Abstract
Osteoarthritis (OA) is degenerative disease, leading to pain and functional disability. It is reported that polydeoxyribonucleotide (PDRN) is a suitable therapy for OA. However, the therapeutic mechanisms of PDRN in OA are not fully understood. To investigate the effect of PDRN in an in vitro model of OA, interleukin (IL)-1β or phosphate-buffered saline (PBS) was used to treat a human chondrocytic cell line in hypoxic conditions for 24 h (IL-1β group or control group). PDRN was then used to treat IL-1β group cells for 24 h (PDRN group). By Label-Based Human Antibody Array 1000, angiopoietin-2 (ANG-2), platelet-derived growth factor (PDGF), angiostatin, and endostatin, which were related to angiogenesis, were chosen for further validation studies. Quantitative real-time reverse transcription polymerase chain reaction and western blot analysis validated that the levels of PDGF and ANG-2, which were related to pro-angiogenesis, were significantly increased in the PDRN group compared with those in the control group or the IL-1β group. However, the levels of endostatin and angiostatin, which were related in anti-angiogenesis, were significantly decreased in the PDRN group compared with those in the control group or the IL-1β group. In the same manner, vascular endothelial growth factor, which was a mediator of angiogenesis, was significantly increased in the PDRN group compared with those in the control group or the IL-1β group. Furthermore, wound closure was significantly increased in the PDRN group compared with the control group or the IL-1β group by in vitro scratch assay. Moreover, PDRN decreased expression of metalloproteinase 13, as a catabolic factor for OA, but increased expression of aggrecan, which was an anabolic factor for OA. These data suggest that PDRN may promote angiogenesis and wound healing via down-regulation of catabolism and up-regulation of anabolism in an in vitro model of OA.
Keywords: polydeoxyribonucleotide, osteoarthritis, angiogenesis, wound healing, catabolism, anabolism
Introduction
Knee osteoarthritis (OA) involves degeneration of the articular cartilage and meniscus in the knee joint, synovial hyperplasia, subchondral bone sclerosis, and edema, leading to joint pain, swelling, stiffness, muscle atrophy, and functional disability1. To date, the pharmacological armamentarium for OA has been limited to symptomatic treatments with a goal to diminish functional impairments and pain severity2. Currently, corticosteroids and hyaluronic acid (HA) are used for intra-articular injection therapy of OA. However, corticosteroid injections only provide short-term pain relief by modulating inflammation. They can lead to further joint destruction3. HA, a large non-sulfated glycosaminoglycan found in synovial fluid, has been injected into arthritic knees to aid and improve articular cartilage lubrication4. Nevertheless, recent clinical practice guidelines, issued by the American Academy of Orthopedic Surgeons in 2013, stated that they were unable to recommend HA for treatment of symptomatic OA in the knee based on the lack of evidence of its effectiveness from high and moderate quality research studies5. Thus, the ideal intra-articular treatment for OA should not only mechanically protect the damaged cartilage surface, but also restore chondrocyte homeostasis by reestablishing the physiological articular micro-environment.
Polydeoxyribonucleotide (PDRN) is extracted from the sperm of trout bred for human food purposes. It is regarded as a source of pyrimidines and purines, thereby stimulating nucleic acid synthesis through the salvage pathway6. By activating the purinergic A2A receptor, PDRN enhances cell proliferation and promotes rapid healing process via stimulating angiogenesis in experimental models of autologous skin graft donor sites, diabetic pressure ulcers, and varicoceles6–9. An in vivo study has investigated the effect of PDRNs on collagen-induced arthritis in mice10. Results of that study showed that PDRN treatment improved clinical signs of arthritis and histological damage, reduced cartilage expression and inflammatory cytokine production from stimulated human chondrocytes, thus representing a valid alternative for treatment of arthritis10.
Angiogenesis, defined as blood vessel outgrowth from pre-existing vasculature, is essential for growth and development, the reproductive cycle, and tissue repair. However, unlike skin wounds or diabetic pressure ulcers, angiogenesis is known to play a key role in the progression of cartilage degradation in OA11. Angiogenesis contributes to synovitis, osteochondral damage, osteophyte formation, and meniscal pathology in patients with OA. Nerve growth along new blood vessels into structures normally not innervated could also contribute to pain in OA11. Hence, whether PDRN exerts an effect on angiogenesis on the chondrocytes of OA is a conflicting issue in the consideration of PDRN as a treatment option of OA.
To our knowledge, the effect of PDRN on angiogenesis in OA has not yet been reported. Therefore, the aim of this study was to investigate the effect of PDRN on factors associated with angiogenesis and determine changes of pro-angiogenic and anti-angiogenic factors after administration of PDRN in an in vitro model of OA.
Materials and Methods
Cell Culture and IL-1β Stimulation
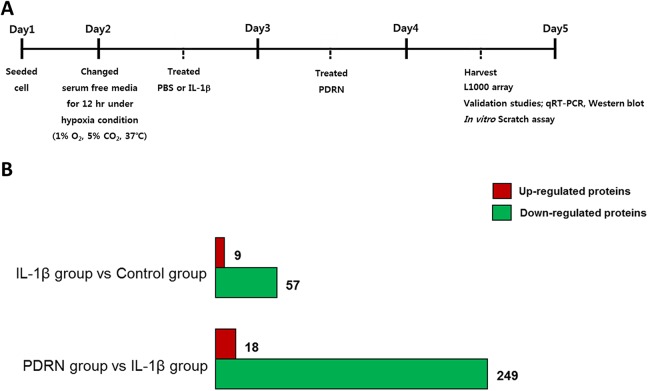
SW1353 cells, which have been reported as human chondrocytic cell line12–14. They were obtained from were obtained from American Type Culture Collection (ATCC; HTB-94; Manassas, VA, USA) and maintained in Dulbecco’s modified Eagle medium high glucose (DMEM-HG; Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Gibco) and 1% 60 U/ml penicilline (Gibco) at 37%. After reaching 80% confluence, cells were harvested using 0.05% trypsin-ethylenediaminetetraacetic acid (EDTA; Gibco). Cells were washed, centrifuged, resuspended and seeded onto new plates. Culture medium was replaced every 2 to 3 days. In each experiment, cells were rendered quiescent for 12 hours by adding DMEM-HG without FBS under hypoxic condition. Cells were stimulated with phosphate-buffered saline (PBS, WELGENE, Gyeongsangbuk-do, South Korea) or 10 ng/ml of interleukin (IL)-1β (R&D Systems, Minneapolis, MN, USA) for 24 hours to establish an in vitro OA model13,15–18. After stimulation with IL-1β, cells were treated with 100 μg/ml of PDRN (Placentex Integro, Mastelli Srl, Italy) for 24 hours and harvested using 0.05% trypsin-EDTA (Gibco) as described above. Three experimental groups were used in this study as follows: PBS treated group (control group); IL-1β treated group (IL-1β group); IL-1β followed by PDRN treatment group (PDRN group). The experimental scheme of this study was shown in Fig. 1A.
