Short abstract
Background
Administrative-claims data enable comparative effectiveness assessment using large numbers of patients treated in real-world settings.
Objective
To evaluate real-world relapses, healthcare costs and resource use in patients with MS newly initiating subcutaneous interferon beta-1a (sc IFNβ-1a) v. oral disease-modifying drugs (DMDs: dimethyl fumarate, fingolimod, teriflunomide).
Methods
Patients from an administrative claims database (1 Jan 2012–31 Dec 2015) were selected if they: were 18–63 years old; had an MS diagnosis; had newly initiated sc IFNβ-1a, dimethyl fumarate, fingolimod, or teriflunomide (first claim = index); had no evidence of DMD 12-months pre-index; and had 12-month eligibility pre- and post-index. Relapse was defined as an MS-related inpatient stay, emergency room visit, or outpatient visit with a corticosteroid prescription ± 7 days. Outcomes were evaluated using logistic regression and generalized linear models.
Results
A total of 4475 patients met inclusion criteria: 21.9% sc IFNβ-1a, 51.0% dimethyl fumarate, 19.7% fingolimod, 7.4% teriflunomide. Teriflunomide patients had 1.357 (95% CI 1.000, 1.831; p = 0.0477) greater odds of 1-year relapse than sc IFNβ-1a patients. Estimated mean all-cause 1-year costs were higher after fingolimod (US$72,376) v. sc IFNβ-1a initiation (US$65,408; p < 0.0001). Non-DMD costs were not significantly different.
Conclusion
Patients initiating sc IFNβ-1a had better relapse outcomes v. teriflunomide, and lower all-cause costs v. fingolimod.
Keywords: Multiple sclerosis, disease-modifying drugs, retrospective database, relapse, cost, resource use
Introduction
The efficacy of self-injectable and oral disease-modifying drugs (DMDs) in multiple sclerosis (MS) has been demonstrated in clinical trials; however, limited real-world evidence (RWE) for the comparative effectiveness of self-injectable and oral DMDs currently exists. Seven published RWE studies comparing subcutaneous interferon beta-1a (sc IFNβ-1a) v. oral DMDs did not evaluate sc IFNβ-1a individually, but rather combined it with other IFNs and/or with glatiramer acetate.1–7 Six of the seven studies compared relapse outcomes,1–6 two compared disability progression,4,5 one compared healthcare resource use and costs,6 and one compared adherence and persistence7 in patients treated with interferons/glatiramer acetate v. patients treated with oral DMDs.2–7 In general, study findings showed that fingolimod and dimethyl fumarate had favourable clinical outcomes compared with IFNs and/or glatiramer acetate.1–7 Only one published RWE study, a retrospective chart review, directly compared sc IFNβ-1a with an oral DMD, dimethyl fumarate.8 Study findings showed that patients treated with sc IFNβ-1a had comparable persistence and relapse outcomes, and better safety outcomes, compared with patients treated with dimethyl fumarate over 2 years.8
Administrative healthcare-claims databases provide an opportunity to assess comparative effectiveness of DMDs using large numbers of patients treated in real-world settings.9,10 The objective of this study was to utilize real-world data to evaluate relapses, healthcare costs and resource use of patients with MS newly initiating sc IFNβ-1a v. oral DMDs (teriflunomide, fingolimod, and dimethyl fumarate).
Materials and methods
Data source
This retrospective database analysis used data from the IQVIA® Health Real-World Data Adjudicated Claims – US database from between January 1, 2012 and December 31, 2015. This anonymous, patient-centric database consists of fully adjudicated medical and pharmacy health plan claims data (costs and descriptive services) and enrollment information for persons with commercial insurance coverage. Data for more than 150 million unique enrollees has been obtained from health plans and self-insured employer groups throughout the USA since 2006. Claims represent provider payments for services rendered to health plan individuals who are covered. The patient-level enrollment information is a record of demographic variables including eligibility status (year of birth, gender, US Census region, eligibility by month). The database enrollee population is generally representative of the < 65 years of age, commercially insured population in the USA with respect to both age and gender. More than 30 million patients have three or more years of continuous enrollment (medical and pharmacy coverage), and the average length of enrollment is ≥39 months. Each contributing plan’s data undergoes rigorous data quality review by IQVIA. No institutional review board approval is necessary as the database is de-identified and compliant with the Health Insurance Portability and Accountability Act of 1996.
