Abstract Abstract
The importance of mountains for plant diversity and richness is underestimated, particularly when transition zones between different bioclimates are present along altitudinal gradients. Here we present the first floristic data for a mountain area in the island of Sardinia (Italy), which exhibits Mediterranean bioclimates at the bottom and temperate bioclimate at the top. We discovered a very high floristic richness, despite the fact that the number of endemic taxa is not high and the number of exclusive taxa is very low. Many of the detected taxa are at their range periphery and/or ecological margin. We conclude that climate transition zones in Mediterranean mountains and especially on islands are key areas regarding plant biodiversity and should be better investigated and protected.
Keywords: bioclimate, biodiversity, Mediterranean mountains, submediterranean, temperate
Introduction
Mountains are a critical landscape and ecosystem; they not only provide water for the lowlands but are a source of well-being and inspiration for numerous people (Korner 2004). The green ‘coat’ of the world’s mountains is composed of specialised biota, all nested in a great variety of microhabitats. Mountains biota are determined by a series of climatically different life zones over short elevational distances (Rahbek 1995, Korner 2000, Hemp 2002, Korner and Paulsen 2004), which often result in areas of high biodiversity of high conservation interest (Korner 2004). However, those areas are also under high threat regarding climate change, as it is expected that they experience drastic changes (Inouye 2008).
Mountain biodiversity can be studied at a multitude of scales in space, time and function (Molau 2004). Even though species richness is usually the focal component in nature conservation, genetic diversity within species is equally important. The small-scale distribution of species in the tropical Andes, as exemplified by the plant genera Calceolaria (Calceolariaceae) and Bartsia (Orobanchaceae), contrasts against the situation in high-latitude mountains, e.g. the Scandes, where species have wide ranges and many are circumpolar (Molau 2004). Several studies on alpine plants, based on molecular data, show that the intraspecific genetic diversity tends to increase with latitude, a situation brought about by glaciation cycles permitting repeated contraction-expansion episodes of species’ distributions (Abbott et al. 2000, Abbott and Brochmann 2003, Gamache et al. 2003, Holderegger and Abbott 2003, Lian et al. 2003, Abbott and Comes 2004). In tropical mountains, species distributions are geographically much narrower, often as a result of relatively recent, local speciation (Deshpande et al. 2001, Friar et al. 2001, Tremetsberger et al. 2003a, 2003b, Zhou et al. 2003). Thus, the classical decrease of genetic diversity observed from the equator toward the pole can eventually be blurred for mountain species. Actually, repeated contraction-expansion of species ranges has led rear edge populations to maintain some genetic diversity, therefore counterbalancing the effect of peripheral isolation (Hampe and Petit 2005). Conjointly, the high genetic differentiation between populations underlines the conservation relevance of those populations.
Mediterranean mountains represent an interesting case, because they often have a relic temperate-like bioclimate at their top (with no or little summer drought) in a context characterised by severe water deficit for at least two consecutive months at lower altitudes. Mediterranean mountains can therefore be considered as climatic islands, where plant diversity patterns are influenced by different factors (or in different ways) with respect to temperate and boreal mountains (Winkler et al. 2016). Furthermore, climatic and land-use changes have different effects on Mediterranean vs Boreal-Temperate mountains of Europe, being detrimental for the floral richness of the first and increasing the species richness of the second (Pauli et al. 2012). Considering that expected climatic trend is an increasing of temperature and a decrease of precipitation (mainly during spring) in Mediterranean mountains, whereas non-Mediterranean European mountains will not experience a reduction of annual and spring precipitation (Bravo et al. 2008), the urgency rises to monitor those mountains at the transition between Temperate and Mediterranean bioclimates. Moreover, before the middle of the century, the expected climatic changes will provoke the disappearance or strong reduction of a suitable habitat in the summit area, where most of the endemic and/or rare species are located (Benito et al. 2011). The most endangered habitats and species are those linked to water availability like streams, wet meadows and temporary ponds (Ghosn et al. 2010, Pérez-Luque et al. 2015). On islands, threats to mountain floras are even more acute compared to mainlands, because narrower spatial scales of habitats and the usually lower mountain altitudes (Vogiatzakis et al. 2016), led some species to have a relic distribution (Petit et al. 2005, Mayol et al. 2015, Fazan et al. 2017). Historical climatic fluctuations and associated ecological constraints are the basis of the fragmented distribution of Boreal-Temperate species on Mediterranean mountains (Mayol et al. 2015, Iszkulo et al. 2016) and determine the presence of plant refugia, climatically stable areas that constitute key areas for the long-term persistence of species and genetic diversity, especially at present and future decades given the threat posed by the extensive environmental change processes operating in the Mediterranean region. These refugia, including large Mediterranean islands, represent ‘phylogeographical hotspots’; that is, significant reservoirs of unique genetic diversity favourable to the evolutionary processes of Mediterranean plants (Médail and Diadema 2009).
The island of Sardinia, the second largest in the whole Mediterranean basin, was already known to have a prevalent Mediterranean bioclimate, with a temperate bioclimate in the two main massifs of the island, the Gennargentu (centre-eastern Sardinian, maximum elevation 1834 m a.s.l.) and the Limbara (north-eastern Sardinia, maximum elevation 1359 m a.s.l.) (Arrigoni 1968). Recent detailed bioclimate analysis (Canu et al. 2015) also showed that the only mountain chain of the island named Marghine-Goceano (located between the Limbara and the Gennargentu massifs, maximum elevation at Mt. Rasu 1259 m a.s.l.) is characterised by a temperate bioclimate (in the sub-Mediterranean variant) along the ranges summit. Although the mountain floras of the Gennargentu and Limbara are well documented (Veri and Bruno 1974, Arrigoni and Camarda 2015), floristic information about the Marghine-Goceano range is lacking (Valsecchi and Corrias 1966).
This paper goes some way to fill this knowledge gap by reporting on the indigenous flora of a forest domain located in the middle of the Marghine-Goceano range. Our aim was to provide a checklist of the flora located in this area to enable future characterisation of the biotic environment of this mountain area of Sardinia. This data will also allow the identification of target species to monitor and understand climate changes in the particular context of Mediterranean islands.
Methods
Study area
The forest domain of Anela is a public property since 1886, at present managed by the Sardinian regional agency Forestas (Fig. 1). The domain covers 1280 hectares of which 1200 ha fall in the municipality of Anela, 55 ha in that of Bultei (to the east) and 25 ha in that of Bono (to the west). The lowest altitude is about 600 m a.s.l. in locality Badu Edras whereas the summit point is at Punta Masiedda 1158 m a.s.l. The geographic coordinates of the forestry station headquarter are 40°27'14"N; 9°01'36"E. At present, the vegetation cover is mainly characterised by coppices and mature shrubs linked to sub-Mediterranean woods Glechomosardoae-Quercetumcongestae and Saniculoeuropaeae-Quercetumilicis above 800 m a.s.l. and meso-Mediterranean Loncomelopyrenaici-Quercetumichnusae and Galioscabri-Quercetumilicis below 800 m a.s.l., as described by Bacchetta et al. (2009). The 2004 forest census determined that 46% of this area was occupied by holm oak (Quercusilex L.) woods, 2.7% by deciduous oak (Q.pubescens Willd.) woods, 23.4% by mixed woods of holm oak and deciduous oak, 0.8% by cork oak (Q.suber L.) woods, 2.8% by plantations with alien trees (Abies, Cedrus, Acer, Fagus, Pinus), 14.7% by shrub communities (with Ericaarborea, Crataegusmonogyna, Rubusulmifolius), 6.2% by dwarf communities (with Helichrysummicrophyllumsubsp.tyrrhenicum, Thymusherba-barona, Genistadesoleana), 0.3% by rocky places and the rest by human activities (including buildings, an artificial lake and firebreaks) (Sechi and Falchi 2013). It should be noted that a large fire destroyed 800 hectares of the domain on 31 July 1945, so the wooded area decreased from 72.4% in 1910 to less than 20% in the 50s (Sechi and Falchi 2013).
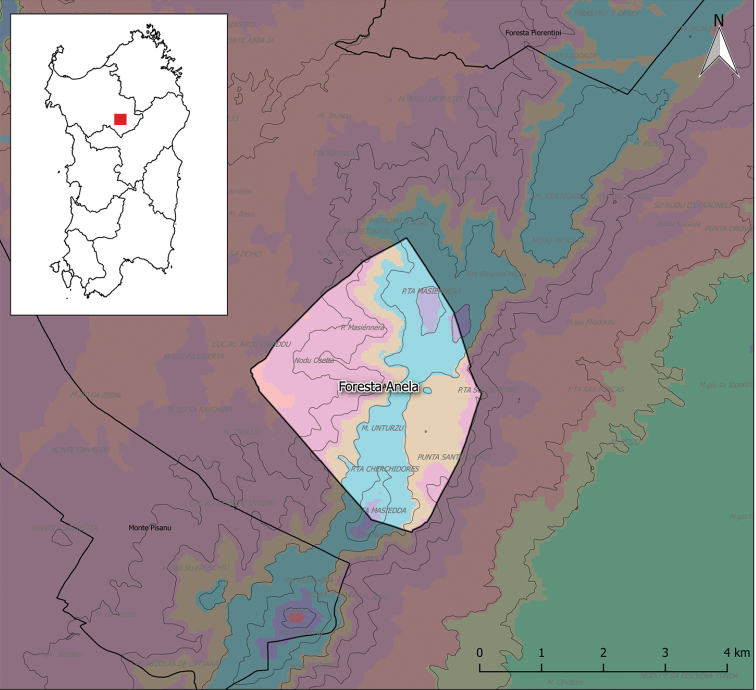
Figure 1.
The study area, Forest of Anela and its location in Sardinia (red rectangle on the inset map). Colours on the map represent different isobioclimates (derived from Canu et al. 2015). In the domain, we can recognise five different isobioclimates: Violet: upper mesotemperate (subMediterranean), lower humid, weak semi-continental; blue: lower supraMediterranean, lower humid, weak semi-continental; orange: upper mesoMediterranean, lower humid, weak semi-continental; lilac: upper mesoMediterranean, upper subhumid, weak semi-continental; pink: upper mesoMediterranean, lower subhumid, weak semi-continental. Thick black lines represent domain limits; thin black lines represent altitude intervals of 100 m.
In the ambit of the Sardinian-Corsican biogeographic province (as defined by Bacchetta et al. 2012), the study area falls in the Goceano-Logudorese sector (Fenu et al. 2014).
The geology of the study area comprises Palaeozoic granites and schists (Madrau 2013). The impermeable nature of these substrates has created a substantial aquifer evident by the presence of 39 springs (half perennial and half seasonal) in the study area (Farris 2013b).
Bioclimate analysis of 1971–2000 data (Canu et al. 2015) showed that 96.9% of the area falls in the Mediterranean Pluviseasonal Oceanic bioclimate, whereas 3.1% in the Temperate Oceanic bioclimate (submediterranean variant). A total of 64.6% of the area is included in the meso-Mediterranean thermotype, 32.3% in the supra-Mediterranean and 3.1% in the meso-Temperate.
Thermo-pluviometric data of the period 1951–1985 showed annual mean temperature of 11.2 °C and annual mean rainfall of 1040 mm; after the year 2000 temperatures did not vary significantly, whereas a reduction of ca. 30% in the annual rainfall was recorded. Late spring and summer rainfall (May-August) decreased even more (more than 50%, see Farris 2013a).
The study area is entirely included in the Natura 2000 site of community importance ITB 011102 ‘‘Catena del Marghine e Goceano’’, extended on 14,984 ha and is also nominated as a Protection Oasis for wildlife “Foresta Anela”, managed by the Province of Sassari.
Floristic research
Floristic research started in the year 2000 and was intensified in the years 2012–17 with regular monthly sampling. Each month, we made one day excursions, which covered three altitudinal ranges (< 800 m a.s.l.; 800–1000 m a.s.l.; > 1000 m a.s.l. on the third). For each excursion, we tried to visit as many habitats as possible in order to capture the highest environmental heterogeneity. Collected plants were stored at the Herbarium SS, where we also searched for specimens collected in previous decades (if present, they are reported in the floristic list).
Plant names were derived from the Euro+Med PlantBase (Euro+Med 2006–2018), except for: a) families not already included in this database for which we referred to the Checklists of Italian Flora (Conti et al. 2005; Bartolucci et al. 2018), APG IV (APG 2016); b) the family Orchidaceae (for which we follow GIROS (2016)); c) the genus Orobanche, for which we follow Domina and Arrigoni (2007); d) the genus Dianthus, for which Bacchetta et al. (2010) is followed; e) and the species Struthiopterisspicant which we use in preference to Blechnumspicant (Gasper et al. 2016); f) for endemics, we also consulted Arrigoni et al. (1976–1991) and Peruzzi et al. (2014). The Italian floras (Pignatti 1982, 2017–2018) and the Sardinian flora (Arrigoni 2006–2015) were also consulted. When other relevant literature was followed, it is specified in the text.
Plant authorities and names were further verified using ‘The Plant List’, ‘The World Checklist of Selected Plant Families’ and ‘The International Plant Names Index’ (IPNI). Herbarium acronyms follow Thiers (2018).
The taxonomic circumscription of orders and families, as well as their sequence in the list was derived from Smith et al. (2006) for Pteridophytes; and APG III (APG 2009), APG IV (APG 2016) and Haston et al. (2009) for Angiosperms. Within each family, genera, species and subspecies are listed in alphabetical order. Species and subspecies are numbered progressively.
For each taxon we report:
Progressive number Scientific name (with authority) Biological type, Chorologic type
Abundance (locality(ies) of collection is(are) specified only for uncommon or range restricted taxa): Habitat
Notes (eventual)
Biological types are in accordance to Raunkiær (1934) and were verified on the collected samples and also in Pignatti (1982, 2017–2018); chorologic types were determined following maps reported in the Euro+Med PlantBase (Euro+Med 2006–2018) and again verified in Pignatti (1982, 2017–2018) and the other bibliographic sources reported in the text.
Geographical abbreviations are:
Atl. Atlantic;
Cauc. Caucasian;
Circumbor. circum-boreal;
Cosmop. cosmopolitan;
Endem. endemic;
Euras. Eurasian;
Eurimedit. euri-Mediterranean;
Europ. European;
Eurosib. Euro-Siberian;
It Italy;
Itc central Italy;
Its northern Italy;
Macaron. Macaronesian;
Medit. Mediterranean;
Medit. Mont. Mediterranean montane;
S. Europ. Orof. Southern European Orophylous;
Paleotemp. paleo-temperate;
Paleotrop. paleo-Tropical;
Sib. Siberian;
Stenomedit. Steno-Mediterranean;
Subatl. sub-Atlantic;
Subcosmop. sub-cosmopolitan;
Submedit. sub-Mediterranean;
Subtrop. sub-Tropical;
Turan. Turanian.
