Abstract
Context
Surrogate indices of muscle and hepatic insulin sensitivity derived from an oral glucose tolerance test (OGTT) are frequently used in clinical studies. However, the predictive accuracy of these indices has not been validated.
Design
In this cross-sectional study, hyperinsulinemic-euglycemic glucose clamp with tritiated glucose infusion and a 75-g OGTT were performed in individuals (n = 659, aged 18 to 49 years, body mass index of 16 to 64 kg/m2) with varying degrees of glucose tolerance. A calibration model was used to assess the ability of OGTT-derived, tissue-specific surrogate indices [hepatic insulin resistance index (HIRI) and muscle insulin sensitivity index (MISI)] to predict insulin sensitivity/resistance indices derived from the reference glucose clamp [Hepatic-IRbasal, a product of fasting plasma insulin and hepatic glucose production (HGP), Hepatic-IRclamp, reciprocal of the percent suppression of HGP during the insulin clamp corrected for plasma insulin concentration, and Muscle-ISclamp, a measure of peripheral glucose disposal]. Predictive accuracy was assessed by root mean squared error of prediction and leave-one-out, cross-validation-type square root of the mean squared error of prediction.
Results
HIRI and MISI were correlated with their respective clamp-derived indices. HIRI was negatively related to Muscle-ISclamp (r = –0.62, P < 0.0001) and MISI correlated with Hepatic-IR derived from the clamp (Hepatic-IRbasal: r = –0.48, P < 0.0001 and Hepatic-IRclamp: r = –0.41, P < 0.0001). However, the accuracy of HIRI and MISI to predict Hepatic-IR (basal or during clamp) was not significantly different. Likewise, the ability of HIRI and MISI to predict Muscle-ISclamp was also similar.
Conclusion
Our findings indicate that the surrogate indices derived from an OGTT are accurate in predicting insulin sensitivity but are not tissue specific.
Insulin resistance is typically defined as decreased sensitivity to metabolic actions of insulin, including peripheral glucose disposal and suppression of hepatic glucose production (HGP) [1–3]. Skeletal muscle and hepatic insulin resistance play a major pathophysiological role in type 2 diabetes and is frequently observed in obesity, hypertension, dyslipidemia, coronary heart disease, and the metabolic syndrome [4, 5]. In insulin-resistant individuals, insulin action is impaired at multiple sites, including the liver, muscle, adipose tissue, and the vasculature [3, 6]. Skeletal muscle insulin resistance is characterized by reduced insulin-mediated glucose disposal and contributes to postprandial hyperglycemia [7]. Impaired insulin-mediated suppression of HGP, a feature of hepatic insulin resistance, is partly due to enhanced gluconeogenesis and reduced inhibition of glycogenolysis that leads to fasting hyperglycemia [8]. In fact, hepatic and muscle insulin resistance differentially affect the development of impaired fasting glucose and postprandial glucose intolerance [9, 10].
Lifestyle interventions and weight reduction improve insulin action, thereby decreasing the progression to type 2 diabetes [11, 12]. Some pharmacological interventions (e.g., metformin and thiazolidinediones) also improve insulin action and reduce the risk of conversion from impaired glucose tolerance (IGT) to type 2 diabetes [11]. However, these interventions appear to modulate hepatic and skeletal muscle insulin sensitivity differently [13–15]. Therefore, accurate tissue-specific metabolic phenotyping and quantitation of the presence and severity of insulin resistance, particularly in nondiabetic/prediabetic subjects, to identify high-risk individuals and initiate intervention programs are important.
The “hyperinsulinemic euglycemic glucose clamp” is widely accepted as the reference method to evaluate insulin sensitivity because it can directly measure insulin-mediated suppression of HGP and whole-body glucose disposal [16]. However, the glucose clamp is labor intensive, technically demanding, and time consuming, thus precluding its use in epidemiological studies. Consequently, surrogate indices are extensively used to quantify insulin action [2]. After an oral glucose challenge, the HGP is maximally suppressed reaching a nadir at approximately 60 minutes and remains suppressed for the duration (~180 minutes) [17, 18]. Therefore, glucose uptake by peripheral tissues (e.g., muscle and adipose tissue) primarily determines the rate of decrease in plasma glucose concentration from its peak value to its nadir during an oral glucose tolerance test (OGTT). Based on this observation, Abdul-Ghani et al. [19] developed surrogate indices of hepatic and muscle insulin sensitivity/resistance from an OGTT that has been widely used. However, the predictive accuracy of these indices has not been examined. In this study, we compared the ability of these surrogate indices to accurately predict tissue-specific insulin sensitivity as determined by the reference glucose clamp method.
