Abstract
Introduction
Transurethral resection of a bladder tumor (TURBT) using a resectoscope has been standard treatment for bladder cancer. However, no treatment method promotes the repair of resected bladder tissue. The aim of this study was to examine the healing process of damaged bladder tissue after a transurethral injection of bone marrow mesenchymal stem cells (MSCs) into the bladder. An injection of magnetic MSCs meant that they accumulated in the damaged area of the bladder. Another aim of this study was to compare the acceleration effect of MSC magnetic delivery on the repair of bladder tissue with that of non-magnetic MSC injection.
Methods
Using the transurethral approach to avoid opening the abdomen, electrofulguration was carried out on the anterior wall of the urinary bladder of white Japanese rabbits to mimic tumor resection. An external magnetic field directed at the injured site was then applied using a 1-tesla (T) permanent magnet. Twelve rabbits were divided into three groups. The 1 × 106 of magnetically labeled MSCs were injected into the urinary bladder with or without the magnetic field (MSC M+ and MSC M-groups, respectively), and phosphate-buffered saline was injected as the control. The effects of the injections in the three groups at 14 days were examined using 4.7-T magnetic resonance imaging (MRI) then macroscopically and histologically. The mRNA expressions of several cytokines in the repair tissues were assessed using real-time polymerase chain reaction.
Results
The macroscopic findings showed the area of repair tissue in the MSC M+ group to be larger than that in either the MSC M-group or control group. MRI clearly depicted the macroscopic findings. The histological study showed that repair of the cauterized area with myofibrous tissue was significantly better in the MSC M+ group than that in either the MSC M-group or control group, although there was no significant difference in several mRNA cytokines among the three groups at 14 days after surgery.
Conclusions
The magnetic delivery of MSCs shows promise as an effective, minimally invasive method of enhancing tissue regeneration after TURBT.
Keywords: Urinary bladder, Cancer, Bone marrow, Mesenchymal stem cell, Transurethral resection, Regeneration
Abbreviations: BC, urinary bladder cancer; NMIBC, non-muscle invasive urinary bladder cancer; TURBT, transurethral resection of bladder tumor; MSC, mesenchymal stem cell; MRI, Magnetic resonance imaging; FBS, fetal bovine serum; SPION, superparamagnetic iron oxide nanoparticle; PBS, phosphate-buffered saline; H&E, hematoxylin and eosin; TR, repetition time; TE, echo time; FA, flip angle; NEX, number of excitations; αSMA, α-smooth muscle actin; PCR, polymerase chain reaction
1. Introduction
The incidence of urinary bladder cancer (BC) varies from country to country, but BC is the fourth most common cancer among men in developed countries [1]. BCs are mainly defined as urothelial cancers originating from the urothelium, and non-muscle invasive BC (NMIBC) is diagnostically limited to the urothelium or underlying lamina propria in three-quarters of cases. Thus, the first line of treatment is a transurethral resection of the bladder tumor (TURBT). When a resected specimen is histopathologically diagnosed as a low-grade Ta tumor, further treatment is not necessary. However, stage T1 is heterogeneous, and the 5-year recurrence rate after TURBT for a high-grade T1 tumor is 72.5% [2]. Such patients need frequent TUR or Bacille de Calmette et Guérin (BCG) instillation, which leads to accumulated damage to the bladder wall. To heal the bladder wall more efficiently, development of methods for tissue degeneration is very important.
Autogenic or allogeneic mesenchymal stem cells (MSCs), hematopoietic stem/progenitor cells such as peripheral blood CD 34⁺and CD 133⁺cells, adipose-derived stem cells, and induced pluripotent stem cells, which can differentiate into mesodermal lineages such as bone, cartilage, fat, muscle, neural tissue, and other tissues, are cell candidates for regeneration therapy. Recent reports indicate that these cells not only differentiate into mesoderm but also endoderm and ectoderm lineages [2], [3], [4], [5], [6]. Among them, autologous bone marrow-derived MSCs have been often used for tissue engineering and cell therapy in clinical situations because of several advantages such as easily increasing the number of MSCs under cultivation and no immunological reaction after implantation. We have recently developed a magnetic delivery system with which injected magnetically labeled cultured MSCs are attracted to the damaged cartilage portion using an external magnet, resulting in good and efficient cartilage repair [7], [8], [9]. We hypothesized that this delivery system may be useful for treating a damaged portion of the urinary bladder after TURBT since the urinary bladder is not a closed cavity, unlike for example the knee joint. Although several small incisions are needed to treat a knee joint with this system, the urinary bladder is accessible without incision via the transurethral approach. Bladder irritation symptoms after TUR and intravesical injection therapy are serious complications after bladder cancer surgery. The aim of this study was to first investigate whether the transurethral approach is feasible for rabbits, second to examine if magnetic resonance imaging (MRI) at 4.7 tesla (T) can depict the regeneration conditions after magnet delivery treatment, and third to evaluate the effect of using an external magnet, compared with not using one, on magnetically labeled MSCs in an injured bladder.