Here we consider as endemics sensu stricto all taxa limited to the Corsican-Sardinian biogeographic province (sensuBacchetta et al. 2012), therefore including the Tuscan Archipelago. Other taxa are considered endemic sensu lato, which includes those present in western Mediterranean islands and continental areas – Calabria in Europe, Kabylies in Africa – as far as the Miocene part of the Hercynian chain (Hercynian endemics sensuMansion et al. 2008). Finally, other endemics sensu lato are ‘administrative endemics’, i.e. taxa confined within Italian national borders (Peruzzi et al. 2014). For endemics, geographic abbreviations are as follows:
Ag Algeria;
AT Tuscan Archipelago;
Bl Balearic Islands;
Co Corsica;
Hy Hyères islands;
Sa Sardinia;
Si Sicily.
Abundance is expressed on the basis of the following criteria:
RR range restricted: taxa present in only one locality within the study area or covering a surface not exceeding 1 hectare, i.e. Mentharequieniisubsp.requienii;
U uncommon: taxa found in 2–5 localities within the study area, or covering a surface not exceeding 1 km2, i.e. Arisarumvulgare;
L localised: taxa present in 6 or more localities within the study area, but covering less than 2.5 km2, i.e. Agrostiscapillaris;
C common: taxa covering more than 2.5 km2, i.e. Quercusilex.
Results
Floristic list
Lycopodiopsida
Isoetales
Isoetaceae
1 Isoeteshistrix Bory G bulb, Stenomedit.-Atl.
U (Zuanne Cane Malu, near Mt. Masiennera): Temporary ponds
Selaginellales
Selaginellaceae
2 Selaginelladenticulata (L.) Spring Ch rept, Stenomedit.
C: Woods, wet cliffs
Polypodiopsida
Osmundales
Osmundaceae
3 Osmundaregalis L. G rhiz, Subcosmop.
L: Alnusglutinosa woods, streams
Polypodiales
Dennstaedtiaceae
4 Pteridiumaquilinum(L.)Kuhnsubsp.aquilinum G rhiz, Cosmop.
C: Woods, meadows, fringes, garrigues, shrublands
Pteridaceae
5 Anogrammaleptophylla (L.) Link T caesp, Cosmop.
L: Shady rocks and cliffs
Aspleniaceae
6 Aspleniumadiantum-nigrum L. H ros, Paleotemp.
C: Shady rocks and cliffs, sometimes woods
Notes: since the taxon has been excluded from the Sardinian flora by Marchetti (2004), Arrigoni (2006–2015) and Bartolucci et al. (2018), here we consider it as new for the Sardinian flora.
7 Aspleniumonopteris L. H ros, Subtrop.
C: Woods, sometimes cliffs
8 AspleniumceterachL.subsp.ceterach H ros, Euras.
L: Walls
9 Aspleniumforeziense Magnier H ros, NW-Medit.-Mont.
U (Badu Edras): Shady rocks and cliffs
Notes: the taxon has been excluded from the Sardinian flora by Marchetti (2004) and Bartolucci et al. (2018), but confirmed by Arrigoni (2006–2015).
10 AspleniumobovatumViv.subsp.obovatum H ros, Stenomedit.
U (Mt. Masiennera): Crevices at the top of the mountain
11 Aspleniumtrichomanessubsp.quadrivalens D.E. Mey. H ros, Cosmop.
C: Shady rocks and cliffs
Woodsiaceae
12 Athyriumfilix-femina (L.) Roth H Ros, Subcosmop.
L: Wet places, mainly Alnusglutinosa woods
Blechnaceae
13 Struthiopterisspicant (L.) F.W.Weiss H ros, Circumbor.
RR (Few individuals in a wet wood near Sos Sauccheddos spring): Alnusglutinosa wood
Dryopteridaceae
14 Polystichumsetiferum (Forssk.) Woyn. G rhiz, Circumbor.
C: Woods
Polypodiaceae
15 PolypodiumcambricumL.subsp.cambricum H ros, Eurimedit.
C: Rocks, big trees
16 Polypodiuminterjectum Shivas H ros, Paleotrop.
U (Bidighinzos): Shady rocks
Magnoliopsida
Alismatales
Araceae
17 Arisarumvulgare O. Targ. Tozz. G rhiz, Stenomedit.
U (Bonu Trau, Badde Cherchi, Badu Edras): Woods and shrubland (lower altutides)
18 ArumitalicumMill.subsp.italicum G rhiz, Stenomedit.
L: Fringes
19 Arumpictum L. f. G rhiz, Endem. Sa-Co-AT-Bl
RR (Su Pizzu Sa Pedra): at the base of a cliff
Notes: this taxon is not considered as an Italian endemic by Peruzzi et al. (2014)
20 Lemnagibba L. I nat, Subcosmop.
L: Wet places, standing water
21 Lemnaminor L. I nat, Subcosmop.
RR (Su Francallossu spring): standing water
Dioscoreales
Dioscoreaceae
22 Dioscoreacommunis (L.) Caddick & Wilkin G rad, Eurimedit.
C: Woods
Liliales
Colchicaceae
23 Colchicumnanum K. Perss. G bulb, Endem. Sa-Co
L: Wet pastures and meadows
Smilacaceae
24 Smilaxaspera L. NP, Subtrop.
C: Woods
Liliaceae
25 Gageabohemica (Zauschn.) Schult. & Schult.f. G bulb, Eurimedit.
C: Pastures
Asparagales
Orchidaceae
26 Anacamptislaxiflora (Lam.) R. M. Bateman, Pridgeon & M. W. Chase G bulb, Eurimedit.
L: Wet meadows
Specimen examined (syn. Orchislaxiflora Lam.): Funtana Arile, Anela, 08 June 1980, B. Corrias, S. Diana (SS)
27 Anacamptislongicornu (Poir.) R. M. Bateman, Pridgeon & M. W. Chase G bulb, W-Stenomedit.
Not found in the field during this research
Specimen examined (syn. Orchislongicornu Poir.): S’Isfundadu, Anela, 13 May 1965, B. Corrias (SS)
28 Anacamptispapilionacea (L.) R. M. Bateman, Pridgeon & M. W. Chase G bulb, Eurimedit.
C: Dry grasslands
Specimen examined (syn. Orchispapilionacea L.): Funtana Arile, Anela, 08 June 1980, B. Corrias, S. Diana (SS)
29 Dactylorhizainsularis (Sommier) Landwehr G bulb, W-Stenomedit.
Not found in the field during this research
Specimen examined (syn. D.sambucina (L.) Soó): S’Isfundadu, Anela, 13 May 1965, B. Corrias (SS)
30 Limodorumabortivum (L.) Sw. G rhiz, Eurimedit.
U (Littu Majore and Minda ‘e Bassu - Minda ‘e Supra): Quercusilex woods
31 Orchisprovincialis Balb. ex Lam. & DC. G bulb, Stenomedit.
L: Clearings, fringes
Specimens examined: S’Isfundadu, Anela, 13 May 1965, B. Corrias (2 specimens, SS)
32 Serapiaslingua L. G bulb, Stenomedit.
L: Wet meadows
Specimen examined: Funtana Arile, Anela, 08 June 1980, B. Corrias, S. Diana (SS)
33 Spiranthesspiralis (L.) Chevall. G rhiz, Europ.-Cauc.
U (Funtana Arile): Wet meadows
Iridaceae
34 Crocusminimus DC. G bulb, Endem. Sa-Co
C: Pastures
35 Irispseudacorus L. G rhiz, Euras.
U (Su Pranu): Flooded meadows, ponds
36 RomuleacolumnaeSebast. & Maurisubsp.columnae G bulb, Stenomedit.
C: Pastures
37 Romulearequienii Parl. G bulb, Endem. Sa-Co
C: Pastures
Asphodelaceae
38 AsphodelusramosusL.subsp.ramosus G rhiz, Stenomedit.
C: Perennial grasslands, pastures, garrigues
Amaryllidaceae
39 AlliumchamaemolyL.subsp.chamaemoly G bulb, W-Stenomedit.
L: Annual grasslands (lower altitudes)
40 Alliumguttatumsubsp.sardoum (Moris) Stearn G bulb, Stenomedit.
C: Pastures, meadows
41 Alliumparciflorum Viv. G bulb, Endem. Sa-Co
L: Garrigues, rocky habitats
42 Alliumsubhirsutum L. G bulb, W-Stenomedit.
C: Perennial grasslands
43 Alliumtriquetrum L. G bulb, W-Stenomedit.
C: Fringes, woods
44 Alliumvineale L. G bulb, Eurimedit.
L: Perennial grasslands
45 Leucojumaestivumsubsp.pulchellum (Salisb.) Briq. G bulb, Endem. Sa-Co-Bl
L: Wet meadows
Notes: This taxon is reported also in the Var (Southern France) (see: Tison and de Foucault 2014, Arrigoni 2006–2015; Pignatti 2017–2018) whereas the Euro+Med Plantbase considers it exclusive only in Sardinia, Corsica and the Balearic Islands.
46 Pancratiumillyricum L. G bulb, Endem. Sa-Co-AT
L: Garrigues
Asparagaceae
47 Asparagusacutifolius L. G rhiz, Stenomedit.
L: Woods and shrubland (lower altitudes)
48 Drimiapancration (Steinh.) J. C. Manning & Goldblatt G bulb, W-Stenomedit.
L: Grasslands
49 Leopoldiacomosa (L.) Parl. G bulb, Eurimedit.
C: Grasslands, pastures
50 Ornithogalumcorsicum Jord. & Fourr. G bulb, Endem. Sa-Co
C: Pastures
51 Ornithogalumpyrenaicum L. G bulb, Eurimedit.
C: Deciduous woods
52 Prosperoautumnale (L.) Speta G bulb, Eurimedit.
C: Grasslands, pastures
53 Ruscusaculeatus L. G rhiz, Eurimedit.
C: Woods
Poales
Typhaceae
54 Typhaangustifolia L. G rhiz, Circumbor.
L: Artificial lake, flooded areas, streams
Juncaceae
55 Juncusarticulatus L. G rhiz, Circumbor.
C: Wet meadows, temporary ponds
56 Juncusbufonius L. T caesp, Cosmop.
C: Temporary ponds, wet soils
57 Juncuscapitatus Weigel T scap, Medit.-Atl.
C: Temporary ponds
58 JuncuseffususL.subsp.effusus H caesp, Cosmop.
C: Wet meadows, temporary ponds
59 Juncushybridus Brot. T caesp, Medit.-Atl.
C: Temporary ponds
60 Luzulaforsteri (Sm.) DC. H caesp, Eurimedit.
C: Woods
Cyperaceae
61 Carexcaryophyllea Latourr. H scap, Euras.
C: Wet pastures and meadows
62 Carexdistachya Desf. H caesp, Stenomedit.
C: Woods
63 Carexdivisa Huds. G rhiz, Medit.-Atl.
C: Wet meadows and pastures, temporary ponds, ditches
64 Carexdivulsa Stockes H caesp, Eurimedit.
C: Fringes
65 Carexmicrocarpa Moris He, Endem. Sa-Co-AT-Itc
L: Alnusglutinosa woods, riparian vegetation
66 Carexremota L. H caesp, Europ.-Cauc.
U (Badu Addes): Alnusglutinosa wood
67 Cyperuslongus L. G rhiz, Paleotemp.
C: Wet meadows, riparian vegetation
Notes: some authors exclude the presence of this species from Sardinia (Desfayes 2004, Arrigoni 2006–2015, Bartolucci et al. 2018) and consider the presence of Cyperusbadius Desf. instead. In the Euro+Med Plantbase, C.badius is considered a heterotypic synonym of C.longus.
68 Eleocharispalustris(L.)Roem. & Schult.subsp.palustris G rhiz, Subcosmop.
L: Wet meadows
Gramineae (nom. altr.Poaceae)
69 Aegilopsgeniculata Roth T scap, Stenomedit.-Turan.
L: Annual grasslands
70 Agrostiscapillaris L. H caesp, Circumbor.
L: Wet pastures and meadows
Notes: this taxon is new for the Sardinian flora following Pignatti (1982), Conti et al. (2005), Arrigoni (2006–2015), Pignatti (2017–2018), Bartolucci et al. (2018) and the Euro+Med PlantBase.
71 AiracaryophylleaL.subsp.caryophyllea T scap, Subtrop.
C: Annual grasslands
72 AlopecurusbulbosusGouansubsp.bulbosus H caesp, Eurimedit.-Subatl.
L: Wet pastures and meadows
73 Anisanthadiandra (Roth) Tutin T scap, Eurimedit.
C: Annual grasslands
74 Anisanthamadritensis(L.)Nevskisubsp.madritensis T scap, Eurimedit.
C: Annual grasslands, pastures
75 Anthoxanthumodoratum L. H caesp, Euras.
C: Wet pastures and meadows
76 Arrhenatherumelatiussubsp.sardoum (Em. Schmid) Gamisans H caesp, W-Stenomedit.
L: Garrigues, rocky habitats (higher altitudes)
