Abstract
Decellularized extracellular matrices have been clinically used for tendon regeneration. However, only a few systematic studies have compared tendon stem/progenitor cells to mesenchymal stromal cells on the tendon-derived decellularized matrix. In the present study, we prepared extracellular matrix derived from porcine tendons and seeded with tendon stem/progenitor cells, embryonic stem cell-derived mesenchymal stromal cells or without stem cells. Then we implanted the mixture (composed of stem cells and scaffold) into the defect of a rat Achilles tendon. Next, 4 weeks post-surgery the regenerated tendon tissue was collected. Histological staining, immunohistochemistry, determination of collagen content, transmission electron microscopy, and biomechanical testing were performed to evaluate the tendon structure and biomechanical properties. Our study collectively demonstrated that decellularized extracellular matrix derived from porcine tendons significantly promoted the regeneration of injured tendons when combined with tendon stem/progenitor cells or embryonic stem cell-mesenchymal stromal cells. Compared to embryonic stem cell-mesenchymal stromal cells, tendon stem/progenitor cells combined with decellularized matrix showed more improvement in the structural and biomechanical properties of regenerated tendons in vivo. These findings suggest a promising strategy for functional tendon tissue regeneration and further studies are warranted to develop a functional tendon tissue regeneration utilizing tendon stem/progenitor cells integrated with a tendon-derived decellularized matrix.
Keywords: Tendon stem/progenitor cells, mesenchymal stem cells, decellularized matrix, tendon tissue engineering, rat, in vivo
Introduction
Tendons are connective tissues that transmit force from muscle to bone. They are injured frequently due to rigorous activities and sports. It is well known that tendons naturally heal with the formation of fibrotic scars rather than regeneration tissue owing to their vascular deficiency and lack of highly differentiated tenocytes, with limited proliferation capability and metabolic rate1. Therefore, the natural tendon healing process always leads to poorer tendon quality and mechanical properties2. A variety of treatment modalities have been employed, such as xenografts3, autografts4, allografts5, suture techniques6, tendon-to-bone fixation7, and tendon prostheses8. However, these options are accompanied by limited donor resource, poor integration graft and high cost of medication.
Tissue engineering provides an alternative with a high potential for treating injured tissue. Cells (stem cells), scaffolds, and bioactive molecules are three fundamental elements; their internal coordination and external interplay with the surrounding tissue of the implantation site induce the native, regenerative healing of injured tissues and organs. Scaffolds play a crucial role during the tissue-regeneration process; the various scaffolds include artificial synthetic and naturally derived materials. Recently, native organs have become a popular alternative as they contain extracellular matrix in which the composition, organization, mechanical properties, and/or geometry support the viability and phenotype of tissue-specific cells. Considering that many native organs are not suitable for transplantation, particularly for xenograft, decellularized organs and scaffolds show enormous potential to work as instructive matrices for tissue-engineering applications. For tendons, many such scaffolds have been approved by the US Food and Drug Administration9.
Currently, stem cells with the capability of multi-lineage commitment and self-renewal are an ideal cell source for tissue engineering. For tendons, tendon stem/progenitor cells (TSPCs) and mesenchymal stem cells (MSCs) are the most popular10,11. Since their discovery in rats in 200712, TSPCs have been isolated from various mammals13–15. As a local stem cell, mounting evidence shows that TSPCs have the potential for tendon tissue engineering16. TSPCs could regulate inflammation in tendon healing via JNK and STAT3 signaling17, and serve as a potential target for the prevention or treatment of tendinopathy via inhibiting the P16 tenogenics of the microRNA signaling pathway18. The shortcomings of TSPCs are the lack of sources and the difficulty in obtaining them. In contrast, MSCs are relatively easy to acquire and can be isolated from a variety of tissues. They can commit toward the expected cell lineages in situ, or secrete trophic molecules, for instance, chemotactic molecules and growth factors, which can recruit extra reparative cells into the lesion site19.
A previous study showed that TSPCs possessed more potential for tenogenic expression compared to MSCs in vitro20; however, there has not yet been any systematic study that compares TSPCs to MSCs on the tendon-derived decellularized matrix. In the present study, we hypothesized that the TSPCs as the resident stem cells of tendons and integrated with a tendon-derived decellularized matrix will express high tenogenics, produce more tendon-like tissue, and improve structural and biomechanical properties of regenerated tendons much better than MSCs or without seed cells in vivo.
