Abstract
Low plasma testosterone (T) levels correlated with metabolic syndrome, cardiovascular diseases, and increased mortality risk. T exerts a significant effect on the regulation of adipose tissue accumulation, and in the glucose and lipids metabolism. Adipocytes are the primary source of the most important adipokines responsible for inflammation and chronic diseases. This review aims to analyze the possible effect of T on the regulation of the proinflammatory cytokines secretion.
A systematic literature search on MEDLINE, Google Scholar, and Cochrane using the combination of the following keywords: “testosterone” with “inflammation,” “cytokines,” “adiponectin, CRP, IL-1B, IL-6, TNFα, leptin” was conducted. Sixteen articles related to the effect of low T level and 18 to the effect of T therapy on proinflammatory cytokine were found.
T exerts a significant inhibitory effect on adipose tissue formation and the expression of various adipocytokines, such as leptin, TNF-α, IL-6, IL-1, and is positively correlated with adiponectin level, whereas a low T level is correlated with increased expression of markers of inflammation. Further studies are necessary to investigate the role of T, integrated with weight loss and physical activity, on its action on the mechanisms of production and regulation of proinflammatory cytokines.
Keywords: testosterone, inflammation, cytokines, adipokines, adiponectin, IL-6
The development and progression of chronic diseases are correlated with low T level and inflammatory biomarkers, but their mechanisms remain poorly understood. T deficiency (also known as hypogonadism) in older men has been associated with metabolic syndrome [1], neurodegeneration [2], and increased risk of cardiovascular diseases (CVDs) and all-cause mortality [3] independently of other numerous risk factors [4, 5]. Similar observations were reported in young men [6]. Before any concurrent manifestations of CVD or other systemic diseases, low T level is correlated with elevated C-reactive protein (CRP) level [7], macrophage inflammatory protein 1-α, macrophage inflammatory protein 1-β, and TNF-α in young and older men [8]. CRP is a sensitive marker of inflammation produced by liver [9] and is correlated with coronary heart disease and deaths from other causes [7].
An inflammatory status due to proinflammatory cytokines is particularly evident in the elderly [10], and in patients with low T levels and obesity [11]. Furthermore, adipokines mediate insulin resistance [12] and the principal adipokines involved are adiponectin, leptin, resistin, visfatin, chemerin, TNF-α, IL-1, IL-6, IL-8, IL-10, plasminogen activator inhibitor-1, monocyte chemoattractant protein-1 (MCP-1), and retinol binding protein-4 (RBP-4) [13]. Higher levels of proinflammatory cytokine play a crucial role in the development of CVD [14], and T therapy provides beneficial effects on the pathophysiological markers and clinical symptoms of coronary heart disease [15].
Furthermore, adipokines are involved in the development and progression of cancer [16]. The etiology of elevated inflammatory markers remains incompletely defined [17], but nutrition and physical inactivity also exert a primary role. Little is known about if and how sex steroid hormone and inflammation pathways may interact to influence the aging process or the development and progression of chronic diseases, including CVD and prostate cancer, in men. This study aims to evaluate the effect of T on proinflammatory cytokines.
1. Methods
A systematic literature search was performed using PubMed, Google Scholar, and Cochrane using the combination of the following keywords: “testosterone” and “inflammation,” “cytokines,” “adiponectin,” “CRP,” “IL-1B, IL-6, TNFα, leptin.” All cross-sectional and longitudinal trials evaluating the incidence of low T in men with moderate to severe inflammation were included. Furthermore, clinical studies that investigated the effect of T administration on inflammatory markers were also considered.
2. Results
Out of 824 retrieved articles, 35 were included in the analysis and had been divided into two groups: one group includes 17 studies evaluating the incidence of inflammatory diseases in men with low T level enrolling 14,658 patients with a mean age of 59.9 ± 12.8 years (Table 1). The other group includes 18 studies that evaluated the effect of T therapy on plasma level of inflammatory markers enrolling 1654 patients with a mean age of 56.4 ± 15.6 years (Table 2). Among the first group, only one study did not find any correlation between T level and CRP, but this study was conducted on healthy middle-aged men, whereas all the other studies found a significant negative correlation between T level and inflammatory markers. Among the studies evaluating the effect of T therapy on proinflammatory markers, six studies found no effect.
Table 1.
Correlation Between T Level and Inflammatory Markers in Hypogonadal Men
| Authors | Subjects | Age | Clinical Picture | Marker of Inflammation |
|---|---|---|---|---|
| Tremellen et al. 2017 [18] | 50 M | 35.1 | Adiposity | Negative correlation between T with CRP, IL-6, and endotoxemia. |
| Wickramatilake et al. 2015 [19] | 153 M | 30–70 | Metabolic syndrome | Low T correlated with high CRP level. |
| Tsilidis et al. 2013 [20] | 1520 M | 44.3 | NHANES population | High androgen and low estrogen level inversely correlate with inflammation markers (CRP and white blood cells). |
| Zhang et al. 2013 [21] | 1989 M | 57 | Population-based cohort | High androgens level correlate with reduced CRP. |
| Chrysohoou et al. 2013 [22] | 467 M | 75 | Metabolic syndrome | T inversely correlated with CRP and insulin level. |
| Soisson et al. 2012 [23] | 350 M | 65 | Carotid intima-media thickness | Low T level correlated with carotid intima-media thickness and high CRP. |
| Haring et al. 2012 [24] | 1344 M | 20–79 | Normal population | TT, SHBG, free T, and DHEAS are inversely correlated with CRP, fibrinogen, and oxidative stress. |
| Brand et al. 2012 [25] | 2418 M | 40–78 | European Prospective Investigation into Cancer | Total T and SHBG are inversely correlated with WBC. |
| Bobjer et al. 2013 [8] | 40 M | 37.4 | Hypogonadism | Significantly elevated levels of the proinflammatory cytokine TNF-α, MIP-1a, and MIP-1B |
| Kupelian et al. 2010 [26] | 2301 M | 30–79 | Urologic symptoms | Inverse dose-response correlation between T and SHBG levels with CRP levels. |
| Kaplan et al. 2010 [27] | 467 M | 52 | Aging men | Inverse relationship between T and CRP. |
| Tang et al. 2007 [28] | 381 M | 78.8 | Nursing home resident | T level correlated negatively with CRP. |
| Bhatia et al. 2006 [29] | 70 M T2D | 56.8 | T2D | Low TT and FT level in T2D patients were correlated with CRP and anemia. |
| Maggio et al. 2006 [30] | 467 M | 65 | Normal older men | IL-6 inversely correlated with total and bioavailable T. |
| Van Pottelbergh et al. 2003 [31] | 715 M | 42.7 | Healthy middle-aged men | No correlation between total and free T with CRP was found. |
| Laaksonen et al. 2003 [32] | 1896 M | 52 | Metabolic syndrome | Total and free T correlated inversely with CRP. |
| Hall et al. 2002 [33] | 30 M HF | 67 | Heart failure | Inverse correlation of T and bio-T with IL-1β, TNF-α, and IL-6. |
Abbreviations: bio-T, bioactive testosterone; CRP, C reactive protein; FT, free testosterone; HF, heart failure; M, men; MIP-1α and -2β, macrophage inflammatory protein-1α and 1β; NHANES, National Health and Nutrition Evaluation Survey; SHBG, sex hormone globulin; STEMI, ST-Elevation Myocardial Infarction; T2D, type 2 diabetes; TT, total testosterone.
Table 2.