Fig. 1.
Experimental scheme and L1000 array analysis. (A) Seeded SW1353 cells were serum-starved under hypoxic conditions for 12 hours and then stimulated with 10 ng/ml of IL-1β or PBS for 24 hours followed by treatment with 100 μg/ml of PDRN. After 24 hours, cells were harvested for the L1000 array. To validate the L1000 array study and OA pathogenesis, qRT-PCR and a western blot were conducted. To examine the migration efficiency, an in vitro scratch assay was performed. (B) The number of enriched proteins is indicated by bar graphs. The number of up-regulated proteins is indicated by a red bar while, the number of down-regulated proteins is indicated by a green bar.
IL: interleukin; OA: osteoarthritis; PBS: phosphate-buffered saline; PDRN: polydeoxyribonucleotide; qRT-PCR: quantitative real-time polymerase chain reaction.
Label-Based Human Antibody Array 1000
To prepare the protein for human L1000 array, whole cell lysates from experimental groups were homogenized and dissolved in radioimmunoprecipitation assay buffer (Thermo Scientific, Rockford, IL, USA) with protease inhibitor (Abcam, Cambridge, MA, USA) and Xpert phosphatase inhibitor cocktail (GenDEPOT, Houston, TX, USA). A human L1000 glass slide array (RayBiotech, Norcross, GA, USA) was performed by eBiogen Inc. (Seoul, South Korea).
Bioinformatics Analysis
To identify protein function, enriched protein-coding genes were used with biological processes in the Database for Annotation, Visualization and Integrated Discovery (DAVID) 6.8 annotation tool. Within several biological processes, enriched protein-coding genes involved in angiogenesis, were identified for further validation studies.
Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
Total RNA was reverse-transcribed into cDNA using ReverTra Ace® qPCR RT Master Mix with gDNA Remover (Toyobo, Osaka, Japan) according to the manufacturer’s instructions. Expression levels of genes of interest were determined using qPCRBIO SyGreen Mix Hi-ROX (PCR BIOSYSTEMS, London, UK) in a StepOnePlus RT-PCR System (Applied Biosystems, Foster City, CA, USA). Data analysis was performed using the 2-ΔΔ cycle threshold (CT) method19. Primers used for qRT-PCR are listed in Table 1.
Table 1.
Primers Used for qRT-PCR.
| Gene symbol | Forward primer (5′→3′) | Reverse primer (5′→3′) |
|---|---|---|
| PDGF | GCA CCG GCT CAT CTT TGT CTA | TTC GGT ACA AGT CTG TGA GGT G |
| ANG-2 | ATA CGA TGA CTC GGT GCA GA | TGC TCC GCT GTT TGG TTC AA |
| Angiostatin | TAA TCC CAG CTT GTC TGC CA | TTC GGT GGA TTG GAC TCT TCC |
| Endostatin | CCC AGC CGT GGC ATT CCT A | TGA TGC GCT CTG AAG ATG GTG G |
| GAPDH | AAG GGT CAT CAT CTC TGC CC | GTG AGT GCA TGG ACT GTG GT |
ANG-2: angiopoietin-2; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; PDGF: platelet-derived growth factor; qRT-PCR: quantitative real-time polymerase chain reaction.
Western Blot Analysis
To assess the protein levels of platelet-derived growth factor (PDGF), angiopoietin-2 (ANG-2), endostatin, angiostatin, vascular endothelial growth factor (VEGF), matrix metalloproteinase 13 (MMP13), and aggrecan, proteins from cell pellets were harvested as described above. Total proteins were quantified using BCA™ Protein Assay Kit (Thermo Scientific). Samples were denatured and separated by 4–12% Bis-Tris gels in 1× NuPage MOPS SDS running buffer (Invitrogen, Eugene, OR, USA). Proteins were transferred onto polyvinylidene difluoride membranes (Invitrogen) in 20% (vol/vol) methanol in NuPage Transfer Buffer (Invitrogen) at 4°C. Membranes were blocked and then incubated at 4°C overnight with the following antibodies: anti-PDGF, anti-ANG-2, anti-endostatin, anti-angiostatin, anti-VEGF, anti-MMP13, anti-aggrecan (1:1000, Abcam), and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:3000, Santa Cruz Biotechnology, Dallas, TX, USA). The next day, blots were washed three times with tris-buffered saline (TBS) plus Tween 20 and incubated with horse-radish peroxidase-conjugated secondary antibodies (1:4000; Santa Cruz) at room temperature for 1 hour. After washing three times with TBS plus Tween 20, proteins were visualized with AmershamTM ECL Western Blotting Detection Reagent (GE Healthcare, Little Chalfont, UK) and West-Q Pico ECL solution (GenDEPOT) systems. The Multi Gauge (version 3.0) software (Fujifilm, Tokyo, Japan) system was used to quantify relative protein expression for the western blot.
In Vitro Scratch Assay
To determine the effect of PDRN treatment on cell spreading and migration capabilities, cells were seeded into six-well tissue culture dishes. A linear wound was then generated in the monolayer of cells with a 200 μl plastic pipette tip. Any cellular debris was removed by washing with PBS (WELGENE), DMEM with PBS (control group), or DMEM with IL-1β (IL-1β group). After 24 hours, PDRN was added (IL-1β+PDRN group). After 24 hours of incubation, images of migrated cells were taken using a digital camera connected to an inverted microscope to observe the closure of wound area. The final gap width of scratch was measured and calculated compared with the initial gap. Wound area was quantified with ImageJ software (NIH, Baltimore, MD, USA). All scratch assays were performed in quadruplicates.
