Abstract
Wharton’s jelly-derived mesenchymal stromal cells (WJ-MSCs) have distinct immunomodulatory and protective effects against kidney, liver, or heart injury. Limited studies have shown that WJ-MSCs attenuates oxygen–glucose deprivation-mediated inflammation in hippocampal slices. The neuroprotective effect of intracerebral WJ-MSC transplantation against stroke has not been well characterized. The purpose of this study was to examine the neuroprotective effect of human WJ-MSC (hWJ-MSC) transplants in an animal model of stroke. Adult male Sprague–Dawley rats were anesthetized and placed in a stereotaxic frame. hWJ-MSCs, pre-labeled with chloromethyl benzamide 1,1’-dioctadecyl-3,3,3’3’- tetramethylindocarbocyanine perchlorate (CM-Dil), were transplanted to the right cerebral cortex at 10 min before a transient (60 min) right middle cerebral artery occlusion (MCAo). Transplantation of hWJ-MSCs significantly reduced neurological deficits at 3 and 5 days after MCAo. hWJ-MSC transplants also significantly reduced brain infarction and microglia activation in the penumbra. Grafted cells carrying CM-Dil fluorescence were identified at the grafted site in the ischemic core; these cells were mostly incorporated into ionized calcium-binding adaptor molecule (+) cells, suggesting these xenograft cells were immuno-rejected by the host. In another set of animals, hWJ-MSCs were transplanted in cyclosporine (CsA)-treated rats. hWJ-MSC transplants significantly reduced brain infarction, improved neurological function, and reduced neuroinflammation. Less phagocytosis of CM-dil-labeled grafted cells was found in the host brain after CsA treatment. Transplantation of hWJ-MSC significantly increased glia cell line-derived neurotrophic factor expression in the host brain. Taken together, our data support that intracerebral transplantation of hWJ-MSCs reduced neurodegeneration and inflammation in the stroke brain. The protective effect did not depend on the survival of grafted cells but may be indirectly mediated through the production of protective trophic factors from the transplants.
Keywords: stroke, transplantation, Wharton’s jelly-derived mesenchymal stromal cells, cyclosporine
Introduction
Stroke is the second leading cause of death in the past decade1 and a leading cause of adult disability worldwide. Various pharmacological therapies have been developed for the treatment of stroke; however, most of these drugs have failed in clinical trials. Cell-based therapy, such as stem cell transplantation, has provided a new approach for stroke patients.
Increasing evidence has supported the immunomodulatory and trophic action of umbilical cord matrix-derived Wharton’s jelly mesenchymal stem cells (WJ-MSCs)2,3. WJ-MSCs express low levels of interferon (IFN)-γR1 and CXCR3 receptors, but higher constitutive expression of brain-derived neurotrophic factor (BDNF), as compared with bone marrow MSCs (BM-MSCs)3. The secretome from WJ-MSCs enhances neurodifferentiation of neural progenitor cells4,5. The protective effect of WJ-MSCs has been examined in cellular or animal models of neurological diseases. Extracellular vesicles isolated from WJ-MSC supernatants suppressed oxygen–glucose deprivation (OGD)-mediated DNA fragmentation and caspase-3 transcription in cultured N2a neuroblastoma cells6. WJ-MSCs reduced cell death and vascular atrophy in the CA1 region after OGD in an ex vivo hippocampal slice model7. Intracerebral or intravenous transplantation of WJ-MSCs improved neurological function in stroke rats8 and antagonized the amphetamine-mediated rotation in unilaterally 6-hydroxydopamine lesioned rats9. Similarly, intracerebral transplantation of WJ tissue at 1 day after traumatic brain injury reduced brain edema, improved neurological function, promoted cognitive functions and increased microtubule associated protein (MAP)2 (+) cells in the lesioned cortex at 2–3 weeks post-injury in rats10. These data suggest that WJ-MSC transplant is neuroprotective against neurodegenerative injury.
Because of the immunomodulatory properties of human WJ-MSCs (hWJ-MSCs), an immunosuppressant was often not used during transplantation in animal models of neurological disease, including Parkinson’s disease11, traumatic brain injury10, epilepsy12, spinal cord injury13, hypoxic-ischemic encephalopathy14, and stroke15. The survival of grafted hWJ-MSCs was demonstrated indirectly by the location of exogenous markers in the host brain. For example, chloromethyl benzamide 1,1’-dioctadecyl-3,3,3’3’- tetramethylindocarbocyanine perchlorate (CM-Dil)-labeled immunofluorescence was found at 35 days in the host cerebral cortex brain after intravenous transplantation of hWJ-MSCs, pre-labeled with CM-Dil, in stroke rats16. However, the pre-labeled markers can be transferred from donor cells to other cells over time by phagocytosis of dying cells17. The presence of exogenous markers is insufficient evidence for the survival of transplants.
In this study, we examined the neuroprotective effects of hWJ-MSCs in a rat model of stroke. We demonstrated that transplantation of WJ-MSCs significantly reduced brain infarction, improved neurological function, and reduced neuroinflammation. CM-Dil-labeled grafted cells were phagocytosed by microglial at the grafted site. Treatment with cyclosporine (CsA) reduced phagocytosis. Our data support that intracerebral transplantation of hWJ-MSCs reduced neurodegeneration and inflammation in the stroke brain. The protective effect did not depend on the survival of grafted cells but may be indirectly mediated through the production of protective trophic factors from the transplants.