Patient population
Inclusion criteria: selected patients aged 18–63 years with at least one medical claim with a diagnosis of MS (International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code: 340) and at least one prescription for sc IFNβ-1a, teriflunomide, fingolimod, or dimethyl fumarate after MS diagnosis. The date of the first DMD prescription claim (i.e. dispensing dates) was defined as the index date. To examine patients new to therapy, any patient with a DMD during the 12 months prior to the index date was excluded. Continuous eligibility for 12 months before and after the index date was also required. A 1-year follow-up period was used in order to maximize capture of adequate numbers of dimethyl fumarate patients, as dimethyl fumarate was only approved by the US Food and Drug Administration (FDA) for use in early 2013.
DMD treatment outcomes and covariates
Primary outcomes were relapses and non-DMD healthcare costs. All-cause healthcare costs and resource utilization were secondary outcomes. Relapse was assessed 12 months following DMD initiation and was defined as presence of ≥1 MS-related (MS in the first diagnosis field) inpatient stay, ≥1 MS-related emergency room (ER) visit, or ≥1 MS-related outpatient visit with a corticosteroid claim ±7 days of that visit. All-cause healthcare costs and all-cause healthcare costs excluding DMD costs were assessed 12 months following DMD initiation. Post-index resource utilization (i.e. visits, laboratory tests and inpatient stays) was evaluated to better understand differences in healthcare costs.
Covariates were qualitatively selected to reflect potential differences in demographics, clinical status and costs. This administrative database only contained data for paid insurance claims. Given that there are no clinical data, an attempt was made to use the available data to develop a range of variables that might be expected to affect utilization or costs; these included variables that reflected patient characteristics, comorbid conditions, MS-related resource use in close proximity to the start of the medication (as a proxy for near-term severity) and 12-month pre-index all-cause healthcare cost (as an indicator of overall severity). Covariates included age, sex, census region, 12-month pre-index comorbidities (depression-related, thyroid and inflammatory/autoimmune, and Charlson Comorbidity Index (CCI) score11,12), 90-day pre-index resource use (neurology visit or magnetic resonance imaging (MRI)), 90-day pre-index relapse and 12-month pre-index all-cause healthcare costs dichotomized as above or below the median. The 90-day pre-index period for the clinical variables was used as a proxy for a patient’s MS disease status near the time of therapy initiation. Pre-index all-cause healthcare costs in the year prior to DMD initiation were used as a proxy for overall health severity. Costs were dichotomized as above and below the median due to the expected skewed distribution. As the study period extended over multiple years, all healthcare costs were discounted to December 2015 using the medical care services component of the Consumer Price Index.13
Univariate analyses
Baseline demographic and clinical characteristics were compared between index DMD cohorts (i.e. sc IFNβ-1a, teriflunomide, fingolimod, or dimethyl fumarate) and included sex, age, census region, 12-month pre-index comorbidities and indicators for having a 90-day pre-index neurology visit, 90-day pre-index MRI, 90-day pre-index relapse, pre-index all-cause costs above the median for the sample and 12-month post-index relapse. Categorical and binary variables were summarized using frequencies and percentages. Continuous variables were summarized using means (with confidence intervals (CIs)), standard deviations and medians. Pairwise Chi-square tests were conducted using sc IFNβ-1a as the reference group (i.e. all pairwise comparisons were with sc IFNβ-1a; no other comparisons were evaluated). Continuous variables were assessed with t-tests using sc IFNβ-1a as the reference group. No correction was made for multiple testing among the univariate tests.
Multivariable analysis
Logistic regression was used to evaluate the likelihood of relapse (i.e. MS-related inpatient stay, MS-related ER visit, or MS-related outpatient visit with a corticosteroid prescription ±7 days of that visit) with sc IFNβ-1a compared with teriflunomide, fingolimod, or dimethyl fumarate as predictors of interest. Covariates included demographics, clinical status and cost variables measured at baseline as listed above. Generalized linear models with a gamma distribution and log link were used to assess post-index healthcare costs using the same model as the logistic regression model for relapse.