Materials and Methods
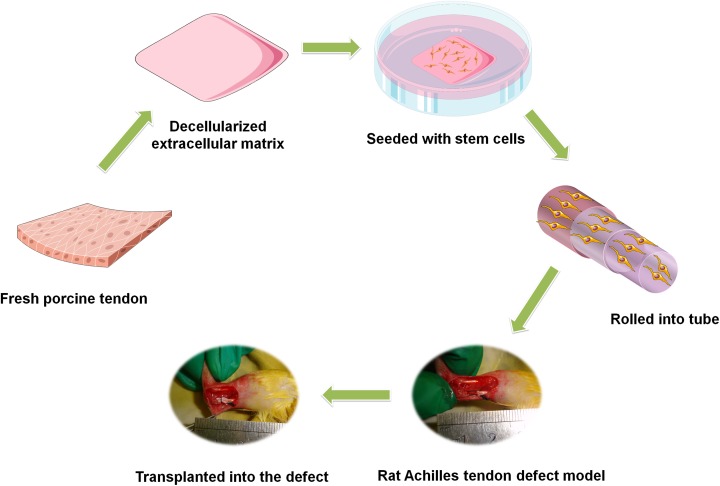
This study was approved by the Zhejiang University Institutional Animal Care and Use Committee, and we strictly followed the current laws for animal experiments. The schematic drawing shown in Fig. 1 was used to illustrate the process of this study.
Fig. 1.
The schematic drawing of the study process.
Preparation of Porcine Tendon Decellularized Matrix
The decellularization protocol we used is similar to our previous study21. Firstly, fresh porcine tendons were cut into 10 mm×10 mm pieces, washed in 50 ml of 0.1 M phosphate buffered saline (PBS) for 15 minutes, then soaked in liquid nitrogen for 2 minutes and immersed in a 37° C solution of 500 ml 0.9% saline for 10 minutes. This procedure was repeated five times. Before being placed in the 24-well plates, the tendon pieces were cut into 80 μm-thick sections with a cryostat microtome (Microm HM550, Waltham, MA, USA). They were then washed three times with ml of 0.1 M PBS, for 30 min each time. Next, 100 μl of 200 unit ml−1 DNase (Roche, Basel, Switzerland) was placed into each well, then they were incubated at 4 % for 14 hours followed by washing for 30 min with 1 ml of 0.1 M PBS at 4 %. Before the experiments, hematoxylin and eosin (H&E) staining confirmed the resident cells were completely removed.
Stem Cell Isolation and Culture
The human TSPCs21 and human embryonic stem cells (ESC)-MSCs22 used in this study were acquired according to our previous study. Briefly, the cells were planted in 10 cm dishes and cultured at 37 %, 5% CO2 with complete medium consisting of Dulbecco’s modified Eagle’s medium, 10% fetal bovine serum, and 1% penicillin-streptomycin (all from Gibco, Carlsbad, CA, USA). The non-adherent cells were removed with PBS and the medium exchanged every 2 to 3 days. Cells at passage 3 were used for experiments. Then 3 days before surgery, the cells at a concentration of 1×105 cells per ml were seeded on the scaffold. Before being used for the experiments, the multi-lineage differentiation potential and clonogenicity of these stem cells were confirmed in this study.
In Situ Rat Achilles Tendon Repair Model
The hind limbs of 18 skeletally mature female Sprague Dawley (SD) rats (Zhejiang University Laboratories, Hangzhou, China) weighing between 200 g and 220 g were used for this experiment. All the rats were treated with cyclophosphamide (150 mg/kg) 24 h before the operation. After general anesthesia, a full tear wound was created and the Achilles tendon was removed to create a defect of 6 mm in length. The decellularized extracellular matrix (ECM) scaffolds (10 × 10 mm, thickness 80 μm), seeded with TSPCs (ECM + TSPCs group, N = 12, 5 × 105 cells per scaffold) or MSCs (ECM + MSCs group, N = 12, 5 × 105 cells per scaffold) or without seeding cells (ECM group, N = 12) were rolled into the gap wound, then sutured to the remaining Achilles tendon using a non-resorbable suture (6-0 nylon). We next irrigated the wound and closed the skin. The animals were allowed free cage activity after surgery. Then 4 weeks post-surgery, specimens were collected successively.
Histological Staining and Immunohistochemistry
The harvested, regenerated tendon tissue was promptly immersed in 10% (v/v) neutral buffered formalin (Xinghan Ltd, Zhengzhou, China) and dehydrated through an alcohol gradient, then embedded in paraffin blocks. For standard histological evaluation, 7 μm sections were stained with H&E (N = 3 per group). To examine the general appearance of the collagen fibers, Masson’s Trichrome staining (N = 3 per group) was also performed according to standard procedures. Polarizing microscopy (N = 3 per group) was used to assess mature collagen fibrils. The general histological score (fiber structure, fiber arrangement, rounding of nuclei, inflammation, vascularity, cell population) of the H&E staining result was calculated in this study and the method was according to Shen et al.23,24 For immunohistochemical analysis (N = 3 per group), mouse anti-rat monoclonal antibody against collagen I (1:200 dilution; Abcam, Cambridge, UK) was used to assess the expression of collagen I in repaired Achilles tendon.
Determination of Collagen Content
The amount of collagen in the repaired tendon was quantified using a collagen assay kit (Jiancheng Ltd, Nanjing, China). We digested the lyophilized tendons (N = 3 per group) with a hydrolysis regent at 95 % for 20 min, and serially diluted acid-soluble collagen type I (provided by the kit) to generate the standard curve according to the manual. The concentration was obtained through absorbance at 550 nm via a microplate reader (Molecular Devices, San Jose, CA, USA).
