Abstract
Purpose
to describe a patient with visual field (VF) defect from an occipital lobe lesion that was found to have macular ganglion cells complex (GCC) quadrantic reduction without significant peripapillary retinal nerve fiber layer (RNFL) loss on optical coherence tomography (OCT). To emphasize that macular GCC loss may be the major ocular manifestation of trans-synaptic optic pathway degeneration in occipital lobe lesions.
Observations
A 15-year-old female was investigated after a VF examination revealed a right homonymous inferior quadrantanopia. Fundoscopic examination was completely normal as were the peripapillary retinal nerve fiber layer (RNFL) thickness measurements on OCT. Macular thickness measurements however, revealed superior homonymous quadrantic GCL reduction evidencing retinal neuronal loss in direct correspondence with her VF defect. Magnetic resonance imaging showed a localized left occipital lobe gliotic lesion as the explanation for her VF defect.
Conclusions and Importance
Small post-geniculate optic pathway lesions may lead to retrograde trans-synaptic degeneration that can be detected on OCT-measured macular GCL measurements despite normal peripapillary RNFL estimates. Awareness of such occurrence in important to avoid diagnostic confusion with other anterior visual pathway diseases.
Keywords: Optical coherence tomography, Retinal ganglion cells layer, Occipital lesions, Retrograde trans-synaptic degeneration
1. Introduction
Macular ganglion cells layer (GCL) along with peripapillary retinal nerve fiber layer (RNFL) reduction on high-resolution optical coherence tomography (OCT) are important manifestations of a number of anterior visual pathway disorders including degenerative, inflammatory, demyelinating, vascular and compressive optic neuropathies.1,2 Since axons originating from retinal GCL form the optic nerve, chiasm and tract to synapse in the lateral geniculate nucleus (LGN), damage to the anterior visual pathway frequently leads to retrograde degeneration of both peripapillary RNFL, with resultant optic disc pallor, and macular GCL thickness reduction. On the other hand, with the exception of large congenital lesions when secondary optic disc anomalies may occur,3 post-geniculate optic pathway lesions were until recently considered to cause homonymous visual field (VF) loss without any clinically observed abnormality in the retina or the optic nerve head.
The development of high-resolution OCT technology however has somehow changed such concept since retinal axonal loss from trans-synaptic degeneration was found to be a more frequent occurrence than previously thought in congenital or even acquired post geniculate lesions.4, 5, 6, 7, 8, 9 In most such cases, however, macular GCL reduction usually occurs in correspondence with significant peripapillary RNFL loss. We report a patient that presented with sectoral VF defect due to an occipital lobe lesion and normal fundus eye examination. On high-resolution OCT examination, she had essentially normal peripapillary RNFL thickness measurements but macular GCL thickness was significantly reduced in direct correspondence with her VF defect. This case is important to emphasize that macular GCL can be the main sign of trans-synaptic degeneration from post geniculate optic pathway lesions.