77 AvenabarbataLinksubsp.barbata T scap, Eurimedit.
C: Annual grasslands
78 Brachypodiumretusum (Pers.) P. Beauv. H caesp, W-Stenomedit.
C: Perennial grasslands on rocky or stony soils
79 Brachypodiumsylvaticum(Huds.)P. Beauv.subsp.sylvaticum H caesp, Paleotemp.
C: Woods, fringes
80 Brizamaxima L. T scap, Subtrop.
C: Annual grasslands, pastures
81 Brizaminor L. T scap, Subcosmop.
U (near Mt. Masiennera): Wet pastures and meadows
82 BromushordeaceusL.subsp.hordeaceus T scap, Subcosmop.
C: Annual grasslands, pastures
83 Bromusscoparius L. T scap, Stenomedit.
U (Top of Mt. Masiennera): Annual grasslands
84 Catabrosaaquatica (L.) P. Beauv. G rhiz, Circumbor.
L: Wet soils
85 Cynodondactylon (L.) Pers. G rhiz, Cosmop.
C: Wet pastures and meadows
86 Cynosuruscristatus L. H caesp, Europ.-Cauc.
C: Wet pastures and meadows
87 Cynosurusechinatus L. T scap, Eurimedit.
C: Annual grasslands, fringes
88 Cynosuruseffusus Link T scap, Stenomedit.
C: Annual grasslands, fringes
89 Dactylisglomeratasubsp.hispanica (Roth) Nyman H caesp, Stenomedit.
C: Perennial grasslands
90 Danthoniadecumbens(L.)DC.subsp.decumbens H caesp, Europ.
L: Wet pastures and meadows
91 Dasypyrumvillosum (L.) P. Candargy T Scap, Eurimedit.-Turan.
L: Annual grasslands
92 FestucamorisianaParl.subsp.morisiana H caesp, Endem. Sa
L: Wet meadows and pastures
93 Glycerianotata Chevall. G rhiz, Subcosmop.
L: Wet habitats
94 HolcuslanatusL.subsp.lanatus H caesp, Circumbor.
C: Wet meadows
95 Hordeumgeniculatum All. T scap, Stenomedit.
C: Wet meadows and pastures, temporary ponds
96 LagurusovatusL.subsp.ovatus T scap, Eurimedit.
C: Annual grasslands, pastures
97 LoliumperenneL.subsp.perenne H caesp, Euras.
C: Wet pastures
98 LoliumrigidumGaudinsubsp.rigidum T scap, Subtrop.
C: Pastures on arid soil
99 MelicaciliataL.subsp.ciliata H caesp, Eurimedit.
U (Mt. Masiennera): Rocky habitats
100 Melicaminuta L. H caesp, Stenomedit.
C: Fringes
101 Melicauniflora Retz. H caesp, Paleotemp.
L: Deciduous woods, fringes
102 Neoschischkiniapourrettii (Willd.) Valdés & H. Scholz T scap, W-Stenomedit.
L: Temporary ponds
103 Piptatherummiliaceum(L.)Coss.subsp.miliaceum H caesp, Stenomedit.
L: Road edges (lower altitudes)
104 PoaannuaL.subsp.annua T caesp, Cosmop.
C: Annual grasslands, pastures
105 Poabalbisii Parl. H caesp, Endem. Sa-Co
U (Mt. Masiennera): Garrigues, rocky habitats
106 PoabulbosaL.subsp.bulbosa H caesp, Paleotemp.
C: Pastures
107 Poainfirma Kunth T caesp, Eurimedit.
C: Mud, wet soils
108 PoanemoralisL.subsp.nemoralis H caesp, Circumbor.
C: Woods
109 PoatrivialisL.subsp.trivialis H caesp, Euras.
C: Wet meadows
110 Vulpialigustica (All.) Link T caesp, Stenomedit.
C: Pastures
111 Vulpiamyuros(L.)C. C. Gmel.subsp.myuros T caesp, Subcosmop.
C: Pastures
112 Vulpiasicula (C. Presl) Link H caesp, W-Medit.-Mont.
C: Pastures, grasslands
Ranunculales
Papaveraceae
113 Fumariabastardii Boreau T scap, Subatl.
C: Annual grasslands, fringes
114 FumariaofficinalisL.subsp.officinalis T scap, Paleotemp.
C: Annual grasslands, fringes
115 PapaverrhoeasL.subsp.rhoeas T scap, E-Medit.
C: Pastures, grasslands
Ranunculaceae
116 AnemonehortensisL.subsp.hortensis G bulb, N-Medit.
RR (Su Tattharesu): Perennial grasslands
117 Clematisvitalba L. P lian, Europ.-Cauc.
C: Woods, mantles
118 FicariavernaHuds.subsp.verna. G bulb, Euras.
C: Woods
119 Ranunculusbulbosussubsp.aleae (Willk.) Rouy & Foucaud H scap, Euras.
C: Grasslands, fringes, woods
120 RanunculusbullatusL.subsp.bullatus H ros, Stenomedit.
C: Annual grasslands
121 RanunculuscordigerViv.subsp.cordiger H scap, Endem. Sa-Co
L: Wet meadows, temporary ponds
122 Ranunculusmacrophyllus Desf. H scap, SW-Medit.
L: Wet meadows
123 Ranunculusmuricatus L. T scap, Eurimedit.
C: Mud, wet meadows
124 Ranunculusophioglossifolius Vill. T scap, Eurimedit.
L: Mud, temporary ponds
125 RanunculuspaludosusPoir.subsp.paludosus H scap, Stenomedit.
C: Pastures
126 Ranunculussardous Crantz T scap, Eurimedit.
C: Mud, temporary ponds
Saxifragales
Paeoniaceae
127 Paeoniacorsica Tausch G rhiz, Endem. Sa-Co
L: Woods, clearings
Saxifragaceae
128 Saxifragatridactylites L. T scap, Eurimedit.
L: Annual grasslands
Crassulaceae
129 Sedumcaeruleum L. T scap, SW-Medit.
C: Rocky habitats, annual grasslands
130 Sedumcepaea L. T scap, Submedit.-Subatl.
C: Rocky habitats, annual grasslands
131 Sedumrubens L. T scap, Eurimedit.-Subatl.
C: Rocky habitats, annual grasslands
132 Sedumstellatum L. T scap, Stenomedit.
C: Rocky habitats, annual grasslands
133 Sedumvillosumsubsp.glandulosum (Moris) P. Fourn. H scap, Endem. Sa-Ag
C: Rocky habitats, annual grasslands
134 Umbilicusrupestris (Salisb.) Dandy subsp. rupestris G bulb, Medit.-Atl.
C: Rocky habitats
Fabales
Leguminosae (nom. altr.Fabaceae)
135 Cytisusvillosus Pourr. P caesp, W-Stenomedit.
C: Shrubland, mantles
136 Dorycniumrectum (L.) Ser. H scap, Stenomedit.
L: Wet habitats
137 Genistacorsica (Loisel.) DC. NP, Endem. Sa-Co
L: Garrigues on rocky soils
138 Genistadesoleana Vals. NP, Endem. Sa-Co-Its
C: Garrigues, dwarf shrubs
Specimens examined: Punta Chelchidores, Anela, 18 July 1972, F. Valsecchi (3 specimens, SS)
139 Lathyrusaphaca L. T scap, Eurimedit.
C: Pastures, fringes
140 Lathyrussphaericus Retz. T Scap, Eurimedit.
L: Pastures
141 Lotusalpinus (DC.) Ramond H scap, Orof. S-Europ.
C: Wet pastures and meadows
142 Lotusangustissimus L. T scap, Eurimedit.
L: Temporary ponds
143 Lotusconimbricensis Brot. T scap, W- Stenomedit.
C: Annual grasslands
144 Lotushispidus DC. T scap, W-Medit.
C: Annual grasslands
145 LupinusangustifoliusL.subsp.angustifolius T scap, Stenomedit.
C: Annual grasslands
146 Medicagopolymorpha L. T scap, Eurimedit.
C: Pastures, annual grasslands
147 OnonisspinosaL.subsp.spinosa Ch suffr, Eurimedit.
C: Grasslands, pastures
148 Ornithopuscompressus L. T scap, Eurimedit.
C: Annual grasslands
149 Ornithopuspinnatus (Mill.) Druce T Scap, Medit.-Atl.
L: Pastures
150 Trifoliumangustifolium L. T scap, Eurimedit.
C: Annual grasslands
151 Trifoliumarvense L. T scap, Paleotemp.
C: Pastures
152 Trifoliumcampestre Schreb. T scap, Paleotemp.
C: Annual grasslands
153 Trifoliumglomeratum L. T Scap, Eurimedit.
L: Pastures
154 Trifoliumincarnatumsubsp.molinerii (Hornem.) Syme T scap, Eurimedit.
C: Grasslands, pastures
155 Trifoliummicranthum Viv. T scap, Paleotemp.
C: Annual grasslands
156 TrifoliumnigrescensViv.subsp.nigrescens T scap, N-Medit.
C: Pastures
157 Trifoliumpratense L. H scap, Eurosib.
C: Wet meadows and pastures
158 Trifoliumrepenssubsp.prostratum Nyman H rept, Eurimedit.
C: Wet meadows and pastures
159 Trifoliumspumosum L. T scap, Stenomedit.
C: Annual grasslands
160 Trifoliumsquarrosum L. T scap, Eurimedit.
L: Pastures
161 Trifoliumstellatum L. T scap, Eurimedit.
C: Annual grasslands, pastures
162 Trifoliumsubterraneumsubsp.yanninicum Katzn. & F. H. W. Morley T rept, E-Medit.
C: Pastures
163 Trifoliumtomentosum L. T rept, Paleotemp.
C: Annual grasslands, pastures
164 ViciacraccaL.subsp.cracca H scap, Euras.
C: Fringes
165 Vicialathyroides L. T scap, Eurimedit.
C: Fringes
166 VicialuteaL.subsp.lutea T scap, Eurimedit.
C: Fringes
167 Viciavillosasubsp.ambigua (Guss.) Kerguélen H Scap, W-Stenomedit.
L: Fringes
168 ViciavillosaRothsubsp.villosa T scap, Eurimedit.
C: Fringes
Rosales
Rosaceae
169 AgrimoniaeupatoriaL.subsp.eupatoria H scap, Subcosmop.
C: Fringes
170 Crataegusmonogyna Jacq. P caesp, Paleotemp.
C: Shrublands, woods, mantles
171 FragariavescaL.subsp.vesca H rept, Eurosib.
C: Deciduous woods, fringes
172 Geumurbanum L. H scap, Circumbor.
C: Deciduous woods, fringes
Specimen examined: Caserma Forestale Anela, sine die, Barba (SS)
173 Maluspumila Mill. P scap, CW-Euras.
L: Woods, mantles
Notes: in accordance with Bagella and Urbani (2006), this is the valid name for Malusdomestica Borkh. (nom. illeg.), also reported in the Euro+Med PlantBase. Yet Galasso et al. (2018) call a taxon Malusdomestica, considering it as a non-native species, while Camarda and Valsecchi (2008), Arrigoni (2006–2015) and Pignatti (2017–2018) still call it M.dasyphylla. Finally, Bartolucci et al. (2018) report the taxon M.sylvestris in Sardinia. Maluspumila is reported as a synonym of M.domestica by Galasso et al. (2018), it is excluded from the Sardinian flora by Arrigoni (2006–2015), finally, it was not mentioned by Camarda and Valsecchi (2008). In the Euro+Med Plantbase, Maluspumila Mill. is the valid name for Malusdomestica Borkh. The populations we have examined in the Marghine-Goceano range (not only the forest domain of Anela) have the characters of Malusdomestica, not M.sylvestris.
174 Potentillareptans L. H ros, Paleotemp.
C: Wet meadows
175 Prunusavium (L.) L. P scap, Pontic
L: Woods
176 Prunusdomesticasubsp.insititia (L.) Bonnier & Layens P scap
U (Su Cantareddu): Mantles
177 PrunusspinosaL.subsp.spinosa P caesp, Europ.-Cauc.
C: Shrublands
178 Pyruscommunissubsp.pyraster (L.) Ehrh. P scap, Euras.
L: Woods, mantles
179 Pyrusspinosa Forssk. P caesp, Stenomedit.
C: Shrublands, mantles, woods
180 Rosacanina L. NP, Paleotemp.
C: Shrublands
181 Rosasempervirens L. NP, Stenomedit.
L: Woods, shrublands (lower altitudes)
182 Rosasubcanina (Christ) Vuk. NP, Europ.
C: Shrublands
183 Rubusulmifolius Schott NP, Eurimedit.
C: Shrublands, woods
184 Sanguisorbaminorsubsp.balearica (Bourg. ex Nyman) Muñoz Garm. & C. Navarro H scap, Eurimedit.
C: Grasslands
Ulmaceae
185 UlmusminorMill.subsp.minor P caesp, Europ.-Cauc.
L: Woods
Cannabaceae
186 CeltisaustralisL.subsp.australis P scap, Eurimedit.
RR (Pedru Addes): Wood edge
Moraceae
187 FicuscaricaL.subsp.carica P scap, Medit.-Turan.
U (Badu Edras): Riparian vegetation
Urticaceae
188 ParietarialusitanicaL.subsp.lusitanica T rept, Stenomedit.
C: Buildings, fringes
189 Urticaatrovirens Loisel. H scap, Endem. Sa-Co-Bl-AT-Itc
L: Ruderal vegetation
190 UrticadioicaL.subsp.dioica H scap, Subcosmop.
C: Ruderal vegetation
Fagales
Fagaceae
191 Quercusilex L. P scap, Stenomedit.
C: Woods
192 Quercuspubescens Willd. agg. P caesp, SE-Europ.
C: Woods
Notes: There are many controversial treatments for describing the variation within Q.pubescens (Mossa et al. 1998, 1999). Until the various treatments are resolved, we prefer to treat this variation as a complex (or aggregate) within Q.pubescens s.l.
193 Quercussuber L. P scap, W-Eurimedit.
L: Woods
Betulaceae
194 Alnusglutinosa(L.)Gaertn.subsp.glutinosa P scap, Paleotemp.
L: Streams, wet places, springs
Oxalidales
Oxalidaceae
195 OxaliscorniculataL.subsp.corniculata H rept, Eurimedit.
L: Walls, buildings
Malpighiales
Guttiferae (nom. altr.Clusiaceae)
196 Hypericumandrosaemum L. NP, W-Eurimedit.-Subatl.
L: Wet habitats, springs
197 HypericumhircinumL.subsp.hircinum NP, Endem. Sa-Co-AT
L: Springs, streams, Alnusglutinosa woods
Notes: H.hircinum includes several subspecies, amongst which the subsp. hircinum is exclusive of Sardinia, Corsica and the Tuscan Archipelago (Carta and Peruzzi 2015)
198 HypericumperforatumL.subsp.perforatum H scap, Paleotemp.
C: Fringes, road edges
Violaceae
199 Violaalbasubsp.dehnhardtii (Ten.) W. Becker H ros, Eurimedit.
C: Woods, fringes
200 Violareichenbachiana Jord. ex Boreau H scap, Eurosib.
C: Deciduous woods
Notes: it was excluded for the Sardinian flora by Arrigoni (2006–2015), but later confirmed by Mereu (2012) for the Gennargentu massif
Salicaceae
201 Salixcinereasubsp.oleifolia Macreight P caesp, W-Medit.-Atl.
L: Streams, springs
202 Salixpurpurea L. P scap, Euras.
L: Ditches
Euphorbiaceae
203 EuphorbiacharaciasL.subsp.characias NP, Stenomedit.
C: Woods, shrublands (lower altitudes)
