Highlights
-
•
IL-12 and IL-23 have established roles during anti-fungal immunity.
-
•
IL-27 promotes regulatory effector responses during fungal infections.
-
•
IL-35 drives T cell differentiation to produce anti-inflammatory responses.
-
•
Increasing evidence for IL-12 family cytokines in maintaining anti-fungal immune homeostasis.
Keywords: Fungal, IL-12, IL-23, IL-27, IL-35
Abstract
Invasive fungal infections cause approximately 1.5 million deaths per year worldwide and are a growing threat to human health. Current anti-fungal therapies are often insufficient, therefore studies into host-pathogen interactions are critical for the development of novel therapies to improve mortality rates. Myeloid cells, such as macrophages and dendritic cells, express pattern recognition receptor (PRRs), which are important for fungal recognition. Engagement of these PRRs by fungal pathogens induces multiple cytokines, which in turn activate T effector responses. Interleukin (IL)-12 family members (IL-12p70, IL-23, IL-27 and IL-35) link innate immunity with the development of adaptive immunity and are also important for regulating T cell responses. IL-12 and IL-23 have established roles during anti-fungal immunity, whereas emerging roles for IL-27 and IL-35 have recently been reported. Here, we discuss the IL-12 family, focusing on IL-27 and IL-35 during anti-fungal immune responses to pathogens such as Candida and Aspergillus.
1. Introduction
Interleukin (IL)-12 family members (IL-12p70, IL-23, IL-27 and IL-35) link innate immunity with the development of adaptive immunity [1]. They are important in regulating T cell responses and while structurally similar, they display different functional activities. IL-12 and IL-23 are both considered to be pro-inflammatory [2], [3]. IL-12 predominantly favors T helper (Th)1 cell differentiation [4], [5], [6], whereas IL-23 enhances Th17 responses and is involved in the activation and expansion of memory T-cells [7], [8]. IL-27 has both pro- and anti-inflammatory properties and is a potent T cell immunomodulator. IL-27 was originally reported to enhance Th1 cell differentiation [9], [10]. However, subsequent studies highlight more nuanced roles linked with suppression of adaptive immunity, where IL-27 inhibits IL-2 signaling, antagonizes Th17 cells and promotes regulatory T cells (Tregs) responses [11], [12], [13], [14], [15], [16]. Lastly, IL-35 induces Tregs and regulatory B cells (Bregs) and suppresses T cell responses [17]. IL-12 and IL-23 have established roles during anti-fungal immunity [7], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29] and new roles for IL-27 and IL-35 have recently been reported [20], [23], [30]. Importantly, the new roles reported for IL-27 as a regulatory cytokine emerged from infection studies suggesting that IL-27 may also have important roles in fungal control. This review will focus on the role of these newer family members during fungal infections, such as Candida and Aspergillus infections.
Candida species (spp). cause various types of disease, from mucosal infections (eg. oral and vaginal thrush) to life threatening invasive candidiasis. Candida spp. bind various Pattern Recognition Receptors (PRRs) such as C-type lectin-like receptors (CLRs) including Dectin-1, Toll-like receptors (TLRs) and Nucleotide-binding Oligomerisation domain-like receptors (NLRs) on myeloid cells [31]. This induces multiple cytokines, which in turn activate Th1 and Th17 responses [32], [33], [34], [35], [36], [37], [38]. The production of IFN-γ by Th1 cells has been shown to be crucial in the control of systemic candidiasis in a murine model [21], [39], [40], [41]. Whereas, the Th17 response protects against oropharyngeal and mucocutaneous models of candidiasis while increasing disease susceptibility in a gastrointestinal model [7], [29], [42], [43].
Aspergillus fumigatus is an airborne fungus that causes various types of disease including invasive pulmonary aspergillosis (IPA) and allergic bronchopulmonary aspergillosis (ABPA). An important role of the host immune response is to limit spore germination and restrict hyphal invasion. Spore swelling, the first step of germination, exposes fungal ligands that bind to CLRs and TLRs resulting in inflammatory cytokine production [44], [45]. This then induces a Th1 response and a much smaller Th17 response in a pulmonary infection model [25], [46] whereas a Th2 response is induced in an allergic model of disease [47], [48].
The T cell responses mediated by these different pathogens (Candida and Aspergillus) are likely controlled by the ligands exposed on their cell surface and by the environment (e.g. systemic, mucosal, pulmonary) of the infection. The yeast form of C. albicans expresses mannans on the cell surface and β-glucans are rapidly exposed at bud scars whereas dormant Aspergillus conidia are covered in a rodlet layer that initially hides β-glucans from the immune system [44], [49], [50]. Mannans present on the yeast form of C. albicans have been shown to induce IL-1β and IL-23 in a Dectin-2 dependent manner [37]. Additionally, both yeast and hyphal forms of C. albicans induced Dectin-2-dependent Th17 differentiation in vitro and Il17a deficient mice were more susceptible to systemic infection with C. albicans [37]. Further to this, dendritic cells (DCs) activated by Dectin-1 engagement by curdlan, a β-glucan preparation, demonstrated Th17 cell differentiation of CD4+ T cells in vitro, and the use of curdlan as an adjuvant in vivo, promoted both Th17 and Th1 responses [34]. We have previously demonstrated differences in T cell differentiation in vitro and in vivo. Heat killed yeast predominantly induced Th17 differentiation in vitro, whereas, mice systemically infected with C. albicans displayed a Th1-biased response in vivo [19], therefore the environment is also important for determining T cell bias. In the context of A. fumigatus infections, Th1 and Th17 CD4+ T cells are driven via TLR/MyD88 and Dectin-1 respectively. TLR2 and TLR4 recognise A. fumigatus conidia [51], whereas Dectin-1 recognises later morphological forms such as swollen conidia and early germlings [44]. It is possible that the TLRs initiate a Th1 response to A. fumigatus and then Dectin-1 induces the Th17 response once germination of A. fumigatus is further underway. As C. albicans and A. fumigatus engage different combinations of PRRs, interactions between these PRRs likely dictate the balance between Th1 and Th17 responses to these two pathogens.
2. IL-12 family cytokines
2.1. Structure
IL-12 family cytokines consist of four heterodimeric proteins (Fig. 1, Fig. 2), which are mainly produced by myeloid cells. This has been extensively reviewed elsewhere [22], [52], [53], [54], [55], [56], [57]. IL-12 is made up of 2 subunits, IL-12p35 and IL-12p40, which together form active IL-12p70. IL-12p70 is a very potent activator of Th1 cell responses [4], [5]. IL-12p40 is also expressed and secreted as a monomer and a homodimer [58], [59]. The p40 monomer has no biological function whereas the homodimer is an antagonist of IL-12p70 by competitively binding the IL-12R [60]. The IL-12p40 homodimer enhances alloantigen-specific Th1 development suggesting similar functional activity to IL-12p70 under certain conditions [61]. IL-23 is composed of IL-23p19 and IL-12p40 (shared with IL-12p70). IL-23 is tightly regulated and is functionally distinct from IL-12. IL-27 is a heterodimer made up of IL-27p28 and Epstein Barr virus induced gene 3 (EBI3) subunits [10], [62]. IL-27p28 has also been shown to be expressed as a monomer, which can inhibit IL-6, IL-27 and IL-11 signalling through the common gp130 signalling receptor [63], [64]. IL-27 modulates pro- and anti-inflammatory signaling [65], [66], [67]. IL-35 consists of the EBI3 subunit (shared with IL-27) and the IL-12p35 subunit (shared with IL-12) [68]. IL-35 promotes Treg responses and suppresses Th1 and Th17 responses. Treg cells deficient in either Ebi3 or Il12a (IL-12p35) display a reduced ability to inhibit effector T cell proliferation [69], [70], [71], [72].
Fig. 1.
IL-12, IL-23 and IL-27 cytokines and their receptors. (A) IL-12 is a heterodimeric cytokine made up of a light chain IL-12p35 and a heavy chain IL-12p40. The IL-12 receptor is made up of IL-12Rβ1 and IL-12Rβ2 and signals through Tyk2 and JAK2 to activate STAT1, 3, 4 and 5. STAT4 induces nuclear translocation of IFN-γ. (B) The IL-12p40 component of IL-23 can dimerise with IL-23p19 to form IL-23. The IL-23 receptor is comprised of IL-12Rβ1 and IL-23R and signals through Tyk2 and JAK2 to activate STAT1, 3, 4 and 5. STAT3 induces nuclear translocation of the IL-23R, IL-17A and IL-22; and STAT4 induces nuclear translocation of IL-17A/F. (C) IL-27 is composed of EBI3 and IL-27p28. IL-27 binds a receptor composed of gp130 and IL-27Rα and signals through JAK1, Tyk2 and JAK2 to activate STAT1, 2, 3, 4 and 5. STAT1 induces nuclear translocation of Tbet and inhibition of GATA3; and STAT3 induces nuclear translocation of IL-10.
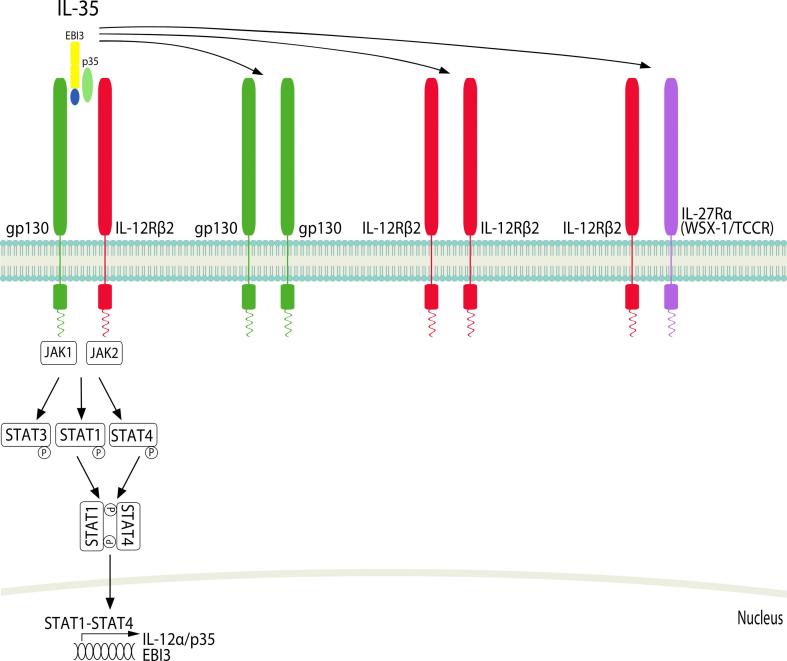
Fig. 2.
IL-35 cytokines and their receptors. IL-35 is a heterodimeric cytokine made up of EBI3 (shared with IL-27) and IL-12p35 (shared with IL-12). gp130 couples with IL-12Rβ2 to form the IL-35 receptor complex and signals through JAK1 and JAK2 to activate STAT1, 3 and 4. STAT-1-STAT4 form a heterodimer to induce nuclear translocation and induction of IL-12α/p35 and EBI3. Additionally, homodimers of gp130 or IL-12Rβ2 have been shown to elicit a partial IL-35 induced response in T cells [86], and IL-12Rβ2 can also couple with IL-27Rα to also form an IL-35 receptor complex in B cells [17].