Models were evaluated for interactions with the DMD treatment variable. Odds ratios (ORs) and 95% CIs for the odds of having a relapse are reported. Generalized linear models, using the same covariates, were also used for evaluation of secondary resource utilization variables including outpatient visits, neurology visits, liver function tests (LFTs), complete blood count tests, MS and all-cause inpatient stay and total inpatient days, and MS and all-cause ER visits. All analyses were performed using SAS for Windows version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline and pre-index characteristics
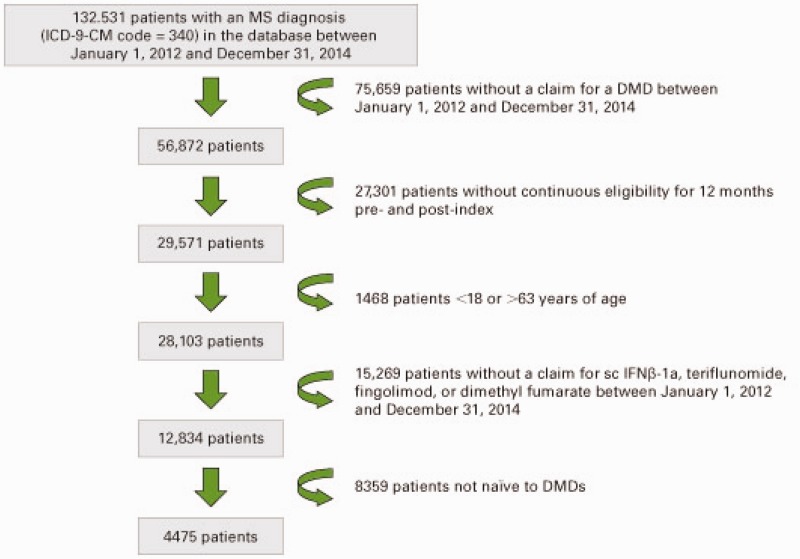
Inclusion criteria were met by 4475 patients (978 (21.9%) sc IFNβ-1a, 330 (7.4%) teriflunomide, 883 (19.7%) fingolimod, 2284 (51.0%) dimethyl fumarate; Figure 1). Patients initiating sc IFNβ-1a were statistically significantly younger, and there was a significantly greater proportion from the Midwest and a significantly lower proportion from the West compared with patients initiating oral DMDs (Table 1). A significantly greater proportion of patients initiating teriflunomide and dimethyl fumarate were from the Northeast compared with patients initiating sc IFNβ-1a. A significantly lower proportion of patients initiating dimethyl fumarate were from the South compared with sc IFNβ-1a patients.
Figure 1.
Patient selection flowchart.
DMD: disease-modifying drug; ICD-9-CM: International Classification of Diseases, Ninth Revision, Clinical Modification; MS: multiple sclerosis; sc IFNβ-1a: subcutaneous interferon beta-1a.
Table 1.
Baseline demographic and clinical characteristics of patients with MS newly initiating sc IFNβ-1a, teriflunomide, fingolimod, or dimethyl fumarate.
| Characteristic | sc IFNβ-1a(n = 978) | Teriflunomide(n = 330) | Fingolimod(n = 883) | Dimethyl fumarate(n = 2284) |
|---|---|---|---|---|
| Age, years, mean (SD) | 42.7 (10.4) | 48.6 (8.5) b | 43.9 (9.7) a | 45.2 (10.3) b |
| Female, n (%) | 716 (73.2) | 255 (77.3) | 668 (75.7) | 1701 (74.5) |
| Census region, n (%) | ||||
| Northeast | 221 (22.6) | 106 (32.1) b | 227 (25.7) | 629 (27.5) b |
| Midwest | 342 (35.0) | 85 (25.8) b | 237 (26.8) b | 682 (29.9) b |
| South | 325 (33.2) | 102 (30.9) | 322 (36.5) | 662 (29.0) a |
| West | 66 (6.7) | 34 (10.3) a | 86 (9.7) a | 274 (12.0) b |
| CCI score, mean (SD) | 0.60 (1.22) | 0.61 (1.06) | 0.46 (0.96) b | 0.59 (1.11) |
| Select comorbidities, n (%) | ||||
| Depression related | 240 (24.5) | 99 (30.0) | 268 (30.4) a | 715 (31.3) b |
| Thyroid | 130 (13.3) | 71 (21.5) b | 118 (13.4) | 356 (15.6) |
| Inflammatory/autoimmune | 33 (3.4) | 21 (6.4) a | 28 (3.2) | 88 (3.9) |
| 90-day pre-index neurology visit, n (%) | 585 (59.8) | 216 (65.5) | 525 (59.5) | 1499 (65.6) b |
| 90-day pre-index MRI, n (%) | 465 (47.5) | 94 (28.5) b | 227 (25.7) b | 957 (41.9) b |
| All-cause pre-index year healthcare cost at or above the median (US$12,158), n (%) | 385 (39.4) | 156 (47.3) a | 482 (54.6) b | 1215 (53.2) b |
| 90-day pre-index relapse, n (%) | ||||
| MS-related inpatient stay | 63 (6.4) | 4 (1.2) b | 8 (0.9) b | 55 (2.4) b |
| MS-related ER visit | 23 (2.4) | 6 (1.8) | 10 (1.1) a | 37 (1.6) |
| MS-related outpatient visit with corticosteroid prescription ±7 days | 223 (22.8) | 68 (20.6) | 183 (20.7) | 484 (21.2) |
| Any relapse, n (%) | 284 (29.0) | 71 (21.5) a | 195 (22.1) b | 558 (24.4) a |
Bold values denote statistically significant differences.