Transmission Electron Microscopy
Achilles tendon specimens (N = 3 per group) were fixed via standard procedures for transmission electron microscopy (TEM; Tecnai G2 F20 S-TWIN, FEI, Hillsboro, Oregon, USA) to assess the diameter and alignment of collagen fibril. Methods and processes are the same as previously described24. To acquire an accurate representation of the fibril diameter distribution, we measured more than 500 collagen fibrils for each specimen.
Mechanical Testing
Mechanical testing (N = 5 per group) was carried out through an Instron tension/compression system with Fast-Track software (Model 5543, Instron, Canton, MA). Measurements of the tendon’s cross-sectional area were performed via two Vernier calipers at 5 mm proximal to the conjunction of the bone and tendon. The bone end of the tendon was secured by a specially designed restraining jig and the tendon end was pinched with a clamp25. The Achilles tendon-calcaneus complex (ACC) was then rigidly fixed to custom-made clamps. After applying a preload of 0.1 N, each ACC underwent pre-conditioning by cyclic elongation of between 0 and 0.5 mm for 20 cycles at 5 mm per min. This was followed by a load-to-failure test at an elongation rate of 5 mm per min. The load-elongation behavior of the ACCs and failure modes were recorded. The structural properties of the ACC were represented by stiffness (N/mm), ultimate load (N), energy absorbed at failure (mJ) and stress at failure. For each ACC, the greatest slope in the linear region of the load-elongation curve over a 0.5 mm elongation interval was used to calculate the stiffness.
Statistical Analysis
Statistical significance between groups was assessed by one-way analysis of variance followed by post-hoc Scheffe test using SPSS 22.0 (IBM, Amun, New York, USA). All values of P < 0.05 were accepted as statistically significant.
Results
Characterization of Decellularized Porcine Tendon
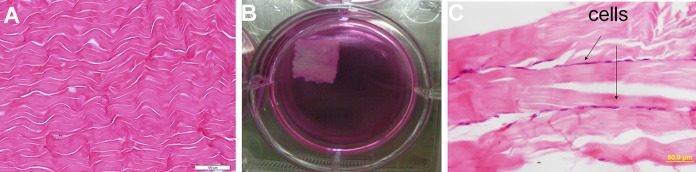
After the decellularization, most of the cells were removed and only the native collagen structure was present on H&E staining (Fig. 2A). MSCs or TSPCs were seeded to the scaffold (Fig. 2B) and kept in an incubator at 37 %, 5% CO2. Then 3 days later, the combination of cells and scaffolds were rolled up, thus an in vitro engineered tendon was generated, in which the cells and scaffolds were alternately layered, as could be observed upon H&E staining (Fig. 2C). Subsequently, the engineered tendons were transplanted into a rat model of an Achilles tendon defect.
Fig. 2.
Porcine Achilles tendon decellularized and sectioned in longitudinal slices with a thickness of 80 μm. (A) The H&E staining of the decellularized matrix; (B) 3 days before surgery, the stem cells were seeded on the bioscaffold, then the mixture was rolled up and transplanted into a defect of rat Achilles tendon; (C) the H&E staining of mixture, cells and scaffolds were alternately layered. Scale bars = 100 μm (A), 50 μm (C).
TSPCs Promoted Tendon Regeneration Structurally
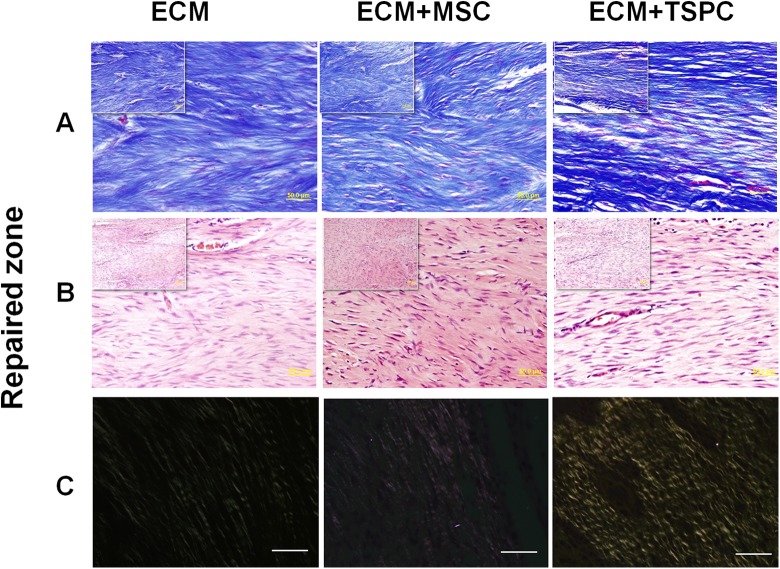
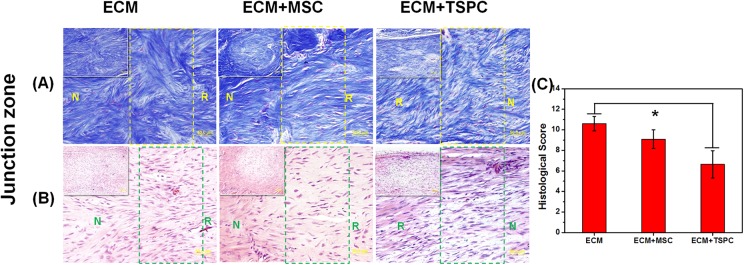