Effect of T Administration on Inflammatory Markers
| Authors | Subjects | Age | Type of Study | T Level | T Therapy | Duration | Marker of Inflammation |
|---|---|---|---|---|---|---|---|
| Dhindsa et al. 2016 [36] | Randomized placebo controlled trial | T = 252 ± 82 ng/dL | T 250 mg/2 wk | 6 mo | Insulin sensitivity increased. Significant reduction of CRP, IL-1β, TNF-α, leptin | ||
| 44 HH, T2D | 54.6 | FT = 4.4 ± 1.2 ng/dL | |||||
| Nasser et al. 2015 [52] | 92 M Crohn disease | 60 | Cumulative, prospective, registry study | T ≤ 12.1 nmol/L | T undecanoate 1000/3 mo | 7 y | Significant reduction in CRP level and Crohn Disease Activity Index |
| Sonmez et al. 2015 [37] | 60 M, CHH | 21.8 | Observational | T = 0.26 ± 0.16 ng/mL | T 250 (Sustanon) every 3 wk | 6 mo | No changes in CRP level. |
| T transdermal 50 mg/d | |||||||
| Maggio et al. 2014 [38] | 109 M | 71.9 | Cohort study | T < 475 ng/dL | T patch 6 mg/24 h | 36 mo | No significant changes in TNF-α, IL-6, PCR. |
| Traish et al. 2013 [39] | 255 M | 58.6 | T = 9.9 ± 1.38 nmol/L | T undecanoate 1000/3 mo | 60 mo | Significant reduction in CRP level. | |
| Basaria et al. 2013 [40] | 179 M | 73 | Double-blind randomized placebo-controlled trial | T = 248 ± 60 | T transdermal gel 100 mg/d | 6 mo | Significant reduction in PAI-1 and increase in IL-6. |
| FT = 4.9 ± 1.2 | |||||||
| Saad et al. 2011 [41] | 110 M | 59.6 | Observational | T = 9.3 ± 1.7 nmol/L | T undecanoate 1000/3 mo | 3–24 mo | Strong decline in BMI and WC; less reduction in CRP. |
| Kalinchenko et al. 2010 [42] | 184 HM MetS | 35–70 | Double-blind, randomized placebo-controlled trial | T < 12.0 nmol/L | T undecanoate 1000 every 6 wk | 18 wk | BMI, leptin, insulin, IL-1β, TNF-α, CPR decreased. IL-6 and IL-10 unchanged. |
| Giltay et al. 2008 [43] | 100 HM | 34–69 | Observational nonrandomized study | T = 5.9–12.1 nmol/L | T undecanoate 1000 mg per 12 wk | 15 mo | Significant decline in CPR. |
| Kapoor et al. 2007 [44] | 20 HM T2D | 63 | Double-blind placebo | T = 7.4 nmol/L | Sustanon 200 mg/2 wk | 3 mo | No significant effect on resistin, TNF-α, IL-6, and CPR. Leptin and adiponectin reduced. |
| FT = 2.4 nmol/L | |||||||
| Nakhai-Pour et al. 2007 [35] | 237 HM | 60–80 | Double-blind randomized placebo-controlled trial | T < 13.7 nmol/L | T undecanoate 160 mg/d | 26 wk | No changes in PCR. |
| Herbst et al. 2006 [45] | 52 W HIV | 18–50 | Placebo-controlled, randomized clinical trial | T < 33 ng/dL | T patches 300 µm daily | 24 wk | No changes in inflammatory markers. |
| Page et al. 2005 [46] | 25 M | 65–85 | Observational | Normal range | T enanthate 600 mg/wk | 3 wk | Adiponectin and leptin level decreased. |
| Malkin et al. 2004 [47] | 27 M | 62 | Single-blind randomized placebo-controlled trial | T < 4.4 nmol/L | Sustanon 100 mg/wk | 4 wk | Reduction in TNF-α, IL-1β. Increase in IL-10. |
| Lanfranco et al. 2004 [48] | 31 HM | 36.5 | Retrospective study | T = 4.4 ± 0.4 nmol/L | 6 mo | Significant decrease in adiponectin. | |
| Singh et al. 2002 [51] | 61 M eugonadal | 18–35 | Double-blind, randomized trial | Normal range | T enanthate/wk | 20 wk | No significant effect on blood lipids, insulin activity, and CRP. |
| 25 mg | |||||||
| 50 mg | |||||||
| 125 mg | |||||||
| 300 mg | |||||||
| 600 mg | |||||||
| Ng et al. 2002 [49] | 33 M (DHT) | <60 | Double-blind placebo-controlled trial | T < 15 nmol/L | DHT 70 mg transdermal/d | 3 mo | Significant changes in CRP, sVCAM-1, or sICAM-1. |
| 20 M (hCG) | hCG 500 µgr/wk | ||||||
| healthy | |||||||
| Sigh et al. 1997 [50] | 15 HM | 68 | Observational | FT < 60 ng/dL | T cypionate 200 mg/biweekly | 12 mo | Significant decrease in leptin level. |
Abbreviations: Bio-T, bioactive testosterone; E2, 17β-estradiol; FT, free testosterone; HCG, human chorionic gonadotropin; HM, hypogonadal men; M, men; T2D, type 2 diabetes; W, women.
3. Discussion
Low T levels in men were significantly associated with high level of inflammatory markers in different clinical conditions such as obesity [18], metabolic syndrome [19, 22, 32], heart failure [33], healthy elderly population [20, 22, 27, 28, 30], carotid atherosclerosis [23], hypogonadism [8], urologic symptoms [26], type 2 diabetes [29], primary care center [34] and are summarized in Table 1. In all studies, a negative correlation was found between low T levels and CRP, whereas only a few studies explored IL-6 [18, 30, 33] and TNF-α [8, 33]. Haring et al. [24] found a negative correlation between sex steroids plasma level in men with markers of inflammation. Bhatia et al. [29] showed that low T was inversely correlated with CRP and may contribute to mild anemia. Maggio et al. [30] found that T level was inversely correlated with IL-6r [35]. An extensive epidemiological study revealed that men with low T level had a higher incidence of obesity, metabolic syndrome, cancer, and acute inflammation [34]. Only a few studies had adjusted the data for other sex hormones [21, 35]. In elderly men, the reduced levels of T were correlated with the incidence of metabolic syndrome, insulin resistance, and inflammation evidencing that high CRP levels can be considered independent predictors of metabolic syndrome [19, 22]. Some studies that evaluated the correlation of CPR with low T level did not adjust for other confounders such as smoking and obesity [22, 23, 25, 27, 34]. A few epidemiologic studies did not find any consistent correlation between sex steroid hormones and inflammatory biomarkers in men [30–32]. Others found a negative correlation between the level of androgens with inflammatory markers [21, 25–28].
The majority of the studies found an evident protecting effect of T against inflammation independently of the clinical condition. However, it is difficult to draw a global evaluation because only a few studies have detected more than one cytokine in addition to CRP [8, 18, 33]. The correlation of T with inflammation should be evaluated on a larger number of biomarkers and adjusted with many confounder factors.
The effect of T therapy on the secretion of inflammatory cytokines levels in men have been investigated by many studies [35–51], which are summarized in Table 2.
Two randomized trials showed that T therapy in hypogonadal men produced a decrease in the concentration of adipokines [42, 47], but other trials did not reach the same results [49]. Singh et al. [51] did not observe any correlation between different doses of T enanthate administered with insulin activity and CPR level in young eugonadal patients (of 18 to 35 years old). Nasser et al. [52] found that T therapy was effective in Crohn disease, determining a reduction in CRP and Crohn Disease Activity Index. Other studies did not find any variation of CRP level after T administration in elderly men with low plasma T (160 mg per day of T undecanoate orally) [35] and on proinflammatory cytokines (Sustanon 200 mg every 2 weeks over 3 months) [44]. Robust clinical evidence reported that T therapy in hypogonadal men had an attenuating effect on inflammatory markers [36, 42, 43, 46–50]. However, others [35, 38, 40, 44, 45] did not find any significant effect [see Table 2]. In the studies reported in this table, some discrepancies are evident, such as the methodology adopted for the trials, the different dose of T administrated, the time of observation, and different clinical conditions. The transdermal administration of T in older men did not show any inhibitory effect on CRP, TNF-α, and IL-6 [38, 45], whereas T undecanoate 1000 mg every 6 weeks seems capable of reducing the inflammatory markers [42, 43]. Differences observed in these studies can be related to different doses of T administration where transdermal way is lighter compared to the injection.
Furthermore, a higher incidence of inflammation and cancer in patients with low T level was reported [53]. Considering the data from these studies, it appears evident that the administration of T is more effective in reducing inflammation in hypogonadal than eugonadal men. In eugonadal men, the effect of T seems to be dose-dependent and that low doses are ineffective as observed with oral and transdermal administration [35, 38, 45] also in young men although hypogonadal [37]. Furthermore, the interaction of T with estradiol in the regulation of systemic inflammation [54], adipose and muscular tissue mass, and other hormones should be considered. More studies are necessary to evaluate the potential effect of T in inhibiting the proinflammatory cytokines expressions.
4. Mechanism of Action of T on Inflammation
A. Effects on Adipose Tissue
T and obesity are interactive, and an inverse correlation between T level and body fat mass has been confirmed [55]. T therapy is effective in determining a sustained loss of body fat mass in hypogonadal men [56]. Androgens are very active in the regulation of adipose tissue metabolism and distribution due to the presence of androgen receptor (AR) on adipocytes [57, 58]. AR is present on preadipocytes with greater expression in visceral than in subcutaneous fat depot, and can partially explain the different adipose tissue distribution [57]. Notably, in adipocytes are also expressed estrogen receptor (ER) α and β [59, 60]. The activation of ERα on dipocytes in males and females has a protective effect against body fat accumulation, inflammation, and fibrosis [61] and the deletion of ERα gene reflects obesity in both sexes [62]. In men, visceral fat deposition is significantly greater than in women due to the low activation of ERα [63]. Visceral fat is correlated with metabolic syndrome [64] and CVDs independently from other measures of adiposity [65].
The most consistent effect of androgen on body fat is the activation of lipolysis [66] and inhibition of adipose tissue lipoprotein lipase activity [67]. Androgens markedly inhibit adipogenesis blocking the differentiation into adipocytes in subcutaneous and visceral preadipocyte in both sexes [68]. Singh et al. [69] showed that T and DHT regulate the pluripotent mesenchymal cells determining their preferential development into the myogenic rather than the adipogenic line. The study demonstrated that pluripotent cells are androgen-dependent and have reciprocal effects on muscle and fat cells. The effect of sex steroids on influencing the preadipocytes differentiation can explain the sexual dimorphism of body fat distribution [70]. Nonaromatizable androgen, such as DHT, has been shown to have a strong inhibitory effect on the differentiation of human mesenchymal stem cells and human preadipocytes, in subcutaneous and visceral (omental and mesenteric) fat deposits in men [71], whereas in women this effect remains unclear. Estrogens favorite the development of fat cells in the subcutaneous fat tissue and inhibiting it in the visceral body fat [72]. A high androgen level inhibits the adipose tissue depots and improves insulin resistance and glucose tolerance in women and men [73]. Then, T administration exerts the primary anti-inflammatory effect in reducing fat mass, which is the source of many inflammatory cytokines.
