Abstract
Background:
People who inject drugs (PWID) experience high HIV incidence, limited access to antiretroviral therapy (ART) and medication-assisted treatment (MAT), and high mortality. We report the effect of an integrated, flexible intervention on HIV and substance use outcomes.
Methods:
HIV Prevention Trials Network 074 was a randomized, controlled vanguard study conducted in Ukraine, Indonesia, and Vietnam and designed to assess the feasibility of a future trial. HIV-infected PWID index participants (indexes) were enrolled with ≥1 HIV-uninfected injection partner. Indexes were randomly assigned (ratio=3:1) to standard of care (SOC) or an intervention comprising systems navigation, psychosocial counseling, and ART at any CD4 count. Local ART and MAT services were used. Outcomes included retention, ART use, viral suppression, MAT use, mortality, and injection partner HIV incidence.
Findings:
502 indexes and 806 partners were enrolled. At 52 weeks, most living indexes (86%) and partners (80%) were retained. At week 52, self-reported ART use was higher among intervention indexes (72%) than SOC indexes (43%) (probability ratio (PR) 1.7, 95% confidence interval (CI): 1.4, 1.9). Viral suppression also increased (intervention: 41%, SOC: 24%; PR: 1.7, 95% CI: 1.3, 2.2). Intervention indexes reported more MAT use at 52 weeks (41%; SOC 25%; PR: 1.7, 95% CI: 1.3, 2.2). Mortality was reduced with the intervention (indexes: hazard ratio (HR): 0.47, 95% CI: 0.22, 0.90; partners: HR: 0.17, 95% CI: 0.01, 0.84). All incident HIV infections occurred in SOC partners (intervention (0 cases): 0.0/100 person-years; 95% CI: 0, 1.7; SOC (7 cases): 1.0/100 person-years, 95% CI: 0.4, 2.1; incidence rate difference: −1.0/100 person-years (95% CI: −2.1, 1.1)).
Interpretation:
This randomized, controlled vanguard trial provides strong evidence that a flexible, scalable intervention increases ART and MAT use and reduces mortality among PWID. The intervention may also reduce HIV transmission to injection partners.
Research in context
Evidence before this study
Our search of PubMed was updated on April 5, 2018 using search terms designed to capture evidence of effectiveness of an integrated intervention of systems navigation and psychosocial counseling to improve the uptake of antiretroviral therapy (ART) and medication assisted therapy (MAT), reduce mortality and possibly HIV incidence among HIV infected people who inject drugs (PWID). We retrieved and reviewed 72 articles. Seek, test, and treat interventions for HIV care and similar harm reduction programs for MAT have been implemented in various settings, often leading to initiation of and increased retention in care and apparent reductions in HIV incidence in observational studies. We found that self-referral and street-based outreach for linkage to universal ART in Vancouver and a multi-level HIV risk and stigma reduction intervention in Vietnam were both associated with reduced mortality in PWID. One randomized controlled trial (RCT) from the US among already in care HIV-infected PWID found that use of a time-limited, cognitive-behavioral therapy intervention increased medication adherence compared to enhanced treatment. To our knowledge, our study is the first to have investigated and rigorously documented the impact of systems navigation and psychosocial counseling among HIV-infected PWID on ART uptake, MAT uptake, mortality, and potentially HIV transmission.
Added value of this study
This randomized, controlled, multi-country vanguard study among HIV infected PWID and their HIV uninfected injection partners produced robust measurements of its outcomes of interest. The flexible, integrated systems navigation and psychosocial counseling intervention had strong effects on ART and MAT uptake and on overall mortality, with a promising effect on HIV seroconversion. The intervention was well grounded on maintenance theory and social cognitive and diffusion of innovation meta-theories of behavior change. Importantly, our rigorous, randomized-controlled design solidifies the largely observational study findings that suggest the uptake of ART (and MAT) among PWID leads to a reduction in mortality and HIV transmission. Furthermore, the 73% uptake of ART at 6 months among those receiving the intervention is a promising result for this highly stigmatized population to help achieve the WHO goal of 90% uptake of ART by 2020. Although this intervention does not differ substantially from activities being implemented at HIV treatment sites around the world, the rigorous assessment of the outcomes is a model of how programmatic data can be used to monitor and adjust activities aimed at increasing the proportion of HIV infected PWID on ART and virally suppressed.
Implications of all the available evidence
HIV-infected PWID have significant barriers to HIV and addiction services leading to high mortality and HIV transmission. This hard-to-reach population needs flexible, innovative, low-cost, scalable programs to support sustained use of ART and MAT. Based on the results of this study along with other studies, increasing the uptake of ART and MAT services combined with standard harm reduction services will likely reduce mortality among HIV-infected PWID, and possibly, among their uninfected injection partners. In addition, as proven with heterosexual HIV transmission and now suggested by this study, ART uptake will likely reduce HIV transmission among PWID. Implementation research will be required on a country level to determine the best approach to bring this intervention to effective scale.
Introduction
People who inject drugs (PWID) experience a disproportionate burden of HIV infection and related morbidity and mortality.1,2 HIV incidence and prevalence remains high among PWID, especially in Southeast Asia, parts of Central Asia, and Eastern Europe.3,4 The PWID HIV epidemic persists because of low rates of HIV testing, insufficient access to and use of HIV prevention services and HIV care, limited access to substance use treatment and harm reduction services, and persistent social barriers, such as stigma and punitive legal systems.5-7
HIV-infected PWID often engage with HIV treatment services late.8-10 Enhancing HIV-infected PWID’s engagement in HIV care may increase ART uptake, viral suppression, and survival, and possibly reduce transmission to HIV-uninfected injection partners.11-13 This potential impact would be augmented if PWID also accessed medication-assisted treatment (MAT) and harm reduction measures.14-17 In HIV Prevention Trials Network 074 (HPTN 074), we assessed the feasibility and effects of a flexible integrated intervention to facilitate the initiation of ART and MAT with the ultimate goal of reducing HIV transmission from HIV-infected PWID to their HIV-uninfected injection partners. This integrated intervention was designed to be scalable and incorporated systems navigation, flexible psychosocial counseling, and access to ART regardless of CD4 cell count. As a vanguard study, the primary objective of HPTN 074 was to evaluate the feasibility of conducting a future, larger HIV transmission prevention trial. Here we report primary results of this vanguard study, including the intervention uptake and its effect on ART use, viral suppression, MAT use, and mortality. We also report HIV incidence among HIV-uninfected injection partners.
Methods
A detailed methods section is provided in Appendix A.
Study settings
HPTN 074 was conducted in three study locations: Kyiv, Ukraine; Thai Nguyen, Vietnam; and Jakarta, Indonesia, site selection was based on ongoing HIV epidemics among PWID with high HIV prevalence and/or incidence, and MAT availability.
Study design and objectives
This multi-site, two-arm, randomized vanguard study was designed to determine the feasibility of a future randomized controlled trial by 1) estimating HIV incidence among injection partners, and 2) assessing the potential for enrollment and retention of HIV-infected PWID and their HIV-uninfected injection partners. The study was also designed to assess the feasibility, barriers, and uptake of the integrated intervention and its effect, as compared to standard of care (SOC), on self-reported ART and MAT initiation and use and viral suppression.
Randomization and masking
Index participants (“indexes”) were randomly assigned to either the SOC or intervention arms at a ratio of 3:1 (SOC:intervention). Randomization was stratified by site and used a permuted-block design. Masking was not feasible due to the nature of the intervention.
Study population
The study population comprised PWID network units with two types of participants: HIV-infected indexes and their HIV-uninfected injection partners. Recruitment began in February 2015 and continued through June 2016. Participants were followed for 12 to 24 months. Inclusion criteria for all participants were: a) age 18 to 60 years (the upper age limit was increased from 45 years to 60 years in September 2015); b) active injection drug use (defined initially as self-report of i) injecting drugs approximately two or more times per week for the past three months and ii) ability to identify the anatomical location of the most recent injection site that was confirmed by study staff; changed in September 2015 to i) injecting 12 times or more in the past three months and at least 6 times in the past month and ii) a PWID in the opinion of site staff); c) having no plans to move outside the study area for at least one year after study enrollment; and d) ability and willingness to provide written informed consent.
Inclusion criteria specific for indexes were: a) HIV-infection at screening based on local HIV test procedures; b) viral load ≥1,000 copies/mL at screening based on local testing with or without self-reported current ART use (people who reported current, stable ART use and daily pill consumption were not screened further); c) willingness and ability to identify, recruit, and enroll at least one HIV-uninfected network injection partner who was eligible for study participation; and d) willingness to participate in intervention activities. On September 10, 2015, a criterion was added requiring CD4 cell count >50 cells/μL due to high early mortality among participants with CD4 cell count ≤50 cells/μL.