Then 4 weeks post-surgery all rats were euthanized, and the inflammatory reaction, immunological reaction or scaffold dislocation were observed in the Achilles tendon repair site. Masson trichrome and H&E staining were employed to evaluate the reparative effect in both the repaired zone and junction zone for each group. In the repaired zone, Masson trichrome staining manifested more organized collagen fiber structures in the ECM + TSPCs group when compared with other two groups (Fig. 3A). Meanwhile, H&E staining also showed more fibroblast-like cells that closely resembled the native tenocyte in the ECM + TSPCs group (Fig. 3B). Around the collagen fibers, vascular components could be found in each group. It showed the good histocompatibility of the ECM scaffold for tissue engineering tendons. Polarized light imaging confirmed the better effect of ECM + TSPCs as there was a notable increase of mature collagen fibers in comparison to the other two (Fig. 3C). At the junction of the repaired tendons, more disarranged collagen fibers and scar tissue could be seen in control groups of both the H&E and Masson trichrome staining (Fig. 4A–B). The histological score (Fig. 4C) was lowest in the ECM + TSPC group (6.64 ± 1.33 vs. 9.09 ± 0.92 in ECM + MSC group and 10.61 ± 0.71 in ECM group). These data collectively demonstrated the combination of decellularized porcine tendon and TSPCs promoted the regeneration of injured tendons to form a better structure.
Fig. 3.
Repaired zone of engineered tendons 4 weeks after surgery. (A) Masson trichrome staining of each group. (B) Typical H&E staining of repaired zones in a section of each group. (C) Polarized light microscopy images of each group. Scale bars = 50 μm (A, B, C), 200 μm (inset of A, B, C).
Fig. 4.
Junction zone (square frame of each picture) of engineered tendons 4 weeks after surgery. (A) Masson trichrome staining of each group (N: normal zone, R: repaired zone). (B) Typical H&E staining of junctional zone of each group. (C) Histological evaluation was performed for each group (ECM vs ECM + TSPC: 10.61± 0.71 vs 6.64 ±1.33, P < 0.05). Scale bars = 50 μm (A, B), 200 μm (inset of A, B).
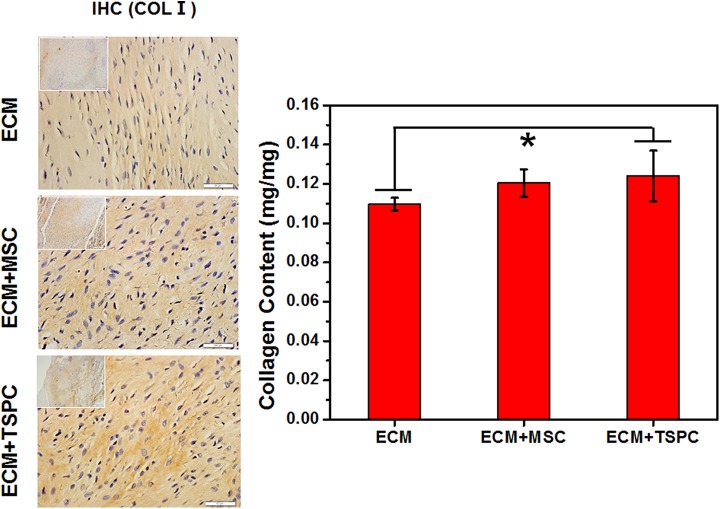
TSPCs Produced More Collagen
We monitored the expression level of the tendon-related collagen content of repaired tendons. Immunohistochemical staining showed the highest density of collagen type I in the ECM + TSPC group (Fig. 5A). Moreover, both TSPCs and MSCs deposited more collagen type I compared to the cell-free group. The collagen content assay (Fig. 5B) showed a similar trend (that had less than 0.124 ± 0.012 mg/mg in ECM + TSPC group vs. 0.12 ± 0.006 mg/mg in the ECM + MSC group and 0.11 ± 0.003 mg/mg in the ECM group). These data suggested stem cells produced more collagen.
Fig. 5.
(A) Histological observation of repaired tendon by immunohistochemical staining specific for collagen I. (B) Collagen content of the repaired tendon in each group (ECM vs ECM + TSPC: 0.110 ± 0.003 vs 0.124 ± 0.013, P < 0.05). Scale bars = 50 μm (A), 200 μm (inset of A). *P < 0.05.
