Abstract
Context
CF is under-diagnosed in Ecuador; one out of every 11,252 live births born in Ecuador could have CF.
Aim
To analyze the clinical findings, based on previously established criteria, with the results of the sweat test, in circumstances where we do not have the routine molecular study.
Methods
Epidemiological, observational, analytic, cross-sectional study. It analyzed 180 patients clinically suspected of CF. Inclusion criteria: children of both sexes older than 30 days and younger than 12 years, who meet at least three clinical criteria suggestive for CF, outpatient and referred by a specialist physician who made a preliminary diagnosis. This is a pilot study.
Results
The combination of criteria pneumonia, chronic cough and chronic obstructive bronchial syndrome is the most frequent, with not a significant relationship with a positive sweat test. On the contrary, a significant relationship was found between the clinical combinations of pneumonia with cough and rhinosinusitis; pneumonia with cough; presence of Pseudomonas aeruginosa; and pneumonia with digital cough and clubbing, so it is recommended to perform the test in all these associations. The most frequent clinical criterion for the reference and performance of the electrolyte test in sweat is pneumonia to repeat for two or more episodes.
Conclusion
Clinical combinations of pneumonia with cough and rhinosinusitis; pneumonia with cough; presence of Pseudomonas aeruginosa; and pneumonia with digital cough and clubbing are pathognomonic for CF and indication for the sweat test. The predictive performance in CF diagnosis, defined as compatible clinical presence plus high values of chloride in sweat test, was 91.1%.
Keywords: Paediatrics, Internal medicine, Evidence-based medicine
1. Introduction
1.1. Context and fundaments
Cystic Fibrosis (CF) is under-diagnosed in Ecuador and the few patients that exist at this moment do not access treatment early, only in advanced stages of the disease can reach proper healthcare, which increases morbidity, complications and reduces the overall survival of these patients [1]. In Ecuador, it is estimate that one of every 11,252 live births born (RNV) can have CF [2], and the prevalence according to ethnic groups has not been establish, considering what the American and Ecuadorian population are genetically diverse [3]. According to this estimated prevalence, in Ecuador there should be at least 29 new cases each year of patients with CF, which means that at least 290 new cases should have been diagnose in the last decade. Currently this does not happen, only a few patients are report in pediatric hospitals. In the other hand, there is not a National Registry of patients with CF to obtain real data [1]. Some studies in Latin America suggest that CF affects to 1 in 1600 to 1 in 14,000 live newborns. The sub-diagnosis could reach 50% in some countries, and it remains an important challenge [1, 4].
Despite the improvement of public health systems [5], this situation exists in our country and is due to three basic problems: lack of diagnosis, late diagnosis, and difficult diagnosis of the disease [6]. The lack of diagnosis is mainly due to there is no awareness of rare diseases in the country, nor public policies aimed at improving diagnosis and timely access to treatment. Many of the patients with clinical suspicion of CF spend a long time in medical consultations, and/or emergency services without a precise diagnosis, which increases their complications, decreases the quality of life, and it saturates the hospital services [7, 8].
The late diagnosis, in turn, is due to three main reasons: 1) there is no qualified and sufficient medical attention; 2) there is no official program for patient recruitment, and 3) laboratory tests are not routinely available [9]. First, in relation to the Ecuadorian health system, it must be said that there are few specialists in pediatric pulmonology available in the country, which are concentrated in the three largest cities in the country, and in tertiary or specialized hospitals. On the other hand, general pediatricians, family doctors or general practitioners who examine the majority of patients in primary care, do not have enough training on this disease to diagnose it early. Second, there is no official and national program that requires the search and recruitment of patients, this disease has not a mandatory notification which makes many patients lose their health system, or take a long time to reach a specialist. In addition, currently a neonatal screening program for CF in Ecuador is not performed even, although there is a national screening program for four other pathologies. Third, there is not a center that offers routinely sweat and molecular tests. The offer of diagnostic services is very limited, disperse, occasional and incomplete, since no center offers comprehensive diagnosis [10]. In addition, due to the low prevalence of the disease, there is little medical interest of physicians to consult these patients; they prefer to devote their efforts to more frequent pathologies. In addition, the molecular tests, especially genomic sequencing, are not available in the country and is limited by the cost, and in some the cases, is paid by pocket money of the relatives of a patient with suspected CF, when they have the funds [11, 12].
Diagnosis of CF is sometimes difficult by the general practitioner due mainly to: 1) imprecision in the clinical diagnosis [13, 14]. 2) Sweat test, when is available, generates confusion when the results shows a low value, or is doubtful [13, 14, 15, 16, 17] and; 3) the molecular diagnosis, when it can be done, does not cover all the minimum mutations that are the most frequent in our population. In the first instance, the great inter-professional clinical variability generates imprecision in the clinical diagnosis, this despite having adequate clinical guidelines. A multidisciplinary diagnosis is not made, which also creates confusion among specialists. In addition, the differential diagnosis is complex because of the large number of pathologies that can be differentiated, and because of the large amount of resources that must be invested for it, resources that are not always available in a country like ours. Second, the sweat test could not be performed with complete certainty in children less than 30 days old, and in children of more than 12 years, where the disease is very likely not present. Despite of that in nowadays, it could confirm the presence of less severe mutations and the CF diagnosis until adult age [17, 18, 19]. Third, in relation to current molecular tests, these are designed for a few mutations only, leaving a group of patients with diagnostic suspicion without complete diagnosis. Several studies showed that with the mutations described until now in Ecuadorian patients, only 70% patients could be cover to diagnosis, these mutations are F508del, G85E, G330E, A455E, G970S, W1098X, R1162X, and N1303K [2, 20, 21, 22, 23]. One study reported by second time the detection of the H609R mutation in Ecuadorian population [24]. It is worth to say that Ecuador is a multi-ethnic and multicultural country, with three major groups such as native Amerindians, mestizos, and Afro-Ecuadorians; these last two with a significant degree of genetic admixture that could change the genetic characterization of the pathology [25, 26].
For all the reasons previously described, a research project was initiated by our research team to improve the early diagnosis, and timely access to the treatment for the patients with CF. Situation appears complicated because several scenarios that can be present. In effect, these situations involve patients with higher values in sweat test and clinical criteria, with higher values in sweat test but who do not meet the clinical criteria, with lower values in sweat test but with one or two mutations in the molecular test.
According with the state of the art, diagnosis standards are a combination of clinical findings, the measurement of chloride through a sweat test by two times, and the presence of two or more known mutations. The presence of salty sweat is still the gold standard test for CF diagnosis. This test consists in the measurement of sweat chloride and sodium concentrations [27, 28, 29]. Indeed, the analysis of electrolytes in sweat by a quantitative method has an accuracy superior to 90% [30, 31]. In the last years, a new method has been develop, the quantitative coulometric test that measure conductivity, especially to neonatal screening of CF [32].