2. Materials and methods
2.1. Animals
Twelve male Japanese white rabbits weighing 2.5–2.99 kg were used in this study. The rabbits were housed singly in cages in the animal experimentation facility. All procedures were approved by the Ethics Committee for Experimental Animals of Hiroshima University.
2.2. Culture of MSCs
The rabbits were anesthetized with an intravenous injection of xylazine (3 mg/kg) and ketamine (10 mg/kg), then 10 mL of bone marrow from the iliac crest was aspirated using an 18-gauge needle on a 10-mL syringe with 1 mL of heparin sodium (1000 U/mL). The aspirated sample was centrifuged for 5 min at 1500 rpm; the subsequent supernatant, including heparin sodium, was disposed of. The extract was resuspended in 7 mL of culture medium composed of Dulbecco`s modified Eagle medium (DMEM; Sigma–Aldrich Corp., St. Louis, MO) with 15% fetal bovine serum (FBS; Sigma–Aldrich Corp., St. Louis, MO) and 1% antibiotics (penicillin, streptomycin, and Amphotericin B; Lonza, Basel, Switzerland). Cells were suspended in 10 mL of culture medium and incubated at 37 °C in a humidified atmosphere of 5% CO2. The medium was not changed for the first 7 days; thereafter, it was changed once every 3 days.
2.3. Magnetic labeling of MSCs
Ferucarbotran (Resovist® Inj.; Fujifilm RI Pharma Co. Ltd., Kanagawa prefecture, Japan) is an intracellular magnetic labeling contrast agent composed of superparamagnetic iron oxide nanoparticles (SPIONs) [0.5 mmol (27.9 mg) Fe/ml]. Ferucarbotran was added to the culture medium, and the MSCs were labeled overnight with ferucarbotran, without transfection of any of the incorporation facilitators such as protamine sulfate or poly-l-lysine. The cells were then washed twice with phosphate-buffered saline (PBS; Wako Pure Chemical Industries Ltd., Osaka prefecture, Japan). After magnetic labeling, the MSCs were referred to as magnetically labeled MSCs.
A paraffin-embedded cell block of MSCs and magnetically labeled MSCs were prepared. 25 mL of 37% formalin and PBS were put into a beaker. The uptaken iron was assessed through hematoxylin and eosin (H&E) staining and Berlin blue staining.
Magnetically labeled MSCs (1× 106/300 μL Dulbecco's PBS) and MSCs were injected into a sink (95 × 95 mm) full of PBS.
2.4. Accumulation capacity of magnetically labeled MSCs under influence of magnetic force
Magnetically labeled MSCs (5× 106/300 μL Dulbecco's PBS) were injected into a tissue culture flask full with PBS. In the magnetic force group, injection of magnetically labeled MSCs was performed under the influence of an external magnetic force. In the control group, cell injection was performed without the influence of a magnetic force.
2.5. Surgical procedure
The rabbits were anesthetized with an intravenous injection of 30 mg/kg of pentobarbital, and a 13 Fr rigid child cystoscope (Olympus Co., Ltd., Tokyo, Japan) was inserted transurethrally into the urinary bladder. In experiments using TUR we thought that the bladder walls of rabbits were thin and made perforation, so we decided to conduct experiments using coagulation. A 20 × 20 mm area of the anterior wall of the urinary bladder's mucosa was cauterized at 20 W for 1 sec using a 1 × 3 mm coagulating device. After cauterization, 1 × 106 magnetically labeled cells were injected into the urinary bladder with and without the magnetic field (MSC M+ and MSC M-groups, respectively). In the control group, phosphate-buffered saline was injected into the urinary bladder. In the MSC M+ group, a 1-T permanent magnet (130× 130 × 47 mm) was placed on the lower abdomen of the rabbit and kept there for 10 min. In the MSC M- and control groups, the rabbits stayed in a supine position for 10 min. After that, urine was removed from the urinary bladder by catheterization in all three groups.
