Abstract
Movement disorders associated with antipsychotic medications are relatively common, stigmatising, and potentially disabling. Their prevalence in people with psychosis who are prescribed second-generation antipsychotics (SGAs) is uncertain, as is their level of recognition by clinicinas. We conducted meta-analyses of randomised controlled trials included in the Cochrane Database of Systematic Reviews on schizophrenia and schizophrenia-like psychoses to estimate the prevalence of new-onset dystonia, akathisia, parkinsonism, and tremor with SGAs (amisulpride, asenapine, aripiprazole, clozapine, olanzapine, paliperidone, quetiapine, risperidone, L-sulpiride, and ziprasidone) approved in Canada and the UK, comparing them with haloperidol and chlorpromazine. We used a random effects model because of the heterogeneity between-studies in drug dosage and method of ascertainment of movement disorders. Our systematic search yielded 37 Cochrane systematic reviews (28 for SGAs), which generated 316 informative randomised controlled trials (243 for SGAs). With respect to SGAs, prevalence estimates ranged from 1.4% (quetiapine) to 15.3% (L-sulpiride) for dystonia, 3.3% (paliperidone) to 16.4% (L-sulpiride) for akathisia, 2.4% (asenapine) to 29.3% (L-sulpiride) for parkinsonism, and 0.2% (clozapine) to 28.2% (L-sulpiride) for tremor. Prevalence estimates were not influenced by treatment duration, the use of a flexible or fixed dosing scheme, or whether studies used validated instruments for the screening/rating of movement disorders. Overall, we found high overlap on the prevalence of new-onset movement disorders across different SGAs precribed for established psychoses. Variations in prevalence figures across antipsychotic medications were observed for the different movement disorders. Differences in pharmacological properties, such as for the dopamine D2 R association rate and serotonin 5-HT2A antagonism, could contribute to this variation.
Keywords: extrapyramidal syndromes, schizophrenia, systematic reviews, tardive dyskinesia
Abstract
Les troubles du mouvement associés aux antipsychotiques sont relativement communs, stigmatisants et potentiellement handicapants. Leur prévalence chez les personnes souffrant de psychose à qui l'on a prescrit des antipsychotiques de la deuxième génération (ADG) est incertaine de même que le niveau de reconnaissance qu'en ont les cliniciens. Nous avons mené des méta-analyses d'essais randomisés contrôlés inclus dans la Base de données de Cochrane de revues systématiques sur la schizophrénie et les psychoses de type schizophrénie, afin d'estimer la prévalence de la dystonie, l'acathisie, le parkinsonisme et le tremblement récemment apparus avec les ADG approuvés au Canada et au Royaume-Uni (amisulpride, asénapine, aripiprazole, clozapine, olanzapine, palipéridone, quétiapine, rispéridone, L-sulpiride, et ziprasidone), en les comparant avec l'halopéridol et la chlorpromazine. Nous avons utilisé un modèle d'effets aléatoires à cause de l'hétérogénéité entre les études de la posologie et de la méthode de détermination des troubles du mouvement. Notre recherche systématique a relevé 37 revues systématiques Cochrane (28 pour les ADG) qui ont produit 316 essais randomisés contrôlés informatifs (243 pour les ADG). En ce qui concerne les ADG, les estimations de prévalence se situaient entre 1,4% (quétiapine) et 15,3% (L-sulpiride) pour la dystonie, entre 3,3% (palipéridone) et 16,4% (L-sulpiride) pour l'acathisie, entre 2,4% (asénapine) et 29,3% (L-sulpiride) pour le parkinsonisme, et entre 0,2% (clozapine) et 28,2% (L-sulpiride) pour le tremblement. Les estimations de prévalence n'étaient pas influencées par la durée du traitement, l'utilisation d'un schéma de dosage flexible ou fixe, ou si les études utilisaient des instruments validés pour le dépistage ou le classement des troubles du mouvement. En général, nous avons constaté un fort chevauchement de la prévalence des troubles du mouvement récemment apparus dans différents ADG prescrits pour une psychose établie. La variation des chiffres de la prévalence entre antipsychotiques a été observée pour les différents troubles du mouvement. Les différences des propriétés pharmacologiques comme la vitesse d'association de D2R et de l'antagonisme 5-HT2A pourraient contribuer à cette variation.
Hypokinetic and hyperkinetic movement disorders in people with psychotic illness are classically linked to antipsychotic medication. These motor symptoms may occur shortly after the introduction of dopamine receptor-blocking antipsychotics (acute akathisia, drug-induced parkinsonism, acute dystonia) or as delayed manifestations (tardive dyskinesia and its variants, tardive dystonia and tardive akathisia).1,2 Acute and tardive movement disorders are believed to result from, respectively, the blockade or denervation supersensitivity of dopamine receptors along nigrostriatal and mesocortical dopaminergic pathways.1 In most patients, acute dystonia presents within 5 days of drug initiation, at variable locations (orofacial, ocular, neck and trunk) and with diverse severity.3 The modulation of acute dystonia or akathisia by anticholinergics or β-adrenergic antagonists suggests, however, the involvement also of other neurotransmitters in these movement disorders.3,4 Drug-induced parkinsonism overlaps substantially with Parkinson’s disease, although the former diagnosis is supported by a highly symmetrical presentation,1 a lack of gait and balance difficulties,5 a negative dopamine transporter single-photon emission computed tomography scan,6 and attenuation with dose reduction or discontinuation of the antipsychotic medication. Tardive involuntary movements (choreiform dyskinesia, dystonia, akathisia, and less commonly myoclonus, tourettism and tremor) are also associated with maladaptive plastic rearrangements of the inferior frontal cortex, which is highly connected to deep subcortical nuclei and in the modulation of motor output selection. In line with this, a proportion of patients show abnormal movements when the offending drug is discontinued or its dosage is reduced (withdrawal dyskinesia).7
Antipsychotic-associated movement disorders can stigmatise patients, exert a negative impact on their quality of life and medication adherence, and confound the clinical assessment of other behavioural symptoms.8,9 A subjective rating of these movement disorders also correlates with health-related quality of life measures; although, these patients may have reduced subjective awareness of their occurrence.10
When second-generation antipsychotic drugs (SGAs) were introduced, there were claims that they had a lower liability for motor side effects than first-generation (FGAs) medications. With reference to acute motor side effects, a meta-analysis of randomised controlled trials11 showed substantial variability in the odds ratios as compared with the placebo across the SGA class. In particular, 5 SGAs (ziprasidone, paliperidone, risperidone, lurasidone, and zotepine) were associated with significantly more acute movement disorders than the placebo, with odds ratios ranging from 1.61 (95% CI, 1.05 to 2.37) for ziprasidone to 3.01 (95% CI, 1.38 to 5.77) for zotepine. The observed variability in motor side effects within the SGA class led these authors to conclude that the dichotomous classification of antipsychotic drugs into FGAs and SGAs cannot be based on differences in their liabilities for movement disorders.