204 EuphorbiahelioscopiaL.subsp.helioscopia T scap, Cosmop.
C: Annual grasslands
205 Euphorbiapithyusasubsp.cupanii (Guss. ex Bertol.) Radcl.-Sm. G rhiz, Endem. Sa-Co-Si
C: Perennial grasslands, pastures
206 Euphorbiasemiperfoliata Viv. G rhiz, Endem. Sa-Co
L: Woods, fringes
Linaceae
207 Linumbienne Mill. H bienn, Eurimedit.
C: Annual grasslands
Geraniales
Geraniaceae
208 Erodiumchium (L.) Willd. T scap, Eurimedit.
L: Pastures
209 Erodiumciconium (L.) L’Hér. T scap, Eurimedit.-Pontic
C: Pastures
210 Erodiumcicutarium (L.) L’Hér. T scap, Subcosmop.
C: Pastures
211 Geraniumpurpureum Vill. T scap, Eurimedit.
C: Woods, fringes
212 Geraniumrobertianum L. T scap, Subcosmop.
C: Woods, fringes
213 Geraniumrotundifolium L. T scap, Paleotemp.
C: Woods, fringes
Myrtales
Lythraceae
214 Lythrumportula (L.) D. A. Webb T rept, S-Europ.-S-Sib.
L: Temporary ponds
Onagraceae
215 Epilobiummontanum L. H scap, Euras.
C: Woods
Sapindales
Sapindaceae
216 AcermonspessulanumL.subsp.monspessulanum P caesp, Eurimedit.
L: Woods and mantles
Malvales
Malvaceae
217 Althaeahirsuta L. T scap, Eurimedit.
L: Annual grasslands
218 Malvaolbia (L.) Alef. P caesp, Stenomedit.
C: Shrublands on wet soils
219 Malvasylvestris L. H scap, Eurosib.
C: Grasslands, fringes
Cistaceae
220 Cistusmonspeliensis L. NP, Stenomedit.
C: Garrigues (lower altitudes)
221 Cistussalviifolius L. NP, Stenomedit.
C: Garrigues
222 Tuberariaguttata (L.) Fourr. T scap, Eurimedit.
C: Annual grasslands
Brassicales
Resedaceae
223 Sesamoidespurpurascenssubsp.spathulata (Moris) Lambinon & Kerguélen H Scap, W-Medit.-Mont.
C: Dirty tracks, trampled places
Cruciferae (nom. altr.Brassicaceae)
224 Arabidopsisthaliana (L.) Heynh. T scap, Paleotemp.
C: Annual grasslands, pastures
225 Capsellabursapastoris(L.)Medik.subsp.bursa-pastoris H bienn, Cosmop.
C: Annual grasslands, pastures
226 Cardamineflexuosa With. H scap, Circumbor.
C: Fringes
227 Cardaminehirsuta L. T scap, Cosmop.
C: Fringes
228 Drabamuralis L. T scap, Circumbor.
L: Cliffs, road edges
229 Erophilavernasubsp.praecox (Steven) Walters T scap, Eurimedit.
C: Annual grasslands
230 Morisiamonanthos (Viv.) Asch. H ros, Endem. Sa-Co
U (Near Mt. Masiennera): Wet meadows
231 Nasturtiumofficinale (L.) R. Br. H scap, Cosmop.
L: Muds, streams
232 RaphanusraphanistrumL.subsp.raphanistrum T scap, Eurimedit.
C: Grasslands
233 Sisymbriumofficinale (L.) Scop. T scap, Paleotemp.
C: Pastures
234 Teesdaliacoronopifolia (J.P. Bergeret) Thell. T scap, Eurimedit.
C: Pastures
Santalales
Santalaceae
235 Osyrisalba L. NP, Eurimedit.
L: Woods, clearings, rocky habitats
Caryophyllales
Plumbaginaceae
236 ArmeriasardoaSpreng.subsp.sardoa Ch suffr, Endem. Sa
L: Garrigues, rocky habitats
Polygonaceae
237 RumexbucephalophorusL.subsp.bucephalophorus T scap, Eurimedit.-Macaron.
C: Annual grasslands
238 Rumexcrispus L. H scap, Subcosmop.
C: Wet meadows
239 RumexpulcherL.subsp.pulcher H scap, Eurimedit.
C: Wet meadows
240 Rumexscutatussubsp.glaucescens (Guss.) Brullo, Scelsi & Spamp. H scap, Endem. Sa-Si
L: Rocky habitats
241 Rumexthyrsoides Desf. H scap, W-Medit.
C: Fringes
Caryophyllaceae
242 Arenariabalearica L. Ch suffr, Endem. Sa-Co-Bl-AT
L: Shady rocks and cliffs
Specimens examined: S’Isfundadu, Anela, 25 May 1966, B. Corrias (2 specimens, SS); S’Isfundadu, Anela, 18 June 1965, F. Valsecchi (1 specimen, SS).
243 Cerastiumgibraltaricum Boiss. Ch suffr, Orof. W-Medit.
L: Garrigues
Notes: in the Euro+Med Plantbase, Cerastiumboissierianum Greuter et Burdet is considered a synonym of C.gibraltaricum
244 Cerastiumglomeratum Thuill. T scap, Eurimedit.
C: Pastures
245 Cerastiumligusticumsubsp.palustre (Moris) P. D. Sell et Whitehead T scap, Endem. Sa-Co
RR (near Mt. Masiennera): Wet pastures and meadows
246 Corrigiolatelephiifolia Pourr. H Ros, W-Medit.
L: Trampled sites, dirty roads
Specimen examined: Badu Addes, Anela, September 1962 (sine die), sine coll. (SS)
247 Dianthusichnusaesubsp.toddei Bacch., Brullo, Casti et Giusso H scap, Endem. Sa
L: Garrigues, rocky habitats
Notes: this taxon is exclusive for the Goceano mountain range (Bacchetta et al. 2010).
248 Moenchiaerecta (L.) P. Gaertn., B. Mey. & Scherb. subsp. erecta T scap, Medit.-Atl.
C: Pastures
249 Petrorhagiadubia (Raf.) G. López & Romo T scap, S-Medit.
C: Pastures
250 Petrorhagiasaxifraga (L.) Link H caesp, Eurimedit.
C: Garrigues, rocky habitats
251 Saginaapetala Ard. T scap, Eurimedit.
L: Annual grasslands, dirty tracks
252 Saginaprocumbens L. H caesp, Subcosmop.
L: Wet places, springs
Specimen seen: Badu Addes, Anela, sine die, Barba (SS)
253 Saginasubulata (Sw.) C. Presl H caesp, Medit.-Atl.
L: Wet meadows, rocky habitats (higher altitudes)
Notes: for this taxon, recently the name S.alexandrae Iamonico has been proposed (Iamonico 2016)
254 Silenegallica L. T scap, Eurimedit.
C: Pastures
255 Silenelaeta (Aiton) Godr. T scap, W-Stenomedit.
L: Muddy places, wet meadows, temporary ponds
256 Silenelatifolia Poir. H bienn, Paleotemp.
C: Fringes
257 Silenevulgaris(Moench)Garckesubsp.vulgaris H scap, Paleotemp.
C: Fringes
258 Spergulaarvensis L. T scap, Subcosmop.
C: Pastures
259 Stellariamedia(L.)Cirillosubsp.media T rept, Cosmop.
C: Ruderal vegetation, woods, fringes
Amaranthaceae
260 ChenopodiumalbumL.subsp.album T Scap, Subcosmop.
Not found in the field during this research
Specimens examined: Badu Addes, Anela, 09 September 1962, Barba (2 specimens, SS).
Portulacaceae
261 Montiafontanasubsp.amporitana Sennen T scap, Medit-Mont. Subatl.
C: Mud, flooded soils
Ericales
Primulaceae
262 Anagallisarvensis L. T rept, Eurimedit.
C: Annual grasslands
263 Asterolinonlinum-stellatum (L.) Duby T Scap, Stenomedit.
L: Annual grasslands, pastures
264 CyclamenrepandumSibth. & Sm.subsp.repandum G bulb, NW-Stenomedit.
C: Woods
Ericaceae
265 Arbutusunedo L. P caesp, Stenomedit.
RR (Littu Majore): Wood
266 Ericaarborea L. P caesp, Stenomedit.
C: Shrublands, woods
Gentianales
Rubiaceae
267 Cruciataglabra (L.) Ehrend. H scap, Euras.
C: Grasslands, pastures
Specimen examined: Badu Addes, Anela, 18 July 1972, B. Corrias, S. Diana, F. Valsecchi (SS).
268 GaliumaparineL.subsp.aparine T scap, Euras.
C: Fringes
269 Galiumcorsicum Spreng. H scap, Endem. Sa-Co
L: Rocky habitats
270 Galiumdebile Desv. H scap, Eurimedit.
L: Wet habitats
271 Galiumrotundifolium L. H scap, Orof.-W-Euras.
L: Woods (higher altitudes)
272 RubiaperegrinaL.subsp.peregrina P lian, Stenomedit.-Macaron.
C: Woods
273 Sherardiaarvensis L. T scap, Eurimedit.
C: Pastures, annual grasslands
274 Theligonumcynocrambe L. T scap, Stenomedit.
C: Annual grasslands, fringes
Gentianaceae
275 Exaculumpusillum (Lam.) Caruel T scap, W-Eurimedit.
RR (Minda ‘e Bassu): Temporary pond
Boraginales
Boraginaceae
276 Anchusahybrida Ten. H scap, Stenomedit.
Not found in the field during this research
Specimens examined: Badu Addes, Anela, 22 October 1963, F. Valsecchi, Barba (3 specimens, SS).
277 Cynoglossumcreticum Mill. H bienn, Eurimedit.
L: Fringes
278 Echiumplantagineum L. T Scap, Eurimedit.
C: Pastures, grasslands
279 Myosotisarvensis(L.)Hillsubsp.arvensis T scap, Europ.-W-Asian
C: Annual grasslands, pastures
280 Myosotissicula Guss. T scap, N-Eurimedit.
L: Wet meadows, temporary ponds
Convolvulaceae
281 Convolvulusalthaeoides L. H scand, Stenomedit.
C: Perennial grasslands
282 Convolvulusarvensis L. G rhiz, Paleotemp.
C: Perennial grasslands
283 Cuscutaepithymumsubsp.corsicana (Yunck.) Lambinon T par, Endem. Sa-Co
L: Garrigues (mainly parasite on Genistadesoleana)
Solanales
Solanaceae
284 Solanumdulcamara L. NP, Paleotemp.
U (Su Pranu): Riparian vegetation
Lamiales
Oleaceae
285 Phillyrealatifolia L. P caesp, Stenomedit.
C: Woods, shrubland (lower altitude)
Plantaginaceae
286 Callitrichestagnalis Scop. I rad, Euras.
L: Temporary ponds, springs, muddy soils
287 Cymbalariaaequitriloba(Viv.)A. Chev.subsp.aequitriloba Ch rept, Endem. Sa-Co-Bl-AT
L: Shady rocks and cliffs
288 DigitalispurpureaL.subsp.purpurea H scap, W-Eurimedit.
C: Fringes, clearings
289 Linariaarvensis (L.) Desf. T scap, Submedit.-Subatl.
C: Annual grasslands
290 Linariapelisseriana (L.) Mill. T scap, Medit.-Atl.
C: Pastures
291 Plantagocoronopus L. T scap, Eurimedit.
C: Grasslands, pastures
292 PlantagolagopusL.subsp.lagopus T scap, Stenomedit.
C: Annual grasslands, pastures
293 Plantagolanceolata L. H ros, Euras.
C: Grasslands
294 PlantagomajorL.subsp.major H ros, Euras.
L: Wet meadows
295 Plantagoweldenii Rchb. T scap, Stenomedit.
C: Annual grasslands
296 Veronicaanagallis-aquaticaL.subsp.anagallis-aquatica H scap, Cosmop.
L: Mud, springs, ditches
Specimen examined: Punta Chelchidores est, Anela, 18 July 1972, B. Corrias, S. Diana, F. Valsecchi (SS)
297 Veronicaarvensis L. T scap, Subcosmop.
C: nitrophilous vegetation
298 VeronicahederifoliaL.subsp.hederifolia T scap, Euras.
C: Woods, fringes
299 Veronicavernasubsp.brevistyla (Moris) Rouy T scap, Endem. Sa-Co
L: Pastures (higher altitudes)
Scrophulariaceae
300 Scrophulariatrifoliata L. H caesp, Endem. Sa-Co-AT
L: Rocky habitats
Specimen examined: Badu Addes, Anela, 18 July 1972, F. Valsecchi (SS)
301 ScrophulariaumbrosaDumort.subsp.umbrosa H Scap, Euras.
Not found in the field during this research
Specimens examined: Badu Addes, Anela, 18 July 1973, F. Valsecchi (3 specimens, SS)
302 Verbascumpulverulentum Vill. H bienn, Europ.
C: Clearings, fringes
Labiatae (nom. altr.Lamiaceae)
303 Clinopodiumnepetasubsp.glandulosum (Req.) Govaert H scap, Stenomedit.
C: Fringes
304 Clinopodiumvulgaresubsp.orientale Bothmer H scap, E-Stenomedit.
C: Fringes
Notes: The Italian Flora Checklists (Conti et al. 2005, Bartolucci et al. 2018) consider the subsp. arundanum (Boiss.) Nyman as present in Sardinia, whereas, the Euro+Med PlantBase considers subsp. arundanum absent from the island (and the whole Italian peninsula) and that, instead, subsp. orientale is present. Our specimens fit well with the diagnostic characters of subsp. orientale as described by Bothmer (1967).
305 Glechomasardoa (Bég.) Bég. H rept, Endem. Sa
L: Woods, fringes
306 Lamiummaculatum (L.) L. H scap, Euras.
U: Forest near forestry headquarters, under Quercusilex
Notes: according to Arrigoni (2006–2015), this taxon was not found in Sardinia in recent years
307 Lamiumpurpureum L. T scap, Euras.
C: Fringes
308 LavandulastoechasL.subsp.stoechas NP, Stenomedit.
C: Garrigues
309 Menthaaquatica L. H scap, Paleotemp.
L: Wet meadows
310 MenthapulegiumL.subsp.pulegium H scap, Eurimedit.
C: Wet meadows, temporary ponds
311 MentharequieniiBenth.subsp.requienii H rept, Endem. Sa-Co
RR (Su Cantareddu spring): Wet rocks, spring
312 Menthasuaveolenssubsp.insularis (Req. ex Gren. & Godr.) Greuter H scap, Endem. Sa-Co-AT-Bl
U (Funtana Arile spring): Fringes
313 Micromeriagraeca(L.)Benth.subsp.graeca Ch suffr, Stenomedit.
C: Garrigues
314 PrunellavulgarisL.subsp.vulgaris H scap, Circumbor.
C: Wet meadows, fringes, clearings
315 Salviaverbenaca L. H scap, Medit.-Atl.
C: Grasslands
Notes: following the Euro+Med PlantBase, in this taxon we include ecotypes referred to Salviaclandestina L.
316 Stachysarvensis (L.) L. T scap, Europ.
L: Annual grasslands, pastures
317 Stachyscorsica Pers. H rept, Endem. Sa-Co
L: Shady rocks and cliffs
Specimens examined: S’Isfundadu, Anela, 18 June 1965, F. Valsecchi (SS); Badu Addes, Anela, 18 July 1972, B. Corrias, S. Diana, F. Valsecchi (SS)
318 Stachysglutinosa L. Ch frut, Endem. Sa-Co-AT
L: Garrigues, rocky habitats
319 TeucriumchamaedrysL.subsp.chamaedrys Ch suffr, Eurimedit.
U (near the helicopter base): Pastures, grasslands
320 Thymusherba-barona Loisel. Ch rept, Endem. Sa-Co-Bl
C: Garrigues
Orobanchaceae
321 Orobanchehederae Duby T par, Eurimedit.
C: Woods
322 Orobancheminor Sm. T par, Paleotemp.
C: Grasslands, pastures
323 Orobanchenana (Reut.) Beck T par, Medit.-Macaron.
L: Grasslands, pastures
324 Orobancheramosa L. T par, Paleotemp.
L: Road sides, pastures
325 Orobancherapum-genistae Thuill. T par, Subatl.
L: Garrigues with Genista sp.