1. Research Design and Study Methods
A total of 659 volunteers participating in a longitudinal study of predictors of type 2 diabetes were included in this study [20, 21]. This subset included individuals in their first visit who had complete data on OGTT, insulin action measured by the gold-standard glucose clamp technique, and body composition measured by either underwater weighing with simultaneous determination of residual lung volume by helium dilution [22] or dual energy x-ray absorptiometry (DPX-L; Lunar Radiation, Madison, WI) [23]. The absorptiometry measures were converged to comparable underwater weighing values, using previously derived equations to calculate body fat percentage [24]. All subjects provided written informed consent for their participation. The study was approved by the Institutional Review Board, National Institute of Diabetes & Digestive & Kidney Diseases. Ethnicity of the participants was identified by self-report. All subjects underwent laboratory testing, history, and physical examination to rule out any other medical disorders. Subjects were not on any medications and were nonsmokers. No subjects had a history of chronic viral hepatitis or liver disease. Subjects were abstinent from alcohol use for at least 2 weeks. Upon admission, subjects were fed a weight-maintaining diet (caloric distribution: 50% carbohydrates, 30% fat, and 20% protein), abstained from strenuous activities, and underwent an OGTT and glucose clamp after at least 3 days of weight-maintaining diet.
A. OGTT
After an overnight fast, a 75-g oral glucose load was given. Blood samples drawn at time 0-, 30-, 60-, 120-, and 180-minute time intervals for measurement of plasma glucose and insulin concentrations. Depending on the results of the OGTT, subjects were categorized as either having normal glucose tolerance (NGT), IGT, or type 2 diabetes mellitus per American Diabetes Association 2003 criteria [25]. Plasma insulin concentrations were measured by three different radioimmunoassays over time: the modified Herbert-Lau assay, Concept 4 (ICN, Costa Mesa, CA), and Access (Beckman Instruments, Fullerton, CA). Through regression equations, all measurements of plasma insulin were normalized to the original radioimmunoassay (modified Herbert-Lau assay). Plasma glucose concentrations were determined by the glucose oxidase method (Beckman Instruments).
B. HIRI and MISI
OGTT-derived, tissue-specific surrogate indices for insulin sensitivity were derived as previously reported [19]. The hepatic insulin resistance index (HIRI) was calculated as the product of the glucose area under the curve (AUC; mg/min–1/dL) and insulin AUC (µU/min–1/mL) during the first 30 minutes during the OGTT. The muscle insulin sensitivity index (MISI) was derived by dividing the rate of decline in plasma glucose concentration, calculated as the slope of the decrease in plasma glucose concentration (dG/dt) from peak to nadir, by the mean plasma insulin concentration and expressed as 10–2 [(mg/dL/min–1)/(µU/mL)].
C. Hyperinsulinemic-Euglycemic Glucose Clamp
Insulin action was evaluated by glucose clamp [21]. After an overnight fast of ≥10 hours, clamp studies were performed the following morning. In postabsorptive states, ambient plasma insulin suppresses hepatic endogenous glucose production (HGP). Impairment in this action indicates hepatic insulin resistance. Thus, the product of basal HGP and plasma insulin concentration is a measure of hepatic insulin resistance. HGP was determined by intravenous 3-[3H] glucose infusion [primed bolus (1.11 MBq) followed by continuous infusion (0.0111 MBq/min) for 2 hours]. Basal HGP was calculated during the fasting state as the 3-[3H] glucose infusion rate divided by the steady-state plasma 3-[3H] glucose-specific activity (measured with Beckman LS6500 scintillation counter; Beckman Instruments). Fasting plasma insulin levels were measured as previously described. Hepatic-IRBasal is thus the product of HGP and fasting plasma insulin [26]. Although performed at the beginning of a clamp procedure, this direct measure of hepatic sensitivity is not strictly clamp based and is not directly dependent on the insulin infusion. Two hours after beginning the infusion of 3-[3H] glucose, a 10-minute priming dose of insulin was administered followed by a continuous infusion at a constant rate (40 mU/m2/min–1). Plasma glucose concentrations from arterialized blood samples were measured at the bedside every 5 minutes with a glucose analyzer. An intravenous infusion of dextrose was adjusted to maintain the plasma glucose concentration ~100 mg/dL. Mean parameter values were used to calculate SIclamp [defined as M/I corrected for estimated metabolic body size (fat free mass + 17.7), where M is the glucose infusion rate (mg/min) and I is the steady-state plasma insulin concentrations (µU/mL)] [27]. The rate of glucose disposal (M) was defined as the average of the glucose infusion rate during the last 40 minutes of the insulin infusion. The measure SIclamp represents insulin-mediated peripheral glucose disposal and signifies predominantly skeletal muscle insulin sensitivity (Muscle-ISclamp). During the insulin clamp, steady-state HGP was the difference between the appearance rates of glucose in the plasma calculated from 3-[3H] glucose measurements during insulin infusion and the glucose infusion rate. Hepatic-IRclamp, an additional index of hepatic insulin resistance during the clamp, was derived as the reciprocal of the percent suppression of HGP during the insulin clamp corrected for plasma insulin concentration ([1/(100 × (HGPbasal–HGPclamp)/HGPbasal ÷ I]).