Statistical Analysis
All results are expressed as mean ± standard error of the mean (SEM) from at least three independent experiments. Statistical analyses were conducted using the Statistical Package for Social Sciences, version 23.0 (SPSS Inc, Chicago, Illinois, USA). A one-way analysis of variance followed by a post-hoc Bonferroni comparison was performed to confirm statistical results. P-value < 0.05 was considered statistically significant.
Results
L1000 Array Analysis
Enriched proteins were categorized according to fold change ratio ≥ |1.5|. Up- and down-regulated proteins at ≥1.5-fold were counted and summarized in Fig. 1B. Enriched protein-coding genes from the IL-1β group compared with the PDRN group were categorized according to the biological process of gene ontology term analysis using the DAVID 6.8 annotation tool and listed in Table 2. These biological process are statistically significant when the false discovery rate (FDR) was less than 0.001. Within several biological processes, proteins coding genes, involved in angiogenesis such as ANG-220,21 and PDGF22,23 related to pro-angiogenesis and angiostatin and endostatin related to anti-angiogenesis, were chosen for further validation studies. These proteins are presented in Table 3.
Table 2.
Biological Process of Gene Ontology Analysis From the IL-1β Group Compared with the PDRN Group.
| Term | Count | p-value | FDR |
|---|---|---|---|
| Immune response | 55 | 6.13E−37 | 1.06E−33 |
| Inflammatory response | 48 | 2.11E−31 | 3.64E−28 |
| Positive regulation of cell proliferation | 47 | 2.24E−26 | 3.86E−23 |
| Signal transduction | 70 | 2.43E−26 | 4.19E−23 |
| Cell–cell signaling | 36 | 5.14E−25 | 8.87E−22 |
| Cytokine-mediated signaling pathway | 28 | 2.73E−24 | 4.71E−21 |
| Response to lipopolysaccharide | 25 | 4.72E−18 | 8.14E−15 |
| Peptidyl-tyrosine phosphorylation | 22 | 2.54E−15 | 4.41E−12 |
| MAPK cascade | 27 | 3.24E−15 | 5.55E−12 |
| Tumor necrosis factor-mediated signaling pathway | 19 | 4.01E−14 | 6.91E−11 |
| Chemotaxis | 19 | 7.28E−14 | 1.26E−10 |
| Positive regulation of ERK1 and ERK2 cascade | 21 | 4.11E−13 | 7.09E−10 |
| Positive regulation of inflammatory response | 15 | 1.12E−12 | 1.93E−09 |
| Regulation of phosphatidylinositol 3-kinase signaling | 15 | 2.92E−12 | 5.03E−09 |
| Positive regulation of phosphatidylinositol 3-kinase signaling | 14 | 4.15E−12 | 7.16E−09 |
| Neutrophil chemotaxis | 14 | 5.11E−12 | 8.81E−09 |
| Positive regulation of MAPK activity | 13 | 2.30E−11 | 3.96E−08 |
| Angiogenesis | 21 | 3.75E−11 | 6.47E−08 |
| Cell chemotaxis | 13 | 7.68E−11 | 1.33E−07 |
| Chemokine-mediated signaling pathway | 13 | 2.27E−10 | 3.92E−07 |
| Phosphatidylinositol phosphorylation | 14 | 5.35E−10 | 9.23E−07 |
| Positive regulation of cell migration | 18 | 7.32E−10 | 1.26E−06 |
| Positive regulation of pathway-restricted SMAD protein phosphorylation | 11 | 8.62E−10 | 1.49E−06 |
| Positive regulation of peptidyl-tyrosine phosphorylation | 13 | 1.29E−09 | 2.22E−06 |
| Phosphatidylinositol-mediated signaling | 14 | 2.44E−09 | 4.21E−06 |
| Positive regulation of interferon-gamma production | 10 | 1.11E−08 | 1.91E−05 |
| Activation of cysteine-type endopeptidase activity involved in apoptotic process | 12 | 1.86E−08 | 3.21E−05 |
| Positive regulation of tyrosine phosphorylation of Stat3 protein | 9 | 4.03E−08 | 6.94E−05 |
| Cell surface receptor signaling pathway | 19 | 5.10E−08 | 8.78E−05 |
| Cellular response to lipopolysaccharide | 13 | 5.26E−08 | 9.07E−05 |
| Transforming growth factor beta receptor signaling pathway | 12 | 5.56E−08 | 9.58E−05 |
| Positive regulation of GTPase activity | 27 | 8.06E−08 | 1.39E−04 |
| Extracellular matrix disassembly | 11 | 8.98E−08 | 1.55E−04 |
| Positive regulation of DNA replication | 9 | 9.27E−08 | 1.60E−04 |
| Extrinsic apoptotic signaling pathway | 9 | 9.27E−08 | 1.60E−04 |
| Positive regulation of MAPK cascade | 11 | 1.66E−07 | 2.87E−04 |
| Platelet degranulation | 12 | 1.80E−07 | 3.11E−04 |
| Negative regulation of interleukin-17 production | 6 | 2.08E−07 | 3.59E−04 |
| Positive regulation of cell division | 9 | 2.33E−07 | 4.01E−04 |
| Regulation of apoptotic process | 16 | 2.81E−07 | 4.84E−04 |
| Regulation of cell proliferation | 15 | 2.95E−07 | 5.08E−04 |
| Positive regulation of JAK–STAT cascade | 7 | 4.04E−07 | 6.97E−04 |
Angiogenesis is relevant to PDRN treatment and is shown in a bold font.
These biological processes are statistically significant and the false discovery rate (FDR) was less than 0.001.
ERK: extracellular signal-regulated kinase; FDR: false discovery rate; GTP: guanosine-5’-triphosphate; DNA: deoxyribonucleic acid; MAPK: mitogen-activated protein kinase; SMAD: small mothers against decapentaplegic; JAK-STAT: Janus kinase-signal transducer and activator of transcription.
Table 3.
L1000 Array Analysis of Protein Related to Angiogenesis.
| Antibody name | IL-1β/control | IL-1β+PDRN/IL-1β |
|---|---|---|
| ANG-2 | 0.818 | 1.717 |
| PDGF | 1.166 | 1.504 |
| Endostatin | 1.219 | 0.640 |
| Angiostatin | 0.834 | 0.607 |
ANG-2: angiopoietin-2; IL: interleukin; PDGF: platelet-derived growth factor; PDRN: polydeoxyribonucleotide.