Inclusion criteria specific for partners were: a) confirmed injection relationship with the index, based on referral identification cards or matching physical description, and b) lack of HIV infection.
Recruitment procedures for index participants
Index recruitment methods included referrals from HIV-testing sites, community outreach, and referrals from others in their injection-networks. Outreach workers were selected from the community and harm reduction programs and were familiar with the PWID communities’ dynamics. They used a prescreening checklist addressing age and active drug use prior to screening visits. Potential indexes were asked to share study information with other PWID in their networks.
Identification of network partners
After HIV infection confirmation, indexes were asked to identify and describe members of their injection network, given referral identification cards, counseled about HIV status disclosure, and encouraged to accompany network partners to the study site to facilitate recruitment. Indexes were free to choose which partners to refer. Up to five concurrent HIV-uninfected injection partners could be enrolled per index participant. Injection partners could be sexual partners, but partners by sexual relationship only were ineligible.
To maintain independence between injection networks: referred network partners who were ineligible because of HIV-infection were not eligible to be screened as an HIV-infected index unless the referring HIV-infected index did not enroll; HIV-uninfected injection partners could not enroll as an index if they seroconverted during the study; partners could enroll for only one index.
Indexes with fewer than five partners enrolled in the study could recruit new, late-entry network partners until the last routine study visit prior to the termination visit. If a network partnership ended, the index participant was asked to recruit a new, late-entry network partner to replace the ending partnership. Indexes received modest monetary compensation for successful partner enrollment. Indexes and network partners were compensated for their time and participation; compensation varied by site and was approved by local institutional review boards.
Standard of care (SOC) and intervention procedures
Indexes in the SOC arm received referrals to existing HIV and MAT (primarily methadone—buprenorphine/naloxone had limited availability in Ukraine and Indonesia) clinics. They also received a standardized harm reduction package, consistent with in-country guidelines and the World Health Organization (WHO) package of care for PWID,18,19 which included HIV testing and counseling, referrals for ART according to national guidelines (see below), and diagnosis and treatment of sexually transmitted infections, hepatitis B virus, hepatitis C virus, and tuberculosis, as appropriate. Harm reduction services at each visit included referral for treatment of substance use/addiction, referral to syringe service programs, injection risk reduction counseling, and sexual risk reduction counseling including access to condoms. No medical care was provided directly by the study.
ART initiation in the SOC arm was based on prevailing country guidelines. After December 2015, PWID in all three sites were eligible for ART regardless of CD4 cell count, based on high risk status. Before December 2015, eligibility in Ukraine was limited to CD4 cell count ≤ 350 cells/μL.
Indexes in the integrated intervention arm received the standard harm reduction package plus: 1) systems navigation to facilitate engagement, retention, and adherence in HIV care and MAT and to negotiate the logistics and, if necessary, cost of any required laboratory testing (e.g., tuberculosis testing) and transportation; 2) psychosocial counseling using motivational interviewing, problem solving, skills building, and goal setting to facilitate initiation of ART and MAT, and if started, medication adherence; 3) and ART initiation regardless of CD4 cell count, guaranteed by each Ministry of Health at study onset. Initially, systems navigators met with intervention arm indexes twice either in-person or by telephone. Subsequent sessions, based on the needs expressed by the participants or observed by the navigators, were conducted in-person, by telephone, or with text messages. The primary goal for navigation was to address individual or systems level barriers to ART and MAT enrollment, such as scheduling ART or MAT initiation appointments, assisting with medical paperwork, and answering health-related questions. The type and frequency of contact varied by participant.
Intervention arm indexes received a minimum of two psychosocial counseling sessions. The first session focused primarily on ART; and the second on ART adherence and MAT. To determine the need for additional sessions, the counselor used a standardized inventory to assess the indexes’ need for counseling on risk reduction, drug treatment entry and retention, HIV-related medical care, adherence for ART and MAT, depression, alcohol dependence, and social support. Intervention indexes were offered additional booster sessions, approximately one month and three months after enrollment. Sessions were tailored to individual need and could focus on more than one topic. Indexes could receive additional counseling sessions through self-referral or referral by the systems navigators. Systems navigators’ and counselors’ responsibilities, as well as counseling session content, were incorporated in an intervention manual (Supplementary Material, Appendix B).
All indexes were asked, but not required, to bring a social support person (family member, sexual partner, friend) with them to their counseling and system navigation sessions. The support person’s role was to help the index initiate and adhere to ART and MAT.
All sites chose to have one person serve both systems navigator and psychosocial counselor roles. Navigator/counselors’ education levels varied from less than a bachelor’s degree to physicians; all had relevant addiction experience.
Network partners in both arms received a standardized harm reduction package with referral for MAT, consistent with national guidelines. Partners in the intervention arm did not receive the intervention directly.
Data collection
Indexes and network partners completed an interview at screening, enrollment, one month post-enrollment, quarterly, and an exit visit. Follow-up duration varied by enrollment date. Network partners were followed until their index withdrew, died, or exited. Trained study staff interviewed participants using non-judgmental techniques. Questions included demographic characteristics, injection risk behavior, substance use, substance use treatment eligibility, HIV testing and treatment history, sexual behavior, barriers and facilitators to substance use and HIV treatment, and social network information.
Deaths were investigated systematically by the local study teams to assess the circumstances and causes of death, when available. Each death was independently categorized by two infectious diseases specialist physicians; discordant categorizations received a third examination.
Indexes and network partners provided blood samples at every study visit and urine samples at every study visit except screening. Urine was stored for centralized testing for substances of abuse and methadone. CD4 cell count testing for indexes was performed locally at screening, 6, 12, 18, and 24 months. HIV viral load testing for indexes was performed locally at screening. Viral load testing at enrollment, 6, 12, 18, and 24 months was performed retrospectively at the HPTN Laboratory Center. The HPTN Laboratory Center retrospectively confirmed local HIV test results, including all cases of seroconversion, with FDA-cleared assays (Appendix A). Timing of seroconversions were assessed by re-evaluating the visit prior to HIV seroconversion with fourth-generation ELISA and HIV PCR.
Statistical Analyses
As a vanguard study, sample size was based on estimating HIV incidence among the SOC partners with reasonable precision; the study was not designed with sufficient power to assess intervention effectiveness on transmission. Assuming a 10% annual loss to follow-up with approximately 563 network partners in the SOC arm, an observed overall HIV incidence rate of 3 cases per 100 person-years (~ 21 HIV seroconversions total) would allow estimation of HIV incidence in the SOC arm with a corresponding exact 95% confidence interval (CI) of 1.8 to 4.5 per 100 person-years.
The main measures of intervention effectiveness were current ART, viral suppression, and MAT, each assessed at 26 and 52 weeks; and time to ART initiation, viral suppression, and MAT initiation. Measures of ART and MAT were based on self-report. Although not a pre-planned endpoint, mortality was assessed and compared across treatment arms. Viral load suppression was considered at two thresholds: <40 copies/mL and <1,000 copies/mL. Although the study was not designed with sufficient power for cross-site statistical comparisons, descriptive cross-site analyses for main measures were planned.
Probability ratios (PR), analogous to prevalence ratios at a specific time point, were used to compare the proportion of indexes alive and self-reporting ART, viral suppression, and MAT at 26 and 52 weeks. The PR were estimated using log-binomial models including study site as a covariate. Indexes who died prior to the 26- and 52-week visits were considered not to be on ART, on MAT, or virally suppressed. Hazard ratios (HR) for time to ART initiation, viral suppression, and MAT initiation were computed over the entire follow-up period using a sub-distribution hazards model stratified by study site, with death treated as a competing risk.20 Participants having already achieved the outcome of interest at baseline (on ART, on MAT, viral suppression) were excluded from time-to-event analyses. Mortality by study arm over the entire study period was assessed using Cox proportional hazard models, with study site as a stratification variable. The Cox models were assessed for significant violations of the proportional hazards assumption; none were identified. An exact 95% CI for the between-arm HIV incidence rate difference was constructed by inverting an exact test assuming that HIV incidence in the two arms was constant over time. A 0.05 significance level and two-sided 95% CIs were used with no adjustment for multiple testing. Analyses were performed using Linux SAS 9.4 (SAS/STAT 14.2, Cary, NC) and R Version 3.4.3.
Ethics Approvals
The study protocol (available at http://clinicaltrials.gov, NCT02935296) was approved by the following institutional review boards: Ukrainian Institute on Public Health Policy (Ukraine); Ethical Review Board for Biomedical Research Hanoi School of Public Health (Vietnam); Ethics Committee of Faculty of Medicine, University of Indonesia/ Cipto Mangunkusumo Hospital (Indonesia); and the University of North Carolina Institutional Review Board. All study participants provided written informed consent in their local languages, or English, if preferred. Each index was informed that his/her recruited HIV-uninfected partner(s) would become aware of the index’s HIV infection during the consent process.