2. Case report
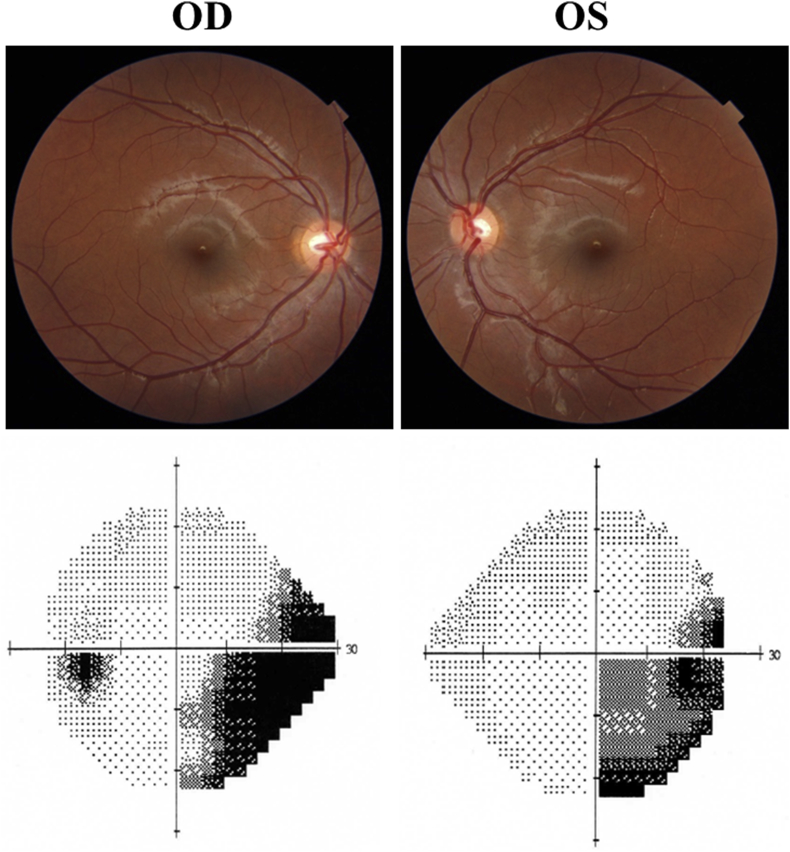
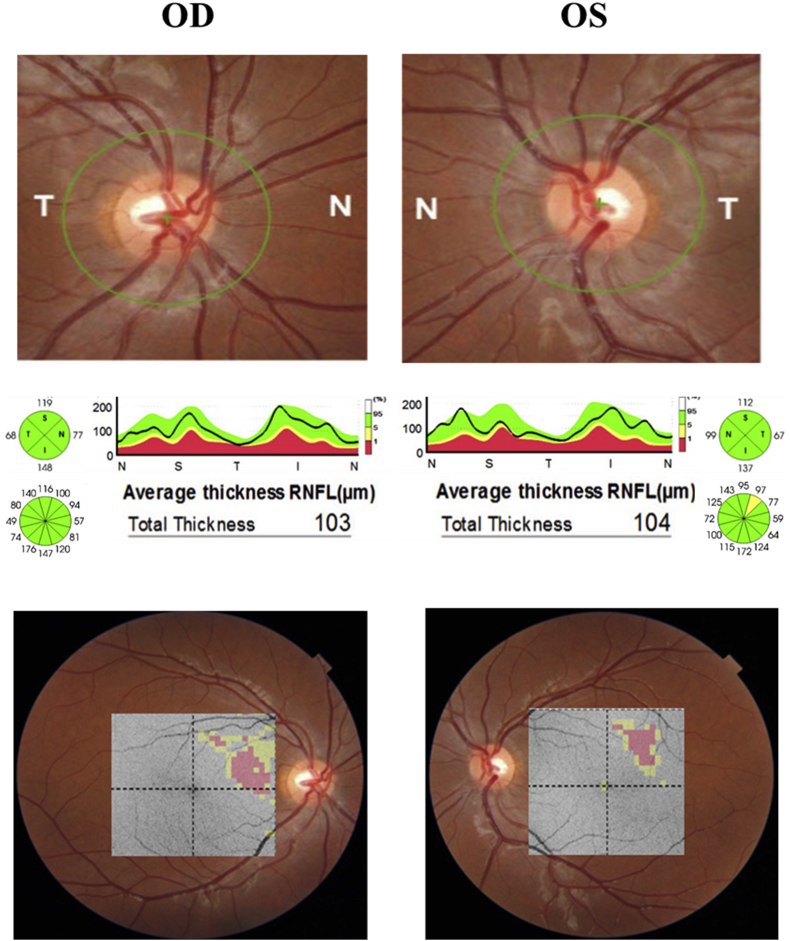
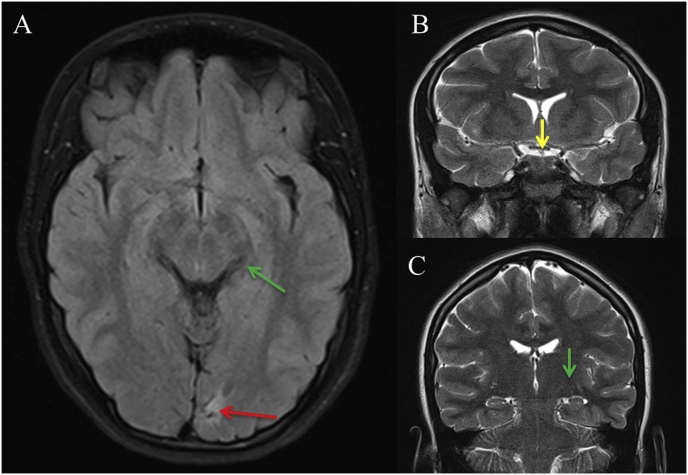
A 15-year-old young girl was referred to our service for investigation of a possible optic neuropathy after her ophthalmologist detected an inferior right defect on confrontation VF examination. The patient did not have any complaint except for mild reading difficulty. On examination, best-corrected visual acuity was 20/20 in both eyes (OU). Ocular motility, pupillary reflexes, biomicroscopy and tonometry were normal, as was the funduscopic examination OU. Standard automated perimetry (Humphrey 24-2 Sita-Standard test) revealed an incomplete right inferior quadrantanopia (Fig. 1). High definition OCT (DRI OCT Triton Plus; Topcon, Inc., Tokyo, Japan) images were acquired of the optic nerve and the macula. Average (360-degree measurement) peripapillary RNFL thickness were within normal limits OU, measuring 103 μm in OD and 104 μm in OS. Quadrantic, 90-degree average peripapillary RNFL thickness measurements were also within normal limits OU (Fig. 2). However, automatically segmented macular thickness measurements disclosed significantly reduction of the macular ganglion cell layer measured together with the inner plexiform layer (GCL-IPL) in the macular superior nasal quadrant in OD and the superior temporal quadrant in OS (Fig. 2). The quadrantic macular GCL-IPL complex reduction had a direct correspondence with the inferior right quadrantanopia found on VF examination. Magnetic resonance imaging revealed a homogeneous hyperintense non-contrast enhancing lesion on T2-weight images compatible with gliosis (Fig. 3). No abnormality was found in the optic chiasm, tract and the LGN.
Fig. 1.
Above: fundus photography depicting normal appearance of the optic nerve in both eyes. Below: incomplete right inferior homonynous hemianopic visual field defect. OD, right eye; OS: left eye.
Fig. 2.
Optic coherence tomography findings (DRI OCT Triton Plus; Topcon, Inc., Tokyo, Japan). Above: fundus image with demarcation of the circular area around the disc chosen for determination of peripapillary retinal nerve fiber layer (RNFL) thickness measurements. Middle: Average and quadrantic RNFL thickness measurements along with a graphic thickness profile representation of the results. Bottom: significance patterns with color-coded grids corresponding to the ganglion cells layer thickness measurements map, plotted on the fundus photography (no color = within normal limits; yellow = outside the 95% normal limit; red = outside the 99% normal limit). OD, right eye; OS: left eye. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
