Abstract
Interleukin (IL)-19, 20 and 24 are the members of IL-10 family. They are also known as IL-20 receptor (IL-20R) cytokines as they all signal through the IL-20RA/IL-20RB receptor complex; IL-20 and IL-24 (but not IL-19) also signal through the IL-20RB/IL22RA1 receptor complex. Despite their protein structure homology and shared use of receptor complexes, they display distinct biological functions in immune regulation, tissue homeostasis, host defense and oncogenesis. IL-20R cytokines can be expressed by both immune cells and epithelial cells, and are important for their interaction. In general, these cytokines are considered to be associated with pathogenesis of chronic inflammation and autoimmune diseases, including psoriasis, rheumatoid arthritis and inflammatory bowel disease. However, a number of studies also highlighted their suppressive functions in regulating both innate and adaptive T cell response and other immune cells, suggesting that the role of IL-20R cytokines in autoimmunity may be complex. In this review, we will discuss the immunobiological functions of IL-20R cytokines and how they are involved in regulating autoimmune diseases.
Keywords: Interleukins, inflammation, leukocytes, epithelial cells
Summary sentence
This review outlines the current view of the biological effects of IL-20 receptor cytokines and the roles of these cytokines in autoimmune diseases.
1. INTRODUCTION
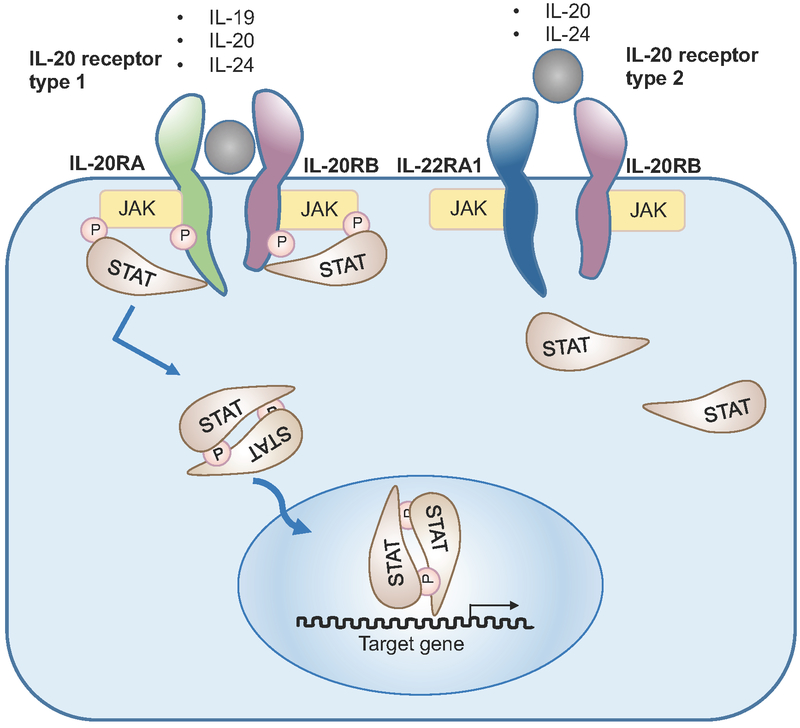
Interleukin (IL)-19, 20, 22, 24 and 26 are members of the interleukin (IL)-20 subfamily, which is part of the IL-10 family. They signal through heterodimeric receptors comprising various combinations of several shared receptor subunits, namely, IL-20 receptor α-subunit (IL-20RA), IL-20 receptor β-subunit (IL-20RB), IL-10 receptor β-subunit (IL-10RB) and IL-20 receptor α1-subunit IL-22RA1 [1]. Of the broader IL-20 subfamily cytokines, IL-19, IL-20 and IL-24 are considered to be the ‘IL-20 receptor cytokines’, because they signal through the same IL-20RA/IL-20RB receptor complex. IL-20 and IL-24 also bind to IL-20RB/IL-22RA1 receptor complex for signal transduction (Fig. 1). IL-22 and IL-26 signal through the IL-22RA1/IL-10RB, and IL-20RA/IL-10RB receptor complex, respectively. These receptors subunits are highly expressed by epithelial cells, indicating the critical roles of the IL-20 subfamily cytokines in regulating tissue inflammation, regeneration and protection [2]. They have been shown to be associated with both pro- and anti-inflammatory responses during autoimmune diseases and chronic inflammation, including psoriasis, inflammatory bowel disease (IBD) and rheumatoid arthritis (RA) [1, 3, 4]. IL-22 can also react with a soluble form of IL-22 inhibitory receptor, known as IL-22-binding protein (IL-22BP, or IL-22RA2), which shares structural homology with IL-22RA1 [5–7]. IL-22BP displays a higher affinity towards IL-22 than the IL-22RA/IL-10RB transmembrane receptor and sequesters IL-22 resulting in suppression of IL-22 signaling [5–7].
Figure 1. The IL-20 receptor cytokines and their receptors.
IL-19, IL-20 and IL-24 signal through the heterodimeric receptor, IL-20RA/IL-20RB complex. In addition, IL-20 and IL-24 have another heterodimeric receptor, which composed of IL-22RA1 and IL-20RB. Both receptors signal through the STAT-JAK signaling pathway.