Most people on continuing antipsychotic medications are prescribed SGAs, and it remains difficult to provide reliable estimates of the prevalence of motor side effects with individual medications when informing patients and their caregivers in routine clinical practice. Estimating the probability of occurrence of acute movement disorders is probably more realistic for clinicians than trying to estimate the likelihood of persistent or later-onset movement disorders. This is due to the greater diversity in the natural history of tardive movement disorders, as well as the greater variety of clinical factors influencing their risk; e.g., baseline psychiatric diagnosis, duration of drug exposure, exposure to multiple antipsychotic agents or to anticholinergic medications, and patient demographics.7,12 Despite extensive evidence for acute movement disorders from clinical trials with antipsychotic drugs, there remains challenges associated with methodological heterogeneity across trials and limitations of existing systematic reviews, meta-analyses and literature overviews. Not all clinical trials use systematic assessments of movement disorders, and others use surrogate indicators of parkinsonism, such as the administration of antiparkinson drugs.11 In many studies, the term ‘extrapyramidal symptoms’ is used as a synonym for parkinsonism, whereas, in others, it may also encompass phenomena such as dystonia, tremor, and akathisia. Moreover, validated cumulative rating instruments that are often used to assess movement disorders may provide scores that do not discriminate clearly across different types of movement disorders.13,14
In this study, we sought to increase knowledge on the prevalence of new-onset movement disorders in people with schizophrenia or established psychosis following exposure to continuing antipsychotic medication, limiting our analyses to those medications approved for use in Canada and the UK. We chose to focus on SGAs because they are most commonly used in current clinical practice, but included 2 FGAs (haloperidol and chlorpromazine) in our analysis for comparative purposes. To this end, we performed meta-analyses selecting exclusively randomised controlled trials included in Cochrane reviews on the treatment of schizophrenia because of their methodological rigor and the homogeneity in terminology for the different types of movement disorders expressed in this patient population.
Methods
Selection of Studies
We performed our search on the 10 SGAs that are approved for use in Canada and the UK (amisulpride, aripiprazole, asenapine, clozapine, olanzapine, paliperidone, quetiapine, risperidone, L-sulpiride, and ziprasidone), and included 2 FGAs (chlopromazine and haloperiodol) for comparison. For each of the 12 selected agents, we searched the Cochrane Database of Systematic Reviews, narrowing the search to the Schizophrenia Group (search date 31 July 2017). We searched for the name of each of the 12 agents separately in the title, abstract, or keywords.
Type of Studies and Participants
To be consistent with the Cochrane methodological approach, we analysed all relevant randomised controlled trials included in the identified Cochrane reviews, and did not consider those that were excluded by the authors of the Cochrane reviews, regardless of the reasons for exclusion. The selected reviews included studies of people with schizophrenia and other types of schizophrenia-like psychoses (schizophreniform and schizoaffective disorders), on the basis that there is no clear evidence that schizophrenia-like psychoses are caused by fundamentally different disease processes or require different treatment approaches.
Outcome Measures
We selected as outcome measures the incidence of the following ‘extrapyramidal’ adverse effects: ‘akathisia’, ‘parkinsonism’, ‘tremor’, and ‘acute dystonia’. We selected only these adverse effects amongst all different types of ‘extrapyramidal’ adverse effects because only these are typically occurring acutely; i.e., after a duration of antipsychotic exposure over a range of weeks. Other adverse effects, such as ‘tardive dyskinesia’ typically occur with more prolonged exposure, and therefore are likely to be underrepresented in short-term clinical trials.
Data Extraction
Data extraction was performed by a single reviewer and checked by a second reviewer for accuracy. For each antipsychotic medication and each outcome (akathisia, parkinsonism, tremor and acute dystonia), we extracted the number of trial participants who received the antipsychotic medication and who experienced an extrapyramidal adverse effect, thereby calculating the study prevalence for each movement disorder. For each trial involving the 10 selected atypical antipsychotic drugs, we also collected treatment duration, type of dosing (flexible or fixed dosing), dose (when flexible dosing was used, mean dose was collected), method of movement disorder ascertainment (whether the authors stated having applied a validated screening/rating instrument for movement disorders), and risk of bias as reported in each selected review. We consulted the full text of each trial to verify the method of movement disorder ascertainment.
Data Analysis
To calculate the overall prevalence of each antipsychotic-induced movement disorder by medication, we performed a meta-analysis of all included studies using a random effects model. We chose to use a random effects model due to the heterogeneity among studies in terms of dosing of medication and ascertainment of movement disorders. The I2 was used to quantify between-study heterogeneity. We directly compared, among medications, the prevalence estimates obtained by the meta-analysis by measuring the overlap of the 95% CIs of estimates. All analyses were performed using Comprehensive Meta-Analysis software.
Results
Our systematic search of the Cochrane Database of Systematic Reviews yielded a total of 37 Cochrane systematic reviews (28 for SGAs),15–50 which generated 316 informative randomised controlled trials (243 for SGAs). Most of these studies did not include children or adolescents; 0% to 5% of studies included patients below 18 years of age, of which fewer than 10% were below the age of 18 years. Table 1 presents the number of reviews and informative studies retrieved for each atypical antipsychotic drug. Most of the reviewed studies (63% to 100% across the 10 SGAs) adopted a flexible dosing methodology. Likewise, for 8 of the 10 SGAs used, most of the studies (56% to 100%) used a validated screening or rating instrument to assess movement disorders; for aripiprazole and L-sulpiride, only 8 of the 85 (9.4%) studies and 2 of the 9 (22%) studies, respectively, employed such an instrument. A complete assessment of risk of bias for different types of biases was available from at least one Cochrane review for all drugs except for L-sulpiride, for which only selection bias associated with allocation concealment was assessed. Risk of bias was variable for aripiprazole, risperidone, haloperidol, chlorpromazine, and quetiapine, whereas it was more homogeneous across studies for amisulpride, olanzapine, paliperidone, and ziprasidone (Supplementary File 1). Many (77%) of the included trials on aripiprazole were from Chinese cohorts, whereas fewer than 15% of the included trials for the other agents were similarly skewed. The Cochrane review by Khanna et al.,17 which represents the source of these Chinese trials, reported a comparable risk of bias across Chinese and non-Chinese studies.