Materials and Methods
Isolation, Expansion, and Labeling of hWJ-MSC
hWJ-MSCs were provided by the HealthBanks Biotech Co., Ltd. (Taiwan). Umbilical cord tissues (UCT) from eight healthy donor mothers were obtained by HealthBanks Biotech Co., Ltd., with informed consent and approval by the Institutional Review Board of the Taipei Wanfang Hospital, Taipei Medical University (PI: Chun-Sen Hsu). Cells were prepared as previously described. UCT was disinfected by 0.22 μm filtered 75% ethanol for 30 seconds. After removal of arteries and veins, UCT was minced into pieces and treated with 1xTrypLE (Gibco, Thermo Fisher Scientific, Waltham, MA, USA.) for 30 min at 37°C. After neutralizing and removing enzymes by centrifugation, tissues were cultured in T75 flasks with complete culture medium composed with α-MEM (Minimum Essential Medium alpha; Gibco, Thermo Fisher Scientific) supplemented with 10% hPL (human platelet lysate) and 100 units penicillin/streptomycin (Gibco, Thermo Fisher Scientific) in a humidified atmosphere of 95% air and 5% CO2 at 37°C. When the primary MSCs were 80% confluent, the cells were harvested using 1×TrypLE and successive sub-culture by 5% hPL complete medium with 100 units penicillin/streptomycin. For cell cryopreservation, harvested cells were resuspended with CryoStor CS10 (BioLife Solutions Inc., Bothell, WA, USA) and placed in a freezing container overnight. After that, cells were transferred into −196°C liquid nitrogen tanks for long-term storage. For MSC characterization, positive markers (CD13, CD29, CD44, CD73, CD90, CD105 from BD Biosciences, San Jose, CA, USA), negative markers (CD31, CD34, CD45, HLA-DR from BD Biosciences) were analyzed by flow cytometry (BD FACSCalibur, San Jose, CA, USA). After thawing, hWJ-MSCs were labeled with CM-Dil (Thermo Fisher Scientific) before injection into rats18. Cell viability was evaluated by trypan blue staining, and cell recovery rate was calculated.
Animals
Adult Sprague–Dawley rats (BioLASCO, Taipei, Taiwan), weighing 250–300 g, were used in this study. The use of animals was approved by the Animal Care and Use Committee (approval number: 104065-A, National Health Research Institutes). The timeline of the experiment is shown in Fig 1. Animals were anesthetized with chloral hydrate (0.4 g/kg, i.p.). Animals received cell transplantation and stroke surgery as previously described19–21. The right middle cerebral artery (MCA) was exposed after a craniotomy. hWJ-MSCs were loaded into a 25-μl Hamilton syringe and were transplanted into three cortical areas adjacent to the bifurcation of the right MCA22. Cells (6.67 × 104 viable cells/μl × 5 μl) or vehicle (culture media) were injected at 1 μl/min into each site using an Ultra MicroPump II (World Precision Instruments, Sarasota, FL, USA). The needle was retained in place for additional 5 min after each transplantation. Approximately 10 min after the last injection of cells, the right distal MCA was occluded by 10-0 suture for 60 min21,23. Body temperature was monitored and maintained at 37°C by a heating pad during surgery and a temperature-controlled incubator after surgery. The animals were returned to their home cages after recovery from the anesthesia. Selective animals received CsA (Novartis Pharmaceuticals, Basel, Switzerland) injection. CsA was given at the dose of 10 mg/kg/d (i.p.). Animals were treated with CsA from 1 day before transplantation to day 4 after MCAo.
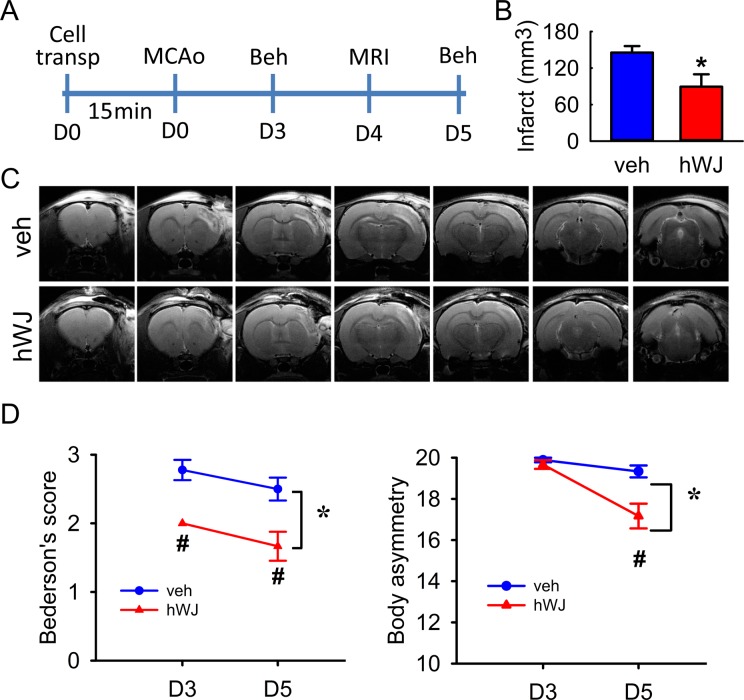
Fig 1.
Transplantation of hWJ-MSCs reduced brain infarction and behavioral deficits in stroke rats. (A) Timeline of the experiment. (B) Averaged infarct volume, measured by T2WI on day 4, was significantly reduced by the hWJ-MSC graft (p = 0.0194). (C) Representative T2WIs. The size of the lesion was reduced in an animal grafted with hWJ-MSCs, as compared with another animal treated with vehicle. (D) Bederson’s neurological and body asymmetry tests were carried out on days 3 and 5 after treatment. Transplantation with hWJ-MSCs significantly reduced the Bederson’s score (p < 0.001) and body asymmetry (p < 0.001, two-way ANOVA).
ANOVA: analysis of variance; hWJ-MSC: Wharton’s jelly-derived mesenchymal stromal cell; T2WI: T2-weighted image.
Behavioral Measurement
Stroke behavior was analyzed by two tests. (a) Body asymmetry or elevated body swing test24. Rats were examined for lateral movements when their bodies were suspended 20 cm above the testing table by lifting their tails. The frequency of initial turning of upper body or head contralateral to the ischemic side was counted in 20 consecutive trials25. (b) Neurological deficits were also evaluated using the Bederson’s test26. The degree of abnormal posture was scored as previously described19.
Magnetic Resonance Imaging (MRI)
In vivo MRI experiments were conducted on a 7 T animal MRI system (Biospec 70/30 AS, Bruker Biospin MRI, Ettlingen, Germany) equipped with an actively shielded gradient coil with the maximum gradient strength of 670 mT/m and the rise time ≤175 ms as we previously described19. A linear volume radio frequency (RF) resonator and an actively decoupled surface coil were used for RF excitation and signal reception, respectively. After being anesthetized using 1% isoflurane, rats were placed in a custom-made head holder with a dedicated water-heated rat bed, maintaining the rat body temperature at 37°C. The respiration rate and rectal temperature were monitored using a small animal monitoring and gating system (SA Instruments, Inc., Stony Brook, NY, USA). The T2-weighted image (T2WI) was acquired as previously described. In brief, a fast spin echo sequence with rapid acquisition with refocused echoes (RARE) was employed. The acquisition parameters were field of view (FOV) = 25 × 25 mm2, matrix size = 256 × 256, repetition time (TR) = 2742 ms, echo time (TE) = 33 ms and slice thickness = 1 mm. A total of 25 slices were acquired to cover the whole brain, resulting in total scan time of 6 min. For brain infarction measurement, the region of interests (ROIs), defining the infarction areas, were manually drawn based on the hyper-intensity regions on T2WI. Infarct volume was calculated by the summation of total ROI areas from all lesion-contaminating slices multiplying the slice thickness.
