Abstract
Background:
The progression to end-stage renal disease (ESRD) is the most important complication of chronic kidney disease (CKD). Patients with ESRD require dialysis or transplantation to survive, incur numerous complications, and have high mortality rates. Slowing the progression of CKD is an important goal. Unfortunately, even when current treatments are appropriately applied, patients with CKD still progress to ESRD. Current treatments do not address the inflammation and fibrosis that mediate progression to ESRD, but micro-particle curcumin, a natural health product, has both anti-inflammatory and anti-fibrotic properties and may be an effective treatment for patients with CKD.
Objective:
Micro-particle curcumin for the treatment of CKD-1 (MPAC-CKD-1) will measure the effect of micro-particle curcumin on 2 important markers of CKD progression: albuminuria and estimated glomerular filtration rate (eGFR). Efficacy in either of these markers will justify a larger, international trial to investigate micro-particle curcumin’s ability to lower the risk of ESRD in patients with CKD.
Design:
MPAC-CKD-1 is a multicenter, double-blind prospective randomized controlled trial.
Setting:
Four kidney disease clinics in Ontario, Canada (3 in London and 1 in Hamilton).
Patients:
We will enroll patients with CKD, defined by an eGFR between 15 and 60 mL/min/1.73 m2 and a daily albumin excretion of more than 300 mg (or a random urine sample albumin-to-creatinine ratio more than 30 mg/mmol).
Measurements:
We will measure changes in the co-primary outcomes of urinary albumin-to-creatinine ratio and eGFR at 3 months and 6 months. We will also measure compliance, safety parameters, and changes in health-related quality of life.
Methods:
Participants will be randomly assigned to receive micro-particle curcumin 90 mg once daily or matching placebo for 6 months. We will enroll at least 500 patients to exclude clinically meaningful 6-month changes in these 2 co-primary outcomes (16% difference in albuminuria, and a 2.3 mL/min/1.73 m2 between-group difference in the 6-month change in eGFR, at a two-tailed alpha of 0.025, power of 0.80).
Results:
Patient enrollment began on October 1, 2015, with 414 participants randomized as of July 2018. We expect to report the results in 2020.
Limitations:
MPAC-CKD-1 is not powered to assess outcomes such as the need for renal replacement therapy or death.
Conclusions:
MPAC-CKD-1 is a multicenter, double-blind prospective randomized controlled trial designed to test whether micro-particle curcumin reduces albuminuria and slows eGFR decline in patients with albuminuric CKD. MPAC-CKD-1 will also test the feasibility of this intervention and inform the need for a future larger scale trial (MPAC-CKD-2).
Trial registration:
MPAC-CKD-1 is registered with U.S. National Institutes of Health at clinicaltrials.gov (NCT02369549). Protocol version 2.0, December 6, 2014.
Keywords: CKD (chronic kidney disease), randomized controlled trial, albuminuria
Abrégé
Contexte:
La progression vers l’insuffisance rénale terminale (IRT) est la plus importante complication de l’insuffisance rénale chronique (IRC). Les patients atteints d’IRT dépendent de la dialyse ou de la transplantation pour survivre. Ces patients subissent de nombreuses complications et font face à des taux de mortalité très élevés. Ralentir la progression de la maladie est un objectif majeur. Malheureusement, même lorsque les traitements sont prodigués correctement, certains patients atteints de néphropathie chronique progressent vers l’IRT. Les traitements actuels ne parviennent pas à réduire l’inflammation et la fibrose qui médient cette progression. Les microparticules de curcumine, un produit de santé naturel qui possède des propriétés anti-inflammatoire et anti-fibrotiques, pourraient s’avérer un traitement efficace pour les patients atteints d’IRC.
Objectif:
L’étude MPAC-CKD-1 mesurera l’effet des microparticules de curcumine sur deux marqueurs importants de la progression de la maladie : l’albuminurie et le débit de filtration glomérulaire estimé (DFGe). L’efficacité des microparticules de curcumine sur l’un ou l’autre de ces marqueurs justifiera la conduite d’un essai international à plus grande échelle qui étudiera leur capacité à réduire le risque de progression vers l’IRT chez les patients atteints d’IRC.
Type d’étude:
L’étude MPAC-CKD-1 est un essai multicentrique prospectif, contrôlé, à répartition aléatoire et à double insu.