MRI with Axial FLAIR (A), coronal T2 (B and C), shows small sequelae in the left calcarine sulcus (red arrow A), and normal morphology and signal of optic chiasm (yellow arrow, B) and lateral geniculate body (green arrow, C, A). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
Structural and functional loss of nerve fibers after injury is named axonal degeneration. The expansion of this process to neurons with which they synapse is called trans-synaptic degeneration and can take place in an anterograde way (Wallerian degeneration) or in a retrograde direction (trans-synaptic retrograde degeneration – TRD).10 While the anterograde degeneration has been well demonstrated,11,12 TRD is still controversial in the human visual pathway, except in cases of cortical injuries occurring early in the development.3,13 In congenital cortical lesions, Hoyt at al3 have identified ophthalmoscopically hypoplasia of optic nerve and retina which they have termed “homonymous hemioptic hypoplasia”. The authors recognized that fetal vascular insufficiency associated with cerebral hemiatrophy could have caused direct or TRD in the optic nerves. On the other hand, acquired post-geniculate lesions are not associated with retina and optic nerve structural damage, at least clinically detectable, even on careful fundus examination. Although previous anatomic studies have demonstrated histologically retinal GCL loss from occipital lesions in humans and animal models14, 15, 16; the detection of TRD on clinical examination in humans has long been questioned. Some older case reports have suggested TRD as possible explanation for optic nerve atrophy in patients with occipital lesions acquired during adult life, but alternative mechanisms for anterior visual pathway damage such as papilledema or traumatic optic nerve injury could be not ruled out.17
The widespread use of OCT in clinical practice has changed the concept about the detection of TRD. Nowadays, using high-definition OCT it is possible to evaluate the inner retinal layers individually, and the detection of subtle peripapillary RNFL and macular CGL loss have been unveiled. Even using time-domain technology, Metha and Plant4 have demonstrated the ability of the OCT to detect RNFL loss in patients with homonymous hemianopia from congenital occipital lobe lesions and visible optic nerve atrophy on ophthalmologic examination. With the advent of high-definition spectral-domain (SD) OCT technique, it became clear that subclinical visual pathway TRD in acquired post-geniculate lesions can occur and be unsuspected on clinical grounds. Jindahra et al.5 reported peripapillary RNFL thinning in patients with congenital and acquired occipital lobe lesions causing homonymous hemianopia although macular thicknesses measurements were not performed. Using a SD-OCT, Goto et al.7 confirmed Jindahra et al.'s5 findings. Also, Park et al.18 studying patients with cerebral infarction have demonstrated reduced RNFL thickness providing evidence for TRD of the RGCs. However, since patients with anterior cerebral artery infarction were included in the study, anterior visual pathway at the level of the optic tract could not be ruled out.