Table 1.
Summary Table of Study Characteristics for the 12 Antipsychotic Medications Reviewed.
| Medication | No. of Systematic Reviews | No. of Informative Studies | Study Duration (weeks) | No. (%) of Studies Adopting a Flexible Dosing Methodology | No. (%) of Informative Studies thatu a Validated Instrument to Assess Movement Disorders |
|---|---|---|---|---|---|
| Amisulpride | 2 | 16 | 3–52 | 10 (63%) | 9 (56%) |
| Aripiprazole | 4 | 85 | 4–52 | 77 (91%) | 8 (9.4%) |
| Asenapine | 1 | 2 | 6–52 | 2 (100%) | 2 (100%) |
| Chlorpromazine | 5 | 57 | 4–24 | 47 (82%) | 9 (16%) |
| Clozapine | 3 | 4 | 16–104 | 3 (75%) | 4 (100%) |
| Haloperidol | 4 | 41 | 4–24 | 39 (95%) | 3 (7%) |
| Olanzapine | 3 | 34 | 6–52 | 27 (79%) | 28 (82%) |
| Paliperidone | 1 | 9 | 2–33 | 7 (78%) | 9 (100%) |
| Quetiapine | 3 | 40 | 2–78 | 38 (95%) | 25 (63%) |
| Risperidone | 6 | 37 | 4–104 | 28 (74%) | 30 (81%) |
| Sulpiride | 3 | 9 | 4–10 | 7 (78%) | 2 (22%) |
| Ziprasidone | 2 | 7 | 4–78 | 5 (71%) | 5 (71%) |
Dystonia
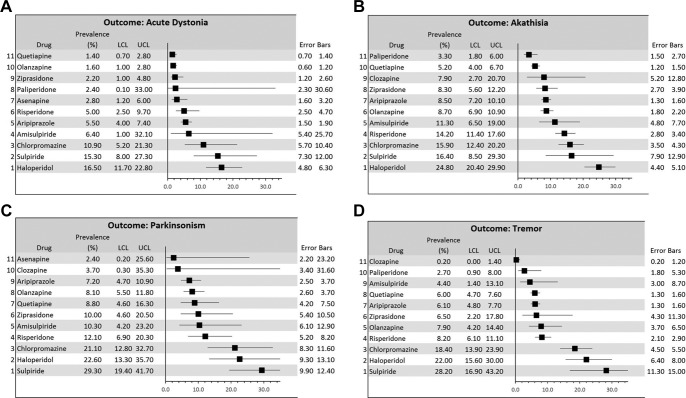
New onset of dystonia, labeled across Cochrane systematic reviews as ‘acute dystonia’, was recorded for all of the investigated agents, with the exception of clozapine, for which no data were available. With respect to SGAs, prevalence estimates of dystonia from our meta-analysis ranged from 1.4% (quetiapine) to 15.3% (sulpiride) (Table 2; Figure 1). The benzamides, L-sulpiride and amisulpride, yielded the highest prevalence estimates, 15.3% and 6.4%, respectively, but their 95% CIs were wide, and the number of studies with amisulpride was small (N=3). Aripiprazole and risperidone yielded very similar prevalence estimates but the CI was wider and study heterogeneity was larger for the 11 studies with risperidone compared with the 41 with aripiprazole. Olanzapine and quetiapine yielded the lowest prevalence estimate, with larger heterogeneity for studies with olanzapine. The 95% CIs of prevalence estimates for the two FGAs substantially overlapped estimates for the benzamides but this overlap was much smaller for aripiprazole and risperidone. Olanzapine, quetiapine and ziprasidone were the only SGAs yielding significantly smaller prevalence estimates than chlorpromazine and haloperidol, using P = 0.05 as the threshold for statistical significance.
Table 2.
Summary Table of Meta-Analysis Prevalence Estimates of New Onset Of Dystonia, Akathisia, Parkinsonism, and Tremor for the 12 Antipsychotic Medications Reviewed.
| Medication | Dystonia Prevalence % (95%CI); Nr of studies and participants; I2 |
Akathisia Prevalence % (95%CI); Nr of studies and participants; I2 |
Parkinsonism Prevalence % (95%CI); Nr of studies and participants; I2 |
Tremor Prevalence % (95%CI); Nr of studies and participants; I2 |
|---|---|---|---|---|
| Amisulpride | 6.4% (1.0 to 32.1); 3 studies, 218 participants; I20 |
11.3% (6.5 to 19.0); 9 studies, 744 participants; I2 29.8 |
10.3% (4.2- to 23.2); 8 studies, 646 participants; I2 33.1 |
4.4% (1.4 to 13.1); 7 studies, 632 participants; I2 0 |
| Aripiprazole | 5.5% (4.0 to 7.4); 41 studies, 1715 participants; I2 0 |
8.5% (7.2 to 10.1); 70 studies, 6552 participants; I2 0 |
7.2% (4.7 to 10.9); 15 studies, 857 participants; I2 0 |
6.1% (4.8 to 7.7); 53 studies, 2397 participants; I2 0 |
| Asenapine | 2.8% (1.2 to 6.0); 1 study, 217 participants; I2 0 |
Not reported | 2.4% (0.2 to 25.6); 2 studies, 411 participants; I2 0 |
Not reported |
| Chlorpromazine | 10.9% (5.2 to 21.3) 12 studies, 825 participants; I2 41 |
15.9% (12.4 to 20.2) 40 studies, 2087 participants; I2 4.8 |
21.1% (12.8 to 32.7) 20 studies, 910 participants; I2 34 |
18.4% (13.9 to 23.9) 29 studies, 1198 participants; I2 22.9 |
| Clozapine | Not reported | 7.9% (2.7 to 20.7); 4 studies, 657 participants; I2 43.9 |
3.7% (0.3 to 35.3); 2 studies, 162 participants; I2 0 |
0.2% (0 to 1.4); 1 study, 490 participants; I2 0 |
| Haloperidol | 16.5% (11.7 to 22.8) 24 studies; 838 participants; I2 7 |
24.8% (20.4 to 29.9) 33 studies, 1278 participants; I2 41 |
22.6% (13.3 to 35.7) 6 studies, 255 participants; I2 0 |
22% (15.6 to 30) 23 studies, 727 participants; I2 22.1 |
| Olanzapine | 1.6% (1.0 to 2.8); 10 studies, 2599 participants; I2 10.5 |
8.7% (6.9 to 10.9); 27 studies, 4569 participants; I2 18.5 |
8.1% (5.5 to 11.8); 15 studies, 2636 participants; I2 37.5 |
7.9% (4.2 to 14.4); 12 studies, 1366 participants; I2 11.5 |
| Paliperidone | 2.4% (0.1 to 33.0); 2 studies, 226 participants; I2 0 |
3.3% (1.8 to 6.0); 6 studies, 1496 participants; I2 0 |
Not reported | 2.7% (0.9 to 8.0); 7 studies, 1701 participants; I2 0 |
| Quetiapine | 1.4% (0.7 to 2.8); 13 studies, 1642 participants; I2 0 |
5.2% (4.0 to 6.7); 35 studies, 3392 participants; I2 0 |
8.8% (4.6 to 16.3); 12 studies, 1585 participants; I2 23.6 |
6.0% (4.7 to 7.6); 20 studies, 1211 participants; I2 0 |
| Risperidone | 5.0% (2.5 to 9.7); 11 studies, 1341 participants; I2 22.5 |