Cadre:
Quatre cliniques spécialisées en néphropathie de l’Ontario, au Canada (trois à London et une à Hamilton).
Sujets:
Seront recrutés les patients atteints d’IRC dont le DFGe se situe entre 15 et 60 ml/min/1,73 m2 et l’excrétion d’albumine quotidienne à plus de 300 mg (ou dont un échantillon d’urine présente un rapport albumine/créatinine de plus de 30 mg/mmol).
Mesures:
Les changements dans les deux principaux résultats (DFGe et rapport albumine/créatinine urinaire) seront mesurés à trois mois et à six mois. Seront également mesurés la conformité, les paramètres relatifs à l’innocuité et les changements dans la qualité de vie du patient en lien avec sa santé.
Méthodologie:
Un traitement d’une durée de six mois (dose quotidienne de 90 mg de curcumine ou un placébo) sera attribué de façon aléatoire aux participants. Un minimum de 500 patients sera inclus à l’étude afin d’exclure les changements cliniquement significatifs survenant au cours des six mois pour les deux principaux résultats étudiés (une différence de 16 % de l’albuminurie et une différence de 2,3 ml/min/1,73 m2 du DFGe dans les six mois entre les deux groupes, avec un alpha bilatéral de 0,025 à la puissance 0,80).
Résultats:
Le recrutement des patients a débuté le 1er octobre 2015 et en date de juillet 2018, 414 participants avaient été répartis. La publication des résultats est prévue en 2020.
Limites:
L’étude MPAC-CKD-1 n’est pas conçue pour mesurer des résultats tels que le besoin de recourir à une thérapie de remplacement rénal ni pour répertorier le taux de mortalité.
Conclusion:
L’étude MPAC-CKD-1 est un essai multicentrique prospectif, contrôlé, à répartition aléatoire et à double insu, conçu pour mesurer l’effet des microparticules de curcumine chez les patients atteints d’IRC albuminurique. On veut pouvoir observer soit une réduction de l’albuminurie, soit un ralentissement du déclin du DFGe. L’étude MPAC-CKD-1 vise également à tester la faisabilité de cette intervention et à éclairer le besoin de procéder à un essai futur à plus grande échelle (MPAC-CKD-2).
What was known before
Curcumin, a component of the spice turmeric, has anti-inflammatory and anti-fibrotic properties. Animal models and small-scale human studies suggest that curcumin may slow the progression of chronic kidney disease.
What this adds
By randomizing at least 500 patients with albuminuric chronic kidney disease to 90 mg per day of micro-particle curcumin or placebo, we will determine its effects on albuminuria and renal function. This trial will also determine the need for, and the feasibility of, a large-scale trial to assess clinically meaningful end points.
Introduction
Chronic kidney disease (CKD) is defined by an estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m2 or evidence of persistent kidney damage, such as excessive albuminuria. CKD is associated with significant morbidity and mortality but the most important complication of CKD is the progression to kidney failure (end-stage renal disease, ESRD). Patients who progress to ESRD require dialysis or transplantation to survive. They experience numerous complications, have high mortality rates,1 and their care is very expensive.2 Therefore, preventing or slowing the progression from CKD to ESRD is an important clinical and research goal. Currently, few treatments have been proven to slow progression, and even when these therapies are appropriately applied, many patients still progress to ESRD.3 This may be explained in part by inflammation and fibrosis, 2 processes that play a role in the progression of CKD,4,5 but are not addressed by current therapies.
Curcumin, a component of the dietary spice turmeric, has proven anti-inflammatory and anti-fibrotic properties (Supplement Figure S1) and has been shown to mitigate renal damage in multiple animal models of CKD (Supplement Table S1).6-9 The ability of curcumin to reduce albuminuria in humans is supported by 2 trials in patients with nephrotic-range proteinuria.10-12 However, these trials were small and patients were treated with turmeric, of which curcumin is only a small component. Furthermore, curcumin in its traditional form has very poor bioavailability.13-15 To increase patients’ exposure to curcumin, we chose to study a micro-particle formulation that is 27 times more bioavailable.16
Micro-particle curcumin for the treatment of CKD-1 (MPAC-CKD-1) is a double-blind, placebo-controlled randomized trial that will measure the effect of micro-particle curcumin on 6-month changes in 2 important markers of CKD progression: albuminuria and eGFR. Positive effects in either of these markers will justify a larger, international trial that will determine micro-particle curcumin’s ability to decrease the risk of ESRD in patients with CKD.