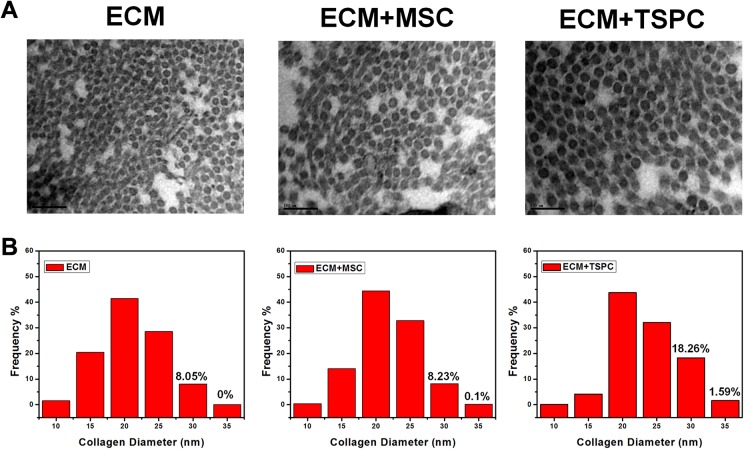
Ultrastructural Morphology
To correlate histological evidence with the ultrastructure morphology of repaired Achilles tendons (ATs), specimens were observed under TEM. The ECM + TSPC group displayed thicker collagen fibrils (Fig. 6A) in comparison to other groups. The fibrils’ average diameter was 23.5 nm (816 fibers) in the ECM + TSPC group, 21.7 nm (984 fibers) in the ECM + MSC group, and 21.1 nm (522 fibers) in ECM group (Fig. 6B). The diameter distribution showed 19.85% of the ECM + TSPC group’s regeneration fibrils were larger than 30 nm, which was higher than that of the ECM + MSC group (8.33%) and the ECM group (8.05%). Those results indicate that TSPCs are beneficial for the generation of larger fibrils.
Fig. 6.
(A) Ultrastructure of tissue-engineered tendons after 4 weeks post-surgery. (B) Histogram of the distribution of collagen fibril diameters (N > 500). Scale bars = 100 nm.
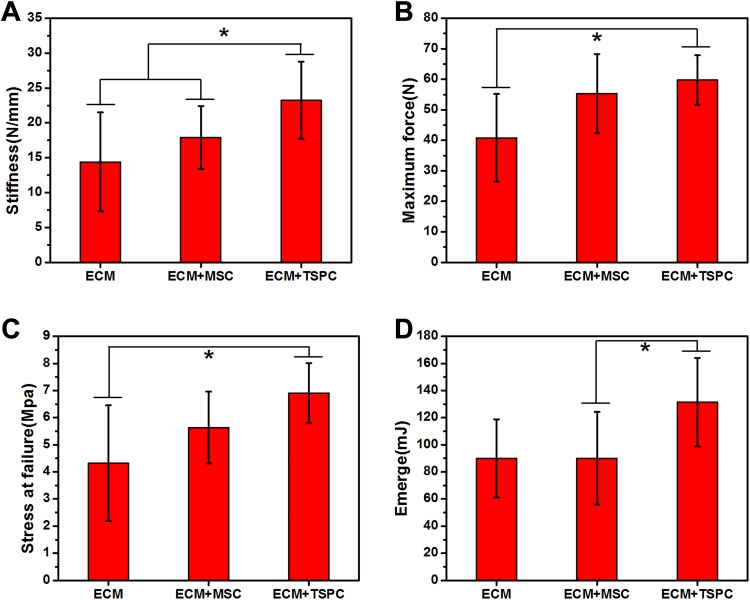
TSPCs Promoted Biomechanical Properties of Repaired AT
To further correlate tissue structural features with their biomechanical properties, we performed mechanical testing. Most of the specimens fractured at the muscle-tendon junction or the tendon-calcaneal interface. Only one of the ECM + MSC group specimens fractured at the middle of the AT. The ECM + TSPC group (23.24 ± 5.54 N/mm) showed significantly more stiffness than the ECM + MSC group (17.89 ± 4.48 N/mm) and the ECM group (14.40 ± 7.09 N/mm) (Fig. 7A). The maximum force (Fig. 7B) of the ECM + TSPC group (59.79 ± 8.05 N) was significantly higher than ECM group (40.83 ± 14.38 N), and stress at failure (Fig. 7C) manifested a similar trend (6.91 ± 1.11 Mpa vs. 4.32 ± 2.13 Mpa, P < 0.05). The assays of energy absorbed at failure (Fig. 7D) of the ECM + TSPC group were obviously higher than the ECM + MSC group (131.44 ± 32.63 mJ vs. 90.10 ± 34.21 mJ, P < 0.05). The lack of statistical significance may be due to the small sample size (N = 5 per group). The ECM + TSPC group exhibited better biomechanical properties than the other two groups.
Fig. 7.
Biomechanical properties of repaired tendons at 4 weeks post-surgery. The stiffness (A), maximum force (B), stress at failure (C), and energy absorbed at failure (D) of the TSPC-treated group was higher than other two groups. *P < 0.05. N = 5 per group.