Immunohistochemistry
Animals were anesthetized and perfused transcardially with saline followed by 4% paraformaldehyde (PFA; Sigma-Aldrich, St. Louis, MO, USA) in phosphate buffer (PB; 0.1 M; pH 7.2, Sigma-Aldrich). The brains were dissected, postfixed in PFA for at least 48 h, and transferred to 20% sucrose in 0.1 M PB for at least 16 h. Serial sections of the entire brain were cut at a 30-μm thickness on a freezing cryostat (model: CM 3050 S; Leica, Heidelberg, Germany). After blocking with 4% bovine serum albumin (BSA; Sigma-Aldrich) and 0.5% Triton X-100 in 0.1 M PB, brain slices were incubated with antibodies against ionized calcium-binding adaptor molecule (IBA1; 1:200; Abcam, Cambridge, MA, USA) at 4°C overnight. Sections were rinsed in 0.1 mol/l PB and were mounted on slides and coverslipped. Control sections were incubated without primary antibody. Confocal analysis was performed using a Nikon D-ECLIPSE 80i microscope (Nikon Instruments, Inc., Tokyo, Japan) and the EZ-C1 3.90 software (Nikon, Tokyo, Japan). The optical density of IBA1 immunoreactivity was quantified in three consecutive brain sections with a visualized anterior commissure in each animal. A total of six photomicrographs were taken along the peri-lesioned region per brain slice. All immunohistochemical measurements were done by blinded observers.
Quantitative Reverse Transcription Polymerase Chain Reaction
Adult rats received hWJ-MSC transplantation or sham surgery. Cerebral cortices were collected at 5 days after transplantation for quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis as described previously20,21,27. Total RNA was isolated using TRIZOL Reagents (Life Technologies, #15596-026, Carlsbad, CA.) and cDNA was synthesized from 1 µg total RNA using a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, #K1622). The TaqMan Gene Expression Assays (primer and probe set) for specifically detecting rat BDNF (#Rn02531967_s1), glyceraldehyde 3-phosphate dehydrogenase (GADPH; #Rn01775763_g1) and beta-actin (Rn00667869_m1) were purchased from Thermo Scientific. The primer and 6-carboxyfluorescein (FAM)-labeled probe used in the qRT-PCR for glial cell-derived neurotrophic factor (GDNF) are as follows: GDNF forward primer (5’-TAAGATGAAGTTATGGGATGTCG); reverse primer (5’-CTTCGAGAAGCCTCTTACCG); probe (mouse/rat universal probe Library #112; Roche). qRT-PCR was carried out using TaqMan Fast Advanced Master Mix (Life Technologies, #4444557) and Applied Biosystems 7500 Fast Real-Time PCR System. Expression and of the target genes GDNF and BDNF was calculated by comparing to two reference genes (beta-actin and GAPDH) using the Applied Biosystems 7500 Real-Time PCR Software (version 2.0.6). The expression of GDNF or BDNF (2-(delta-delta-Ct)) after transplantation or CsA injection was further normalized to the vehicle control. All experiments were duplicated.
Statistics
Values are means ± SEM. Two-tailed unpaired Student’s t-test, two-way analysis of variance (ANOVA), and post-hoc Newman–Keuls test were used for statistical analysis. A statistically significant difference was defined as p < 0.05. All analyses were performed with Sigma plot version 12.5 software (Systat Software Inc., Chicago, IL, USA).
Results
Brain Infarction
A total of 15 stroke rats (6 receiving hWJ-MSCs and 9 receiving vehicles) were used to examine brain infarction by MRI on day 4 after MCAo (see Timeline, Fig 1A). Typical T2-weighted images (T2WIs) from two stroke rats receiving hWJ-MSCs or vehicle are shown in Fig 1C. In both animals, an increase in signal intensity of T2WI was found in the cortex of the lesioned side. The ROIs were manually determined by two of the authors (CWC and LWK) to enclose the infarct regions in T2WI after contrast adjustment. The volume of the lesion (infarction) was quantified using Cavalieri’s method. hWJ-MSC grafts significantly reduced the infarct volume, as compared with the vehicle (hWJ-MSCs: 89.3 ± 20.4 mm3 versus vehicle: 145.5 ± 10.6 mm3, p = 0.0194, t-test, Fig 1B).
Transplantation of hWJ-MSCs Improved Motor Behavior in Stroke Rats
Two behavioral tests were used to evaluate the functional recovery on days 3 or 5 after cell transplantation in 15 rats (please see timeline in Fig 1A). The Bederson’s neurological score was significantly reduced by cell transplantation (Fig 1D, F1,26 = 24.948, p < 0.001, two-way ANOVA). No significant interaction was found between the transplantation group and days after stroke (F1,16 = 0.0295, p = 0.865, two-way ANOVA). A post-hoc Newman–Keuls test indicated that hWJ-MSC transplants significantly reduced neurological score on day 3 (p = 0.002) and day 5 (p = 0.001), compared with the vehicle. Neurological deficit was also examined by an elevated body asymmetry test. The frequency of initial turning of the head or upper body contralateral to the ischemic side was counted in 20 consecutive trials. The maximum impairment in body asymmetry in stroke animals is 20 contralateral turns/20 trials. A significant reduction in body asymmetry was seen in cell transplantation animals (Fig 1D, F1,26 = 14.016, p < 0.001) and days after stroke (F1,26 = 22.930, p < 0.001). A significant interaction was found between the transplantation and days after stroke (F1,36 = 9.286, p = 0.005, two-way ANOVA). A post-hoc Newman–Keuls test indicated that hWJ-MSC transplants significantly reduced body asymmetry on day 5 (p < 0.002, Fig 1D).