Methods
This protocol is presented according to the SPIRIT guidelines (see supplemental materials).17
Study Setting
We will coordinate this trial through the Lilibeth Caberto Kidney Clinical Research Unit at London Health Sciences Centre in London, Ontario. The trial steering committee is comprised of AX Garg and MA Weir, who will also provide outcome and adverse event adjudication. We will identify patients through 3 clinics in London, Ontario, Canada, and 1 clinic in Hamilton, Ontario, Canada. In each clinic, attending physicians will introduce the study and interested patients will meet with research coordinators to discuss the trial in detail. Those who are eligible and willing to participate will provide written, informed consent prior to randomization.
Eligibility Criteria
We will recruit patients with advanced CKD but who have not yet progressed to an irreparable stage. We will enroll patients with an eGFR between 15 and 60 mL/min/1.73 m2 and overt albuminuria, defined by a 24-hour urine collection with more than 300 mg of protein or a random urine albumin-to-creatinine ratio greater than 30 mg/mmol (265.2 mg/g). We will exclude patients with conditions that may potentially be exacerbated by the use of micro-particle curcumin (active peptic ulcer disease, hepatobiliary disease, history of significant bleeding) or who take medications that may interact with micro-particle curcumin (Table 1).
Table 1.
Inclusion and Exclusion Criteria.
| Inclusion criteria | 18 years of age and older | |
| Chronic kidney disease (eGFR 15 to 60 mL/min/1.73 m2) | ||
| Albuminuria (24-hour urine protein ⩾300 mg, or urinary albumin-to-creatinine ratio ⩾30 mg/mmol) | ||
| For those with diabetes mellitus, willing to measure and record blood glucose concentrations | ||
| Stable dose of ACE inhibitor or ARB | ||
| Exclusion criteria | Life expectancy <1 year | |
| Renal replacement therapy in the prior 3 months | ||
| Plans for renal transplantation during the study period | ||
| Active peptic ulcer disease | ||
| Recent hepatobiliary disease | ||
| Evidence of recent acute kidney injury (>50% increase in serum creatinine in the preceding 30 days) | ||
| Significant bleeding history in the last 6 months | Gastrointestinal bleed or retroperitoneal bleed requiring transfusion, or intracranial hemorrhage | |
| Ongoing use of drugs that may interact with curcumin | Oral anticoagulants | |
| Chemotherapeutic agents: cyclophosphamide, camptothecin, mechlorethamine, or doxorubicin | ||
| Anti-psychotic medications: haloperidol, aripiprazole, risperidone, ziprasidone, pimozide, quetiapine | ||
| Allergy to turmeric or its derivatives (ginger, cumin, cardamom) | ||
| Allergy to components of the investigational product | ||
Note. eGFR = estimated glomerular filtration rate; ACE = angiotensin converting enzyme; ARB = angiotensin receptor blocker.
Interventions
We will randomly assign patients to receive either micro-particle curcumin or matching placebo. Micro-particle curcumin will be administered at 90 mg per day (three 30 mg capsules once daily) for 6 months. Our rationale for the dosage selection is presented in the supplemental materials. The dose will remain constant over the study period. Randomization to micro-particle curcumin or matching placebo will occur in a 1:1 ratio. Balanced block randomization will be conducted using a computerized algorithm with variable block sizes and treatment allocation will be stratified by site (3 sites in London, 1 site in Hamilton) and by baseline diabetes mellitus status. After providing written informed consent, patients will be allocated randomly by means of a 24-hour online computerized system to maintain allocation concealment. Participants, investigators, and all research staff will remain unaware of the treatment allocation. Unblinding will occur in the setting of a severe adverse reaction in which the treating physician believes the investigational product may have a role in the patient’s condition or treatment.
Concomitant Care
Hypertension and proteinuria will be managed according to the Canadian Hypertension Education Program guidelines,18 which suggest treatment with an angiotensin-converting-enzyme inhibitor or an angiotensin receptor blocker. Patients not taking one of these medications require the reason why to be documented in the medical record. The doses of these medications may be reduced or the drug may be stopped during the course of the study, but the medical indication prompting that decision (eg, hyperkalemia) must be documented. We will not impose dietary restrictions because dietary sources of curcumin provide exceedingly small amounts of curcumin19,20; however, we will ask patients to refrain from using over-the-counter curcumin or turmeric supplements and document any use at each study visit.