Indeed, international recommendations suggest that after neonatal screening, newborns with two mutations of the CFTR gene could be diagnose with CF; but should also undergo a sweat test with chloride to confirm the diagnosis. Newborns with an identified CFTR only mutation require a chloride sweat test to distinguish healthy carriers from affected newborns with a second, unidentified mutation [48].
1.2. Objective
The purpose of this research is to analyze the clinical findings, based on previously established criteria, with the results of the sweat test, in circumstances where we do not have the routine molecular study. This research it could be applied in countries of low and middle resources.
2. Methods
2.1. Research design
Epidemiological, observational, analytic, and cross-sectional with one cohort of patients.
2.2. Context
This project had three phases: 1) Identification of patients with clinical criteria and suspicion of CF, in the largest pediatric hospitals in the country, being two hospitals that concentrate the majority of patients, the Carlos Andrade Marín Hospital and the Baca Ortiz Hospital but there are patients from other different medical centers also through the country. First contact physician diagnosed the patient only with regular clinical criteria; previously they were train. 2) The selected patients were send to the Translational Medicine Unit of the Faculty of Medical Sciences of the Central University, where a new clinical screening was carried out, through a survey which includes 25 diagnostic criteria, and those that meet at least three of the criteria are sent to the sweat test. This second clinical screening is only confirmatory. 3) Patients with a positive test twice were send back to the hospital, where they enter a CF care program, and receive the proper treatment.
2.3. Subjects
It analyzed 180 (n= 180). Patients clinically suspected of CF. Inclusion criteria were: children of both sexes, older than 30 days and younger than 12 years, who meet at least three clinical criteria suggestive for the disease, ambulatory and that were referred by a specialist doctor who made a preliminary diagnosis, at major pediatric hospitals of Quito. Exclusion criteria were children under 30 days or older than 12 years, who were not under steroidal treatment, who have an acute illness or are hospitalized. Elimination criteria were deceased in the study period. Exposed: patients diagnosed by clinical criteria and high values of sweat test, Non-exposed: patients diagnosed only by clinical criteria.
2.4. Variables
The patient had to meet at least three (3) of 25 clinical criteria to perform the sweat test, and/or one of the major criteria. The clinical diagnostic criteria were: recurrent pneumonia with two or more episodes with hospitalization in one year, hyponatremic dehydration, brother deceased due to an unidentified respiratory cause, distal intestinal obstruction, digital clubbing, bronchial obstructive syndrome refractory to treatment, anemia, edema, hypoproteinemia in the infant, persistent or reticular radiological images, hepatomegaly and/or liver disease, nasal polyps, chronic cough of unknown cause, bilateral congenital absence of vas deferens, presence of Pseudomonas aeruginosa, brother with CF diagnosis, exocrine pancreatic insufficiency, chronic diarrhea, steatorrhea, asthma, bronchiectasis, hyponatremic dehydration with hypochloremic metabolic alkalosis, severe lower respiratory tract infections, chronic malnutrition, meconium ileus, rectal prolapse, chronic rhinosinusitis s in a certain etiology, prolonged neonatal illness. The major criteria were recurrent pneumonia (two or more episodes with hospitalization in a year); presence of Pseudomonas aeruginosa aeruginosa infection; exocrine pancreatic insufficiency; and digital clubbing. Survey also included a socio-demographic data, family history and past pathological history. The criteria were evaluated as presence or absence of them.
2.5. Laboratory method
Iontophoresis was perform through sweat test using sweat (Macroduct®, Wescor Inc., Logan, UT, USA) and analysis of osmolarity of sodium chloride through Sweat Check®. The sweat test system is composed of the sweat inducer (Webster Sweat Inducer) that produces iontophoresis with sweat, the sweat collector (Macroduct® Sweat collector), and the conductive analyzer (Sweat Check Analyzer). Pilogel® iontophoretic discs are sweat ion gel deposits that bring simplicity and safety to the iontophoretic stimulation of sweat. A pilogel disc is simply inserted into each of the stainless steel recessed electrodes. The Webster Sweat Inducer Model 3700 performs sweat iontophoresis; it automatically provides the amount of sweat for the stimulation of the glands (equivalent to five minutes of iontophoresis at 1.5 mA). Some authors think that the Gibson and Cooke test could be a better choice than Macroduct® sweat collection system but Macroduct shows be easily performed, accurate method, and it can run in small quantity of samples, with shorter performance time and lower cost [18, 19].
2.6. Data source
Previous application of a patient survey with clinical criteria, for performance of the electrolyte test in sweat. The test is performed only in patients with clinical suspicion of CF. Prior to the test, anamnesis and physical examination of the patient by a physician trained in this pathology was performed.
2.7. Sampling
All the available patients, with a clinical suspicious, at the pediatric hospitals through the country were included, during a period of two years.
2.8. Avoided biases
It was always the same doctor who performed the physical examination, the operators of the equipment were always the same and received training to avoid variability; the equipment was calibrated before each sampling.
2.9. Quantitative variables
A test with less 60 mmol/L, it was a negative result; between 60 to 80 mmol/L, a doubtful result, and more than 80 mmol/L a positive result.
2.10. Statistical analysis
The statistical program R was used, and the packets knitr, epiR, Hmisc, caTools and ROCR. Patients with positive sweat test plus presence of three diagnostic clinical criteria were consider as “cases”. It performed bivariate and multivariate analysis.
3. Results
3.1. Subjects
It studied 180 patients, 88 (48.9%) of who were men. According to the ethnic distribution, most of the patients were self-define as mestizos, 173 (96.1%). The majority of patients were born and reside in the province of Pichincha (65.6%, n = 118); 159 patients (88.3%) were referred by health facilities belonging to the Integrated Public Health Network (RPIS); the remaining 11.7% were from private hospitals.
3.2. Descriptive data
Of the 180 patients, 30 (16.7%) presented a combination of clinical criteria suggestive of CF with positive sweat test; 158 (87.8%) only completed the clinical criteria, and the sweat test was negative.
3.2.1. Main results
Table 1 shows distribution of clinical findings according to standardized clinical criteria (n = 180). Most common findings were repeated pneumonia with more than two episodes (61.7%), chronic cough of unspecified cause (58.9%), bronchial obstructive syndrome refractory to treatment (36.7%) and asthma (36.7%). The most frequent clinical criterion for the reference and performance of sweat test is persistent pneumonia for two or more episodes with hospitalization; this means that the respiratory cause is predominant in some patients with CF.
Table 1.