Localization of hWJ-MSCs Transplant in the Ischemic Core
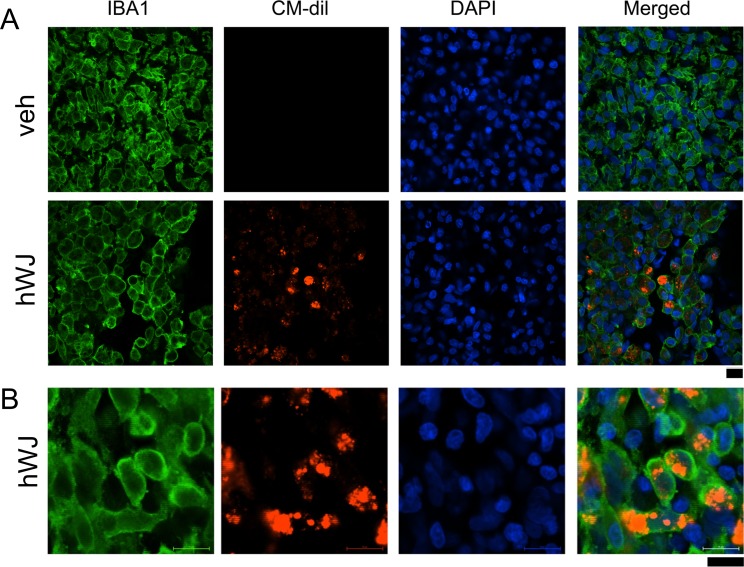
hWJ-MSCs were labeled with CM-Dil fluorescence before transplantation. Animals receiving hWJ-MSCs (n = 6) or vehicle (n = 9) were perfused on day 5 after transplantation and stroke surgery. As seen in the representative photomicrograph in Fig 2A, CM-Dil (red) fluorescence was confined to the graft sites in the cerebral cortex. No red fluorescence was found in animals receiving vehicle (Fig 2A). The density of IBA1 immunoreactivity was increased in the ischemic core. The IBA immunoreactivity in the core region was further quantified. Transplantation of hWJ-MSCs did not significantly alter the density of IBA1 immunoreactivity in the lesioned core (p = 0.270, Student’s t-test). Most of this microglia had an amoeboid morphology (Fig 2B), suggesting that hWJ-MSC transplants did not reduce the activation of microglia in the core region. Using confocal imaging, we found that CM-Dil fluorescence was mainly present within the IBA1 (+) cells (Fig 2B), suggesting that the graft cells were phagocytized by the activated microglia in the core of lesion.
Fig 2.
Transplantation of hWJ-MSCs did not reduce microglial activation in the ischemic core area. hWJ-MSCs were pre-labeled with the fluorescent dye CM-Dil before grafting to stroke rats. Animals were perfused on day 5 after transplantation and stroke surgery. (A) Enhanced IBA1 immunoreactivity (green fluorescence) was found in the ischemic core in animals receiving vehicle or hWJ-MSCs. (A) Red CM-Dil fluorescence (+) cells were confined to the graft sites. No red fluorescence was found in animals receiving vehicle. (B) High-magnification confocal photomicrographs indicated that grafted cells (red fluorescence) were mainly in the microglia. Calibration = 10 μm.
CM-Dil: chloromethyl benzamide 1,1’-dioctadecyl-3,3,3’3’- tetramethylindocarbocyanine perchlorate; hWJ-MSC: Wharton’s jelly-derived mesenchymal stromal cell.
Transplantation of hWJ-MSCs Reduced Microglia Activation in the Peri-Lesioned Area
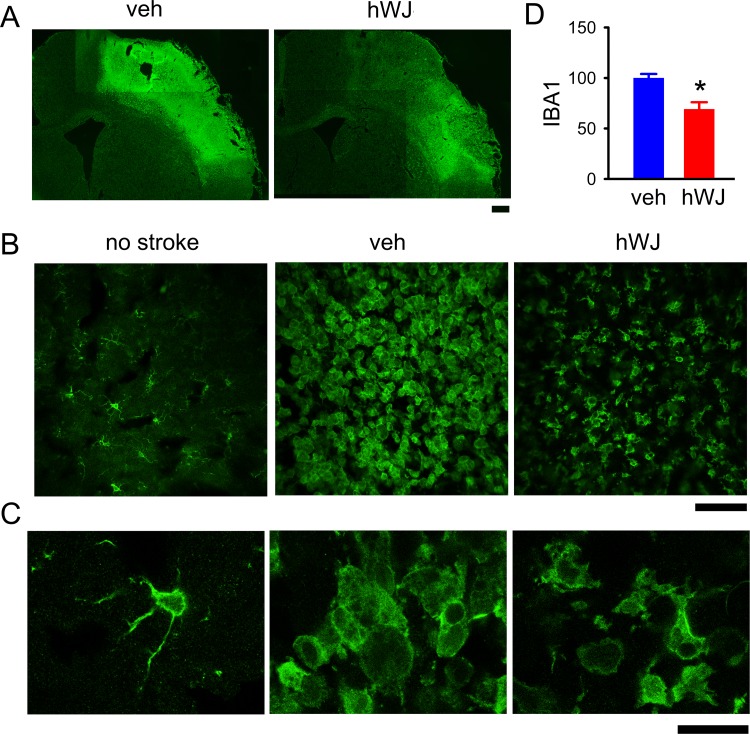
Microglial activation was also found in the peri-lesioned area (Fig 3). Transplantation of hWJ-MSCs reduced IBA1 immunoreactivity in this region (Fig 3 A and B). The optical density of IBA1 immunoreactivity was quantified in the six different peri-lesioned areas in three consecutive brain sections with a visualized anterior commissure in each animal. Averaged IBA1 optical density in the peri-lesioned zone, normalized to the mean of vehicle controls, was significantly reduced by the hWJ-MSC transplant (p < 0.001, Student’s t-test, Fig 3D). At a higher magnification, we found that most of the microglia were de-ramified or in amoeboid morphology in the peri-lesioned area in animals receiving vehicle (Fig 3C). In contrast, resting microglia exhibiting ramified morphology were found in the non-lesioned side cortex (Fig 3C). Some ramified microglia were found in the peri-lesioned area in animals receiving hWJ-MSC grafting (Fig 3C). Taken together, these data suggest that hWJ-MSC transplants reduced the IBA1 immunoreactivity as well as morphological activation of microglia in the peri-lesioned area.