Outcomes
Primary outcomes
We will assess the 6-month change in 2 co-primary outcomes: the change in albuminuria, and the change in eGFR. Albuminuria will be measured using albumin-to-creatinine ratios from first morning urine samples. The eGFR will be calculated using the CKD-EPI formula, which includes patient age, sex, race (African or non-African), and the serum creatinine concentration.21
Secondary outcomes
Glycemic control
We will assess glycemic control using the percentage of glycated hemoglobin at baseline, 3 months, and 6 months among patients with diabetes mellitus (diabetes mellitus was a stratification variable in the randomization). Curcumin use has been associated with improved glycemic control in animal models,22 and human studies.23
Study agent discontinuation and safety
Because MPAC-CKD-1 may inform the launch of subsequent larger trial, a better understanding of the tolerability of micro-particle curcumin in the CKD population is necessary. We will test protocol compliance through pill counts and interviews at each follow-up visit. Side effects will be assessed using standardized case report forms at each visit.
Renal failure composite (eGFR loss of ⩾30%, or ESRD, or death)
We expect less than 10% of participants will experience these outcomes by 6 months. Although we will not have adequate statistical power to detect a meaningful effect of curcumin on these outcomes, we will document any trends to inform the expected event rate for future studies. We will define ESRD as an eGFR <15 mL/min/1.73 m2 or the initiation of renal replacement therapy, which includes dialysis or transplantation.
Health-related quality of life
We will compare health-related quality of life scores determined by the RAND version of the Short Form-36 (SF-36) questionnaire administered at baseline and 6 months. We will compare changes in both the physical composite score and the mental composite score. Beyond preservation of kidney function are several other potential mechanisms by which curcumin may benefit quality of life, including potential benefits on depression and chronic pain.24,25
Additional outcomes
Serum curcumin levels
The strength of previously identified relationships between traditional curcumin exposure and clinical outcomes has been limited by the difficulty in achieving measurable serum curcumin levels. To confirm the improved bioavailability reported with micro-particle curcumin,16 and to strengthen any relationship between micro-particle curcumin and any outcomes we identify, we will measure serum trough levels of curcumin and its major metabolites in the first 25 participants randomized.14
Recruitment
To ensure we can achieve this recruitment goal, we have assessed the number of provisionally eligible patients managed in the 4 clinics involved in this trial and have gauged the success of a recently conducted trial with similar inclusion criteria.26,27 In addition, we have worked closely with research coordinators to remove possible barriers to enrollment and streamline the data collection process.
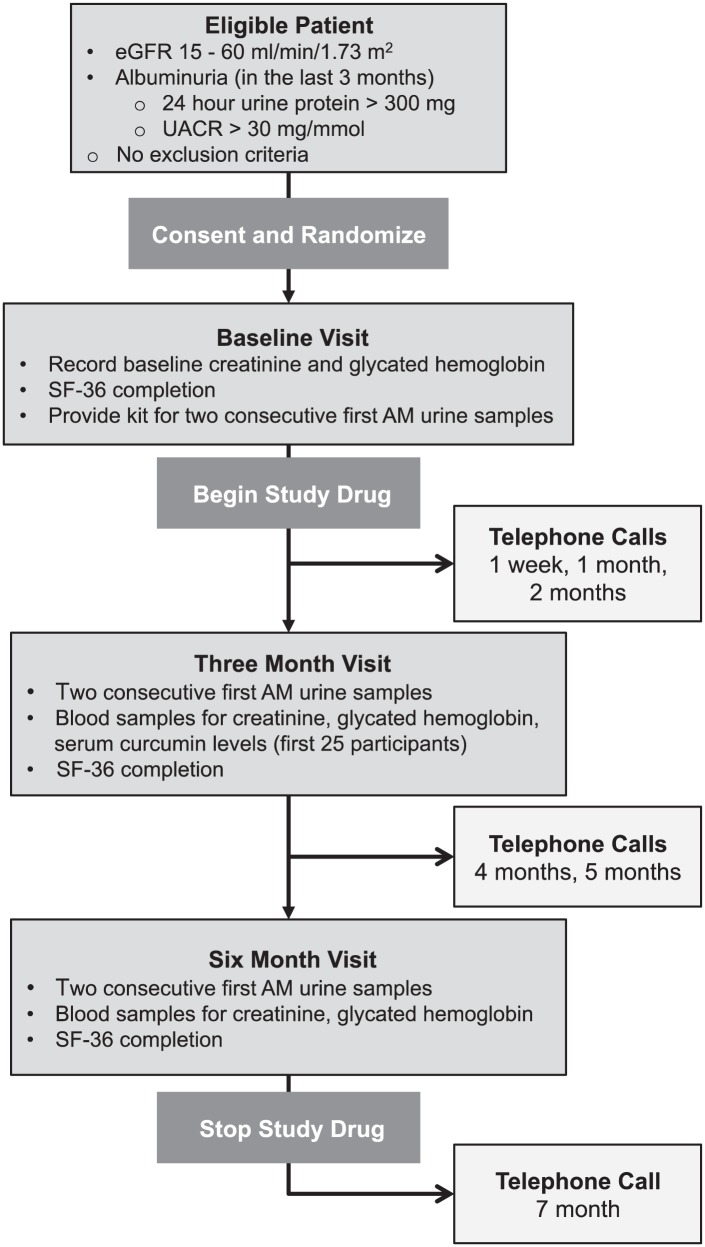
Figure 1.
Participant timeline.
Note. eGFR = estimated glomerular filtration rate; SF-36 = Short Form-36; UACR = urinary albumin-to-creatinine ratio.
Data Collection Methods