Validation for L1000 Array
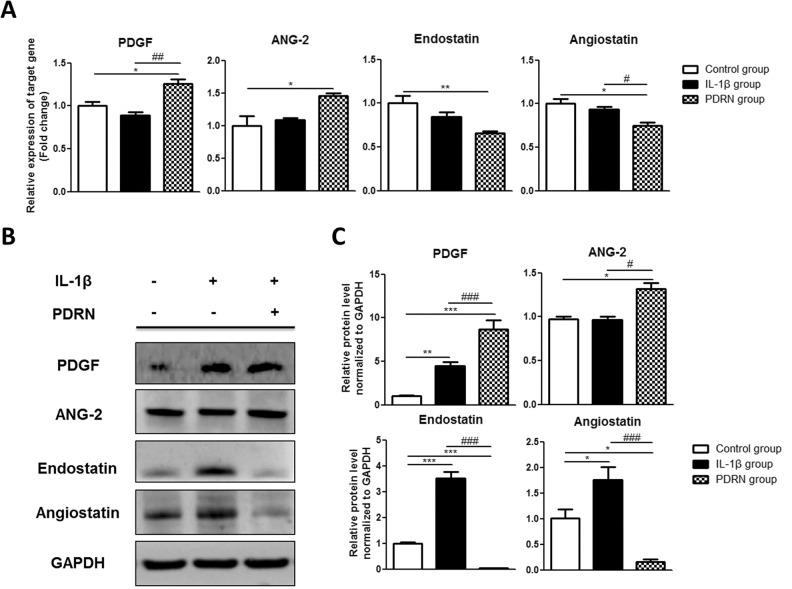
The qRT-PCR and western blot analysis were conducted to validate L1000 array results for proteins involved in angiogenesis. By qRT-PCR, there were no significant changes in expression in the IL-1β group compared with the control group (Fig. 2A). Expression change results for the four selected proteins were as follows: PDGF, 0.887-fold; ANG-2, 1.089-fold; endostatin, 0.842-fold; and angiostatin, 0.937-fold. In the PDRN group, PDGF and ANG-2 were significantly increased, whereas endostatin and angiostatin were significantly decreased compared with those in the control group. Their expression changes were as follows: PDGF, 1.259-fold (p < 0.05); ANG-2, 1.454-fold (p < 0.05); endostatin, 0.654-fold (p < 0.01); and angiostatin, 0.746-fold (p < 0.05). The PDGF level was significantly increased (p < 0.01) whereas the angiostatin level was significantly decreased (p < 0.05) in the PDRN group compared with those in the IL-1β group.
Fig. 2.
Effects of PDRN on mRNA and protein levels of angiogenesis. (A) PDGF, ANG-2, endostatin, and angiostatin expression levels were validated by qRT-PCR. Relative expression levels of target genes were calculated with the 2−ΔΔCt method. (B) Western blot analysis was performed with anti-PDGF, anti-ANG-2, anti-endostatin, anti-angiostatin, and anti-GAPDH antibody (as a control). (C) Relative protein expression levels in the IL-1β group and PDRN group compared with those in the control group by western blot. All results are expressed as mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. The control group. #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. The IL-1β group.
ANG-2: angiopoietin-2; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; IL: interleukin; PDGF: platelet-derived growth factor; PDRN: polydeoxyribonucleotide; qRT-PCR: quantitative real-time polymerase chain reaction; SEM: standard error of the mean.
Expression levels of these proteins in the IL-1β group compared with those in the control group by western blot were as follows: PDGF, 4.444-fold (p < 0.01); ANG-2, 0.965-fold; endostatin, 3.526-fold (p < 0.001); and angiostatin, 1.754-fold (p < 0.05; Fig. 2B and 2C). In the PDRN group, PDGF and ANG-2 levels were significantly increased whereas endostatin and angiostatin levels were significantly decreased compared with those in the control group. Their expression values were as follows: PDGF, 8.659-fold (p < 0.001); ANG-2, 1.320-fold (p < 0.05); endostatin, 0.041-fold (p < 0.001); and angiostatin, 0.165-fold (p < 0.05).
In the PDRN group, PDGF and ANG-2 levels were significantly increased whereas endostatin and angiostatin levels were significantly decreased compared with those in the IL-1β group. Their expression values were as follows: PDGF (p < 0.001), ANG-2 (p < 0.05), endostatin (p < 0.001), and angiostatin (p < 0.001).
Effects of PDRN on Expression of VEGF and Wound Healing
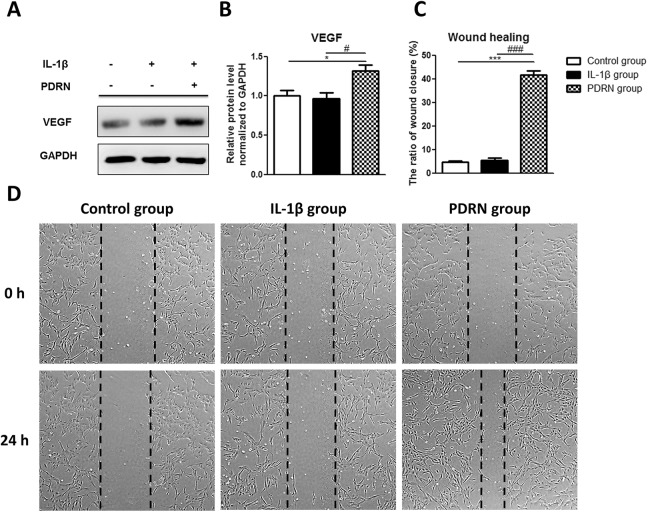
It has been reported that PDRN, an adenosine receptor A2A agonist, can induce stimulation of VEGF expression and facilitate wound healing24. VEGF is reported as one of the potent pro-angiogenic growth factors and can affect wound healing as a mediator of angiogenesis25. To determine the effect of PDRN on wound healing, the expression of VEGF was examined by western blot and in vitro scratch assay.