2.6. MRI assessment
Morphological evaluation of the urinary bladder was carried out using a 4.7-T superconducting magnet system (BioSpec47/40USR, Bruker BioSpin, Ettlingen, Germany) with a transmit/receive quadrature volume coil (154 mm inner diameter). We used MRI in order to follow up the regeneration of the bladder wall longitudinally. The rabbits were anesthetized with sustained inhalation of 2.0% isoflurane and placed on an animal bed in a prone position and held on the inside of the magnet center. The sagittal and axial MRI sequence involved proton density-weighted imaging with the rapid acquisition of the refocused echo technique (repetition time [TR], 1338 msec; echo time [TE], 28.5 msec; flip angle [FA], 90–131 deg; echo train length, 8; matrix, 192*192; number of excitations [NEX], 8; and acquisition time; 4 min 16 sec) and T2*-weighted gradient recalled echo images (TR, 364 msec; TE, 11 msec; FA, 30; matrix, 256*256; NEX, 1; and acquisition time, 2 min 40 sec). Each sequence was obtained using the respiratory synchronization technique with a field of view of 110 *100 mm; thickness of 3 mm; and slice of 20, and both sequences incorporated chemical shift selective radiofrequency pulses for effective fat suppression.
The regenerated bladder was evaluated using 4.7-T MRI in each group at 14 days after treatment. The rabbits were anesthetized with sustained inhalation of 2.0% isoflurane and injected with 20 mL of saline, after which urethral catheterization was carried out. The rabbits were placed on the animal bed in a prone position and held on the inside of the magnet center. The sagittal and axial MRI sequence was carried out on the rabbit's lower abdomen, and the regeneration tissue was measured at the cross section of its maximum area. The size of the regenerated tissue in each group was compared.
2.7. Histological evaluation
The rabbits were sacrificed at 14 days after transplantation. Urinary bladders were fixed in 10% paraformaldehyde phosphate-buffered solution (Wako Pure Chemical Industries Ltd.) for 24 h. The samples were embedded in paraffin blocks then cut into 4-μm sections sagittally. For histological evaluation, sections were stained with H&E (Muto Pure Chemicals Co. Ltd., Tokyo, Japan) and immunostained with α-smooth muscle actin (αSMA; Abcam, Cambridge, UK). The reaction for visualization was carried out using an avidin-biotin peroxidase system (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA).
2.8. Real-time PCR
The mRNA expressions of repair tissue were analyzed at 14 days after surgery. Total RNA was isolated from repair tissue using ISOGEN (Nippon Gene, Tokyo, Japan) according to the manufacturer's protocol. Total RNA (2.0 μg) was reversed-transcribed into cDNA using the SuperScript VILO cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA). Real-time polymerase chain reaction (PCR) analysis was conducted using the CFX96 real-time PCR system (Bio-Rad Laboratories, Hercules, CA), and KOD SYBR qPCR Mix (Toyobo, Osaka, Japan) was used to detect levels of the mRNA. Because one of the most representative previous study (Imamura et al.) using cell sheets of MSCs showed elevated expression of FGFs and IL-2 as the marker of tissue regeneration, the expressions of these molecular were assessed [10]. The mRNA expression levels of these samples were normalized to β-actin (PrimePCR™ SYBR® Green Assay: ACTB, Rabbit, Bio-Rad) as an endogenous control. Data were analyzed using the comparative ΔΔCT method. The primer sequences are shown in Table 1.
Table 1.
The characteristics of primers utilized for real-time polymerase chain reaction.
| Genes | Primer sequence forward | Primer sequence reverse |
|---|---|---|
| FGF1 | 5′-GTAAGCAACGCGCTTCTACG-3′ | 5′-GGGTTTGAGCCATCTCGGAA-3′ |
| FGF2 | 5′-GCTGTACTGCAAAAACGGGG-3′ | 5′-TTGCCTGGCCATTCAACAAAG-3′ |
| IL2 | 5′-GGCAAAAACTCTCATGGGGGA-3′ | 5′- ACTCGATGCTGAGATGATGCT-3′ |
2.9. Statistical analysis
Results were expressed as mean-standard deviation. Statistical differences were determined using the Excel Statistics program (Esumi Co., Ltd. Tokyo, Japan). The Kruskal Wallis test followed by the Steel-Dwass post-hoc test were conducted for multiple comparisons. The p-values less than 0.05 were considered statistically significant.
3. Results
3.1. Magnetic labeling of MSCs
Hematoxylin and eosin staining and Berlin blue staining of cell block showed the uptake of iron particles in the magnetically labeled MSCs, while no stainable iron was detected in the MSCs without magnetic labeling (Fig.1A–D).
Fig. 1.
Microscopic findings of cell block. In the MSC cells, there was no brown iron stained with (A, MSC M-x40) H&E, and no blue iron stained with (B, MSC M-x40) Berlin blue stain. There was brown iron stained with (C, MSC M+ x40) H&E and blue iron stained with (D, MSC M+ x40) Berlin blue stain.