The broader IL-20 subfamily cytokines are produced mainly by immune cells, including T cells, B cells, NK cells, innate lymphoid cells, monocytes, macrophages and dendritic cells [1]. IL-20R cytokines, IL-19, IL-20 and IL-24, are expressed by various immune cells, particularly Th2 cells (Table 1). In contrast to IL-22 and IL-26, IL-20R cytokines can also be expressed by tissue cells, such as keratinocytes [12, 13], airway epithelial cells [14, 15], and other endothelial cells [16], suggesting the possible reciprocal interaction between immune cells and tissue cells through the production of IL-20R cytokines (Table 2). IL-22 can be co-expressed with interferon (IFN)-γ in Th1 cells and with IL-17A in Th17 cells [4, 8], however more recent studies identified Th22 cells, which only produce IL-22 but not IFN-γ or IL-17A [9, 10]. IL-26 is expressed only in humans but not in mice, and its main cellular sources include Th1 and Th17 cells, NK cells and alveolar macrophages [1, 11]. The biological effects of IL-20 subfamily plays a critical role in host defense, tissue protection and regulation of autoimmune response. Among them, IL-22 is the most studied cytokine because it acts as the effector cytokine of Th17 and Th22 cells, as well as several lineages of innate lymphoid cells [4, 17, 18]. The biological functions and the association in human diseases of the broader IL-20 subfamily have been reviewed previously [1, 4, 11, 17, 19]. However, most of them only focus on IL-22, while the discussion on the roles of IL-20R cytokines in immunoregulation is limited. In this review, we will emphasize the functions of the IL-20R cytokines in regulating immune cells and serving as a potential bridge between the immune cells and the tissues. Their possible roles in regulating autoimmune diseases will also be highlighted.
Table 1.
The cellular sources of IL-20R cytokines.
| IL20-R members |
Cellular Sources | |
|---|---|---|
| Immune cells | Tissue cells | |
| IL-19 | •T cells •B cells •Monocytes •Macrophages |
•Keratinocytes •Fibroblasts •Endothelial cells •Airway epithelial cells |
| IL-20 | •T cells •Monocytes •Granulocytes •DCs |
•Keratinocytes •Fibroblasts |
| IL-24 | •T cells •B cells •NK cells •Monocytes •Macrophages |
•Endothelial cells •Melanocytes •Colonic subepithelial myofibroblasts |
Table 2.
The common and distinct roles of IL-20R cytokines in regulating immune cells.
| Common | Distinct | ||
|---|---|---|---|
| IL-19, IL-20 | •Inhibit production of IL-17A by γδT cells | IL-19 | •Promotes Th2 cells •Promotes M2 monocytes in humans and M1 monocytes in mice |
| IL-19, IL-24 | •Inhibit production of IFN-γ and IL-17A by CD4+ and CD8+ T cells | IL-20 | •Promotes DC maturation |
| IL-24 | •Promotes production of pro-inflammatory cytokines by PBMC •Promotes memory B cells but inhibits plasma cells |
2. IMMUNOBIOLOGY OF THE IL-20R CYTOKINES
IL-19, IL-20 and IL-24 mediate their functions by binding IL-20RA/IL-20RB or IL-20RB/IL-22RA1 receptor complex, and induce intracellular signaling through the Janus kinase (JAK)-signal transducer and through the activator of transcription (STAT) pathways, particularly STAT1[20], STAT3 [21] and STAT5 [22], that are critical for T cell activation. Indeed, several studies identified the potential of IL-20R cytokines in regulating both innate and adaptive T cell responses. Recent studies also identified the possible roles of IL-20R in regulating the cytokine production of Th1, Th2 and Th17 cells, which will be discussed in the later section.
The biological effects of IL-20R cytokines in inhibiting T cells responses have been demonstrated by knocking down their common receptor subunit, IL-20RB. IL-20RB deficient CD4+ and CD8+ T cells express elevated levels of IL-2 and IFN-γ, accompanied by diminished level of IL-10, upon in-vitro stimulation [23]. In line with this, antigen specific IFN-γ producing CD4+ and CD8+ T cells are significantly increased in IL-20RB deficient mice after immunized with a DNA vaccine for hepatitis S-Antigen or OVA [23]. However, it is unclear which IL-20R cytokines, i.e. IL-19, IL-20 and IL-24, is/are responsible for these observations.
In addition to regulating adaptive T cells, IL-20R cytokines can also affect the function of γδT cells. IL-20RB deficient mice show enhanced protection from Staphylococcus aureus infection, as IL-20R cytokines, including IL-19 and IL-20, inhibit the expression of IL-17A from γδT cells, reducing the recruitment of neutrophils and dampening the anti-bacterial inflammatory response [24]. While IL-24 was not included in this study, it would be interesting to investigate whether it can also regulate γδ T cells similarly to IL-19 and IL-20. Thus, IL-20R cytokines can contribute to host defense and tissue homeostasis by regulating both innate and adaptive lymphoid T cell compartment.
It should be noted that although IL-19, IL-20 and IL-24 share structural homology as well as receptors and some of the signaling pathways, they have both common and unique biological functions as pertains to immune cells (Table 2). Their distinct associations with autoimmune diseases will be discussed ahead.