Role of the funding source
The funder of the study reviewed and approved the protocol and protocol revisions. The sponsor participated in study design and reviewed the final report, but had no role in data collection or data analysis. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
Results
Overall, 3343 screening evaluations were performed; 504 HIV-infected PWID were enrolled as indexes (Figure 1). Two enrolled indexes were excluded: one was virally suppressed at screening based on retrospective testing and one had no confirmed eligible partners. The 502 eligible indexes were enrolled in Vietnam (194/502, 39%), Ukraine (187/502, 37%), and Indonesia (121/502, 24%). Indexes were assigned to the SOC arm (376/502, 75%) or intervention arm (126/502, 25%).
Figure 1:
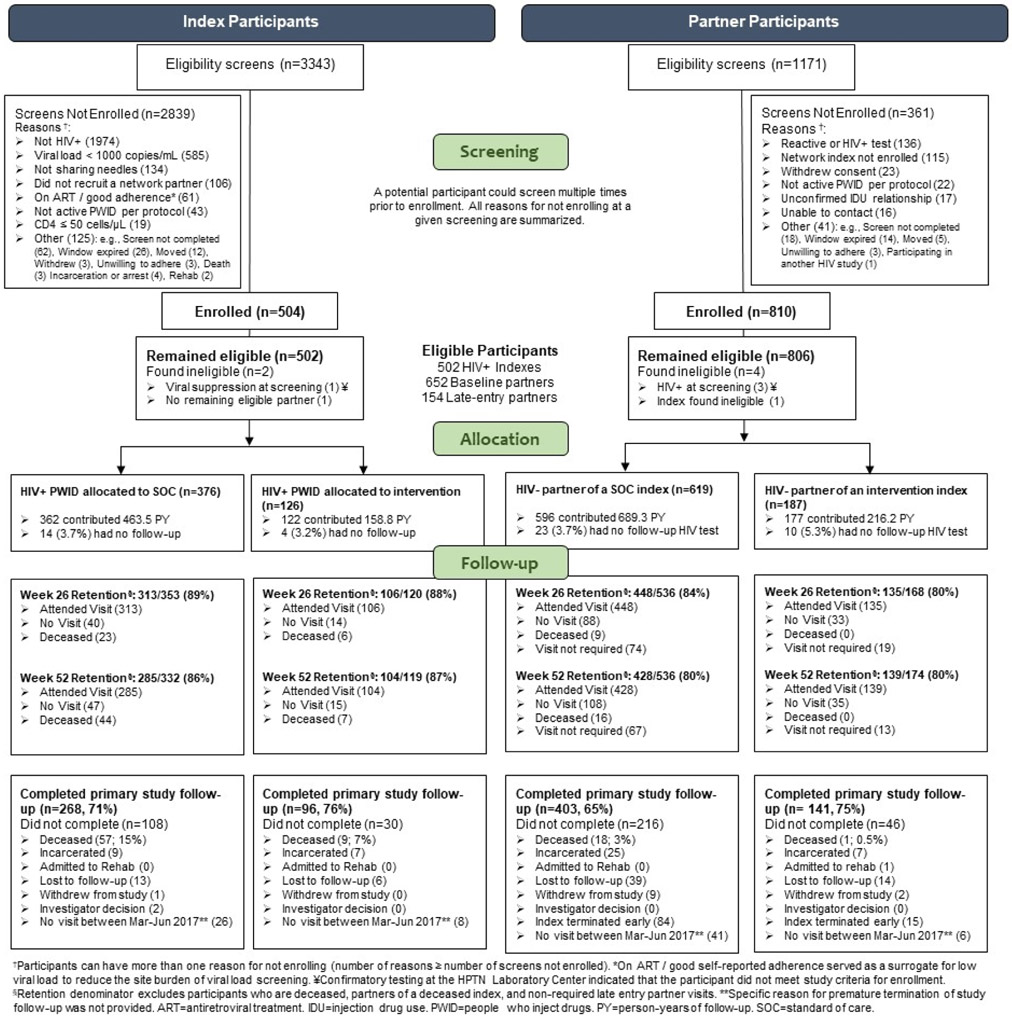
CONSORT diagram. Enrollment and retention for index and partner participants are shown, with indexes on the left and partners on the right.
Of the referred partners, 810 were identified as HIV-uninfected and enrolled. Four partners were excluded: three were retrospectively identified as HIV infected at screening (see Appendix A for details); the other had the ineligible index due to viral suppression. Thus, 806 partners were included for analysis. Indexes enrolled an average of 1.6 partners each. Most partners (652/806, 81%) enrolled at baseline; 19% (154/806) enrolled during the index’s follow-up.
Of surviving indexes, 89% (419/473) and 86% (389/451) completed 26 and 52 weeks of follow-up, respectively. Of surviving partners, 83% (583/704) and 80% (567/710) completed 26 and 52 weeks of follow-up, respectively.
Baseline characteristics
Demographic and clinical characteristics at baseline were comparable across the intervention and SOC arms (Table 1). Among indexes, 85% (427/502) were men. Most enrolled women were Ukrainian (63/75, 84%). The median age was 35 years (intraquartile range (IQR): 31,38).
Table 1:
Baseline characteristics of index and partner participants, by arm
| Index Participants |
Partner Participants |
|||||
|---|---|---|---|---|---|---|
| Overall |
Intervention |
SOC |
Overall |
Intervention |
SOC |
|
| Characteristic | n=502 n (%) |
n=126 n (%) |
n=376 n (%) |
n=806 n (%) |
n=187 n (%) |
n=619 n (%) |
| Self-identified gender | ||||||
| Female | 75 (14.9%) | 16 (12.7%) | 59 (15.7%) | 90 (11.2%) | 17 (9.1%) | 73 (11.8%) |
| Male | 427 (85.1%) | 110 (87.3%) | 317 (84.3%) | 716 (88.8%) | 170 (90.9%) | 546 (88.2%) |
| Age at enrollment (years) | ||||||
| 18–19 | 1 (0.2%) | 0 (0.0%) | 1 (0.3%) | 5 (0.6%) | 1 (0.5%) | 4 (0.6%) |
| 20–29 | 81 (16.1%) | 21 (16.7%) | 60 (16.0%) | 229 (28.4%) | 42 (22.5%) | 187 (30.2%) |
| 30–39 | 328 (65.3%) | 85 (67.5%) | 243 (64.6%) | 402 (49.9%) | 100 (53.5%) | 302 (48.8%) |
| 40+ | 92 (18.3%) | 20 (15.9%) | 72 (19.1%) | 170 (21.1%) | 44 (23.5%) | 126 (20.4%) |
| Highest education level | ||||||
| No schooling | 4 (0.8%) | 1 (0.8%) | 3 (0.8%) | 4 (0.5%) | 1 (0.5%) | 3 (0.5%) |
| At least some primary school | 55 (11.0%) | 12 (9.5%) | 43 (11.4%) | 41 (5.1%) | 9 (4.8%) | 32 (5.2%) |
| At least some secondary school | 256 (51.0%) | 61 (48.4%) | 195 (51.9%) | 418 (51.9%) | 86 (46.0%) | 332 (53.6%) |
| At least some technical training | 122 (24.3%) | 33 (26.2%) | 89 (23.7%) | 211 (26.2%) | 51 (27.3%) | 160 (25.8%) |
| At least some college or university | 65 (12.9%) | 19 (15.1%) | 46 (12.2%) | 132 (16.4%) | 40 (21.4%) | 92 (14.9%) |
| Relationship status | ||||||
| Married | 171 (34.1%) | 45 (35.7%) | 126 (33.5%) | 293 (36.4%) | 68 (36.4%) | 225 (36.3%) |
| Living with sexual partner, not married | 75 (14.9%) | 17 (13.5%) | 58 (15.4%) | 107 (13.3%) | 15 (8.0%) | 92 (14.9%) |
| Separated, Divorced, Widowed | 104 (20.7%) | 24 (19.0%) | 80 (21.3%) | 142 (17.6%) | 40 (21.4%) | 102 (16.5%) |
| Single | 152 (30.3%) | 40 (31.7%) | 112 (29.8%) | 264 (32.8%) | 64 (34.2%) | 200 (32.3%) |
| Unemployed (last 3 months) | ||||||
| Yes | 305 (60.8%) | 78 (61.9%) | 227 (60.4%) | 451 (56.0%) | 103 (55.1%) | 348 (56.2%) |
| No | 197 (39.2%) | 48 (38.1%) | 149 (39.6%) | 355 (44.0%) | 84 (44.9%) | 271 (43.8%) |
| Employment status | ||||||
| Employed full-time | 149 (29.7%) | 36 (28.6%) | 113 (30.1%) | 269 (33.4%) | 69 (36.9%) | 200 (32.3%) |
| Employed part-time | 134 (26.7%) | 34 (27.0%) | 100 (26.6%) | 231 (28.7%) | 48 (25.7%) | 183 (29.6%) |
| Unemployed, seeking work | 152 (30.3%) | 40 (31.7%) | 112 (29.8%) | 225 (27.9%) | 52 (27.8%) | 173 (27.9%) |
| Unemployed, not seeking work | 63 (12.5%) | 14 (11.1%) | 49 (13.0%) | 79 (9.8%) | 18 (9.6%) | 61 (9.9%) |
| Retired | 3 (0.6%) | 1 (0.8%) | 2 (0.5%) | 2 (0.2%) | 0 (0.0%) | 2 (0.3%) |
| ART status (self-report) | ||||||
| Currently on ART | 54 (10.8%) | 15 (11.9%) | 39 (10.4%) | |||
| Previously on ART | 46 (9.2%) | 9 (7.1%) | 37 (9.8%) | |||
| ART naive | 402 (80.1%) | 102 (81.0%) | 300 (79.8%) | |||
| Currently on MAT (baseline) | ||||||
| Yes | 109 (21.7%) | 28 (22.2%) | 81 (21.5%) | 150 (18.6%) | 47 25.1%) | 103 (16.6%) |
| No | 393 (78.3%) |
98 (77.8%) |
295 (78.5%) |
656 (81.4%) | 140 (74.9%) | 516 (83.4%) |
| Median (IQR) |
Median (IQR) |
Median (IQR) |
||||
| Years since HIV diagnosis | 1.4 (0.07, 6.4) | 2.1 (0.08, 8.4) | 0.8 (0.07, 5.9) | |||
| Years since ART initiation | 0.7 (0.05, 4.2) | 1.2 (0.03, 5.2) | 0.7 (0.09, 4.2) | |||
| HIV-1 RNA (log10 copies/mL) | 4.6 (4.0, 5.0) | 4.6 (4.0, 5.0) | 4.6 (4.0, 5.0) | |||
| CD4 cell count (cells/μL) | 293 (166, 463) | 295 (174,492) | 291 (165,462) | |||
Abbreviations: SOC: standard of care, MAT: medication-assisted treatment, ART: antiretroviral therapy, mL: milliliter, μL: microliter; IQR: interquartile range.