B. Effects on the Expression of Inflammatory Adipokines
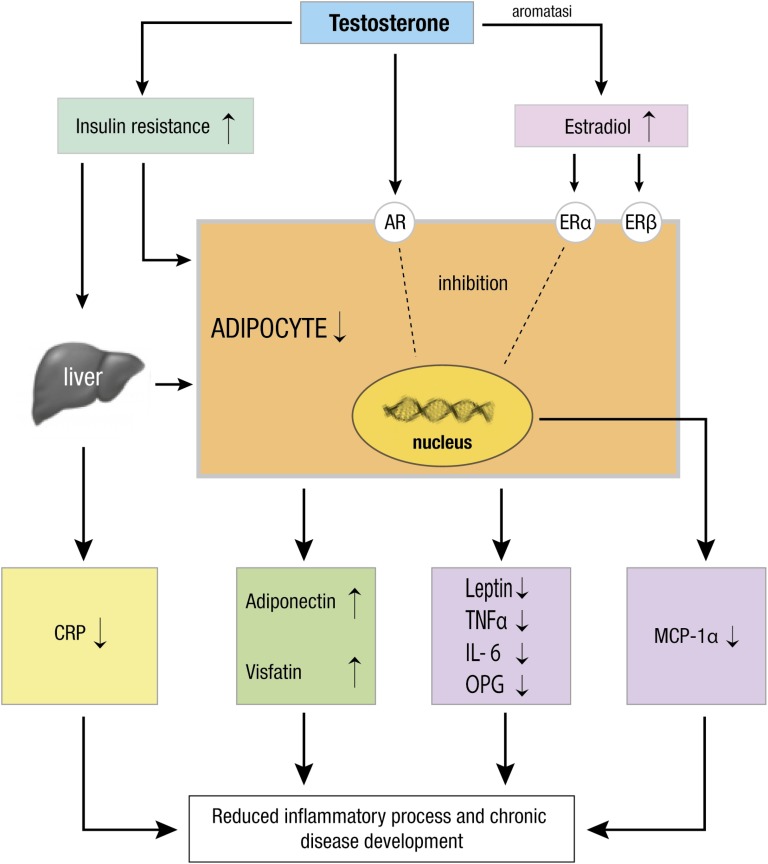
The effects of androgen on body fat reflect on many adipocytokines releasing, and the mechanisms are summarized in Fig. 1.
Figure 1.
T exerts its anti-inflammatory activity through different mechanisms. Firstly, T inhibits body fat expansion and reduces adipocytes size and metabolism. After its aromatization in estradiol, T can activate AR and ERα and ERβ, which contribute to adipocytes regulation decreasing the release of adipokines (leptin, IL-6, TNF-α, OPG, MCP-1α) and improving adiponectin and visfatin production, which possess an anti-inflammatory effect. Furthermore, T improves insulin activity and reduces the CRP from the liver. Altogether, it results in a reduction of inflammation and development of chronic disease.
B-1. Leptin
T level interacts profoundly with many proinflammatory cytokines. Leptin, the most specific hormone secreted by adipocytes [74], is associated with adipose tissue expansion and with body mass index (BMI) [75]. Leptin concentration is significantly higher in obese than in lean individuals and for any given BMI more in women than in men [76]. Leptin level reduces T secretion from rodent testes in vitro [77], inhibiting leptin receptor isoform present in Leydig cells. From the other side, T level in men inhibits leptin secretion, independently by BMI, suggesting that T exerts an inhibitory effect on adipocyte [78]. In men with metabolic syndrome, the leptin level is higher, whereas T level is lower than in normal subjects [79]. Conversely, in women, androgen levels are positively correlated with high free and total leptin level [80] also in polycystic ovary syndrome (PCOS) [81], evidencing a sexual dimorphism of T on leptin secretion. There is a bidirectional effect between leptin and T secretion. The lack of leptin or leptin receptors in humans and mice develop profound obesity and infertility [82]. Leptin has a modulatory effect on Leydig cells function [83], inhibiting basal T production [84]. Behre et al. demonstrated a significant inverse correlation between serum leptin levels with T and BMI in males, whereas serum estradiol had no influence [85]. The administration of T to young men suppressed serum leptin secretion, which returned to the pretreatment level after cessation of T injections [86]. The short-term T administration, in boys with pubertal delay, decreased leptin and insulin concentrations [87] and therefore obesity [86]. Restoring the physiological level of serum T in men with metabolic syndrome can discontinue the vicious circle of metabolic disorders resulting in a T deficiency and clinical complications [88]. The antiobesity effect of T may be mediated by the leptin suppression.
B-2. Adiponectin
Adiponectin, the highest expressed cytokine in adipocytes [89], is inversely correlated with metabolic disorders and, CVD [90], with waist-to-hip ratio, and visceral fat [91, 92]. A higher level of adiponectin is expressed in lean subjects, both in men and women, and is correlated with better insulin sensitivity and lower TNF-α level [93]. Adiponectin level is lower in obese compared with healthy subjects who have higher adiponectin level and a reduced risk of type 2 diabetes [94]. Type 2 diabetic patients with CVD had a lower level of adiponectin compared to diabetic patients without CVD [95]. Furthermore, the plasma adiponectin level increases relevantly following a reduction in body weight in the diabetic subjects as well as the nondiabetic subjects [95].
Circulating level of adiponectin has a sexual dimorphism because adiponectin levels are normally higher in females than in males [96, 97]. In hyperandrogenic PCOS obese women, adiponectin level was significantly lower compared with normal women, whereas in thin women, no difference has been observed between women with or without PCOS [98]. In PCOS women, the level of adiponectin is reduced and more correlated with insulin resistance and adipose tissue than androgen levels [99]. So it seems that adiponectin in women is more influenced prevalently by the body mass. In young men, acute T treatment determines a reduction of adiponectin high molecular weight (HMW) levels and low T level is associated with increased adiponectin HMW levels [100]. T therapy has been shown to exert a direct suppressive effect on adiponectin secretion in men with type 2 diabetes [44], in elderly men [46] and in transsexual female patients [101]. Frederiksen et al. [102] found a decrease in subcutaneous fat and adiponectin level after 6 months of T administration in aging men. Estradiol has an opposite effect on T, determining the stimulation of adiponectin and its receptors expression in skeletal muscle [103].
In rats, T controlled directly the sex differences in adiponectin by the activation of androgen-mediated effects that regulates the secretion and metabolism of adiponectin [104]. The changes in circulating adiponectin level are highly correlated with the androgen levels, but not with estradiol level. A nonestrogenic and nonaromatizable androgen such as trenbolone determines a reduction in adiponectin level and visceral fat similar to that caused by T. Both T and trenbolone increased the HMW adiponectin in males and females and reduced the lower molecular weight adiponectin [104] showing that aromatizable and nonaromatizable androgen have similar effects on the isoforms of adiponectin.
B-3. Osteoprotegerin
Osteoprotegerin (OPG) is a cytokine of the TNF superfamily [105], which regulates bone resorption [106], and calcium metabolism in both bone and vascular tissues [107]. Body fat is a potential source of OPG [108]. OPG has been proposed as a mediator of vascular calcification [109]. High serum OPG levels were correlated with greater incidence of CVD mortality [110], vascular calcification at coronary and aortic level [109, 111], and arterial disease in type 2 diabetes [112]. OPG level is inhibited by androgens, whereas estradiol shows an opposite effect [113]. The different action T vs estradiol on OPG secretion may explain why T is less efficient than estradiol on inhibiting bone resorption in humans [114]. In hypogonadal men, an increased RANKL activity and an increased bone turnover-related OPG has been observed [115], whereas T administration in men significantly decreased OPG level [116]. The decreased OPG level following T therapy reduced the incidence of cardiovascular risk [116]. In women, OPG levels are positively correlated with T level [117]. In premenopausal women, obesity favorites an increase of serum OPG levels, whereas weight loss favorites a decrease of serum OPG levels [118]. In PCOS women, serum OPG level is lower compared with nonhyperandrogenic control women [119], whereas Glintborg et al. [117] showed that OPG levels were more correlated with bone mineral density in PCOS than with T level. In conclusion, OPG production is inhibited by T in men, less evident in women, where body fat mass seems to have a prevalent effect.
B-4. Tumor necrosis factor-α
TNF-α is a potent cytokine prevalently secreted by macrophages after they have infiltrated into adipose tissue in obese humans [120]. TNF-α mediates apoptosis, insulin resistance, and lipolysis [121], inducing serine phosphorylation of insulin receptor substrates [122]. Furthermore, TNF-α determines the alteration of endothelial permeability to immune cells and small particles like low-density lipoprotein [123], promoting the first stage of atherosclerosis increasing the transport of low-density lipoprotein across endothelial cells [124]. TNF-α downregulates the mRNA level of adiponectin, a cytokine which contributes to maintaining glucose and lipids homeostasis [125]. The effect of T on TNF-α secretion is poorly investigated. Recently, Chen et al. [126] showed that T significantly attenuated the release of TNF-α in a dose-dependent manner and might reduce the inflammatory responses and modulate the immune system. Withdrawal of T administration in hypogonadal men determined significant increases in IL-6 and TNF-α [44]. Corrales et al. [127] evidenced that T therapy in type 2 diabetic men caused a reduction or complete abrogation of natural ex vivo production of IL-1β, IL-6, and TNF-α. In young overweight and obese women with PCOS, higher TNF-α has a positive correlation with androgen level and insulin resistance [128].