2.2. Signaling
IL-12 binds to IL-12Rβ1 and IL-12Rβ2 and signals via Janus associated kinase 2 (JAK2) and tyrosine kinase 2 (Tyk2) to activate various signal transducer and activator of transcription (STAT) family members [3], [73] (Fig. 1). Patients with defective IL12B or IL12RB1 [74] and cells from mice deficient for Tyk2 or STAT4 showed reduced IFN-γ production in T cells and natural killer (NK) cells [75], [76], [77]. IL-23 binds IL-12Rβ1 and IL-23R [78], [79] and signals in a similar manner to IL-12 [79], [80] (Fig. 1). JAK2 and Tyk2 constitutively associate with IL-23R and IL-12R-β1 chains respectively [79]. IL-23 activated STAT3 and STAT4 is critical for IL-17 induction from Th17 cells [81], [82]. The IL-23R and IL-12R-β1 chains alone lack intrinsic enzymatic activity [2], [3], [79], [83].
IL-27 signals through IL-27Rα (WSX-1/TCCR) paired with glycoprotein (gp)130 [84] (Fig. 1). IL-27 can bind WSX-1 with low affinity in the absence of gp130, however both IL-27R subunits are required for effective signal transduction [10], [84]. IL-27 signaling activates various JAK and STAT family members in a cell-specific manner. For example, IL-27 activates both STAT1 and STAT3 in naïve CD4+ T cells but in fully active T cells only STAT3 activation is retained [85]. IL-35 signaling is unconventional as it utilizes different receptor combinations including IL-12Rβ2 and gp130 homodimers and heterodimers (Fig. 2). STAT1 and/or STAT4 are activated depending on which receptor homodimer/heterodimer is engaged. In B cells IL-35 signals through IL-12Rβ2 and WSX-1 to activate STAT1 and STAT4. More work is required to fully understand IL-35 signaling downstream of the different homodimers or heterodimers [17], [86].
3. IL-12 family cytokines in anti-fungal host defense
3.1. IL-12
IL-12 is induced by microbial products in monocytes, macrophages and DCs and acts on NK and T cells to induce IFN-γ. IFN-γ in turn activates monocytes and macrophages to induce further IL-12 production [87]. This positive feedback mechanism helps protect against certain pathogens that induce low IL-12 levels [88]. Deficiency of IL-12 or IL-12R genes leads to impaired cell mediated immunity and enhanced disease susceptibility [89], [90], [91], [92]. We will only briefly discuss the role of IL-12 during Candida infections as this has been reviewed elsewhere [22], [93].
Several C. albicans infection experiments have been performed in mice deficient in different components of the IL-12 family (Table 1). However, due to subunit sharing between different family members, interpretation of these results is rather complex. Overall, IL-12 appears to be important during systemic infection but less important during mucocutaneous infections. Il12p40-/- mice were susceptible to oral infection with C. albicans, however, this was mainly attributed to IL-23 and Th17 responses [29]. Interestingly, Il12p35-/- mice demonstrated disseminated infection following oral infection with C. albicans suggesting that while IL-12 is not overly important in the local response during an oral infection, it controls systemic dissemination [29]. In addition, Il12p35-/- and IFN-γ-/- mice displayed enhanced susceptibility to systemic infection with C. albicans [7], [39], [40], [41], indicating an important role for IL-12/Th1 responses during systemic infection. Il12p40-/- mice displayed variable susceptibility to systemic infection in different studies [7], [26], [94]. Additionally, in a mouse model where mannoproteins from the fungal pathogen Cryptococcus neoformans were shown to protect against systemic C. albicans infection, this protective response was reversed upon IL-12 blockade [95]. Further, when IL-12 was fused to enolase (a C. albicans antigen) and administered during systemic infection with C. albicans, mice demonstrated increased survival and decreased fungal burden in their kidneys [27]. However, administration of IL-12 during systemic infection increased disease severity due to IFN-γ mediated pathology [96]. These data suggest that while IL-12 and IFN-γ are important for controlling systemic infections with C. albicans, this response needs to be finely tuned for optimal fungal clearance while minimising pathology.
Table 1.
Effects of different Candida and Aspergillus infections on Il12p40-/-, Il12p35-/-, Il23p19-/- and Il27ra-/- mice.
| Genotype | Pathogen | Outcome | References |
|---|---|---|---|
| Il12p40-/- | C. albicans | Susceptible to oral challenge | Farah et al. [26] |
| C. albicans | Reduced fungal burden during systemic challenge (1, 3 days post infection) but increased fungal burden following re-infection | Mencacci et al. [94] | |
| C. albicans | No effect during systemic challenge | Netea et al. 2003 [170] | |
| C. albicans | No effect during systemic challenge (5 days post infection) | Farah et al. 2006 [26] | |
| C. albicans | Increased fungal burden during gastrointestinal challenge (3, 10 days post infection) | Zelante et al. [7] | |
| C. albicans | Susceptible to cutaneous challenge | Kagami et al. [112] | |
| C. albicans | Increased fungal burden during gastrointestinal challenge | Mencacci et al. [94] | |
| A. fumigatus | Increased fungal burden during IPA in immunosuppressed mice | Cenci et al. [99] | |
| A. fumigatus | Reduced fungal burden during IPA | Zelante et al. [7] | |
| Il12p35-/- | C. albicans | Systemic dissemination during oral challenge | Conti et al. [29] |
| C. albicans | Susceptible to gastrointestinal challenge | Zelante et al. [7] | |
| C. albicans | No effect during cutaneous challenge | Kagami et al. [112] | |
| A. fumigatus | Reduced fungal burden during IPA | Zelante et al. [7] | |
| Il23p19-/- | C. albicans | Susceptible to oral challenge | Conti et al. [29] |
| C. albicans | No effect during gastrointestinal challenge | Zelante et al. [7] | |
| C. albicans | No effect during vulvovaginal challenge | Yano et al. [111] | |
| C. albicans | Susceptible to cutaneous challenge | Kagami et al. [112] | |
| A. fumigatus | Reduced fungal burden during IPA | Zelante et al. [7] | |
| Il27ra-/- | C. albicans | No significant effect during systemic challenge | Patin et al. [30] |
| C. parapsilosis | Enhanced fungal clearance and reduced fungal burden in the kidneys during systemic challenge (6 weeks post infection) | Patin et al. [30] | |
The importance of IL-12 has also been demonstrated during A. fumigatus infections. Human DCs infected with A. fumigatus induced IL-12p70 production and promoted IFN-γ production from CD4+ T cells [20]. Rivera et al. [25] showed that Th1 and Th17 responses are induced during an in vivo model of A. fumigatus lung infection. The authors showed that TLR and Dectin-1 pathways modulate the balance between Th1 and Th17 populations by controlling production of IL-12 family members. In addition, IL-12 has been shown to induce human monocytes to damage A. fumigatus hyphae via an oxidative burst response in an IFN-γ-independent manner [97].
During an in vivo infection model with A. fumigatus, Cenci et al. [98] compared the immune response of resistant (intact DBA/2 mice) and susceptible (leukopenic DBA/2 mice) mice. Interestingly, the authors demonstrated that resistant mice produced high levels of IL-12, TNF and IFN-γ while susceptible mice produced an IL-10 and IL-4 skewed response. In addition, immunosuppressed Il12p40-/- and Ifng-/- mice were more susceptible to invasive pulmonary aspergillosis [99]. In agreement with this, Nagai et al. [100] showed that administration of IFN-γ protected mice from invasive aspergillosis. Furthermore, Delsing et al. [101] showed that adjunctive therapy with IFN-γ and an antifungal drug partially restored immune function in patients with invasive Candida and/or Aspergillus infections indicating that further clinical studies are warranted to fully determine the potential clinical benefits of IFN-γ therapy during invasive fungal infections.
3.2. IL-23
IL-23 is involved in the maintenance of Th17 cells and the activation of memory T-cells [78], [102]. It induces CD4+ Th17 cells to produce IL-17 and IL-22 [80], [103], [104], [105]. IL-17A is a proinflammatory cytokine that promotes neutrophil recruitment via upregulation of other cytokines and chemokines [106]. While the IL-23-IL-17 pathway is important for protective responses against infection, it can also contribute to autoimmunity and increased pathology [107], [108]. IL-23 is also important for regulation of innate lymphocyte cells (e.g. innate lymphoid, NK, NKT and γδ T-cells [109]).
C. albicans induces IL-23p19 and/or IL-23 in cultured macrophages and DCs and at sites of inflammation during infection [19], [24]. Interestingly, human monocyte derived DCs produced IL-23 when challenged with C. albicans hyphae, whereas C. albicans yeast induced IL-12 production and IL-23 was only produced with high concentrations of yeast [110]. This suggests that the hyphal form of C. albicans triggers the Th17 response in vivo.
During systemic infection with C. albicans, IL-23 was dispensable, however the IL-23/Th17 pathway is important during chronic muccocutaneous candidiasis (CMC) [7]. Il23p19-/-, and Il17ra-/- mice were more susceptible to oral C. albicans infection [29]. However, Yano et al. (2012) found that fungal burden and S100A8/S100A9 alarmin-mediated neutrophil recruitment was normal in Il23p19-/-, Il17ra-/- and Il22-/- mice in a mouse vaginal infection model suggesting that the vaginal alarmin S100 response is independent of the IL-23/Th17 axis [111]. In contrast, several other studies have found important roles for Th17 cells, IL-17 and IL-22 in protecting patients against CMC [29], [43], [112]. Patients with impaired Th17 and Th17-associated cytokines due to mutations in genes such as STAT3 (hyper IgE syndrome, [113], [114]), IL-17RA [115], IL-17F [115], ACT1 [116], STAT1 [117], [118], AIRE [119], [120], IRF8 [121], CARD9 and DECTIN-1 [122], [123], [124] are prone to CMC. In addition, two Mexican patients with a mutation in IL12RB1, suffered from infections with Baccille Calmette-Guérin (BCG) and C. albicans and they both died early at ages 4 and 16 [125]. Furthermore, in an international study of 141 patients, 23% of patients lacking IL12RB1were found to suffer from CMC [126]. However, as both IL-12 and IL-23 signal through IL-12Rβ1, this could be due to a lack of responsiveness to either cytokine. IL-23 and IL-6 were detected in vaginal secretions from women infected with vaginal candidiasis [24], [127]. Additionally, healthy PBMCs stimulated with C. albicans induced a Th17 response demonstrating that Th17 cells are involved in the defense against C. albicans [128]. Furthermore, reduced Th17 responses have been reported in human PBMCs stimulated with C. albicans from patients with hyper IgE syndrome, demonstrating that Th17 cells play an important role in the defense against C. albicans [113]. These data indicate a protective role for the IL-23/Th17 pathway during C. albicans infection, however, heightened IL-17 and IL-23 responses can also negatively regulate Th1 responses to C. albicans resulting in increased inflammation and susceptibility to candidiasis [7]. Therefore, similar to IL-12 and IFN-γ, the IL-23/Th17 response needs to be tightly controlled. Th17 cells express IL-10Rα and IL-10 signalling has been shown to control IL-17A producing CD4+ T cells in a colitis model [129], suggesting that IL-10 may be an important regulator of IL-23/Th17 responses during anti-fungal immunity.