At baseline, the median viral load was 4.6 log10 copies/mL (IQR: 4.0, 5.0); the median CD4 cell count was 293 cells/μL (IQR: 166, 463). Most indexes (402/502, 80%) reported being ART-naïve at baseline. Only 22% (109/502) of indexes reported current MAT use at enrollment.
Partner demographic characteristics were comparable to those of the indexes (Table 1). Most enrolled partners (716/806, 89%) were men; the median age was 33 years (IQR: 29, 39). Few enrolled partners reported being on MAT (19%, 150/806).
Intervention uptake
Nearly all index participants in the intervention arm (123/126, 98%) completed the initial systems navigation encounter, which occurred a median of 7 days (IQR: 2, 14) after enrollment. The initial encounters were brief—28% (34/123) lasted 5 minutes and 84% (103/123) lasted 30 minutes or less. The primary focus of the initial sessions was providing referrals or making appointments for HIV care (80/123, 65%), ART initiation/management (9/123, 7%), and MAT (6/123, 5%). The median number of systems navigator encounters in the first 8 weeks was 3 (IQR: 2, 5). Considering all encounters, 75% (1205/1605) of systems navigation encounters were 10 minutes or less; 41% (653/1605) occurred in person and 56% (892/1605) over the telephone. Contact occurred most commonly in person in Indonesia (56%; 313/560) and Ukraine (80%; 158/197), and by telephone in Vietnam (78%; 661/848).
All but one index in the intervention arm (125/126, 99%) completed their first psychosocial counseling encounter. The median time until the first counseling encounter was four days (IQR: 2, 8). Most initial encounters were 31-60 minutes in duration (72/125, 58%); only 4% (5/125) lasted >60 minutes. Two-thirds (87/126, 69%) of intervention indexes completed two or more counseling sessions within four weeks of enrollment; 83% (104/126) completed two or more sessions within 60 days. Overall, 870 psychosocial counseling sessions were provided (median 7 per index, IQR: 5, 8). These sessions addressed ART adherence (497/870, 57%), care engagement (423/870, 49%), risk reduction (361/870, 42%), HIV literacy (284/870, 33%), ART adherence communication skills (271/870, 31%), dealing with HIV infection (234/870, 27%), and injection risk communication skills (169/870, 19%). Most indexes (98/125, 78%) were accompanied by a support person to at least one session. Support persons were primarily family members (47/98, 48%) or spouses/partners/boyfriends/girlfriends (40/98, 41%).
ART
At week 26, 73% (82/112) of the intervention indexes were alive and reported being on ART, compared to 36% (121/336) of SOC indexes (probability ratio (PR): 1.9, 95% CI: 1.6, 2.3) (Figure 2). The effect persisted at week 52 (72% vs 43%; PR: 1.7, 95% CI: 1.4, 1.9). Transitions between ART use and non-use are described in Appendix C, Supplemental Table 1.
Figure 2:
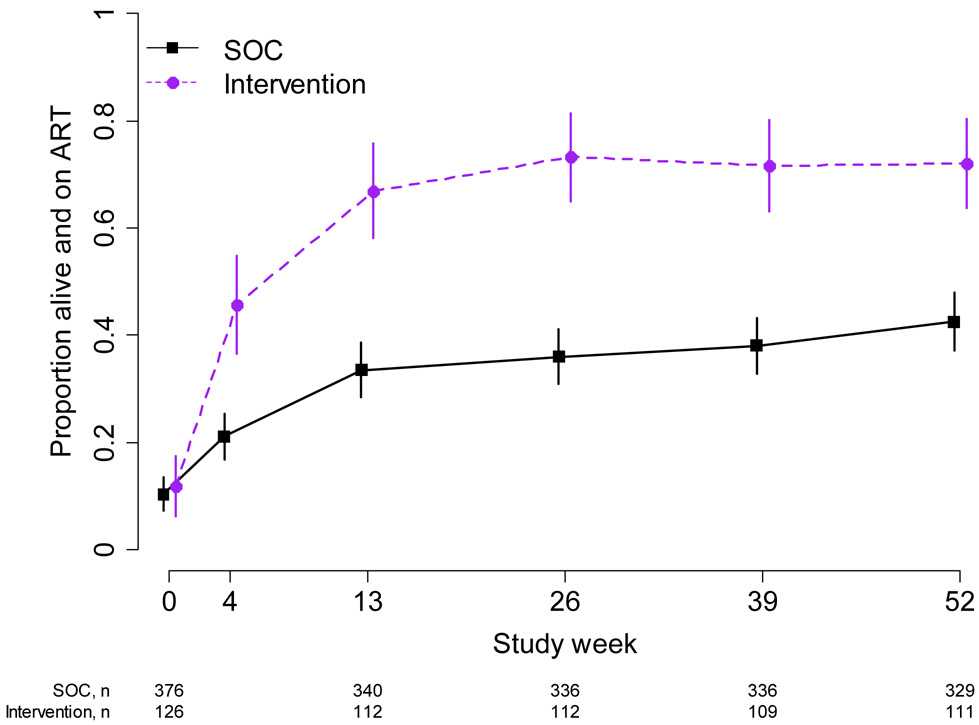
Proportion Alive and on ART – Index Participants. The figure shows the proportion of indexes who were alive at each study week and reported that they were on ART. 95% confidence intervals are calculated with the Wald method. Purple dashed line: intervention; black solid line: standard of care.
The hazard ratio for ART initiation over the entire follow-up period comparing intervention to SOC index participants was 3.6 (95% CI: 2.7, 4.7) (Supplemental Figure 1).
Viral suppression
At 26 weeks, 36% (40/112) of intervention indexes were alive and virally suppressed (<40 copies/mL), compared to 16% (55/336) in the SOC arm (PR: 2.2, 95% CI: 1.6, 3.0). At 52 weeks, 41% (45/111) of intervention indexes and 24% (80/328) of SOC index participants were alive and virally suppressed (PR: 1.7, 95% CI: 1.3, 2.2). (Table 2, Figure 3). Intervention indexes achieved viral suppression (<40 copies/mL) more rapidly than SOC indexes (HR: 1.8, 95% CI: 1.3, 2.4). (Supplemental Figure 2). Additional results for viral suppression, including use of the alternative threshold of <1000 copies/mL, viral load distributions for those who were not suppressed, and transitions from 26 to 52 weeks, are shown in Supplemental Tables 2-5, Supplemental Figures 3-4.