B-5. Monocyte chemoattractant protein-1
MPC-1 is a cytokine secreted by adipocytes in obese subjects with the effect to promote the infiltration of monocytes/macrophages into adipose tissue [129]. The MCP-1 level is significantly raised in obese subjects suggesting the concept that chronic inflammation is due to excess adiposity [130]. Low T and high estradiol levels have a direct adverse effect on MCP-1 in vivo [131], showing that the action of T is regulated by estrogen level. Morooka et al. [132] demonstrated in adipocytes cocultured with monocytes that the activation of AR determined the suppression of MCP-1 release, particularly suppressed by DHT and chronic inflammation in adipose tissue. High androgen level in women, as observed in PCOS, is correlated with a significant increase in MCP-1 level and with abdominal obesity [133].
B-6. Interleukin-6
IL-6 is a cytokine that plays a fundamental role in inflammation, immune response, and hematopoiesis [134]. IL-6 is secreted prevalently by white adipose tissue (for one-third), and by skeletal muscles and liver [135]. The IL-6 expression is correlated, similarly to TNF-α, with BMI, abdominal obesity, and free fatty acids level [136]. In adipose tissue and liver, IL-6 exerts proinflammatory activity responsible for insulin resistance [137]. IL-6 is also produced by skeletal muscle during exercise mediating muscle hypertrophy and myogenesis [138]. There is consistent evidence that IL-6 plasma level increases in response to exercise [139] and the production of IL-6 is stimulated by ROS from skeletal myotubes [140]. The IL-6 produced by skeletal muscles affects white adipose tissue mass regulating glucose uptake capacity and the lipogenic and lipolytic factors [141]. After weight loss, plasma level of IL-6 is reduced and improves insulin sensitivity [142]. Adrenal androgens, particularly DHEA, had an inhibitory effect on different cell types such as leukocytes and decreased IL-6 secretion [143], and the suppressive effect was greater than that of DHT and estrogen [144]. In aged orchidectomized male rats, at the supra-physiological level of T the inflammatory ILs, specifically IL-2, IL-6, IL-10, IL-12, and IL-13, were elevated, whereas T supplementation decreased plasma IL level [145]. In PCOS women, high androgen levels, in both lean and obese women, were correlated with IL-6 and with insulin resistance [146]. However, IL-6 levels were found to be higher as compared with controls, although IL-6 levels might be more dependent on nutritional status [147]. These effects are inhibited by the neutralization of IL-6 with the anti-IL-6 antibody [148]. After intense physical exercise, the IL-6 production is inversely correlated with T level [149]. T treatment (150 mg every two weeks) of aging type 2 diabetic men after 12 months reduced or abrogate the production of proinflammatory cytokines (IL-1b, IL-6, TNF-α) entirely by monocytes and dendritic cells observed after stimulation with lipopolysaccharide plus recombinant human interferon-γ. [150].
B-7. Resistin
Resistin is a proinflammatory cytokine that has the greatest effect on promoting atherosclerosis and CVD diseases [151, 152] and is a marker of heart failure [153]. Plasma resistin level is positively correlated with coronary artery disease and mortality risk [154] and predictors of all-cause mortality independent of other risk factors [155, 156]. Resistin showed significant correlation with BMI, insulin resistance, obesity, and inflammation in patients with type 2 diabetes [157, 158]. Resistin may be a link between insulin resistance and androgens [159]. Although T therapy in hypogonadal men with type 2 diabetes decreases leptin and adiponectin levels, no significant effect on resistin level has been observed [44]. Further research is necessary to clarify the effects of androgens on the regulation of resistin plasma level and function.
B-8. Visfatin
Plasma visfatin concentration is increased in subjects with overweight/obesity, type 2 diabetes mellitus, metabolic syndrome, and CVDs [160]. In patients with metabolic syndrome, visfatin is correlated with adiponectin [161], whereas in patients without metabolic syndrome, circulating visfatin levels were significantly correlated with glucose, insulin, and triglyceride levels. A meta-analysis indicates that high-circulating visfatin level is an intrinsic characteristic of PCOS, suggesting visfatin as a potential biomarker for PCOS [162].
C. Future Directions
With advancing age, the decline of sex hormones patronizes the development of the inflammatory processes that represent the basic mechanism for the development of chronic diseases. Adipokines production increases in the condition of low T level. However, more specific and well-controlled clinical trials analyzing the interaction of sex hormones on a wider number of adipokines that interact with other risk factors. A low T level in men represents an important risk factor for health, but its effect is modulated by estrogen level that should always be detected.
5. Conclusions
T level is determinant in the regulation of the inflammatory processes by inhibiting adipocytes expansion, differentiation, function, and suppressing cytokines formation (leptin, IL-6, TNF-α, MCP-1, resistin) while stimulating the adiponectin secretion. Low T level has implications for metabolic health in both males and females and should be considered a risk factor because of its correlation with metabolic syndrome and all-cause mortality [1]. The inhibitory effect of androgens on adipokines secretion can also interfere in cancerogenesis reducing the progression and diffusion of the diseases. Low T level is correlated with a high level of adipokines and inflammation, and T therapy is necessary to restore the physiological, hormonal level. However, T administration in hypogonadal men on the inflammatory markers has shown conflicting results, probably related to the different dose and duration time of T administration and the limited evaluation to a small number of markers. Furthermore, not all studies were corrected for the many confounder factors, such as fat mass distribution, nutritional intake, and muscle mass. Diet restriction and physical exercise are important in the regulation of metabolic disorders. Finally, androgen therapy in older men with T deficiency improves physical efficiency and reduces the risk of rehospitalization [163]. Chronic diseases in aging have a great impact on the lifestyle of patients and public health cost due to the frequency of hospitalization. The reduction of the inflammatory state is relevant, and further investigations are required to evaluate the mechanisms of proinflammatory cytokines regulation.
Acknowledgments
Disclosure Summary: The author has nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- CRP
C-reactive protein
- CVD
cardiovascular disease
- ER
estrogen receptor
- HMW
high molecular weight
- MCP-1
monocyte chemoattractant protein
- OPG
osteoprotegerin
- PCOS
polycystic ovary syndrome
- T
testosterone
References and Notes
- 1. Bianchi V. Metabolic syndrome, obesity paradox and testosterone level. Endocrinol Metab Syndr. 2015;4(172):1–16. [Google Scholar]
- 2. Kurth F, Luders E, Sicotte NL, Gaser C, Giesser BS, Swerdloff RS, Montag MJ, Voskuhl RR, Mackenzie-Graham A. Neuroprotective effects of testosterone treatment in men with multiple sclerosis. Neuroimage Clin. 2014;4:454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(10):3007–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haring R, Völzke H, Steveling A, Krebs A, Felix SB, Schöfl C, Dörr M, Nauck M, Wallaschofski H. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20-79. Eur Heart J. 2010;31(12):1494–1501. [DOI] [PubMed] [Google Scholar]
- 5. Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169(6):725–733. [DOI] [PubMed] [Google Scholar]
- 6. Sinclair M, Grossmann M, Angus PW, Hoermann R, Hey P, Scodellaro T, Gow PJ. Low testosterone as a better predictor of mortality than sarcopenia in men with advanced liver disease. J Gastroenterol Hepatol. 2016;31(3):661–667. [DOI] [PubMed] [Google Scholar]
- 7. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J; Emerging Risk Factors Collaboration . C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bobjer J, Katrinaki M, Tsatsanis C, Lundberg Giwercman Y, Giwercman A. Negative association between testosterone concentration and inflammatory markers in young men: a nested cross-sectional study. PLoS One. 2013;8(4):e61466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105(6):2294–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107. [DOI] [PubMed] [Google Scholar]
- 12. Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front Endocrinol (Lausanne). 2013;4:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;53(Suppl 1):S143–S151. [DOI] [PubMed] [Google Scholar]