Distribution of clinical findings according to standardized clinical criteria (n = 180), descriptive analysis.
| Ítem | Criteria | n= | % |
|---|---|---|---|
| 1 | Repeated pneumonia (more than 2 episodes) | 111 | 61.7 |
| 2 | Chronic cough of unspecified cause | 106 | 58.9 |
| 3 | Bronchial obstructive syndrome refractory to treatment | 66 | 36.7 |
| 4 | Asthma | 66 | 36.7 |
| 5 | Bronchiectasis | 51 | 28.3 |
| 6 | Chronic rhinosinusitis | 49 | 27.2 |
| 7 | Chronic malnutrition | 41 | 22.8 |
| 8 | Persistent or reticular radiological images | 40 | 22.2 |
| 9 | Prolonged neonatal jaundice | 29 | 16.1 |
| 10 | Anemia, edema and hypoproteinemia in the infant | 24 | 13.3 |
| 11 | Digital hypocrisy | 23 | 12.8 |
| 12 | Severe lower respiratory tract infections | 22 | 12.2 |
| 13 | Chronic diarrhea, steatorrhea | 21 | 11.7 |
| 14 | Presence of Pseudomonas aeruginosa | 20 | 11.1 |
| 15 | Hyponatremic dehydration | 12 | 6.7 |
| 16 | Meconial ileus | 11 | 6.1 |
| 17 | Distal intestinal obstruction | 11 | 6.1 |
| 18 | Brother with CF diagnosis | 11 | 6.1 |
| 19 | Brother deceased due to respiratory cause | 9 | 5.0 |
| 20 | Hepatomegaly and/or liver disease | 8 | 4.4 |
| 21 | Excrine pancreatic insufficiency | 7 | 3.9 |
| 22 | Nasal polyps | 6 | 3.3 |
| 23 | Hyponatremic dehydration with hypochloremic metabolic alkalosis | 2 | 1.1 |
Source: database of the patients analyzed. Elaboration: author.
Table 2 shows the distribution of the most frequent combinations of clinical criteria, with at least three findings in the same patient (n = 98). First combination was pneumonia + cough + digital clubbing (62.5%), followed by pneumonia + cough + asthma (43.8%), and, pneumonia + cough + Pseudomonas aeruginosa (60.0%). However, they do not have a statistically significant relationship with higher values in the sweat test. On contrary, we found a statistically significant relationship between the clinical combinations of pneumonia + cough + rhinosinusitis; pneumonia + cough + Pseudomonas aeruginosa; and pneumonia + cough + digital clubbing, in those cases the sweat test is indicated.
Table 2.
Distribution of the most frequent combinations of clinical criteria (n = 98).
| Criteria combination | Cases with 3 or more criteria n = 37 | % | Cases with 1 or 2 criteria only n = 61 | % | Total n = 98 | Chi-square | IC 95% | |
|---|---|---|---|---|---|---|---|---|
| 1 | pneumonia + cough + digital clubbing | 5 | 62.5 | 3 | 37.5 | 8 | 0.009 | 6.667 (1.431–31.07) |
| 2 | pneumonia + cough + asthma | 7 | 43.8 | 9 | 56.3 | 16 | 0.02 | 3.565 (1.108–11.47) |
| 3 | pneumonia + cough + pseudomonas aeruiginosa | 3 | 60.0 | 2 | 40.0 | 5 | 0.04 | 5.382 (0.8311–34.86) |
| 4 | pneumonia + cough + rhinosinusitis | 5 | 45.5 | 6 | 54.5 | 11 | 0.05 | 1.15 (0.84–11.81) |
| 5 | pneumonia + cough + persistent or reticular Rx images | 6 | 33.3 | 12 | 66.7 | 18 | 0.16 | 1.395 (0.39–4.56) |
| 6 | pneumonia + cough + bronchiectasis | 5 | 27.8 | 13 | 72.2 | 18 | 0.29 | 1.401 (0.4267, 4.6) |
| 7 | pneumonia + cough + obstructive bronchial syndrome | 6 | 27.3 | 16 | 72.7 | 22 | 0.39 | 1.15 (0.38–3.47) |
Source: database of the patients analyzed. Elaboration: author.
Table 3 showed the distribution of other related factors and use of medications in positive cases. They were growth retardation (53.3%), acropachies characterized by swelling and clubbing of fingers and toes and sometimes periostitis of the hands and feet (13.3%), inhaled corticosteroid (38.0%), oxygen saturation ≤90% (14.7%) and, bronchodilators use (7.3%). The delay in growth, hypoxemia with oxygen saturation less than 90% and digital clubbing are statistically significant findings for perform the sweat test. The history of use of diuretic medications, systemic corticosteroids, bronchodilators, antibiotics and antihistamines had no statistical relationship with the result of the sweat test. The use of topical corticosteroid medications is significantly associated with a negative result in the sweat test.
Table 3.
Distribution of other associated factors and use of medications.
| Non- exposed n = 150 | % | Exposed n = 30 | % | Total with finding | % | X2 | p < 0.05 | CI (95%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Growth retardation | 80 | 53.3 | 23 | 76.7 | 103 | 57 | 16.43 | 0.001 | 9.29 (2.89–40.67) |
| 2 | Acropachies | 20 | 13.3 | 10 | 33.3 | 30 | 17 | 17.77 | 0.001 | 7.16 (2.54–20.48) |
| 3 | Inhaled corticosteroid | 57 | 38.0 | 2 | 6.7 | 59 | 33 | 11.71 | 0.001 | 0.11 (0.01–0.042) |
| 4 | Oxygen saturation ≤90% | 22 | 14.7 | 8 | 26.7 | 30 | 17 | 6.83 | 0.005 | 3.54 (1.25–9.72) |
| 5 | Bronchodilators | 11 | 7.3 | 4 | 13.3 | 15 | 8 | 3.15 | 0.030 | 3.08 (0.73–11.59) |
| 6 | Antibiotics | 9 | 6.0 | 3 | 10.0 | 12 | 7 | 1.81 | 0.080 | 2.6 (0.5–11.28) |
| 7 | Systemic corticoid | 6 | 4.0 | 0 | 0.0 | 6 | 3 | 1.27 | 0.120 | 0 |
| 8 | Diuretics | 5 | 33 | 0 | 0.0 | 5 | 3 | 1.05 | 0.150 | 0 |
| 9 | Productive cough | 42 | 28.0 | 9 | 30.0 | 51 | 28 | 0.8 | 0.180 | 1.5 (0.58–3.69) |
| 10 | Antihistamines | 7 | 4.7 | 1 | 3.3 | 8 | 4 | 0.03 | 0.400 | 0.8 (0.093–6.99) |
Source: database of the patients analyzed. Elaboration: author.
Table 4 shows the distribution of results by risk analysis according to bivariate analysis using simple 2 × 2 tables, where infection with Pseudomonas aeruginosa has 26.91 times more risk of presentation than others, digital clubbing 8.42 times, and 54.5 times more meconium ileus.