Table 2:
Status at 26 and 52 weeks and time to event for self-reported ART, viral suppression, and self-reported MAT
| Status1 at 26 weeks |
Status1 at 52 weeks |
Time to Event2 |
|||||
|---|---|---|---|---|---|---|---|
| Intervention Percentage |
SOC Percentage |
PR (95% CI) | Intervention Percentage |
SOC Percentage |
PR (95% CI) | HR (95% CI) | |
| Indexes | |||||||
| ART | 73% | 36% | 1.9 (1.6, 2.3) | 72% | 43% | 1.7 (1.4, 1.9) | 3.6 (2.7, 4.8) |
| Viral suppression (<40 copies/mL) |
36% | 16% | 2.2 (1.6, 3.0) | 41% | 24% | 1.7 (1.3, 2.2) | 1.8 (1.3, 2.4) |
| MAT | 38% | 24% | 1.7 (1.2, 2.2) | 41% | 25% | 1.7 (1.3, 2.2) | 2.4 (1.6, 3.7) |
| Partners | |||||||
| MAT | 30% | 25% | 1.2 (0.84, 1.6) | 34% | 26% | 1.3 (0.96, 1.7) | 1.3 (0.87, 2.0) |
Abbreviations: SOC: standard of care, PR: probability ratio, HR: hazard ratio, CI: confidence intervals, ART: antiretroviral therapy, mL: milliliter, MAT: medication-assisted treatment;
Status reflects self-report of current ART or MAT at time interval noted;
Time to event reflects time to ART or MAT uptake
Figure 3:
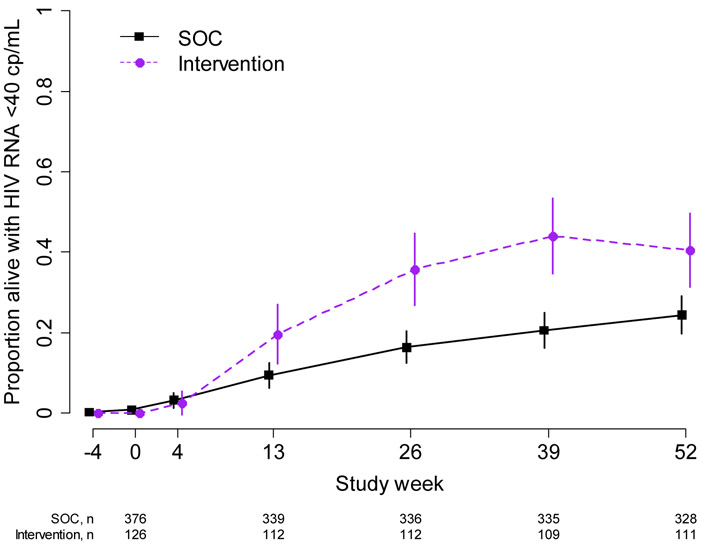
Proportion of indexes alive and virally suppressed with HIV RNA <40 copies/mL. 95% confidence intervals are calculated with the Wald method. Purple dashed line: intervention; black solid line: standard of care. cp/ml = copies/mL
MAT: Indexes and partners
At 26 weeks, 38% (42/111) of intervention indexes were alive and reported being on MAT, compared to 24% (80/336) in the SOC arm (PR: 1.7, 95% CI: 1.2, 2.2) with similar results at 52 weeks (intervention: 41% (45/111), SOC: 25% (81/329); PR: 1.7, 95% CI: 1.3, 2.2) (Figure 4). Over the entire study period, intervention indexes reported initiation of MAT sooner than SOC indexes (HR: 2.4, 95% CI: 1.6, 3.7); Supplemental Figure 5).
Figure 4:
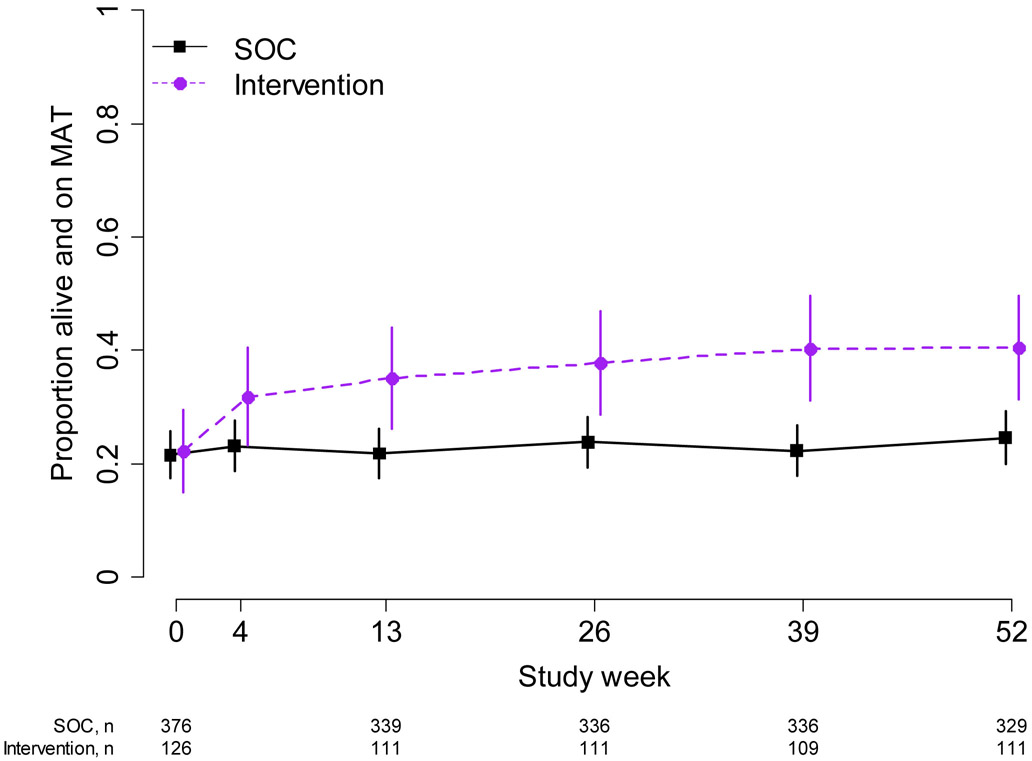
Medication-assisted treatment status among index participants. The proportion of index participants who were alive and reported that they were on medication-assisted treatment (MAT) at each study visit is shown. 95% confidence intervals are calculated with the Wald method.Purple dashed line: intervention; black solid line: standard of care.
Partners in the intervention arm reported current MAT use slightly more often than partners in the SOC arm (26 weeks: intervention: 30% (44/146) versus SOC: 25% (119/485), PR: 1.2, 95% CI: 0.84, 1.6; 52 weeks: intervention: 34% (44/129) versus SOC: 26% (107/416); PR: 1.3, 95% CI: 0.96, 1.7) (Supplemental Figure 6). Partners in the intervention arm initiated MAT slightly, but not significantly, more often than partners in the SOC arm ((HR: 1.3, 95% CI: 0.87, 2.0); Supplemental Figure 7.
Mortality: Indexes and Partners
Overall, 13% (66/502) of indexes died during follow-up. More indexes died in Indonesia (26/121, 21%) than in Vietnam (24/194, 12%) or Ukraine (16/187, 9%). Overall, 26% (17/66) of index deaths were considered HIV-related, 24% (16/66) had unknown cause with CD4 cell count <200 cells/mL, and 18% (12/66) had unknown cause with CD4 cell count ≥200 cells/mL (Supplemental Table 6).
Mortality among indexes was reduced by 53% in the intervention arm (mortality rates: intervention: 5.6/100 person-years (95% CI: 2.6, 10.6) versus SOC: 12.1/100 person-years (95% CI: 9.1, 15.6); HR 0.47, 95% CI: 0.22, 0.90) (Figure 5).