Fig 3.
Transplantation of hWJ-MSCs reduced the microglial activation in the peri-lesioned area. (A) IBA1 immunoreactivity was greatly increased in the peri-lesioned area of the ischemic cortex. (B) Transplantation of hWJ-MSCs reduced the IBA1 immunoreactivity in stroke brains (vehicle versus hWJ). (C) High-magnification images demonstrate de-ramified or amoeboid microglial cells in the peri-lesioned cortex in animals receiving vehicle, as compared with the resting microglia with ramified morphology in the non-lesioned side cortex. Ramified microglia were partially restored in the peri-lesioned area in animals receiving hWJ-MSCs. (D) The optical density of IBA1 immunoreactivity was quantified in the six different peri-lesioned areas in three consecutive brain sections with a visualized anterior commissure in each animal. Averaged IBA1 optical density in the peri-lesioned zone was significantly reduced by hWJ-MSCs transplantation. Calibration: (A) 1000 μm, (B) 50 μm, (C) 10 μm.
hWJ-MSC: Wharton’s jelly-derived mesenchymal stromal cell; IBA1: ionized calcium-binding adaptor molecule.
CsA Reduced the Immunorejection of hWJ-MSC Transplants
Stroke rats receiving (A) CsA and hWJ-MSC transplant (hWJ + CsA, n = 8), (B) hWJ-MSC transplants only (hWJ, n = 6), or (C) vehicle (n = 9) were used in this study. Transplantation of hWJ + CsA, similar to hWJ, significantly reduced the Bederson’s neurological score (Fig 4A F3,52 = 14.134, p < 0.001) and body asymmetry (Fig 4B, F3,52 = 4.837, p = 0.005, two-way ANOVA). No difference was found between the animals receiving hWJ + CsA and hWJ. Brain infarction was examined on day 4 by MRI. The area of infarction in brain slices was quantified every 2 mm from the rostral end (Fig 4I). hWJ or hWJ + CsA significantly reduced brain infarction (p < 0.001, F2,126 = 14.910, two-way ANOVA, Fig 4I). No significant difference was found between hWJ + CsA and hWJ groups (Fig 4I, p = 0.111, post-hoc Newman–Keuls test).
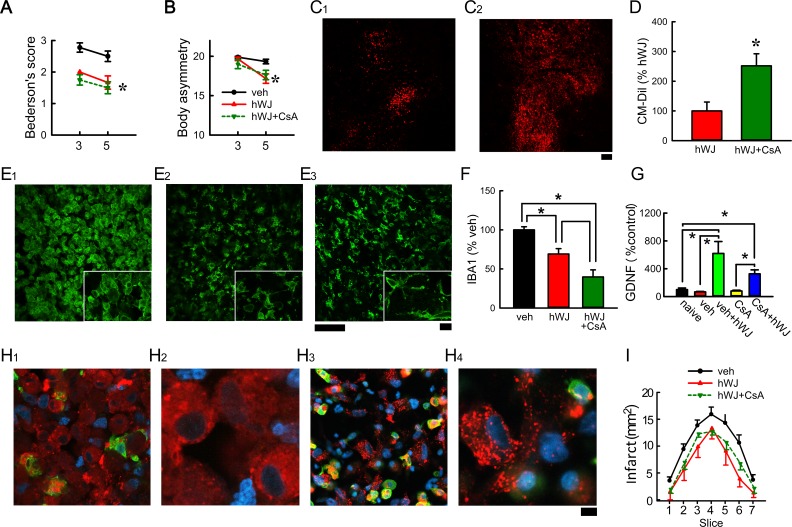
Fig 4.
Treatment with CsA reduced immunorejection of grafted hWJ-MSCs in stroke brain. Transplantation of hWJ-MSCs with CsA (hWJ + CsA) or without CsA (hWJ) significantly reduced (A) Bederson’s neurological score and (B) body asymmetry. Animals were perfused on day 5 after the MCAo. (C) CM-Dil (red fluorescence) was found at the graft sites (C1: hWJ versus C2: hWJ + CsA). (D) A significant increase in CM-Dil fluorescence at the graft site was found in animals receiving CsA at the level of anterior commissure. Compared with vehicle (E1), hWJ + CsA (E3) and hWJ (E2) significantly attenuated IBA1 immunoreactivity in the peri-lesioned cortex (F). High-magnification images (insets) demonstrate ameboid microglial cells in the peri-lesioned cortex in animals receiving vehicle (E1). Ramified microglia were found in the peri-lesioned area in animals receiving hWJ (E2) or hWJ with CsA (E3). (H) CM-Dil (+) cells were found in the lesioned core. Higher magnification confocal photomicrographs indicated that some CM-Dil (+) cells were not contained by the microglia in animals receiving CsA (H2, H4). (I) Brain infarction was examined on day 4 by MRI. The area of infarction in brain slices was quantified every 2 mm from the rostral end. hWJ or hWJ + CsA significantly reduced brain infarction. Calibration C1-2:100 µm; E1–3: 50 µm; E1–3 (insert) 10 µm; H1 and H3 = 10 µm; H2 and H4: 3.2 µm. (G) Transplantation of hWJ-MSCs, with or without CsA, significantly increased GDNF mRNA expression in the grafted side (right) cortex, as compared with the no-transplant controls. The expression of GDNF in the contralateral side (left) cortex was not altered by the transplant.
CM-Dil: chloromethyl benzamide 1,1’-dioctadecyl-3,3,3’3’- tetramethylindocarbocyanine perchlorate; CsA: cyclosporine; hWJ-MSC: human Wharton’s jelly-derived mesenchymal stromal cell; MCAo: middle cerebral artery occlusion; MRA: magnetic resonnace imaging.