In the IL-1β group, the VEGF expression was not significantly different compared with the control group by western blot as follows: VEGF (0.964-fold; Fig. 3A and B). In the PDRN group, the VEGF expression was significantly increased compared with the control group and the expression value as follows: VEGF (1.319-fold, p < 0.05; Fig. 3A and B). In the PDRN group, the VEGF expression was also significantly increased compared with the IL-1β group (p < 0.05; Fig. 3A and B). In vitro cell migration ability was determined by the number of cells that migrated across scratch parts of cells (Fig. 3C and D). The percentage of wound closure was as follows: Control group, 4.68%; IL-1β group, 5.51%; and PDRN group, 41.62% (Fig. 3C). As shown in Fig. 3C and D, the wound closure ratio of the PDRN group was significantly increased compared to the IL-1β group (p < 0.001) or the control group (p < 0.001). As shown in Fig. 3D, a significant increase in cell migration was observed in the PDRN group compared with the control group or the IL-1β group.
Fig. 3.
Effects of PDRN on protein levels of VEGF and wound healing. (A) Western blot analysis was performed with anti-VEGF and anti-GAPDH (as a control). (B) The relative protein expression in the IL-1β group and PDRN group compared with the control group by western blot. (C) The area of the wound closure was quantified, and the ratio of wound closure was expressed as a percentage of recovered wound compared with the area at 0 h of each group. All results are expressed as mean ± SEM. *p < 0.05, and ***p < 0.001 vs. The control group. #p < 0.05, and ###p < 0.001 vs. The IL-1β group. (D) Representative data of wound healing experiment. The beginning of the experiment is before treatment with PDRN and indicated as 0 h in the figure. After treatment with PDRN for 24 hours is indicated as 24 h in the figure.
GAPDH: glyceraldehyde-3-phosphate dehydrogenase; IL: interleukin; PDRN: polydeoxyribonucleotide; SEM: standard error of the mean; VEGF: vascular endothelial growth factor.
Effect of PDRN on Expression of MMP13 and Aggrecan
It has been reported that imbalance of anabolic and catabolic activity is crucial for OA pathogenesis26–29. Thus, we examined the effect of PDRN on expression of anabolic and catabolic factors.
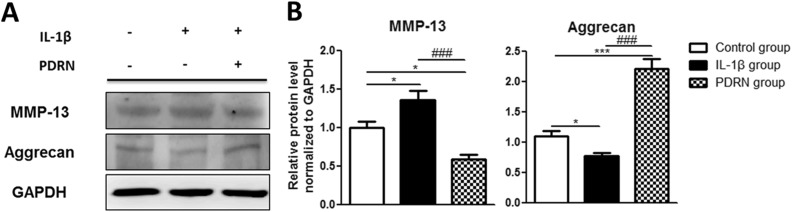
MMP13 and aggrecan, which were components of cartilage and can regulate cartilage destruction. It is reported that the expression of MMP-13, one of the catabolic genes for OA, was increased, whereas the expression of aggrecan, one of the anabolic genes for OA, was down-regulated in IL-1β treated chondrocytes30. Thus, we examined the expression levels of MMP13 and aggrecan in this study.
In the IL-1β group, MMP13 expression was statistically increased compared with that in the control group (Fig. 4A). On the other hand, aggrecan expression was significantly decreased in the IL-1β group compared with that in the control group (Fig. 4A). Their expression values were as follows: MMP13, 1.359-fold (p < 0.05); and aggrecan, 0.765-fold (p < 0.05; Fig. 4B). MMP13 expression was significantly decreased while aggrecan expression was significantly increased in the PDRN group compared with those in the control group (Fig. 4A). Their expression values were as follows: MMP13, 0.589-fold (p < 0.05); and aggrecan, 2.211-fold (p < 0.001; Fig. 4B). MMP13 expression was significantly (p < 0.001) decreased while aggrecan expression was significantly (p < 0.001) increased in the PDRN group compared with those in the control group (Fig. 4A and B).
Fig. 4.
Effects of PDRN on protein levels of MMP13 and aggrecan. (A) Western blot analysis using anti-MMP13, anti-aggrecan, and anti-GAPDH (as a control). (B) Relative protein expression in the IL-1β group and the PDRN group compared with the control group by western blot. All results are expressed as mean ± SEM. *p < 0.05, and ***p < 0.001 vs. The control group. ###p < 0.001 vs. The IL-1β group.
GAPDH: glyceraldehyde-3-phosphate dehydrogenase; IL: interleukin; MMP13: matrix metalloproteinase 13; PDRN: polydeoxyribonucleotide; SEM: standard error of the mean.
Discussion
Findings from this study indicate that PDRN could promote the expression of pro-angiogenic factors and inhibit the expression of anti-angiogenic factors in an in vitro model of OA. This is the first study that compares changes in factors influencing angiogenesis after PDRN treatment in this model.
To detect relative expression levels of 1000 human proteins including angiogenic factors, the Label-Based Human Antibody Array 1000 was used. Among the angiogenic factors evaluated, PDGF, ANG-2, and VEGF levels were significantly increased, whereas endostatin and angiostatin levels were significantly decreased in the PDRN group compared with those in the IL-1β group, based on western blot analysis. These factors play important roles in angiogenesis. Activation of angiogenesis is basically the result of an imbalance between pro- and anti-angiogenic factors31. PDGF32,33, ANG-234, and VEGF34 are known as pro-angiogenic factors whereas endostatin35,36 and angiostatin37,38 are known as anti-angiogenic factors.
The mechanism underlying preservation of avascularity in articular cartilage remains unclear. However, it has been suggested that hyaline cartilage contains high concentrations of endogenous inhibitors of angiogenesis39. Angiogenic and anti-angiogenic factors might be up-regulated in the osteoarthritic joint. However, when vascular growth predominates, articular cartilage will lose its resistance to vascularization11. Blood vessel and nerve growth are linked by common pathways involving the release of pro-angiogenic factors. As sensory nerves grow along new blood vessels in osteoarthritic joints, they eventually penetrate non-calcified articular cartilage, osteophytes, and inner regions of menisci. Consequently, angiogenesis can contribute to structural damage and pain in OA, thus providing potential targets for new OA treatments40.