Studies have demonstrated a role for the IL-23/Th17/IL-17 pathway during A. fumigatus infection [7], [130]. Il12p40-/-, Il12p35-/- and Il23p19-/- mice displayed reduced fungal burden in the lungs of A. fumigatus infected mice, indicating that a heightened IL-23/Th17 response is associated with susceptibility [7]. However, Dectin-1 and TLR9 deficient mice displayed reduced IL-23, IL-17A and/or IL-22 responses and increased fungal burden in the lungs of A. fumigatus challenged mice, indicating that the IL-17 pathway is involved in a protective response to A. fumigatus [131], [132]. In contrast, an immunopathogenic role for Dectin-1 and IL-22 has been shown during chronic fungal allergy [104], once again demonstrating the importance for tight regulation of these pathways. IL-17 has been shown to inhibit neutrophil mediated killing of A. fumigatus and in vivo clearance [7]. Th1 cells, Treg cells, and Th17 cells are all important in producing both an inflammatory and anti-inflammatory response against A. fumigatus [7], [48], [133]. The balance between Th17 cells and Treg cells has been suggested to be important in determining whether commensal C. albicans causes infection and the same could be true for Aspergillus infections [134], [135].
3.3. IL-27
The ability of IL-27 to regulate T cell responses has been reviewed elsewhere [136], [137]. Briefly, IL-27 was shown to enhance in vitro Th1 cell differentiation [138]. However, more recently, anti-inflammatory properties of IL-27 have been discovered. IL-27 has been shown to inhibit T cell responses by negatively regulating IL-2 signaling and by inducing IL-10 [11], [12], [66], [139], [140], [141], [142]. IL-10 has been shown to inhibit both CD4+ and CD8+ T cell responses [134], [143]. Additionally, a role in inhibiting Th17 cell differentiation has been demonstrated to protect against Th17 associated disease, such as encephalomyelitis [11], [12], [13], [14]. Th17 cells in encephalomyelitis promotes the development of ectopic lymphoid structures in the central nervous system [144]. Interestingly, IL-27 inhibits the development of ectopic lymphoid structures in inflamed tissues [145]. These functions of IL-27 help protect against T-cell driven pathology. IL-27 has shown contrasting effects on regulatory T cells. It has been shown to promote Treg growth and survival at sites of infection [16] but also to indirectly inhibit Treg development by inhibiting IL-2 production [15], [16].
IL-27 has been reported to be involved in the immune response to various pathogens including Trypanosoma cruzi [146], Leishmania major [147], [148], [149], Mycobacteria tuberculosis [11] and Toxoplasma gondii [12], [14], [150]. In addition, a limited number of studies reviewed here, have recently examined the role of IL-27 in response to fungal pathogens. The first report in a fungal model demonstrated that EBI3 levels were significantly induced in human monocyte derived DCs following stimulation with A. fumigatus while IL27 levels only showed a slight increase, demonstrating that A. fumigatus-induced IL-27 production is minimal [20]. Indeed, it is possible that the increased EBI3 levels could actually reflect IL-35 induction rather than IL-27 production, as human monocyte derived tolerogenic DCs express and produce IL12A and EBI3 [151], although this remains to be determined in a fungal model. However, in a later study these authors went on to show that IFN-β treated DCs challenged with A. fumigatus resulted in increased IL27 mRNA levels, IL-12p70 production and increased expression of CD86 and CD83 [152]. While it is well established that IFN-β induces IL-27 [153], this report describes a new role for A. fumigatus in IL-27 production [152]. Further, DCs stimulated with IFN-β and challenged with A. fumigatus resulted in increased Th1-mediated IFN-γ production [152]. This suggests that IFN-β could be used as a possible therapy to enhance a beneficial Th1 response during infection with A. fumigatus.
Type 1 regulatory T cells (Tr1) are instrumental in protecting against Th1/Th17-mediated autoimmunity in mice and reactions to common allergens in humans [154], [155], and may also play an important role during A. fumigatus infections [156]. Tr1 cells are Foxp3- regulatory T cells that produce high IL-10 levels and suppress T cell expansion in vitro and in vivo [157]. Bedke et al. [156] identified Crf-1/p41-specific latent associated peptide+ and IL-10+ Tr1 cells in healthy humans and in mice following vaccination with an Aspergillus peptide (Crf-1/p41) and zymosan. In agreement with previous studies [158], [159], Tr1 cell differentiation in vivo was found to be dependent on the aryl hydrocarbon receptor, c-Maf and IL-27. These data indicate that IL-27 plays a role in maintaining anti-fungal immune homeostasis via induction of Tr1 cell differentiation [156].
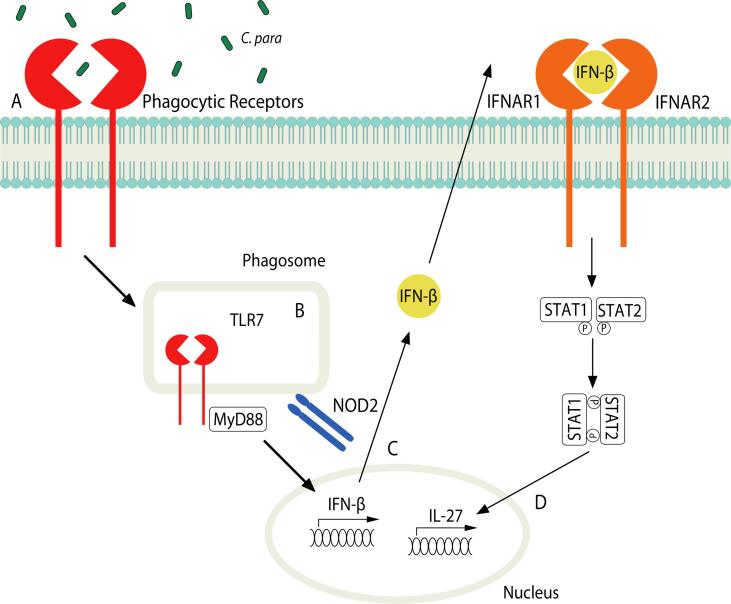
Several inflammatory cytokines/molecules, such as lipopolysaccharide (LPS) [10], [22], [160], [161], IL-1β [162], Poly(I:C) [161] and IFN-β [152], [153], [163], have been reported to induce IL-27 as a protective response to prevent excess inflammation. Interestingly, increased expression of IL27 and EBI3 has been shown in THP-1 cells challenged with heat killed C. albicans compared with LPS [22]. In contrast, IL-27 production was reduced in the RAW264.7 macrophage cell line when treated with bakers yeast (saccharomyces cerevisiae)-derived β-glucan (BBG) [160]. In addition, we showed that β-glucan alone does not induce IL-27 in bone marrow derived macrophages (BMDMs) [30]. We also showed that select Candida spp. induce IL-27 production, such as C. parapsilosis, C. glabrata and C. tropicalis but not C. albicans. IL-27 production by C. parapsilosis was dependent on phagocytosis, TLR7/MyD88 and nucleotide-binding oligomerisation domain-containing protein (NOD)2 signaling. Further, C. parapsilosis induced IFN-β production, which then signaled through the IFN-α/β receptor (IFNAR) and STAT1/2 to induce IL-27 (Fig. 3). Interestingly, while we found that C. albicans did not induce IL-27, likely due to the absence of IFN-β induction, it also actively blocked C. parapsilosis-induced IL-27 via a soluble mediator [30]. This soluble mediator has yet to be identified, but it is tempting to speculate that BBG might induce the same soluble mediator to block IL-27 production [160]. However, it is interesting to note that live C. albicans blocked C. parapsilosis-induced IL-27 in mouse BMDMs [30] while heat killed C. albicans enhanced LPS-induced IL-27 in the human THP-1 cell line [22]. These discrepancies could be due to differences in mouse vs human IL-27/cells or due to exposure of different ligands on live vs heat killed C. albicans and requires further study to unravel these differences.
Fig. 3.
IL-27 induction by C. parapsilosis. (A) C. parapsilosis engages cell surface receptors on BMDM. (B) C. parapsilosis is phagocytosed and TLR7/MyD88 and NOD2 signaling is activated resulting in the induction of nuclear translocation of IFN-β. (C) IFN-β signals through IFNAR1/2-STAT1/2 pathway to (D) induce IL-27 [30].
Similar to infections with other pathogens such as M. tuberculosis, P. berghei and T. gondii [11], [164], we demonstrated improved clearance of systemic C. parapsilosis infection in WSX-1 (Il27ra) deficient mice. This was accompanied by increased IFN-γ and IL-17 T cell responses in addition to increased serum pro-inflammatory cytokine levels [30]. While we observed improved fungal clearance and only a modest increase in inflammation, other groups have shown improved pathogen clearance in WSX-1 deficient mice accompanied by fatal organ pathogenesis due to highly elevated pro-inflammatory responses [11], [12], [164]. Interestingly, some studies showed that organ pathology could be prevented by depleting CD4+ T cells but not CD8+ T cells [12], [164]. As C. parapsilosis induced a CD8+ T cell biased response rather than a CD4+ T cell response, this could account for the improved fungal clearance without inducing fatal organ pathology [30]. These data suggest that blocking IL-27 during infection with C. parapsilosis could have therapeutic benefits.
In this review, it has already been mentioned that Th17 cells, IL-17, IL-22 and IL-23 cytokines have important role in protecting patients against CMC [7], [24], [29], [43], [112]. Patients with a gain of function mutation in STAT1 have higher susceptibility to Candida infections [165]. Further, increased STAT1 function showed increased IL-27, IFN-γ and IFN-α production but reduced IL-17 responses [165], [166], [167]. In a separate study, it has been reported that reduced IFN-γ and IL-27 responses from impaired STAT1-expression was observed in two unrelated patients (from Japan and Saudi Arabia) with heterozygous missense mutations in the STAT1 SH2 domain [168]. Further studies in both mice and humans are required to fully unravel the role of IL-27 during anti-fungal immunity.
3.4. IL-35
IL-35 is mainly produced by Bregs and Tregs [169]. Stimulation of naïve effector T cells with recombinant IL-35 suppressed T cell proliferation in a murine model and ectopic expression of IL-35 on naïve T cells conferred regulatory activity [71]. IL-35 has been shown to suppress immune responses important for the induction of inflammatory disease in murine models such as collagen-induced arthritis [69], [71]. However, human T regulatory cells do not express IL-35 and as result a role for IL-35 in humans remains unclear [72]. As cytokine subunits are shared between IL and 35, IL-12 and IL-27, mechanistic studies to determine specific roles for IL-35 in the regulation of anti-fungal immunity are challenging.
Il12p35-/- mice have been shown to have lower fungal burden during oral candidiasis. As IL-12p35 is a subunit of IL-35, it is possible that IL-35 may play an important role in reducing tissue damage during oral infection [29]. Recently, heat killed C. albicans was shown to suppress LPS-induced IL-12p70 production in M2 macrophages due to increased Ebi3 expression, a subunit of IL-35. Further, the authors demonstrated that C. albicans induced IL-35 production, which inhibited an LPS-induced M2 (anti-inflammatory) to M1 (inflammatory) phenotype change in BMDMs, thereby supressing an inflammatory response. These results demonstrate a possible mechanism for how C. albicans is able to evade immune detection [23].
4. Conclusions
IL-12, IL-23, IL-27 and IL-35 are critical cytokines in innate and adaptive immune responses against fungal infections. They are important in regulating T cell responses and exert their effects on the Th1, Th17 and Treg immune pathways of T cell differentiation. These cytokines are important in regulating the development of inflammation and disease progression. A better understanding of the IL-12 receptor cytokine family, particularly the newer members (IL-27 and IL-35) is invaluable to fully unravel the importance of targeting this family to develop immunotherapies to help fight fungal infections.
Acknowledgments
Acknowledgements
We would like to thank Gareth Jones and Ian Humphreys for critically reviewing this manuscript.
Funding
SJO is funded by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant Number 099953/Z/12/Z).