- 14. Kritchevsky SB, Cesari M, Pahor M. Inflammatory markers and cardiovascular health in older adults. Cardiovasc Res. 2005;66(2):265–275. [DOI] [PubMed] [Google Scholar]
- 15. Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis. 2009;207(2):318–327. [DOI] [PubMed] [Google Scholar]
- 16. Liu ST, Pham H, Pandol SJ, Ptasznik A. Src as the link between inflammation and cancer. Front Physiol. 2014;4:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10(3):319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tremellen K, McPhee N, Pearce K. Metabolic endotoxaemia related inflammation is associated with hypogonadism in overweight men. Basic Clin Androl. 2017;27(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wickramatilake CM, Mohideen MR, Pathirana C. Association of metabolic syndrome with testosterone and inflammation in men. Ann Endocrinol (Paris). 2015;76(3):260–263. [DOI] [PubMed] [Google Scholar]
- 20. Tsilidis KK, Rohrmann S, McGlynn KA, Nyante SJ, Lopez DS, Bradwin G, Feinleib M, Joshu CE, Kanarek N, Nelson WG, Selvin E, Platz EA. Association between endogenous sex steroid hormones and inflammatory biomarkers in US men. Andrology. 2013;1(6):919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y, Gao Y, Tan A, Yang X, Zhang H, Zhang S, Wu C, Lu Z, Wang M, Liao M, Qin X, Li L, Hu Y, Mo Z. Endogenous sex hormones and C-reactive protein in healthy Chinese men. Clin Endocrinol (Oxf). 2013;78(1):60–66. [DOI] [PubMed] [Google Scholar]
- 22. Chrysohoou C, Panagiotakos D, Pitsavos C, Siasos G, Oikonomou E, Varlas J, Patialiakas A, Lazaros G, Psaltopoulou T, Zaromitidou M, Kourkouti P, Tousoulis D, Stefanadis C. Low total testosterone levels are associated with the metabolic syndrome in elderly men: the role of body weight, lipids, insulin resistance, and inflammation; the Ikaria study. Rev Diabet Stud. 2013;10(1):27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soisson V, Brailly-Tabard S, Helmer C, Rouaud O, Ancelin ML, Zerhouni C, Guiochon-Mantel A, Scarabin PY. A J-shaped association between plasma testosterone and risk of ischemic arterial event in elderly men: the French 3C cohort study. Maturitas. 2013;75(3):282–288. [DOI] [PubMed] [Google Scholar]
- 24. Haring R, Baumeister SE, Völzke H, Dörr M, Kocher T, Nauck M, Wallaschofski H. Prospective inverse associations of sex hormone concentrations in men with biomarkers of inflammation and oxidative stress. J Androl. 2012;33(5):944–950. [DOI] [PubMed] [Google Scholar]
- 25. Brand JS, van der Schouw YT, Dowsett M, Folkerd E, Luben RN, Wareham NJ, Khaw KT. Testosterone, SHBG and differential white blood cell count in middle-aged and older men. Maturitas. 2012;71(3):274–278. [DOI] [PubMed] [Google Scholar]
- 26. Kupelian V, Chiu GR, Araujo AB, Williams RE, Clark RV, McKinlay JB. Association of sex hormones and C-reactive protein levels in men. Clin Endocrinol (Oxf). 2010;72(4):527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaplan SA, Johnson-Levonas AO, Lin J, Shah AK, Meehan AG. Elevated high sensitivity C-reactive protein levels in aging men with low testosterone. Aging Male. 2010;13(2):108–112. [DOI] [PubMed] [Google Scholar]
- 28. Tang YJ, Lee WJ, Chen YT, Liu PH, Lee MC, Sheu WH. Serum testosterone level and related metabolic factors in men over 70 years old. J Endocrinol Invest. 2007;30(6):451–458. [DOI] [PubMed] [Google Scholar]
- 29. Bhatia V, Chaudhuri A, Tomar R, Dhindsa S, Ghanim H, Dandona P. Low testosterone and high C-reactive protein concentrations predict low hematocrit in type 2 diabetes. Diabetes Care. 2006;29(10):2289–2294. [DOI] [PubMed] [Google Scholar]
- 30. Maggio M, Basaria S, Ble A, Lauretani F, Bandinelli S, Ceda GP, Valenti G, Ling SM, Ferrucci L. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab. 2006;91(1):345–347. [DOI] [PubMed] [Google Scholar]
- 31. Van Pottelbergh I, Braeckman L, De Bacquer D, De Backer G, Kaufman JM. Differential contribution of testosterone and estradiol in the determination of cholesterol and lipoprotein profile in healthy middle-aged men. Atherosclerosis. 2003;166(1):95–102. [DOI] [PubMed] [Google Scholar]
- 32. Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Salonen R, Rauramaa R, Salonen JT. Sex hormones, inflammation and the metabolic syndrome: a population-based study. Eur J Endocrinol. 2003;149(6):601–608. [DOI] [PubMed] [Google Scholar]
- 33. Hall J, Pugh PJ, Jones RD, Corlett G, Channer KS, Jones TH. Testosterone and pro-inflammatory cytokines in men with chronic heart failure. Endocrine Abstracts. 2002;3:350. [Google Scholar]
- 34. Schneider HJ, Sievers C, Klotsche J, Böhler S, Pittrow D, Lehnert H, Wittchen HU, Stalla GK. Prevalence of low male testosterone levels in primary care in Germany: cross-sectional results from the DETECT study. Clin Endocrinol (Oxf). 2009;70(3):446–454. [DOI] [PubMed] [Google Scholar]
- 35. Nakhai-Pour HR, Grobbee DE, Emmelot-Vonk MH, Bots ML, Verhaar HJ, van der Schouw YT. Oral testosterone supplementation and chronic low-grade inflammation in elderly men: a 26-week randomized, placebo-controlled trial. Am Heart J. 2007;154(6):1228.e1–1228.e7. [DOI] [PubMed] [Google Scholar]
- 36. Dhindsa S, Ghanim H, Batra M, Kuhadiya ND, Abuaysheh S, Sandhu S, Green K, Makdissi A, Hejna J, Chaudhuri A, Punyanitya M, Dandona P. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care. 2016;39(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sonmez A, Haymana C, Aydogdu A, Tapan S, Basaran Y, Meric C, Baskoy K, Dinc M, Yazici M, Taslipinar A, Barcin C, Yilmaz MI, Bolu E, Azal O. Endothelial dysfunction, insulin resistance and inflammation in congenital hypogonadism, and the effect of testosterone replacement. Endocr J. 2015;62(7):605–613. [DOI] [PubMed] [Google Scholar]
- 38. Maggio M, Snyder PJ, De Vita F, Ceda GP, Milaneschi Y, Lauretani F, Luci M, Cattabiani C, Peachey H, Valenti G, Cappola AR, Longo DL, Ferrucci L. Effects of transdermal testosterone treatment on inflammatory markers in elderly males. Endocr Pract. 2014;20(11):1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Traish AM, Haider A, Doros G, Saad F. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract. 2014;68(3):314–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Basaria S, Davda MN, Travison TG, Ulloor J, Singh R, Bhasin S. Risk factors associated with cardiovascular events during testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2013;68(2):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saad F, Haider A, Giltay EJ, Gooren LJ. Age, obesity and inflammation at baseline predict the effects of testosterone administration on the metabolic syndrome. Horm Mol Biol Clin Investig. 2011;6(1):193–199. [DOI] [PubMed] [Google Scholar]
- 42. Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJ, Giltay EJ, Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf). 2010;73(5):602–612. [DOI] [PubMed] [Google Scholar]
- 43. Giltay EJ, Haider A, Saad F, Gooren LJ. C-reactive protein levels and ageing male symptoms in hypogonadal men treated with testosterone supplementation. Andrologia. 2008;40(6):398–400. [DOI] [PubMed] [Google Scholar]
- 44. Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007;156(5):595–602. [DOI] [PubMed] [Google Scholar]
- 45. Herbst KL, Calof OM, Hsia SH, Sinha-Hikim I, Woodhouse LJ, Buchanan TA, Bhasin S. Effects of transdermal testosterone administration on insulin sensitivity, fat mass and distribution, and markers of inflammation and thrombolysis in human immunodeficiency virus-infected women with mild to moderate weight loss. Fertil Steril. 2006;85(6):1794–1802. [DOI] [PubMed] [Google Scholar]
- 46. Page ST, Herbst KL, Amory JK, Coviello AD, Anawalt BD, Matsumoto AM, Bremner WJ. Testosterone administration suppresses adiponectin levels in men. J Androl. 2005;26(1):85–92. [PubMed] [Google Scholar]
- 47. Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89(7):3313–3318. [DOI] [PubMed] [Google Scholar]
- 48. Lanfranco F, Zitzmann M, Simoni M, Nieschlag E. Serum adiponectin levels in hypogonadal males: influence of testosterone replacement therapy. Clin Endocrinol (Oxf). 2004;60(4):500–507. [DOI] [PubMed] [Google Scholar]
- 49. Ng MK, Liu PY, Williams AJ, Nakhla S, Ly LP, Handelsman DJ, Celermajer DS. Prospective study of effect of androgens on serum inflammatory markers in men. Arterioscler Thromb Vasc Biol. 2002;22(7):1136–1141. [DOI] [PubMed] [Google Scholar]
- 50. Sih R, Morley JE, Kaiser FE, Perry HM III, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82(6):1661–1667. [DOI] [PubMed] [Google Scholar]
- 51. Singh AB, Hsia S, Alaupovic P, Sinha-Hikim I, Woodhouse L, Buchanan TA, Shen R, Bross R, Berman N, Bhasin S. The effects of varying doses of T on insulin sensitivity, plasma lipids, apolipoproteins, and C-reactive protein in healthy young men. J Clin Endocrinol Metab. 2002;87(1):136–143. [DOI] [PubMed] [Google Scholar]