3.2. Accumulation capacity of magnetically labeled MSCs under influence of magnetic force
In the control group, magnetically labeled MSCs fell down vertically with gravity (Fig. 2A). In the magnetic force group (Fig. 2B), magnetically labeled MSCs fell down not vertically but diagonally, moved in the direction of the generated magnetic force, and accumulated to the side wall of the flask under the influence of gravity and the magnetic force.
Fig. 2.
A. The magnetic labeled MSCs dropping into PBS in a flask fell straight down by the by the force of gravity. B. When a neodymium magnet was set on one side of a flask, most of dropped MSCs was attracted to a magnet.
3.3. MRI
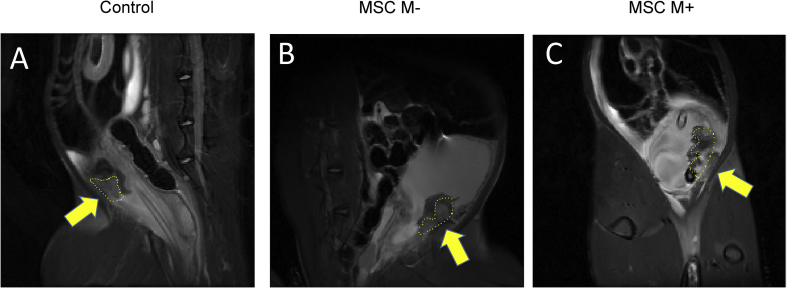
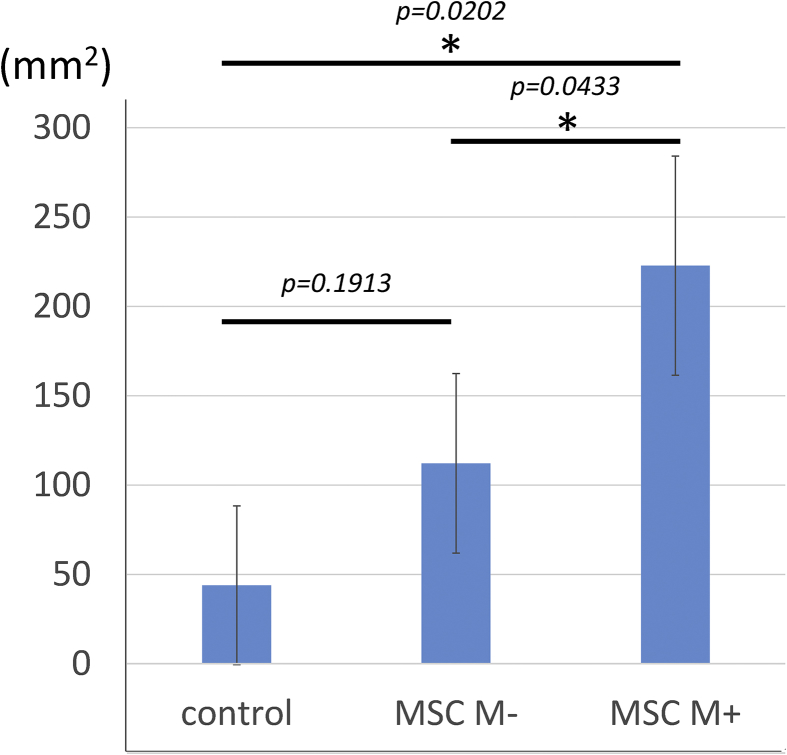
The volume of repaired bladder tissue was assessed using the sagittal area view of MRI at 14 days after treatment (Fig. 3). The mean values of the sagittal area of the repaired tissue was 222.9 ± 61.4 mm2 in the MSC M+ group (Fig. 3C), 112.1 ± 50.2 mm2 in the MSC M-group (Fig. 3B), and 43.9 ± 44.4 mm2 in the control group (Fig. 3A). The area of repaired tissue in the MSC M-group was significantly larger than that in the control group. In addition, that in MSC M+ group was significantly larger than those in the MSC M- and control groups (see Fig. 4).
Fig. 3.
MRI findings of the rabbit bladder. The regeneration areas were surrounded by yellow dotted lines (arrows). In the control and MSC M-groups, there was a small amount regenerated tissue (A, control SAG, B MSC M- SAG). In the MSC M+ group, there was more regenerated tissue than in the other two groups (C, MSC M+).
Fig. 4.
Comparison of regenerated areas among three groups. In the MSC M+ group, the regenerated area was significantly larger than that in the control and MSC M-groups.