Table 4.
Distribution of results by risk analysis according to bivariate analysis using simple 2 × 2 tables.
| Diagnostic criteria | Exposed n= | % | Non exposed n= | % | OR | IC (95%) | p < 0.05 | |
|---|---|---|---|---|---|---|---|---|
| 1 | Infection with Pseudomonas aeruginosa | 8 | 80.0 | 22 | 12.90 | 26.91 | 5.36–135.01 | 0.0001 |
| 2 | Digital clubbing | 12 | 52.2 | 18 | 11.50 | 8.42 | 3.24–21.87 | 0.0001 |
| 3 | Meconial ileus | 6 | 54.5 | 24 | 14.20 | 7.25 | 2.05–25.64 | 0.0010 |
| 4 | Obstructive bronchial syndrome | 18 | 27.3 | 12 | 10.50 | 3.19 | 1.42–7.14 | 0.0040 |
| 5 | Malnutrition | 15 | 29.4 | 15 | 11.60 | 3.17 | 1.41–7.10 | 0.0040 |
| 6 | severe lower respiratory infections | 8 | 36.4 | 22 | 13.90 | 3.53 | 1.33–9.40 | 0.0080 |
| 7 | Nasal polyps | 3 | 50.0 | 27 | 15.50 | 5.44 | 1.04–28.41 | 0.0300 |
| 8 | Pancreatic insufficiency | 3 | 42.9 | 27 | 15.60 | 4.06 | 0.86–19.15 | 0.0580 |
| 9 | Brother with CF | 4 | 36.4 | 26 | 15.40 | 3.14 | 0.86–11.50 | 0.0700 |
| 10 | Rhinosinusitis | 12 | 24.5 | 18 | 13.70 | 2.04 | 0.90–4.62 | 0.0850 |
| 11 | Reticular images in X rays | 12 | 24.0 | 18 | 13.80 | 1.96 | 0.87–4.45 | 0.1000 |
| 12 | Dehydration | 4 | 33.3 | 26 | 15.50 | 2.73 | 0.77–9.73 | 0.1100 |
| 13 | Hepatomegaly | 3 | 37.5 | 27 | 15.70 | 3.22 | 0.73–14.29 | 0.1100 |
| 14 | Bronchiectasis | 11 | 21.6 | 19 | 14.70 | 1.59 | 0.70–3.64 | 0.2700 |
| 15 | Neonatal jaundice | 3 | 10.3 | 27 | 17.90 | 0.53 | 0.15–1.88 | 0.3200 |
| 16 | Intestinal obstruction | 1 | 9.1 | 29 | 17.16 | 0.48 | 0.06–3.92 | 0.4900 |
| 17 | Anemia | 3 | 12.5 | 27 | 17.30 | 0.68 | 0.19–2.45 | 0.5600 |
| 18 | Chronic cough | 19 | 17.9 | 11 | 14.90 | 1.25 | 0.56–2.81 | 0.5900 |
| 19 | Asthma | 12 | 18.2 | 18 | 15.80 | 1.19 | 0.53–2.65 | 0.6800 |
| 20 | Pneumonia | 19 | 17.1 | 11 | 15.90 | 1.09 | 0.48–2.45 | 0.8400 |
| 21 | Chronic diarrhea | 5 | 16.1 | 25 | 16.80 | 0.95 | 0.33–2.72 | 0.9300 |
Source: database of the patients analyzed. Elaboration: author.
In Table 5, it saw the distribution of the results of the risk analysis according to bivariate analysis considering the presence or absence of CF. Patients with positive sweat test plus presence of three diagnostic clinical criteria were considered as cases. Infection with Pseudomonas aeruginosa were 26.91 times more frequent than other findings, digital clubbing 8.42 times, and meconium ileus 7.25 times. The bivariate analysis showed an association between the positive sweat test with the bronchial obstructive syndrome; chronic malnutrition; and very importantly with the presence of infection by Pseudomonas aeruginosa in sputum cultures; and also, with the presence of digital clubbing, meconium ileus, the development of severe lower respiratory infections, and the presence of nasal polyposis. Borderline association was observe between the positive sweat test with a sibling with CF diagnosis, and diagnosis of exocrine pancreatic insufficiency also. NO significant association were found with presence of rhino-sinusitis, images of reticular alterations in X rays, pneumonia, chronic diarrhea, with hyponatremic dehydration, anemia report, edema and hypoproteinemia in the infant, the presence of bronchiectasis, asthma, a history of neonatal jaundice and hepatomegaly.
Table 5.
Distribution of the results of the risk analysis according to bivariate analysis. Patients with high values in sweat test plus presence of three diagnostic clinical criteria were considere as cases.
| Exposed |
Non-exposed |
OR | IC (95%) | p < 0.05 | ||||
|---|---|---|---|---|---|---|---|---|
| n = 30 | % | n = 150 | % | |||||
| 1 | Infection with Pseudomonas aeruginosa | 8 | 26.7 | 2 | 1.3 | 26.91 | 5.36–135.01 | 0.0001 |
| 2 | Digital clubbing | 12 | 40.0 | 11 | 7.3 | 8.42 | 3.24–21.87 | 0.0001 |
| 3 | Meconial ileus | 6 | 20.0 | 5 | 3.3 | 7.25 | 2.05–25.64 | 0.0010 |
| 4 | Bronchial obstructive syndrome | 18 | 60.0 | 48 | 32.0 | 3.19 | 1.42–7.14 | 0.0040 |
| 5 | Malnutrition | 15 | 50.0 | 36 | 24.0 | 3.17 | 1.41–7.10 | 0.0040 |
| 6 | Severe lower respiratory infections | 8 | 26.7 | 14 | 9.3 | 3.53 | 1.33–9.40 | 0.0080 |
| 7 | Nasal polyps | 3 | 10.0 | 3 | 2.0 | 5.44 | 1.04–28.41 | 0.0300 |
| 8 | Pancreatic insufficiency | 3 | 10.0 | 4 | 2.7 | 4.06 | 0.86–19.15 | 0.0580 |
| 9 | Brother with CF | 4 | 13.3 | 7 | 4.7 | 3.14 | 0.86–11.50 | 0.0700 |
| 10 | Rhinosinusitis | 12 | 40.0 | 37 | 24.7 | 2.04 | 0.90–4.62 | 0.0850 |
| 11 | Reticular images in X rays | 12 | 40.0 | 38 | 25.3 | 1.96 | 0.87–4.45 | 0.1000 |
| 12 | Dehydration | 4 | 13.3 | 8 | 5.3 | 2.73 | 0.77–9.73 | 0.1100 |
| 13 | Hepatomegaly | 3 | 10.0 | 5 | 3.3 | 3.22 | 0.73–14.29 | 0.1100 |
| 14 | Bronchiectasis | 11 | 36.7 | 40 | 26.7 | 1.59 | 0.70–3.64 | 0.2700 |
| 15 | Neonatal jaundice | 3 | 10.0 | 26 | 17.3 | 0.53 | 0.15–1.88 | 0.3200 |
| 16 | Intestinal obstruction | 1 | 3.3 | 10 | 6.7 | 0.48 | 0.06–3.92 | 0.4900 |
| 17 | Anemia | 3 | 10.0 | 21 | 14.0 | 0.68 | 0.19–2.45 | 0.5600 |
| 18 | Chronic cough | 19 | 63.3 | 87 | 58.0 | 1.25 | 0.56–2.81 | 0.5900 |
| 19 | Asthma | 12 | 40.0 | 54 | 36.0 | 1.19 | 0.53–2.65 | 0.6800 |
| 20 | Pneumonia | 19 | 63.3 | 92 | 61.3 | 1.09 | 0.48–2.45 | 0.8400 |
| 21 | Chronic diarrhea | 5 | 16.7 | 26 | 17.3 | 0.95 | 0.33–2.72 | 0.9300 |
Source: database of the patients analyzed. Elaboration: author.