Because about 40% of retinal ganglion cells are present in the inner area of macula, the measurement of GCL thickness in the macular area is used to evaluate diseases related to RGC damage. Moon et al.19 have demonstrated considerable reduction of GCL and inner plexiform layer in patients with various kinds of brain lesions. It is worthy to note that the study included pre LGN lesions such as pituitary tumors and optic chiasm glioma. Yamashita et al.20 demonstrated GCL thinning in both eyes of patients with posterior cerebral artery (PCA). However, doubt remains about possible LGN ischemia in patients with PCA infarction and direct axonal damage.
Our case is interesting because macular GCL thinning was clearly able to demonstrate RTD although while no definite abnormality of peripapillary RNFL loss could be established even using high-definition OCT measurements (Fig. 2). Therefore, it shows that GCL measurements are more sensitive parameters than peripapillary RNFL for detecting retinal neural loss. The better performance of macular GCL to detect sectoral neuronal loss as compared to the peripapillary RNFL is most likely due to the complex distribution of the nerve fibers around the disc. For example, the superior, temporal and inferior quadrants of the disc receive retinal nerve fibers from both the nasal and the temporal hemiretina. The result is a lack of specificity between VF loss and optic disc sectors particularly in patients with small quadrantic lesions. On the other hand, with macular thickness measurements a quadrantic superior VF defect may be related to reduced quadrantic inferior macular thickness measurements, resulting in greater sensitivity of detection.2 Our case is also interesting because detailed neuroimaging provided evidence that the lesion was in fact confined to the occipital lobe, ruling-out possible confounding lesions of the anterior visual pathway that might also lead to direct neuronal damage. Michell et al.21 evaluated GCL thickness in patients with homonymous hemianopia and occipital lobe lesions from several etiologies and found out that 15 of 22 patients had TRD of the GCL. Three of their patients were similar to ours, with normal peripapillary RFNL and reduced macular GCL. Although the causes of VF loss in these three patients were not defined in the study it is most likely that they support our finding that macular GCL loss may be more sensitive to detect TRD than peripapillary RNFL, particularly in small quadrantic lesions.
4. Conclusion
High-definition OCT may detect trans-synaptic degeneration in lesions restricted to occipital lobe even when peripapillary RNFL thickness measurements are within normal limits.
Conflicts of interest
The following authors have no financial disclosures: K. H., M.L.R.M.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Patient consent
The patient consented to publication of the case in writing.
Acknowledgments and Disclosures
No funding or grant support.
References
- 1.Cunha L.P., Lopes L.C., Costa-Cunha L.V. Macular thickness measurements with frequency domain-OCT for quantification of retinal neural loss and its correlation with cognitive impairment in alzheimer's disease. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0153830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monteiro M.L., Costa-Cunha L.V., Cunha L.P., Malta R.F. Correlation between macular and retinal nerve fibre layer Fourier-domain OCT measurements and visual field loss in chiasmal compression. Eye (Lond) 2010;24(8):1382–1390. doi: 10.1038/eye.2010.48. [DOI] [PubMed] [Google Scholar]
- 3.Hoyt W.F., Rios-Montenegro E.N., Behrens M.M., Eckelhoff R.J. Homonymous hemioptic hypoplasia. Fundoscopic features in standard and red-free illumination in three patients with congenital hemiplegia. Br J Ophthalmol. 1972;56(7):537–545. doi: 10.1136/bjo.56.7.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta J.S., Plant G.T. Optical coherence tomography (OCT) findings in congenital/long-standing homonymous hemianopia. Am J Ophthalmol. 2005;140(4):727–729. doi: 10.1016/j.ajo.2005.03.059. [DOI] [PubMed] [Google Scholar]
- 5.Jindahra P., Petrie A., Plant G.T. Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain. 2009;132(Pt 3):628–634. doi: 10.1093/brain/awp001. [DOI] [PubMed] [Google Scholar]