We will gather outcome measures 3 months and 6 months after randomization. To reduce the variability in the urinary albumin-to-creatinine ratio, we will take the mean of first morning urine samples collected on 2 consecutive days. In our previous work, we determined that the mean of two samples substantially reduced the standard deviation of the log-transformed percentage change from 0.59 to 0.49.28 We will calculate the eGFR rates using the measured plasma creatinine concentrations. These will be measured at central hospital-based laboratories using standardized enzymatic colorimetric methods.
Nonadherence
Patients who miss taking the investigational study medication over 7 or more days during any 4-week period will meet with an investigator to discuss ways of improving adherence. We will catalogue the reasons for nonadherence and the strategies used to overcome them. Patients who do not attend study visits will be contacted and encouraged to comply with the protocol in whatever way they can. Until the study ends or the participant withdraws consent, we will attempt to reach them 3 times within each 4-week period to determine their status. In extenuating circumstances, we will contact the patient’s family physician to determine the patient’s vital status.
Statistical Methods
Sample size
Most physicians would view a 15% to 25% reduction in albuminuria as evidence of a promising treatment effect. Such was the case for ACE inhibitors and angiotensin receptor blockers, which showed a reduction in albuminuria of 15% to 50% and were later proven to reduce the risk of mortality and ESRD.29-32 In our preliminary work on urinary albumin-to-creatinine ratio testing, we found patients eligible for MPAC-CKD-1 had a mean initial urinary albumin-to-creatinine ratio of 128.3 mg/mmol.28 Over 3 months, we observed a mean increase in the urinary albumin-to-creatinine ratio of 30.7 mg/mmol and found the standard deviation of the change to be 69.2 mg/mmol. Using these data, enrolling 250 patients per group will allow an 87% power (= 0.025) to exclude a difference of 21 mg/mmol. To allow for loss to follow-up, we expect to recruit up to an additional 75 patients to reach a minimum of 250 patients per arm with complete follow-up data. For changes in eGFR, we will have 90% power (α = 0.025) to exclude a difference of 2.3 mL/min/1.73 m2 between treatment and control groups (using a standard deviation of 4 mL/min/1.73 m2). This estimate is based on a reported average decline in eGFR in albuminuric CKD patients of 2 to 4 mL/min/1.73 m2/year (with a standard deviation of 3-4 mL/min/1.73 m2/year).33,34
Primary outcome
We will conduct all primary analyses according to the intention-to-treat principle. Significance testing will be conducted with a two-sided alpha level of 0.05/2 using Hochberg adjustment for 2 simultaneous primary end points.35 We will use two-sample t tests to compare changes in the co-primary outcomes.32,36 Missing data will be handled using model-based multiple imputation methods (and sensitivity analyses will be performed to confirm that conclusions are not sensitive to assumptions about the missing-data mechanism)37; the albumin-to-creatinine ratio data collected at the 3-month visit will be included in the imputation model.