D. Statistical Analysis
All indices were log transformed to approximate normality. Subject characteristics are depicted as mean ± SD or median (25th to 75th percentile). Normally distributed variables were analyzed by Student t test and for multiple groups by one-way ANOVA. Linear regression models were used to calculate least square means and 95% CI for various insulin sensitivity/resistance indices after adjusting for age and sex. We used a calibration model to assess the ability of these tissue-specific surrogate indices derived from an OGTT to predict insulin sensitivity measures derived from a clamp [28, 29]. Using calibration models is particularly appropriate when an accurate measurement method, such as the glucose clamp, is compared with an indirect method such as MISI and HIRI. Calibration is inverse regression in which the surrogate method is regressed on the accurate measurement method and new x* (clamp derived) is predicted for a given y* (surrogate derived). A calibration model, (SIclamp)i = α + β(OGTT-surrogate)i + εi, where εi is random error for the ith subject, was fitted. Random error having a Gaussian distribution with μ = 0 and constant variance was assumed. Two types of predicted residuals were considered: The first type of residual was the difference between measured clamp variable (xi for the ith subject) and fitted SIclamp (xˆi = α + βˆyi) with all subjects included in the estimation of model parameters α and β. The second type of residual considered is a cross-validation-type predicted residual e(i) = xi – xˆ(i), where xi is still the measured clamp but xˆ(i) is the predicted clamp from the calibration model that excludes the ith subject. Two measures of predictive accuracy were calculated from these residuals: square root of the mean squared error of prediction (RMSE) and leave-one-out cross-validation-type root mean squared error of prediction (CVPE). Smaller values indicate better predictive power, and CVPE is more robust than RMSE because CVPE uses an estimate that excludes the ith subject when predicting results for the ith subject. To compare predictive accuracy of the surrogates in terms of CVPE/RMSE, the one-sided alternative hypotheses that HIRI had a smaller RMSE/CVPE than MISI for hepatic insulin resistance and vice versa for muscle insulin resistance were established. We used a bootstrap percentile method with 60,000 replications performed for each comparison. The RMSEs (or CVPEs) corresponding to surrogate indices were derived from the same group of subjects and thus the bootstrap method is appropriate. The P values calculated from comparisons of RMSE and CVPE were for pairwise comparisons.
2. Results
Baseline demographics, clinical characteristics, glucose metabolism indices, and measurements of insulin sensitivity of our study subjects are summarized in Table 1. In models adjusted for age and sex, basal HGP was higher in patients with type 2 diabetes mellitus, but hepatic insulin resistance as determined by Hepatic-IRbasal, Hepatic-IRclamp, and HIRI was significantly higher in subjects with IGT and type 2 diabetes mellitus. Similarly, muscle insulin sensitivity as determined by the glucose clamp was significantly lower in IGT and type 2 diabetes mellitus when compared with NGT individuals. MISI was lower in the IGT group but not significantly different than NGT. In terms of racial composition, our study population was comprised of American Indians (n = 516), African Americans (n = 33), and white individuals (n = 110). Hepatic-IRbasal, Hepatic-IRclamp, and HIRI were significantly higher, and Muscle-ISclamp and MISI were significantly lower, in American Indians when compared with African Americans and white individuals (data not shown).
Table 1.
Characteristics of Study Participants
| NGT (n = 446) | IGT (n = 188) | T2DM (n = 25) | P Value | |
|---|---|---|---|---|
| Age, y | 27 ± 6 | 29 ± 7 | 30 ± 7 | <0.05 |
| Female (%, n) | 36% (n = 159) | 54% (n = 102) | 64% (n = 16) | <0.0001 |
| Body mass index, kg/m2 | 32 ± 8a | 37 ± 8b | 39 ± 9c | <0.0001 |
| Body fat (%) | 30 ± 9a | 36 ± 8b | 38 ± 8b | <0.0001 |
| Metabolic parameters | ||||
| Fasting plasma glucose, mg/dL | 86 ± 7a | 97 ± 10b | 128 ± 36c | <0.0001 |
| Fasting plasma insulin, pmol/L | 30 (22)a | 47 (30)b | 57 (41)b | <0.0001 |
| 2-h plasma glucose, mg/dL | 105 ± 20a | 150 ± 23b | 239 ± 54c | <0.0001 |
| Indices of insulin sensitivity | ||||
| Basal HGP (mg/kgEMBS/min) | 1.9 (0.3)a | 1.9 (0.3)a | 2.1 (0.8)b | <0.0001 |
| Hepatic-IRbasal (mg/kg–1/min–1/µU/mL) | 55 (40)a | 82 (56)b | 112 (74)c | <0.0001 |
| Hepatic-IRclamp | 1.47 (0.93)a | 1.85 (1.03)b | 2.29 (1.79)b | <0.0001 |
| HIRI | 11.40 (10.26)a | 15.66 (10.54)b | 15.18 (13.23)b | <0.0001 |
| Muscle-ISclamp 10–3 (mg/kg–1/min–1)/(µU/mL) | 21.13 (18.43)a | 15.74 (9.87)b | 12.87 (9.87)b | <0.0001 |
| MISI | 14.93 (15.30)a | 12.87 (11.27)b | 15.57 (16.31)a | <0.0001 |
Data are presented as mean ± SD or median (interquartile range). HIRI is measured as 106 [glucose AUC (mg/min–1/dL) × insulin AUC (µU/min–1/mL) during the first 30 min of the OGTT], and MISI is measured as 10–2 (mg/dL/min–1)/(µU/mL). One-way ANOVA followed by Tukey post hoc test was used to compare differences between groups. P values are adjusted for age and sex. Means or medians sharing the same superscript letter are not significantly different from each other across glucose tolerance status (P < 0.05)
Abbreviations: EMBS, estimated metabolic body size (fat free mass + 17.7); T2DM, type 2 diabetes mellitus.