3.4. Macroscopical and histological evaluation
Macroscopically, ulcerisation and edema were confirmed in the cauterization area of the control group (Fig. 5A) 14 days after treatment. In the MSC M-group, a small area of regenerated tissue was confirmed, and in the MSC M+ group a larger area of regenerated tissue was confirmed (Fig. 5B and C).
Fig. 5.
Macroscopic findings of the rabbit bladder in representative cases of control (A), MSC M- (B), and MSC M+(C) groups. In the control group, ulcer and edema were confirmed at the cauterization area (A). In the MSC M- and MSC M+ groups, ulcerative regions were replaced with regenerative tissues (B) and (C).
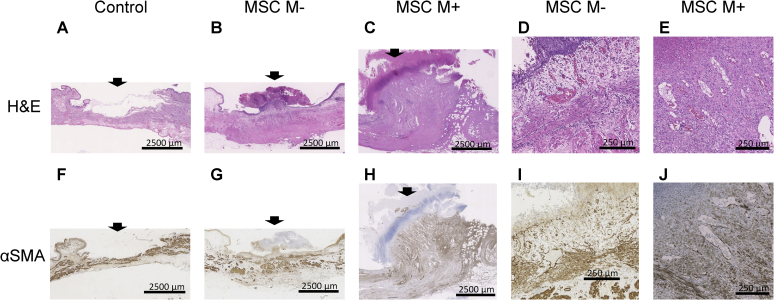
Histological staining with H&E did not show the presence of any urothelial cells, and necrotic tissue adhered to the cauterization area in the control group (Fig. 6A and F). Histological staining with H&E and immunohistochemical staining with αSMA showed several vessels and myofibrous tissue in the MSC M-group (Fig. 6B, D, G, and I). The mean numbers of vessels in the control, MSC M-, and MSC M+ groups were 10 ± 7.87, 21.8 ± 6.30, and 23.3 ± 4.21, respectively. There were more vessels and myofibrous tissue was regenerated in the MSC M+ group (Fig. 6C, E, H, and J) but there was no significant difference among the three groups. Concerning the comparison of the size of the repaired myofibrous tissue among the three groups, the width x height of the area of regenerated tissue was calculated. The mean areas in the control, MSC M-, and MSC M+ groups were (4.76)±(3.67), (10.8)±(6.70), and (24.7)±(6.23) mm2, respectively. Although there was no significant difference in area between the control and MSC M-groups, there was a statistically significant difference between the areas in the MSC M+ and those in the MSC M- and control groups (Fig. 7).
Fig. 6.
Histological findings of the rabbit bladder tissues stained with H&E (A–E) and αSMA (F–J). (A) There was a lack of urothelial cells, and the adhered necrotic tissue was stained with H&E (A). Smooth muscle stained with αSMA (F) was exposed at the surface of the bladder wall in the control group. There were several vessels stained with H&E (D), and myofibrous tissue stained with αSMA (G and I) was regenerated in the MSC M-group. Both vessels and fibrous tissue were abundant in the MSC M+ and MSC M-groups. Scale bar: A-C and F-H 2500 μm; D, E, I, and J 250 μm.
Fig. 7.
Size of the histological regenerated tissue in each group at 14 days. The regeneration areas (length x depth) were measured and compared. In the MSC M+ group, the regeneration area was significantly larger than those in the control and MSC M-groups (p = 0.0433, p = 0.0209).
3.5. Expressions of mRNA in repair tissues
To elucidate the factors contributing to bladder tissue repair, the mRNA expressions of FGF-1, FGF-2, and IL-2 in the repair tissues were analyzed at 14 days after surgery using real-time PCR. There was no significantly difference in the mRNA expressions among the three groups (FGF-1: control 1.00 ± 0.47, MSC M- 0.94 ± 0.61, MSC M+ 0.75 ± 0.66; FGF-2: control 1.00 ± 0.66, MSC M- 1.11 ± 0.85, MSC M+ 0.49 ± 0.58; IL-2: control 1.00 ± 0.69, MSC M- 2.35 ± 0.66, MSC M+ 0.97 ± 0.52) (Fig. 8).
Fig. 8.
The mRNA expressions of FGF-1, FGF-2, and IL-2 in the repair tissues at 14 days after surgery. There was no significantly difference among the three groups.
4. Discussion
The technical feasibility of our study was clear, both in terms of the transurethral approach, which created partial burn damage of a rabbit bladder as a mimic of tumor resection, and regarding the treatment of the damaged area by injection of autologous MSCs. In addition, the MSC M+ group was demonstrated to induce more effective myofibrous tissue regeneration than the MSC M-group. The regenerated tissue was clearly depicted through MRI. This trial was the first application in the world to use magnetically labeled MSCs for the repair of the urinary bladder with a minimally invasive procedure.