CCI: Charlson Comorbidity Index; ER: emergency room; MRI: magnetic resonance imaging; MS: multiple sclerosis; sc IFNβ-1a: subcutaneous interferon beta-1a; SD: standard deviation.
ap < 0.05 using pairwise Chi-square test or independent sample t-test v. sc IFNβ-1a as the standard (no adjustment for multiplicity).
bp < 0.005 using pairwise Chi-square test or independent sample t-test v. sc IFNβ-1a as the standard (no adjustment for multiplicity).
Patients initiating sc IFNβ-1a had a statistically significantly higher mean CCI score during the year prior to the index date compared with patients initiating fingolimod. A significantly smaller proportion of patients initiating sc IFNβ-1a had depression-related comorbidities compared with patients initiating fingolimod and dimethyl fumarate. A significantly smaller proportion of patients initiating sc IFNβ-1a had thyroid disease and inflammatory or autoimmune disease compared with patients initiating teriflunomide. A significantly greater proportion of patients initiating dimethyl fumarate had a 90-day pre-index neurology visit compared with patients initiating sc IFNβ-1a. A significantly greater proportion of patients initiating sc IFNβ-1a had a 90-day pre-index MRI compared with patients initiating teriflunomide, fingolimod and dimethyl fumarate. A greater proportion of patients initiating teriflunomide, fingolimod, and dimethyl fumarate had all-cause pre-index year healthcare costs at or above the median compared with patients initiating sc IFNβ-1a. A greater percentage of sc IFNβ-1a patients had a relapse in the 90-day pre-index period compared with patients initiating teriflunomide, fingolimod, or dimethyl fumarate. Among the components of relapse in the 90-day pre-index period, statistically significant differences were observed for presence of an MS-related inpatient stay or presence of an MS-related ER visit (Table 1).
DMD treatment outcomes: relapse
Unadjusted analysis
In the 1-year post-index period, there were no statistically significant differences (unadjusted) in the percentages of patients with a relapse or in the components of relapse (Table 2).
Table 2.
Unadjusted relapse rates 1 year after DMD initiation among treatment groups (MS-related inpatient stays, ER visits and outpatient relapses).
| Characteristic | sc IFNβ-1a(n = 978) | Teriflunomide(n = 330) | Fingolimod(n = 883) | Dimethyl fumarate(n = 2284) |
|---|---|---|---|---|
| 1-year post-index relapse, n (%) | ||||
| MS-related inpatient stay | 21 (2.1) | 6 (1.8) | 16 (1.8) | 34 (1.5) |
| MS-related ER visit | 24 (2.5) | 13 (3.9) | 16 (1.8) | 37 (1.6) |
| MS-related outpatient visit with corticosteroid prescription ±7 days | 205 (21.0) | 83 (25.2) | 182 (20.6) | 483 (21.1) |
| Any relapse | 216 (22.1) | 87 (26.4) | 192 (21.7) | 515 (22.5) |
DMD: disease-modifying drug; ER: emergency room; sc IFNβ-1a: subcutaneous interferon beta-1a.
Multivariable analysis
A multivariable analysis was used to control for baseline differences among the treatment groups. The logistic regression showed an adequate fit based on the Hosmer–Lemeshow statistic (p = 0.2161; values > 0.05 are acceptable). The Hosmer–Lemeshow goodness of fit test is frequently used in risk prediction models to assess whether observed event rates match expected event rates in deciles of fitted risk values.14 The C-statistic, a measure of predictive accuracy, was 0.655 (values > 0.6 are acceptable), and the maximum R-squared for the model was 7.5%. These statistics support the predictive ability of the model.
After controlling for covariates in a logistic regression, initiation of teriflunomide was associated with a statistically significantly higher likelihood of relapse (OR 1.357; p = 0.0477) relative to sc IFNβ-1a (Table 3). There was no statistically significant difference for fingolimod or dimethyl fumarate relative to sc IFNβ-1a. The only covariate associated with statistically significantly lower odds of 1-year post-index relapse was age. With each year of increased age, the odds of 1-year post-index relapse were 1.1% lower (OR 0.989; 95% CI 0.982, 0.996; p = 0.0028). Statistically significant covariates associated with increased odds of relapse included: Midwest region (reference: West; OR 1.353, 95% CI 1.032, 1.789; p = 0.0310), South region (reference: West; OR 1.324, 95% CI 1.010, 1.749; p = 0.0450), depression-related comorbidities (reference: depression not present; OR 1.226, 95% CI 1.048, 1.433; p = 0.0107), neurology visit 90 days pre-index (reference: no visit; OR 1.064, 95% CI 1.020, 1.116; p = 0.0091), relapse 90 days pre-index (reference: no relapse; OR 2.146, 95% CI 1.823, 2.525; p < 0.0001), and pre-index all-cause healthcare costs above the median (reference: costs below median; OR 1.673, 95% CI 1.436, 1.950; p < 0.0001). A significant interaction was observed between census region in the East and the DMD variable (p = 0.0080).