When we compared relationships between tissue-specific surrogate indices and clamp-derived indices, simple linear regression analysis showed modest correlations between Hepatic-IR and HIRI (Hepatic-IRbasal vs HIRI: r = 0.57, P < 0.0001 and Hepatic-IRclamp vs HIRI: r = 0.49, P < 0.001) and between Muscle-ISclamp and MISI (r = 0.50, P < 0.0001). Indices of hepatic insulin resistance were negatively associated with indices of muscle insulin sensitivity (Muscle-ISclamp vs Hepatic-IRbasal: r = –0.78, P < 0.0001; Muscle-ISclamp vs Hepatic-IRclamp: r = –0.83, P < 0.0001; Muscle-ISclamp vs HIRI: r = –0.62, P < 0.0001; MISI vs Hepatic-IRbasal: r = –0.48, P < 0.0001; MISI vs Hepatic-IRclamp: r = –0.41, P < 0.0001; and MISI vs HIRI: r = –0.53, P < 0.0001). Sex did not modulate the strength of these relationships. A significant portion of the study cohort (~78%) are American Indians who are well known to be insulin resistant [30]. The primary purpose of these surrogate indices of insulin sensitivity is to recognize and characterize tissue-specific insulin resistance. Therefore, we examined if the insulin resistance status affected the relationship between clamp-derived measure and surrogate index. Based on prior clamp studies that use an insulin infusion rate of 40 mU/m2/min–1, we defined insulin resistance as an M value expressed as a function of metabolic size (FFM + 17.7) < 3.6 mg/FFM + 17.7*min [31, 32]. Based on this criterion, 170 and 489 subjects were characterized as insulin sensitive and insulin resistant, respectively. We then examined if the insulin resistance status modified the linear relationships between Muscle-ISClamp and MISI. The correlation coefficients between Muscle-ISclamp and MISI were similar in the insulin-resistant (r = 0.39, P < 0.0001) and insulin-sensitive groups (r = 0.35, P < 0.0001).
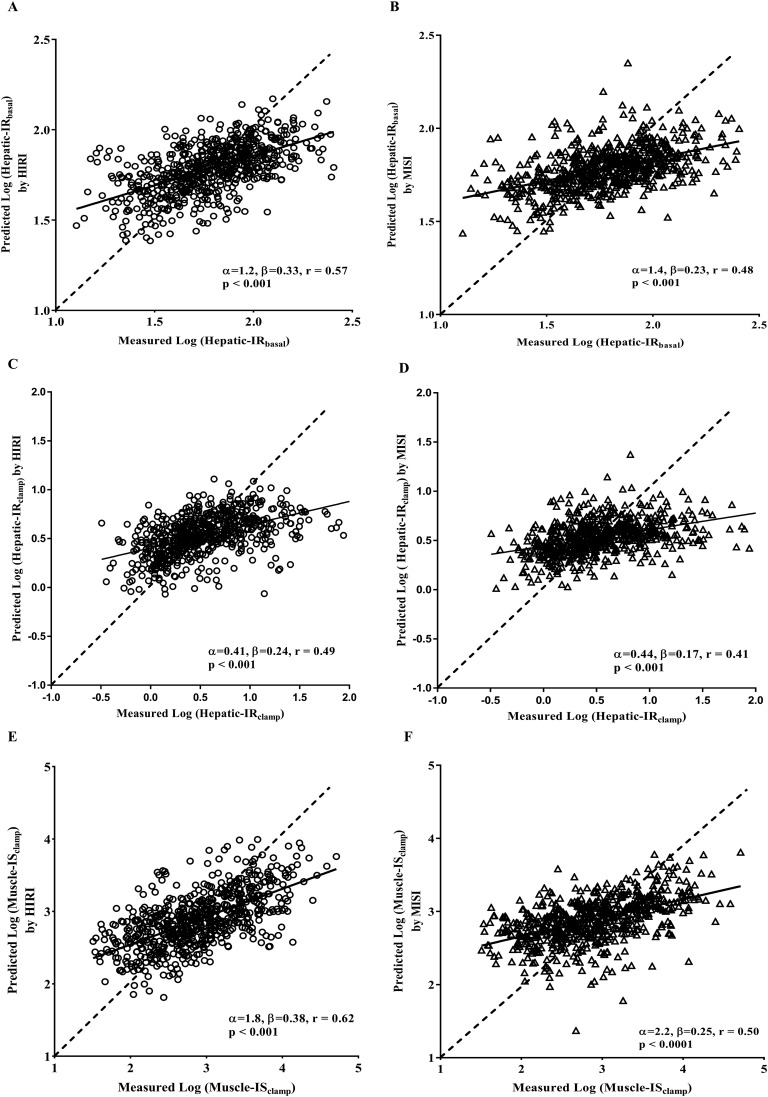
The absolute accuracy of the tissue-specific surrogate indices derived from OGTT were assessed using the calibration model. Experimentally determined hepatic IR and muscle IS from the clamp studies were regressed on each surrogate index, and data were fit with a calibration model. Using the leave-one-out cross-validation analysis, we used the fitted calibration model to generate plots for each surrogate index (HIRI and MISI), comparing predicted clamp values (generated from each surrogate index) with actual values for each subject (Fig. 1). Perfect prediction by a surrogate index would result in predicted values along a straight line with a slope of 1 and a y-intercept of 0. Both HIRI and MISI predict their respective clamp-derived indices reasonably well. However, as seen in Fig. 1(B), 1(D), and 1(E), MISI and HIRI predicted Hepatic-IRclamp and Muscle-ISclamp, respectively, as well. These results suggest that although the surrogate indices accurately predict insulin sensitivity/resistance, they are not tissue specific.