14.2% (11.4 to 17.6); 28 studies, 3263 participants; I2 6.9 |
12.1% (6.9 to 20.3); 16 studies, 1865 participants; I2 51.2 |
8.2% (6.1 to 11.1); 22 studies, 2699 participants; I2 16.6 |
| Sulpiride | 15.3% (8.0 to 27.3); 6 studies, 146 participants; I2 11.9 |
16.4% (8.5 to 29.3); 8 studies, 211 participants; I2 30.3 |
29.3% (19.4 to 41.7); 7 studies, 193 participants; I2 14.7 |
28.2% (16.9 to 43.2); 4 studies, 93 participants; I2 21.0 |
| Ziprasidone | 2.2% (1.0 to 4.8); 1 study, 271 participants; I2 0 |
8.3% (5.6 to 12.2); 6 studies, 832 participants; I2 1 |
10% (4.6 to 20.5); 1 study, 60 participants; I2 0 |
6.5 (2.2 to 17.8); 2 studies, 240 participants; I2 0 |
Figure 1A-D.
Forest plots of the meta-analysis results on the prevalence of acute dystonia, akathisia, parkinsonism and tremor for each of the 12 antipsychotic drugs investigated. LCL: lower limit of the 95% CIs. UCL: upper limit of the 95% CIs.
Akathisia
A new occurrence of akathisia was recorded for all of the investigated agents, with the exception of asenapine, for which no data were available. With respect to SGAs, our meta-analysis yielded prevalence estimates ranging from 3.3% (paliperidone) to 16.4% (L-sulpiride) (Table 2; Figure 1). Like for dystonia, benzamides (L-sulpiride and amisulpride) yielded relatively higher prevalence estimates for akathisia (16.4% and 11.3%, respectively) but these were associated with wider CIs than most of the other agents and relatively large study heterogeneity. Risperidone yielded a prevalence estimate of akathisia (14.2%) that was comparable to that of the benzamides, with relatively small study heterogeneity, whereas the prevalence estimate for the risperidone derivative, paliperidone, was almost 5 times lower (3.3%). Estimates for olanzapine, aripiprazole, and ziprasidone were all between 8% and 9%, with minimal overlap to risperidone and with higher study heterogeneity observed for olanzapine (I2 = 18.5). The prevalence observed from the 4 studies with clozapine was 7.9%, but the CI was very wide and study heterogeneity was large (I2 = 43.9). The 95% CI of prevalence estimates for haloperidol overlapped only that of L-sulpiride, whereas there was no significant overlap between haloperidol and any of the other agents explored, including chlorpromazine. Aripiprazole, olanzapine, paliperidone, quetiapine, and ziprasidone all yielded significantly smaller prevalence estimates compared with chlorpromazine, using P = 0.05 as the threshold for statistical significance.
Parkinsonism
A new occurrence of parkinsonism was recorded for all of the investigated agents, with the exception of paliperidone, for which no data were available. With respect to SGAs, our meta-analysis yielded prevalence estimates ranging from 2.4% (asenapine) to 29.3% (L-sulpiride) (Table 2; Figure 1). With the exception of the relatively high prevalence estimated for L-sulpiride, most of the remaining agents yielded rates ranging from 7% to 12%, with highly overlapping CIs. The 12.1% estimate for risperidone had a wide CI, and the heterogeneity of the 16 studies with risperidone with respect to parkinsonism frequency was the highest detected by our analysis (I2=51.2). The 15 studies with aripiprazole yielded a prevalence estimate of 7.2%, associated with the narrowest CI and extremely low study heterogeneity. The low estimates for clozapine and asenapine were based on only 2 studies for each of these 2 medications, their CIs also being very wide. Aripiprazole and olanzapine were the only SGAs yielding significantly smaller prevalence estimates compared with chlorpromazine and haloperidol, using P = 0.05 as the threshold for statistical significance.
Tremor
Tremor as a side effect was recorded for all of the investigated agents, with the exception of asenapine, for which no data were available. With respect to SGAs, our meta-analysis yielded prevalence estimates ranging from 0.2% (clozapine) to 28.2% (L-sulpiride; Table 2; Figure 1). The 95% CIs of tremor rate for L-sulpiride, based on only 4 studies with moderate study heterogeneity, did not overlap with those of any of the other investigated SGAs, with the exception of a small overlap with ziprasidone. All the other SGAs had prevalence estimates with highly overlapping CIs. The most robust estimates for number of studies, CIs and study heterogeneity were obtained for aripiprazole and quetiapine. Tremor with clozapine was reported in only one study, and the CIs for prevalence were substantially below those yielded for the other agents investigated. The 95% CIs of prevalence estimates for FGAs markedly overlapped those for L-sulpiride, and only slightly overlapped the CIs yielded by olanzapine and ziprasidone. All the other SGAs, including amisulpride, yielded significantly lower CIs compared with FGAs, using P = 0.05 as threshold for statistical significance.