326 Orobancherigens Loisel. T par, Endem. Sa-Co
L: Garrigues with Genista sp.
327 Parentucellialatifolia(L.)Caruelsubsp.latifolia T scap, Eurimedit.
C: Pastures
328 Parentucelliaviscosa (L.) Caruel T scap, Medit.-Atl.
C: Annual grasslands
Aquifoliales
Aquifoliaceae
329 Ilexaquifolium L. P caesp, Submedit.-Subatl.
C: Woods
Asterales
Campanulaceae
330 Jasionemontana L. H scap, Europ.-Cauc.
C: Pastures and rocky habitats
Compositae (nom. altr.Asteraceae)
331 Achillealigustica All. H scap, W-Stenomedit.
C: Fringes
332 AnthemisarvensisL.subsp.arvensis T scap, Stenomedit.
C: Pastures
333 Arctiumminus (Hill) Bernh. H bienn, Eurimedit.
C: Fringes, clearings
334 BellisannuaL.subsp.annua T scap, Stenomedit.
C: Annual grasslands on wet soils
335 Bellisperennis L. H ros, Europ.-Cauc.
C: Wet meadows
336 Bellissylvestris Cirillo H ros, Stenomedit.
L: Perennial grasslands (lower altutides)
337 Belliumbellidioides L. H ros, Endem. Sa-Co-Bl-AT
C: Temporary ponds, wet soils
338 Carlinacorymbosa L. H scap, Stenomedit.
C: Pastures
339 CarthamuslanatusL.subsp.lanatus T scap, Eurimedit.
C: Pastures, nitrophilous vegetation near sheep pens
340 CentaureacalcitrapaL.subsp.calcitrapa H bienn, Eurimedit.
C: Pastures
341 Chamaemelumfuscatum (Brot.) Vasc. T scap, W-Stenomedit.
L: Temporary ponds
342 Chondrillajuncea L. H scap, S-Europ.-S-Sib.
C: Pastures
343 CichoriumintybusL.subsp.intybus H scap, Paleotemp.
L: Perennial grasslands
344 Cirsiumscabrum (Poir.) Bonnet & Barratte H scap, SW-Medit.
L: Fringes, road edges (lower altitudes)
345 Cirsiumvulgaresubsp.silvaticum (Tausch) Arènes H bienn, Eurimedit.
C: Fringes, road edges
346 Crepisbellidifolia Loisel. T scap, W-Stenomedit.
L: Pastures
347 Crepisleontodontoides All. H ros, W-Medit.-Mont.
C: Pastures
348 CrepisvesicariaL.subsp.vesicaria T scap, Submedit.-Subatl.
C: Pastures
349 Crupinavulgaris Cass. T scap, S-Sib.-Eurimedit.
L: Pastures, perennial grasslands
350 Filagogallica L. T scap, Eurimedit.
C: Annual grasslands
351 Filagogermanica (L.) Huds. T scap, Paleotemp.
U (S. Giorgio): Annual grasslands
352 Galactitestomentosus Moench H bienn, Stenomedit.
C: Pastures
353 Glebioniscoronaria (L.) Spach. T scap, Stenomedit.
L: Pastures, annual grasslands (lower altitudes)
354 Helichrysumitalicumsubsp.tyrrhenicum (Bacch., Brullo et Giusso) Herrando, J.M. Blanco, L. Sáez & Galbany Ch frut., Endem. Sa-Co-Bl
C: Garrigues
Notes: for this taxon, we follow Herrando-Moraira et al. (2016)
355 Hyoserisradiata L. H ros, Stenomedit.
C: Pastures, meadows
356 Hypochaerisachyrophorus L. T scap, Stenomedit.
C: Annual grasslands
357 Hypochaeriscretensis (L.) Bory & Chaub. H scap, NE-Medit.-Mont.
L: Dry pastures and rocky habitats
358 Hypochaerisglabra L. T scap, Eurimedit.
C: Pastures, meadows
359 HypochaerisradicataL.subsp.radicata H ros, Europ.-Cauc.
C: Pastures, meadows
360 Hypochaerisrobertia (Sch. Bip.) Fiori H ros, Endem. Sa-Co-Si-It
L: Wet rocks and cliffs
361 Lactucamuralis (L.) Gaertn. H scap, Europ.-Cauc.
C: Woods, fringes
362 Leontodontuberosus L. H ros, Stenomedit.
C: Grasslands, pastures
363 Pilosellaziziana (Tausch) F. W. Schultz & Sch. Bip. H scap, Europ. (?)
L: Grasslands
364 Ptilostemoncasabonae (L.) Greuter H scap, Endem. Sa-Co-AT-Hy
U (Entrance of the Domain): Road edge
365 Pulicariaodora (L.) Rchb. H scap, Eurimedit.
C: Woods, fringes (lower altitude)
366 Reichardiapicroides (L.) Roth H scap, Stenomedit.
L: Rocky habitats (lower altitudes)
367 Rhagadiolusstellatus (L.) Gaertn. T scap, Eurimedit.
C: Annual grasslands
368 ScolymushispanicusL.subsp.hispanicus H bienn, Eurimedit.
C: Pastures
369 SeneciovulgarisL.subsp.vulgaris T scap, Eurimedit.
C: Pastures, ruderal vegetation
370 Sonchusasper(L.)Hillsubsp.asper T scap, Euras.
C: Ruderal vegetation
371 Sonchusoleraceus L. T scap, Euras.
C: Ruderal vegetation
372 Silybummarianum (L.) Gaertn. H bienn, Medit.-Turan.
C: Ruderal vegetation, pastures
373 Taraxacumsect.Erythrosperma (H. Lindb.) Dahlst. or Taraxacumsect.Scariosa Hand.-Mazz. H ros, Circumbor.
C: Wet meadows
374 Urospermumdalechampii (L.) F.W. Schmidt H scap, Eurimedit.
C: Grasslands
Dipsacales
Adoxaceae
375 Sambucusebulus L. G rhiz, Eurimedit.
L: Streams
376 Sambucusnigra L. P caesp, Europ.-Cauc.
C: Woods, shrublands
Caprifoliaceae
377 Dipsacusferox Loisel. H bienn, Endem. Sa-Co-Itc
C: Pastures
378 Valerianellaeriocarpa Desv. T scap, Stenomedit.
C: Annual grasslands
Apiales
Araliaceae
379 Hederahelix L. P lian, Eurimedit.
C: Woods
Umbelliferae (nom. altr.Apiaceae)
380 Buniumcorydalinum DC. G bulb, Endem. Sa-Co
C: Garrigues, rocky habitats
381 Chaerophyllumtemulum L. T scap, Euras.
L: Woods, fringes
382 Eryngiumcampestre L. H scap, Eurimedit.
C: Pastures
383 FerulacommunisL.subsp.communis H scap, S-Eurimedit.
L: Pastures, clearings (lower altitudes)
384 Oenanthecrocata L. H scap, Medit.-Atl.
L: Alnusglutinosa woods, streams
385 Oenanthelisae Moris H scap, Endem. Sa
U (Funtana Arile spring): Wet meadows
Specimen examined: Funtana Arile, Anela, 08 June 1980, B. Corrias, S. Diana (SS)
386 Oenanthepimpinelloides L. H scap, Medit.-Atl.
C: Woods, fringes
387 Saniculaeuropaea L. H scap, Paleotemp.
C: Woods, fringes
388 Smyrniumperfoliatumsubsp.rotundifolium (Mill.) Bonnier & Layens H bienn, Stenomedit.
C: Fringes, woods
389 ThapsiagarganicaL.subsp.garganica H scap, S-Medit.
C: Pastures, grasslands
390 Torilisafricana Spreng. T scap, Medit.-Macaron.
C: Pastures, annual grasslands
391 Torilisnodosa (L.) Gaertn. T scap, Medit.-Turan.
C: Pastures, annual grasslands
Ecological and biogeographical analysis of the indigenous flora of Anela
Here we assess the presence in the forest domain of Anela of 391 taxa, belonging to 32 orders and 74 families.
Of the listed taxa, 5 (Anacamptislongicornu (Orchidaceae), Anchusahybrida (Boraginaceae), Chenopodiumalbumsubsp.album (Amaranthaceae), Dactylorhizainsularis (Orchidaceae), Scrophulariaumbrosa (Scrophulariaceae)) were not found during our investigation. Excluding these species, then we recorded a total of 386 indigenous taxa within the domain. Two species are new for the Sardinian flora (Agrostiscapillaris, Aspleniumadiantum-nigrum) and, for 17 taxa, our findings determine an important enlargement of their known range on the island (Arrhenatherumelatiussubsp.sardoum, Aspleniumforeziense, Clinopodiumvulgaresubsp.orientale, Colchicumnanum, Danthoniadecumbenssubsp.decumbens, Euphorbiasemiperfoliata, Exaculumpusillum, Festucamorisianasubsp.morisiana, Lamiummaculatum, Mentharequieniisubsp.requienii, Morisiamonanthos, Poabalbisii, Prunusdomesticasubsp.insititia, Ranunculuscordigersubsp.cordiger, Rosasubcanina, Veronicavernasubsp.brevistyla, Violareichenbachiana).
Overall, we found 141 hemicryptophytes (36.1%), 137 therophytes (35.0%), 56 geophytes (14.3%), 27 phanaerophytes (6.9%), 15 nano-phanaerophytes (3.8%), 11 chamaephytes (2.8%), 3 hydrophytes (0.8%), and 1 helophyte (0.3%).
A total of 239 taxa belong to the Mediterranean element (61.1%), 53 are Eurasian sensu lato (including the true Eurasian, plus European, Euro-Siberian, Euro-Caucasian and Pontic district: overall 13.6%), 42 are Boreal-Temperate taxa (paleotemperate + circumboreal: 10.7%), 36 are widespread (cosmopolitan, sub-cosmopolitan and sub-tropical: 9.2%) and 19 are Atlantic (4.9%). We were not able to assign a geographical category to Prunusdomesticasubsp.insititia.
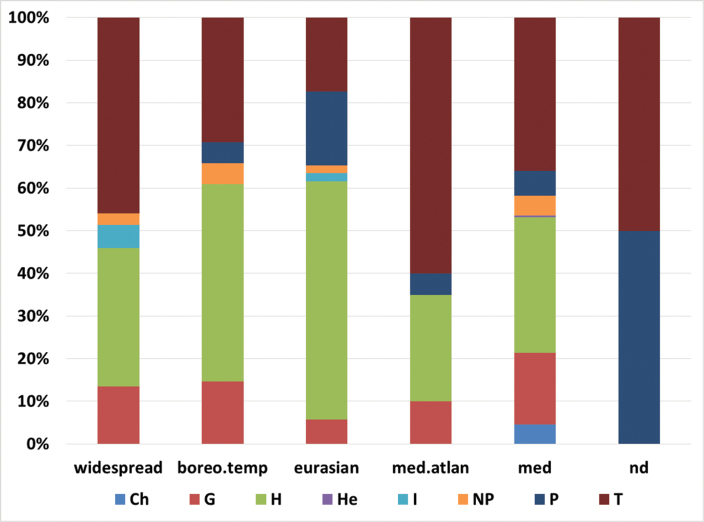
Hemicryptophytes dominate within the Boreal-Temperate and the Eurasian components; annual species prevail within the widespread and the Mediterranean-Atlantic groups. The Mediterranean component hosts similar percentages of annuals and hemicryptophytes (Fig. 2).
Figure 2.
Percentage of biological types for each chorologic element detected in the vascular flora of Anela (390 taxa). boreo.temp = Boreal-temperate taxa; med.atlan = Mediterranean-Atlantic taxa; med = Mediterranean; nd = not determined.
The Mediterranean component is dominated by the euri-Mediterranean sub-element (94 taxa, 24.0% of the whole flora), followed by the steno-Mediterranean (77 taxa, 19.7%) and the endemics (45 entities, 11.5%). A total of 23 Mediterranean taxa belonged to other chorotypes (mountain-Mediterranean, Mediterranean-Turanian, Mediterranen-Macaronesian).
The endemic component of the flora of Anela is dominated by those of the Sardinian-Corsican biogeographic province (sensuBacchetta et al. 2012) accounting for 28 taxa (endemics sensu stricto, 7.4%), of which 19 taxa are Sardinian-Corsican (42.2% of the endemic component), followed by Sardinian entities (5, 11.1%) and those present on Sardinia, Corsica and the Tuscan Archipelago (4, 8.9%). Tyrrhenian or Hercynian endemics (those present in Sardinia, Corsica, Tuscan Archipelago, the Balearic and Hyeres Islands and Sicily) account 12 (26.7%) and, finally, 11.1% is constituted by 5 entities with larger ranges including some continental areas (Sardinia and northern Africa or Sardinia and Italy).
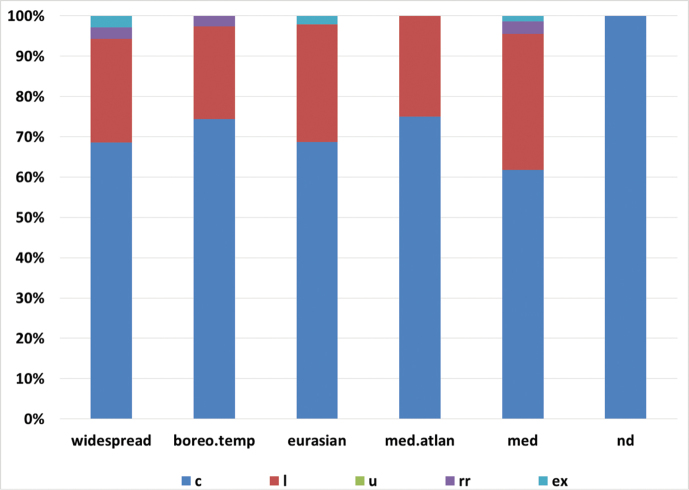
On the basis of our criteria, 241 taxa (61.6%) can be considered common at the local level, 113 (28.9%) are localised, 23 (5.9%) are uncommon, 9 (2.3%) are range restricted and 5 (1.3%) are locally extinct in the last 50 years. Common taxa are the dominant category in all the geographic groups, whereas range restricted taxa are found only in the widespread, Boreal-Temperate and the Mediterranean groups (Fig. 3).
Figure 3.
Percentage of abundance categories for each chorologic element detected in the vascular flora of Anela (390 taxa). c = common; l = localized; u = uncommon; rr = range restricted; ex = extinct. ; boreo.temp = Boreal-temperate taxa; med.atlan = Mediterranean-Atlantic taxa; med = Mediterranean; nd = not determined.