Traditional bladder reconstruction uses an autologous intestinal graft, sometimes resulting in several postoperative complications such as infection, malignant transformation, and stone formation, while widely studied tissue-engineered bladders have become an established alternative for patients needing cystoplasty. Tissue-engineered bladders are composed of several different types of cells and scaffolds, including synthetic and biodegradable materials. Studies have proven a more positive effect of a scaffold seeded by cells than that of a scaffold without cells.
Atala et al. reported in 2006 on the successful implantation of tissue-engineered autologous bladders composed of biodegradable bladder-shaped scaffolds made of collagen and a composite of collagen and polyglycolic acid seeded by urothelial and muscle cells. That study focused on seven patients who had suffered from high-pressure or poorly compliant bladders due to myelomenigocele [11]. Hence, the implantation of such tissue-engineered autologous bladders offers a promising alternative to traditional reconstruction for patients who are unable to undergo the latter procedure.
These procedures require an abdominal opening to enable the grafting of a scaffold. It is a reasonable to conduct open surgery for the total resection of a urinary bladder due to an invasive malignant tumor or a poorly compliant bladder to replace it with a newly formed substitute. However, in the case of localized tumor formation in a bladder, a less invasive treatment is more appropriate and beneficial, whereby the tumor is resected through the TU approach and injected with MSCs that can differentiate into several types of mature cells. Several cell types are considered potential candidates for cell therapy, for example, hematopoietic stem/progenitor cells, MSCs from bone marrow, synovial tissue or adipose tissue, a combination of hematopoietic stem cells and MSCs, and induced pluripotent stem cells. To avoid an immunological reaction, the use of autologous cells is ideal. In addition, bone-marrow-derived MSCs can be easily cultured to increase the number of MSCs and have been safely and widely used not only in experimental but also in clinical settings for tissue engineering and cell therapy.
Since the final goal of this experimental study was on clinical use, we selected autologous bone-marrow-derived MSCs as the cell source and used white Japanese rabbits that were subjected to partial injury and injection of the cells through the TU approach. Although the partial injury was artificially inflicted to the anterior wall of the bladder before the study, it was demonstrated to be successfully done. This experimental approach will become an animal model in future studies for the partial regeneration of the bladder due to its minimally invasive treatment.
According to a 2010 report by Imamura et al., the injected autologous MSCs in cryo-injured urethral sphincters differentiated into muscle-like cells and formed contacts with similar cells, creating a layered muscle structure by 14 days [12]. We previously observed relatively good repair in the control group at 28 days, and at 14 days after surgery we investigated MRI and the histology of the bladders with partial injury in the three groups. Through our investigation into the effects of injected magnetically labeled cells on repair with an external magnet, we also learned that even the injection of cells without the use of such a magnet could induce larger amount of regenerated tissue at the injured site.
Agung et al. demonstrated that injection of many cells into the knee cavity of rats with cartilage, meniscal, and ligamentous injuries, induced scar formation in the knee joint, although the injured portions healed [13]. Under non-magnet conditions, the injected cells were scattered in the urinary bladder with some of the cells attracted to the injured portion. However, there was no evidence that a concentration of 2 × 10⁶cells induced scar formation. There was a significant difference in the volume of regenerated tissue through MRI, but the magnetic cells were not visible from T2-weighted gradient-echo MR imaging. There was also a significant difference in the amount of regenerated tissue at the injured site between the two groups through MRI. Since the MRI findings were demonstrated to be similar to the microscopic findings, T2-weighted gradient-echo MR imaging may be of use when evaluating regenerated tissue over a follow-up period during rabbit experimental studies. In our study, no iron was observed, either through MRI or at histology 14 days after injection. Song et al. demonstrated that SPIO-labeled human MSCs were observed during 1.5-T MRI and histologically even 12 weeks after injection in rat and rabbit bladders [14]. The difference between our findings and theirs may be due to different injection methods. They injected cells into the smooth muscle of the bladder wall, whereas we injected them into the bladder cavity.
Since Imamura et al. in 2009 demonstrated that the expression of growth-related mRNAs is significantly upregulated in the freeze-injured urinary bladder three days after freezing the urinary bladder [15], Although we hypothesized a significant difference in the expression of growth-related mRNAs among the three groups at 14 days, there was no significant difference among the three groups. The upregulated expression of mRNAs at the early stage after injury might decrease to the standard level 14 days after surgery, although the expression of mRNAs should be examined at the early stage of between one and three days.
In 2011, Imamura et al. injected autologous MSCs into dysfunctional urethral sphincters that had been cryo-injured 7 days before and demonstrated that sphincter function and muscle regeneration were significantly better than those of the control, which was treated with cell-free solutions [15].