Summary
The detailed meta-analyses for each medication and type of movement disorder are presented in Supplementary File 2. Across all 12 of the antipsychotic medications investigated, we did not observe any substantial difference in movement disorder prevalence estimates based on treatment duration, after excluding studies that did not apply a flexible dosing scheme, or after excluding studies that did not use or provide sufficient information on the use of validated instruments for the screening or rating of movement disorders (data not shown). Importantly, however, of the 41 clinical trials using haloperidol, it is worth noting that 26 allowed the use of anticholinergics as a rescue intervention in the case of ‘extrapyramidal symptoms’, 7 allowed their use either as prophylaxis or without specifying whether they were used prophylactically or as rescue treatment, and the remaining 8 did not specify whether anticholinergic use was allowed.
Discussion
Our overview of Cochrane systematic reviews showed a considerable degree of overlap across SGAs used in Canada and the UK in the prevalence estimates of new onset movement disorders in schizophrenia-like psychoses. Some of these SGAs yielded CIs of prevalence estimates that overlap those of 2 FGAs, chlorpromazine and haloperidol. Overall, our results on 12 antipsychotic agents indicate a distribution of movement disorders prevalence across 4 main groups (Table 3): ‘high prevalence’, represented by the two FGAs, haloperidol and chlorpromazine, and the FGA/SGA agent L-sulpiride; ‘moderate–high prevalence’, represented by ziprasidone, aripiprazole, risperidone, and amisulpride; ‘moderate–low prevalence’, represented by olanzapine, paliperidone and asenapine; and ‘low prevalence’, represented by clozapine and quetiapine.
Table 3.
Summary Table of the Classification of Antipsychotic Agents into Four Groups and Mode of Action Based on Prevalence Estimates of Acute Movement Disorders.
| Prevalence of Movement Disorders | Medication | NbN Classification |
|---|---|---|
| High | Haloperidol Chlorpromazine L-sulpiride |
D2 receptor antagonist D2 and 5-HT2 receptor antagonist D2 receptor antagonist |
| Moderate-to-high | Ziprasidone Aripiprazole Risperidone Amisulpride |
D2 and 5-HT2 receptor antagonist D2 and 5-HT1A partial agonist and 5-HT2A receptor antagonist D2, 5HT2A and NE-alpha 2 receptor antagonist D2 receptor antagonist |
| Moderate-to-low | Olanzapine Paliperidone Asenapine |
D2 and 5-HT2A receptor antagonist D2, 5HT2A and NE-alpha 2 receptor antagonist D2, 5HT2A and NE-alpha 2 receptor antagonist |
| Low | Clozapine Quetiapine |
D2, 5HT2A and NE-alpha 2 receptor antagonist D2 and 5HT2A receptor antagonist – norepinephrine transporter inhibitor |
The table indicates also their classification based on Principal Mode of Action, according to the neuroscience-based nomenclature (NbN).
For the investigated antipsychotic drugs, the differences in prevalence estimates were relatively similar across the four different types of acute movement disorders. This suggests that the pathogenesis of movement disorders as a whole may be related to specific pharmacokinetic and pharmacodynamic properties that differentiate these medications among each other. The relative motor toxicity observed in our analysis may reflect both different antagonistic potency on the serotonergic 5-HT2A receptor,51,52 and different association kinetics for the dopamine D2 R receptor.4,53 Higher 5-HT2A antagonism may have a balancing effect on striatal dopamine signaling, eventually protecting from motor side effects.54 Although our prevalence estimates parallel to a certain degree this pharmacodynamic property, an important exception is amisulpride, which has relatively high D2 R selectivity and lower 5-HT2A antagonistic potential55 but yielded a movement disorder prevalence that was substantially lower than the FGAs.
On the other hand, the observed prevalence distribution broadly reflects also the difference across agents in affinity to D2 R. Earlier observations suggested that some SGAs have lower affinity for the D2 R than FGAs resulting from a faster dissociation rate (“fast off hypothesis”), whereas it was widely assumed that association rates for antipsychotic drugs are comparable because they are diffusion-limited.53 According to this hypothesis, rapid dissociation by an antagonist would allow a greater binding of D2 R by transiently high local concentrations of released dopamine, whereas antagonists dissociating slowly from this receptor would determine a much more prolonged antagonism. This hypothesis has recently been questioned by a kinetics study that revealed a range of association and dissociation rates across antipsychotics. The report showed a positive correlation between D2 R association rate (but not dissociation rate) and frequency of movement disorders from a meta-analysis of randomized controlled trials with atypical antipsychotics.53 Interestingly, the prevalence estimates observed in our meta-analysis appear to reflect drug differences in the D2 R association rates, but not in the D2 R dissociation rates, thus supporting this recent kinetic study (see, for a comparison, Figures 3a and 3b from Sykes et al.53).
Differences in the D2 R association rates may be only one of several factors influencing the variability of movement disorder prevalence across SGAs. The D2 R association rate of L-sulpiride is very similar to that of olanzapine,53 and only slightly higher than that of clozapine. Nevertheless, in our analysis, L-sulpiride was associated with the highest prevalence of parkinsonism and tremor, even higher than that seen with haloperidol. Moreover, the prevalence of acute dystonia and akathisia with L-sulpiride was second only to haloperidol. This may be explained by the very high selectivity of L-sulpiride for D2 R and its negligible affinity for 5-HT2A receptors, with both of these pharmacological characteristics contributing to the risk of developing movement disorders in patients with schizophrenia and schizophrenia-like psychoses. Another counterintuitive finding is related to asenapine, which has a high D2 R association rate,53 but to date has not been associated with a significantly higher prevalence of movement disorders compared with placebo.11 The limited number of randomised controlled trials with asenapine reporting this category of side effects prevents, at present, any definitive conclusion on its liability for movement disorders.11
It is interesting to read our results also in the context of the recently devised nomenclature system dubbed Neuroscience-based Nomenclature (NbN; http://nbnomenclature.org), which proposes to name psychotropic drugs after their pharmacological domain and mode of action, rather than their indication (see Table 3). Using the principal mode of action according to NbN, there is unsurprisingly an association between pure D2 receptor antagonists (i.e., with weak or absent antagonistic effect on other receptors) and a high frequency of movement disorders, whereas multireceptor antagonists, particularly D2/5-HT2A receptor antagonists and D2/5-HT2A/NE-alpha2 receptor antagonists, span across the different prevalence subgroups. Our results support, at least in part, the usefulness of the NbN approach, even if the prevalence of motor side effects does not appear to separate different types of NbN drug categories in a clear-cut fashion.