Table 3.
Logistic regression predictions of odds of relapse in the 1 year following treatment initiation.
| Factors in multivariable analysis | Estimate | p value | OR | 95% CI |
|---|---|---|---|---|
| Teriflunomide (reference: sc IFNβ-1a) | 0.044 | 0.0477 | 1.357 | 1.000, 1.831 |
| Fingolimod (reference: sc IFNβ-1a) | –0.009 | 0.7284 | 0.960 | 0.762, 1.209 |
| Dimethyl fumarate (reference: sc IFNβ-1a) | 0.006 | 0.8316 | 1.021 | 0.846, 1.234 |
| Sex, female (reference: male) | –0.040 | 0.0595 | 0.847 | 0.712, 1.005 |
| Age | –0.063 | 0.0028 | 0.989 | 0.982, 0.996 |
| Census region (reference: West) | ||||
| East | 0.044 | 0.2056 | 1.198 | 0.909, 1.592 |
| Midwest | 0.077 | 0.0310 | 1.353 | 1.032, 1.789 |
| South | 0.072 | 0.0450 | 1.324 | 1.010, 1.749 |
| Unknown | –0.011 | 0.6472 | 0.852 | 0.412, 1.639 |
| Comorbidities (reference: not present) | ||||
| Depression related | 0.051 | 0.0107 | 1.226 | 1.048, 1.433 |
| Thyroid | 0.026 | 0.1991 | 1.139 | 0.932, 1.388 |
| Inflammatory/autoimmune disease | 0.034 | 0.0712 | 1.375 | 0.966, 1.933 |
| CCI score | 0.026 | 0.1790 | 1.044 | 0.979, 1.112 |
| 90-day pre-index neurology visit (reference: no visit) | 0.069 | 0.0091 | 1.064 | 1.020, 1.116 |
| 90-day pre-index MRI (reference: no MRI claim) | –0.030 | 0.1625 | 0.901 | 0.778, 1.042 |
| 90-day pre-index relapse (reference: no relapse) | 0.178 | <0.0001 | 2.146 | 1.823, 2.525 |
| All-cause healthcare costs above the median (reference: below median) | 0.142 | <0.0001 | 1.673 | 1.436, 1.950 |
Bold values denote statistically significant differences.
CCI: Charlson Comorbidity Index; CI: confidence interval; MRI: magnetic resonance imaging; OR: odds ratio; sc IFNβ-1a: subcutaneous interferon beta-1a.
DMD treatment outcomes: healthcare costs and resource use
After adjustment for baseline characteristics, least squares mean estimated all-cause healthcare costs 1 year after fingolimod initiation (US$72,376) were statistically significantly greater than least squares mean estimated all-cause healthcare costs 1 year after sc IFNβ-1a initiation (US$65,408; p < 0.0001; Table 4). All-cause healthcare costs did not statistically significantly differ between sc IFNβ-1a (US$65,408) and teriflunomide (US$64,203; p = 0.5849) or dimethyl fumarate (US$67,784; p = 0.0809). All-cause healthcare costs excluding DMD costs did not statistically significantly differ between sc IFNβ-1a (US$13,404) and teriflunomide (US$15,205; p = 0.0704), fingolimod (US$13,681; p = 0.6890), or dimethyl fumarate (US$14,455; p = 0.0818). An interaction between DMD and neurology visit 90 days pre-index was significant in the all-cause cost model with and without DMD costs. sc IFNβ-1a patients had lower costs than teriflunomide or dimethyl fumarate patients in the group that did not have a neurology visit in the 90 days pre-index. There was also an interaction between DMD and 90-day pre-index MRI in the all-cost model with DMD costs removed. sc IFNβ-1a patients had lower costs than fingolimod patients in the group that had a MRI claim in the 90 days pre-index.
Table 4.