Figure 5:
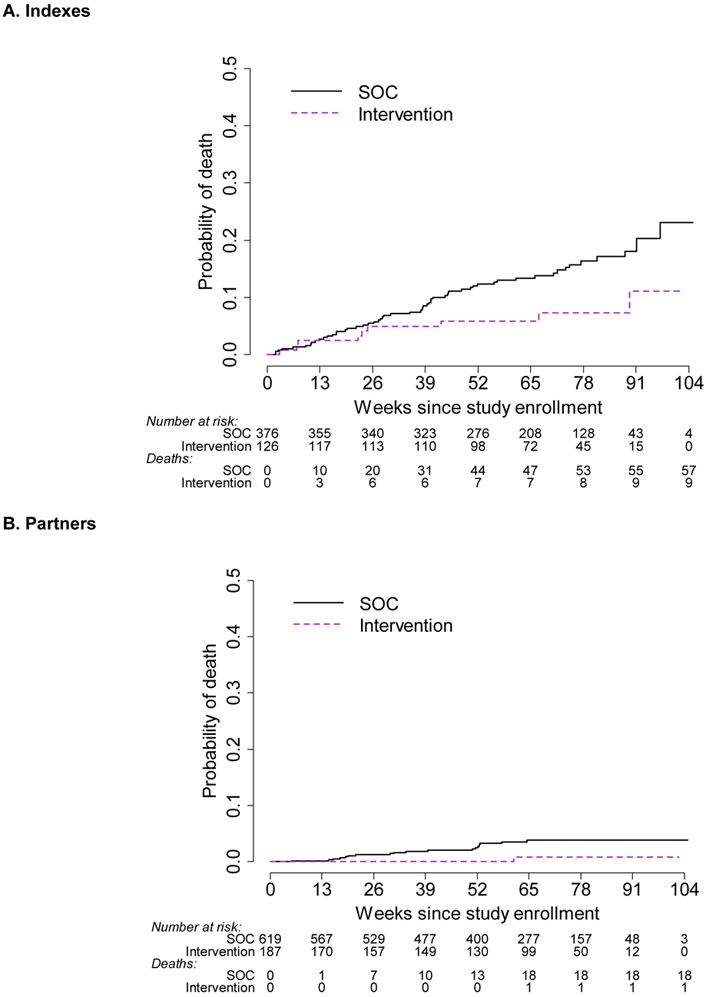
A. Time to death for index participants. B. Time to death for partner participants. Cumulative probability plots of death for indexes (A) and partners (B). At each time point below the x-axis, the number remaining at risk is provided, as well as the number of accumulated deaths. Note that the number of participants with follow-up beyond 91 weeks is limited. Purple dashed line: intervention; black solid line: standard of care.
Mortality was also reduced among partners in the intervention arm (mortality rates: intervention: 0.46/100 person-years (95% CI: 0.01, 2.6) versus SOC: 2.6/100 person-years (95% CI: 1.5, 4.1; HR: 0.17, 95% CI: 0.01, 0.84) (Figure 5).
HIV incidence among network partners
No network partners in the intervention arm acquired HIV infection (0 cases/216.2 person-years; incidence rate: 0/100 person-years; 95% CI: 0.0, 1.7), compared to seven partners who acquired HIV infection in the SOC arm (7 cases/684.1 person-years; incidence rate: 1.0/100 person-years; 95% CI: 0.41, 2.1). The incidence rate difference for HIV transmission was −1.0/100 person-years (95% CI: −2.1, 1.1).
Site comparisons
At baseline, self-reported ART use varied by site. In Indonesia, 69% (84/121) of indexes reported they were ART naïve, compared to 83% (156/187) in Ukraine and 84% (162/194) in Vietnam. The median reported duration of ART also varied (Indonesia 5.0 years, IQR: 1.2, 6.9; Ukraine 0.05 years, IQR: 0.01, 0.12; Vietnam 0.15 years, IQR: 0.04, 2.0). The baseline median viral loads and CD4 cell counts were comparable across sites (Supplemental Table 7).
No meaningful differences in implementation and uptake of systems navigator sessions were identified across sites.
Site-specific differences in psychosocial counseling encounters were observed. In Vietnam, 86% (42/49) of indexes completed two psychosocial counseling encounters within four weeks compared to 64% (30/47) in Ukraine and 50% (15/30) in Indonesia. More counseling encounters were completed with a support person present in Vietnam (48/48, 100.0%) and Ukraine (35/47, 74.5%) than in Indonesia (15/30, 50.0%). The duration and content of counseling sessions was similar across sites.
The effect of the intervention was not uniform across the three sites. In Vietnam and Ukraine, the intervention effect was positive for self-reported ART and time to ART initiation, viral suppression, self-reported MAT initiation, and mortality (Table 3, Supplemental Figures 8-11). But, in Indonesia, the intervention effect appeared to be limited.
Table 3:
Status at 26 and 52 weeks and time to event for self-reported ART, viral suppression, and self-reported MAT uptake, by site
| Status1 at 26 weeks |
Status1 at 52 weeks |
Time to Event2 |
|||||
|---|---|---|---|---|---|---|---|
| Intervention Percentage |
SOC Percentage |
PR (95% CI) | Intervention Percentage |
SOC Percentage |
PR (95% CI) | HR (95% CI) | |
| Indexes | |||||||
| ART | |||||||
| Ukraine | 71% (31/44) | 17% (22/127) | 4.1 (2.7, 6.2) | 73% (30/41) | 34% (43/125) | 2.1 (1.6, 2.9) | 4.9 (3.2, 7.6) |
| Vietnam | 88% (35/40) | 51% (64/125) | 1.7 (1.4, 2.1) | 88% (37/42) | 56% (67/119) | 1.6 (1.3, 1.9) | 4.7 (2.8, 7.8) |
| Indonesia | 57% (16/28) | 42% (35/84) | 1.4 (0.91, 2.1) | 46% (13/28) | 35% (30/85) | 1.3 (0.81, 2.2) | 1.3 (0.75, 2.3) |
| Viral suppression (<40 copies/mL) |
|||||||
| Ukraine | 41% (18/44) | 11% (14/127) | 3.7 (2.0, 6.8) | 49% (20/41) | 26% (32/125) | 1.9 (1.2, 2.9) | 2.3 (1.5, 3.8) |
| Vietnam | 48% (19/40) | 26% (32/125) | 1.9 (1.2, 2.9) | 50% (21/42) | 30% (35/118) | 1.7 (1.1, 2.5) | 1.7 (1.1, 2.7) |
| Indonesia | 11% (3/28) | 11% (9/84) | 1.0 (0.29, 3.4) | 14% (4/28) | 15% (13/85) | 0.93 0.33, 2.6) | 0.93 (0.40, 2.1) |
| MAT | |||||||
| Ukraine | 21% (9/44) | 16% (20/127) | 1.3 (0.64, 2.6) | 27% (11/41) | 18% (23/125) | 1.5 (0.78, 2.7) | 2.6 (1.3, 5.3) |
| Vietnam | 62% (24/39) | 30% (37/125) | 2.1 (1.4, 3.0) | 67% (28/42) | 35% (41/119) | 1.9 (1.4, 2.7) | 2.9 (1.7, 5.0) |
| Indonesia | 32% (9/28) | 27% (23/84) | 1.2 (0.62, 2.2) | 21% (6/28) | 20% (17/85) | 1.1 (0.47, 2.5) | 0.43 (0.05, 3.4) |
| Partners | |||||||
| MAT | |||||||
| Ukraine | 17% (10/60) | 12% (23/197) | 1.2 (0.48, 2.8) | 18% (10/56) | 13% (23/172) | 1.2 (0.49, 2.8) | 1.0 (0.41, 2.6) |
| Vietnam | 44% (21/48) | 35% (67/192) | 1.3 (0.86, 1.8) | 55% (24/44) | 41% (63/154) | 1.3 (0.94, 1.8) | 1.4 (0.83, 2.3) |
| Indonesia | 34% (13/38) | 30% (29/96) | 0.91 (0.48, 1.7) | 35% (10/29) | 23% (21/90) | 1.4 (0.70. 2.7) | 1.4 (0.52, 4.0) |
Abbreviations: SOC: standard of care, PR: probability ratio, CI: confidence intervals, HR: hazard ratio, ART: antiretroviral therapy, mL: milliliter, MAT: medication-assisted treatment.
Status reflects self-report of current ART or MAT at time interval noted;
Time to event reflects time to ART or MAT uptake
Discussion
The integrated intervention of HPTN 074 combined systems navigation and tailored, brief psychosocial counseling for PWID. ART was ensured to be available at any CD4 cell count. This intervention led to increased ART and MAT use; viral suppression also increased. The intervention also reduced mortality among the HIV-infected indexes and their HIV-uninfected partners. All HIV seroconversions among partners occurred in the SOC arm, although the overall HIV incidence was low. These findings have important implications for HIV prevention and treatment among PWID worldwide.
PWID experience disproportionate morbidity and mortality, which is amplified by HIV infection.21 The HIV-related excess morbidity is due, in part, to the inability of HIV-infected PWID to engage in HIV care, initiate ART, and adhere to ART over the long term.10,22 PWID face numerous barriers to engagement in HIV prevention, care and treatment, including their personal circumstances and behaviors, providers’ attitudes and behaviors, and punitive laws and prevailing stigma and discrimination.5 In HPTN 074, we observed that many barriers can be overcome, leading to increased viral suppression and decreased mortality.
HPTN 074 provides direct evidence of expediting linkage to care and treatment for HIV and substance use. Seek, test, and treat interventions for HIV care have often led to apparent reductions in HIV incidence in observational studies conducted in diverse settings.17,23,24 In the United States, a strengths-based case management intervention administered by masters-level social workers was associated with increased viral suppression23 and a time-limited cognitive behavioral therapy intervention among HIV-infected PWID in care increased ART adherence.25 In Greece, expedited ART linkage for PWID identified by respondent-driven sampling surveys led to an apparent decline in HIV incidence.16 In Vancouver, universal ART availability in the provincial health care system was associated with fewer new HIV diagnoses and reduced mortality among PWID.24 Mortality has been reduced through stigma reduction coupled with multi-level HIV risk reduction in Vietnam.17 HPTN 074 extends these previous findings by showing direct effects on ART use, MAT use, viral suppression, mortality, and potentially HIV transmission.