In a previous meta-analysis, Leucht et al.11 compared the tolerability profile of 15 antipsychotic drugs with placebo, reporting that haloperidol, chlorpromazine, risperidone, paliperidone, zotepine, and lurasidone produced significantly more ‘extrapyramidal side-effects’ than other agents like clozapine, olanzapine, quetiapine, aripiprazole, amisulpride, and asenapine. Our findings are not directly comparable with those of Leucht et al., because we did not assess the odds ratios with respect to placebo. However, the relative movement disorder prevalence estimates that we report here still show similarities with those found in their earlier meta-analysis. Unlike our report, though, Leucht et al. measured ‘extrapyramidal side effects’ as a whole and adopted ‘use of antiparkinson drugs’ as a surrogate measure of movement disorders. This approach provided odds ratios that most likely reflect only the occurrence of parkinsonism, dystonia and possibly tremor, whereas an important motor side effect like akathisia, which differs from other movement disorders with respect to pharmacological management,1,4 might have been under-ascertained. We also acknowledge that, in our study, the rescue or prophylactic use of anticholinergic drugs in older clinical trials of FGAs, such as haloperidol, may have led to an underestimation of the prevalence of movement disorders for this agent.
The difference in prevalence of movement disorders across the explored antipsychotic agents may also depend on differences in study design and study population. Although heterogeneity across agents with respect to dosages may influence prevalence, such an effect is very difficult to tease out because most studies adopt a flexible dosing system, and do not specifically report any association between dosage and frequency of movement disorders. This could partly reflect that movement disorders were secondary outcomes in these studies, and post hoc analyses addressing this safety characteristic are scant in the literature on SGAs. Differences in the ascertainment of movement disorders may have also played a role, particularly with L-sulpiride and aripiprazole, for which details on the ascertainment methodology is provided only in a few studies. Most of the studies covered in these Cochrane systematic reviews included chronically ill patients with a history of exposure to antipsychotics, which is known to modify D2 R availability,56 and therefore potentially also the risk of developing movement disorders. A recent systematic review with meta-analyses on SGAs in patients treated for a first episode of schizophrenia57 reported that olanzapine is associated with less frequent use of antiparkinson drugs than haloperidol and risperidone, and with less akathisia than haloperidol, aripiprazole and risperidone. In the same study,57 quetiapine was associated with the less-frequent use of antiparkinson drugs than haloperidol, and with less akathisia than haloperidol, aripiprazole, risperidone, and olanzapine. Except for the equivalence in akathisia prevalence between olanzapine and aripiprazole, the findings from our study are remarkably similar to this recent meta-analysis,57 despite the differences in illness duration and previous exposure to antipsychotics.
Finally, it is worth noting that the geographical source of the included trials on aripiprazole is markedly skewed towards Chinese institutions (77%), clearly different from the source distribution of all the other agents analyzed. Aripiprazole appears to be heavily marketed in China, and this is most likely the main reason for this skewed distribution. In their Cochrane review, Khanna et al.17 state that the new data obtained from the Chinese studies included after the previous version of the review increased precision of the comparisons whilst not clearly affecting the overall quality of data, based on the risk of bias classification as per Cochrane methodology. However, it needs to be emphasised that an important difference between Chinese and non-Chinese studies is that most of the participants in the Chinese studies were diagnosed using the Chinese Classification of Mental Disorders (CCMD-3) and not the DSM. Regardless of whether Chinese and non-Chinese trials on aripiprazole differ in methodological quality or reproducibility, the narrower CI of prevalence estimates for aripiprazole is strongly influenced by the larger number of included trials for this agent, and this numerosity is undoubtedly linked to their geographical source.
In conclusion, our overview of Cochrane systematic reviews confirms a high degree of overlap in the prevalence of new-onset movement disorders across SGAs. Because this overview focused on schizophrenia and schizophrenia-like psychoses, our results cannot be generalised to the whole spectrum of diagnoses in which antipsychotics are part of the treatment armamentarium; e.g., major depressive disorder, Tourette syndrome, among others. The differences in pharmacological properties, like the D2 R association rate and 5-HT2A antagonism, could contribute to the variable prevalence of acute movement disorders observed among different medications. Future randomized controlled trials and observational studies should focus on teasing out the specific pathophysiological role of kinetics and the pharmacodynamic profile of different antipsychotic agents for movement disorders, and increase our understanding of the relationship between dosage and movement disorder risk. Our refinement of the prevalence estimates of acute movement disorders following exposure to SGAs in established psychotic illness offers clinicians practical information on the risk profile of this class of drug that can be readily presented to patients when discussing therapeutic options.
Supplemental Material
Supplementary_File_1 for Movement Disorders Associated With Antipsychotic Medication in People With Schizophrenia: An Overview of Cochrane Reviews and Meta-Analysis by Davide Martino, Vikram Karnik, Sydney Osland, Thomas R. E. Barnes, and Tamara M. Pringsheim in The Canadian Journal of Psychiatry
Supplemental Material
Supplementary_File_2 for Movement Disorders Associated With Antipsychotic Medication in People With Schizophrenia: An Overview of Cochrane Reviews and Meta-Analysis by Davide Martino, Vikram Karnik, Sydney Osland, Thomas R. E. Barnes, and Tamara M. Pringsheim in The Canadian Journal of Psychiatry
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Materials: Supplemental material for this article is available online.