Animals were perfused on day 5 after the MCAo. hWJ-MSCs (CM-Dil, red fluorescence) were found at the graft sites (Fig 4C1: hWJ only versus C2: hWJ + CsA). A significant increase in CM-Dil fluorescence at the graft site was found in animals receiving CsA at the level of anterior commissure (Fig 4C and D, p = 0.0151, Student’s t-test). Both hWJ + CsA and hWJ significantly attenuated IBA1 immunoreactivity in the peri-lesioned cortex (Fig 4 E1: vehicle, E2: hWJ, and E3: hWJ + CsA; Fig 4F: hWJ + CsA versus vehicle, p < 0.001, F2,22 = 23.972, one-way ANOVA; p < 0.001, post-hoc Newman–Keuls test; hWJ only versus vehicle, p = 0.004, post-hoc Newman–Keuls test). A significant difference was found between hWJ + CsA and hWJ (Fig 4F, p = 0.009, post-hoc Newman–Keuls test). In the lesioned core, a number of CM-Dil (+) cells were found outside of IBA1 (+) cell s in the lesioned site (Fig 4H1, H3). Higher magnification confocal photomicrographs indicated that these CM-Dil (+) cells were not contained in the microglia in animals receiving CsA (Fig 4H2, H4). In contrast, the grafted hWJ-MSCs were mainly located within the microglia in the recipients receiving vehicle (Fig 2). These data suggest that administration of CsA reduces the phagocytic action of microglia and may prolong the survival of hWJ-MSC transplants.
hWJ-MSC Transplants Upregulated GDNF Expression in the Host Brain
A total of eight rats were transplanted with hWJ-MSCs. Of these, four rats were treated with CsA and four received the vehicle. Another four rats received sham surgery (no transplantation) and vehicle injection were used as controls. Right (with transplant) and left (without transplant) cortices were collected at 5 days after transplantation. Transplantation of hWJ-MSCs, with or without CsA, significantly increased GDNF mRNA expression in the grafted side (right) cortex, as compared with the no-transplant controls (Fig 4G, p = 0.006, H = 14.557, one-way ANOVA on ranks). The expression of GDNF in the contralateral side (left) cortex was not altered by the transplant. Transplantation of hWJ-MSCs did not significantly alter BDNF expression (p = 0.0176, H = 6.329, one-way ANOVA on ranks).
Discussion
In this study, we examined the protective effect of hWJ-MSC xenografts in rodents. Intracortical WJ-MSC grafts significantly reduced body asymmetry and neurological score in stroke rats. Using a T2WI, we demonstrated that transplantation of hWJ-MSCs significantly reduced infarction in stroke rats. Our data support a neuroprotective role of WJ-MSC transplants in an animal model of stroke.
Human umbilical cord blood cells (hUCBCs) have unique immunomodulatory potential. hUCBCs have counteracted the proinflammatory T-helper cell response in an animal model of stroke28. In this study, transplantation of hWJ-MSCs significantly reduced IBA1 immunoreactivity and morphological activation of microglia in the peri-lesioned area. In contrast, the density of IBA1 immunoreactivity was not altered by the hWJ-MSC transplants in the lesioned core. The microglia in the core region were in amoeboid morphology. These data suggest that the anti-inflammatory action induced by hWJ-MSC transplant is limited to the peri-lesioned region. hWJ-MSCs did not reduce the activation of microglia in the core region.
The survival of grafted cells has often been examined by exogenously labeled markers in host tissue16. In this study, hWJ-MSCs were labeled with CM-Dil before transplantation. We found that CM-Dil fluorescence was found at the site of transplantation in the core of ischemic area. However, using confocal imaging, we found that CM-Dil was present mainly in microglia at the graft site in animals, indicating that grafted cells were phagocytosed by activated microglia after intracerebral transplantation. We found that treatment with CsA increased the CM-Dil fluorescence and reduced the phagocytosis of grafted cells. This finding was supported by previous reports that CsA inhibits activation of resident microglia in animals receiving oligodendrocyte progenitor cell transplantation29 and improved the survival of human xenografts in an animal model of Parkinson’s disease30. It is thus possible that CsA is needed to suppress the immunoreaction and prolong the survival of the grafted hWJ-MSCs.
The neuroprotective effects of human cord blood cell (hUCBC) transplant have been examined in experimental animals. Most of these studies were conducted without CsA treatment31–33. The recipients demonstrated a functional improvement after transplantation, similar to our current findings in animals without CsA treatment. For example, intravenous injection of hUCBCs reduced brain infarction, improved behavioral function, and upregulated the neuroprotective trophic factor GDNF in the stroke rats34. No grafted cells were found in the host brain. In the current study, we found that transplantation of hWJ-MSCs improved neurological function; however, most of the grafted cells were phagocytosed by the activated microglia. These data suggest that the functional recovery does not require the presence of grafted cells in the host brain. It is possible that the constituents in the grafted cells may improve neurological outcomes. We previously reported that administration of GDNF protein23 or overexpression GDNF by HSV-GDNF35 reduced brain infarction and restored locomotor activity in stroke rats. A similar protective response can be found after transplantation of GDNF containing cells, such as fetal kidney cells22, to the stroke brain. In the current study, we found that the hWJ-MSC graft significantly increased GDNF expression in the host brain. It is possible that behavioral improvement and anti-inflammatory effects after transplantation may indirectly derive from neuroprotective molecules or trophic factors released from the graft cells.
Our data support that the exogenous MSCs transplanted can be rejected by an inflammatory response in the host brain. Similar effects have shown that neuronal-primed hMSCs can only survive in host rodent brain for 7 days despite the reported immunosuppressive properties of MSCs36. Coyne et al. reported that a pre-labeled marker, such as BrdU, can be transferred from donor cells to other cells over time by phagocytosis of dying cells17. They cautioned against the use of BrdU as donor cell labels. The presence of CM-Dil at days after transplantation may not accurately reflect donor cells survival, and the improved functional recovery may not correlate with the survival of grafted cells in the host. Immunosuppressive agents are required to prolong the survival of the grafted cells, which may play significant roles in neuroregeneration.
In conclusion, our data support that intracerebral transplantation of hWJ-MSCs reduced neurodegeneration and inflammation in the stroke brain. The protective mechanisms of hWJ-MSCs in stroke brain is summarized in Fig 5. The protective responses may not require the survival of cells, but the constituents in the grafted cells.
Fig 5.