Secondary outcomes
We will use a two-sample t test to compare changes in hemoglobin A1c between the 6-month and baseline values. The risk of the composite outcome of loss of ⩾30% of baseline eGFR, ESRD, or death will be compared between groups using logistic regression analysis. The proportions of patients discontinuing study capsules and experiencing adverse events will be compared between groups using the chi-square test.
Baseline characteristics
Randomization reliably removes random differences between treatment groups when at least 1000 participants are included.38 With the size of our sample, it is possible that imbalances may arise between the treatment groups on important characteristics that may influence outcomes. We will record baseline characteristics pertinent to the progression of CKD and adjust the final point estimates of risk for these variables using linear regression analysis. Characteristics that will be included in the multivariable regression model are the following: age, sex, tobacco use, blood pressure, glycemic control, use of angiotensin-converting-enzyme inhibitors or angiotensin receptor blockers, use of aldosterone antagonists. Sodium-glucose co-transporter-2 (SGLT-2) inhibitors were approved for use in Canada in May 2014, and gained provincial formulary coverage in 2015. Because these medications have been shown to reduce albuminuria, we will record these use39,40; however, given their minimum eGFR cut off of 45 mL/min/1.73 m2, we anticipate that they will not be commonly used in our population.
Other analyses
We will conduct an exploratory per-protocol analysis consisting of patients with no major protocol violations and who were exposed to their randomly assigned treatment for a minimum of 3 months. We will also conduct a subgroup analysis based on a higher or lower estimated risk of kidney failure (using the kidney failure risk equation).41
Results
MPAC-CKD-1 began enrollment on October 1, 2015, and has randomized 414 participants as of July 2018. We expect to complete enrollment in 2019 and report the results publicly in 2020.
Discussion
Micro-particle curcumin holds a great deal of promise as a treatment to slow the progression of CKD because of its anti-inflammatory and anti-fibrotic properties.
In vitro experiments using a variety of cell lines have shown that curcumin exposure attenuates the activation of nuclear factor-kappa B (NF-κB). Activation of NF-κB is a pivotal regulatory step in the inflammatory process that leads to the elaboration of interleukin-1 (IL-1), IL-2, IL-6, tumor necrosis factor-alpha (TNF-α), and monocyte chemotactic protein-1 (MCP-1).42 By blocking its activation, curcumin acts as a potent anti-inflammatory agent.43-45 The conversion of functional tissue to scar is a hallmark of progressive CKD. Transforming growth factor-beta (TGF-β) is one of the most important mediators in this process.46,47 In a variety of in vitro settings, curcumin has been shown to lessen the effect of TGF-β through inhibition of its molecular signaling,48,49 activation of endogenous inhibitors,50 thereby resulting in less scar formation.51,52
Animal models of CKD also support the effectiveness of curcumin exposure. Soetikno et al reported that compared with placebo, diabetic rats fed with curcumin 100 mg/kg per day for 8 weeks had far less scar tissue in their kidneys and expressed lower levels of TGF-β.6 In two related studies, Ghosh et al showed that rats with 5/6 nephrectomies who consumed 75 mg/kg curcumin per day had less proteinuria, better kidney function, more normal appearing renal histology, and lower levels of NF-κB and TNF-α than those on a placebo diet.7 Follow-up study of Ghosh et al showed that this benefit was realized even when curcumin treatment was delayed until after the appearance of proteinuria (a more clinically relevant timing of curcumin exposure).8 Sharma et al found similar effects in diabetic rats and demonstrated a dose-response.9
Two small trials have tested curcumin supplementation in patients with kidney impairment.10,11 In the study most applicable to MPAC-CKD-1, Khajehdehi et al randomized 40 patients with diabetic CKD to receive placebo or turmeric (0.5 g 3 times daily; approximately equivalent to 66 mg of curcumin per day) for 2 months. Both groups began the study with approximately 4.5 g of daily proteinuria. After only 2 months of treatment, the turmeric group had a 39% reduction in proteinuria while the placebo group’s proteinuria remained unchanged.10 The treatment group experienced significant reductions in serum levels of TGF-β and TNF-α. In the second trial of patients with lupus nephritis, turmeric supplementation again decreased proteinuria significantly compared with placebo.11
MPAC-CKD-1 is the next step in assessing micro-particle curcumin’s potential to slow the progression of CKD. If meaningful changes in our surrogate outcome measure are observed, this will justify a larger, international trial equipped to test curcumin’s ability to lower the risk of ESRD.