References
- 1.Ma X., Trinchieri G. Regulation of interleukin-12 production in antigen-presenting cells. Adv. Immunol. 2001;79:55–92. doi: 10.1016/s0065-2776(01)79002-5. [DOI] [PubMed] [Google Scholar]
- 2.Yen D., Cheung J., Scheerens H., Poulet F., McClanahan T., McKenzie B., Kleinschek M.A., Owyang A., Mattson J., Blumenschein W., Murphy E., Sathe M., Cua D.J., Kastelein R.A., Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 2006;116(5):1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacon C.M., McVicar D.W., Ortaldo J.R., Rees R.C., O'Shea J.J., Johnston J.A. Interleukin 12 (IL-12) induces tyrosine phosphorylation of JAK2 and TYK2: differential use of Janus family tyrosine kinases by IL-2 and IL-12. J. Exp. Med. 1995;181(1):399–404. doi: 10.1084/jem.181.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsieh C.S., Macatonia S.E., Tripp C.S., Wolf S.F., O'Garra A., Murphy K.M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 5.Manetti R., Parronchi P., Giudizi M.G., Piccinni M.P., Maggi E., Trinchieri G., Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J. Exp. Med. 1993;177(4):1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humphreys I.R., Edwards L., Walzl G., Rae A.J., Dougan G., Hill S., Hussell T. OX40 ligation on activated T cells enhances the control of Cryptococcus neoformans and reduces pulmonary eosinophilia. J. Immunol. 2003;170(12):6125–6132. doi: 10.4049/jimmunol.170.12.6125. [DOI] [PubMed] [Google Scholar]
- 7.Zelante T., De Luca A., Bonifazi P., Montagnoli C., Bozza S., Moretti S., Belladonna M.L., Vacca C., Conte C., Mosci P., Bistoni F., Puccetti P., Kastelein R.A., Kopf M., Romani L. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur. J. Immunol. 2007;37(10):2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 8.Haines C.J., Chen Y., Blumenschein W.M., Jain R., Chang C., Joyce-Shaikh B., Porth K., Boniface K., Mattson J., Basham B., Anderton S.M., McClanahan T.K., Sadekova S., Cua D.J., McGeachy M.J. Autoimmune memory T helper 17 cell function and expansion are dependent on interleukin-23. Cell Rep. 2013;3(5):1378–1388. doi: 10.1016/j.celrep.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 9.Lucas S., Ghilardi N., Li J., de Sauvage F.J. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. PNAS. 2003;100(25):15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pflanz S., Timans J.C., Cheung J., Rosales R., Kanzler H., Gilbert J., Hibbert L., Churakova T., Travis M., Vaisberg E., Blumenschein W.M., Mattson J.D., Wagner J.L., To W., Zurawski S., McClanahan T.K., Gorman D.M., Bazan J.F., de Waal Malefyt R., Rennick D., Kastelein R.A. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16(6):779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 11.Holscher C., Holscher A., Ruckerl D., Yoshimoto T., Yoshida H., Mak T., Saris C., Ehlers S. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J. Immunol. 2005;174(6):3534–3544. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 12.Villarino A., Hibbert L., Lieberman L., Wilson E., Mak T., Yoshida H., Kastelein R.A., Saris C., Hunter C.A. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19(5):645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 13.Batten M., Li J., Yi S., Kljavin N.M., Danilenko D.M., Lucas S., Lee J., de Sauvage F.J., Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 2006;7(9):929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 14.Stumhofer J.S., Laurence A., Wilson E.H., Huang E., Tato C.M., Johnson L.M., Villarino A.V., Huang Q., Yoshimura A., Sehy D., Saris C.J., O'Shea J.J., Hennighausen L., Ernst M., Hunter C.A. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 2006;7(9):937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 15.Neufert C., Becker C., Wirtz S., Fantini M.C., Weigmann B., Galle P.R., Neurath M.F. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur. J. Immunol. 2007;37(7):1809–1816. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- 16.Hall A.O., Beiting D.P., Tato C., John B., Oldenhove G., Lombana C.G., Pritchard G.H., Silver J.S., Bouladoux N., Stumhofer J.S., Harris T.H., Grainger J., Wojno E.D., Wagage S., Roos D.S., Scott P., Turka L.A., Cherry S., Reiner S.L., Cua D., Belkaid Y., Elloso M.M., Hunter C.A. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37(3):511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang R.X., Yu C.R., Dambuza I.M., Mahdi R.M., Dolinska M.B., Sergeev Y.V., Wingfield P.T., Kim S.H., Egwuagu C.E. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat. Med. 2014;20(6):633–641. doi: 10.1038/nm.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyazato A., Nakamura K., Yamamoto N., Mora-Montes H.M., Tanaka M., Abe Y., Tanno D., Inden K., Gang X., Ishii K., Takeda K., Akira S., Saijo S., Iwakura Y., Adachi Y., Ohno N., Mitsutake K., Gow N.A., Kaku M., Kawakami K. Toll-like receptor 9-dependent activation of myeloid dendritic cells by Deoxynucleic acids from Candida albicans. Infect. Immun. 2009;77(7):3056–3064. doi: 10.1128/IAI.00840-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orr S.J., Burg A.R., Chan T., Quigley L., Jones G.W., Ford J.W., Hodge D., Razzook C., Sarhan J., Jones Y.L., Whittaker G.C., Boelte K.C., Lyakh L., Cardone M., O'Connor G.M., Tan C., Li H., Anderson S.K., Jones S.A., Zhang W., Taylor P.R., Trinchieri G., McVicar D.W. LAB/NTAL facilitates fungal/PAMP-induced IL-12 and IFN-gamma production by repressing beta-catenin activation in dendritic cells. PLoS Pathogens. 2013;9(5):e1003357. doi: 10.1371/journal.ppat.1003357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gafa V., Lande R., Gagliardi M.C., Severa M., Giacomini E., Remoli M.E., Nisini R., Ramoni C., Di Francesco P., Aldebert D., Grillot R., Coccia E.M. Human dendritic cells following Aspergillus fumigatus infection express the CCR7 receptor and a differential pattern of interleukin-12 (IL-12), IL-23, and IL-27 cytokines, which lead to a Th1 response. Infect. Immun. 2006;74(3):1480–1489. doi: 10.1128/IAI.74.3.1480-1489.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romani L., Mencacci A., Tonnetti L., Spaccapelo R., Cenci E., Puccetti P., Wolf S.F., Bistoni F. IL-12 is both required and prognostic in vivo for T helper type 1 differentiation in murine candidiasis. J. Immunol. 1994;153(11):5167–5175. [PubMed] [Google Scholar]
- 22.Wei X.Q., Rogers H., Lewis M.A., Williams D.W. The role of the IL-12 cytokine family in directing T-cell responses in oral candidosis. Clin. Dev. Immunol. 2011;2011:697340. doi: 10.1155/2011/697340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng X.F., Hong Y.X., Feng G.J., Zhang G.F., Rogers H., Lewis M.A., Williams D.W., Xia Z.F., Song B., Wei X.Q. Lipopolysaccharide-induced M2 to M1 macrophage transformation for IL-12p70 production is blocked by Candida albicans mediated up-regulation of EBI3 expression. PloS One. 2013;8(5):e63967. doi: 10.1371/journal.pone.0063967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Y. Wu, Z. Tan, Z. Liu, D. Xia, J. Li, Local IL-23 expression in murine vaginal candidiasis and its relationship with infection and immune status, Journal of Huazhong University of Science and Technology. Medical sciences = Hua zhong ke ji da xue xue bao. Yi xue Ying De wen ban = Huazhong keji daxue xuebao. Yixue Yingdewen ban 26(2) (2006) 245–247. [DOI] [PubMed]
- 25.Rivera A., Hohl T.M., Collins N., Leiner I., Gallegos A., Saijo S., Coward J.W., Iwakura Y., Pamer E.G. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J. Exp. Med. 2011;208(2):369–381. doi: 10.1084/jem.20100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farah C.S., Hu Y., Riminton S., Ashman R.B. Distinct roles for interleukin-12p40 and tumour necrosis factor in resistance to oral candidiasis defined by gene-targeting. Oral Microbiol. Immunol. 2006;21(4):252–255. doi: 10.1111/j.1399-302X.2006.00288.x. [DOI] [PubMed] [Google Scholar]
- 27.Montagnoli C., Sandini S., Bacci A., Romani L., La Valle R. Immunogenicity and protective effect of recombinant enolase of Candida albicans in a murine model of systemic candidiasis. Med. Mycol. 2004;42(4):319–324. doi: 10.1080/13693780310001644653. [DOI] [PubMed] [Google Scholar]
- 28.Huppler A.R., Conti H.R., Hernandez-Santos N., Darville T., Biswas P.S., Gaffen S.L. Role of neutrophils in IL-17-dependent immunity to mucosal candidiasis. J. Immunol. 2014;192(4):1745–1752. doi: 10.4049/jimmunol.1302265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conti H.R., Shen F., Nayyar N., Stocum E., Sun J.N., Lindemann M.J., Ho A.W., Hai J.H., Yu J.J., Jung J.W., Filler S.G., Masso-Welch P., Edgerton M., Gaffen S.L. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009;206(2):299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patin E.C., Jones A.V., Thompson A., Clement M., Liao C.T., Griffiths J.S., Wallace L.E., Bryant C.E., Lang R., Rosenstiel P., Humphreys I.R., Taylor P.R., Jones G.W., Orr S.J. IL-27 Induced by Select Candida spp. via TLR7/NOD2 Signaling and IFN-beta Production Inhibits Fungal Clearance. J. Immunol. 2016;197(1):208–221. doi: 10.4049/jimmunol.1501204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patin E.C., Thompson A., Orr S.J. Pattern recognition receptors in fungal immunity. Semin Cell Dev Biol. 2018 doi: 10.1016/j.semcdb.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells C.A., Salvage-Jones J.A., Li X., Hitchens K., Butcher S., Murray R.Z., Beckhouse A.G., Lo Y.L., Manzanero S., Cobbold C., Schroder K., Ma B., Orr S., Stewart L., Lebus D., Sobieszczuk P., Hume D.A., Stow J., Blanchard H., Ashman R.B. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J. Immunol. 2008;180(11):7404–7413. doi: 10.4049/jimmunol.180.11.7404. [DOI] [PubMed] [Google Scholar]
- 33.Robinson M.J., Osorio F., Rosas M., Freitas R.P., Schweighoffer E., Gross O., Verbeek J.S., Ruland J., Tybulewicz V., Brown G.D., Moita L.F., Taylor P.R., Reis e Sousa C. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J. Exp. Med. 2009;206(9):2037–2051. doi: 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LeibundGut-Landmann S., Gross O., Robinson M.J., Osorio F., Slack E.C., Tsoni S.V., Schweighoffer E., Tybulewicz V., Brown G.D., Ruland J. C. Reis e Sousa, Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 2007;8(6):630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 35.Bourgeois C., Majer O., Frohner I.E., Lesiak-Markowicz I., Hildering K.S., Glaser W., Stockinger S., Decker T., Akira S., Muller M., Kuchler K. Conventional dendritic cells mount a type I IFN response against Candida spp. requiring novel phagosomal TLR7-mediated IFN-beta signaling. J. Immunol. 2011;186(5):3104–3112. doi: 10.4049/jimmunol.1002599. [DOI] [PubMed] [Google Scholar]