2.2.1. IL-19
IL-19 regulates functions of different immune cell populations, including T cells and myeloid cells. IL-19 is considered to be a Th2 cytokine as it is not only produced by Th2 cells, but also exerts a positive feedback on Th2 cells for further Th2 cell differentiation and cytokine production, including IL-4, IL-5, IL-10 and IL-13 [25]. Reiss-Mandel et al. demonstrated that IL-19 suppresses Staphylococcus aureus-induced IL-17A production from human peripheral blood mononuclear cells (PBMC) [26]; in line with this finding, Anuradha et al. found that blocking IL-19 increases production of IFN-γ and IL-17A by CD4+ and CD8+ T cells in whole blood culture from filaria-infected individuals [27]. In contrast, Myles et al. reported that Staphylococcus aureus infection in mice induces keratinocytes to produce IL-19, which significantly suppresses the production of IL-17A from γδ T cells [24]. These reports indicate that IL-19 likely affects the functions of adaptive and innate T cell in different manners. A similar dichotomy in response to IL-19 may also occur in non-T cells. In monocytes, IL-19 induced human monocytes with M2 phenotype to produce IL-10 [28], whereas it induced mouse M1 monocytes to express IL-6 and TNF-α [29]. These studies suggest that IL-19 (i) plays pleiotropic roles in both pro- and anti-inflammatory responses among different types of immune cells; and/or (ii) has distinct effects on different populations of human and mouse immune cells. Further investigations will be needed to clarify the effects of IL-19 in different species, such as human and mouse, and in different types of immune cells.
Importantly, IL-19 is also involved in interaction between immune cells and the tissue. It induces CD8+ T cells to produce keratinocyte growth factor (KGF) for keratinocyte activation and as such is associated with psoriasis [30]. Furthermore, Th17 and Th2 cytokines, including IL-17A, IL-4 and IL-13, induce IL-19 expression in airway epithelial cells [15], where it may be involved in asthma and tissue fibrosis as part of a complex cellular interactions between Th17 cells, Th2 cells and airway epithelial cells. IL-19 is also involved in tissue injury and inflammation, as it induces angiogenesis by promoting proangiogenic genes expression in both endothelial cells and macrophages [31, 32].
2.2.2. IL-20
There are very limited reported in describing the role of IL-20 in adaptive T cell responses. Similarly to IL-19, IL-20 suppresses the production of IL-17A from γδ T cells [24]. However, IL-20 is unique in being able to induce DCs maturation [38]. IL-20 also increases the expression of CD86 on human monocyte-derived DCs [38], pointing to a possible role during antigen presentation in regulating T cell activation in a indirect manner. Finally, IL-20 may affect DCs migration, due to its effects on the shedding of the integrin CD18 [38], This could potentially influence the ability of DC to migrate to lymphoid organs and secondarily affect their effectiveness as antigen presenting cells for elicitation of the adaptive immune response. However, more detail in-vivo studies will be required to clarify this hypothesis.
IL-20 has been described as a pro-inflammatory cytokine in autoimmune diseases, including psoriasis and RA, due to its effects on keratinocytes and synovial fibroblasts [13, 33–35]. IL-20 treatment leads to psoriasis-like morphological changes in a human epidermis model, which associates with the inhibition of terminal differentiation in keratinocytes [34]. IL-20 also induces synovial fibroblasts to produce pro-inflammatory molecules, i.e., TNF-α, IL-1β, matrix metalloproteinase (MMP)-1, MMP-13 and also monocyte chemoattractant protein (MCP)-1. This in turns recruits activated T cells, monocytes, dendritic cells (DC) and neutrophils and results in tissue damage [35, 36]. Apart from its association with RA through activating synovial fibroblasts, IL-20 is also associated with the pathogenesis of nephritis, owing to its capacity to stimulate kidney mesangial cells to express various pro-inflammatory molecules, such as MCP-1, RANTES, IP-10, and IL-6 [37].
2.2.3. IL-24
IL-24 is an immunoregulatory cytokine that has been associated with various diseases, including autoimmunity, infection and cancer. Its role in suppressing tumor development has been studied particularly intensively [39]. IL-24 was first identified from human H0–1 melanoma cell as having an anti-proliferative property [40]. Later studies confirmed that overexpression of IL-24, achieved mostly by use of adenoviral vectors, leads to cell growth inhibition and apoptosis in a number of tumor lines [39, 41]. Interestingly, IL-24 can induce apoptosis in some tumor cell lines through the MAPK pathway, instead of the more often seen JAK/STAT pathway. This underscores the complexity of IL-24 signaling in controlling oncogenesis [20].Despite encouraging results in cancer therapy, the broader physiological functions of IL-24 are largely unknown.
One of the distinguishing functions of IL-24 is its ability to regulate immune cells. IL-24 is mainly produced by activated immune cells, including monocytes, macrophages, NK cells, T cells and B cells [45]. Caudell et al. reported that IL-24 is an inflammatory cytokine, which stimulates human PBMC to produce higher levels of IL-6, IFN-γ and TNF-α [46]. Further studies identified IL-24 as a Th2 cytokine with immunoregulatory properties: IL-24 was found to be produced by activated T cells, particularly Th2 cells [47]. It suppresses the production of IFN-γ and IL-17A in filarial antigen stimulated-CD4+ and CD8+ T cells from individuals with filaria infection [27]. A transcriptome study on T cells in different phases of Th17 differentiation had identified the expression of IL-24 in the late-differentiation phase[49]. Taken together with its the suppressive effect on CD4+ and CD8+ T cells [27], it is conceivable that IL-24 in differentiated Th17 cells could have a role in regulation and/or immunocontraction as a means to control the Th17 response, which is involved in the pathologies of various autoimmune diseases [49]. IL-24 has also been described in regulating B cell response. It is strongly expressed by CD5+ B cells and memory B cells, but not in plasma cells [50]. In vitro studies reveal that IL-24 promotes differentiation of memory B cells from germinal center B cells, but inhibits the formation of plasma cells as well as the expression of IgG [50].