Generalized linear models predicting patients’ healthcare costs (in US$) 1 year post-index
| sc IFNβ-1a (n = 978) | Teriflunomide (n = 330) | Fingolimod (n = 883) | Dimethyl fumarate (n = 2284) | |
|---|---|---|---|---|
| All-cause healthcare costs, LS mean (95% CI) | 65,408(63,253, 67,637) | 64,203(60,628, 67,989) | 72,376 (69,881, 74,960) | 67,784(66,330, 69,269) |
| All-cause healthcare costs excluding DMDs, LS mean (95% CI) | 13,404(12,513, 14,358) | 15,205 (13,523, 17,096) | $13,681(12,729, 14,705) | 14,455(13,827, 15,113) |
Healthcare expenditures adjusted to December 2015 US$ using the Medical Services component of the Consumer Pricing Index.
Bold values denote statistically significant differences compared with the reference (sc IFNβ-1a).
CI; confidence interval; DMD: disease-modifying drug; LS: least squares; sc IFNβ-1a: subcutaneous interferon beta-1a.
sc IFNβ-1a initiation was also associated with lower use of several outpatient management-related healthcare resources compared with the initiation of oral DMDs, with the exception of inpatient stays and LFTs for dimethyl fumarate (Table 5).
Table 5.
Generalized linear models predicting patients’ healthcare resource use 1 year post-index
| sc IFNβ-1a (n = 978) | Teriflunomide (n = 330) | Fingolimod (n = 883) | Dimethyl fumarate (n = 2284) | |
|---|---|---|---|---|
| All-cause healthcare resource use, LS mean (95% CI) | ||||
| All-cause outpatient visits | 18.0 (17.1, 18.9) | 20.8 (19.1, 22.6) | 19.7 (18.7, 20.8) | 18.3 (17.7, 18.9) |
| All-cause inpatient stays | 0.11 (0.10, 0.13) | 0.14 (0.11, 0.19) | 0.08 (0.07, 0.10) | 0.10 (0.09, 0.11) |
| All-cause inpatient days | 0.67 (0.56, 0.80) | 0.57 (0.42, 0.77) | 0.50 (0.41, 0.61) | 0.58 (0.51, 0.65) |
| All-cause ER visits | 0.27 (0.23, 0.32) | 0.40 (0.31, 0.52) | 0.34 (0.29, 0.40) | 0.33 (0.30, 0.36) |
| MS healthcare resource use, LS mean (95% CI) | ||||
| MS-related outpatient visit with corticosteroid prescription ±7 days | 5.6 (5.2, 6.0) | 6.1 (5.5, 6.9) | 6.2 (5.8, 6.6) | 5.8 (5.5, 6.0) |
| Neurology visits | 3.7 (3.3, 4.1) | 4.8 (4.0, 5.7) | 4.8 (4.3, 5.4) | 4.0 (3.8, 4.3) |
| MS inpatient stay | 0.021 (0.018, 0.023) | 0.012 (0.009, 0.015) | 0.018 (0.016, 0.021) | 0.016 (0.014, 0.017) |
| Total MS inpatient days | 0.083 (0.071, 0.097) | 0.030 (0.022, 0.041) | 0.073 (0.062, 0.087) | 0.084 (0.075, 0.093) |
| MS ER visits | 0.021 (0.019, 0.025) | 0.043 (0.034, 0.053) | 0.018 (0.015, 0.020) | 0.016 (0.015, 0.018) |
| Laboratory tests and procedures, LS mean (95% CI) | ||||
| MRIs | 0.57 (0.50, 0.64) | 0.55 (0.45, 0.68) | 0.67 (0.59, 0.76) | 0.64 (0.59, 0.69) |
| Liver function tests | 0.57 (0.49, 0.66) | 1.13 (0.87, 1.46) | 0.57 (0.48, 0.67) | 0.36 (0.32, 0.39) |
| CBC tests | 1.4 (1.3, 1.6) | 1.9 (1.6, 2.3) | 1.6 (1.4, 1.8) | 1.6 (1.5, 1.7) |
Bold values denote statistically significant differences compared with the reference (sc IFNβ-1a).
CBC: complete blood count; CI: confidence interval; ER: emergency room; LS: least squares; MRI: magnetic resonance imaging; MS: multiple sclerosis; sc IFN β-1a: subcutaneous interferon beta-1.