- 36.Wagener J., Malireddi R.K., Lenardon M.D., Koberle M., Vautier S., MacCallum D.M., Biedermann T., Schaller M., Netea M.G., Kanneganti T.D., Brown G.D., Brown A.J., Gow N.A. Fungal chitin dampens inflammation through IL-10 induction mediated by NOD2 and TLR9 activation. PLoS Pathogens. 2014;10(4):e1004050. doi: 10.1371/journal.ppat.1004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saijo S., Ikeda S., Yamabe K., Kakuta S., Ishigame H., Akitsu A., Fujikado N., Kusaka T., Kubo S., Chung S.H., Komatsu R., Miura N., Adachi Y., Ohno N., Shibuya K., Yamamoto N., Kawakami K., Yamasaki S., Saito T., Akira S., Iwakura Y. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32(5):681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Taylor P.R., Tsoni S.V., Willment J.A., Dennehy K.M., Rosas M., Findon H., Haynes K., Steele C., Botto M., Gordon S., Brown G.D. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol. 2007;8(1):31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balish E., Wagner R.D., Vazquez-Torres A., Pierson C., Warner T. Candidiasis in interferon-gamma knockout (IFN-gamma-/-) mice. J. Infect. Dis. 1998;178(2):478–487. doi: 10.1086/515645. [DOI] [PubMed] [Google Scholar]
- 40.Cenci E., Mencacci A., Del Sero G., d'Ostiani C.F., Mosci P., Bacci A., Montagnoli C., Kopf M., Romani L. IFN-gamma is required for IL-12 responsiveness in mice with Candida albicans infection. J. Immunol. 1998;161(7):3543–3550. [PubMed] [Google Scholar]
- 41.Kaposzta R., Tree P., Marodi L., Gordon S. Characteristics of invasive candidiasis in gamma interferon- and interleukin-4-deficient mice: role of macrophages in host defense against Candida albicans. Infect. Immun. 1998;66(4):1708–1717. doi: 10.1128/iai.66.4.1708-1717.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang W., Na L., Fidel P.L., Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis. 2004;190(3):624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 43.Eyerich K., Foerster S., Rombold S., Seidl H.P., Behrendt H., Hofmann H., Ring J., Traidl-Hoffmann C. Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J. Invest. Dermatol. 2008;128(11):2640–2645. doi: 10.1038/jid.2008.139. [DOI] [PubMed] [Google Scholar]
- 44.Steele C., Rapaka R.R., Metz A., Pop S.M., Williams D.L., Gordon S., Kolls J.K., Brown G.D. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathogens. 2005;1(4):e42. doi: 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carrion Sde J., Leal S.M., Jr., Ghannoum M.A., Aimanianda V., Latge J.P., Pearlman E. The RodA hydrophobin on Aspergillus fumigatus spores masks dectin-1- and dectin-2-dependent responses and enhances fungal survival in vivo. J. Immunol. 2013;191(5):2581–2588. doi: 10.4049/jimmunol.1300748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rivera A., Ro G., Van Epps H.L., Simpson T., Leiner I., Sant'Angelo D.B., Pamer E.G. Innate immune activation and CD4+ T cell priming during respiratory fungal infection. Immunity. 2006;25(4):665–675. doi: 10.1016/j.immuni.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 47.Kreindler J.L., Steele C., Nguyen N., Chan Y.R., Pilewski J.M., Alcorn J.F., Vyas Y.M., Aujla S.J., Finelli P., Blanchard M., Zeigler S.F., Logar A., Hartigan E., Kurs-Lasky M., Rockette H., Ray A., Kolls J.K. Vitamin D3 attenuates Th2 responses to Aspergillus fumigatus mounted by CD4+ T cells from cystic fibrosis patients with allergic bronchopulmonary aspergillosis. J. Clin. Invest. 2010;120(9):3242–3254. doi: 10.1172/JCI42388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murdock B.J., Shreiner A.B., McDonald R.A., Osterholzer J.J., White E.S., Toews G.B., Huffnagle G.B. Coevolution of TH1, TH2, and TH17 responses during repeated pulmonary exposure to Aspergillus fumigatus conidia. Infect. Immun. 2011;79(1):125–135. doi: 10.1128/IAI.00508-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hohl T.M., Van Epps H.L., Rivera A., Morgan L.A., Chen P.L., Feldmesser M., Pamer E.G. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS pathogens. 2005;1(3):e30. doi: 10.1371/journal.ppat.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gersuk G.M., Underhill D.M., Zhu L., Marr K.A. Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J. Immunol. 2006;176(6):3717–3724. doi: 10.4049/jimmunol.176.6.3717. [DOI] [PubMed] [Google Scholar]
- 51.Netea M.G., Warris A., Van der Meer J.W., Fenton M.J., Verver-Janssen T.J., Jacobs L.E., Andresen T., Verweij P.E., Kullberg B.J. Aspergillus fumigatus evades immune recognition during germination through loss of toll-like receptor-4-mediated signal transduction. J. Infect. Dis. 2003;188(2):320–326. doi: 10.1086/376456. [DOI] [PubMed] [Google Scholar]
- 52.Vignali D.A., Kuchroo V.K. IL-12 family cytokines: immunological playmakers. Nat. Immunol. 2012;13(8):722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gee K., Guzzo C., Che Mat N.F., Ma W., Kumar A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm. Allergy Drug Targets. 2009;8(1):40–52. doi: 10.2174/187152809787582507. [DOI] [PubMed] [Google Scholar]
- 54.Hunter C.A. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 2005;5(7):521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 55.Beadling C., Slifka M.K. Regulation of innate and adaptive immune responses by the related cytokines IL-12, IL-23, and IL-27. Arch. Immunol. Therapiae Exp. 2006;54(1):15–24. doi: 10.1007/s00005-006-0002-6. [DOI] [PubMed] [Google Scholar]
- 56.Trinchieri G., Pflanz S., Kastelein R.A. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19(5):641–644. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 57.Sun L., He C., Nair L., Yeung J., Egwuagu C.E. Interleukin 12 (IL-12) family cytokines: Role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine. 2015;75(2):249–255. doi: 10.1016/j.cyto.2015.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gillessen S., Carvajal D., Ling P., Podlaski F.J., Stremlo D.L., Familletti P.C., Gubler U., Presky D.H., Stern A.S., Gately M.K. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur. J. Immunol. 1995;25(1):200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 59.Podlaski F.J., Nanduri V.B., Hulmes J.D., Pan Y.C., Levin W., Danho W., Chizzonite R., Gately M.K., Stern A.S. Molecular characterization of interleukin 12. Arch. Biochem. Biophys. 1992;294(1):230–237. doi: 10.1016/0003-9861(92)90162-p. [DOI] [PubMed] [Google Scholar]
- 60.Ling P., Gately M.K., Gubler U., Stern A.S., Lin P., Hollfelder K., Su C., Pan Y.C., Hakimi J. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J. Immunol. 1995;154(1):116–127. [PubMed] [Google Scholar]
- 61.Piccotti J.R., Chan S.Y., Goodman R.E., Magram J., Eichwald E.J., Bishop D.K. IL-12 antagonism induces T helper 2 responses, yet exacerbates cardiac allograft rejection. Evidence against a dominant protective role for T helper 2 cytokines in alloimmunity. J. Immunol. 1996;157(5):1951–1957. [PubMed] [Google Scholar]
- 62.Devergne O., Hummel M., Koeppen H., Le Beau M.M., Nathanson E.C., Kieff E., Birkenbach M. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J. Virol. 1996;70(2):1143–1153. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimozato O., Sato A., Kawamura K., Chiyo M., Ma G., Li Q., Tagawa M. The secreted form of p28 subunit of interleukin (IL)-27 inhibits biological functions of IL-27 and suppresses anti-allogeneic immune responses. Immunology. 2009;128(1 Suppl):e816–e825. doi: 10.1111/j.1365-2567.2009.03088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stumhofer J.S., Tait E.D., Quinn W.J., 3rd, Hosken N., Spudy B., Goenka R., Fielding C.A., O'Hara A.C., Chen Y., Jones M.L., Saris C.J., Rose-John S., Cua D.J., Jones S.A., Elloso M.M., Grotzinger J., Cancro M.P., Levin S.D., Hunter C.A. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat. Immunol. 2010;11(12):1119–1126. doi: 10.1038/ni.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goriely S., Goldman M. Interleukin-12 family members and the balance between rejection and tolerance. Curr. Opin. Organ Transplant. 2008;13(1):4–9. doi: 10.1097/MOT.0b013e3282f406c4. [DOI] [PubMed] [Google Scholar]
- 66.Awasthi A., Carrier Y., Peron J.P., Bettelli E., Kamanaka M., Flavell R.A., Kuchroo V.K., Oukka M., Weiner H.L. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 2007;8(12):1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 67.Yamanaka A., Hamano S., Miyazaki Y., Ishii K., Takeda A., Mak T.W., Himeno K., Yoshimura A., Yoshida H. Hyperproduction of proinflammatory cytokines by WSX-1-deficient NKT cells in concanavalin A-induced hepatitis. J. Immunol. 2004;172(6):3590–3596. doi: 10.4049/jimmunol.172.6.3590. [DOI] [PubMed] [Google Scholar]
- 68.Devergne O., Birkenbach M., Kieff E. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. PNAS. 1997;94(22):12041–12046. doi: 10.1073/pnas.94.22.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Niedbala W., Wei X.Q., Cai B., Hueber A.J., Leung B.P., McInnes I.B., Liew F.Y. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur. J. Immunol. 2007;37(11):3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 70.Collison L.W., Chaturvedi V., Henderson A.L., Giacomin P.R., Guy C., Bankoti J., Finkelstein D., Forbes K., Workman C.J., Brown S.A., Rehg J.E., Jones M.L., Ni H.T., Artis D., Turk M.J., Vignali D.A. IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 2010;11(12):1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Collison L.W., Workman C.J., Kuo T.T., Boyd K., Wang Y., Vignali K.M., Cross R., Sehy D., Blumberg R.S., Vignali D.A. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450(7169):566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 72.Bardel E., Larousserie F., Charlot-Rabiega P., Coulomb-L'Hermine A., Devergne O. Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J. Immunol. 2008;181(10):6898–6905. doi: 10.4049/jimmunol.181.10.6898. [DOI] [PubMed] [Google Scholar]
- 73.Gollob J.A., Murphy E.A., Mahajan S., Schnipper C.P., Ritz J., Frank D.A. Altered interleukin-12 responsiveness in Th1 and Th2 cells is associated with the differential activation of STAT5 and STAT1. Blood. 1998;91(4):1341–1354. [PubMed] [Google Scholar]
- 74.Ozbek N., Fieschi C., Yilmaz B.T., de Beaucoudrey L., Demirhan B., Feinberg J., Bikmaz Y.E., Casanova J.L. Interleukin-12 receptor beta 1 chain deficiency in a child with disseminated tuberculosis. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2005;40(6):e55–e58. doi: 10.1086/427879. [DOI] [PubMed] [Google Scholar]