Considering these points, it is very difficult to interpret whether the angiogenic effect of PDRN has a positive effect on OA. Angiogenic effects of PDRN on the recovery of chondrocytes in OA cannot be determined by the results of this study. At the present time, there are two reasonable hypotheses. First, as is known, increased angiogenesis by PDRN may adversely affect chondrocytes in OA. Nevertheless, the positive effects of PDRN on OA patients such as the anti-inflammatory and cell repair effects are greater than those of angiogenesis. Consequently, PDRN may be a valuable regenerative medicine to help wound healing in OA patients. Second, it can be assumed that the angiogenesis effect of PDRN itself does not have a bad influence on OA, in contrast with what was known in the past. If the first hypothesis is correct, PDRN will be considered as a treatment for OA without violating the existing theory that angiogenesis plays a key role in the progression of cartilage degradation in OA11. For the second hypothesis to be persuasive, it is necessary to redefine the concept of angiogenesis in OA, especially in the early stages of OA.
The phenomenon of OA may be defined clinically, radiologically, and pathologically. However, its etiology remains poorly understood. The precise contribution of angiogenesis to symptoms and pathology of OA is currently unclear. Angiogenesis is a complex multistep process controlled by a wide range of positive and negative regulatory factors. Angiogenesis occurs during essential physiological processes such as embryogenesis and wound repair. However, angiogenesis can also contribute to a variety of pathological conditions, including unwanted vessel growth in chronic inflammatory diseases and growth or metastasis of tumors41. In OA, angiogenesis interacts closely with inflammation, compressive forces, and hypoxia. However, studies regarding the role of angiogenesis in early stages of OA, especially as a defense mechanism against the degenerative process, are insufficient.
Recently published studies have reported that PDRN may represent a new and safe injection therapy for knee OA. Gennero et al.42 have estimated the efficacy of PDRNs on cartilage degradation and found that PDRNs are suitable for long-term cultivation of in vitro cartilage, with therapeutic effects on chondrocytes by protecting cartilage. A randomized and double-blind clinical trial published in 2014 on 75 patients assessed the efficacy and safety profiles of intra-articular PDRN injection in the treatment of knee OA associated with persistent pain and showed a reduction of pain and an increase in the function in daily living and sport activity from the baseline values comparable to those obtained with the use of HA43. Another study that followed up 95 patients with knee OA or chondropathy (grade III or IV) for 60 days after injecting PDRN found that intra-articular administration of PDRN in patients with both severe knee arthritis and chondropathy can reverse the short and medium-term symptoms and function, with a significant improvement in quality of life44. Chondrocytes have a pivotal role during OA. They are mainly responsible for the anabolic–catabolic balance required for matrix maintenance and tissue function41. On the other hand, OA chondrocytes are characterized by accelerated catabolic processes as well as suppression of anabolic processes. An imbalance in the expression of catabolic and anabolic factors can eventually lead to osteoarthritic cartilage destruction45. In the in vitro scratch assay in our study, a significant increase in cell migration was observed in the PDRN group compared with either the control group or the IL-1β group. The percentage of wound closure values was as follows: control group, 4.68%; IL-1β group, 5.51%; and PDRN group, 41.62%. These results also support that PDRN might have therapeutic effect on OA chondrocyte regardless of whether the effect of angiogenesis on OA is positive or negative. Apart from the effect of PDRN on angiogenesis, the possibility that PDRN has therapeutic effect on OA cartilage is still high. Therefore, angiogenesis induced by PDRN on OA cartilage should be clarified.
This study has some limitations. Observations reported here should be interpreted with caution since factors involved in angiogenesis were not demonstrating real angiogenesis. However, the activation of angiogenesis is basically the result of an imbalance between pro- and anti-angiogenic factors. Increased expression of angiogenesis activators can lead to angiogenesis switch and act positively on neovascularization46,47. In addition, this study was based on cell line model of OA instead of human patients. The cell line model of OA might differ from the chronic OA condition. We conducted human chondrocytic cell line with the addition of DMEM-HG under hypoxic condition for 12 hours and stimulation with 10 ng/ml of IL-1β for 24 hours. This process might not be sufficient enough to reflect chronic status of OA. Additional study on chronic in vitro or in vivo model of OA is required.
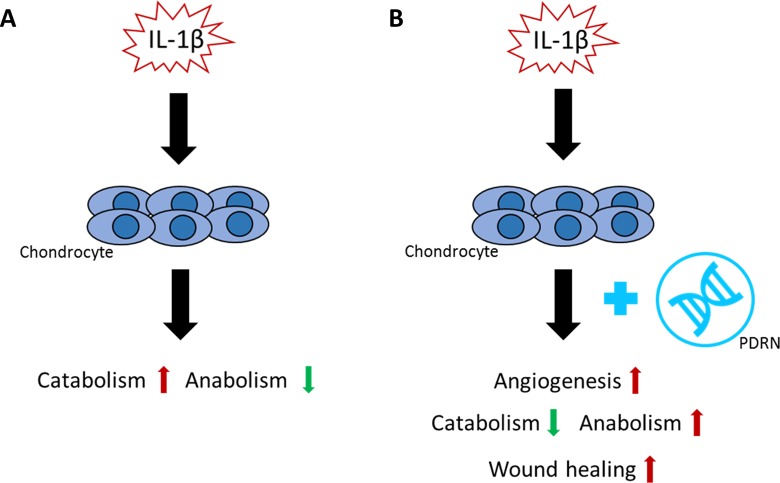
In conclusion, PDRN may promote angiogenesis in an in vitro OA model. PDRN significantly increased the levels of pro-angiogenic factors (PDGF, ANG-2, and VEGF) and decreased the levels of anti-angiogenic factors (endostatin and angiostatin) in an in vitro model of OA. PDRN also significantly facilitated cell migration regardless of angiogenesis through up-regulation of anabolism and down-regulation of catabolism (Fig. 5). Upon these findings, the role of PDRN, as novel regenerative medicine for wound healing in OA, needs to be considered and verified in various aspects.
Fig. 5.
Effects of PDRN on in vitro OA model. (A) IL-1β induces the pathogenesis of OA in chondrocytes through up-regulation of catabolism and down-regulation of anabolism. (B) PDRN inhibit the pathogenesis of OA via up-regulation of angiogenesis and wound healing.
IL: interleukin; OA: osteoarthritis; PDRN: polydeoxyribonucleotide.