Discussion
Randomized controlled trials (RCTs) remain the gold standard for assessing efficacy; however, they are inadequate for addressing questions about real-world comparative effectiveness of interventions.15,16 Comparative effectiveness research supports optimal decision making by stakeholders in the healthcare system.17 No single study captures all of the data required to make the best care decision for an individual patient.18 Inferences are based on linking together findings from an array of studies to determine the strength of evidence to support clinical decision making.18
There were some pre-index differences among the treatment groups, suggesting that patients initiating oral DMD treatment differed from patients initiating sc IFNβ-1a treatment. These differences supported the use of a multivariable model. The logistic regression model of the primary outcome of relapse showed that patients with MS newly initiating treatment with teriflunomide had a small but statistically significantly greater likelihood of relapse in the first year compared with patients initiating sc IFNβ-1a. The comparisons of sc IFNβ-1a with dimethyl fumarate and fingolimod did not reach statistical significance. Findings of this study are consistent with a recently published retrospective chart review that showed that patients treated with sc IFNβ-1a had similar relapse outcomes compared with dimethyl fumarate-treated patients over 2 years.8
To our knowledge, this is the first published study to use real-world data to compare costs between sc IFNβ-1a and oral DMDs. All-cause healthcare costs were greater for fingolimod v. sc IFNβ-1a. When DMD costs were removed from the cost comparisons, absolute differences between products were much smaller, as would be expected given the magnitude of the drug v. medical costs. The medical costs were numerically lowest for sc IFNβ-1a; however, statistically significant differences among the DMDs did not persist. Increased non-DMD costs may be potential indicators of increased disease activity. Evidence has shown that disease severity has a correlation with quality of life in patients with MS and with costs associated with the disease.19,20 Also, for all-cause healthcare costs excluding DMD costs, significant interactions existed for having a neurology visit in the 90 days pre-index or having a MRI claim in the 90 days pre-index. These differences may reflect differences in how the index drugs were prescribed, and the types of follow-up care that were required.
There was greater resource use for some variables in teriflunomide and fingolimod patients compared with sc IFNβ-1a patients, with the exception of inpatient stays. The most notable results are that sc IFNβ-1a had lower estimated 1-year post-index least square mean numbers of all-cause outpatient visits, all-cause neurology visits, and all-cause ER visits v. teriflunomide and fingolimod. Most differences for dimethyl fumarate were not statistically significant. The number of inpatient stays in this sample were relatively small and costs associated with these stays represented a small proportion of all-cause healthcare costs.
While more research is needed to assess the differences in these outcomes, possible explanations for the findings include differences in types of physicians managing these patients, unmeasured confounding differences, or differences in monitoring requirements as specified in the prescribing information for the individual DMDs.21
Limitations of this study include that the ICD-9-CM code for systemic MS does not distinguish between different MS types. Although the analysis aimed to identify patients new to DMDs by requiring no DMD for 12 months pre-index, it is possible that patients received DMDs prior to this time and subsequently discontinued treatment. Additionally, challenges associated with patients changing health plans or who are no longer in the database are present. Covariates were used to adjust for baseline differences and were checked for interactions with the DMD variables; however, they were limited to what was available in the database. Administrative databases lack information regarding indication for use, clinical variables and physician characteristics that could be important. As this dataset includes administrative claims data for patients with commercial health insurance, these findings may not be generalizable to patients with other types of healthcare coverage. A 1-year follow-up period was used in order to maximize capture of adequate numbers of dimethyl fumarate patients after its approval by the FDA; however, this is a short time horizon for assessing effectiveness, tolerability and costs of DMDs in MS. A longer follow-up duration would be important to confirm these findings.
Conclusion
As RCTs evaluate very limited patient populations under highly regulated clinical trial protocols, it is also important to evaluate the comparative effectiveness of clinical interventions. In this real-world evaluation, patients initiating sc IFNβ-1a had better relapse outcomes compared with patients initiating teriflunomide, and lower all-cause healthcare costs compared with patients initiating fingolimod over a 1-year follow-up. As more data on oral DMDs become available, a longer follow-up would be of interest for assessing comparative effectiveness over a longer time horizon.
Acknowledgements
The authors thank Natalie Edwards of Health Services Consulting Corporation, Boxborough, MA, USA (supported by EMD Serono, Inc., Rockland, MA, USA (a business of Merck KGaA, Darmstadt, Germany)) for editorial assistance.
Conflict of Interests
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: James D. Bowen received personal compensation from Acorda Therapeutics, Biogen IDEC, EMD Serono, Genzyme, Genentech, Novartis and Teva; held stock options in Amgen; and received research support from Acorda Therapeutics, Alexion, Alkermes, Allergan, Biogen IDEC, Genzyme, Genentech, GlaxoSmithKline, Novartis, Roche and Sanofi-Aventis. Chris M. Kozma is an employee of CK Consulting, Inc., which received funding from EMD Serono, Inc., Rockland, MA, USA (a business of Merck KGaA, Darmstadt, Germany) to conduct the analyses. Megan M. Grosso and Amy L. Phillips are employees of EMD Serono, Inc., Rockland, MA, USA (a business of Merck KGaA, Darmstadt, Germany). The authors received no funding for their authorship responsibilities in the development and writing of this manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study and manuscript development were supported by EMD Serono, Inc., Rockland, MA, USA (a business of Merck KGaA, Darmstadt, Germany).