Supplemental Material
Supplemental material, Supplementary_Materials_(1) for Micro-Particle Curcumin for the Treatment of Chronic Kidney Disease-1: Study Protocol for a Multicenter Clinical Trial by Matthew A. Weir, Michael Walsh, Meaghan S. Cuerden, Jessica M. Sontrop, Laura C. Chambers and Amit X. Garg in Canadian Journal of Kidney Health and Disease
Acknowledgments
We would like to thank Darek Gozdzik for the development and maintenance of the trial database and Virginia Schumann for her financial management and assistance in obtaining Health Canada approval for this trial.
Footnotes
Availability of Data and Materials: The study investigators will have access to the final trial data set. We do not plan to make data sets available to the public.
Data Monitoring: The Data Safety Monitoring Committee (DSMB) will be comprised of external content experts with experience in clinical trials. The DSMB will review unblinded safety data once 50% of patients have completed the study and make recommendations to the trial Steering Committee. The unblinded statistician associated with the DSMB will make no contribution to the trial design or final analysis of the study. No interim analyses are planned. The Lawson Health Research Institute will be responsible for conducting periodic audits of the conduct of MPAC-CKD-1 (Micro-Particle Curcumin for the Treatment of Chronic Kidney Disease-1).
Dissemination Policy: We plan to disseminate the results of MPAC-CKD-1 (Micro-Particle Curcumin for the Treatment of Chronic Kidney Disease-1) through peer-reviewed publication. We will not employ professional writers and each author on the final article will have fulfilled the requirements set out by the International Committee of Medical Journal Editors.
Ethics Approval and Consent to Participate: We have obtained approval for the conduct of MPAC-CKD-1 (Micro-Particle Curcumin for the Treatment of Chronic Kidney Disease-1) from both the Western University research ethics board and the McMaster University research ethics board. Any modifications to the protocol that may impact the conduct of the study or affect patient safety, including changes of study objectives, study design, patient population, sample sizes, study procedures, or significant administrative aspects will prompt a formal amendment. Revisions will be forwarded to each participating site with direction for submission to each respective research ethics board. The protocol and prespecified statistical analysis plan were approved by all authors and the data safety and monitoring board.
Consent to Participate is not required section for a Protocol publication type.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: No investigator or research personnel has a financial or other competing interest in MPAC-CKD-1. The study design and collection, management, analysis, and interpretation of the study data have not and will not involve the funders. The trial funders will have no role in the reporting of the results.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Kidney Foundation of Canada (KFOC130029), and the Canadian Institute of Health Research (CIHR365629). Natural Factors Nutritional Products Ltd (Coquitlam, BC, Canada) supplied investigational product and matching placebo as in-kind support. Michael Walsh holds a New Investigator Award from the Canadian Institutes of Health Research. Amit X. Garg is supported by a Clinician Investigator Award from the Canadian Institutes of Health Research and the Adam Linton Chair in Kidney Health Analytics.