- 52. Nasser M, Haider A, Saad F, Kurtz W, Doros G, Fijak M, Vignozzi L, Gooren L. Testosterone therapy in men with Crohn’s disease improves the clinical course of the disease: data from long-term observational registry study. Horm Mol Biol Clin Investig. 2015;22(3):111–117. [DOI] [PubMed] [Google Scholar]
- 53. Burney BO, Hayes TG, Smiechowska J, Cardwell G, Papusha V, Bhargava P, Konda B, Auchus RJ, Garcia JM. Low testosterone levels and increased inflammatory markers in patients with cancer and relationship with cachexia. J Clin Endocrinol Metab. 2012;97(5):E700–E709. [DOI] [PubMed] [Google Scholar]
- 54. van Koeverden ID, de Bakker M, Haitjema S, van der Laan SW, de Vries JPM, Hoefer IE, de Borst GJ, Pasterkamp G, den Ruijter HM. Testosterone to estradiol ratio reflects systemic and plaque inflammation and predicts future cardiovascular events in men with severe atherosclerosis. Cardiovasc Res. 2018;7:20. [DOI] [PubMed] [Google Scholar]
- 55. De Maddalena C, Vodo S, Petroni A, Aloisi AM. Impact of testosterone on body fat composition. J Cell Physiol. 2012;227(12):3744–3748. [DOI] [PubMed] [Google Scholar]
- 56. Traish AM. Testosterone and weight loss: the evidence. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Joyner J, Hutley L, Cameron D. Intrinsic regional differences in androgen receptors and dihydrotestosterone metabolism in human preadipocytes. Horm Metab Res. 2002;34(5):223–228. [DOI] [PubMed] [Google Scholar]
- 58. Blouin K, Richard C, Brochu G, Hould FS, Lebel S, Marceau S, Biron S, Luu-The V, Tchernof A. Androgen inactivation and steroid-converting enzyme expression in abdominal adipose tissue in men. J Endocrinol. 2006;191(3):637–649. [DOI] [PubMed] [Google Scholar]
- 59. Crandall DL, Busler DE, Novak TJ, Weber RV, Kral JG. Identification of estrogen receptor beta RNA in human breast and abdominal subcutaneous adipose tissue. Biochem Biophys Res Commun. 1998;248(3):523–526. [DOI] [PubMed] [Google Scholar]
- 60. Mizutani T, Nishikawa Y, Adachi H, Enomoto T, Ikegami H, Kurachi H, Nomura T, Miyake A. Identification of estrogen receptor in human adipose tissue and adipocytes. J Clin Endocrinol Metab. 1994;78(4):950–954. [DOI] [PubMed] [Google Scholar]
- 61. Davis KE, D Neinast M, Sun K, M Skiles W, D Bills J, A Zehr J, Zeve D, D Hahner L, W Cox D, M Gent L, Xu Y, V Wang Z, A Khan S, Clegg DJ. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab. 2013;2(3):227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cooke PS, Heine PA, Taylor JA, Lubahn DB. The role of estrogen and estrogen receptor-alpha in male adipose tissue. Mol Cell Endocrinol. 2001;178(1-2):147–154. [DOI] [PubMed] [Google Scholar]
- 63. Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Després JP. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr. 1993;58(4):463–467. [DOI] [PubMed] [Google Scholar]
- 64. Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, Jerosch-Herold M, Lima JA, Ding J, Allison MA. Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA Study. JACC Cardiovasc Imaging. 2014;7(12):1221–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Franssens BT, Westerink J, van der Graaf Y, Nathoe HM, Visseren FLJ; SMART study group . Metabolic consequences of adipose tissue dysfunction and not adiposity per se increase the risk of cardiovascular events and mortality in patients with type 2 diabetes. Int J Cardiol. 2016;222:72–77. [DOI] [PubMed] [Google Scholar]
- 66. Bélanger C, Hould FS, Lebel S, Biron S, Brochu G, Tchernof A. Omental and subcutaneous adipose tissue steroid levels in obese men. Steroids. 2006;71(8):674–682. [DOI] [PubMed] [Google Scholar]
- 67. Mårin P, Lönn L, Andersson B, Odén B, Olbe L, Bengtsson BA, Björntorp P. Assimilation of triglycerides in subcutaneous and intraabdominal adipose tissues in vivo in men: effects of testosterone. J Clin Endocrinol Metab. 1996;81(3):1018–1022. [DOI] [PubMed] [Google Scholar]
- 68. Blouin K, Nadeau M, Perreault M, Veilleux A, Drolet R, Marceau P, Mailloux J, Luu-The V, Tchernof A. Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clin Endocrinol (Oxf). 2010;72(2):176–188. [DOI] [PubMed] [Google Scholar]
- 69. Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144(11):5081–5088. [DOI] [PubMed] [Google Scholar]
- 70. Allan CA, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17(3):224–232. [DOI] [PubMed] [Google Scholar]
- 71. Gupta V, Bhasin S, Guo W, Singh R, Miki R, Chauhan P, Choong K, Tchkonia T, Lebrasseur NK, Flanagan JN, Hamilton JA, Viereck JC, Narula NS, Kirkland JL, Jasuja R. Effects of dihydrotestosterone on differentiation and proliferation of human mesenchymal stem cells and preadipocytes. Mol Cell Endocrinol. 2008;296(1-2):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Escobar-Morreale HF, Alvarez-Blasco F, Botella-Carretero JI, Luque-Ramírez M. The striking similarities in the metabolic associations of female androgen excess and male androgen deficiency. Hum Reprod. 2014;29(10):2083–2091. [DOI] [PubMed] [Google Scholar]
- 74. Sahin-Efe A, Katsikeris F, Mantzoros CS. Advances in adipokines. Metabolism. 2012;61(12):1659–1665. [DOI] [PubMed] [Google Scholar]
- 75. McConway MG, Johnson D, Kelly A, Griffin D, Smith J, Wallace AM. Differences in circulating concentrations of total, free and bound leptin relate to gender and body composition in adult humans. Ann Clin Biochem. 2000;37(Pt 5):717–723. [DOI] [PubMed] [Google Scholar]
- 76. Mantzoros C, Flier JS, Lesem MD, Brewerton TD, Jimerson DC. Cerebrospinal fluid leptin in anorexia nervosa: correlation with nutritional status and potential role in resistance to weight gain. J Clin Endocrinol Metab. 1997;82(6):1845–1851. [DOI] [PubMed] [Google Scholar]
- 77. Pinilla L, Gonzalez D, Tena-Sempere M, Aguilar R, Aguilar E. Mechanisms of inhibitory action of kainic acid on prolactin secretion in male rats. J Endocrinol. 1996;151(1):159–167. [DOI] [PubMed] [Google Scholar]
- 78. Vettor R, De Pergola G, Pagano C, Englaro P, Laudadio E, Giorgino F, Blum WF, Giorgino R, Federspil G. Gender differences in serum leptin in obese people: relationships with testosterone, body fat distribution and insulin sensitivity. Eur J Clin Invest. 1997;27(12):1016–1024. [DOI] [PubMed] [Google Scholar]
- 79. Grosman H, Fabre B, Lopez M, Scorticati C, Lopez Silva M, Mesch V, Mazza O, Berg G. Complex relationship between sex hormones, insulin resistance and leptin in men with and without prostatic disease. Aging Male. 2016;19(1):40–45. [DOI] [PubMed] [Google Scholar]
- 80. Rizk NM, Sharif E. Leptin as well as free leptin receptor is associated with polycystic ovary syndrome in young women. Int J Endocrinol. 2015;2015:927805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chakrabarti J. Serum leptin level in women with polycystic ovary syndrome: correlation with adiposity, insulin, and circulating testosterone. Ann Med Health Sci Res. 2013;3(2):191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Donato J Jr, Cravo RM, Frazão R, Elias CF. Hypothalamic sites of leptin action linking metabolism and reproduction. Neuroendocrinology. 2011;93(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Giovambattista A, Suescun MO, Nessralla CC, França LR, Spinedi E, Calandra RS. Modulatory effects of leptin on leydig cell function of normal and hyperleptinemic rats. Neuroendocrinology. 2003;78(5):270–279. [DOI] [PubMed] [Google Scholar]
- 84. Herrid M, Xia Y, O’Shea T, McFarlane JR. Leptin inhibits basal but not gonadotrophin-stimulated testosterone production in the immature mouse and sheep testis. Reprod Fertil Dev. 2008;20(4):519–528. [DOI] [PubMed] [Google Scholar]
- 85. Behre HM, Simoni M, Nieschlag E. Strong association between serum levels of leptin and testosterone in men. Clin Endocrinol (Oxf). 1997;47(2):237–240. [DOI] [PubMed] [Google Scholar]
- 86. Luukkaa V, Pesonen U, Huhtaniemi I, Lehtonen A, Tilvis R, Tuomilehto J, Koulu M, Huupponen R. Inverse correlation between serum testosterone and leptin in men. J Clin Endocrinol Metab. 1998;83(9):3243–3246. [DOI] [PubMed] [Google Scholar]
- 87. Adan L, Bussières L, Trivin C, Souberbielle JC, Brauner R. Effect of short-term testosterone treatment on leptin concentrations in boys with pubertal delay. Horm Res. 1999;52(3):109–112. [DOI] [PubMed] [Google Scholar]
- 88. Janjgava S, Zerekidze T, Uchava L, Giorgadze E, Asatiani K. Influence of testosterone replacement therapy on metabolic disorders in male patients with type 2 diabetes mellitus and androgen deficiency. Eur J Med Res. 2014;19(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab. 2013;2(3):133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55(9):2319–2326. [DOI] [PubMed] [Google Scholar]
- 91. Ryo M, Nakamura T, Kihara S, Kumada M, Shibazaki S, Takahashi M, Nagai M, Matsuzawa Y, Funahashi T. Adiponectin as a biomarker of the metabolic syndrome. Circ J. 2004;68(11):975–981. [DOI] [PubMed] [Google Scholar]