In 2015, Imamura et al. conducted an experiment on bladder regeneration using cell sheets of MSCs [10]. The cell sheets were patched onto the irradiated anterior bladder wall. The cell-sheet-transplanted bladders had smooth muscle layers and acetylcholinesterase-positive nerve fibers, and bladder function improved significantly. Many researchers have successfully regenerated the bladder by injecting MSCs into the bladder wall [15], [17], [18] and using MSC cell sheets [10], [16]. Their procedure required an abdominal incision to expose the bladder. A less invasive procedure is more desirable for a clinical setting.
The injection of many cells may run the risk of scar formation. Thus, a relatively small number of cells should ideally be accumulated in the injured area rather than in the intact area to guarantee successful and complication-free repair of the injured site. Magnet therapy was developed according to this concept. Using magnetically labeled autologous MSCs and a 1-T permanent magnet, five patients with a cartilage defect in the knee joint demonstrated to have improved clinically and arthroscopically at 1 year after microfracture of the defect area and cell implantation.
There were several limitations of this study. The burned necrotic tissue was not able to be completely removed, resulting in incomplete exposure of the raw surface, although saline injection flush was tried several times to remove the necrotic tissue. We need to investigate the mechanism on more myofibrous tissue in the MSC M+ group by analyzing mRNAs at the early stage after surgery. We also need to investigate the appropriate minimum number of magnetically labeled MSCs for a certain injured site. In this study, the tissue repair was assessed at 14 days after operation because the repair of urothelium was observed in the control group similar to MSC M+ group at 4 weeks after operation in our pilot study. The potential of urothelial tissue for spontaneous repair might be different between rabbit and human. Although the survival duration of transplanted MSCs was unclear in this study, our previous reports showed that the magnetic targeting of MSCs enhance the survival of transplanted MSCs at the lesion using in vivo bioluminescence imaging [17], [18]. The improvement of the survival rate of transplanted MSCs by the magnetic targeting might contribute to the enhancement of tissue repair. Another limitation was about the mechanism of regeneration in this procedure. In the previous study, Imamura et al. showed that regenerated tissue was derived from injected MSCs themselves rather than tissue of host. This mechanism has not been made sure in this study. Another study is required to clarify this point in the near future.
In this study, we wanted to focus on the bladder tissue repair after TURBT and to show the possibility of application of magnetic targeting method for regenerative therapy in the field of urinary tract as the future direction of the treatment to enhance repair process, which was the first report. We prepare the animal model with damaged bladder using electrocoagulation as the simple and reproducible model. Further studies using more sophisticated models are needed to clarify the precise mechanism of the healing enhancement in the magnetic therapy and to confirm the usefulness of magnetic targeting for regenerative therapy for the damaged bladder.
5. Conclusion
We have shown the ability to deliver magnetically labelled MSCs to a cauterization area in the bladder. We suggested that the clinical application of this novel stem-cell delivery system using m-MSCs and an external magnetic force is a potential therapeutic option for treating bladder cancer.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgements
We would like to acknowledge Prof. Ochi's contribution in providing the concept of the magnetic cell delivery system. This work was supported by the Grant-in-Aid for Scientific Research (C) to S.I. (Grant no. 16K11050) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) in Japan. Additional support was obtained from the Grant by Ryokufu-kai. Fujii Seturo Memorial Osaka Basic Science Grant, Astellas Academic support (Grant No.RS2016A000714 and RS2017A000971), and Takeda Research Support (Grant No. TKDS20170619043).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Haggstrom C., Liedberg F., Hagberg O., Aljabery F., Strock V., Hosseini A. Cohort profile: the Swedish National register of urinary bladder cancer (SNRUBC) and the bladder cancer data base Sweden (BladderBaSe) BMJ Open. 2017;7(9):e016606. doi: 10.1136/bmjopen-2017-016606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeng G., Lai K., Li J., Zou Y., Huang H., Liang J. A rapid and efficient method for primary culture of human adipose-derived stem cells. Organogenesis. 