Figure 1.
Comparison between measured and predicted clamp insulin sensitivity/resistance measures (Hepatic-IRbasal, Hepatic-IRclamp, and Muscle-ISClamp) from tissue-specific surrogate indexes of insulin sensitivity/resistance. Predicted liver or muscle reference measures shown for each surrogate index were calculated using the leave-one-out cross-validation analysis of the calibration model as described in the research design and methods. The dashed line indicates ideal predictive accuracy. The solid line indicates the linear least-squares fit of measured vs predicted liver or muscle measure. Correlation coefficients (r) and respective P values are shown in each panel. (A, C, and E) Results derived from HIRI. (B, D, and F) Results derived from MISI.
Further, we have calculated cross-validation-type error (CVPE) and root mean square analysis (RMSE) from calibration analysis to quantitatively assess prediction errors of these surrogate indices of insulin sensitivity/resistance. RMSE and CVPE analysis summarized in Table 2 reveals that both MISI and HIRI equally predict the insulin resistance at the liver and muscle tissues, with the same precision. These results together suggest that although the surrogate indices accurately predict insulin sensitivity/resistance, they are not tissue specific.
Table 2.
CVPE and RMSE Calculated From Calibration Analysis of Tissue-Specific Surrogate Indices of Insulin Sensitivity/Resistance
| RMSE Hepatic-IRbasal | CVPE Hepatic-IRbasal | RMSE Hepatic-IRclamp | CVPE Hepatic-IRclamp | RMSE Muscle-ISclamp | CVPE Muscle-ISclamp | |
|---|---|---|---|---|---|---|
| HIRI | 0.20 | 0.20 | 0.37 | 0.37 | 0.48 | 0.48 |
| MISI | 0.21 | 0.21 | 0.39 | 0.39 | 0.54 | 0.54 |
| P value | 0.96 | 1.00 | 0.95 | 1.00 | 0.99 | 0.48 |
CVPE and RMSE were calculated from calibration analysis of tissue-specific surrogate indices of insulin sensitivity derived from an OGTT as described in the research design and methods. P values correspond to comparisons between the surrogate indexes.
3. Discussion
In this study, we examined the absolute predictive accuracy of tissue-specific insulin sensitivity/resistance indices derived from an OGTT by comparing it with corresponding glucose clamp estimates. HIRI and MISI are reasonably accurate in predicting Hepatic-IRclamp and Muscle-ISclamp, respectively. However, MISI was as accurate as HIRI in predicting Hepatic-IRclamp. Likewise, HIRI and MISI predicted Muscle-ISclamp with similar accuracy. These findings suggest that surrogate indices derived from an OGTT, HIRI, and MISI may not be tissue-specific as originally proposed [19].
Insulin resistance, a characteristic feature of type 2 diabetes [33] [34], is associated with obesity and atherosclerotic cardiovascular disease [4]. As impaired insulin action is the cornerstone for the development of type 2 diabetes and metabolic syndrome, there has been widespread interest in the development of techniques and methods to assess insulin sensitivity [2]. Simple, feasible, reliable, and accurate methods for quantifying insulin sensitivity is necessary to identify insulin-resistant individuals [2]. Hepatic and muscle insulin sensitivity can be simultaneously measured during a glucose clamp when used in combination with radiolabeled glucose [35]. Because of the nature of the clamp study, it is not a feasible option for large studies. Therefore, to quantitate insulin sensitivity, several simpler methods were derived from the OGTT [35], a commonly used test to assess glucose homeostasis in clinical practice, epidemiological studies, and research settings. Based on the kinetics of HGP and peripheral glucose disposal during an OGTT, Abdul-Ghani et al. [19] developed indices of hepatic and skeletal muscle insulin sensitivity in nondiabetic subjects. The study has been cited over 200 times and these indices have been used in multiple studies and conclusions are based on the assumed tissue “selectivity” of these indices [36–40].