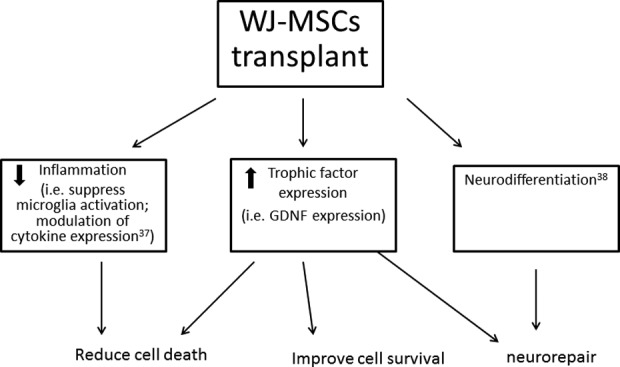
A schematic diagram illustrating the protective and regenerative action of hWJ-MSC transplant in stroke brain.
hWJ-MSC: human Wharton’s jelly-derived mesenchymal stromal cell.
Acknowledgments
This research was supported by (i) National Health Research Institutes, Taiwan, (ii) Central Government S & T grant, Taiwan (107-1901-01-19-02), and (iii) HealthBanks Biotech Co., Ltd., Taiwan.
Footnotes
Author Contributions: K.J. Wu (surgery, behavior and data analysis); Seong-Jin Yu (immunohistochemistry, data analysis, manuscript preparation); C.W. Chiang and L.W. Kuo (MRI studies, data analysis), C.S. Hsu and P.C. Tseng (WJ-MSC dissection and characterization), Y.W. Lee (stem cell in vitro preparation), B.L. Yen (experimental design, manuscript preparation), Y. Wang (experimental design, manuscript preparation).
Ethical Approval: Umbilical cord tissues (UCT) from eight healthy donor mothers were obtained by HealthBanks Biotech Co., Ltd., with informed consent and approval by the Institutional Review Board of the Taipei Wanfang Hospital, Taipei Medical University (PI: Chun-Sen Hsu).
Statement of Human and Animal Rights: hWJ-MSCs were provided by the HealthBanks Biotech Co., Ltd. (Taiwan).
Statement of Informed Consent: Umbilical cord tissues (UCT) from eight healthy donor mothers were obtained by HealthBanks Biotech Co., Ltd.
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Pei-Chi Tseng is an employee of HealthBanks Biotech Co., Ltd., Taipei, Taiwan. The National Health Research Institute and HealthBanks Biotech Co. have a cooperative research and development agreement to develop WJ-MSCs as a treatment strategy for neurodegenerative disorders.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. WHO. The top 10 causes of death. 2018. [cited ]. Available from www.who.int/mediacentre/factsheets/fs310/en/index.html. Online Source. 24 May, 2018.
- 2. Watson N, Divers R, Kedar R, Mehindru A, Mehindru A, Borlongan MC, Borlongan CV. Discarded Wharton jelly of the human umbilical cord: a viable source for mesenchymal stromal cells. Cytotherapy. 2015;17(1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Donders R, Bogie JFJ, Ravanidis S, Gervois P, Vanheusden M, Marée R, Schrynemackers M, Smeets HJM, Pinxteren J, Gijbels K, Walbers S, Mays RW, Deans R, Van Den Bosch L, Stinissen P, Lambrichts I, Gyselaers W, Hellings N. Human Wharton’s jelly-derived stem cells display a distinct immunomodulatory and proregenerative transcriptional signature compared with bone marrow-derived stem cells. Stem Cells Dev. 2018;27(2):65–84. [DOI] [PubMed] [Google Scholar]
- 4. Oppliger B, Joerger-Messerli MS, Simillion C, Mueller M, Surbek DV, Schoeberlein A. Mesenchymal stromal cells from umbilical cord Wharton’s jelly trigger oligodendroglial differentiation in neural progenitor cells through cell-to-cell contact. Cytotherapy. 2017;19(7):829–838. [DOI] [PubMed] [Google Scholar]
- 5. Teixeira FG, Carvalho MM, Neves-Carvalho A, Panchalingam KM, Behie LA, Pinto L, Sousa N, Salgado AJ. Secretome of mesenchymal progenitors from the umbilical cord acts as modulator of neural/glial proliferation and differentiation. Stem Cell Rev. 2015;11(2):288–297. [DOI] [PubMed] [Google Scholar]
- 6. Joerger-Messerli MS, Oppliger B, Spinelli M, Thomi G, di Salvo, I, Schneider P, Schoeberlein A. Extracellular vesicles derived from Wharton’s jelly mesenchymal stem cells prevent and resolve programmed cell death mediated by perinatal hypoxia-iIschemia in neuronal cells. Cell Transplant. 2018;27(1):168–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Obtulowicz P, Lech W, Strojek L, Sarnowska A, Domanska-Janik K. Induction of endothelial phenotype from Wharton’s jelly-derived MSCs and comparison of their vasoprotective and neuroprotective potential with primary WJ-MSCs in CA1 hippocampal region ex vivo. Cell Transplant. 2016;25(4):715–727. [DOI] [PubMed] [Google Scholar]
- 8. Ding DC, Shyu WC, Chiang MF, Lin SZ, Chang YC, Wang HJ, Su CY, Li H. Enhancement of neuroplasticity through upregulation of beta1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol Dis. 2007;27(3):339–53. [DOI] [PubMed] [Google Scholar]
- 9. Fu YS, Cheng YC, Lin MY, Cheng H, Chu PM, Chou SC, Shih YH, Ko MH, Sung MS. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006;24(1):115–24. [DOI] [PubMed] [Google Scholar]
- 10. Cheng T, Yang B, Li D, Ma S, Tian Y, Qu R, Zhang W, Zhang Y, Hu K, Guan F, Wang J. Wharton’s jelly transplantation improves neurologic function in a rat model of traumatic brain injury. Cell Mol Neurobiol. 2015;35(5):641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells. 2006;24(3):781–792. [DOI] [PubMed] [Google Scholar]
- 12. Huang PY, Shih YH, Tseng YJ, Ko TL, Fu YS, Lin YY. Xenograft of human umbilical mesenchymal stem cells from Wharton’s jelly as a potential therapy for rat pilocarpine-induced epilepsy. Brain Behav Immun. 2016;54:45–58. [DOI] [PubMed] [Google Scholar]
- 13. Zhang L, Zhang HT, Hong SQ, Ma X, Jiang XD, Xu RX. Cografted Wharton’s jelly cells-derived neurospheres and BDNF promote functional recovery after rat spinal cord transection. Neurochem Res. 2009;34(11):2030–2039. [DOI] [PubMed] [Google Scholar]
- 14. Zhang X, Zhang Q, Li W, Nie D, Chen W, Xu C, Yi X, Shi J, Tian M, Qin J, Jin G, Tu W. Therapeutic effect of human umbilical cord mesenchymal stem cells on neonatal rat hypoxic-ischemic encephalopathy. J Neurosci Res. 2014;92(1):35–45. [DOI] [PubMed] [Google Scholar]