The distribution of risk factors included in the predictive model and associated with the diagnosis of CF according to multivariate models, performed with binary logistic regression, it was adjusted for age and the presence of allergies as confounding factors, was showed in Table 6. Hyponatremic dehydration, exocrine pancreatic insufficiency, meconium ileus, brother with CF diagnosis, and infection with Pseudomonas aeruginosa had a statistical difference with the other factors. The OR for hyponatremic dehydration was 191 times more and for exocrine pancreatic insufficiency 152 times more.
Table 6.
Distribution of risk factors included in the predictive model and associated with the diagnosis of CF according to multivariate models, performed with binary logistic regression. It was adjusted for age and the presence of allergies as confounding factors.
| OR | IC (95%) | p < 0.05 | ||
|---|---|---|---|---|
| 1 | Hyponatremic dehydration | 191.03 | 14.14–4505.61 | 0.0001 |
| 2 | Exocrine pancreatic insufficiency | 152.77 | 5.27–8064.36 | 0.006 |
| 3 | Meconial ileus | 65.73 | 6.16–1179.40 | 0.001 |
| 4 | Brother with CF diagnosis | 32.19 | 1.45–1401.39 | 0.041 |
| 5 | Infection with Pseudomonas aeruginosa | 24.37 | 2.08–487.07 | 0.019 |
| 6 | Digital clubbing | 5.46 | 1.24–26.46 | 0.027 |
| 7 | Chronic rhinosinusitis | 4.57 | 1.05–22.72 | 0.049 |
| 8 | Chronic diarrhea | 0.09 | 0.01–0.71 | 0.039 |
| 9 | Intestinal obstruction | 0.01 | 0.00–0.35 | 0.048 |
Source: database of the patients analyzed. Elaboration: author.
In the multivariate analysis with logistic regression, it found that factors associated with the diagnosis of clinical CF with higher values in sweat test and digital clubbing. At some time with Pseudomonas aeruginosa infection, with a history of having a brother diagnosed by CF, presence of meconium ileus, exocrine pancreatic insufficiency and hyponatremic dehydration. Factors that were inversely related with CF were the presence of chronic diarrhea or intestinal obstruction, all of which showed a statistically significant association and an adequate predictive power.
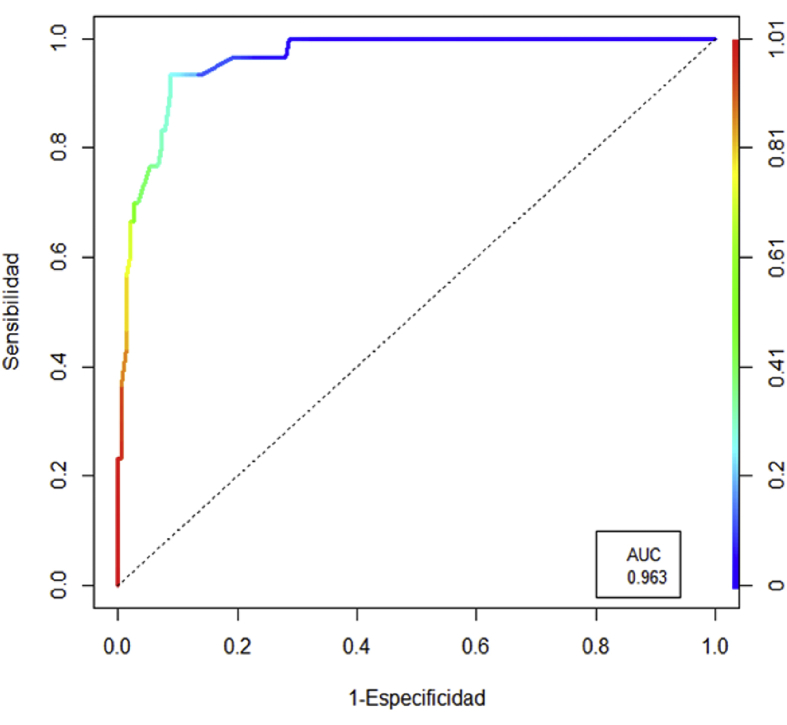
Fig. 1 showed a ROC curve for predictive performance in CF diagnosis, defined as compatible clinical presence plus positive sweat test, model evaluated; the total yield reached 91.1%, with an AUC: 0.963. The best cut point was reached with a probability p ≥ 0.25, the prediction model reached a total yield of 91.1%, with a sensitivity of 93.3% (95% CI: 77.9% to 99.2%); specificity of 90.7% (95% CI: 84.8% to 94.8%); a positive predictive value (PPV) of 66.7% (95% CI: 54.6% to 76.9%) and a negative predictive value of 98.6% (95% CI: 94.7% a 99.6%), the area under the curve (AUC) was calculated at 0.963.
Fig. 1.
ROC curve for predictive performance in CF diagnosis, defined as compatible clinical presence plus positive pilocarpine test, model evaluated; the total yield reached 91.1%, with an AUC: 0.963. The best cut point was reached with a probability p ≥ 0.25, the prediction model reached a total yield of 91.1%, with a sensitivity of 93.3% (95% CI: 77.9% to 99.2%); specificity of 90.7% (95% CI: 84.8% to 94.8%); a positive predictive value (PPV) of 66.7% (95% CI: 54.6% to 76.9%) and a negative predictive value of 98.6% (95% CI: 94.7% a 99.6%), total area under the curve (AUC) was calculated at 0.963.