As a vanguard study, HPTN 074 was designed to guide the development of a future, larger trial. Primary objectives focused on HIV incidence among the partners in the SOC arm and participant retention. Retention was acceptable at week 52, exceeding 80% in the surviving indexes and partners. Thus, following a large cohort of PWID is feasible, but HIV incidence was too low for an efficient HIV-prevention trial among injection partners.
The HIV incidence rate was lower than expected among the uninfected partners,4,26 despite selecting study sites based on high HIV incidence or prevalence among PWID.27-29 The observed low incidence may be due to a Hawthorne effect30 or direct interactions with interviewers, leading to reduced risk behaviors regardless of study arm. These effects may have been augmented by awareness of the HIV status of the index and his/her partner(s) within an injection network. In addition, the WHO-recommended SOC package may have served as an intervention for PWID in the SOC arm, due to greater awareness of needle exchange availability, counseling regarding needle sharing risk reduction, and initial and repeated HIV tests. Finally, PWID in the SOC arm may also have benefited from the presence of the systems navigators and counselors at the study sites.
All seven new HIV infections occurred in partners in the SOC arm. This vanguard study was not designed with sufficient power to confirm an effect of the study intervention on HIV acquisition, but the absence of any HIV infections among 187 partners in the intervention arm supports the possibility that the intervention may reduce HIV incidence among injection partners.
MAT uptake was less than ART uptake, but was still substantially higher among intervention indexes compared to the SOC arm. The modest uptake of MAT may have several contributing factors. Although designed to address both ART and MAT, ART initiation was emphasized as a first priority in the intervention. In addition, local government restrictions on MAT access, such as limited numbers of MAT ciinics (Indonesia, Ukraine) and substantial travel distance (Vietnam), often complicated MAT initiation and retention. Finally, many PWID expressed a reluctance to initiate MAT, despite counseling (qualitative interviews, data not shown).
The HPTN 074 intervention was designed to be scalable, focusing on assisted engagement in care through systems navigation and flexible, but brief, psychosocial counseling. The intervention should be implementable in many settings that serve PWID, including HIV counseling and testing centers and nonprofit organizations. The role of systems navigator can be fulfilled by peers, social workers, counselors, or ciinicians—the key feature is that navigators understand the local health care system and are able to facilitate entry and retention in care. The HPTN 074 counselors had previous training as addiction counselors, peer counselors, social workers, or physicians. The counselor role did not require a high level of education—some counselors in Ukraine and Indonesia did not have bachelor’s degrees. The roles are conceptually distinct, but in all three sites, the same people served both roles, reducing the personnel necessary to implement the intervention.
The three study sites had variable responses to the intervention. The effects were broadly positive in Ukraine and Vietnam, but weak in Indonesia. The reasons for these differences are unclear. Part of the explanation may be that Indonesian indexes were less likely to complete two counseling sessions within four weeks and less likely to involve a social support person in those counseling sessions. Also, nearly a third of Indonesian indexes reported previous or current ART use at enrollment—about twice the proportion in Ukraine or Vietnam. Indonesian PWID on ART had been on ART longer than Ukrainian or Vietnamese PWID on ART. These ART-experienced Indonesian PWID may have been more likely to have antiretroviral resistance and less likely to restart ART or become adherent, despite navigation and counseling.
We unexpectedly observed reduced mortality among partners in the intervention arm. Mortality was reduced despite absence of direct service provision to partners and a minimal impact on partners’ MAT use. One plausible explanation may be the intervention for the indexes helped reduce barriers to health care for their injection partners. The intervention may also have had indirect general life benefits, given that, among the indexes, only about a half of deaths were considered HIV-related or occurred with unknown cause and low CD4 cell count.
ART and MAT were assessed using self-report. Given the emphasis on ART and MAT in the intervention, participants may have overstated actual ART and MAT. But the validity of the intervention’s effect on these self-reported measures is supported by the impact of the intervention on objectively-measured outcomes, including viral suppression and mortality.
The relative effects of the intervention were sustained through 52 weeks, but the absolute effects in the intervention arm were less than optimal—only about half of indexes were virally suppressed at 52 weeks. Further efforts to enhance the intervention and reduce structural barriers are needed to maximize ART, viral suppression, and MAT. But, in the meantime, the integrated intervention evaluated in HPTN 074 has the potential to substantially reduce morbidity and mortality in PWID globally.
In summary, a scalable, integrated intervention combining systems navigation and flexible psychosocial counseling increased self-reported ART, viral suppression, and self-reported MAT, and reduced mortality. The intervention may have reduced HIV incidence, but HIV incidence was low in the HIV-uninfected partners, which presents a challenge for any similar future trial. Based on the strength of the intervention’s impact on the HIV-infected indexes and the likely effect of expanded ART use on HIV transmission, widespread implementation of this intervention should be considered in other populations of PWID.
Supplementary Material
Acknowledgements
A study of this size could not be completed without the hard work and dedication of many people behind the scenes. We would like to especially thank Dr. Myron Cohen for his assistance in the earliest stages of this study, including its conceptualization and securing HPTN funding. We would also like to thank Dr. Wafaa El-Sadr for her support throughout the study and critical review of the manuscript. We thank Katherine Davenny for her advocacy and assistance in the entire study process. We would like to thank Ilana Trumble and Laura McKinstry for their contributions to data management and analysis. We appreciate the critical role of FHI 360 in study implementation and Bonnie Dye and Jonathan Lucas, in particular. We would like to thank Teerada Sripaipan for her support throughout the study. We would like to acknowledge
Dr Bui Duc Duong of the Vietnam Administration of HIV/AIDS Control and Dr Nguyen Duc Vuong, Director of Pho Yen Health Center for his role in implementing the study in Vietnam. Finally, we wish to thank all of the HPTN Protocol Team, the site staff in Indonesia, Vietnam, and Ukraine for their dedication to the study, and the participants for their willingness to share their experiences through the study.
Funding: National Institutes of Health.
Sources of support: This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute of Mental Health (NIMH), and the National Institute on Drug Abuse (NIDA) of the National Institutes of Health (NIH); award numbers UM1AI068619 [HPTN Leadership and Operations Center], UM1AI068617 [HPTN Statistical and Data Management Center], UM1AI068613 [HPTN Laboratory Center], and the University of North Carolina at Chapel Hill Center for AIDS Research (P30 AI50410). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Declaration of Interests
This study was supported by the National Institutes of Health (NIH) and multiple investigators received grants or support from NIH (WCM, IFH, BSH, TVH, CAL, DSM, KEL, VFG, KRM, SAR, EMP-M, PR, MGH, JS, SHE, SAS). DNB is an employee of NIH. KRM received grants from Merck; SHE received support from Abbott Diagnostics/Molecular; JS received personal fees and non-financial support from Merck KGaA and IQVIA; all these activities were outside the scope of the current work.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
William C. Miller, Division of Epidemiology, College of Public Health, The Ohio State University, Columbus, Ohio, USA
Irving F. Hoffman, Division of Infectious Diseases, School of Medicine, University of North Carolina – Chapel Hill, Chapel Hill, North Carolina, USA
Brett S. Hanscom, Statistical Center for HIV/AIDS Research and Prevention (SCHARP), Fred Hutchinson Cancer Research Center, Seattle, Washington, USA.
Tran V. Ha, UNC Vietnam, Lot E2, Duong Dinh Nghe Street, Yen Hoa, Cau Giay District, Hanoi, Vietnam, Department of Health Behavior, Gillings School of Global Public Health, University of North Carolina – Chapel Hill, Chapel Hill, North Carolina, USA.
Kostyantyn Dumchev, Ukrainian Institute on Public Health Policy, Kyiv, Ukraine.
Zubairi Djoerban, Faculty of Medicine, University of Indonesia/Cipto Mangunkusumo Hospital
Scott M. Rose, Science Facilitation Department, FHI 360, Durham, North Carolina, USA.
Carl A. Latkin, Department of Health Policy and Management, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, USA
David S. Metzger, HIV Prevention Research Division, Department of Psychiatry, School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania,- Chapel Hill, Chapel Hill, North Carolina, USA
Kathryn E. Lancaster, Division of Epidemiology, College of Public Health, The Ohio State University, Columbus, Ohio, USA.
Vivian F. Go, Department of Health Behavior, Gillings School of Global Public Health, University of North Carolina – Chapel Hill, Chapel Hill, North Carolina, USA
Sergii Dvoria, Academy of Labor, Social Relations and Tourism, Kyiv, Ukraine.