- 15. Sabbaghziarani F, Mortezaee K, Akbari M, Kashani IR, Soleimani M, Moini A, Ataeinejad N, Zendedel A, Hassanzadeh G. Retinoic acid-pretreated Wharton’s jelly mesenchymal stem cells in combination with triiodothyronine improve expression of neurotrophic factors in the subventricular zone of the rat ischemic brain injury. Metab Brain Dis. 2017;32(1):185–193. [DOI] [PubMed] [Google Scholar]
- 16. Zhang L, Wang LM, Chen WW, Ma Z, Han X, Liu CM, Cheng X, Shi W, Guo JJ, Qin JB, Yang XQ, Jin GH, Zhang XH. Neural differentiation of human Wharton’s jelly-derived mesenchymal stem cells improves the recovery of neurological function after transplantation in ischemic stroke rats. Neural Regen Res. 2017;12(7):1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coyne TM, Marcus AJ, Woodbury D, Black IB. Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells. 2006;24(11):2483–2492. [DOI] [PubMed] [Google Scholar]
- 18. Ting CH, Ho PJ, Yen BL. Age-related decreases of serum-response factor levels in human mesenchymal stem cells are involved in skeletal muscle differentiation and engraftment capacity. Stem Cells Dev. 2014;23(11):1206–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu KJ, Yu SJ, Chiang CW, Cho KH, Lee YW, Yen BL, Kuo LW, Wang Y. Transplantation of human placenta-derived multipotent stem cells reduces ischemic brain injury in adult rats. Cell Transplant. 2015;24(3):459–470. [DOI] [PubMed] [Google Scholar]
- 20. Luo Y, Shen H, Liu HS, Yu SJ, Reiner DJ, Harvey BK, Hoffer BJ, Yang Y, Wang Y. CART peptide induces neuroregeneration in stroke rats. J Cereb Blood Flow Metab. 2013;33:300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luo Y, Kuo CC, Shen H, Chou J, Greig NH, Hoffer BJ, Wang Y. Delayed treatment with a p53 inhibitor enhances recovery in stroke brain. Ann Neurol. 2009;65(5):520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chiang YH, Lin SZ, Borlongan CV, Hoffer BJ, Morales MF, Wang Y. Transplantation of fetal kidney tissue reduces cerebral infarction induced by middle cerebral artery ligation. J Cerebral Blood Flow Metab. 1999;19(12):1329–1335. [DOI] [PubMed] [Google Scholar]
- 23. Wang Y, Lin SZ, Chiou AL, Williams LR, Hoffer BJ. Glial cell line-derived neurotrophic factor protects against ischemia-induced injury in the cerebral cortex. J Neurosci. 1997;17(11):4341–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Borlongan CV, Hida H, Nishino H. Early assessment of motor dysfunctions aids in successful occlusion of the middle cerebral artery. Neuroreport. 1998;9(16):3615–3621. [DOI] [PubMed] [Google Scholar]
- 25. Chang CF, Morales M, Chou J, Chen HL, Hoffer BJ, Wang Y. Bone morphogenetic proteins are involved in fetal kidney tissue transplantation -induced neuroprotection in stroke rats. Neuropharmacology. 2002;43(3):418–426. [DOI] [PubMed] [Google Scholar]
- 26. Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17(3):472–476. [DOI] [PubMed] [Google Scholar]
- 27. Shen H, Luo Y, Kuo CC, Deng X, Chang CF, Harvey BK, Hoffer BJ, Wang Y. 9-Cis-Retinoic acid reduces ischemic brain injury in rodents via bone morphogenetic protein. J Neurosci Res. 2009;87(2):545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vendrame M, Cassady J, Newcomb J, Butler T, Pennypacker KR, Zigova T, Sanberg CD, Sanberg PR, Willing AE. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35(10):2390–2395. [DOI] [PubMed] [Google Scholar]
- 29. Lu HZ, Wang YX, Zhou JS, Wang FC, Hu JG. Cyclosporin A increases recovery after spinal cord injury but does not improve myelination by oligodendrocyte progenitor cell transplantation. BMC Neurosci. 2010;11:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Y, Lin JC, Chiou AL, Liu JY, Liu JC, Zhou FC. Human ventromesencephalic grafts restore dopamine release and clearance in hemiparkinsonian rats. Exp Neurol. 1995;136(2):98–106. [DOI] [PubMed] [Google Scholar]
- 31. Huang L, Liu Y, Lu J, Cerqueira B, Misra V, Duong TQ. Intraarterial transplantation of human umbilical cord blood mononuclear cells in hyperacute stroke improves vascular function. Stem Cell Res Ther. 2017;8(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shahaduzzaman MD, Mehta V, Golden JE, Rowe DD, Green S, Tadinada R, Foran EA, Sanberg PR, Pennypacker KR, Willing AE. Human umbilical cord blood cells induce neuroprotective change in gene expression profile in neurons after ischemia through activation of Akt pathway. Cell Transplant. 2015;24(4):721–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yan T, Venkat P, Chopp M, Zacharek A, Ning R, Cui Y, Roberts C, Kuzmin-Nichols N, Sanberg CD, Chen J. Neurorestorative therapy of stroke in type 2 diabetes mellitus rats treated with human umbilical cord blood cells. Stroke. 2015;46(9):2599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Borlongan CV, Hadman M, Sanberg CD, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35(10):2385–2389. [DOI] [PubMed] [Google Scholar]
- 35. Harvey BK, Chang CF, Chiang YH, Bowers WJ, Morales M, Hoffer BJ, Wang Y, Federoff HJ. HSV amplicon delivery of glial cell line-derived neurotrophic factor is neuroprotective against ischemic injury. Exp Neurol. 2003;183(1):47–55. [DOI] [PubMed] [Google Scholar]
- 36. Khoo ML, Tao H, Meedeniya AC, Mackay-Sim A, Ma DD. Transplantation of neuronal-primed human bone marrow mesenchymal stem cells in hemiparkinsonian rodents. PLoS One. 2011;6(5):e19025. [DOI] [PMC free article] [PubMed] [Google Scholar]