- 75.Thierfelder W.E., van Deursen J.M., Yamamoto K., Tripp R.A., Sarawar S.R., Carson R.T., Sangster M.Y., Vignali D.A., Doherty P.C., Grosveld G.C., Ihle J.N. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382(6587):171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 76.Shimoda K., Kato K., Aoki K., Matsuda T., Miyamoto A., Shibamori M., Yamashita M., Numata A., Takase K., Kobayashi S., Shibata S., Asano Y., Gondo H., Sekiguchi K., Nakayama K., Nakayama T., Okamura T., Okamura S., Niho Y., Nakayama K. Tyk2 plays a restricted role in IFN alpha signaling, although it is required for IL-12-mediated T cell function. Immunity. 2000;13(4):561–571. doi: 10.1016/s1074-7613(00)00055-8. [DOI] [PubMed] [Google Scholar]
- 77.Shimoda K., Tsutsui H., Aoki K., Kato K., Matsuda T., Numata A., Takase K., Yamamoto T., Nukina H., Hoshino T., Asano Y., Gondo H., Okamura T., Okamura S., Nakayama K., Nakanishi K., Niho Y., Harada M. Partial impairment of interleukin-12 (IL-12) and IL-18 signaling in Tyk2-deficient mice. Blood. 2002;99(6):2094–2099. doi: 10.1182/blood.v99.6.2094. [DOI] [PubMed] [Google Scholar]
- 78.Oppmann B., Lesley R., Blom B., Timans J.C., Xu Y., Hunte B., Vega F., Yu N., Wang J., Singh K., Zonin F., Vaisberg E., Churakova T., Liu M., Gorman D., Wagner J., Zurawski S., Liu Y., Abrams J.S., Moore K.W., Rennick D., de Waal-Malefyt R., Hannum C., Bazan J.F., Kastelein R.A. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13(5):715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 79.Parham C., Chirica M., Timans J., Vaisberg E., Travis M., Cheung J., Pflanz S., Zhang R., Singh K.P., Vega F., To W., Wagner J., O'Farrell A.M., McClanahan T., Zurawski S., Hannum C., Gorman D., Rennick D.M., Kastelein R.A., de Waal Malefyt R., Moore K.W. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 2002;168(11):5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 80.Aggarwal S., Ghilardi N., Xie M.H., de Sauvage F.J., Gurney A.L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003;278(3):1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 81.Yang X.O., Panopoulos A.D., Nurieva R., Chang S.H., Wang D., Watowich S.S., Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007;282(13):9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 82.Mathur A.N., Chang H.C., Zisoulis D.G., Stritesky G.L., Yu Q., O'Malley J.T., Kapur R., Levy D.E., Kansas G.S., Kaplan M.H. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J. Immunol. 2007;178(8):4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 83.Zou J., Presky D.H., Wu C.Y., Gubler U. Differential associations between the cytoplasmic regions of the interleukin-12 receptor subunits beta1 and beta2 and JAK kinases. J. Biol. Chem. 1997;272(9):6073–6077. doi: 10.1074/jbc.272.9.6073. [DOI] [PubMed] [Google Scholar]
- 84.Pflanz S., Hibbert L., Mattson J., Rosales R., Vaisberg E., Bazan J.F., Phillips J.H., McClanahan T.K., de Waal Malefyt R., Kastelein R.A. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 2004;172(4):2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 85.Yoshimura T., Takeda A., Hamano S., Miyazaki Y., Kinjyo I., Ishibashi T., Yoshimura A., Yoshida H. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J. Immunol. 2006;177(8):5377–5385. doi: 10.4049/jimmunol.177.8.5377. [DOI] [PubMed] [Google Scholar]
- 86.Collison L.W., Delgoffe G.M., Guy C.S., Vignali K.M., Chaturvedi V., Fairweather D., Satoskar A.R., Garcia K.C., Hunter C.A., Drake C.G., Murray P.J., Vignali D.A. The composition and signaling of the IL-35 receptor are unconventional. Nat. Immunol. 2012;13(3):290–299. doi: 10.1038/ni.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lederer J.A., Perez V.L., DesRoches L., Kim S.M., Abbas A.K., Lichtman A.H. Cytokine transcriptional events during helper T cell subset differentiation. J. Exp. Med. 1996;184(2):397–406. doi: 10.1084/jem.184.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reiner S.L., Zheng S., Wang Z.E., Stowring L., Locksley R.M. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J. Exp. Med. 1994;179(2):447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cooper A.M., Magram J., Ferrante J., Orme I.M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J. Exp. Med. 1997;186(1):39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Muller U., Kohler G., Mossmann H., Schaub G.A., Alber G., Di Santo J.P., Brombacher F., Holscher C. IL-12-independent IFN-gamma production by T cells in experimental Chagas' disease is mediated by IL-18. J. Immunol. 2001;167(6):3346–3353. doi: 10.4049/jimmunol.167.6.3346. [DOI] [PubMed] [Google Scholar]
- 91.Mattner F., Magram J., Ferrante J., Launois P., Di Padova K., Behin R., Gately M.K., Louis J.A., Alber G. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur. J. Immunol. 1996;26(7):1553–1559. doi: 10.1002/eji.1830260722. [DOI] [PubMed] [Google Scholar]
- 92.Altare F., Durandy A., Lammas D., Emile J.F., Lamhamedi S., Le Deist F., Drysdale P., Jouanguy E., Doffinger R., Bernaudin F., Jeppsson O., Gollob J.A., Meinl E., Segal A.W., Fischer A., Kumararatne D., Casanova J.L. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280(5368):1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 93.Ashman R.B., Vijayan D., Wells C.A. IL-12 and related cytokines: function and regulatory implications in Candida albicans infection. Clin. Dev. Immunol. 2011;2011:686597. doi: 10.1155/2011/686597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mencacci A., Cenci E., Del Sero G., Fe d'Ostiani C., Mosci P., Trinchieri G., Adorini L., Romani L. IL-10 is required for development of protective Th1 responses in IL-12-deficient mice upon Candida albicans infection. J. Immunol. 1998;161(11):6228–6237. [PubMed] [Google Scholar]
- 95.Pietrella D., Lupo P., Bistoni F., Vecchiarelli A. An early imbalance of interleukin 12 influences the adjuvant effect of mannoproteins of Cryptococcus neoformans. Cell. Microbiol. 2004;6(9):883–891. doi: 10.1111/j.1462-5822.2004.00411.x. [DOI] [PubMed] [Google Scholar]
- 96.Lavigne L.M., Schopf L.R., Chung C.L., Maylor R., Sypek J.P. The role of recombinant murine IL-12 and IFN-gamma in the pathogenesis of a murine systemic Candida albicans infection. J. Immunol. 1998;160(1):284–292. [PubMed] [Google Scholar]
- 97.Roilides E., Tsaparidou S., Kadiltsoglou I., Sein T., Walsh T.J. Interleukin-12 enhances antifungal activity of human mononuclear phagocytes against Aspergillus fumigatus: implications for a gamma interferon-independent pathway. Infect. Immun. 1999;67(6):3047–3050. doi: 10.1128/iai.67.6.3047-3050.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cenci E., Mencacci A., Fe d'Ostiani C., Del Sero G., Mosci P., Montagnoli C., Bacci A., Romani L. Cytokine- and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. J. Infect. Dis. 1998;178(6):1750–1760. doi: 10.1086/314493. [DOI] [PubMed] [Google Scholar]
- 99.Cenci E., Mencacci A., Del Sero G., Bacci A., Montagnoli C., d'Ostiani C.F., Mosci P., Bachmann M., Bistoni F., Kopf M., Romani L. Interleukin-4 causes susceptibility to invasive pulmonary aspergillosis through suppression of protective type I responses. J. Infect. Dis. 1999;180(6):1957–1968. doi: 10.1086/315142. [DOI] [PubMed] [Google Scholar]
- 100.Nagai H., Guo J., Choi H., Kurup V. Interferon-gamma and tumor necrosis factor-alpha protect mice from invasive aspergillosis. J. Infect. Dis. 1995;172(6):1554–1560. doi: 10.1093/infdis/172.6.1554. [DOI] [PubMed] [Google Scholar]
- 101.Delsing C.E., Gresnigt M.S., Leentjens J., Preijers F., Frager F.A., Kox M., Monneret G., Venet F., Bleeker-Rovers C.P., van de Veerdonk F.L., Pickkers P., Pachot A., Kullberg B.J., Netea M.G. Interferon-gamma as adjunctive immunotherapy for invasive fungal infections: a case series. BMC Infect. Dis. 2014;14:166. doi: 10.1186/1471-2334-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stritesky G.L., Yeh N., Kaplan M.H. IL-23 promotes maintenance but not commitment to the Th17 lineage. J. Immunol. 2008;181(9):5948–5955. doi: 10.4049/jimmunol.181.9.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Happel K.I., Zheng M., Young E., Quinton L.J., Lockhart E., Ramsay A.J., Shellito J.E., Schurr J.R., Bagby G.J., Nelson S., Kolls J.K. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J. Immunol. 2003;170(9):4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lilly L.M., Gessner M.A., Dunaway C.W., Metz A.E., Schwiebert L., Weaver C.T., Brown G.D., Steele C. The beta-glucan receptor dectin-1 promotes lung immunopathology during fungal allergy via IL-22. J. Immunol. 2012;189(7):3653–3660. doi: 10.4049/jimmunol.1201797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kreymborg K., Etzensperger R., Dumoutier L., Haak S., Rebollo A., Buch T., Heppner F.L., Renauld J.C., Becher B. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J. Immunol. 2007;179(12):8098–8104. doi: 10.4049/jimmunol.179.12.8098. [DOI] [PubMed] [Google Scholar]
- 106.Yang X.O., Pappu B.P., Nurieva R., Akimzhanov A., Kang H.S., Chung Y., Ma L., Shah B., Panopoulos A.D., Schluns K.S., Watowich S.S., Tian Q., Jetten A.M., Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Langrish C.L., Chen Y., Blumenschein W.M., Mattson J., Basham B., Sedgwick J.D., McClanahan T., Kastelein R.A., Cua D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen Y., Langrish C.L., McKenzie B., Joyce-Shaikh B., Stumhofer J.S., McClanahan T., Blumenschein W., Churakovsa T., Low J., Presta L., Hunter C.A., Kastelein R.A., Cua D.J. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J. Clin. Invest. 2006;116(5):1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Takatori H., Kanno Y., Watford W.T., Tato C.M., Weiss G. Ivanov, II, D.R. Littman, J.J. O'Shea, Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 2009;206(1):35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Acosta-Rodriguez E.V., Rivino L., Geginat J., Jarrossay D., Gattorno M., Lanzavecchia A., Sallusto F., Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007;8(6):639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 111.Yano J., Kolls J.K., Happel K.I., Wormley F., Wozniak K.L., Fidel P.L., Jr. The acute neutrophil response mediated by S100 alarmins during vaginal Candida infections is independent of the Th17-pathway. PloS one. 2012;7(9):e46311. doi: 10.1371/journal.pone.0046311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kagami S., Rizzo H.L., Kurtz S.E., Miller L.S., Blauvelt A. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J. Immunol. 