Our study cohort was large (n = 659) with normal healthy subjects and subjects with varying degrees of obesity and glucose intolerance. In addition, there was a wide range of hepatic insulin resistance and muscle insulin sensitivity. The utility of the OGTT-derived surrogate indices is primarily in insulin-resistant subjects, especially following interventions or in large cross-sectional studies. Therefore, examining the accuracy of these indices in this cohort with a substantial portion of insulin-resistant subjects does not affect robustness of the calibration analysis.
The study by Abdul-Ghani et al. [19] included Mexican-American subjects (n = 155) who received a euglycemic-hyperinsulinemic clamp (40 mU/m2/min–1) and a 75-g OGTT. In that study, OGTT-derived MISI strongly correlated with insulin sensitivity, measured with the hyperinsulinemic-euglycemic clamp (r = 0.78, P < 0.0001). Similarly, HIRI was significantly related to a direct measure of hepatic insulin resistance (r = 0.64, P < 0.001). In a sample of nondiabetic individuals (n = 368) from the Relationship between Insulin Sensitivity and Cardiovascular Disease (RISC) study, Vangipurapu et al. [41] have also examined the relationship between HIRI and Hepatic-IRbasal. They report that HIRI was directly related to Hepatic-IRbasal with a correlation coefficient of r = 0.58. The correlation coefficient (r = 0.57) between Hepatic-IRbasal and HIRI in our study is similar in magnitude to reported correlation coefficients in studies by Abdul-Ghani et al. [19] and Vangipurapu et al. [41]. The study by Vangipurapu et al. did not examine the relationship between MISI and insulin sensitivity as measured with the hyperinsulinemic-euglycemic clamp technique. However, Bastard et al. [42] report a modest but significant association between MISI and a glucose clampderived insulin sensitivity measure (r = 0.54) in older, nondiabetic postmenopausal women (n = 113). The magnitude of the relationship between Muscle-ISclamp and MISI observed in our study (r = 0.49, P < 0.0001) is not significantly different than Bastard et al. [42]. In the study by Ghani et al. [19], HIRI correlated not only with Hepatic-IRbasal (r = 0.64), but also with Muscle-ISclamp (r = –0.55). Similarly, MISI was related to Muscle-ISclamp (r = 0.78) and Hepatic-IRbasal (r = 0.46). Based on the differences in correlation coefficients, r = 0.78 vs r = 0.46 for MISI and r = 0.64 vs r = 0.55 for HIRI, the authors concluded that the proposed indices had “greater selectivity” in detecting muscle and hepatic insulin sensitivity, respectively [19].
Magnitude of correlation coefficients are not particularly informative about predictive ability, because it is possible to observe a very strong correlation with almost zero predictive accuracy. Furthermore, if the sample is not normally distributed, the observed correlation overestimates the predictive accuracy. Measures of insulin sensitivity/resistance are typically not normally distributed, and the results of simple correlational analyses in such a sample do not inform predictive ability. Thus, assessing predictive accuracy is an important aspect of validating a surrogate index. The relationships between surrogate indices and the clamp-derived measures were modest in our current study as well as by the study of Abdul-Ghani et al. [19]. However, using calibration model analysis, we found that CVPE and RMSE, criterion functions measuring predictive accuracy, were not different among the surrogate indices (HIRI and MISI). These findings clearly suggest that these surrogate indices are not tissue specific. Furthermore, insulin resistance in liver and muscle are strongly associated [1]. Therefore, the cross-correlation between hepatic and muscle indices may reflect the underlying physiology rather than limitations of the indices themselves. Nevertheless, the use of HIRI and MISI to evaluate changes in insulin sensitivity “specifically” in the liver or skeletal muscle is not supported by our findings.
There are many strengths to our study. The analyses are from a large data set, which includes American Indians, African Americans, and white individuals and thus is more diverse. Both American Indians and African Americans are at high risk for developing type 2 diabetes mellitus. The study cohort also includes individuals with a wide range of body fat content, glucose tolerance, and insulin sensitivity. Insulin sensitivity was measured using the reference clamp technique along with the use of a isotope dilution technique to measure HGP. When comparing Muscle-ISclamp indices from different glucose clamp studies, it is imperative that the insulin doses used in the clamps are comparable. The original study by Abdul-Ghani et al. [19] in which these surrogate indices were first proposed used an insulin dose of 40 mU/m2/min–1, similar to the dosage in our study. HIRI is calculated in an insulin-stimulated state (i.e., during an OGTT), whereas Hepatic-IRbasal is measured during fasting. Therefore, we also examined the relationship between the clamp-derived measure of hepatic insulin sensitivity and HIRI. Lastly, we used a robust calibration model to validate these indices and assess predictive accuracy. Among the weaknesses, our cohort is mainly comprised of Pima Indians who are highly insulin resistant. Therefore, one could argue that lower glucose disposal rates especially with a clamp insulin dose of 40 mU/m2/min–1 may thus have contributed to the less-than-robust relationship between the surrogate index and Muscle-ISclamp. However, the strength of the relationship between MISI and Muscle-ISclamp did not differ in subjects classified as “insulin sensitive” or “insulin resistant,” suggesting that there was no procedural bias. Furthermore, our cohort was more heterogeneous, whereas the population was comprised of Mexican Americans in the original study by Abdul-Ghani et al. [19] and of postmenopausal white women in the Canadian study [42]. OGTT-derived indices generally have high within-subject variability [43]. Calculation of MISI involves multiple time points for glucose and insulin and thus is more prone for more measurement error. This is clearly evident in the rank order of tissue-specific insulin resistance using the clamp or surrogate index in the subjects with NGT, IGT, and type 2 diabetes mellitus (Table 1). MISI was clearly lower in the IGT group, but was unable to distinguish NGT and type 2 diabetes mellitus groups. Perhaps the small sample size of type 2 diabetes mellitus in our cohort contributed to this finding. Finally, because insulin assays are not standardized, there are no cutoff points for these tissue-specific surrogate indices of insulin resistance.