Dysregulation of IL-24 expression is associated with a number of autoimmune diseases, including psoriasis, IBD and RA [39] and infections. Similar to IL-20, IL-24 induces the production of MCP-1 from keratinocytes and synovial fluid mononuclear cells for cellular infiltration and tissue inflammation [42, 43]. Unlike IL-20, IL-24 may suppress mucosal inflammation by inducing suppressor of cytokine signaling (SOCS) 3 expression in HT-29 colonic epithelial cells and membrane-bound mucin (MUC)-1, −3, and −4 in human colonic subepithelial myofibroblasts obtained from patients with IBD [44]. IL-24 also exacerbates Pseudomonas aeruginosa infection in mouse corneas by promoting SOCS3 expression, which leads to the suppression of anti-bacterial molecules expression, including S100A8/A9 and IL-1β [48].
Of note, the IL-24 gene can be transcribed into different splice variants with distinct biological functions. Sahoo et al. reported that a IL-24 splice variant with 29-nucleotides deletion in exon 4 can antagonize IL-24 by dimerization [51]. Whitaker et al. identified five alternatively spliced variants transcribed from IL-24 gene, which display different capacity to induce apoptosis in tumor cells [52].
3. IL-20R CYTOKINES IN AUTOIMMUNE DISEASES
3.1. Psoriasis
Psoriasis is a chronic inflammation in the skin that is characterized by excessive growth and abnormal differentiation of keratinocytes. The role of IL-20R cytokines in psoriasis is well established. Elevated levels of IL-19, IL-20 and IL-24 are observed in the inflamed psoriatic skin and it has been reported that they can trigger pathological changes in keratinocytes [53, 54]. In addition, genetic overexpression of IL-19, IL-20 or IL-24 leads to the development of paresis-like disease in mice [1]. IL-19 induces CD8+ T cells to produce KGF for keratinocyte activation [30]. IL-20 has been shown to be critical in the development of psoriasis in the human xenograft transplantation model [55]. Briefly, blockade of IL-20 by neutralizing antibody resolves the lesions in animals that have received psoriasis plaque transplant. Conversely, continuous injection of recombinant IL-20 induces psoriasis-like lesion in the animals transplanted with non-lesional skin [55]. Kumari et al. demonstrated that TNF signalling induces the expression of IL-24 in keratinocytes and leads to the development of psoriasis-like skin inflammation in mice [56]. These studies confirmed that IL-20R cytokines are directly involved in the pathogenesis of psoriasis. Furthermore, their promoting effects on angiogenesis, cellular infiltration and tissue remodeling, suggest that they may also involve in promoting psoriasis through other mechanisms.
3.2. Rheumatoid arthritis
RA is mediated by an autoinflammatory/autoimmune response that is a cause of inflammation and tissue damage in the joints. It is characterized by massive infiltration of immune cells and activation of synovial cells. The first evidence of association of IL-20R cytokines in RA is that they are all up-regulated in the synovial fluid from patients with RA [57]. Later studies confirmed their pathogenic roles in RA by showing that they promote the proliferation of synovial cells and induce the expression of pro-inflammatory molecules, including IL-6 and MCP1 from these cells [35, 58].
In animal studies, blockade of IL-20R cytokines attenuates autoimmune arthritis. Hsu et al. demonstrated that administration of anti-IL-19 neutralizing antibody prevents bone destruction in collagen-induced arthritis (CIA) and inhibits production of pro-inflammatory cytokines, i.e. TNF-α, IL-1β, IL-6 and RANKL, by synovial cells [59]. Similarly, anti-IL-20 antibody treatment could resolve CIA in rat and reduced expression of IL-1β, IL-6, RANKL, and MMPs in synovial tissue [60]. A phase IIa clinical trial confirmed that anti-IL-20 antibody treatment is effective in patients with RA [61]. However, there is a concern that targeting only one member from IL-20R cytokines might not be sufficient as a therapeutic approach, as they have overlapping expression patterns and display redundant biological effects. Blocking of the IL-20RB maybe a better approach in treating RA as all three signal through the same IL-20RA/IL-20RB and IL-20RB/IL-22RA1 receptor complexes [62].
3.3. Inflammatory bowel disease
IBD is an immune-mediated inflammatory disease affecting the gastrointestinal tract that includes Crohn’s disease and ulcerative colitis. Although IL-22 in IBD has been intensively studied, the functions of IL-20R cytokines in this disease are not as well characterized. Studies in patients revealed that elevated levels of IL-19, IL-20 and IL-24, at the transcription as well as the protein levels, are associated with IBD [44, 63, 64]. While this might hint at a pathogenic role, despite these associations, IL-19 and IL-24 have been shown to play a suppressive role in IBD. In the dextran sulfate sodium (DSS)-induced colitis model, IL-19-deficient mice are more prone than wild type mice to develop active colitis with increased accumulation of macrophages and production of pro-inflammatory cytokines, including IFN-γ, IL-1β, IL-6, IL-12 and TNF-α [65]. IL-24 induces the expression of SOCS3 to suppress inflammatory cytokine signaling, and enhances intestinal barrier function by enhancing production of membrane-bound mucins from colonic epithelial cells and human colonic subepithelial myofibroblasts [44]. These suggest that IL-20R cytokines may be harnessed to play protective and suppressive roles in IBD.
3.4. Inflammatory diseases of the central nervous system (CNS)
Virtually nothing is known about the role of IL-20R cytokines in the context of inflammatory diseases of the CNS, uveitis and multiple sclerosis, or their respective animal models. Non-infectious uveitis that targets the retina and uvea of the eye is believed to be a T cell-mediated autoimmune disease. Li et al reported upregulated IL-19 and IL-20 gene expression in PBMC of patients with uveitis [66]. IL-24 was not included in this study, but Muls et al. [67] reported that IL-24 (and IL-26) mRNA levels are comparable in relapsing or stable MS, and similar to healthy controls. Since autoreactive T cells are believed to participate in pathogenesis of both these diseases [68, 69], and in view of the reported ability of IL-20R cytokines to regulate T cells responses, further studies exploring the potential roles of IL-20R cytokines in CNS diseases would be of interest.