2010;185(9):5453–5462. doi: 10.4049/jimmunol.1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Milner J.D., Brenchley J.M., Laurence A., Freeman A.F., Hill B.J., Elias K.M., Kanno Y., Spalding C., Elloumi H.Z., Paulson M.L., Davis J., Hsu A., Asher A.I., O'Shea J., Holland S.M., Paul W.E., Douek D.C. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452(7188):773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Conti H.R., Baker O., Freeman A.F., Jang W.S., Holland S.M., Li R.A., Edgerton M., Gaffen S.L. New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome. Mucosal Immunol. 2011;4(4):448–455. doi: 10.1038/mi.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Puel A., Cypowyj S., Bustamante J., Wright J.F., Liu L., Lim H.K., Migaud M., Israel L., Chrabieh M., Audry M., Gumbleton M., Toulon A., Bodemer C., El-Baghdadi J., Whitters M., Paradis T., Brooks J., Collins M., Wolfman N.M., Al-Muhsen S., Galicchio M., Abel L., Picard C., Casanova J.L. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332(6025):65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boisson B., Wang C., Pedergnana V., Wu L., Cypowyj S., Rybojad M., Belkadi A., Picard C., Abel L., Fieschi C., Puel A., Li X., Casanova J.L. An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity. 2013;39(4):676–686. doi: 10.1016/j.immuni.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van de Veerdonk F.L., Plantinga T.S., Hoischen A., Smeekens S.P., Joosten L.A., Gilissen C., Arts P., Rosentul D.C., Carmichael A.J., Smits-van der Graaf C.A., Kullberg B.J., van der Meer J.W., Lilic D., Veltman J.A., Netea M.G. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. New Engl. J. Med. 2011;365(1):54–61. doi: 10.1056/NEJMoa1100102. [DOI] [PubMed] [Google Scholar]
- 118.Smeekens S.P., Plantinga T.S., van de Veerdonk F.L., Heinhuis B., Hoischen A., Joosten L.A., Arkwright P.D., Gennery A., Kullberg B.J., Veltman J.A., Lilic D., van der Meer J.W., Netea M.G. STAT1 hyperphosphorylation and defective IL12R/IL23R signaling underlie defective immunity in autosomal dominant chronic mucocutaneous candidiasis. PloS one. 2011;6(12):e29248. doi: 10.1371/journal.pone.0029248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kisand K., Boe Wolff A.S., Podkrajsek K.T., Tserel L., Link M., Kisand K.V., Ersvaer E., Perheentupa J., Erichsen M.M., Bratanic N., Meloni A., Cetani F., Perniola R., Ergun-Longmire B., Maclaren N., Krohn K.J., Pura M., Schalke B., Strobel P., Leite M.I., Battelino T., Husebye E.S., Peterson P., Willcox N., Meager A. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J. Exp. Med. 2010;207(2):299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Puel A., Doffinger R., Natividad A., Chrabieh M., Barcenas-Morales G., Picard C., Cobat A., Ouachee-Chardin M., Toulon A., Bustamante J., Al-Muhsen S., Al-Owain M., Arkwright P.D., Costigan C., McConnell V., Cant A.J., Abinun M., Polak M., Bougneres P.F., Kumararatne D., Marodi L., Nahum A., Roifman C., Blanche S., Fischer A., Bodemer C., Abel L., Lilic D., Casanova J.L. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 2010;207(2):291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hambleton S., Salem S., Bustamante J., Bigley V., Boisson-Dupuis S., Azevedo J., Fortin A., Haniffa M., Ceron-Gutierrez L., Bacon C.M., Menon G., Trouillet C., McDonald D., Carey P., Ginhoux F., Alsina L., Zumwalt T.J., Kong X.F., Kumararatne D., Butler K., Hubeau M., Feinberg J., Al-Muhsen S., Cant A., Abel L., Chaussabel D., Doffinger R., Talesnik E., Grumach A., Duarte A., Abarca K., Moraes-Vasconcelos D., Burk D., Berghuis A., Geissmann F., Collin M., Casanova J.L., Gros P. IRF8 mutations and human dendritic-cell immunodeficiency. New Engl. J. Med. 2011;365(2):127–138. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Glocker E.O., Hennigs A., Nabavi M., Schaffer A.A., Woellner C., Salzer U., Pfeifer D., Veelken H., Warnatz K., Tahami F., Jamal S., Manguiat A., Rezaei N., Amirzargar A.A., Plebani A., Hannesschlager N., Gross O., Ruland J., Grimbacher B. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. New Engl. J. Med. 2009;361(18):1727–1735. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Drewniak A., Gazendam R.P., Tool A.T., van Houdt M., Jansen M.H., van Hamme J.L., van Leeuwen E.M., Roos D., Scalais E., de Beaufort C., Janssen H., van den Berg T.K., Kuijpers T.W. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood. 2013;121(13):2385–2392. doi: 10.1182/blood-2012-08-450551. [DOI] [PubMed] [Google Scholar]
- 124.Ferwerda B., Ferwerda G., Plantinga T.S., Willment J.A., van Spriel A.B., Venselaar H., Elbers C.C., Johnson M.D., Cambi A., Huysamen C., Jacobs L., Jansen T., Verheijen K., Masthoff L., Morre S.A., Vriend G., Williams D.L., Perfect J.R., Joosten L.A., Wijmenga C., van der Meer J.W., Adema G.J., Kullberg B.J., Brown G.D., Netea M.G. Human dectin-1 deficiency and mucocutaneous fungal infections. New Engl. J. Med. 2009;361(18):1760–1767. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pedraza-Sanchez S., Herrera-Barrios M.T., Aldana-Vergara R., Neumann-Ordonez M., Gonzalez-Hernandez Y., Sada-Diaz E., de Beaucoudrey L., Casanova J.L., Torres-Rojas M. Bacille Calmette-Guerin infection and disease with fatal outcome associated with a point mutation in the interleukin-12/interleukin-23 receptor beta-1 chain in two Mexican families. Int. J. Infect. Dis.: IJID: Off. Publ. Int. Soc. Infect. Dis. 2010;14(Suppl 3):e256–e260. doi: 10.1016/j.ijid.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 126.de Beaucoudrey L., Samarina A., Bustamante J., Cobat A., Boisson-Dupuis S., Feinberg J., Al-Muhsen S., Janniere L., Rose Y., de Suremain M., Kong X.F., Filipe-Santos O., Chapgier A., Picard C., Fischer A., Dogu F., Ikinciogullari A., Tanir G., Al-Hajjar S., Al-Jumaah S., Frayha H.H., AlSum Z., Al-Ajaji S., Alangari A., Al-Ghonaium A., Adimi P., Mansouri D., Ben-Mustapha I., Yancoski J., Garty B.Z., Rodriguez-Gallego C., Caragol I., Kutukculer N., Kumararatne D.S., Patel S., Doffinger R., Exley A., Jeppsson O., Reichenbach J., Nadal D., Boyko Y., Pietrucha B., Anderson S., Levin M., Schandene L., Schepers K., Efira A., Mascart F., Matsuoka M., Sakai T., Siegrist C.A., Frecerova K., Bluetters-Sawatzki R., Bernhoft J., Freihorst J., Baumann U., Richter D., Haerynck F., De Baets F., Novelli V., Lammas D., Vermylen C., Tuerlinckx D., Nieuwhof C., Pac M., Haas W.H., Muller-Fleckenstein I., Fleckenstein B., Levy J., Raj R., Cohen A.C., Lewis D.B., Holland S.M., Yang K.D., Wang X., Wang X., Jiang L., Yang X., Zhu C., Xie Y., Lee P.P., Chan K.W., Chen T.X., Castro G., Natera I., Codoceo A., King A., Bezrodnik L., Di Giovani D., Gaillard M.I., de Moraes-Vasconcelos D., Grumach A.S., da Silva Duarte A.J., Aldana R., Espinosa-Rosales F.J., Bejaoui M., Bousfiha A.A., Baghdadi J.E., Ozbek N., Aksu G., Keser M., Somer A., Hatipoglu N., Aydogmus C., Asilsoy S., Camcioglu Y., Gulle S., Ozgur T.T., Ozen M., Oleastro M., Bernasconi A., Mamishi S., Parvaneh N., Rosenzweig S., Barbouche R., Pedraza S., Lau Y.L., Ehlayel M.S., Fieschi C., Abel L., Sanal O., Casanova J.L. Revisiting human IL-12Rbeta1 deficiency: a survey of 141 patients from 30 countries. Medicine. 2010;89(6):381–402. doi: 10.1097/MD.0b013e3181fdd832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lev-Sagie A., Nyirjesy P., Tarangelo N., Bongiovanni A.M., Bayer C., Linhares I.M., Giraldo P.C., Ledger W.J., Witkin S.S. Hyaluronan in vaginal secretions: association with recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 2009;201(2):206 e1-5. doi: 10.1016/j.ajog.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 128.Zhou M., Yang B., Ma R., Wu C. Memory Th-17 cells specific for C. albicans are persistent in human peripheral blood. Immunol. Lett. 2008;118(1):72–81. doi: 10.1016/j.imlet.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 129.Huber S., Gagliani N., Esplugues E., O'Connor W., Jr., Huber F.J., Chaudhry A., Kamanaka M., Kobayashi Y., Booth C.J., Rudensky A.Y., Roncarolo M.G., Battaglia M., Flavell R.A. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(-) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34(4):554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Romani L., Fallarino F., De Luca A., Montagnoli C., D'Angelo C., Zelante T., Vacca C., Bistoni F., Fioretti M.C., Grohmann U., Segal B.H., Puccetti P. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451(7175):211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 131.Werner J.L., Metz A.E., Horn D., Schoeb T.R., Hewitt M.M., Schwiebert L.M., Faro-Trindade I., Brown G.D., Steele C. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J. Immunol. 2009;182(8):4938–4946. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ramaprakash H., Ito T., Standiford T.J., Kunkel S.L., Hogaboam C.M. Toll-like receptor 9 modulates immune responses to Aspergillus fumigatus conidia in immunodeficient and allergic mice. Infect. Immun. 2009;77(1):108–119. doi: 10.1128/IAI.00998-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zelante T., De Luca A., D'Angelo C., Moretti S., Romani L. IL-17/Th17 in anti-fungal immunity: what's new? Eur. J. Immunol. 2009;39(3):645–648. doi: 10.1002/eji.200839102. [DOI] [PubMed] [Google Scholar]
- 134.Bonifazi P., Zelante T., D'Angelo C., De Luca A., Moretti S., Bozza S., Perruccio K., Iannitti R.G., Giovannini G., Volpi C., Fallarino F., Puccetti P., Romani L. Balancing inflammation and tolerance in vivo through dendritic cells by the commensal Candida albicans. Mucosal Immunol. 2009;2(4):362–374. doi: 10.1038/mi.2009.17. [DOI] [PubMed] [Google Scholar]
- 135.De Luca A., Montagnoli C., Zelante T., Bonifazi P., Bozza S., Moretti S., D'Angelo C., Vacca C., Boon L., Bistoni F., Puccetti P., Fallarino F., Romani L. Functional yet balanced reactivity to Candida albicans requires TRIF, MyD88, and IDO-dependent inhibition of Rorc. J. Immunol. 2007;179(9):5999–6008. doi: 10.4049/jimmunol.179.9.5999. [DOI] [PubMed] [Google Scholar]
- 136.Yoshida H., Hunter C.A. The immunobiology of interleukin-27. Ann. Rev. Immunol. 2015;33:417–443. doi: 10.1146/annurev-immunol-032414-112134. [DOI] [PubMed] [Google Scholar]
- 137.Hunter C.A., Kastelein R. Interleukin-27: balancing protective and pathological immunity. Immunity. 2012;37(6):960–969. doi: 10.1016/j.immuni.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Owaki T., Asakawa M., Morishima N., Hata K., Fukai F., Matsui M., Mizuguchi J., Yoshimoto T. A role for IL-27 in early regulation of Th1 differentiation. J. Immunol. 2005;175(4):2191–2200. doi: 10.4049/jimmunol.175.4.2191. [DOI] [PubMed] [Google Scholar]
