai Y.M., Wang C.Y., Liu S.H., Hamushan M., Wu F., Cai W.J., Han P. Resection arthrodesis using distraction osteogenesis then plating as a hybrid surgical technique for the management of bone sarcomas of the distal tibia. Int. Orthopaed. 2018;42(3):705–711. doi: 10.1007/s00264-018-3811-4. [DOI] [PubMed] [Google Scholar]
- 22.Atkins D., Best D., Briss P.A., Eccles M., Falck-Ytter Y., Flottorp S., Guyatt G.H., Harbour R.T., Haugh M.C., Henry D., Hill S., Jaeschke R., Leng G., Liberati A., Magrini N., Mason J., Middleton P., Mrukowicz J., O'Connell D., Oxman A.D., Phillips B., Schunemann H.J., Edejer T., Varonen H., Vist G.E., Williams J.W., Zaza S., Jr., Group G.W. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan J., Li S.Z., Mei J., Yu G.R. Reconstruction with double pedicel fibular graft and ankle arthrodesis for aggressive chondroblastoma in the distal tibia. World J. Surg. Oncol. 2016;14:143. doi: 10.1186/s12957-016-0839-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamada K., Naka N., Murata Y., Yasui Y., Joyama S., Araki N. Prosthetic reconstruction for tumors of the distal tibia. Report of two cases. Foot (Edinb) 2011;21(3):157–161. doi: 10.1016/j.foot.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Mizoshiri N., Shirai T., Terauchi R., Tsuchida S., Mori Y., Katsuyama Y., Hayashi D., Oka Y., Kubo T. Limb saving surgery for Ewing's sarcoma of the distal tibia: a case report. BMC Cancer. 2018;18(1):503. doi: 10.1186/s12885-018-4372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saglik Y., Yildiz Y., Atalar H., Gunay C. The use of fibular autograft and ankle arthrodesis for aggressive giant cell tumor in the distal tibia: a case report. Foot Ankle Int. 2008;29(4):438–441. doi: 10.3113/FAI.2008.0438. [DOI] [PubMed] [Google Scholar]
- 27.Yang P., Evans S., Khan Z., Abudu A., Jeys L., Grimer R. Reconstruction of the distal tibia following resection of aggressive bone tumours using a custom-made megaprosthesis. J. Orthopaed. 2017;14(3):406–409. doi: 10.1016/j.jor.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han K., Dang P., Bian N., Chen X., Yang T., Fan Q., Zhou Y., Zhao T., Wang P. Is limb salvage with microwave-induced hyperthermia better than amputation for osteosarcoma of the distal tibia? Clin. Orthopaed. Related Res. 2017;475(6):1668–1677. doi: 10.1007/s11999-017-5273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ajit Singh V., Nasirudin N., Bernatt M. Endoprosthetic reconstruction for giant cell tumors of the distal tibia: a short term review. Asia Pac. J. Clin. Oncol. 2013;9(2):182–189. doi: 10.1111/j.1743-7563.2012.01553.x. [DOI] [PubMed] [Google Scholar]
- 30.Casadei R., Ruggieri P., Giuseppe T., Biagini R., Mercuri M. Ankle resection arthrodesis in patients with bone tumors. Foot Ankle Int. 1994;15(5):242–249. doi: 10.1177/107110079401500503. [DOI] [PubMed] [Google Scholar]
- 31.El-Sherbiny M. Long term behavior of pedicled vascularized fibular grafts in reconstruction of middle and distal tibia after resection of malignant bone tumors. J. Egypt. Nat. Cancer Inst. 2008;20(2):187–195. [PubMed] [Google Scholar]
- 32.Gebhardt M.C., Lord F.C., Rosenberg A.E., Mankin H.J. The treatment of adamantinoma of the tibia by wide resection and allograft bone transplantation. J. Bone Joint Surg. Am. 1987;69(8):1177–1188. [PubMed] [Google Scholar]
- 33.Gede E.W.I., Ida Ayu A.A., Setiawan I.G.Y., Aryana Ign W., S I.K., SK I.K., Putu A. Outcome of bone recycling using liquid nitrogen as bone reconstruction procedure in malignant and recurrent benign aggressive bone tumour of distal tibia: A report of four cases. J. Orthopaed. Surg. (Hong Kong) 2017;25(2) doi: 10.1177/2309499017713940. [DOI] [PubMed] [Google Scholar]
- 34.Ozaki T., Hillmann A., Wuisman P., Winkelmann W. Reconstruction of tibia by ipsilateral vascularized fibula and allograft. 12 cases with malignant bone tumors. Acta Orthop. Scand. 1997;68(3):298–301. doi: 10.3109/17453679708996706. [DOI] [PubMed] [Google Scholar]
- 35.Scaglioni M.F., Arzi R.Y., Gur E., Ben Amotz O., Barnea Y., Kollender Y., Meller I., Bickels J., Dadia S., Zaretski A. Free fibula reconstruction of distal tibial defects after sarcoma surgery. Ann. Plast. Surg. 2015;74(6):680–683. doi: 10.1097/01.SAP.0000435595.24360.d0. [DOI] [PubMed] [Google Scholar]
- 36.Zeytoonjian T., Mankin H.J., Gebhardt M.C., Hornicek F.J. Distal lower extremity sarcomas: frequency of occurrence and patient survival rate. Foot Ankle Int. 2004;25(5):325–330. doi: 10.1177/107110070402500509. [DOI] [PubMed] [Google Scholar]
- 37.Allsopp B.J., Hunter-Smith D.J., Rozen W.M. Vascularized versus nonvascularized bone grafts: what is the evidence? Clin. Orthopaed. Relat. Res. 2016;474(5):1319–1327. doi: 10.1007/s11999-016-4769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.