4. Discussion
4.1. Clue results
According to Table 1 the standardized clinical criteria was a very useful tool to guide diagnostic; these criteria are widely used [33]. All unspecific symptoms were eliminated of these criteria. Respiratory symptoms were the most common in early stages. Age of patients could vary the clinical manifestations age at presentation. Meconium ileus may be present in neonates, in children younger than one year may be observed wheezing, coughing, and/or recurring respiratory infections and pneumonias; in this study two or more hospitalizations were required as inclusion criteria. In early infancy gastrointestinal symptoms could be appear like steatorrhea, failure to thrive, and malnutrition as consequence [34]. In older children may diagnose pancreatic sufficiency and with chronic cough and sputum production. It is estimates that 10% of patients with CF show always meconium ileus [35]. At birth these patients could have abdominal distension, that progress to failure to pass meconium, bilious vomiting, and progressive abdominal distension. In children with complicated meconium ileus can present severe abdominal distention, abdominal wall erythema and edema occasional. Respiratory distress may be a consequence of abdominal. Differential diagnosis includes asthma, bronchiectasis, acute sinusitis, celiac disease, bronchiolitis, and short stature, failure to thrive, aspergillosis, and primary ciliary dyskinesia.
In Table 2 it could see the most common combination with pneumonia plus cough and digital clubbing. The mechanism of digital clubbing is still unknown, with some theories proposed but none conclusive [36]. The combination of symptoms can be useful to identify patterns of disease, sometimes could be consider pathognomonic findings [29].
In Table 3 it can see that delay in growth, hypoxemia with oxygen saturation less than 90% and digital clubbing most important related findings. The first one implies that it exist chronicity and general decay. The others two are related with the lack of tissues oxygen. Clubbing is due to distal digital vasodilation, and an in increased blood flow to the digits [37]. The only important related factor is the use of oral corticosteroid medications; which is significantly associated with low values result in the sweat test in this study. Oral corticosteroids at a dose equivalent to prednisolone of 1 to 2 mg/kg every other day seem to slow the progression of lung disease in CF [38].
Table 4 shows that infection with Pseudomonas aeruginosa, digital clubbing, and meconium ileus is most likely diagnosis in all cases, without age difference. Pseudomonas aeruginosa infections are due to chronicity caused by bacterial persistence, and some bacterial strains have a phenotypic change that carries a major production of a polysaccharide called alginate [39]. Prognosis is poor when Pseudomonas aeruginosa colonization is associated with Staphylococcus aureus [40].
The high values of sweat test was related with bronchial obstructive syndrome, chronic malnutrition, infection by Pseudomonas aeruginosa, digital clubbing, meconium ileus, the development of severe lower respiratory infections, and nasal polyposis, as it shown in Table 5. According with some authors [41, 42, 43], levels of sweat chloride could be higher adrenal insufficiency, atopic dermatitis, glycogen storage disease, hypothyroidism, type 1 fucosidosis, vasopressin-resistant diabetes insipidus, ectodermal dysplasia, malnutrition, mucopolysaccharidosis, pan hypopituitarism, familial cholestasis, familial hypoparathyroidism, and iatrogenic causes. CFTR gene mutations may be the cause of sweat test variability, with variations by time, environmental, residual factors, and individual factors mostly genetics [44].
Hyponatremic dehydration, exocrine pancreatic insufficiency, meconium ileus, brother with CF diagnosis, and infection with Pseudomonas aeruginosa were most important factors in clinical diagnosis. The cause of exocrine pancreatic insufficiency is accumulation of secretions within the pancreatic ducts that leads to interstitial fibrosis [45, 46].
In relation with Fig. 1 the ROC curve for predictive performance in CF diagnosis, defined as compatible clinical presence plus high values in sweat test, the total yield reached was 91.1%; with a sensitivity of 93.3% and a specificity of 90.7%. It means that one of the most common clinical combinations described before plus high values of chloride in sweat test, is sufficient to diagnose 91% of the patients.
It is important to say that sweat tests should be performed only in children older than two weeks and at least two kg of weight. Following standard recommendations [47, 48] sweat testing should be delayed in patients when conditions is acutely unwell, dehydrated, edematous, malnourished or does not have a suitable skin site free of eczema. This guideline also refers, that to chloridimetery, inductively coupled plasma mass spectrometry (ICP-MS) and ion chromatography/high performance liquid chromatography (IC-HPLC) are appropriate to perform analysis of sweat chloride analysis [48].
4.1.1. Limitations
Some limitations were present, it was not possible to correlate phenotypes with mutation because there was not availability of the molecular tests, only a very few patients has molecular results. Second, this study was a pilot study performed in patients of a few provinces.
4.1.2. Generalization
It is possible to generalize results to other countries with similar conditions, and no availability of routine molecular tests. For the author this project was very successful, and it received some petitions to move the idea to neighbors' countries.
4.1.3. Conclusion
Clinical combinations of pneumonia with cough and rhinosinusitis; pneumonia with cough; presence of Pseudomonas aeruginosa; and pneumonia with digital cough and clubbing are most common combinations of clinical presentation of CF that indicate sweat test analysis. Predictive performance of CF diagnosis, defined as compatible clinical presence plus high values of chloride in sweat test, was 91.1%.
Declarations
Author contribution statement
Fabricio Gonzalez-Andrade:conceived and designed the experiments; performed the experiments; analyzed and interpreted the data; contributed reagents, materials, analysis tools or data; wrote the paper.
Funding statement
This work was supported by the Faculty of Medical Sciences of the Central University of Ecuador, and a donation of reactants from Roche Ecuador SA.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Author thank to all members of the Translational Medicine Unit. To Gabriela Aguinaga, Alicia Rodríguez, Hernán Vinelli, Matías Hernández, Juan José Pérez, Henry Cuevas, and others collaborators, for their support in this project. Also thanks to Gustavo Del Pozo for the support in the statistical analysis. For the logistic support thank to Francisco Guijarro, Elizabeth Arboleda, and Mauricio Terán. Special thanks to all patients and practitioners involved in the Cystic Fibrosis program at Hospital Carlos Andrade Marín and Hospital Baca Ortiz. Finally, author thank also to Ramiro López-Pulles and Fausto Coello for the support, as faculty authorities.