- 92. Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects [published correction appears in Nature. 2004;431(7012):1123]. Nature. 2003;423(6941):762–769. [DOI] [PubMed] [Google Scholar]
- 93. Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52(7):1779–1785. [DOI] [PubMed] [Google Scholar]
- 94. Spranger J, Kroke A, Möhlig M, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361(9353):226–228. [DOI] [PubMed] [Google Scholar]
- 95. Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20(6):1595–1599. [DOI] [PubMed] [Google Scholar]
- 96. Riestra P, Garcia-Anguita A, Ortega L, Garcés C. Relationship of adiponectin with sex hormone levels in adolescents. Horm Res Paediatr. 2013;79(2):83–87. [DOI] [PubMed] [Google Scholar]
- 97. Amengual-Cladera E, Lladó I, Gianotti M, Proenza AM. Retroperitoneal white adipose tissue mitochondrial function and adiponectin expression in response to ovariectomy and 17β-estradiol replacement. Steroids. 2012;77(6):659–665. [DOI] [PubMed] [Google Scholar]
- 98. Chen CI, Hsu MI, Lin SH, Chang YC, Hsu CS, Tzeng CR. Adiponectin and leptin in overweight/obese and lean women with polycystic ovary syndrome. Gynecol Endocrinol. 2015;31(4):264–268. [DOI] [PubMed] [Google Scholar]
- 99. Toulis KA, Goulis DG, Farmakiotis D, Georgopoulos NA, Katsikis I, Tarlatzis BC, Papadimas I, Panidis D. Adiponectin levels in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Hum Reprod Update. 2009;15(3):297–307. [DOI] [PubMed] [Google Scholar]
- 100. Høst C, Gormsen LC, Hougaard DM, Christiansen JS, Pedersen SB, Gravholt CH. Acute and short-term chronic testosterone fluctuation effects on glucose homeostasis, insulin sensitivity, and adiponectin: a randomized, double-blind, placebo-controlled, crossover study. J Clin Endocrinol Metab. 2014;99(6):E1088–E1096. [DOI] [PubMed] [Google Scholar]
- 101. Berra M, Armillotta F, D’Emidio L, Costantino A, Martorana G, Pelusi G, Meriggiola MC. Testosterone decreases adiponectin levels in female to male transsexuals. Asian J Androl. 2006;8(6):725–729. [DOI] [PubMed] [Google Scholar]
- 102. Frederiksen L, Højlund K, Hougaard DM, Mosbech TH, Larsen R, Flyvbjerg A, Frystyk J, Brixen K, Andersen M. Testosterone therapy decreases subcutaneous fat and adiponectin in aging men. Eur J Endocrinol. 2012;166(3):469–476. [DOI] [PubMed] [Google Scholar]
- 103. Capllonch-Amer G, Lladó I, Proenza AM, García-Palmer FJ, Gianotti M. Opposite effects of 17-β estradiol and testosterone on mitochondrial biogenesis and adiponectin synthesis in white adipocytes. J Mol Endocrinol. 2014;52(2):203–214. [DOI] [PubMed] [Google Scholar]
- 104. Yarrow JF, Beggs LA, Conover CF, McCoy SC, Beck DT, Borst SE. Influence of androgens on circulating adiponectin in male and female rodents. PLoS One. 2012;7(10):e47315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Schoppet M, Preissner KT, Hofbauer LC. RANK ligand and osteoprotegerin: paracrine regulators of bone metabolism and vascular function. Arterioscler Thromb Vasc Biol. 2002;22(4):549–553. [DOI] [PubMed] [Google Scholar]
- 106. Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–319. [DOI] [PubMed] [Google Scholar]
- 107. Augoulea A, Vrachnis N, Lambrinoudaki I, Dafopoulos K, Iliodromiti Z, Daniilidis A, Varras M, Alexandrou A, Deligeoroglou E, Creatsas G. Osteoprotegerin as a marker of atherosclerosis in diabetic patients. Int J Endocrinol. 2013;2013:182060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pérez de Ciriza C, Moreno M, Restituto P, Bastarrika G, Simón I, Colina I, Varo N. Circulating osteoprotegerin is increased in the metabolic syndrome and associates with subclinical atherosclerosis and coronary arterial calcification. Clin Biochem. 2014;47(18):272–278. [DOI] [PubMed] [Google Scholar]
- 109. Reid P, Holen I. Pathophysiological roles of osteoprotegerin (OPG). Eur J Cell Biol. 2009;88(1):1–17. [DOI] [PubMed] [Google Scholar]
- 110. Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, Santer P, Smolen J, Poewe W, Willeit J. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109(18):2175–2180. [DOI] [PubMed] [Google Scholar]
- 111. Abedin M, Omland T, Ueland T, Khera A, Aukrust P, Murphy SA, Jain T, Gruntmanis U, McGuire DK, de Lemos JA. Relation of osteoprotegerin to coronary calcium and aortic plaque (from the Dallas Heart Study). Am J Cardiol. 2007;99(4):513–518. [DOI] [PubMed] [Google Scholar]
- 112. Niu Y, Zhang W, Yang Z, Li X, Wen J, Wang S, Zhang H, Wang X, Zhou H, Fang W, Qin L, Su Q. Association of plasma osteoprotegerin levels with the severity of lower extremity arterial disease in patients with type 2 diabetes. BMC Cardiovasc Disord. 2015;15(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hofbauer LC, Hicok KC, Chen D, Khosla S. Regulation of osteoprotegerin production by androgens and anti-androgens in human osteoblastic lineage cells. Eur J Endocrinol. 2002;147(2):269–273. [DOI] [PubMed] [Google Scholar]
- 114. Khosla S, Atkinson EJ, Dunstan CR, O’Fallon WM. Effect of estrogen versus testosterone on circulating osteoprotegerin and other cytokine levels in normal elderly men. J Clin Endocrinol Metab. 2002;87(4):1550–1554. [DOI] [PubMed] [Google Scholar]
- 115. Pepene CE, Crişan N, Coman I. Elevated serum receptor activator of nuclear factor kappa B ligand and osteoprotegerin levels in late-onset male hypogonadism. Clin Invest Med. 2011;34(4):E232. [DOI] [PubMed] [Google Scholar]
- 116. Frederiksen L, Glintborg D, Højlund K, Hougaard DM, Brixen K, Rasmussen LM, Andersen M. Osteoprotegerin levels decrease during testosterone therapy in aging men and are associated with changed distribution of regional fat. Horm Metab Res. 2013;45(4):308–313. [DOI] [PubMed] [Google Scholar]
- 117. Glintborg D, Hermann AP, Rasmussen LM, Andersen M. Plasma osteoprotegerin is associated with testosterone levels but unaffected by pioglitazone treatment in patients with polycystic ovary syndrome. J Endocrinol Invest. 2013;36(7):460–465. [DOI] [PubMed] [Google Scholar]
- 118. Holecki M, Zahorska-Markiewicz B, Janowska J, Nieszporek T, Wojaczyńska-Stanek K, Zak-Gołab A, Wiecek A. The influence of weight loss on serum osteoprotegerin concentration in obese perimenopausal women. Obesity (Silver Spring). 2007;15(8):1925–1929. [DOI] [PubMed] [Google Scholar]
- 119. Escobar-Morreale HF, Botella-Carretero JI, Martínez-García MA, Luque-Ramírez M, Alvarez-Blasco F, San Millán JL. Serum osteoprotegerin concentrations are decreased in women with the polycystic ovary syndrome. Eur J Endocrinol. 2008;159(3):225–232. [DOI] [PubMed] [Google Scholar]
- 120. Cildir G, Akıncılar SC, Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends Mol Med. 2013;19(8):487–500. [DOI] [PubMed] [Google Scholar]
- 121. Palomer X, Salvadó L, Barroso E, Vázquez-Carrera M. An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy. Int J Cardiol. 2013;168(4):3160–3172. [DOI] [PubMed] [Google Scholar]
- 122. Kanety H, Feinstein R, Papa MZ, Hemi R, Karasik A. Tumor necrosis factor alpha-induced phosphorylation of insulin receptor substrate-1 (IRS-1). Possible mechanism for suppression of insulin-stimulated tyrosine phosphorylation of IRS-1. J Biol Chem. 1995;270(40):23780–23784. [DOI] [PubMed] [Google Scholar]
- 123. Steyers CM, III, Miller FJ Jr. Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci. 2014;15(7):11324–11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhang Y, Yang X, Bian F, Wu P, Xing S, Xu G, Li W, Chi J, Ouyang C, Zheng T, Wu D, Zhang Y, Li Y, Jin S. TNF-α promotes early atherosclerosis by increasing transcytosis of LDL across endothelial cells: crosstalk between NF-κB and PPAR-γ. J Mol Cell Cardiol. 2014;72:85–94. [DOI] [PubMed] [Google Scholar]
- 125. Hector J, Schwarzloh B, Goehring J, Strate TG, Hess UF, Deuretzbacher G, Hansen-Algenstaedt N, Beil FU, Algenstaedt P. TNF-alpha alters visfatin and adiponectin levels in human fat. Horm Metab Res. 2007;39(4):250–255. [DOI] [PubMed] [Google Scholar]
- 126. Chen CW, Jian CY, Lin PH, Chen CC, Lieu FK, Soong C, Hsieh CC, Wan CY, Idova G, Hu S, Wang SW, Wang PS. Role of testosterone in regulating induction of TNF-α in rat spleen via ERK signaling pathway. Steroids. 2016;111:148–154. [DOI] [PubMed] [Google Scholar]
- 127. Corrales JJ, Almeida M, Burgo R, Mories MT, Miralles JM, Orfao A. Androgen-replacement therapy depresses the ex vivo production of inflammatory cytokines by circulating antigen-presenting cells in aging type-2 diabetic men with partial androgen deficiency. J Endocrinol. 2006;189(3):595–604. [DOI] [PubMed] [Google Scholar]