4. CONCLUDING REMARKS
Despite sharing the structural homology and signaling through the same receptors, it is clear that IL-19, IL-20 and IL-24 display distinct biological effects on their target cells. One of the explanations could be different binding affinities to their shared receptor complexes, which could induce distinct cellular signaling and regulation. To date, most of the studies highlighted the similar pathological roles of these cytokine in autoimmune diseases, underemphasizing their distinct functions, especially towards different immune cells. For example, IL-20R cytokines play an important role in the crosstalk between immune cells and epithelial cells, to facilitate host defense and tissue homeostasis. Defects in this crosstalk increase susceptibility to infection, chronic inflammation and autoimmune diseases. While numerous studies have addressed the role of IL-20R cytokines in regulating epithelial cells during inflammatory and autoimmune diseases (keratinocytes in psoriasis, synovial cells in RA), much less is known about their biological effects on the participating innate and adaptive immune cells, including CD4+ and CD8+ T cells. Increased understanding how antigen-presenting cells, CD4+ and CD8+ T cells can be regulated by IL-20R cytokines could open new avenues for the development of novel therapies for T cell-mediated autoimmune diseases.
ACKNOWLEDGEMENTS
W.P.C. and J.C. are supported by the Natural Science Foundation of Guangdong Province (Grants 2014A030313048 and 2017A030313836), the Guangzhou Science and Technology Program key projects (Grant 201604020082) and the Fundamental Research Funds of the State Key Laboratory of Ophthalmology (Grants 2015KF01 and 30306020240020). RRC is supported by NIH/NEI Intramural funding, Project Number EY000184.
Abbreviations:
- IL-20RA
IL-20 receptor α-subunit
- IL-20RB
IL-20 receptor β-subunit
- IL-10RB
IL-10 receptor β-subunit
- IL-22RA1
IL-20 receptor α1-subunit
- IBD
inflammatory bowel disease
- RA
rheumatoid arthritis
- IL-22BP
IL-22-binding protein
- ILCs
Innate lymphoid cells
- JAK
Janus kinase
- STAT
signal transducer and activator of transcription
- PBMC
peripheral blood mononuclear cell
- KGF
keratinocyte growth factor
- MMP
matrix metalloproteinase
- MCP
monocyte chemoattractant protein
- MUC
membrane-bound mucin
- SOCS
suppressor of cytokine signaling
- Ig
immunoglobulin
- CIA
collagen-induced arthritis
- DSS
dextran sulfate sodium
References
- 1.Rutz S, Wang X, Ouyang W (2014) The IL-20 subfamily of cytokines--from host defence to tissue homeostasis. Nat Rev Immunol 14, 783–95. [DOI] [PubMed] [Google Scholar]
- 2.Wirtz MK and Keller KE (2016) The Role of the IL-20 Subfamily in Glaucoma. Mediators Inflamm 2016, 4083735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wegenka UM (2010) IL-20: biological functions mediated through two types of receptor complexes. Cytokine Growth Factor Rev 21, 353–63. [DOI] [PubMed] [Google Scholar]
- 4.Shabgah AG, Navashenaq JG, Shabgah OG, Mohammadi H, Sahebkar A (2017) Interleukin-22 in human inflammatory diseases and viral infections. Autoimmun Rev 16, 1209–1218. [DOI] [PubMed] [Google Scholar]
- 5.Dumoutier L, Lejeune D, Colau D, Renauld JC (2001) Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J Immunol 166, 7090–5. [DOI] [PubMed] [Google Scholar]
- 6.Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S (2001) Identification, cloning, and characterization of a novel soluble receptor that binds IL-22 and neutralizes its activity. J Immunol 166, 7096–103. [DOI] [PubMed] [Google Scholar]
- 7.Xu W, Presnell SR, Parrish-Novak J, Kindsvogel W, Jaspers S, Chen Z, Dillon SR, Gao Z, Gilbert T, Madden K, Schlutsmeyer S, Yao L, Whitmore TE, Chandrasekher Y, Grant FJ, Maurer M, Jelinek L, Storey H, Brender T, Hammond A, Topouzis S, Clegg CH, Foster DC (2001) A soluble class II cytokine receptor, IL-22RA2, is a naturally occurring IL-22 antagonist. Proc Natl Acad Sci U S A 98, 9511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutz S, Eidenschenk C, Ouyang W (2013) IL-22, not simply a Th17 cytokine. Immunol Rev 252, 116–32. [DOI] [PubMed] [Google Scholar]
- 9.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F (2009) Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol 10, 857–63. [DOI] [PubMed] [Google Scholar]
- 10.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H (2009) Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol 10, 864–71. [DOI] [PubMed] [Google Scholar]
- 11.Stephen-Victor E, Fickenscher H, Bayry J (2016) IL-26: An Emerging Proinflammatory Member of the IL-10 Cytokine Family with Multifaceted Actions in Antiviral, Antimicrobial, and Autoimmune Responses. PLoS Pathog 12, e1005624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, Kasman I, Winer J, Modrusan Z, Danilenko DM, Ouyang W (2007) The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol 178, 2229–40. [DOI] [PubMed] [Google Scholar]