A total of 176 out of 387 taxa were found mainly in grasslands habitats (45.5%) including dry pastures (61 taxa), annual and perennial grasslands (52 and 31 taxa, respectively) and wet pastures and meadows (32 taxa). Woodland habitats hosted 97 taxa (25.1%), comprising woods (57 taxa), fringes and clearings (30 taxa) and shrubs (10 taxa). Wet habitats (including Alnusglutinosa woods, springs, temporary ponds, ditches, muds, streams) hosted 53 taxa (13.7%). Rocky habitats (cliffs, rocks, screes) harbour 24 taxa (6.2%), then the garrigues hosted 21 taxa (5.4%) and finally the anthropogenic habitats (ruderal vegetation, buildings, walls, trampled sites, road edges) were the main habitat for 15 taxa (3.9%).
Discussion
Biogeographical description of the mountain
Our research discovered a high species density at the study area (30.6 taxa km-2), that is one of the highest ever documented in the Sardinian mountain floras (Table 1). Even if there is a clear inverse relationship between the area investigated and species’ density, we should note that, for areas having a comparable surface (~ 10 km2), the floristic density recorded at our study area is second only to the Mt. Gonare complex (Camarda 1984a, 1984b). It is noteworthy that the summit area of Sardinia (> 1500 m a.s.l.), having a surface of 16.8 km2, hosts “only” 214 taxa of which 66 are considered endemics (Arrigoni and Camarda 2015). So we can argue that areas at the edge between the Mediterranean and the temperate bioclimates, like Foresta Demaniale Anela and Mt. Gonare, host floristic components from both the two bioclimatic – biogeographic regions, having therefore more abundant floras than areas located in coastal or summit zones.
Table 1.
Synthetic data on mountain floras from Sardinia and the regional flora, based on different sources (see notes below).
| Site | Altitudinal interval | Area (km2) | No. taxa | Taxa / km2 | H/T | No. endemics | % endemics | Source |
|---|---|---|---|---|---|---|---|---|
| Anela forest domain | 600–1158 | 12.8 | 391 | 30.6 | 1.03 | 45 | 11.5 | This work |
| Gennargentu | 1500–1834 | 16.8 | 214 | 12.7 | 2.5 | 66 | 30.8 | Arrigoni and Camarda 2015 |
| Gennargentu | 1000–1834 | 240 | 675 | 2.8 | 1.25 | 105 | 15.6 | Arrigoni and Camarda 2015 |
| Gennargentu | 1000–1834 | 500 | 897† | 1.8 | 1.03‡ | n.d. | 28§ | Bacchetta et al. 2013 |
| Supramontes | 0–1463 | 335 | n.d. | n.d. | n.d. | 138 | 30 § | Fenu et al. 2010 |
| Mt. Albo | 900–1127 | 68 | 659 | 9.7 | 0.61 | 48 | 7.3 | Camarda 1984a |
| Mt. Gonare | 538–1083 | 10 | 520 | 52 | 0.85 | 23 | 4.4 | Camarda 1984b |
| Mt. Limbara | 160–1359 | 166.24 | 923 | 5.5 | 0.75 | 80 | 8.7 | Calvia and Ruggero unpublished |
| Mt. Limbara | 800–1359 | 49.46 | 687 | 13.9 | 0.84 | 72 | 10.5 | Calvia and Ruggero unpublished |
| Mt. Limbara | 500–1359 | n.r. | 506 | n.d. | 1.18 | 55 | 10.9 | Veri and Bruno 1974 |
| Sardinia | 0–1834 | 24090 | 2028 | 0.084 | 0.70 | n.d. | 7.1 | Pignatti 1995 |
| Sardinia | 0–1834 | 24090 | 2400 | 0.099 | n.d. | n.d. | n.d. | Arrigoni (2006–15) |
| Sardinia | 0–1834 | 24090 | 2408| | 0.1 | 0.74¶ | 290# | 12 | Various (see notes) |
| Sardinia | 0–1834 | 24090 | 2149 | 0.09 | n.r. | 290 | 13.5 | Médail 2017, table 2 |
| Sardinia | 0–1834 | 24090 | 2301 | 0.095 | n.r. | 331 | 14.4 | Bartolucci et al. 2018 |
†Bacchetta et al. (2013) list 948 entities, including 10 varieties, 3 hybrids and 38 aliens: here we therefore consider 897 native taxa;
‡calculated by Arrigoni and Camarda 2015;
#Fenu et al. 2014; n.r. not reported; n.d. not determined.
The hemicryptophytes/therophytes (H/T) ratio, as previously noted by Arrigoni and Camarda (2015), underlines the co-presence of two main elements, the perennial and the annual herbs, having very different life-cycles and summing 71.1% of our flora.The H/T ratio, that in Sardinia peaks at 2.5 at the summit of Gennargentu (Arrigoni and Camarda 2015), but decreases to 0.74 as the regional average, is at Anela 1.03. Limestone mountains like Mt. Albo, with a karst geology and consequently a pronounced summer drought, have a H/T ratio even lower than the regional average, whereas mountain complexes with impermeable substrates (plutonic, volcanic, metamorphic) approaching 1000 m a.s.l. have a H/T ratio ~ 1 gradually increasing with elevation (Table 1). This means that at 1000 m a.s.l., the co-presence of two large groups of non-woody plants, having an annual or perennial life cycle, has been detected: the annuals have a greater prevalence at lower altitudes, the perennials at higher altitudes and their ratio ~ 1 at 1000 m a.s.l. underlines the transition character of this altimetric level in Sardinia.
Important differences with the regional (Sardinian) value (Pignatti 1995) have also been detected for the Mediterranean floristic component, particularly the steno-Mediterranean taxa having a 28.9% regional percentage and 19.7% at the Anela forest domain; contrarily, the euri-Mediterranean component has 16.1% regional average but increases to 24% at our study area, the same percentage (24.3%) reached by the sum of the Boreal-Temperate and the Eurasian floristic components. Whereas lower altitude floras have a dominant steno-Mediterranean component and the floras at the summit of Mediterranean mountains show the prevalence of southern-European and Mediterranean orophytes and narrow endemics (Cañadas et al. 2014; Arrigoni and Camarda 2015), our flora is a good example of transition areas, having the 80% of taxa quite equally distributed amongst steno-Mediterranean, euri-Mediterranean, Boreal-Temperate and Eurasian and the endemic contingents. High species density, H/T ratio ~ 1, balance amongst different chorologic groups and endemic percentage ~ 10% can be considered characteristic features of mountain areas at the transition between the Mediterranean and the temperate bioclimates.
The composition of the flora of the Forest Domain of Anela is also peculiar because it is one of the few examples, not only in Sardinia but in the whole Mediterranean area, with no native Gymnosperms. Junipers (Juniperusphoeniceasubsp.turbinata (Guss.) Nym. and J.oxycedrussubsp.macrocarpa (Sibth. & Sm.) Neilr.) in NW Sardinia are mainly confined in coastal areas (Farris et al. 2017), but Yew (Taxusbaccata L.) and Prikly Juniper (JuniperusoxycedrusL.subsp.oxycedrus) are usually present in high hills and mountains. However junipers are not present in NW Sardinia inland areas (Farris et al. 2017), but the Yew is occurring in all the massifs and mountain ranges, including the two forest domains bordering Anela, the Fiorentini Forest Domain to the east (municipality of Bultei) and the Mt. Pisanu Forest Domain to the west (municipality of Bono, see Farris and Filigheddu 2008). The total absence of Gymnosperms in the native flora of the Anela forest domain is therefore surprising, most probably anomalous and it seems likely to be linked to the management history of the area rather than a natural pattern (Sechi and Falchi 2013).
Despite the fact that in 2004 (last forest census) 90.4% of the domain area was covered by forest or shrub communities (Sechi and Falchi 2013), it is striking that the 45% of the detected taxa were linked mainly to herbaceous habitats (annual and perennial grasslands, dry and wet pastures and meadows), already described for their peculiar and original floristic composition (Farris et al. 2013). Traditional grazing, particularly ovine pastoralism characterised by low flock density and transhumance, has been proven to be beneficial for the plant biodiversity of Mediterranean silvo-pastoral systems, whereas abandonment is detrimental even at short temporal scales (Farris et al. 2010a). The forest domain of Anela is a typical case where ovine stocks had a dramatic decrease in a short period: between 1990 and 2007, a decrease from 0.77 sheep ha-1 to 0.13 sheep ha-1 has been recorded (-83%, Farris et al. 2010a), whereas wood and shrub communities linked to potential natural vegetation (sensuFarris et al. 2010b) are recovering very fast, following a trend common to all Italy (Falcucci et al. 2007) and particularly to Sardinia (Puddu et al. 2012).
Conservation issues of this Flora
Even if rarity is not always linked to threat (de Lange and Norton 1998, Bacchetta et al. 2012), it is an important feature to consider when setting conservation priorities within long lists of taxa (Bacchetta et al. 2012, Le Berre et al. 2018), as in the case of the flora of the Anela forest domain. Additionally, 14 out of 32 uncommon and range-restricted taxa found in this flora are linked to wet habitats: some belong to the Mediterranean and endemic contingents (Cerastiumligusticumsubsp.palustre, Exaculumpusillum, Isoeteshystrix, Menthasuaveolenssubsp.insularis, Mentharequieniisubsp.requienii, Morisiamonanthos, Oenanthelisae), others to the Eurasian and Boreal-Temperate contingents (Struthiopterisspicant, Carexremota, Irispseudacorus, Solanumdulcamara, Spiranthesspiralis). Those habitats are supposed to be highly vulnerable (Filipe et al. 2013), as changes in land use and modification of water balance (because of climate change or human use) are amongst the most important threats to wetlands. Moreover, little is known about the resilience of associated plant communities, a threat increased by the high spatial isolation of such places within a Mediterranean context. At the study site, we detected several species having a contraction of range or local extinctions caused by the capture of surface or underground water for human use, as for example Struthiopterisspicant, Cerastiumligusticumsubsp.palustre, Mentharequieniisubsp.requienii and the localized fern Osmundaregalis for which we documented a local decrease > 50% in the last 20 years. Other species had a decrease directly caused by drainage of temporary ponds (Exaculumpusillum, Isoeteshystrix, Morisiamonanthos). Water management in a climatic changing scenario is and will increasingly be a key issue for the conservation of biodiversity in the Mediterranean basin (Casazza et al. 2014), a climatic change hotspot at the global scale (Giorgi 2006, Giorgi and Lionello 2008), where wet habitats and the species linked are amongst the most threatened (Ghosn et al. 2010, Pérez-Luque et al. 2015).
The 5 taxa, locally extinct, have no relationship with a particular habitat or human use from which they are (were) dependent for their survival in the area, with the exception of Chenopodiumalbum whose disappearance could be explained with the above-mentioned abandonment of pastoral activities, as it is a nitrophilous species. Their disappearance in the last decades, inferred from herbarium records, can be therefore a normal turnover in the composition of the local indigenous flora or an artifact derived from our sampling method (in the sense that these taxa are maybe still present in the area but we were not able to find them during our monthly sampling excursions).
Amongst the flora we inventoried, it is worth mentioning that several populations represent peripheral populations regarding the overall distribution of the taxa. First, a group of uncommon or range restricted species in the domain, are common plants in the Mediterranean bioclimate areas of Sardinia and sometimes in the whole basin. They are here confined to warm niches in the mountain area under study (Anemonehortensis, Arbutusunedo, Arisarumvulgare, Arumpictum, Celtisaustralis, Ficuscarica, Ptilostemoncasabonae), places relatively scattered through this mountain landscape. Oppositely, several Boreal-Temperate and Eurasian taxa confined in this sub-Mediterranean bioclimate island represent peripheral populations isolated sometimes by over 1000 km of their northern range. Those constitute rear edge populations (Hampe and Petit 2005) which may contain unique genetic variation, inherited from ancient species distribution and particular ecological conditions. These two contrasted situations have been highlighted several times within the Mediterranean flora (Lavergne et al. 2005, 2006) and are characteristic of those climatic transition areas. These plants all share the characteristic of occurring as fragmented, disjunct and often highly isolated populations, which restrain gene flow with central population (Pironon et al. 2017) and enhance amongst-population differentiation (Papuga et al. 2018). Thus, the relative isolation associated with potentially marginal ecological conditions highlight their evolutionary potential (Thompson 1999, Anacker and Strauss 2014), as it has recently been shown in Sardinia and Corsica for some marginal and peripheral populations of Cyclamenrepandum (Thompson et al. 2018). Additionally, these groups of taxa are often found in different macro-habitats which have very different links with human activities, therefore leading to different threats and management issues (Lavergne et al. 2006). Thus, conservation policies need to integrate such complex entities within their framework (Lesica and Allendorf 1995, Brunnell et al. 2004, Leppig and White 2006). Finally, those transition areas also contain numerous endemics, which render those places original and of high value for conservation.
Even if biodiversity hot-spots definition at multiple spatial scales is commonly based on the presence, density and distribution of endemic taxa (Myers et al. 2000, Cañadas et al. 2014), the data here presented support that other parameters should also be taken into account to more precisely define priority areas for conservation, as taxonomic complexity (Ennos et al. 2005) of floras and evolutionary potential of populations (Thompson et al. 2010), detected within continuous schemes of biodiversity monitoring (Marignani et al. 2014). This is particularly urgent in southern European mountains, whose biodiversity is threatened by both climate and land use changes (Bravo et al. 2008, Benito et al. 2011, Pauli et al. 2012, Vogiatzakis et al. 2016).
Acknowledgements
E.F. acknowledges the Amministrazione Provinciale di Sassari – Settore Ambiente e Agricoltura Nord Ovest, for funding the project “Monitoraggio di flora, vegetazione e habitat delle oasi di protezione faunistica del Goceano per la gestione delle popolazioni di animali selvatici” for the years 2014–17.
P.d.L. and E.F. thank the project Visiting Professor (Sardinian regional laws 3/2008 and 2/2009) for funding P.d.L. journey to Sardinia in 2013 (Protocol No. 13574 of the 07.05.2012 of the University of Sassari).
Giacomo Calvia and Alessandro Ruggero kindly offered their unpublished quantitative data on the flora of Mt. Limbara for Table 1.
Authors thank all the workers and staff of the forest station of Foresta Demaniale Anela belonging to the Sardinian regional agency Forestas for their help and genuine hospitality given throughout the entire research period.