In conclusion, our findings indicate that HIRI and MISI surrogate indices are not tissue specific as previously proposed. Our study findings caution the use and interpretation of OGTT-derived surrogate markers specifically to evaluate hepatic or muscle insulin sensitivity in large epidemiological studies. Finally, further research on surrogate indices that are feasible and accurately predict tissue-specific insulin sensitivity/resistance are needed for use in clinical studies.
Acknowledgments
Financial Support: This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Author Contributions: R.M. conceived and designed the study, acquired and analyzed data, and drafted and reviewed the manuscript. S.H.T., S.S., S.G., R.M., and B.S.A. analyzed data and drafted and reviewed the manuscript. R.M. and S.A. performed the statistical analyses. M.C.S. drafted and reviewed the manuscript. D.C.C. and J.K. designed the study, acquired data, and drafted and reviewed the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AUC
area under the curve
- CVPE
leave-one-out cross-validation-type root mean squared error of prediction
- HGP
hepatic glucose production
- HIRI
hepatic insulin resistance index
- IGT
impaired glucose tolerance
- MISI
muscle insulin sensitivity index
- NGT
normal glucose tolerance
- OGTT
oral glucose tolerance test
- RMSE
square root of the mean squared error of prediction
References and Notes
- 1. Abdul-Ghani MA, Matsuda M, DeFronzo RA. Strong association between insulin resistance in liver and skeletal muscle in non-diabetic subjects. Diabet Med. 2008;25(11):1289–1294. [DOI] [PubMed] [Google Scholar]
- 2. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15–E26. [DOI] [PubMed] [Google Scholar]
- 3. Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84(1):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–194. [DOI] [PubMed] [Google Scholar]
- 5. Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106(4):453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. 2007;28(5):463–491. [DOI] [PubMed] [Google Scholar]
- 7. DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S157–S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jeng CY, Sheu WH, Fuh MM, Chen YD, Reaven GM. Relationship between hepatic glucose production and fasting plasma glucose concentration in patients with NIDDM. Diabetes. 1994;43(12):1440–1444. [DOI] [PubMed] [Google Scholar]
- 9. Meyer C, Pimenta W, Woerle HJ, Van Haeften T, Szoke E, Mitrakou A, Gerich J. Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care. 2006;29(8):1909–1914. [DOI] [PubMed] [Google Scholar]
- 10. Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29(5):1130–1139. [DOI] [PubMed] [Google Scholar]
- 11. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M; Finnish Diabetes Prevention Study Group . Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. [DOI] [PubMed] [Google Scholar]
- 13. Cusi K, Consoli A, DeFronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1996;81(11):4059–4067. [DOI] [PubMed] [Google Scholar]
- 14. Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, Shulman GI. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338(13):867–872. [DOI] [PubMed] [Google Scholar]
- 15. Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54(3):603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. [DOI] [PubMed] [Google Scholar]
- 17. Ferrannini E, Bjorkman O, Reichard GA Jr, Pilo A, Olsson M, Wahren J, DeFronzo RA. The disposal of an oral glucose load in healthy subjects. A quantitative study. Diabetes. 1985;34(6):580–588. [DOI] [PubMed] [Google Scholar]
- 18. Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Järvinen H, Van Haeften T, Renn W, Gerich J. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000;23(3):295–301. [DOI] [PubMed] [Google Scholar]
- 19. Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care. 2007;30(1):89–94. [DOI] [PubMed] [Google Scholar]
- 20. Jumpertz R, Thearle MS, Bunt JC, Krakoff J. Assessment of non-insulin-mediated glucose uptake: association with body fat and glycemic status. Metabolism. 2010;59(10):1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lillioja S, Mott DM, Howard BV, Bennett PH, Yki-Järvinen H, Freymond D, Nyomba BL, Zurlo F, Swinburn B, Bogardus C. Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. N Engl J Med. 1988;318(19):1217–1225. [DOI] [PubMed] [Google Scholar]
- 22. Goldman R, Buskirk E. A method for underwater weighing and the determination of body density In: Brozek JHA, ed. Techniques for Measuring Body Composition. Washington, DC: National Academy of Sciences National Research Council; 1961:78–89. [Google Scholar]