Footnotes
Author Contributions: Ahreum Baek and Yoon Kim contributed equally to this work.
The contributions to this study were: AB, conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; YK, data analysis and interpretation, manuscript writing; JWL, data analysis and interpretation; SCL and SRC conception and design, data analysis and interpretation, manuscript writing, final approval of the manuscript. All authors read and approved the manuscript.
Ethical Approval: Ethical Approval is not applicable for this article.
Statement of Human and Animal Rights: Statement of Human and Animal Rights is not applicable for this article.
Statement of Informed Consent: Statement of Informed Consent is not applicable for this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a new faculty research seed money grant of Yonsei University College of Medicine for 2015 (32-0025); the National Research Foundation (NRF-2017R1D1A1B03028855 and 2018R1A6A3A01013415); and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (HI16C1012).
References
- 1. Sakata R, Reddi AH. Platelet-rich plasma modulates actions on articular cartilage lubrication and regeneration. Tissue Eng Part B Rev. 2016;22(5):408–419. [DOI] [PubMed] [Google Scholar]
- 2. Daouti S, Latario B, Nagulapalli S, Buxton F, Uziel-Fusi S, Chirn GW, Bodian D, Song C, Labow M, Lotz M, Quintavalla J, Kumar C. Development of comprehensive functional genomic screens to identify novel mediators of osteoarthritis. Osteoarthritis Cartilage. 2005;13(6):508–518. [DOI] [PubMed] [Google Scholar]
- 3. Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ. 2004;328(7444):869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rutjes AW, Juni P, da Costa BR, Trelle S, Nuesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157(3):180–191. [DOI] [PubMed] [Google Scholar]
- 5. Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):577–579. [DOI] [PubMed] [Google Scholar]
- 6. Galeano M, Bitto A, Altavilla D, Minutoli L, Polito F, Calo M, Lo Cascio P, Stagno d’Alcontres F, Squadrito F. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair Regen. 2008;16(2):208–217. [DOI] [PubMed] [Google Scholar]
- 7. Arena S, Minutoli L, Arena F, Nicotina PA, Romeo C, Squadrito F, Altavilla D, Morgia G, Magno C. Polydeoxyribonucleotide administration improves the intra-testicular vascularization in rat experimental varicocele. Fertil Steril. 2012;97(1):165–168. [DOI] [PubMed] [Google Scholar]
- 8. Rubegni P, De Aloe G, Mazzatenta C, Cattarini L, Fimiani M. Clinical evaluation of the trophic effect of polydeoxyribonucleotide (PDRN) in patients undergoing skin explants. A Pilot Study. Curr Med Res Opin. 2001;17(2):128–131. [PubMed] [Google Scholar]
- 9. Valdatta L, Thione A, Mortarino C, Buoro M, Tuinder S. Evaluation of the efficacy of polydeoxyribonucleotides in the healing process of autologous skin graft donor sites: a pilot study. Curr Med Res Opin. 2004;20(3):403–408. [DOI] [PubMed] [Google Scholar]
- 10. Bitto A, Polito F, Irrera N, D’Ascola A, Avenoso A, Nastasi G, Campo GM, Micali A, Bagnato G, Minutoli L, Marini H, Rinaldi M, Squadrito F, Altavilla D. Polydeoxyribonucleotide reduces cytokine production and the severity of collagen-induced arthritis by stimulation of adenosine A((2)A) receptor. Arthritis Rheum. 2011;63(11):3364–3371. [DOI] [PubMed] [Google Scholar]
- 11. Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8(7):390–398. [DOI] [PubMed] [Google Scholar]
- 12. Santoro A, Conde J, Scotece M, Abella V, Lopez V, Pino J, Gomez R, Gomez-Reino JJ, Gualillo O. Choosing the right chondrocyte cell line: focus on nitric oxide. J Orthop Res. 2015;33(12):1784–1788. [DOI] [PubMed] [Google Scholar]
- 13. Gebauer M, Saas J, Sohler F, Haag J, Soder S, Pieper M, Bartnik E, Beninga J, Zimmer R, Aigner T. Comparison of the chondrosarcoma cell line SW1353 with primary human adult articular chondrocytes with regard to their gene expression profile and reactivity to IL-1beta. Osteoarthritis Cartilage. 2005;13(8):697–708. [DOI] [PubMed] [Google Scholar]
- 14. Tew SR, Clegg PD, Brew CJ, Redmond CM, Hardingham TE. SOX9 transduction of a human chondrocytic cell line identifies novel genes regulated in primary human chondrocytes and in osteoarthritis. Arthritis Res Ther. 2007;9(5):R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeng L, Wang W, Rong XF, Zhong Y, Jia P, Zhou GQ, Li RH. Chondroprotective effects and multi-target mechanisms of Icariin in IL-1 beta-induced human SW 1353 chondrosarcoma cells and a rat osteoarthritis model. Int Immunopharmacol. 2014;18(1):175–181. [DOI] [PubMed] [Google Scholar]
- 16. Jia P, Chen G, Zhou G, Zhong Y, Li R. Fuyuan Decoction inhibits nitric oxide production via inactivation of nuclear factor-kappaB in SW1353 chondrosarcoma cells. J Ethnopharmacol. 2013;146(3):853–858. [DOI] [PubMed] [Google Scholar]
- 17. Bao G, Xu L, Xu X, Zhai L, Duan C, Xu D, Song J, Liu Z, Tao R, Cui Z, Yang H. SGTB Promotes the Caspase-Dependent Apoptosis in Chondrocytes of Osteoarthritis. Inflammation. 2016;39(2):601–610. [DOI] [PubMed] [Google Scholar]
- 18. Baek A, Kim M, Kim SH, Cho SR, Kim HJ. Anti-inflammatory effect of DNA polymeric molecules in a cell model of osteoarthritis. Inflammation. 2018;41(2):677–688. [DOI] [PubMed] [Google Scholar]