Citation
Farris E, Carta M, Circosta S, Falchi S, Papuga G, de Lange P (2018) The indigenous vascular flora of the forest domain of Anela (Sardinia, Italy). PhytoKeys 113: 97–143. https://doi.org/10.3897/phytokeys.113.28681
References
- Abbott RJ, Brochmann C. (2003) History and evolution of the arctic flora: In the footsteps of Eric Hulten. Molecular Ecology 12(2): 299–313. 10.1046/j.1365-294X.2003.01731.x [DOI] [PubMed] [Google Scholar]
- Abbott RJ, Comes HP. (2004) Evolution in the Arctic: A phylogeographic analysis of the circumarctic plant, Saxifragaoppositifolia (Purple saxifrage). The New Phytologist 161(1): 211–224. 10.1046/j.1469-8137.2003.00953.x [DOI] [Google Scholar]
- Abbott RJ, Smith LC, Milne RI, Crawford RMM, Wolff K, Balfour J. (2000) Molecular analysis of plant migration and refugia in the Arctic. Science 289(5483): 1343–1346. 10.1126/science.289.5483.1343 [DOI] [PubMed] [Google Scholar]
- Anacker BL, Strauss SY. (2014) The geography and ecology of plant speciation: Range overlap and niche divergence in sister species. Proceedings. Biological Sciences 281(1778): 20132980 10.1098/rspb.2013.2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angiosperm Phylogeny Group (2009) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society 161(2): 105–121. 10.1111/j.1095-8339.2009.00996.x [DOI] [Google Scholar]
- Angiosperm Phylogeny Group (2016) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181(1): 1–20. 10.1111/boj.12385 [DOI] [Google Scholar]
- Arrigoni PV. (1968) Fitoclimatologia della Sardegna. Webbia 23(1): 1–100. 10.1080/00837792.1968.10669879 [DOI] [Google Scholar]
- Arrigoni PV. (2006–2015) Flora dell’isola di Sardegna, volumes I–VI. Carlo Delfino Editore, Sassari.
- Arrigoni PV, Camarda I. (2015) La flora del Gennargentu (Sardegna centrale). Quaderni di Botanica Ambientale e Applicata 25: 3–109. [Google Scholar]
- Arrigoni PV, Camarda I, Corrias B, Diana S, Nardi E, Raffaelli M, Valsecchi F. (1976–1991) Le piante endemiche della Sardegna 1–202. Bollettino della Società Sarda di Scienze Naturali 16–28.
- Bacchetta G, Bagella S, Biondi E, Farris E, Filigheddu R, Mossa L. (2009) Vegetazione forestale e serie di vegetazione della Sardegna (con rappresentazione cartografica alla scala 1:350.000). Fitosociologia 46(1, suppl. 1): 3–82.
- Bacchetta G, Brullo S, Casti M, Giusso del Galdo GP. (2010) Taxonomic revision of the Dianthussylvestris group (Caryophyllaceae) in central-southern Italy, Sicily and Sardinia. Nordic Journal of Botany 28(2): 137–173. 10.1111/j.1756-1051.2009.00459.x [DOI] [Google Scholar]
- Bacchetta G, Farris E, Pontecorvo C. (2012) A new method to set conservation priorities in biodiversity hotspots. Plant Biosystems 146: 638–648. 10.1080/11263504.2011.642417 [DOI] [Google Scholar]
- Bacchetta G, Fenu F, Guarino R, Mandis G, Mattana E, Nieddu G, Scudu C. (2013) Floristic traits and biogeographic characterization of the Gennargentu massif (Sardinia). Candollea 68(2): 209–220. 10.15553/c2012v682a4 [DOI] [Google Scholar]
- Bagella S, Urbani M. (2006) Vascular flora of the calcareous outcrops in North-Western Sardinia (Italy). Webbia 61(1): 95–132. 10.1080/00837792.2006.10670796 [DOI] [Google Scholar]
- Bartolucci F, Peruzzi L, Galasso G, Albano A, Alessandrini A, Ardenghi NMG, Astuti G, Bacchetta G, Ballelli S, Banfi E, Barberis G, Bernardo L, Bouvet D, Bovio M, Cecchi L, Di Pietro R, Domina G, Fascetti S, Fenu G, Festi F, Foggi B, Gallo L, Gottschlich G, Gubellini L, Iamonico D, Iberite M, Jiménez-Mejías P, Lattanzi E, Marchetti D, Martinetto E, Masin RR, Medagli P, Passalacqua NG, Peccenini S, Pennesi R, Pierini B, Poldini L, Prosser F, Raimondo FM, Roma-Marzio F, Rosati L, Santangelo A, Scoppola A, Scortegagna S, Selvaggi A, Selvi F, Soldano A, Stinca A, Wagensommer RP, Wilhalm T, Conti F. (2018) An updated checklist of the vascular flora native to Italy. Plant Biosystems 152(2): 179–303. 10.1080/11263504.2017.1419996 [DOI] [Google Scholar]
- Benito B, Lorite J, Penas J. (2011) Simulating potential effects of climatic warming on altitudinal patterns of key species in Mediterranean-alpine ecosystems. Climatic Change 108(3): 471–483. 10.1007/s10584-010-0015-3 [DOI] [Google Scholar]
- Bothmer RV. (1967) Intraspecific variation in Clinopodiumvulgare L. (Labiatae). Botaniska Notiser 120(2): 202–208. [Google Scholar]
- Bravo DN, Araujo MB, Lasanta T, Lopez Moreno JI. (2008) Climate change in Mediterranean mountains during the 21st century. Ambio 37(4): 280–285. 10.1579/0044-7447(2008)37[280:CCIMMD]2.0.CO;2 [DOI] [PubMed]
- Brunnell FL, Campbell RW, Squires KA. (2004) Conservation priorities for peripheral species: The example of British Columbia. Canadian Journal of Forest Research 34(11): 2240–2247. 10.1139/x04-102 [DOI] [Google Scholar]
- Camarda I. (1984a) Studi sulla flora e sulla vegetazione del Monte Albo (Sardegna centro-orientale): 1. La flora. Webbia 37(2): 283–327. 10.1080/00837792.198.10670281 [DOI] [Google Scholar]
- Camarda I. (1984b) Studi sulla flora e sulla vegetazione del Monte Gonare (Sardegna centrale): 1. la flora. Bollettino della Società Sarda di Scienze Naturali 23: 173–211. [Google Scholar]
- Camarda I, Valsecchi F. (2008) Alberi e arbusti spontanei della Sardegna. Carlo Delfino Editore, Sassari.
- Cañadas EM, Fenu G, Penas J, Lorite J, Mattana E, Bacchetta G. (2014) Hotspots within hotspots: Endemic plant richness, environmental drivers, and implications for conservation. Biological Conservation 170: 282–291. 10.1016/j.biocon.2013.12.007 [DOI] [Google Scholar]
- Canu S, Rosati L, Fiori M, Motroni A, Filigheddu R, Farris E. (2015) Bioclimate map of Sardinia (Italy). Journal of Maps 11(5): 711–718. 10.1080/17445647.2014.988187 [DOI] [Google Scholar]
- Carta A, Peruzzi L. (2015) Contributo alla conoscenza della flora vascolare endemica di Toscana ed aree contermini. 6. Hypericumhircinumsubsp.hircinum (Hypericaceae). Informatore Botanico Italiano 47(1): 27–31. [Google Scholar]
- Casazza G, Giordani P, Benesperi R, Foggi B, Viciani D, Filigheddu R, Farris E, Bagella S, Pisanu S, Mariotti MG. (2014) Climate change hastens the urgency of conservation for range-restricted plant species in the central-northern Mediterranean region. Biological Conservation 179: 129–138. 10.1016/j.biocon.2014.09.015 [DOI] [Google Scholar]
- Conti F, Abbate G, Alessandrini A, Blasi C. (2005) An annotated checklist of the Italian vascular flora. Palombi Editori, Roma, 420 pp. [Google Scholar]
- de Lange PJ, Norton DA. (1998) Revisiting rarity: a botanical perspective on the meanings of rarity and the classification of New Zealand’s uncommon plants. Royal Society of New Zealand Miscellaneous Series 48: 145–159. [Google Scholar]
- Desfayes M. (2004) Flore vasculaire herbacée des eaux douces et des milieux humides de la Sardaigne. Flora Mediterranea 18: 247–231. http://www.herbmedit.org/flora18.html [Google Scholar]
- Deshpande AU, Apte GS, Bahulikar RA, Lagu MD, Kulkarni BG, Suresh HS, Singh NP, Rao MKV, Gupta VS, Pant A, Ranjekar PK. (2001) Genetic diversity across natural populations of three montane plant species from the Western Ghats, India revealed by intersimple sequence repeats. Molecular Ecology 10(10): 2397–2408. 10.1046/j.0962-1083.2001.01379.x [DOI] [PubMed] [Google Scholar]
- Domina G, Arrigoni PV. (2007) The genus Orobanche (Orobanchaceae) in Sardinia. Flora Mediterranea 17: 115–136. [Google Scholar]
- Ennos RA, French GC, Hollingsworth PM. (2005) Conserving taxonomic complexity. Trends in Ecology & Evolution 20(4): 164–168. 10.1016/j.tree.2005.01.012 [DOI] [PubMed] [Google Scholar]
- Euro+Med (2006–2018) Euro+Med PlantBase – the information resource for Euro-Mediterranean plant diversity. http://ww2.bgbm.org/EuroPlusMed/ [accessed on 22 October 2018]
- Falcucci A, Maiorano L, Boitani L. (2007) Changes in land-use/land-cover patterns in Italy and their implications for biodiversity conservation. Landscape Ecology 22(4): 617–631. 10.1007/s10980-006-9056-4 [DOI] [Google Scholar]
- Farris E. (2013a) La biodiversità vegetale associata ai fontanili della Foresta Demaniale di Anela (Sardegna, Italia). In: Farris GA. (Ed.) Le fontane della Foresta Demaniale di Anela (Sassari).Tipolitografia Il Torchietto, Ozieri, 47–67.
- Farris GA. (Ed.) (2013b) Le fontane della Foresta Demaniale di Anela (Sassari). Tipolitografia Il Torchietto, Ozieri, 1–256.
- Farris E, Filigheddu R. (2008) Effects of browsing in relation to vegetation cover on common yew (Taxusbaccata L.) recruitment in Mediterranean environments. Plant Ecology 199(2): 309–318. 10.1007/s11258-008-9434-x [DOI] [Google Scholar]
- Farris E, Filigheddu R, Deiana P, Farris GA, Garau G. (2010a) Short-term effects on sheep pastureland due to grazing abandonment in a Western Mediterranean island ecosystem: A multidisciplinary approach. Journal for Nature Conservation 18(4): 258–267. 10.1016/j.jnc.2009.11.003 [DOI] [Google Scholar]
- Farris E, Filibeck G, Marignani M, Rosati L. (2010b) The power of potential natural vegetation (and of spatial-temporal scale) – a response to Carrión & Fernández (2009). Journal of Biogeography 37(11): 2211–2213. 10.1111/j.1365-2699.2010.02323.x [DOI] [Google Scholar]
- Farris E, Rosati L, Secchi Z, Filigheddu R. (2013) Are all pastures eligible for conservation? A phytosociological survey of the Sardinian-Corsican province as a basic tool for the Habitats Directive. Plant Biosystems 147(4): 931–946. 10.1080/11263504.2013.778911 [DOI] [Google Scholar]
- Farris E, Canopoli L, Cucca E, Landi S, Maccioni A, Filigheddu R. (2017) Foxes provide a direct dispersal service to Phoenician junipers in Mediterranean coastal environments: Ecological and evolutionary implications. Plant Ecology and Evolution 150(2): 117–128. 10.5091/plecevo.2017.1277 [DOI] [Google Scholar]
- Fazan L, Guillet S, Corona C, Kozlowski G, Stoffel M. (2017) Imprisoned in the Cretan mountains: How relict Zelkovaabelicea (Ulmaceae) trees cope with Mediterranean climate. The Science of the Total Environment 599: 797–805. 10.1016/j.scitotenv.2017.04.047 [DOI] [PubMed] [Google Scholar]
- Fenu G, Fois M, Cañadas EM, Bacchetta G. (2014) Using endemic-plant distribution, geology and geomorphology in biogeography: The case of Sardinia (Mediterranean Basin). Systematics and Biodiversity 12(2): 181–193. 10.1080/14772000.2014.894592 [DOI] [Google Scholar]
- Filipe AF, Lawrence JE, Bonada N. (2013) Vulnerability of stream biota to climate change in mediterranean climate regions: A synthesis of ecological responses and conservation challenges. Hydrobiologia 719: 331–351. 10.1007/s10750-012-1244-4 [DOI] [Google Scholar]
- Friar EA, Boose DL, LaDoux T, Roalson EH, Robichaux RH. (2001) Population structure in the endangered Mauna Loa silversword, Argyroxiphiumkauense (Asteraceae), and its bearing on reintroduction. Molecular Ecology 10(7): 1657–1663. 10.1046/j.1365-294X.2001.01315.x [DOI] [PubMed] [Google Scholar]
- Galasso G, Conti F, Peruzzi L, Ardenghi NMG, Banfi E, Celesti-Grapow L, Albano A, Alessandrini A, Bacchetta G, Ballelli S, Bandini Mazzanti M, Barberis G, Bernardo L, Blasi C, Bouvet D, Bovio M, Cecchi L, Del Guacchio E, Domina G, Fascetti S, Gallo L, Gubellini L, Guiggi A, Iamonico D, Iberite M, Jiménez-Mejías P, Lattanzi E, Marchetti D, Martinetto E, Masin RR, Medagli P, Passalacqua NG, Peccenini S, Pennesi R, Pierini B, Podda L, Poldini L, Prosser F, Raimondo FM, Roma-Marzio F, Rosati L, Santangelo A, Scoppola A, Scortegagna S, Selvaggi A, Selvi F, Soldano A, Stinca A, Wagensommer RP, Wilhalm T, Bartolucci F. (2018) An updated checklist of the vascular flora alien to Italy. Plant Biosystems 152(3): 556–592. 10.1080/11263504.2018.1441197 [DOI] [Google Scholar]
- Gamache I, Jaramillo-Correa JP, Payette S, Bousquet J. (2003) Diverging patterns of mitochondrial and nuclear DNA diversity in subarctic black spruce: Imprint of a founder effect associated with postglacial colonization. Molecular Ecology 12(4): 891–901. 10.1046/j.1365-294X.2003.01800.x [DOI] [PubMed] [Google Scholar]
- Gasper AL, de Oliveira Dittrich VA, Smith AR, Salino A. (2016) A classification for Blechnaceae (Polypodiales: Polypodiopsida): New genera, resurrected names, and combinations. Phytotaxa 275(3): 191–227. 10.11646/phytotaxa.275.3.1 [DOI] [Google Scholar]
- Ghosn D, Vogiatzakis IN, Kazakis G, Dimitriou E, Moussoulis E, Maliaka V, Zacharias I. (2010) Ecological changes in the highest temporary pond of western Crete (Greece): Past, present and future. Hydrobiologia 648(1): 3–18. 10.1007/s10750-010-0143-9 [DOI] [Google Scholar]
- Giorgi F. (2006) Climate change hot-spots. Geophysical Research Letters 33(8): L08707. 10.1029/2006GL025734 [DOI]
- Giorgi F, Lionello P. (2008) Climate change projections for the Mediterranean region. Global and Planetary Change 63(2–3): 90–104. 10.1016/j.gloplacha.2007.09.005 [DOI] [Google Scholar]
- GIROS (Gruppo Italiano per la Ricerca sulle Orchidee Spontanee) (2016) Orchidee d’Italia, second edition. Il Castello srl, Cornaredo, 1–368.