References
- 1. Mehta SH, Morgan JC, Sethi KD. Drug-induced movement disorders. Neurol Clin. 2015;33:153–174. [DOI] [PubMed] [Google Scholar]
- 2. Solmi M, Murru A, Pacchiarotti I, et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag. 2017;13:757–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keepers GA, Clappison VJ, Casey DE. Initial anticholinergic prophylaxis for neuroleptic-induced extrapyramidal syndromes. Arch Gen Psychiatry. 1983;40:1113–1117. [DOI] [PubMed] [Google Scholar]
- 4. Lima AR, Bacalcthuk J, Barnes TR, et al. Central action beta-blockers versus placebo for neuroleptic-induced acute akathisia (meta-analysis review). Cochrane Database Syst Rev. 2004;(4):CD001946 doi: 10.1002/14651858.CD001946.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giladi N, Kao R, Fahn S. Freezing phenomenon in patients with parkinsonian syndromes. Mov Disord. 1997;12:302–305. [DOI] [PubMed] [Google Scholar]
- 6. Tinazzi M, Ottaviani S, Isaias IU, et al. [123I]FP-CIT SPET imaging in drug-induced parkinsonism. Mov Disord. 2008;23:1825–1829. [DOI] [PubMed] [Google Scholar]
- 7. Correll CU, Kane JM, Citrome LL. Epidemiology, prevention, and assessment of tardive dyskinesia and advances in treatment. J Clin Psychiatry. 2017;78:1136–1147. [DOI] [PubMed] [Google Scholar]
- 8. Adrianzén C, Arango-Dávila C, Araujo DM, et al. Relative association of treatment-emergent adverse events with quality of life of patients with schizophrenia: post hoc analysis from a 3-year observational study. Hum Psychopharmacol. 2010;25:439–447. [DOI] [PubMed] [Google Scholar]
- 9. Gervin M, Barnes TRE. Assessment of drug-related movement disorders in schizophrenia. Advances in Psychiatric Treatment. 2000;6:332–341. [Google Scholar]
- 10. Bebbington PE, Angermeyer M, Azorin JM, Marwaha S, Marteau F, Toumi M. Side-effects of antipsychotic medication and health-related quality of life in schizophrenia. Acta Psychiatr Scand Suppl. 2009;(438):22–28. [DOI] [PubMed] [Google Scholar]
- 11. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. Lancet. 2013;382:951–962. [DOI] [PubMed] [Google Scholar]
- 12. Tarsy D, Baldessarini RJ. Epidemiology of tardive dyskinesia: Is risk declining with modern antipsychotics? Mov Disord. 2006;21:589–598. [DOI] [PubMed] [Google Scholar]
- 13. Martino D, Morgante F. Movement disorders and chronic psychosis: Five new things. Neurol Clin Pract. 2017;7:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pope A, Adams C, Paton C, Weaver T, Barnes TRE. Assessment of adverse effects in clinical studies of antipsychotic medication: Survey of methods used. Br J Psychiatry. 2010:197:67–72. [DOI] [PubMed] [Google Scholar]
- 15. Silveira da Mota Neto JI, Soares BGO, Silva de Lima M. Amisulpride for schizophrenia. Cochrane Database Syst Rev. 2002;(2):CD001357 doi: 10.1002/14651858.CD001357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Komossa K, Rummel-Kluge C, Hunger H, et al. Amisulpride versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev 2010; (1):CD006624 doi:10.1002/14651858.CD006624.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khanna P, Suo T, Komossa K, et al. Aripiprazole versus other atypical antipsychotics for schizophrenia. Cochrane Database of Systematic Rev. 2014;(1):CD006569 doi:10.1002/14651858.CD006569.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Belgamwar RB, El-Sayeh HGG. Aripiprazole versus placebo for schizophrenia. Cochrane Database Syst Rev. 2011;(8):CD006622 doi:10.1002/14651858.CD006622.pub2. [DOI] [PubMed] [Google Scholar]
- 19. Bhattacharjee J, El-Sayeh HG. Aripiprazole versus typical antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev 2008;(3):CD006617 doi:10.1002/14651858.CD006617.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El-Sayeh HG, Morganti C. Aripiprazole for schizophrenia. Cochrane Database Syst Rev. 2006;(2):CD004578 doi: 10.1002/14651858.CD004578.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hay A, Byers A, Sereno M, Basra MK, Dutta S. Asenapine versus placebo for schizophrenia. Cochrane Database Syst Rev. 2015;(11):CD011458 doi:10.1002/14651858.CD011458.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mazhari S, Esmailian S, Shah-Esmaeili A, Goughari AS, Bazrafshan A, Zare M. Chlorpromazine versus clotiapine for schizophrenia. Cochrane Database Syst Rev. 2017;(4):CD011810 doi:10.1002/14651858.CD011810.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zare M, Bazrafshan A. Chlorpromazine versus metiapine for schizophrenia. Cochrane Database Syst Rev. 2017;(3):CD011655 doi:10.1002/14651858.CD011655.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saha KB, Bo L, Zhao S, Xia J, Sampson S, Zaman RU. Chlorpromazine versus atypical antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. 2016;(4):CD010631 doi:10.1002/14651858.CD010631.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adams CE, Awad GA, Rathbone J, Thornley B, Soares-Weiser K. Chlopromazine versus placebo for schizophrenia. Cochrane Database Syst Rev. 2014;(1):CD000284 doi:10.1002/14651858.CD000284.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Essali A, Al-Haj Haasan N, Li C, Rathbone J. Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database Syst Rev. 2009;(1):CD000059 doi:10.1002/14651858.CD000059.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barber S, Olotu U, Corsi M, Cipriani A. Clozapine combined with different antipsychotic drugs for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2017; (3):CD006324 doi:10.1002/14651858.CD006324.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Asenjo Lobos C, Komossa K, Rummel-Kluge C, et al. Clozapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010;(11):CD006633 doi:10.1002/14651858.CD006633.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dold M, Samara MT, Li C, Tardy M, Leucht S. Haloperidol versus first-generation antipsychotics for the treatment of schizophrenia and other psychotic disorders. Cochrane Database Syst Rev. 2015;(1):CD009831 doi:10.1002/14651858.CD009831.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tardy M, Huhn M, Kissling W, Engel RR, Leucht S. Haloperidol versus low-potency first-generation antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. 2014;(7):CD009268 doi:10.1002/14651858.CD009268.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adam CE, Bergma H, Irvin CB, Lawri S. Haloperidol versus placebo for schizophrenia. Cochrane Database Syst Rev. 2013;(11):CD003082 doi:10.1002/14651858.CD003082.pub2. [DOI] [PubMed] [Google Scholar]