Discussion
In this study, we used a decellularized mammalian tendon extracellular matrix scaffold with TSPCs or MSCs to repair injured tendons, and demonstrated that: (1) the tendon ECM scaffold easily acquires and promotes tendon regeneration especially when combined with TSPCs or MSCs, which can be used as a suitable bioscaffold for tendon tissue engineering; and (2) seeded TSPCs showed enhanced improvement of biomechanical properties and morphological structure in regenerated tendons compared to MSCs.
In recent years, bioscaffolds’ physical properties have been given more attention and various sources of decellularized matrix have been used in tendon tissue engineering26. In our engineered tendons with stem cells, the cells and decellularized tendon matrix were arranged in layers and were in close contact with each other. Such an intimate contact may be a key inducer in directing the site-specific differentiation of stem cells because the biophysical and biochemical properties of the decellularized matrix is relatively native, such as the native parallel arrangement of collagen fibers of the ECM leading to the right direction of engraftment stem cells24,27 and suppressing the wrong direction of commitment. Our previous study showed the tendon ECM not only enhanced tenogenic differentiation of TSPCs, but also significantly suppressed osteogenic-lineage differentiation21. In comparison to the study by Kryger et al. 28 , both studies proved tenocytes and MSCs could be used to successfully repopulate acellularized tendons for tendon repair. The present study goes deeper into the in vivo application of these cells for tissue engineering. Furthermore, the bioactive factors of tendon extracellular matrix could quickly release into the microenvironment of stem cells and promote cell proliferation. Zhang et al. recently reported tendon extracellular matrix effectively stimulated the proliferation of rabbit patellar tendon stem cells in vitro29.
Plenty of evidence has documented that tissue-specific stem cells are best for tissue engineering. Numerous studies demonstrated that TSPCs promoted tissue regeneration in vivo30–32. As the local resident stem cells, TSPCs showed higher potential in repairing injured tendons compared to MSCs20. This is consistent with our study, wherein the ECM + TSPCs group possessed a better anatomical structure, synthesized more collagen, and had more powerful biomechanical properties than ECM + MSCs. The regenerated tendons were closer to normal tendon tissue structure and properties, and regulated inflammation in tendon healing via JNK and STAT3 signaling17. However, it is known that the tenogenic differentiation capacity of TSPCs significantly decreases with advancing age18. The expression of transcript factor Fos observably decreased in early post-natal rat Achilles tendons with time11. Loss of tenomodulin (Tnmd) possibly resulted in augmented senescence and reduced self-renewal of TSPCs33. Only an allogeneic cell source of TSPCs can be used for tissue repair because it is hard to obtain autologous cells without causing morbidity of the donor site. Hence, the aging and the small quantity will limit the development of TSPCs in the future.
The MSCs’ specialty of directed differentiation and paracrine mechanisms has important implications for tendon repair and regeneration34. Many kinds of tissue-derived MSCs used to repair injured tendons have achieved good curative effects35,36. In this study, the ECM + MSCs group possessed better functional healing of injured tendons than ECM group. This is consistent with previous results that show the tendon tissue matrix promotes the tenogenic-lineage differentiation of MSCs37. Even though their therapeutic effect is lower than TSPCs, their wide variety of sources, ease of isolation and culture, and suitability for large-scale industrial development underline their huge potential.
The selection of suitable seed cells is critical to tendon tissue engineering. Although stem cells perform similarly to some extent, there are some unique properties of tissue-specific stem cells that may influence the tissue engineering outcome38. TSPCs expressed a higher level of adipogenic, osteogenic, and chondrogenic besides tenogenic genes, suggesting they possessed the capacities of adipogenesis, osteogenesis, and chondrogenesis differentiation20. When an optimized condition is obtained, the complete capability of TSPCs in tendon tissue engineering is achieved, which shows its potential. Large animal models may be required, with long-term follow up and cell tracking to appreciate the functional benefits in future.
Our study collectively demonstrated that decellularized extracellular matrix derived from porcine tendon significantly promoted injured tendon regeneration when combined with TSPCs or ESC-MSCs. Compared with ESC-MSCs, TSPCs combined with decellularized matrix showed more improvement in the structural and biomechanical properties of regenerated tendons in vivo. This study developed a practical strategy for functional tendon tissue regeneration and further studies are warranted to utilize TSPCs integrated with a tendon-derived decellularized matrix for tendon regeneration.
Acknowledgements
This work was supported by Natural Science Foundation grants of Zhejiang Province, China (NO. LY16H170001). The authors thank Qingmei Wang from the Stroke Biological Recovery Laboratory, Spaulding Rehabilitation Hospital, Harvard Medical School for her contributions and English editing of this manuscript. The authors declare no conflicts of interest.
Footnotes
Author Contribution: Haixin Song and Zi Yin contributed equally to this study.
Ethical Approval: This study was approved by the Zhejiang University Institutional Animal Care and Use Committee.