- Hampe A, Petit RJ. (2005) Conserving biodiversity under climate change: The rear edge matters. Ecology Letters 8(5): 461–467. 10.1111/j.1461-0248.2005.00739.x [DOI] [PubMed] [Google Scholar]
- Haston E, Richardson JE, Stevens PF, Chase MW, Harris DJ. (2009) The Linear Angiosperm Phylogeny Group (LAPG) III: A linear sequence of the families in APGIII. Botanical Journal of the Linnean Society 161(2): 128–131. 10.1111/j.1095-8339.2009.01000.x [DOI] [Google Scholar]
- Hemp A. (2002) Ecology of the pteridophytes on the southern slopes of Mt. Kilimanjaro – I. Altitudinal distribution. Plant Ecology 159(2): 211–239. 10.1023/A:1015569125417 [DOI] [Google Scholar]
- Herrando-Moraira S, Blanco-Moreno JM, Sáez L, Galbany-Casals M. (2016) Re-evaluation of the Helichrysumitalicum complex (Compositae: Gnaphalieae): a new species from Majorca (Balearic Islands). Collectanea Botanica 35: e009. 10.3989/collectbot.2016.v35.009 [DOI]
- Holderegger R, Abbott RJ. (2003) Phylogeography of the Arctic-Alpine Saxifragaoppositifolia (Saxifragaceae) and some related taxa based on cpDNA and its sequence variation. American Journal of Botany 90(6): 931–936. 10.3732/ajb.90.6.931 [DOI] [PubMed] [Google Scholar]
- Iamonico D. (2016) A new name in Sagina, Saginaalexandrae (Caryophyllaceae). Phytotaxa 282(2): 164–165. 10.11646/phytotaxa.282.2.8 [DOI] [Google Scholar]
- Inouye DW. (2008) Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89(2): 353–362. 10.1890/06-2128.1 [DOI] [PubMed] [Google Scholar]
- Iszkulo G, Pers-Kamczyc E, Nalepka D, Rabska M, Walas Ł, Dering M. (2016) Postglacial migration dynamics helps to explain current scattered distribution of Taxusbaccata. Dendrobiology (Poznan) 76: 81–89. 10.12657/denbio.076.008 [DOI] [Google Scholar]
- Korner C. (2000) Why are there global gradients in species richness? Mountains might hold the answer. Trends in Ecology & Evolution 15(12): 513–514. 10.1016/S0169-5347(00)02004-8 [DOI] [Google Scholar]
- Korner C. (2004) Mountain biodiversity, its causes and function. Ambio Special report 13: 11–17. [PubMed] [Google Scholar]
- Korner C, Paulsen J. (2004) A world-wide study of high altitude treeline temperatures. Journal of Biogeography 31(5): 713–732. 10.1111/j.1365-2699.2003.01043.x [DOI] [Google Scholar]
- Lavergne S, Thuiller W, Molina J, Debussche M. (2005) Environmental and human factors influencing rare plant local occurrence, extinction and persistence: A 115-year study in the Mediterranean region. Journal of Biogeography 32(5): 799–811. 10.1111/j.1365-2699.2005.01207.x [DOI] [Google Scholar]
- Lavergne S, Molina J, Debussche M. (2006) Fingerprints of environmental change on the rare Mediterranean flora: A 115-year study. Global Change Biology 12(8): 1466–1478. 10.1111/j.1365-2486.2006.01183.x [DOI] [Google Scholar]
- Le Berre M, Noble V, Pires M, Casazza G, Minuto L, Mariotti M, Abdulhak S, Fort N, Médail F, Diadema K. (2018) Applying a hierarchisation method to a biodiversity hotspot: Challenges and perspectives in the South-Western Alps flora. Journal for Nature Conservation 42: 19–27. 10.1016/j.jnc.2018.01.007 [DOI] [Google Scholar]
- Leppig G, White JW. (2006) Conservation of peripheral plant populations in California. Madrono 253(3): 264–274. 10.3120/0024-9637(2006)53[264:COPPPI]2.0.CO;2 [DOI]
- Lesica P, Allendorf FW. (1995) When Are Peripheral Populations Valuable for Conservation? Conservation Biology 9(4): 753–760. 10.1046/j.1523-1739.1995.09040753.x [DOI]
- Lian CL, Oishi R, Miyashita N, Nara K, Nakaya H, Wu BY, Zhou ZH, Hogetsu T. (2003) Genetic structure and reproduction dynamics of Salixreinii during primary succession on Mount Fuji, as revealed by nuclear and chloroplast microsatellite analysis. Molecular Ecology 12(3): 609–618. 10.1046/j.1365-294X.2003.01756.x [DOI] [PubMed] [Google Scholar]
- Madrau S. (2013) Caratteristiche pedologiche della Foresta Demaniale di Anela. In: Farris GA. (Ed.) Le fontane della Foresta Demaniale di Anela (Sassari).Tipolitografia Il Torchietto, Ozieri, 30–46.
- Mansion G, Rosenbaum G, Schoenenberger N, Bacchetta G, Rossellò JA, Conti E. (2008) Phylogenetic analysis informed by geological history supports multiple, sequential invasions of the Mediterranean Basin by the Angiosperm family Araceae. Systematic Biology 57(2): 269–285. 10.1080/10635150802044029 [DOI] [PubMed] [Google Scholar]
- Marchetti D. (2004) Le Pteridofite d’Italia. Annali Museo Civico di Rovereto – Sezione Archeologia, Storia. Science and Nature 19: 71–231. http://www.museocivico.rovereto.tn.it/UploadDocs/325_art05_marchetti.pdf [Google Scholar]
- Marignani M, Bacchetta G, Bagella S, Caria MC, Delogu F, Farris E, Fenu G, Filigheddu R, Blasi C. (2014) Is time on our side? Strengthening the link between field efforts and conservation needs. Biodiversity and Conservation 23(2): 421–431. 10.1007/s10531-013-0610-5 [DOI] [Google Scholar]
- Mayol M, Riba M, Gonzalez-Martinez SC, Bagnoli F, de Beaulieu J-L, Berganzo E, Burgarella C, Dubreuil M, Krajmerová D, Paule L, Romšáková I, Vettori C, Vincenot L, Vendramin GG. (2015) Adapting through glacial cycles: Insights from a long-lived tree (Taxusbaccata). The New Phytologist 208(3): 973–986. 10.1111/nph.13496 [DOI] [PubMed] [Google Scholar]
- Médail F, Diadema K. (2009) Glacial refugia influence plant diversity patterns in the Mediterranean Basin. Journal of Biogeography 36(7): 1333–1345. 10.1111/j.1365-2699.2008.02051.x [DOI] [Google Scholar]
- Mereu G. (2012) Notulae alla check-list della flora vascolare italiana – notula 1944. Informatore Botanico Italiano 44(2): 395 http://www.societabotanicaitaliana.it/ibi?c=IBI%2044%20(2)%202012 [Google Scholar]
- Molau U. (2004) Mountain biodiversity patterns at low and high latitudes. AMBIO Special report 13: 24–28. [PubMed] [Google Scholar]
- Mossa L, Bacchetta G, Brullo S. (1998) Considerazioni tassonomiche sulle querce caducifoglie della Sardegna. Monti e Boschi 1998(2): 41–46. [Google Scholar]
- Mossa L, Bacchetta G, Brullo S. (1999) Quercusichnusae (Fagaceae), a new species from Sardinia. Israel. Le Journal de Botanique 47: 199–207. 10.1080/07929978.1999.10676774 [DOI]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kents J. (2000) Biodiversity hotspots for conservation priorities. Nature 403(6772): 853–858. 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Papuga G, Gauthier P, Pons V, Farris E, Thompson JD. (2018) Ecological niche differentiation in peripheral populations: A comparative analysis of eleven Mediterranean plant species. Ecography 41(10): 1650–1664. 10.1111/ecog.03331 [DOI] [Google Scholar]
- Pauli H, Gottfried M, Dullinger S, Abdaladze O, Akhalkatsi M, Alonso JLB, Coldea G, Dick J, Erschbamer B, Calzado RF, Ghosn D, Holten JI, Kanka R, Kazakis G, Kollar J, Larsson P, Moiseev P, Moiseev D, Molau U, Mesa JM, Nagy L, Pelino G, Puscas M, Rossi G, Stanisci A, Syverhuset AO, Theurillat J-P, Tomaselli M, Unterluggauer P, Villar L, Vittoz P, Grabherr G. (2012) Recent Plant Diversity Changes on Europe’s Mountain Summits. Science 336(6079): 353–355. 10.1126/science.1219033 [DOI] [PubMed] [Google Scholar]
- Pérez-Luque AJ, Sànchez-Rojas CP, Zamora R, Pérez-Pérez R, Bonet FJ. (2015) Dataset of phenology of Mediterranean high-mountain meadows flora (Sierra Nevada, Spain). PhytoKeys 46: 89–107. 10.3897/phytokeys.46.9116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruzzi L, Conti F, Bartolucci F. (2014) An inventory of vascular plants endemic to Italy. Phytotaxa 168(1): 1–75. 10.11646/phytotaxa.168.1.1 [DOI] [Google Scholar]
- Petit RJ, Hampe A, Cheddadi R. (2005) Climate changes and tree phylogeography in the Mediterranean. Taxon 54(4): 877–885. 10.2307/25065474 [DOI] [Google Scholar]
- Pignatti S. (1982) Flora d’Italia, volumes I–III. Edagricole, Bologna.
- Pignatti S. (1995) Ecologia Vegetale. UTET, Torino, 1–532.
- Pignatti S. (2017–2018) Flora d’Italia, volumes I–III. Edagricole-New Business Media, Bologna.
- Pironon S, Papuga G, Villellas J, Angert AL, Garcia MB, Thompson JD. (2017) Geographic variation in genetic and demographic performance: New insights from an old biogeographical paradigm. Biological Reviews of the Cambridge Philosophical Society 92(4): 1877–1909. 10.1111/brv.12313 [DOI] [PubMed] [Google Scholar]
- Puddu G, Falcucci A, Majorano L. (2012) Forest changes over a century in Sardinia: Implications for conservation in a Mediterranean hotspot. Agroforestry Systems 85(3): 319–330. 10.1007/s10457-011-9443-y [DOI] [Google Scholar]
- Rahbek C. (1995) The elevational gradient of species richness – a uniform pattern. Ecography 18(2): 200–205. 10.1111/j.1600-0587.1995.tb00341.x [DOI] [Google Scholar]
- Raunkiær CC. (1934) The Life Forms of Plants and Statistical Plant Geography, Oxford University Press, Oxford, 1–632.
- Sechi C, Falchi S. (2013) La gestione pubblica delle foreste demaniali del Goceano, sezione Anela (Sardegna, Italia). In: Farris GA. (Ed.) Le fontane della Foresta Demaniale di Anela (Sassari).Tipolitografia Il Torchietto, Ozieri, 21–29.
- Smith AR, Pryer KM, Schuettpelz E, Korall P, Schneider H, Wolf PG. (2006) A classification for extant ferns. Taxon 55(3): 705–731. 10.2307/25065646 [DOI] [Google Scholar]
- Thiers B. (2018) Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih/ [accessed 23.01.2018].
- Thompson JD. (1999) Population differentiation in Mediterranean plants: Insights into colonization history and the evolution and conservation of endemic species. Heredity 82(3): 229–236. 10.1038/sj.hdy.6885040 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gauthier P, Debussche M. (2010) Conservation value of sites of hybridization in peripheral populations of rare plant species. Conservation Biology 24(1): 236–245. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gauthier P, Papuga G, Pons V, Debussche M, Farris E. (2018) The conservation significance of natural hybridisation in Mediterranean plants: From a case study on Cyclamen (Primulaceae) to a general perspective. Plant Biology 20(Suppl. 1): 128–138. 10.1111/plb.12595 [DOI] [PubMed]
- Tison J-M, de Foucault B. (2014) Flora Gallica. Biotope Editions, Mèze.
- Tremetsberger K, Stuessy TF, Samuel RM, Baeza CM, Fay MF. (2003a) Genetics of colonization in Hypochaeristenuifolia (Asteraceae, Lactuceae) on Volcan Lonquimay, Chile. Molecular Ecology 12(10): 2649–2659. 10.1046/j.1365-294X.2003.01956.x [DOI] [PubMed] [Google Scholar]
- Tremetsberger K, Stuessy TF, Guo YP, Baeza CM, Weiss H, Samuel RM. (2003b) Amplified fragment length polymorphism (AFLP) variation within and among populations of Hypochaerisacaulis (Asteraceae) of Andean southern South America. Taxon 52(2): 237–245. 10.2307/3647392 [DOI] [Google Scholar]
- Valsecchi F, Corrias B. (1966) La vegetazione di Monte Rasu. I: Flora cacuminale. Studi Sassaresi, sezione III. Annali Facoltà di Agraria Università di Sassari 14(2): 498–504. [Google Scholar]
- Veri L, Bruno E. (1974) La Flora del massiccio del Limbara (Sardegna settentrionale). Annali di Botanica 33: 83–139. [Google Scholar]
- Vogiatzakis IN, Mannion AM, Sarris D. (2016) Mediterranean island biodiversity and climate change: The last 10,000 years and the future. Biodiversity and Conservation 25(13): 2597–2627. 10.1007/s10531-016-1204-9 [DOI] [Google Scholar]
- Winkler M, Lamprecht A, Steinbauer K, Hülber K, Theurillat J-P, Breiner F, Choler P, Ertl S, Gutiérrez Girón A, Rossi G, Vittoz P, Akhalkatsi M, Bay C, Benito Alonso J-L, Bergström T, Carranza ML, Corcket E, Dick J, Erschbamer B, Fernández Calzado R, Fosaa AM, Gavilán RG, Ghosn D, Gigauri K, Huber D, Kanka R, Kazakis G, Klipp M, Kollar J, Kudernatsch T, Larsson P, Mallaun M, Michelsen O, Moiseev P, Moiseev D, Molau U, Molero Mesa J, Morra di Cella U, Nagy L, Petey M, Pușcaș M, Rixen C, Stanisci A, Suen M, Syverhuset AO, Tomaselli M, Unterluggauer P, Ursu T, Villar L, Gottfried M, Pauli H. (2016) The rich sides of mountain summits - a pan-European view on aspect preferences of alpine plants. Journal of Biogeography 43(11): 2261–2273. 10.1111/jbi.12835 [DOI] [Google Scholar]
- Zhou ZH, Miwa M, Nara K, Wu BY, Nakaya H, Lian CL, Miyashita N, Oishi R, Maruta E, Hogetsu T. (2003) Patch establishment and development of a clonal plant, Polygonumcuspidatum, on Mount Fuji. Molecular Ecology 12(6): 1361–1373. 10.1046/j.1365-294X.2003.01816.x [DOI] [PubMed] [Google Scholar]