References
- 1.Bergvall N, Makin C, Lahoz R, et al. Comparative effectiveness of fingolimod versus interferons or glatiramer acetate for relapse rates in multiple sclerosis: a retrospective US claims database analysis. Curr Med Res Opin 2013; 29: 1647–1656. [DOI] [PubMed] [Google Scholar]
- 2.Bergvall N, Makin C, Lahoz R, et al. Relapse rates in patients with multiple sclerosis switching from interferon to fingolimod or glatiramer acetate: a US claims database study. PLoS One 2014; 9: e88472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boster A, Nicholas J, Wu N, et al. Comparative effectiveness research of disease-modifying therapies for the management of multiple sclerosis: analysis of a large health insurance claims database. Neurol Ther 2017; 6: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He A, Spelman T, Jokubaitis V, et al. Comparison of switch to fingolimod or interferon beta/glatiramer acetate in active multiple sclerosis. JAMA Neurol 2015; 72: 405–413. [DOI] [PubMed] [Google Scholar]
- 5.Iaffaldano P, Lucisano G, Pozzilli C, et al. Fingolimod versus interferon beta/glatiramer acetate after natalizumab suspension in multiple sclerosis. Brain 2015; 138: 3275–3286. [DOI] [PubMed] [Google Scholar]
- 6.Nicholas J, Boster A, Wu N, et al. Comparison of disease-modifying therapies for the management of multiple sclerosis: analysis of healthcare resource utilization and relapse rates from US insurance claims data. PharmacoEconomics – open 2018; 2: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergvall N, Petrilla AA, Karkare SU, et al. Persistence with and adherence to fingolimod compared with other disease-modifying therapies for the treatment of multiple sclerosis: a retrospective US claims database analysis. J Med Econ 2014; 17: 696–707. [DOI] [PubMed] [Google Scholar]
- 8.Ernst FR, Barr P, Elmor R, et al. Relapse outcomes, safety, and treatment patterns in patients diagnosed with relapsing-remitting multiple sclerosis and initiated on subcutaneous interferon b-1a or dimethyl fumarate: a real-world study. Curr Med Res Opin 2017; 33: 2099–2106. [DOI] [PubMed] [Google Scholar]
- 9.Capkun G, Lahoz R, Verdun E, et al. Expanding the use of administrative claims databases in conducting clinical real-world evidence studies in multiple sclerosis. Curr Med Res Opin 2015; 31: 1029–1039. [DOI] [PubMed] [Google Scholar]
- 10.Chastek BJ, Oleen-Burkey M, Lopez-Bresnahan MV. Medical chart validation of an algorithm for identifying multiple sclerosis relapse in healthcare claims. J Med Econ 2010; 13: 618–625. [DOI] [PubMed] [Google Scholar]
- 11.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45: 613–619. [DOI] [PubMed] [Google Scholar]
- 12.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 13.US Department of Labor. Bureau of Labor Statistics Consumer Price Index. http://www.bls.gov/cpi/ (2016, accessed 8 November 2017).
- 14.Hosmer DW, Jr, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd ed New York, NY: Wiley, 2013. [Google Scholar]
- 15.Dreyer NA, Schneeweiss S, McNeil BJ, et al. GRACE principles: recognizing high-quality observational studies of comparative effectiveness. Am J Manag Care 2010; 16: 467–471. [PubMed] [Google Scholar]
- 16.Schneeweiss S, Gagne JJ, Glynn RJ, et al. Assessing the comparative effectiveness of newly marketed medications: methodological challenges and implications for drug development. Clin Pharmacol Ther 2011; 90: 777–790. [DOI] [PubMed] [Google Scholar]
- 17.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA 2003; 290: 1624–1632. [DOI] [PubMed] [Google Scholar]
- 18.Edwards NC, Skelly AC, Ziewacz JE, et al. The role of decision analytic modeling in the health economic assessment of spinal intervention. Spine (Phila Pa 1976) 2014; 39: S16–S42. [DOI] [PubMed] [Google Scholar]
- 19.Henriksson F, Fredrikson S, Masterman T, et al. Costs, quality of life and disease severity in multiple sclerosis: a cross-sectional study in Sweden. Eur J Neurol 2001; 8: 27–35. [DOI] [PubMed] [Google Scholar]
- 20.Kobelt G, Berg J, Lindgren P, et al. Costs and quality of life of patients with multiple sclerosis in Europe. J Neurol Neurosurg Psychiatry 2006; 77: 918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Remington G, Rodriguez Y, Logan D, et al. Facilitating medication adherence in patients with multiple sclerosis. Int J MS Care 2013; 15: 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]