Statement of Human and Animal Rights: This study was approved by the Zhejiang University Institutional Animal Care and Use Committee, and we strictly followed the current laws for animal experiments.
Statement of Informed Consent: Statement of Informed Consent is not applicable for this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Natural Science Foundation grants of Zhejiang Province, China (NO. LY16H170001).
References
- 1. Veronesi F, Torricelli P, Della Bella E, Pagani S, Fini M. In vitro mutual interaction between tenocytes and adipose-derived mesenchymal stromal cells. Cytotherapy. 2015;17(2):215–223. [DOI] [PubMed] [Google Scholar]
- 2. Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004;6:303–329. [DOI] [PubMed] [Google Scholar]
- 3. Milthorpe BK. Xenografts for tendon and ligament repair. Biomaterials. 1994;15(10):745–752. [DOI] [PubMed] [Google Scholar]
- 4. Bach BR, Tradonsky S, Bojchuk J, Levy ME, Bush-Joseph CA, Khan NH. Arthroscopically assisted anterior cruciate ligament reconstruction using patellar tendon autograft. Am J Sports Med. 1998;26(1):20–29. [DOI] [PubMed] [Google Scholar]
- 5. Aynardi M, Zahoor T, Mitchell R, Loube J, Feltham T, Manandhar L, Paudel S, Schon L, Zhang Z. Orthotopic transplantation of Achilles tendon allograft in rats: with or without incorporation of autologous mesenchymal stem cells. Cell Transplant. 2018;27(2):245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ketchum Lynn D. Suture materials and suture techniques used in tendon repair. Hand Clin. 1985;1(1):43–53. [PubMed] [Google Scholar]
- 7. Burkhart SS, Danaceau SM, Pearce CE., Jr Arthroscopic rotator cuff repair: Analysis of results by tear size and by repair technique-margin convergence versus direct tendon-to-bone repair. Arthroscopy. 2001;17(9):905–912. [DOI] [PubMed] [Google Scholar]
- 8. Kato YP, Dunn MG, Zawadsky JP, Tria AJ, Silver FH. Regeneration of Achilles tendon with a collagen tendon prosthesis. Results of a one-year implantation study. J Bone Joint Surg Am. 1991;73(4):561–574. [PubMed] [Google Scholar]
- 9. Ricchetti ET, Aurora A, Iannotti JP, Derwin KA. Scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2012;21(2):251–265. [DOI] [PubMed] [Google Scholar]
- 10. Park SH, Choi YJ, Moon SW, Lee BH, Shim JH, Cho DW, Wang JH. Three-dimensional bio-printed scaffold sleeves with mesenchymal stem cells for enhancement of tendon-to-bone healing in anterior cruciate ligament reconstruction using soft-tissue tendon graft. Arthroscopy. 2018;34(1):166–179. [DOI] [PubMed] [Google Scholar]
- 11. Chen J, Zhang E, Zhang W, Liu Z, Lu P, Zhu T, Yin Z, Backman LJ, Liu H, Chen X, Ouyang H. Fos promotes early stage teno-lineage differentiation of tendon stem/progenitor cells in tendon. Stem Cells Transl Med. 2017;6(11):2009–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13(10):1219–1227. [DOI] [PubMed] [Google Scholar]
- 13. Rui YF, Lui PP, Li G, Fu SC, Lee YW, Chan KM. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng Part A. 2010;16(5):1549–1558. [DOI] [PubMed] [Google Scholar]
- 14. Zhang J, Wang JHC. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord. 2010;11(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou Z, Akinbiyi T, Xu L, Ramcharan M, Leong DJ, Ros SJ, Colvin AC, Schaffler MB, Majeska RJ, Flatow EL, Sun HB. Tendon-derived stem/progenitor cell aging: Defective self-renewal and altered fate. Aging Cell. 2010;9(5):911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu K, Al-Ani MK, Sun Y, Xu W, Pan L, Song Y, Xu Z, Pan X, Yang L. Platelet-rich plasma activates tendon-derived stem cells to promote regeneration of Achilles tendon rupture in rats. J Tissue Eng Regen Med. 2017;11(4):1173–1184. [DOI] [PubMed] [Google Scholar]
- 17. Tarafder S, Chen E, Jun Y, Kao K, Sim KH, Back J, Lee FY, Lee CH. Tendon stem/progenitor cells regulate inflammation in tendon healing via JNK and STAT3 signaling. FASEB J. 2017;31(9):3991–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han W, Wang B, Liu J, Chen L. The p16/miR-217/EGR1 pathway modulates age-related tenogenic differentiation in tendon stem/progenitor cells. Acta Biochim Biophys Sin (Shanghai). 2017;49(11):1015–1021. [DOI] [PubMed] [Google Scholar]
- 19. Caplan AI. Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11(7–8):1198–1211. [DOI] [PubMed] [Google Scholar]