- 6.Jindahra P., Petrie A., Plant G.T. The time course of retrograde trans-synaptic degeneration following occipital lobe damage in humans. Brain. 2012;135(Pt 2):534–541. doi: 10.1093/brain/awr324. [DOI] [PubMed] [Google Scholar]
- 7.Goto K., Miki A., Yamashita T. Sectoral analysis of the retinal nerve fiber layer thinning and its association with visual field loss in homonymous hemianopia caused by post-geniculate lesions using spectral-domain optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2016;254(4):745–756. doi: 10.1007/s00417-015-3181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meier P.G., Maeder P., Kardon R.H., Borruat F.X. Homonymous ganglion cell layer thinning after isolated occipital lesion: macular OCT demonstrates transsynaptic retrograde retinal degeneration. J Neuro Ophthalmol. 2015;35(2):112–116. doi: 10.1097/WNO.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 9.Shin H.Y., Park H.Y., Choi J.A., Park C.K. Macular ganglion cell-inner plexiform layer thinning in patients with visual field defect that respects the vertical meridian. Graefes Arch Clin Exp Ophthalmol. 2014;252(9):1501–1507. doi: 10.1007/s00417-014-2706-3. [DOI] [PubMed] [Google Scholar]
- 10.Wang J.T., Medress Z.A., Barres B.A. Axon degeneration: molecular mechanisms of a self-destruction pathway. J Cell Biol. 2012;196(1):7–18. doi: 10.1083/jcb.201108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu L., Yin X., Dai C. Morphologic changes in the anterior and posterior subregions of V1 and V2 and the V5/MT+ in patients with primary open-angle glaucoma. Brain Res. 2014;1588:135–143. doi: 10.1016/j.brainres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Goldby F. A note on transneuronal atrophy in the human lateral geniculate body. J Neurol Neurosurg Psychiatry. 1957;20(3):202–207. doi: 10.1136/jnnp.20.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fletcher W.A., Hoyt W.F., Narahara M.H. Congenital quadrantanopia with occipital lobe ganglioglioma. Neurology. 1988;38(12):1892–1894. doi: 10.1212/wnl.38.12.1892. [DOI] [PubMed] [Google Scholar]
- 14.Cowey A., Alexander I., Stoerig P. Transneuronal retrograde degeneration of retinal ganglion cells and optic tract in hemianopic monkeys and humans. Brain. 2011;134(Pt 7):2149–2157. doi: 10.1093/brain/awr125. [DOI] [PubMed] [Google Scholar]
- 15.Beatty R.M., Sadun A.A., Smith L., Vonsattel J.P., Richardson E.P., Jr. Direct demonstration of transsynaptic degeneration in the human visual system: a comparison of retrograde and anterograde changes. J Neurol Neurosurg Psychiatry. 1982;45(2):143–146. doi: 10.1136/jnnp.45.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanburen J.M. Trans-synaptic retrograde degeneration in the visual system of primates. J Neurol Neurosurg Psychiatry. 1963;26:402–409. doi: 10.1136/jnnp.26.5.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haddock J.N., Berlin L. Transsynaptic degeneration in the visual system; report of a case. Arch Neurol Psychiatr. 1950;64(1):66–73. doi: 10.1001/archneurpsyc.1950.02310250072006. [DOI] [PubMed] [Google Scholar]
- 18.Park H.Y., Park Y.G., Cho A.H., Park C.K. Transneuronal retrograde degeneration of the retinal ganglion cells in patients with cerebral infarction. Ophthalmology. 2013;120(6):1292–1299. doi: 10.1016/j.ophtha.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Moon H., Yoon J.Y., Lim H.T., Sung K.R. Ganglion cell and inner plexiform layer thickness determined by spectral domain optical coherence tomography in patients with brain lesions. Br J Ophthalmol. 2015;99(3):329–335. doi: 10.1136/bjophthalmol-2014-305361. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita T., Miki A., Iguchi Y., Kimura K., Maeda F., Kiryu J. Reduced retinal ganglion cell complex thickness in patients with posterior cerebral artery infarction detected using spectral-domain optical coherence tomography. Jpn J Ophthalmol. 2012;56(5):502–510. doi: 10.1007/s10384-012-0146-3. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell J.R., Oliveira C., Tsiouris A.J., Dinkin M.J. Corresponding ganglion cell atrophy in patients with postgeniculate homonymous visual field loss. J Neuro Ophthalmol. 2015;35(4):353–359. doi: 10.1097/WNO.0000000000000268. [DOI] [PubMed] [Google Scholar]