2013;9(4):287–295. doi: 10.4161/org.27153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J.H., Lee H.J., Song Y.S. Treatment of bladder dysfunction using stem cell or tissue engineering technique. Kor J Urol. 2014;55(4):228–238. doi: 10.4111/kju.2014.55.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bury M.I., Fuller N.J., Wethekam L., Sharma A.K. Bone marrow derived cells facilitate urinary bladder regeneration by attenuating tissue inflammatory responses. Cent Eur J Urol. 2015;68(1):115–120. doi: 10.5173/ceju.2015.01.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snow-Lisy D.C., Diaz E.C., Bury M.I., Fuller N.J., Hannick J.H., Ahmad N. The role of genetically modified mesenchymal stem cells in urinary bladder regeneration. PLoS One. 2015;10(9):e0138643. doi: 10.1371/journal.pone.0138643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan Y.Y., Sandlin S.K., Kurzrock E.A., Osborn S.L. The current use of stem cells in bladder tissue regeneration and bioengineering. Biomedicines. 2017;5(1) doi: 10.3390/biomedicines5010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi T., Ochi M., Yanada S., Ishikawa M., Adachi N., Deie M. A novel cell delivery system using magnetically labeled mesenchymal stem cells and an external magnetic device for clinical cartilage repair. Arthroscopy. 2008;24(1):69–76. doi: 10.1016/j.arthro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Kamei G., Kobayashi T., Ohkawa S., Kongcharoensombat W., Adachi N., Takazawa K. Articular cartilage repair with magnetic mesenchymal stem cells. Am J Sports Med. 2013;41(6):1255–1264. doi: 10.1177/0363546513483270. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoud E.E., Kamei G., Harada Y., Shimizu R., Kamei N., Adachi N. Cell magnetic targeting system for repair of severe chronic osteochondral defect in a rabbit model. Cell Transplant. 2016;25(6):1073–1083. doi: 10.3727/096368915X689613. [DOI] [PubMed] [Google Scholar]
- 10.Imamura T., Ogawa T., Minagawa T., Yokoyama H., Nakazawa M., Nishizawa O. Engineered bone marrow-derived cell sheets restore structure and function of radiation-injured rat urinary bladders. Tissue Eng Part A. 2015;21(9–10):1600–1610. doi: 10.1089/ten.TEA.2014.0592. [DOI] [PubMed] [Google Scholar]
- 11.Atala A., Bauer S.B., Soker S., Yoo J.J., Retik A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. The Lancet. 2006;367(9518):1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 12.Minagawa T., Imamura T., Igawa Y., Aizawa N., Ishizuka O., Nishizawa O. Differentiation of smooth muscle cells from human amniotic mesenchymal cells implanted in the freeze-injured mouse urinary bladder. Eur Urol. 2010;58(2):299–306. doi: 10.1016/j.eururo.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Agung M., Ochi M., Yanada S., Adachi N., Izuta Y., Yamasaki T. Mobilization of bone marrow-derived mesenchymal stem cells into the injured tissues after intraarticular injection and their contribution to tissue regeneration. Knee Surg Sports Traumatol Arthrosc. 2006;14(12):1307–1314. doi: 10.1007/s00167-006-0124-8. [DOI] [PubMed] [Google Scholar]
- 14.Song Y.S., Ku J.H. Monitoring transplanted human mesenchymal stem cells in rat and rabbit bladders using molecular magnetic resonance imaging. Neurourol Urodyn. 2007;26(4):584–593. doi: 10.1002/nau.20351. [DOI] [PubMed] [Google Scholar]
- 15.Imamura T., Yamamoto T., Ishizuka O., Gotoh M., Nishizawa O. The microenvironment of freeze-injured mouse urinary bladders enables successful tissue engineering. Tissue Eng Part A. 2009;15(11):3367–3375. doi: 10.1089/ten.TEA.2009.0038. [DOI] [PubMed] [Google Scholar]
- 16.Sharma A.K., Hota P.V., Matoka D.J., Fuller N.J., Jandali D., Thaker H. Urinary bladder smooth muscle regeneration utilizing bone marrow derived mesenchymal stem cell seeded elastomeric poly(1,8-octanediol-co-citrate) based thin films. Biomaterials. 2010;31(24):6207–6217. doi: 10.1016/j.biomaterials.2010.04.054. [DOI] [PubMed] [Google Scholar]
- 17.Kodama A., Kamei N., Kamei G., Kongcharoensombat W., Ohkawa S., Nakabayashi A. In vivo bioluminescence imaging of transplanted bone marrow mesenchymal stromal cells using a magnetic delivery system in a rat fracture model. J Bone Joint Surg Br. 2012;94(7):998–1006. doi: 10.1302/0301-620X.94B7.28521. [DOI] [PubMed] [Google Scholar]
- 18.Nakabayashi A., Kamei N., Sunagawa T., Suzuki O., Ohkawa S., Kodama A. In vivo bioluminescence imaging of magnetically targeted bone marrow-derived mesenchymal stem cells in skeletal muscle injury model. J Orthop Res. 2013;31(5):754–759. doi: 10.1002/jor.22282. [DOI] [PubMed] [Google Scholar]