- 13.Wolk K, Witte E, Warszawska K, Schulze-Tanzil G, Witte K, Philipp S, Kunz S, Docke WD, Asadullah K, Volk HD, Sterry W, Sabat R (2009) The Th17 cytokine IL-22 induces IL-20 production in keratinocytes: a novel immunological cascade with potential relevance in psoriasis. Eur J Immunol 39, 3570–81. [DOI] [PubMed] [Google Scholar]
- 14.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK (2008) IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med 14, 275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang F, Wachi S, Thai P, Loukoianov A, Tan KH, Forteza RM, Wu R (2008) Potentiation of IL-19 expression in airway epithelia by IL-17A and IL-4/IL-13: important implications in asthma. J Allergy Clin Immunol 121, 1415–21, 1421 e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain S, Gabunia K, Kelemen SE, Panetti TS, Autieri MV (2011) The anti-inflammatory cytokine interleukin 19 is expressed by and angiogenic for human endothelial cells. Arterioscler Thromb Vasc Biol 31, 167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG (2011) Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 29, 71–109. [DOI] [PubMed] [Google Scholar]
- 18.Dudakov JA, Hanash AM, van den Brink MR (2015) Interleukin-22: immunobiology and pathology. Annu Rev Immunol 33, 747–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leng RX, Pan HF, Tao JH, Ye DQ (2011) IL-19, IL-20 and IL-24: potential therapeutic targets for autoimmune diseases. Expert Opin Ther Targets 15, 119–26. [DOI] [PubMed] [Google Scholar]
- 20.Sauane M, Gopalkrishnan RV, Lebedeva I, Mei MX, Sarkar D, Su ZZ, Kang DC, Dent P, Pestka S, Fisher PB (2003) Mda-7/IL-24 induces apoptosis of diverse cancer cell lines through JAK/STAT-independent pathways. J Cell Physiol 196, 334–45. [DOI] [PubMed] [Google Scholar]
- 21.Parrish-Novak J, Xu W, Brender T, Yao L, Jones C, West J, Brandt C, Jelinek L, Madden K, McKernan PA, Foster DC, Jaspers S, Chandrasekher YA (2002) Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor-ligand interactions mediate unique biological functions. J Biol Chem 277, 47517–23. [DOI] [PubMed] [Google Scholar]
- 22.Tritsaris K, Myren M, Ditlev SB, Hubschmann MV, van der Blom I, Hansen AJ, Olsen UB, Cao R, Zhang J, Jia T, Wahlberg E, Dissing S, Cao Y (2007) IL-20 is an arteriogenic cytokine that remodels collateral networks and improves functions of ischemic hind limbs. Proc Natl Acad Sci U S A 104, 15364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wahl C, Muller W, Leithauser F, Adler G, Oswald F, Reimann J, Schirmbeck R, Seier A, Weiss JM, Prochnow B, Wegenka UM (2009) IL-20 receptor 2 signaling down-regulates antigen-specific T cell responses. J Immunol 182, 802–10. [DOI] [PubMed] [Google Scholar]
- 24.Myles IA, Fontecilla NM, Valdez PA, Vithayathil PJ, Naik S, Belkaid Y, Ouyang W, Datta SK (2013) Signaling via the IL-20 receptor inhibits cutaneous production of IL-1beta and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat Immunol 14, 804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao SC, Cheng YC, Wang YC, Wang CW, Yang SM, Yu CK, Shieh CC, Cheng KC, Lee MF, Chiang SR, Shieh JM, Chang MS (2004) IL-19 induced Th2 cytokines and was up-regulated in asthma patients. J Immunol 173, 6712–8. [DOI] [PubMed] [Google Scholar]
- 26.Reiss-Mandel A, Rubin C, Zayoud M, Rahav G, Regev-Yochay G (2018) S. aureus colonization induces strain-specific suppression of IL-17. Infect Immun [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anuradha R, Munisankar S, Dolla C, Kumaran P, Nutman TB, Babu S (2016) Modulation of CD4+ and CD8+ T-Cell Function by Interleukin 19 and Interleukin 24 During Filarial Infections. J Infect Dis 213, 811–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan WJ, Eskdale J, Boniotto M, Lennon GP, Peat J, Campbell JD, Gallagher G (2005) Human IL-19 regulates immunity through auto-induction of IL-19 and production of IL-10. Eur J Immunol 35, 1576–82. [DOI] [PubMed] [Google Scholar]
- 29.Liao YC, Liang WG, Chen FW, Hsu JH, Yang JJ, Chang MS (2002) IL-19 induces production of IL-6 and TNF-alpha and results in cell apoptosis through TNF-alpha. J Immunol 169, 4288–97. [DOI] [PubMed] [Google Scholar]
- 30.Li HH, Lin YC, Chen PJ, Hsiao CH, Lee JY, Chen WC, Tzung TY, Wu JC, Chang MS (2005) Interleukin-19 upregulates keratinocyte growth factor and is associated with psoriasis. Br J Dermatol 153, 591–5. [DOI] [PubMed] [Google Scholar]