References
- 1.Silva Filho L.V., Castaños C., Ruíz H.H. Cystic fibrosis in Latin America-improving the awareness. J. Cyst. Fibros. 2016 Nov;15(6):791–793. doi: 10.1016/j.jcf.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Pérez M.M., Luna M.C., Pivetta O.H., Keyeux G. CFTR gene analysis in Latin American CF patients: heterogeneous origin and distribution of mutations across the continent. J. Cyst. Fibros. 2007 May;6(3):194–208. doi: 10.1016/j.jcf.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Roewer L., Nothnagel M., Gusmão L., Gomes V., González M., Corach D. Continent-wide decoupling of Y-chromosomal genetic variation from language and geography in native South Americans. PLoS Genet. 2013 Apr;9(4):e1003460. doi: 10.1371/journal.pgen.1003460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raskin S., Pereira-Ferrari L., Reis F.C. Incidence of cystic fibrosis in five different states of Brazil as determined by screening of p.F508del, mutation at the CFTR gene in newborns and patients. J. Cyst. Fibros. 2008;7(1):15–22. doi: 10.1016/j.jcf.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Chiriboga S.R. Incremental health system reform policy: Ecuador's law for the provision of free maternity and child care. J. Ambul. Care Manag. 2009 Apr–Jun;32(2):80–90. doi: 10.1097/JAC.0b013e3181994306. [DOI] [PubMed] [Google Scholar]
- 6.López-Cevallos D., Chi C., Ortega F. Equity-based considerations for transforming the Ecuadorian health system. Rev. Salud Publica (Bogota) 2014 May–Jun;16(3):347–359. [PubMed] [Google Scholar]
- 7.Espinosa V., de la Torre D., Acuña C., Cadena C. Human resources for health in Ecuador's new model of care. Rev. Panam. Salud Públic. 2017 Jun 8;41:e52. doi: 10.26633/RPSP.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luna C., Emanuele C.A., Torre D. Positioning Ecuador in the global health agenda as a result of sector reform. Rev. Panam. Salud Públic. 2017 Jun 8;41:e55. doi: 10.26633/RPSP.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malo-Serrano M., Malo-Corral N. Health reform in Ecuador: never again the right to health as a privilege. Rev. Peru. Med. Exp. Salud Pública. 2014 Oct-Dec;31(4):754–761. [PubMed] [Google Scholar]
- 10.Lucio R., Villacrés N., Henríquez R. The health system of Ecuador. Salud Publica Mex. 2011;53(Suppl. 2):s177–s187. [PubMed] [Google Scholar]
- 11.Aldulaimi S., Mora F.E. A primary care system to improve health care efficiency: lessons from Ecuador. J. Am. Board Fam. Med. 2017 May–Jun;30(3):380–383. doi: 10.3122/jabfm.2017.03.160304. [DOI] [PubMed] [Google Scholar]
- 12.Ecuador: public health genomics. Publ Health Genom. 2010;13(3):171–180. doi: 10.1159/000249817. [DOI] [PubMed] [Google Scholar]
- 13.Smyth A.R., Bell S.C., Bojcin S., European Cystic Fibrosis Society European cystic fibrosis society standards of care: best practice guidelines. J. Cyst. Fibros. 2014 May;13(Suppl. 1):S23–S42. doi: 10.1016/j.jcf.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Villanueva G., Marceniuk G., Murphy M.S., Walshaw M., Cosulich R., Guideline Committee Diagnosis and management of cystic fibrosis: summary of NICE guidance. BMJ. 2017 Oct 26;359:j4574. doi: 10.1136/bmj.j4574. [DOI] [PubMed] [Google Scholar]
- 15.Elborn J.S. Cystic fibrosis. Lancet. 2016 Nov 19;388(10059):2519–2531. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- 16.Farrell P.M., White T.B., Ren C.L. Diagnosis of cystic fibrosis: consensus guidelines from the cystic fibrosis foundation. J. Pediatr. 2017 Feb;181S:S4–S15.e1. doi: 10.1016/j.jpeds.2016.09.064. [DOI] [PubMed] [Google Scholar]
- 17.Pagaduan J.V., Ali M., Dowlin M., Suo L., Ward T., Ruiz F., Devaraj S. Revisiting sweat chloride test results based on recent guidelines for diagnosis of cystic fibrosis. Pract. Lab. Med. 2018 Jan 3;10:34–37. doi: 10.1016/j.plabm.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattar A.C., Gomes E.N., Adde F.V., Leone C., Rodrigues J.C. Comparison between classic Gibson and Cooke technique and sweat conductivity test in patients with and without cystic fibrosis. J. Pediatr. (Rio J). 2010 Mar–Apr;86(2):109–114. doi: 10.2223/JPED.1979. [DOI] [PubMed] [Google Scholar]
- 19.Mattar A.C., Leone C., Rodrigues J.C., Adde F.V. Sweat conductivity: an accurate diagnostic test for cystic fibrosis? J. Cyst. Fibros. 2014 Sep;13(5):528–533. doi: 10.1016/j.jcf.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Moya-Quiles M.R., Glover G., Mondéjar-López P., Pastor-Vivero M.D., Fernández-Sánchez A., Sánchez-Solís M. CFTR H609R mutation in Ecuadorian patients with cystic fibrosis. J. Cyst. Fibros. 2009 Jul;8(4):280–281. doi: 10.1016/j.jcf.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Valle E.P., Burgos R.I., Valle J.R., Egas Béjar D., Ruiz-Cabezas J.C. Analysis of CFTR gene mutations and cystic fibrosis incidence in the Ecuadorian population. Invest. Clin. 2007 Mar;48(1):91–98. [PubMed] [Google Scholar]
- 22.Paz-y-Miño C., Pérez J.C., Burgos R., Dávalos M.V., Leone P.E. The DeltaF508 mutation in Ecuador, South America. Hum. Mutat. 1999;14(4):348–350. doi: 10.1002/(SICI)1098-1004(199910)14:4<348::AID-HUMU11>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz S.C., Aguirre S.J., Flores S., Maldonado C., Mejía J., Salinas L. Spectrum of CFTR gene mutations in Ecuadorian cystic fibrosis patients: the second report of the p.H609R mutation. Mol. Genet. Genomic Med. 2017 Nov;5(6):751–757. doi: 10.1002/mgg3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santangelo R., González-Andrade F., Børsting C., Torroni A., Pereira V., Morling N. Analysis of ancestry informative markers in three main ethnic groups from Ecuador supports a trihybrid origin of Ecuadorians. Forensic Sci. Int. Genet. 2017 Nov;31:29–33. doi: 10.1016/j.fsigen.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 25.González-Andrade F., Sánchez D., González-Solórzano J., Gascón S., Martínez-Jarreta B. Sex-specific genetic admixture of Mestizos, Amerindian Kichwas, and Afro-Ecuadorans from Ecuador. Hum. Biol. 2007 Feb;79(1):51–77. doi: 10.1353/hub.2007.0024. [DOI] [PubMed] [Google Scholar]