- 32. Leucht C, Kitzmantel M, Kane J, Leucht S, Chua WLLC. Haloperidol versus chlorpromazine for schizophrenia. Cochrane Database Syst Rev. 2008;(1):CD004278 doi:10.1002/14651858.CD004278.pub2. [DOI] [PubMed] [Google Scholar]
- 33. Komossa K, Rummel-Kluge C, Hunger H, et al. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010;(3):CD006654 doi:10.1002/14651858.CD006654.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jayaram MB, Hosalli P, Stroup TS. Risperidone versus olanzapine for schizophrenia. Cochrane Database Syst Rev. 2006;(2):CD005237 doi:10.1002/14651858.CD005237.pub2. [DOI] [PubMed] [Google Scholar]
- 35. Duggan L, Fenton M, Rathbone J, Dardennes R, El-Dosoky A, Indran S. Olanzapine for schizophrenia. Cochrane Database Syst Rev. 2005;(2):CD001359 doi:10.1002/14651858.CD001359.pub2. [DOI] [PubMed] [Google Scholar]
- 36. Nussbaum AM, Stroup TS. Paliperidone palmitate for schizophrenia. Cochrane Database Syst Rev. 2012;(6):CD008296 doi:10.1002/14651858.CD008296.pub2. [DOI] [PubMed] [Google Scholar]
- 37. Asmal L, Flegar SJ, Wang J, Rummel-Kluge C, Komossa K, Leucht S. Quetiapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2013;(11):CD006625 doi:10.1002/14651858.CD006625.pub3. [DOI] [PubMed] [Google Scholar]
- 38. Suttajit S, Srisurapanont M, Xia J, Suttajit S, Maneeton B, Maneeton N. Quetiapine versus typical antipsychotic medications for schizophrenia. Cochrane Database Syst Rev. 2013;(5):CD007815 doi:10.1002/14651858.CD007815.pub2. [DOI] [PubMed] [Google Scholar]
- 39. Srisurapanont M, Maneeton B, Maneeton N, Lankappa S, Gandhi R. Quetiapine for schizophrenia. Cochrane Database Syst Rev. 2004;(2):CD000967 doi:10.1002/14651858.CD000967.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rattehalli RD, Zhao S, Li BG, Jayaram MB, Xia J, Sampson S. Risperidone versus placebo for schizophrenia. Cochrane Database Syst Rev. 2016;(12):CD006918 doi:10.1002/14651858.CD006918.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sampson S, Hosalli P, Furtado VA, Davis JM. Risperidone (depot) for schizophrenia. Cochrane Database Syst Rev. 2016;(4):CD004161 doi:10.1002/14651858.CD004161.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011;(1):CD006626 doi:10.1002/14651858.CD006626.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li C, Xia J, Wang J. Risperidone dose for schizophrenia. Cochrane Database Syst Rev. 2009;(4):CD007474 doi:10.1002/14651858.CD007474.pub2. [DOI] [PubMed] [Google Scholar]
- 44. Jayaram MB, Hosalli P, Stroup TS. Risperidone versus olanzapine for schizophrenia. Cochrane Database Syst Rev. 2006;(2):CD005237 doi:10.1002/14651858.CD005237.pub2. [DOI] [PubMed] [Google Scholar]
- 45. Hunter R, Kennedy E, Song F, Gadon L, Irving CB. Risperidone versus typical antipsychotic medication for schizophrenia. Cochrane Database Syst Rev. 2003; (2):CD000440 doi: 10.1002/14651858.CD000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang J, Sampson S. Sulpiride versus placebo for schizophrenia. Cochrane Database Syst Rev. 2014;(4):CD007811 doi:10.1002/14651858.CD007811.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang J, Omori IM, Fenton M, Soares BGO. Sulpiride augmentation for schizophrenia. Cochrane Database Syst Rev. 2010;(1):CD008125 doi:10.1002/14651858.CD008125.pub2. [DOI] [PubMed] [Google Scholar]
- 48. Soares BGO, Fenton M, Chue P. Sulpiride for schizophrenia. Cochrane Database Syst Rev. 1999;(1):CD001162 doi:10.1002/14651858.CD001162. [DOI] [PubMed] [Google Scholar]
- 49. Komossa K, Rummel-Kluge C, Hunger H, et al. Ziprasidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2009;(4):CD006627 doi:10.1002/14651858.CD006627.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bagnall AM, Kleijnen J, Leitner M, Lewis R. Ziprasidone for schizophrenia and severe mental illness. Cochrane Database Syst Rev. 2000;(4):CD001945 doi:10.1002/14651858.CD001945. [DOI] [PubMed] [Google Scholar]
- 51. Janssen PA, Niemegeers CJ, Awouters F, Schellekens KH, Megens AA, Meert TF. Pharmacology of risperidone (R 64 766), a new antipsychotic with serotonin-S2 and dopamine-D2 antagonistic properties. J Pharmacol Exp Ther. 1988;244:685–693. [PubMed] [Google Scholar]
- 52. Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov. 2004;3:353–359. [DOI] [PubMed] [Google Scholar]
- 53. Sykes DA, Moore H, Stott L, et al. Extrapyramidal side effects of antipsychotics are linked to their association kinetics at dopamine D2 receptors. Nat Commun. 2017;8:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D1, D2 and serotonin2 pKi values. J Pharmacol Exp Ther. 1998;251:238–246. [PubMed] [Google Scholar]
- 55. Kapur S, Seeman P. Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics?: a new hypothesis. Am J Psychiatry. 2001;158:360–369. [DOI] [PubMed] [Google Scholar]
- 56. Tarazi FI, Zhang K, Baldessarini RJ. Long-term effects of olanzapine, risperidone, and quetiapine on dopamine receptor types in regions of rat brain: implications for antipsychotic drug treatment. J Pharmacol Exp Ther. 2001;297:711–717. [PubMed] [Google Scholar]
- 57. Zhu Y, Krause M, Huhn M, et al. Antipsychotic drugs for the acute treatment of patients with a first episode of schizophrenia: a systematic review with pairwise and network meta-analyses. Lancet Psychiatry. 2017;4:694–705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_File_1 for Movement Disorders Associated With Antipsychotic Medication in People With Schizophrenia: An Overview of Cochrane Reviews and Meta-Analysis by Davide Martino, Vikram Karnik, Sydney Osland, Thomas R. E. Barnes, and Tamara M. Pringsheim in The Canadian Journal of Psychiatry
Supplementary_File_2 for Movement Disorders Associated With Antipsychotic Medication in People With Schizophrenia: An Overview of Cochrane Reviews and Meta-Analysis by Davide Martino, Vikram Karnik, Sydney Osland, Thomas R. E. Barnes, and Tamara M. Pringsheim in The Canadian Journal of Psychiatry