- 31.Kako F, Gabunia K, Ray M, Kelemen SE, England RN, Kako B, Scalia RG, Autieri MV (2016) Interleukin-19 induces angiogenesis in the absence of hypoxia by direct and indirect immune mechanisms. Am J Physiol Cell Physiol 310, C931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richards J, Gabunia K, Kelemen SE, Kako F, Choi ET, Autieri MV (2015) Interleukin-19 increases angiogenesis in ischemic hind limbs by direct effects on both endothelial cells and macrophage polarization. J Mol Cell Cardiol 79, 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei CC, Hsu YH, Li HH, Wang YC, Hsieh MY, Chen WY, Hsing CH, Chang MS (2006) IL-20: biological functions and clinical implications. J Biomed Sci 13, 601–12. [DOI] [PubMed] [Google Scholar]
- 34.Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, Vom Baur E, Witte K, Warszawska K, Philipp S, Johnson-Leger C, Volk HD, Sterry W, Sabat R (2009) IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med (Berl) 87, 523–36. [DOI] [PubMed] [Google Scholar]
- 35.Kragstrup TW, Andersen MN, Schiottz-Christensen B, Jurik AG, Hvid M, Deleuran B (2017) Increased interleukin (IL)-20 and IL-24 target osteoblasts and synovial monocytes in spondyloarthritis. Clin Exp Immunol 189, 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu YH, Yang YY, Huwang MH, Weng YH, Jou IM, Wu PT, Lin TY, Wu LW, Chang MS (2017) Anti-IL-20 monoclonal antibody inhibited inflammation and protected against cartilage destruction in murine models of osteoarthritis. PLoS One 12, e0175802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li HH, Cheng HH, Sun KH, Wei CC, Li CF, Chen WC, Wu WM, Chang MS (2008) Interleukin-20 targets renal mesangial cells and is associated with lupus nephritis. Clin Immunol 129, 277–85. [DOI] [PubMed] [Google Scholar]
- 38.Bech R, Jalilian B, Agger R, Iversen L, Erlandsen M, Otkjaer K, Johansen C, Paludan SR, Rosenberg CA, Kragballe K, Vorup-Jensen T (2016) Interleukin 20 regulates dendritic cell migration and expression of co-stimulatory molecules. Mol Cell Ther 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Persaud L, De Jesus D, Brannigan O, Richiez-Paredes M, Huaman J, Alvarado G, Riker L, Mendez G, Dejoie J, Sauane M (2016) Mechanism of Action and Applications of Interleukin 24 in Immunotherapy. Int J Mol Sci 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB (1995) Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene 11, 2477–86. [PubMed] [Google Scholar]
- 41.Dash R, Bhutia SK, Azab B, Su ZZ, Quinn BA, Kegelmen TP, Das SK, Kim K, Lee SG, Park MA, Yacoub A, Rahmani M, Emdad L, Dmitriev IP, Wang XY, Sarkar D, Grant S, Dent P, Curiel DT, Fisher PB (2010) mda-7/IL-24: a unique member of the IL-10 gene family promoting cancer-targeted toxicity. Cytokine Growth Factor Rev 21, 381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kragstrup TW, Otkjaer K, Holm C, Jorgensen A, Hokland M, Iversen L, Deleuran B (2008) The expression of IL-20 and IL-24 and their shared receptors are increased in rheumatoid arthritis and spondyloarthropathy. Cytokine 41, 16–23. [DOI] [PubMed] [Google Scholar]
- 43.He M and Liang P (2010) IL-24 transgenic mice: in vivo evidence of overlapping functions for IL-20, IL-22, and IL-24 in the epidermis. J Immunol 184, 1793–8. [DOI] [PubMed] [Google Scholar]
- 44.Andoh A, Shioya M, Nishida A, Bamba S, Tsujikawa T, Kim-Mitsuyama S, Fujiyama Y (2009) Expression of IL-24, an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in inflammatory bowel disease. J Immunol 183, 687–95. [DOI] [PubMed] [Google Scholar]
- 45.Poindexter NJ, Walch ET, Chada S, Grimm EA (2005) Cytokine induction of interleukin-24 in human peripheral blood mononuclear cells. J Leukoc Biol 78, 745–52. [DOI] [PubMed] [Google Scholar]
- 46.Caudell EG, Mumm JB, Poindexter N, Ekmekcioglu S, Mhashilkar AM, Yang XH, Retter MW, Hill P, Chada S, Grimm EA (2002) The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J Immunol 168, 6041–6. [DOI] [PubMed] [Google Scholar]
- 47.Schaefer G, Venkataraman C, Schindler U (2001) Cutting edge: FISP (IL-4-induced secreted protein), a novel cytokine-like molecule secreted by Th2 cells. J Immunol 166, 5859–63. [DOI] [PubMed] [Google Scholar]
- 48.Ross BX, Gao N, Cui X, Standiford TJ, Xu J, Yu FX (2017) IL-24 Promotes Pseudomonas aeruginosa Keratitis in C57BL/6 Mouse Corneas. J Immunol 198, 3536–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yosef N, Shalek AK, Gaublomme JT, Jin H, Lee Y, Awasthi A, Wu C, Karwacz K, Xiao S, Jorgolli M, Gennert D, Satija R, Shakya A, Lu DY, Trombetta JJ, Pillai MR, Ratcliffe PJ, Coleman ML, Bix M, Tantin D, Park H, Kuchroo VK, Regev A (2013) Dynamic regulatory network controlling TH17 cell differentiation. Nature 496, 461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maarof G, Bouchet-Delbos L, Gary-Gouy H, Durand-Gasselin I, Krzysiek R, Dalloul A (2010) Interleukin-24 inhibits the plasma cell differentiation program in human germinal center B cells. Blood 115, 1718–26. [DOI] [PubMed] [Google Scholar]