- 26.Somayaji R., Ramos K.J., Kapnadak S.G., Aitken M.L., Goss C.H. Common clinical features of CF (respiratory disease and exocrine pancreatic insufficiency) Presse. Med. 2017 Jun;46(6 Pt 2):e109–e124. doi: 10.1016/j.lpm.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 27.Servidoni M.F., Gomez C.C.S., Marson F.A.L., Grupo Colaborativo de Estudos em Fibrose Cística Sweat test and cystic fibrosis: overview of test performance at public and private centers in the state of São Paulo, Brazil. J. Bras. Pneumol. 2017 Mar-Apr;43(2):121–128. doi: 10.1590/S1806-37562016000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Başaran A.E., Karataş-Torun N. Normal sweat chloride test does not rule out cystic fibrosis. Turk. J. Pediatr. 2017;59(1):68–70. doi: 10.24953/turkjped.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Raina M.A., Khan M.S., Malik S.A. Assessment of correlation between sweat chloride levels and clinical features of cystic fibrosis patients. J. Clin. Diagn. Res. 2016 Dec;10(12):BC01–BC06. doi: 10.7860/JCDR/2016/21526.8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Awasthi S., Dixit P., Maurya N. Higher sweat chloride levels in patients with asthma: a case-control study. Indian J. Pediatr. 2015 Feb;82(2):114–118. doi: 10.1007/s12098-014-1432-5. [DOI] [PubMed] [Google Scholar]
- 31.Collaco J.M., Blackman S.M., Raraigh K.S. Sources of variation in sweat chloride measurements in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2016 Dec 1;194(11):1375–1382. doi: 10.1164/rccm.201603-0459OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gokdemir Y., Vatansever P., Karadag B., Seyrekel T., Baykan O., Bas Ikızoglu N., Ersu R., Karakoc F., Haklar G. Performance evaluation of a new coulometric endpoint method in sweat testing and its comparison with classic Gibson & Cooke and chloridometer methods in cystic fibrosis. Front. Pediatr. 2018 May 22;6:133. doi: 10.3389/fped.2018.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farrell P.M., Rosenstein B.J., White T.B., Accurso F.J., Castellani C., Cutting G.R. Cystic Fibrosis Foundation. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: cystic Fibrosis Foundation consensus report. J. Pediatr. 2008 Aug;153(2):S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dipasquale V., Corica D., Gramaglia S.M.C., Valenti S., Romano C. Gastrointestinal symptoms in children: primary care and specialist interface. Int. J. Clin. Pract. 2018 Jun;72(6):e13093. doi: 10.1111/ijcp.13093. [DOI] [PubMed] [Google Scholar]
- 35.Abbott J., Morton A.M., Hurley M.A., Conway S.P. Longitudinal impact of demographic and clinical variables on health-related quality of life in cystic fibrosis. BMJ Open. 2015 May 19;5(5):e007418. doi: 10.1136/bmjopen-2014-007418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Callemeyn J., Van Haecke P., Peetermans W.E., Blockmans D. Clubbing and hypertrophic osteoarthropathy: insights in diagnosis, pathophysiology, and clinical significance. Acta Clin. Belg. 2016 Jun;71(3):123–130. doi: 10.1080/17843286.2016.1152672. [DOI] [PubMed] [Google Scholar]
- 37.Van Ginderdeuren F., Van Cauwelaert K., Malfroot A. Influence of digital clubbing on oxygen saturation measurements by pulse-oximetry in cystic fibrosis patients. J. Cyst. Fibros. 2006 May;5(2):125–128. doi: 10.1016/j.jcf.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Cheng K., Ashby D., Smyth R.L. Oral steroids for long-term use in cystic fibrosis. Cochrane Database Syst. Rev. 2015 Dec 9;(12):CD000407. doi: 10.1002/14651858.CD000407.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langan K.M., Kotsimbos T., Peleg A.Y. Managing Pseudomonas aeruginosa respiratory infections in cystic fibrosis. Curr. Opin. Infect. Dis. 2015 Dec;28(6):547–556. doi: 10.1097/QCO.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 40.Mayer-Hamblett N., Ramsey B.W., Kulasekara H.D., Wolter D.J., Houston L.S., Pope C.E. Pseudomonas aeruginosa phenotypes associated with eradication failure in children with cystic fibrosis. Clin. Infect. Dis. 2014 Sep 1;59(5):624–631. doi: 10.1093/cid/ciu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siwamogsatham O., Alvarez J.A., Tangpricha V. Diagnosis and treatment of endocrine comorbidities in patients with cystic fibrosis. Curr. Opin. Endocrinol. Diabetes Obes. 2014 Oct;21(5):422–429. doi: 10.1097/MED.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Préville-Ratelle S., Coriati A., Ménard A., Bourdeau I., Tremblay F., Berthiaume Y. Adrenal insufficiency in cystic fibrosis: a rare phenomenon? Can. Respir. J. 2018 Mar 13;2018:3629031. doi: 10.1155/2018/3629031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christiansen A.L., Nybo M.v. Lack of harmonization in sweat testing for cystic fibrosis- a national survey. Scand. J. Clin. Lab. Invest. 2014 Nov;74(8):708–712. doi: 10.3109/00365513.2014.953992. [DOI] [PubMed] [Google Scholar]
- 44.McKone E.F., Velentgas P., Swenson A.J., Goss C.H. Association of sweat chloride concentration at time of diagnosis and CFTR genotype with mortality and cystic fibrosis phenotype. J. Cyst. Fibros. 2015 Sep;14(5):580–586. doi: 10.1016/j.jcf.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Dominguez-Muñoz J.E. Diagnosis and treatment of pancreatic exocrine insufficiency. Curr. Opin. Gastroenterol. 2018 Jun 7 doi: 10.1097/MOG.0000000000000459. [DOI] [PubMed] [Google Scholar]
- 46.Othman M.O., Harb D., Barkin J.A. Introduction and practical approach to exocrine pancreatic insufficiency for the practicing clinician. Int. J. Clin. Pract. 2018 Feb;72(2) doi: 10.1111/ijcp.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greaves R.F., Jolly L., Massie J., Scott S., Wiley V.C., Metz M.P., Mackay R.J., Australasian Association of Clinical Biochemists Sweat Test Working Party in association with the Royal Australasian College of Pathologists Quality Assurance Programs Laboratory performance of sweat conductivity for the screening of cystic fibrosis. Clin. Chem. Lab. Med. 2018 Mar 28;56(4):554–559. doi: 10.1515/cclm-2017-0530. [DOI] [PubMed] [Google Scholar]
- 48.Massie J., Greaves R., Metz M., Wiley V., Graham P., Shepherd S., Mackay R. Australasian guideline (2nd edition): an annex to the CLSI and UK guidelines for the performance of the sweat test for the diagnosis of cystic fibrosis. Clin. Biochem. Rev. 2017 Nov;38(3):115–130. [PMC free article] [PubMed] [Google Scholar]