- 23. Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51(6):1106–1112. [DOI] [PubMed] [Google Scholar]
- 24. Tataranni PA, Ravussin E. Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr. 1995;62(4):730–734. [DOI] [PubMed] [Google Scholar]
- 25. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. [DOI] [PubMed] [Google Scholar]
- 26. Choukem SP, Gautier JF. How to measure hepatic insulin resistance? Diabetes Metab. 2008;34(6 Pt 2):664–673. [DOI] [PubMed] [Google Scholar]
- 27. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–2410. [DOI] [PubMed] [Google Scholar]
- 28. Muniyappa R, Irving BA, Unni US, Briggs WM, Nair KS, Quon MJ, Kurpad AV. Limited predictive ability of surrogate indices of insulin sensitivity/resistance in Asian-Indian men. Am J Physiol Endocrinol Metab. 2010;299(6):E1106–E1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen H, Sullivan G, Quon MJ. Assessing the predictive accuracy of QUICKI as a surrogate index for insulin sensitivity using a calibration model. Diabetes. 2005;54(7):1914–1925. [DOI] [PubMed] [Google Scholar]
- 30. Lillioja S, Mott DM, Zawadzki JK, Young AA, Abbott WG, Knowler WC, Bennett PH, Moll P, Bogardus C. In vivo insulin action is familial characteristic in nondiabetic Pima Indians. Diabetes. 1987;36(11):1329–1335. [DOI] [PubMed] [Google Scholar]
- 31. Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev. 1985;6(1):45–86. [DOI] [PubMed] [Google Scholar]
- 32. Stern SE, Williams K, Ferrannini E, DeFronzo RA, Bogardus C, Stern MP. Identification of individuals with insulin resistance using routine clinical measurements. Diabetes. 2005;54(2):333–339. [DOI] [PubMed] [Google Scholar]
- 33. DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37(6):667–687. [DOI] [PubMed] [Google Scholar]
- 34. Groop LC, Bonadonna RC, Shank M, Petrides AS, DeFronzo RA. Role of free fatty acids and insulin in determining free fatty acid and lipid oxidation in man. J Clin Invest. 1991;87(1):83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. [DOI] [PubMed] [Google Scholar]
- 36. Leon-Acuña A, Alcala-Diaz JF, Delgado-Lista J, Torres-Peña JD, Lopez-Moreno J, Camargo A, Garcia-Rios A, Marin C, Gomez-Delgado F, Caballero J, Van-Ommen B, Malagon MM, Perez-Martinez P, Lopez-Miranda J. Hepatic insulin resistance both in prediabetic and diabetic patients determines postprandial lipoprotein metabolism: from the CORDIOPREV study. Cardiovasc Diabetol. 2016;15(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blanco-Rojo R, Alcala-Diaz JF, Wopereis S, Perez-Martinez P, Quintana-Navarro GM, Marin C, Ordovas JM, van Ommen B, Perez-Jimenez F, Delgado-Lista J, Lopez-Miranda J. The insulin resistance phenotype (muscle or liver) interacts with the type of diet to determine changes in disposition index after 2 years of intervention: the CORDIOPREV-DIAB randomised clinical trial [published online ahead of print 16 October 2015]. Diabetologia. doi: 10.1007/s00125-015-3776-4. [DOI] [PubMed]
- 38. Fízel’ová M, Cederberg H, Stančáková A, Jauhiainen R, Vangipurapu J, Kuusisto J, Laakso M. Markers of tissue-specific insulin resistance predict the worsening of hyperglycemia, incident type 2 diabetes and cardiovascular disease. PLoS One. 2014;9(10):e109772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wlazlo N, van Greevenbroek MM, Ferreira I, Feskens EJ, van der Kallen CJ, Schalkwijk CG, Bravenboer B, Stehouwer CD. Complement factor 3 is associated with insulin resistance and with incident type 2 diabetes over a 7-year follow-up period: the CODAM Study. Diabetes Care. 2014;37(7):1900–1909. [DOI] [PubMed] [Google Scholar]
- 40. Haufe S, Haas V, Utz W, Birkenfeld AL, Jeran S, Böhnke J, Mähler A, Luft FC, Schulz-Menger J, Boschmann M, Jordan J, Engeli S. Long-lasting improvements in liver fat and metabolism despite body weight regain after dietary weight loss. Diabetes Care. 2013;36(11):3786–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vangipurapu J, Stančáková A, Kuulasmaa T, Paananen J, Kuusisto J, Ferrannini E, Laakso M; EGIR-RISC Study Group . A novel surrogate index for hepatic insulin resistance. Diabetologia. 2011;54(3):540–543. [DOI] [PubMed] [Google Scholar]
- 42. Bastard JP, Faraj M, Karelis AD, Lavasseur J, Garrel D, Prud’homme D, Rabasa-Lhoret R. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test: response to Abdul-Ghani et al. Diabetes Care. 2007;30(7):e83–e84. [DOI] [PubMed] [Google Scholar]
- 43. Mooy JM, Grootenhuis PA, de Vries H, Kostense PJ, Popp-Snijders C, Bouter LM, Heine RJ. Intra-individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: the Hoorn Study. Diabetologia. 1996;39(3):298–305. [DOI] [PubMed] [Google Scholar]