- 20. Tan Q, Lui PP, Rui YF, Wong YM. Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Eng Part A. 2012;18(7–8):840–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yin Z, Chen X, Zhu T, Hu JJ, Song HX, Shen WL, Jiang LY, Heng BC, Ji JF, Ouyang HW. The effect of decellularized matrices on human tendon stem/progenitor cell differentiation and tendon repair. Acta Biomater. 2013;9(12):9317–9329. [DOI] [PubMed] [Google Scholar]
- 22. Chen JL, Yin Z, Shen WL, Chen X, Heng BC, Zou XH, Ouyang HW. Efficacy of hESC-MSCs in knitted silk-collagen scaffold for tendon tissue engineering and their roles. Biomaterials. 2010;31(36):9438–9451. [DOI] [PubMed] [Google Scholar]
- 23. Shen W, Chen J, Yin Z, Chen X, Liu H, Heng BC, Chen W, Ouyang HW. Allogenous tendon stem/progenitor cells in silk scaffold for functional shoulder repair. Cell Transplant. 2012;21(5):943–958. [DOI] [PubMed] [Google Scholar]
- 24. Yin Z, Chen X, Song HX, Hu JJ, Tang QM, Zhu T, Shen WL, Chen JL, Liu H, Heng BC, Ouyang HW. Electrospun scaffolds for multiple tissues regeneration in vivo through topography dependent induction of lineage specific differentiation. Biomaterials. 2015;44:173–185. [DOI] [PubMed] [Google Scholar]
- 25. Shen W, Chen X, Chen J, Yin Z, Heng BC, Chen W, Ouyang HW. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials. 2010;31(28):7239–7249. [DOI] [PubMed] [Google Scholar]
- 26. Cen L, Liu W, Cui L, Zhang W, Cao Y. Collagen tissue engineering: Development of novel biomaterials and applications. Pediatr Res. 2008;63(5):492–496. [DOI] [PubMed] [Google Scholar]
- 27. Shi Y, Zhou K, Zhang W, Zhang Z, Zhou G, Cao Y, Liu W. Microgrooved topographical surface directs tenogenic lineage specific differentiation of mouse tendon derived stem cells. Biomed Mater. 2017;12(1):015013. [DOI] [PubMed] [Google Scholar]
- 28. Kryger GS, Chong AK, Costa M, Pham H, Bates SJ, Chang J. A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J Hand Surg Am. 2007;32(5):597–605. [DOI] [PubMed] [Google Scholar]
- 29. Zhang J, Li B, Wang JH. The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo. Biomaterials. 2011;32(29):6972–6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lui PP, Wong OT, Lee YW. Transplantation of tendon-derived stem cells pre-treated with connective tissue growth factor and ascorbic acid in vitro promoted better tendon repair in a patellar tendon window injury rat model. Cytotherapy. 2016;18(1):99–112. [DOI] [PubMed] [Google Scholar]
- 31. Yang Z, Cao H, Gao S, Yang M, Lyu J, Tang K. Effect of tendon stem cells in chitosan/beta-glycerophosphate/collagen hydrogel on Achilles tendon healing in a rat model. Med Sci Monit. 2017;23:4633–4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang D, Xu B, Yang M, Zhao Z, Zhang Y, Li Z. Efficacy of tendon stem cells in fibroblast-derived matrix for tendon tissue engineering. Cytotherapy. 2014;16(5):662–673. [DOI] [PubMed] [Google Scholar]
- 33. Alberton P, Dex S, Popov C, Shukunami C, Schieker M, Docheva D. Loss of tenomodulin results in reduced self-renewal and augmented senescence of tendon stem/progenitor cells. Stem Cells Dev. 2015;24(5):597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang B, Luo Q, Halim A, Ju Y, Morita Y, Song G. Directed differentiation and paracrine mechanisms of mesenchymal stem cells: Potential implications for tendon repair and regeneration. Curr Stem Cell Res Ther. 2017;12(6):447–454. [DOI] [PubMed] [Google Scholar]
- 35. Lee SY, Kwon B, Lee K, Son YH, Chung SG. Therapeutic mechanisms of human adipose-derived mesenchymal stem cells in a rat tendon injury model. Am J Sports Med. 2017;45(6):1429–1439. [DOI] [PubMed] [Google Scholar]
- 36. Aktas E, Chamberlain CS, Saether EE, Duenwald-Kuehl SE, Kondratko-Mittnacht J, Stitgen M, Lee JS, Clements AE, Murphy WL, Vanderby R. Immune modulation with primed mesenchymal stem cells delivered via biodegradable scaffold to repair an Achilles tendon segmental defect. J Orthop Res. 2017;35(2):269–280. [DOI] [PubMed] [Google Scholar]
- 37. Tong WY, Shen W, Yeung CW, Zhao Y, Cheng SH, Chu PK, Chan D, Chan GC, Cheung KM, Yeung KW, Lam YW. Functional replication of the tendon tissue microenvironment by a bioimprinted substrate and the support of tenocytic differentiation of mesenchymal stem cells. Biomaterials. 2012;33(31):7686–7698. [DOI] [PubMed] [Google Scholar]
- 38. Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414(6859):118–121. [DOI] [PubMed] [Google Scholar]