- 139.Fitzgerald D.C., Zhang G.X., El-Behi M., Fonseca-Kelly Z., Li H., Yu S., Saris C.J., Gran B., Ciric B., Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat. Immunol. 2007;8(12):1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 140.Stumhofer J.S., Silver J.S., Laurence A., Porrett P.M., Harris T.H., Turka L.A., Ernst M., Saris C.J., O'Shea J.J., Hunter C.A. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 2007;8(12):1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 141.Murugaiyan G., Mittal A., Lopez-Diego R., Maier L.M., Anderson D.E., Weiner H.L. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J. Immunol. 2009;183(4):2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Batten M., Kljavin N.M., Li J., Walter M.J., de Sauvage F.J., Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J. Immunol. 2008;180(5):2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 143.Clement M., Marsden M., Stacey M.A., Abdul-Karim J., Gimeno Brias S., Costa Bento D., Scurr M.J., Ghazal P., Weaver C.T., Carlesso G., Clare S., Jones S.A., Godkin A., Jones G.W., Humphreys I.R. Cytomegalovirus-specific IL-10-producing CD4+ T cells are governed by type-I IFN-induced IL-27 and promote virus persistence. PLoS Pathogens. 2016;12(12):e1006050. doi: 10.1371/journal.ppat.1006050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Peters A., Pitcher L.A., Sullivan J.M., Mitsdoerffer M., Acton S.E., Franz B., Wucherpfennig K., Turley S., Carroll M.C., Sobel R.A., Bettelli E., Kuchroo V.K. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 2011;35(6):986–996. doi: 10.1016/j.immuni.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jones G.W., Bombardieri M., Greenhill C.J., McLeod L., Nerviani A., Rocher-Ros V., Cardus A., Williams A.S., Pitzalis C., Jenkins B.J., Jones S.A. Interleukin-27 inhibits ectopic lymphoid-like structure development in early inflammatory arthritis. J. Exp. Med. 2015;212(11):1793–1802. doi: 10.1084/jem.20132307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hamano S., Himeno K., Miyazaki Y., Ishii K., Yamanaka A., Takeda A., Zhang M., Hisaeda H., Mak T.W., Yoshimura A., Yoshida H. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19(5):657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 147.Zahn S., Wirtz S., Birkenbach M., Blumberg R.S., Neurath M.F., von Stebut E. Impaired Th1 responses in mice deficient in Epstein-Barr virus-induced gene 3 and challenged with physiological doses of Leishmania major. Eur. J. Immunol. 2005;35(4):1106–1112. doi: 10.1002/eji.200425926. [DOI] [PubMed] [Google Scholar]
- 148.Yoshimoto T., Yoshimoto T., Yasuda K., Mizuguchi J., Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J. Immunol. 2007;179(7):4415–4423. doi: 10.4049/jimmunol.179.7.4415. [DOI] [PubMed] [Google Scholar]
- 149.Yoshida H., Hamano S., Senaldi G., Covey T., Faggioni R., Mu S., Xia M., Wakeham A.C., Nishina H., Potter J., Saris C.J., Mak T.W. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15(4):569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 150.Villarino A.V., Stumhofer J.S., Saris C.J., Kastelein R.A., de Sauvage F.J., Hunter C.A. IL-27 limits IL-2 production during Th1 differentiation. J. Immunol. 2006;176(1):237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 151.Dixon K.O., van der Kooij S.W., Vignali D.A., van Kooten C. Human tolerogenic dendritic cells produce IL-35 in the absence of other IL-12 family members. Eur. J. Immunol. 2015;45(6):1736–1747. doi: 10.1002/eji.201445217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gafa V., Remoli M.E., Giacomini E., Severa M., Grillot R., Coccia E.M. Enhancement of anti-Aspergillus T helper type 1 response by interferon-beta-conditioned dendritic cells. Immunology. 2010;131(2):282–288. doi: 10.1111/j.1365-2567.2010.03302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Molle C., Nguyen M., Flamand V., Renneson J., Trottein F., De Wit D., Willems F., Goldman M., Goriely S. IL-27 synthesis induced by TLR ligation critically depends on IFN regulatory factor 3. J. Immunol. 2007;178(12):7607–7615. doi: 10.4049/jimmunol.178.12.7607. [DOI] [PubMed] [Google Scholar]
- 154.Pacciani V., Gregori S., Chini L., Corrente S., Chianca M., Moschese V., Rossi P., Roncarolo M.G., Angelini F. Induction of anergic allergen-specific suppressor T cells using tolerogenic dendritic cells derived from children with allergies to house dust mites. J. Allergy Clin. Immunol. 2010;125(3):727–736. doi: 10.1016/j.jaci.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 155.Duan W., So T., Mehta A.K., Choi H., Croft M. Inducible CD4+LAP+Foxp3- regulatory T cells suppress allergic inflammation. J. Immunol. 2011;187(12):6499–6507. doi: 10.4049/jimmunol.1101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bedke T., Iannitti R.G., De Luca A., Giovannini G., Fallarino F., Berges C., Latge J.P., Einsele H., Romani L., Topp M.S. Distinct and complementary roles for Aspergillus fumigatus-specific Tr1 and Foxp3+ regulatory T cells in humans and mice. Immunol. Cell Biol. 2014;92(8):659–670. doi: 10.1038/icb.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Meiler F., Zumkehr J., Klunker S., Ruckert B., Akdis C.A., Akdis M. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J. Exp. Med. 2008;205(12):2887–2898. doi: 10.1084/jem.20080193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Apetoh L., Quintana F.J., Pot C., Joller N., Xiao S., Kumar D., Burns E.J., Sherr D.H., Weiner H.L., Kuchroo V.K. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 2010;11(9):854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pot C., Jin H., Awasthi A., Liu S.M., Lai C.Y., Madan R., Sharpe A.H., Karp C.L., Miaw S.C., Ho I.C., Kuchroo V.K. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J. Immunol. 2009;183(2):797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xu X., Yasuda M., Mizuno M., Ashida H. beta-Glucan from Saccharomyces cerevisiae reduces lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages. Biochim. et Biophys. Acta. 2012;1820(10):1656–1663. doi: 10.1016/j.bbagen.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 161.Pirhonen J., Siren J., Julkunen I., Matikainen S. IFN-alpha regulates Toll-like receptor-mediated IL-27 gene expression in human macrophages. J. Leukocyte Biol. 2007;82(5):1185–1192. doi: 10.1189/jlb.0307157. [DOI] [PubMed] [Google Scholar]
- 162.Wirtz S., Becker C., Fantini M.C., Nieuwenhuis E.E., Tubbe I., Galle P.R., Schild H.J., Birkenbach M., Blumberg R.S., Neurath M.F. EBV-induced gene 3 transcription is induced by TLR signaling in primary dendritic cells via NF-kappa B activation. J. Immunol. 2005;174(5):2814–2824. doi: 10.4049/jimmunol.174.5.2814. [DOI] [PubMed] [Google Scholar]
- 163.Remoli M.E., Gafa V., Giacomini E., Severa M., Lande R., Coccia E.M. IFN-beta modulates the response to TLR stimulation in human DC: involvement of IFN regulatory factor-1 (IRF-1) in IL-27 gene expression. Eur. J. Immunol. 2007;37(12):3499–3508. doi: 10.1002/eji.200737566. [DOI] [PubMed] [Google Scholar]
- 164.Findlay E.G., Greig R., Stumhofer J.S., Hafalla J.C., de Souza J.B., Saris C.J., Hunter C.A., Riley E.M., Couper K.N. Essential role for IL-27 receptor signaling in prevention of Th1-mediated immunopathology during malaria infection. J. Immunol. 2010;185(4):2482–2492. doi: 10.4049/jimmunol.0904019. [DOI] [PubMed] [Google Scholar]
- 165.Depner M., Fuchs S., Raabe J., Frede N., Glocker C., Doffinger R., Gkrania-Klotsas E., Kumararatne D., Atkinson T.P., Schroeder H.W., Jr., Niehues T., Duckers G., Stray-Pedersen A., Baumann U., Schmidt R., Franco J.L., Orrego J., Ben-Shoshan M., McCusker C., Jacob C.M., Carneiro-Sampaio M., Devlin L.A., Edgar J.D., Henderson P., Russell R.K., Skytte A.B., Seneviratne S.L., Wanders J., Stauss H., Meyts I., Moens L., Jesenak M., Kobbe R., Borte S., Borte M., Wright D.A., Hagin D., Torgerson T.R., Grimbacher B. The extended clinical phenotype of 26 patients with chronic mucocutaneous candidiasis due to gain-of-function mutations in STAT1. J. Clin. Immunol. 2016;36(1):73–84. doi: 10.1007/s10875-015-0214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu L., Okada S., Kong X.F., Kreins A.Y., Cypowyj S., Abhyankar A., Toubiana J., Itan Y., Audry M., Nitschke P., Masson C., Toth B., Flatot J., Migaud M., Chrabieh M., Kochetkov T., Bolze A., Borghesi A., Toulon A., Hiller J., Eyerich S., Eyerich K., Gulacsy V., Chernyshova L., Chernyshov V., Bondarenko A., Grimaldo R.M., Blancas-Galicia L., Beas I.M., Roesler J., Magdorf K., Engelhard D., Thumerelle C., Burgel P.R., Hoernes M., Drexel B., Seger R., Kusuma T., Jansson A.F., Sawalle-Belohradsky J., Belohradsky B., Jouanguy E., Bustamante J., Bue M., Karin N., Wildbaum G., Bodemer C., Lortholary O., Fischer A., Blanche S., Al-Muhsen S., Reichenbach J., Kobayashi M., Rosales F.E., Lozano C.T., Kilic S.S., Oleastro M., Etzioni A., Traidl-Hoffmann C., Renner E.D., Abel L., Picard C., Marodi L., Boisson-Dupuis S., Puel A., Casanova J.L. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J. Exp. Med. 2011;208(8):1635–1648. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hirahara K., Onodera A., Villarino A.V., Bonelli M., Sciume G., Laurence A., Sun H.W., Brooks S.R., Vahedi G., Shih H.Y., Gutierrez-Cruz G., Iwata S., Suzuki R., Mikami Y., Okamoto Y., Nakayama T., Holland S.M., Hunter C.A., Kanno Y., O'Shea J.J. Asymmetric action of STAT transcription factors drives transcriptional outputs and cytokine specificity. Immunity. 2015;42(5):877–889. doi: 10.1016/j.immuni.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tsumura M., Okada S., Sakai H., Yasunaga S., Ohtsubo M., Murata T., Obata H., Yasumi T., Kong X.F., Abhyankar A., Heike T., Nakahata T., Nishikomori R., Al-Muhsen S., Boisson-Dupuis S., Casanova J.L., Alzahrani M., Shehri M.A., Elghazali G., Takihara Y., Kobayashi M. Dominant-negative STAT1 SH2 domain mutations in unrelated patients with Mendelian susceptibility to mycobacterial disease. Hum. Mutation. 2012;33(9):1377–1387. doi: 10.1002/humu.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Egwuagu C.E., Yu C.R. Interleukin 35-producing B Cells (i35-Breg): A new mediator of regulatory B-Cell functions in CNS autoimmune diseases. Critical Rev. Immunol. 2015;35(1):49–57. doi: 10.1615/critrevimmunol.2015012558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Netea M.G., Vonk A.G., van den Hoven M., Verschueren I., Joosten L.A., van Krieken J.H., van den Berg W.B., Van der Meer J.W., Kullberg B.J. Differential role of IL-18 and IL-12 in the host defense against disseminated Candida albicans infection. Eur. J. Immunol. 2003;33(12):3409–3417. doi: 10.1002/eji.200323737. [DOI] [PubMed] [Google Scholar]