- 128. Pawelczak M, Rosenthal J, Milla S, Liu YH, Shah B. Evaluation of the pro-inflammatory cytokine tumor necrosis factor-α in adolescents with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2014;27(6):356–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lim JP, Leung BP, Ding YY, Tay L, Ismail NH, Yeo A, Yew S, Chong MS. Monocyte chemoattractant protein-1: a proinflammatory cytokine elevated in sarcopenic obesity. Clin Interv Aging. 2015;10:605–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ruige JB, Bekaert M, Lapauw B, Fiers T, Lehr S, Hartwig S, Herzfeld de Wiza D, Schiller M, Passlack W, Van Nieuwenhove Y, Pattyn P, Cuvelier C, Taes YE, Sell H, Eckel J, Kaufman JM, Ouwens DM. Sex steroid-induced changes in circulating monocyte chemoattractant protein-1 levels may contribute to metabolic dysfunction in obese men. J Clin Endocrinol Metab. 2012;97(7):E1187–E1191. [DOI] [PubMed] [Google Scholar]
- 132. Morooka N, Ueguri K, Yee KKL, Yanase T, Sato T. Androgen-androgen receptor system improves chronic inflammatory conditions by suppressing monocyte chemoattractant protein-1 gene expression in adipocytes via transcriptional regulation. Biochem Biophys Res Commun. 2016;477(4):895–901. [DOI] [PubMed] [Google Scholar]
- 133. Glintborg D, Andersen M, Richelsen B, Bruun JM. Plasma monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1alpha are increased in patients with polycystic ovary syndrome (PCOS) and associated with adiposity, but unaffected by pioglitazone treatment. Clin Endocrinol (Oxf). 2009;71(5):652–658. [DOI] [PubMed] [Google Scholar]
- 134. Eder K, Baffy N, Falus A, Fulop AK. The major inflammatory mediator interleukin-6 and obesity. Inflamm Res. 2009;58(11):727–736. [DOI] [PubMed] [Google Scholar]
- 135. Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Role of interleukin-6 in human nonalcoholic steatohepatitis. Response to Dogru et al. Am J Gastroenterol. 2009;104(3):author reply 788. [DOI] [PubMed] [Google Scholar]
- 136. Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res. 2001;9(7):414–417. [DOI] [PubMed] [Google Scholar]
- 137. Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, Furlanetto RW, Mooney RA. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem. 2003;278(16):13740–13746. [DOI] [PubMed] [Google Scholar]
- 138. Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17(8):884–886. [DOI] [PubMed] [Google Scholar]
- 139. Rohde T, MacLean DA, Richter EA, Kiens B, Pedersen BK. Prolonged submaximal eccentric exercise is associated with increased levels of plasma IL-6. Am J Physiol. 1997;273(1 Pt 1):E85–E91. [DOI] [PubMed] [Google Scholar]
- 140. Kosmidou I, Vassilakopoulos T, Xagorari A, Zakynthinos S, Papapetropoulos A, Roussos C. Production of interleukin-6 by skeletal myotubes: role of reactive oxygen species. Am J Respir Cell Mol Biol. 2002;26(5):587–593. [DOI] [PubMed] [Google Scholar]
- 141. Knudsen JG, Bertholdt L, Joensen E, Lassen SB, Hidalgo J, Pilegaard H. Skeletal muscle interleukin-6 regulates metabolic factors in iWAT during HFD and exercise training. Obesity (Silver Spring). 2015;23(8):1616–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, Vidal H, Hainque B. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85(9):3338–3342. [DOI] [PubMed] [Google Scholar]
- 143. Kim SK, Shin MS, Jung BK, Shim JY, Won HS, Lee PR, Kim A. Effect of dehydroepiandrosterone on lipopolysaccharide-induced interleukin-6 production in DH82 cultured canine macrophage cells. J Reprod Immunol. 2006;70(1-2):71–81. [DOI] [PubMed] [Google Scholar]
- 144. Gordon CM, LeBoff MS, Glowacki J. Adrenal and gonadal steroids inhibit IL-6 secretion by human marrow cells. Cytokine. 2001;16(5):178–186. [DOI] [PubMed] [Google Scholar]
- 145. Freeman BM, Mountain DJ, Brock TC, Chapman JR, Kirkpatrick SS, Freeman MB, Klein FA, Grandas OH. Low testosterone elevates interleukin family cytokines in a rodent model: a possible mechanism for the potentiation of vascular disease in androgen-deficient males. J Surg Res. 2014;190(1):319–327. [DOI] [PubMed] [Google Scholar]
- 146. Peng Z, Sun Y, Lv X, Zhang H, Liu C, Dai S. Interleukin-6 levels in women with polycystic ovary syndrome: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0148531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Küçük M, Altınkaya SO, Nergiz S, Sezer SD, Yüksel H, Bağlı İ, Yıldız G. Interleukin-6 levels in relation with hormonal and metabolic profile in patients with polycystic ovary syndrome. Gynecol Endocrinol. 2014;30(6):423–427. [DOI] [PubMed] [Google Scholar]
- 148. Coward J, Kulbe H, Chakravarty P, Leader D, Vassileva V, Leinster DA, Thompson R, Schioppa T, Nemeth J, Vermeulen J, Singh N, Avril N, Cummings J, Rexhepaj E, Jirström K, Gallagher WM, Brennan DJ, McNeish IA, Balkwill FR. Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res. 2011;17(18):6083–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Vingren JL, Budnar RG Jr, McKenzie AL, Duplanty AA, Luk HY, Levitt DE, Armstrong LE. The acute testosterone, growth hormone, cortisol and interleukin-6 response to 164-km road cycling in a hot environment. J Sports Sci. 2016;34(8):694–699. [DOI] [PubMed] [Google Scholar]
- 150. Corrales JJ, Almeida M, Miralles JM, Orfao A. Persistence of androgenic effects on the production of proinflammatory cytokines by circulating antigen-presenting cells after withdrawal of testosterone treatment in aging type 2 diabetic men with partial androgen deficiency. Fertil Steril. 2009;92(1):311–319. [DOI] [PubMed] [Google Scholar]
- 151. Pischon T, Bamberger CM, Kratzsch J, Zyriax BC, Algenstaedt P, Boeing H, Windler E. Association of plasma resistin levels with coronary heart disease in women. Obes Res. 2005;13(10):1764–1771. [DOI] [PubMed] [Google Scholar]
- 152. Langheim S, Dreas L, Veschini L, Maisano F, Foglieni C, Ferrarello S, Sinagra G, Zingone B, Alfieri O, Ferrero E, Maseri A, Ruotolo G. Increased expression and secretion of resistin in epicardial adipose tissue of patients with acute coronary syndrome. Am J Physiol Heart Circ Physiol. 2010;298(3):H746–H753. [DOI] [PubMed] [Google Scholar]
- 153. Cheng JM, Akkerhuis KM, Battes LC, van Vark LC, Hillege HL, Paulus WJ, Boersma E, Kardys I. Biomarkers of heart failure with normal ejection fraction: a systematic review. Eur J Heart Fail. 2013;15(12):1350–1362. [DOI] [PubMed] [Google Scholar]
- 154. Lubos E, Messow CM, Schnabel R, Rupprecht HJ, Espinola-Klein C, Bickel C, Peetz D, Post F, Lackner KJ, Tiret L, Münzel T, Blankenberg S. Resistin, acute coronary syndrome and prognosis results from the AtheroGene study. Atherosclerosis. 2007;193(1):121–128. [DOI] [PubMed] [Google Scholar]
- 155. Lee SH, Ha JW, Kim JS, Choi EY, Park S, Kang SM, Choi D, Jang Y, Chung N. Plasma adiponectin and resistin levels as predictors of mortality in patients with acute myocardial infarction: data from infarction prognosis study registry. Coron Artery Dis. 2009;20(1):33–39. [DOI] [PubMed] [Google Scholar]
- 156. Fontana A, Spadaro S, Copetti M, Spoto B, Salvemini L, Pizzini P, Frittitta L, Mallamaci F, Pellegrini F, Trischitta V, Menzaghi C. Association between resistin levels and all-cause and cardiovascular mortality: a new study and a systematic review and meta-analysis. PLoS One. 2015;10(3):e0120419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Mojiminiyi OA, Abdella NA. Associations of resistin with inflammation and insulin resistance in patients with type 2 diabetes mellitus. Scand J Clin Lab Invest. 2007;67(2):215–225. [DOI] [PubMed] [Google Scholar]
- 158. Menzaghi C, Bacci S, Salvemini L, Mendonca C, Palladino G, Fontana A, De Bonis C, Marucci A, Goheen E, Prudente S, Morini E, Rizza S, Kanagaki A, Fini G, Mangiacotti D, Federici M, De Cosmo S, Pellegrini F, Doria A, Trischitta V. Serum resistin, cardiovascular disease and all-cause mortality in patients with type 2 diabetes. PLoS One. 2013;8(6):e64729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. González Hernández A, Cabrera de León A, Dominguez Coello S, Almeida González D, Rodríguez Pérez MC, Brito Díaz B, Aguirre-Jaime A, Díaz-Chico BN. Serum resistin and polymorphisms of androgen receptor GAGn and GGNn and aromatase TTTAn. Obesity (Silver Spring). 2008;16(9):2107–2112. [DOI] [PubMed] [Google Scholar]
- 160. Chang YH, Chang DM, Lin KC, Shin SJ, Lee YJ. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: a meta-analysis and systemic review. Diabetes Metab Res Rev. 2011;27(6):515–527. [DOI] [PubMed] [Google Scholar]
- 161. Esteghamati A, Morteza A, Zandieh A, Jafari S, Rezaee M, Nakhjavani M, Jamali A, Esteghamati AR, Khalilzadeh O. The value of visfatin in the prediction of metabolic syndrome: a multi-factorial analysis. J Cardiovasc Transl Res. 2012;5(4):541–546. [DOI] [PubMed] [Google Scholar]
- 162. Sun Y, Wu Z, Wei L, Liu C, Zhu S, Tang S. High-visfatin levels in women with polycystic ovary syndrome: evidence from a meta-analysis. Gynecol Endocrinol. 2015;31(10):808–814. [DOI] [PubMed] [Google Scholar]
- 163. Baillargeon J, Deer RR, Kuo YF, Zhang D, Goodwin JS, Volpi E. Androgen therapy and rehospitalization in older men with testosterone deficiency. Mayo Clin Proc. 2016;91(5):587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]