- 51.Sahoo A, Jung YM, Kwon HK, Yi HJ, Lee S, Chang S, Park ZY, Hwang KC, Im SH (2008) A novel splicing variant of mouse interleukin (IL)-24 antagonizes IL-24-induced apoptosis. J Biol Chem 283, 28860–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitaker EL, Filippov V, Filippova M, Guerrero-Juarez CF, Duerksen-Hughes PJ (2011) Splice variants of mda-7/IL-24 differentially affect survival and induce apoptosis in U2OS cells. Cytokine 56, 272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romer J, Hasselager E, Norby PL, Steiniche T, Thorn Clausen J, Kragballe K (2003) Epidermal overexpression of interleukin-19 and −20 mRNA in psoriatic skin disappears after short-term treatment with cyclosporine a or calcipotriol. J Invest Dermatol 121, 1306–11. [DOI] [PubMed] [Google Scholar]
- 54.Kunz S, Wolk K, Witte E, Witte K, Doecke WD, Volk HD, Sterry W, Asadullah K, Sabat R (2006) Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp Dermatol 15, 991–1004. [DOI] [PubMed] [Google Scholar]
- 55.Stenderup K, Rosada C, Worsaae A, Dagnaes-Hansen F, Steiniche T, Hasselager E, Iversen LF, Zahn S, Woldike H, Holmberg HL, Romer J, Kragballe K, Clausen JT, Dam TN (2009) Interleukin-20 plays a critical role in maintenance and development of psoriasis in the human xenograft transplantation model. Br J Dermatol 160, 284–96. [DOI] [PubMed] [Google Scholar]
- 56.Kumari S, Bonnet MC, Ulvmar MH, Wolk K, Karagianni N, Witte E, Uthoff-Hachenberg C, Renauld JC, Kollias G, Toftgard R, Sabat R, Pasparakis M, Haase I (2013) Tumor necrosis factor receptor signaling in keratinocytes triggers interleukin-24-dependent psoriasis-like skin inflammation in mice. Immunity 39, 899–911. [DOI] [PubMed] [Google Scholar]
- 57.Kragstrup TW (2016) The IL-20 receptor axis in immune-mediated inflammatory arthritis: novel links between innate immune recognition and bone homeostasis. Scand J Rheumatol 45, 53–57. [DOI] [PubMed] [Google Scholar]
- 58.Sakurai N, Kuroiwa T, Ikeuchi H, Hiramatsu N, Maeshima A, Kaneko Y, Hiromura K, Nojima Y (2008) Expression of IL-19 and its receptors in RA: potential role for synovial hyperplasia formation. Rheumatology (Oxford) 47, 815–20. [DOI] [PubMed] [Google Scholar]
- 59.Hsu YH, Hsieh PP, Chang MS (2012) Interleukin-19 blockade attenuates collagen-induced arthritis in rats. Rheumatology (Oxford) 51, 434–42. [DOI] [PubMed] [Google Scholar]
- 60.Hsu YH and Chang MS (2010) Interleukin-20 antibody is a potential therapeutic agent for experimental arthritis. Arthritis Rheum 62, 3311–21. [DOI] [PubMed] [Google Scholar]
- 61.Senolt L, Leszczynski P, Dokoupilova E, Gothberg M, Valencia X, Hansen BB, Canete JD (2015) Efficacy and Safety of Anti-Interleukin-20 Monoclonal Antibody in Patients With Rheumatoid Arthritis: A Randomized Phase IIa Trial. Arthritis Rheumatol 67, 1438–48. [DOI] [PubMed] [Google Scholar]
- 62.Liu X, Zhou H, Huang X, Cui J, Long T, Xu Y, Liu H, Yu R, Zhao R, Luo G, Huang A, Liang JG, Liang P (2016) A Broad Blockade of Signaling from the IL-20 Family of Cytokines Potently Attenuates Collagen-Induced Arthritis. J Immunol 197, 3029–3037. [DOI] [PubMed] [Google Scholar]
- 63.Fonseca-Camarillo G, Furuzawa-Carballeda J, Granados J, Yamamoto-Furusho JK (2014) Expression of interleukin (IL)-19 and IL-24 in inflammatory bowel disease patients: a cross-sectional study. Clin Exp Immunol 177, 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fonseca-Camarillo G, Furuzawa-Carballeda J, Llorente L, Yamamoto-Furusho JK (2013) IL-10-- and IL-20--expressing epithelial and inflammatory cells are increased in patients with ulcerative colitis. J Clin Immunol 33, 640–8. [DOI] [PubMed] [Google Scholar]
- 65.Azuma YT, Matsuo Y, Kuwamura M, Yancopoulos GD, Valenzuela DM, Murphy AJ, Nakajima H, Karow M, Takeuchi T (2010) Interleukin-19 protects mice from innate-mediated colonic inflammation. Inflamm Bowel Dis 16, 1017–28. [DOI] [PubMed] [Google Scholar]
- 66.Li Z, Liu B, Maminishkis A, Mahesh SP, Yeh S, Lew J, Lim WK, Sen HN, Clarke G, Buggage R, Miller SS, Nussenblatt RB (2008) Gene expression profiling in autoimmune noninfectious uveitis disease. J Immunol 181, 5147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muls N, Nasr Z, Dang HA, Sindic C, van Pesch V (2017) IL-22, GM-CSF and IL-17 in peripheral CD4+ T cell subpopulations during multiple sclerosis relapses and remission. Impact of corticosteroid therapy. PLoS One 12, e0173780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caspi RR (2010) A look at autoimmunity and inflammation in the eye. J Clin Invest 120, 3073–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weiner HL (2004) Multiple sclerosis is an inflammatory T-cell-mediated autoimmune disease. Arch Neurol 61, 1613–5. [DOI] [PubMed] [Google Scholar]