Katie R. Mollan, Center for AIDS Research, University of North Carolina – Chapel Hill, Chapel Hill, North Carolina, USA.
Sarah A. Reifeis, Center for AIDS Research, University of North Carolina – Chapel Hill, Chapel Hill, North Carolina, USA.
Estelle M. Piwowar-Manning, Department of Pathology, Johns Hopkins Univ. School of Medicine, Baltimore, MD, USA.
Paul Richardson, Department of Pathology, Johns Hopkins Univ. School of Medicine, Baltimore, MD, USA.
Michael G. Hudgens, Center for AIDS Research, University of North Carolina – Chapel Hill, Chapel Hill, North Carolina, USA, Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina – Chapel Hill, Chapel Hill, North Carolina, USA
Erica L. Hamilton, Science Facilitation Department, FHI 360, Durham, North Carolina, USA.
Jeremy Sugarman, Berman Institute of Bioethics and Department of Medicine, School of Medicine, and Bloomberg School of Public Health; Johns Hopkins University, Baltimore, Maryland USA
Susan H. Eshleman, Department of Pathology, Johns Hopkins Univ. School of Medicine, Baltimore, MD, USA
Hepa Susami, Faculty of Medicine, University of Indonesia/Cipto Mangunkusumo Hospital, Jakarta, Indonesia.
Viet Anh Chu, UNC Vietnam, Lot E2, Duong Dinh Nghe Street, Yen Hoa, Cau Giay District, Hanoi, Vietnam.
Samsuridjal Djauzi, Faculty of Medicine, University of Indonesia/Cipto Mangunkusumo Hospital, Jakarta, Indonesia
Tetiana Kiriazova, Ukrainian Institute on Public Health Policy, Kyiv, Ukraine.
Bui Due Duong, Vietnam Administration of HIV/AIDS Control, Hanoi, Vietnam.
Steffanie A. Strathdee, Department of Medicine, School of Medicine, University of California San Diego, La Jolla, California, USA
David N Burns, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
References
- 1.Degenhardt L, Charlson F, Stanaway J, et al. Estimating the burden of disease attributable to injecting drug use as a risk factor for HIV, hepatitis C, and hepatitis B: findings from the Global Burden of Disease Study 2013. The Lancet Infectious diseases 2016; 16(12): 1385–98. [DOI] [PubMed] [Google Scholar]
- 2.Mathers BM, Degenhardt L, Phillips B, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet (London, England) 2008; 372(9651): 1733–45. [DOI] [PubMed] [Google Scholar]
- 3.Beyrer C, Abdool Karim Q. The changing epidemiology of HIV in 2013. Current opinion in HIV and AIDS 2013; 8(4): 306–10. [DOI] [PubMed] [Google Scholar]
- 4.DeHovitz J, Uuskula A, El-Bassel N. The HIV epidemic in Eastern Europe and Central Asia. Current HIV/AIDS reports 2014; 11 (2): 168–76. [DOI] [PubMed] [Google Scholar]
- 5.Strathdee SA, Shoptaw S, Dyer TP, Quan VM, Aramrattana A, Substance Use Scientific Committee of the HIV Prevention Trials Network. Towards combination HIV prevention for injection drug users: addressing addictophobia, apathy and inattention. Current opinion in HIV and AIDS 2012; 7(4): 320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boltaev AA, El-Bassel N, Deryabina AP, et al. Scaling up HIV prevention efforts targeting people who inject drugs in Central Asia: a review of key challenges and ways forward. Drug Alcohol Depend 2013; 132 Suppl 1: S41–7. [DOI] [PubMed] [Google Scholar]
- 7.Larney S, Peacock A, Leung J, et al. Global, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: a systematic review. The Lancet Global Health 2017; 5(12): e1208–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heimer R, Usacheva N, Barbour R, Niccolai LM, Uuskula A, Levina OS. Engagement in HIV care and its correlates among people who inject drugs in St Petersburg, Russian Federation and Kohtla-Jarve, Estonia. Addiction (Abingdon, England) 2017; 112(8): 1421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoots BE, Finlayson TJ, Broz D, Paz-Bailey G. Antiretroviral therapy use among HIV-infected people who inject drugs-20 cities, United States, 2009–2015. Journal of acquired immune deficiency syndromes (1999) 2017; 75 Suppl 3: S392–s6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiriazova TK, Postnov Ov, Perehinets IB, Neduzhko OO Association of injecting drug use and late enrolment in HIV medical care in Odessa Region, Ukraine. HIV medicine 2013; 14 Suppl 3: 38–41. [DOI] [PubMed] [Google Scholar]
- 11.Lazarus L, Patel S, Shaw A, et al. Uptake of Community-Based Peer Administered HIV Point-of-Care Testing: Findings from the PROUD Study. PloS one 2016; 11(12): e0166942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Go VF, Frangakis C, Le Minh N, et al. Increased Survival Among HIV-Infected PWID Receiving a Multi-Level HIV Risk and Stigma Reduction Intervention: Results From a Randomized Controlled Trial. Journal of acquired immune deficiency syndromes (1999) 2017; 74(2): 166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joseph B, Wood E, Hayashi K, et al. Factors associated with initiation of antiretroviral therapy among HIV-positive people who use injection drugs in a Canadian setting. AIDS (London, England) 2016; 30(6): 925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC). Integrated prevention services for HIV infection, viral hepatitis, sexually transmitted diseases, and tuberculosis for persons who use drugs illicitly: summary guidance from CDC and the U.S. Department of Health and Human Services. MMWR Recomm Rep 2012; 61(Rr-5): 1–40. [PubMed] [Google Scholar]
- 15.Low AJ, Mburu G, Welton NJ, et al. Impact of opioid substitution therapy on antiretroviral therapy outcomes: a systematic review and meta-analysis. clinical infectious diseases: an official publication of the Infectious Diseases Society of America 2016; 63(8): 1094–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sypsa V, Psichogiou M, Paraskevis D, et al. Rapid decline in HIV incidence among persons who inject drugs during a fast-track combination prevention program after an HIV outbreak in Athens. The Journal of infectious diseases 2017; 215(10): 1496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zelaya CE, Le Minh N, Lau B, et al. The effect of a multi-level intervention on the initiation of antiretroviral therapy (ART) among HIV-infected men who inject drugs and were diagnosed late in Thai Nguyen, Vietnam. PloS one 2016; 11(8): e0161718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beyrer C, Malinowska-Sempruch K, Kamarulzaman A, Kazatchkine M, Sidibe M, Strathdee SA. Time to act: a call for comprehensive responses to HIV in people who use drugs. Lancet (London, England) 2010; 376(9740): 551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization; UNAIDS technical guide for countries to set targets for universal access to HIV prevention, treatment and care for injecting drug users. Geneva, Switzerland, 2009. [Google Scholar]
- 20.Zhou B, Latouche A, Rocha V, Fine J. Competing risks regression for stratified data. Biometrics 2011; 67: 661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta-analysis. Bulletin of the World Health Organization 2013; 91(2): 102–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesko CR, Edwards JK, Moore RD, Lau B. A longitudinal, HIV care continuum: 10-year restricted mean time in each care continuum stage after enrollment in care, by history of IDU. AIDS (London, England) 2016; 30(14): 2227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kral AH, Lambdin BH, Comfort M, et al. A Strengths-Based Case Management Intervention to Reduce HIV Viral Load Among People Who Use Drugs. AIDS and behavior 2018; 22: 146–53. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi K, Dong H, Kerr T, et al. Declining Mortality Rates in HIV-Infected People Who Inject Drugs During a Seek-and-Treat Initiative in Vancouver, Canada, 1996–2014: A Prospective Cohort Study. The Journal of infectious diseases 2017; 217(1): 64–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safren SA, O’Cleirigh CM, Bulli JR, Otto MW, Stein MD, Pollack MH. Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected injection drug users: a randomized controlled trial. J Consult Clin Psychol 2012; 80(3): 404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jolley E, Rhodes T, Platt L, et al. HIV among people who inject drugs in Central and Eastern Europe and Central Asia: a systematic review with implications for policy. Bmj Open 2012; 2(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berleva G, Dumchev K, Kasianchuk M, et al. Estimation of the Size of the Populations Most-at-Risk for HIV Infection in Ukraine. Ukraine, 2012. [Google Scholar]
- 28.National AIDS Commission. Indonesia Country Progress Report 2014. Indonesia, 2014. [Google Scholar]
- 29.Quan VM, Minh NL, Ha TV, et al. Mortality and HIV transmission among male Vietnamese injection drug users. Addiction (Abingdon, England) 2011; 106(3): 583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. Journal of clinical epidemiology 2014; 67(3): 267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