- 19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 20. Yoshiji H, Kuriyama S, Noguchi R, Yoshii J, Ikenaka Y, Yanase K, Namisaki T, Kitade M, Uemura M, Masaki T, Fukui H. Angiopoietin 2 displays a vascular endothelial growth factor dependent synergistic effect in hepatocellular carcinoma development in mice. Gut. 2005;54(12):1768–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coffelt SB, Chen YY, Muthana M, Welford AF, Tal AO, Scholz A, Plate KH, Reiss Y, Murdoch C, De Palma M, Lewis CE. Angiopoietin 2 stimulates TIE2-expressing monocytes to suppress T cell activation and to promote regulatory T cell expansion. J Immunol. 2011;186(7):4183–4190. [DOI] [PubMed] [Google Scholar]
- 22. Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5(1):e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ding W, Knox TR, Tschumper RC, Wu W, Schwager SM, Boysen JC, Jelinek DF, Kay NE. Platelet-derived growth factor (PDGF)-PDGF receptor interaction activates bone marrow-derived mesenchymal stromal cells derived from chronic lymphocytic leukemia: implications for an angiogenic switch. Blood. 2010;116(16):2984–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bitto A, Galeano M, Squadrito F, Minutoli L, Polito F, Dye JF, Clayton EA, Calo M, Venuti FS, Vaccaro M, Altavilla D. Polydeoxyribonucleotide improves angiogenesis and wound healing in experimental thermal injury. Crit Care Med. 2008;36(5):1594–1602. [DOI] [PubMed] [Google Scholar]
- 25. Johnson KE, Wilgus TA. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv Wound Care (New Rochelle). 2014;3(10):647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mueller MB, Tuan RS. Anabolic/Catabolic balance in pathogenesis of osteoarthritis: identifying molecular targets. PM R. 2011;3(6 Suppl 1):S3–S11. [DOI] [PubMed] [Google Scholar]
- 27. Kim JH, Jeon J, Shin M, Won Y, Lee M, Kwak JS, Lee G, Rhee J, Ryu JH, Chun CH, Chun JS. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell. 2014;156(4):730–743. [DOI] [PubMed] [Google Scholar]
- 28. Caterson B, Hughes CE, Roughley P, Mort JS. Anabolic and catabolic markers of proteoglycan metabolism in osteoarthritis. Acta Orthop Scand Suppl. 1995;266:121–124. [PubMed] [Google Scholar]
- 29. Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. [DOI] [PubMed] [Google Scholar]
- 31. Pesesse L, Sanchez C, Delcour JP, Bellahcene A, Baudouin C, Msika P, Henrotin Y. Consequences of chondrocyte hypertrophy on osteoarthritic cartilage: potential effect on angiogenesis. Osteoarthritis Cartilage. 2013;21(12):1913–1923. [DOI] [PubMed] [Google Scholar]
- 32. Edelberg JM, Aird WC, Wu W, Rayburn H, Mamuya WS, Mercola M, Rosenberg RD. PDGF mediates cardiac microvascular communication. J Clin Invest. 1998;102(4):837–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277(5323):242–245. [DOI] [PubMed] [Google Scholar]
- 34. Distler JH, Hirth A, Kurowska-Stolarska M, Gay RE, Gay S, Distler O. Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q J Nucl Med. 2003;47(3):149–161. [PubMed] [Google Scholar]
- 35. Sasaki T, Larsson H, Kreuger J, Salmivirta M, Claesson-Welsh L, Lindahl U, Hohenester E, Timpl R. Structural basis and potential role of heparin/heparan sulfate binding to the angiogenesis inhibitor endostatin. EMBO J. 1999;18(22):6240–6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamaguchi N, Anand-Apte B, Lee M, Sasaki T, Fukai N, Shapiro R, Que I, Lowik C, Timpl R, Olsen BR. Endostatin inhibits VEGF-induced endothelial cell migration and tumor growth independently of zinc binding. EMBO J. 1999;18(16):4414–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Claesson-Welsh L, Welsh M, Ito N, Anand-Apte B, Soker S, Zetter B, O’Reilly M, Folkman J. Angiostatin induces endothelial cell apoptosis and activation of focal adhesion kinase independently of the integrin-binding motif RGD. Proc Natl Acad Sci U S A. 1998;95(10):5579–5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79(2):315–328. [DOI] [PubMed] [Google Scholar]
- 39. Moses MA, Sudhalter J, Langer R. Identification of an inhibitor of neovascularization from cartilage. Science. 1990;248(4961):1408–1410. [DOI] [PubMed] [Google Scholar]
- 40. Ashraf S, Mapp PI, Walsh DA. Contributions of angiogenesis to inflammation, joint damage, and pain in a rat model of osteoarthritis. Arthritis Rheum. 2011;63(9):2700–2710. [DOI] [PubMed] [Google Scholar]
- 41. Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford). 2005;44(1):7–16. [DOI] [PubMed] [Google Scholar]
- 42. Gennero L, Denysenko T, Calisti GF, Vercelli A, Vercelli CM, Amedeo S, Mioletti S, Parino E, Montanaro M, Melcarne A, Juenemann C, De Vivo E, Longo A, Cavallo G, De Siena R. Protective effects of polydeoxyribonucleotides on cartilage degradation in experimental cultures. Cell Biochem Funct. 2013;31(3):214–227. [DOI] [PubMed] [Google Scholar]
- 43. Giarratana LS, Marelli BM, Crapanzano C, De Martinis SE, Gala L, Ferraro M, Marelli N, Albisetti W. A randomized double-blind clinical trial on the treatment of knee osteoarthritis: the efficacy of polynucleotides compared to standard hyaluronian viscosupplementation. Knee. 2014;21(3):661–668. [DOI] [PubMed] [Google Scholar]
- 44. Saggini R, Di Stefano A, Cavezza T, Saladino G, Bellomo RG. Intrarticular treatment of osteoartropaty knee with polynucleotides: a pilot study with medium-term follow-up. J Biol Regul Homeost Agents. 2013;27(2):543–549. [PubMed] [Google Scholar]
- 45. Kim H, Kang D, Cho Y, Kim JH. Epigenetic regulation of chondrocyte catabolism and anabolism in osteoarthritis. Mol Cells. 2015;38(8):677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ribatti D, Vacca A, Presta M. The discovery of angiogenic factors: a historical review. Gen Pharmacol. 2000;35(5):227–231. [DOI] [PubMed] [Google Scholar]
- 47. Ribatti D, Nico B, Crivellato E, Roccaro AM, Vacca A. The history of the angiogenic switch concept. Leukemia. 2007;21(1):44–52. [DOI] [PubMed] [Google Scholar]