ORCID iD: Jessica M. Sontrop  https://orcid.org/0000-0001-7784-2028
https://orcid.org/0000-0001-7784-2028
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Collins AJ, Foley RN, Herzog C, et al. Excerpts from the US Renal Data System 2009 Annual Data Report. Am J Kidney Dis. 2010;55(suppl 1):S1-A7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kerr M, Bray B, Medcalf J, O’Donoghue DJ, Matthews B. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant. 2012;27(suppl 3):iii73-iii80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003;63:225-232. [DOI] [PubMed] [Google Scholar]
- 4. Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116:288-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev. 2008;4:39-45. [DOI] [PubMed] [Google Scholar]
- 6. Soetikno V, Watanabe K, Sari FR, et al. Curcumin attenuates diabetic nephropathy by inhibiting PKC-α and PKC-β1 activity in streptozotocin-induced type I diabetic rats. Mol Nutr Food Res. 2011;55:1655-1665. [DOI] [PubMed] [Google Scholar]
- 7. Ghosh SS, Massey HD, Krieg R, et al. Curcumin ameliorates renal failure in 5/6 nephrectomized rats: role of inflammation. Am J Physiol Renal Physiol. 2009;296:F1146-F1157. [DOI] [PubMed] [Google Scholar]
- 8. Ghosh SS, Krieg R, Massey HD, et al. Curcumin and enalapril ameliorate renal failure by antagonizing inflammation in 5/6 nephrectomized rats: role of phospholipase and cyclooxygenase. Am J Physiol Renal Physiol. 2012;302:F439-F454. [DOI] [PubMed] [Google Scholar]
- 9. Sharma S, Kulkarni SK, Chopra K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2006;33:940-945. [DOI] [PubMed] [Google Scholar]
- 10. Khajehdehi P, Pakfetrat M, Javidnia K, et al. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double-blind and placebo-controlled study. Scand J Urol Nephrol. 2011;45:365-370. [DOI] [PubMed] [Google Scholar]
- 11. Khajehdehi P, Zanjaninejad B, Aflaki E, et al. Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: a randomized and placebo-controlled study. J Ren Nutr. 2012;22:50-57. [DOI] [PubMed] [Google Scholar]
- 12. Shoskes D, Lapierre C, Cruz-Correa M, et al. Beneficial effects of the bioflavonoids curcumin and quercetin on early function in cadaveric renal transplantation: a randomized placebo controlled trial. Transplantation. 2005;80:1556-1559. [DOI] [PubMed] [Google Scholar]
- 13. Shoba G, Joy D, Joseph T, et al. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353-356. [DOI] [PubMed] [Google Scholar]
- 14. Vareed SK, Kakarala M, Ruffin MT, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:1411-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shehzad A, Wahid F, Lee YS. Curcumin in cancer chemoprevention: molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch Pharm (Weinheim). 2010;343:489-499. [DOI] [PubMed] [Google Scholar]
- 16. Sasaki H, Sunagawa Y, Takahashi K, et al. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull. 2011;34:660-665. [DOI] [PubMed] [Google Scholar]
- 17. Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013:200-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33:557-576. [DOI] [PubMed] [Google Scholar]
- 19. Tayyem RF, Heath DD, Al-Delaimy WK, et al. Curcumin content of turmeric and curry powders. Nutr Cancer. 2006;55:126-131. [DOI] [PubMed] [Google Scholar]
- 20. Kwon Y. Estimation of curcumin intake in Korea based on the Korea National Health and Nutrition Examination Survey (2008-2012). Nutr Res Pract. 2014;8:589-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pari L, Murugan P. Antihyperlipidemic effect of curcumin and tetrahydrocurcumin in experimental type 2 diabetic rats. Ren Fail. 2007;29:881-889. [DOI] [PubMed] [Google Scholar]
- 23. de Meloa ISV, Santosb Dos AF, Buenoc NB. Curcumin or combined curcuminoids are effective in lowering the fasting blood glucose concentrations of individuals with dysglycemia: systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2018;128:137-144. doi: 10.1016/j.phrs.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 24. Sahebkar A, Henrotin Y. Analgesic efficacy and safety of curcuminoids in clinical practice: a systematic review and meta-analysis of randomized controlled trials. Pain Med. 2016;17:1192-1202. [DOI] [PubMed] [Google Scholar]
- 25. Ng QX, Koh SSH, Chan HW, Ho CYX. Clinical use of curcumin in depression: a meta-analysis. J Am Med Dir Assoc. 2017;18:503-508. [DOI] [PubMed] [Google Scholar]
- 26. Clark WF, Sontrop JM, Huang S-H, et al. The chronic kidney disease Water Intake Trial (WIT): results from the pilot randomised controlled trial. BMJ Open. 2013;3:e003666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clark WF, Sontrop JM, Huang S-H, et al. Effect of coaching to increase water intake on kidney function decline in adults with chronic kidney disease: the CKD WIT randomized clinical trial. JAMA. 2018;319:1870-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sontrop JM, Garg AX, Li L, et al. Consecutive first-morning urine samples to measure change in the albumin-to-creatinine ratio: a pilot study of a home urine collection protocol. Can J Kidney Health Dis. 2016;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456-1462. [DOI] [PubMed] [Google Scholar]
- 30. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851-860. [DOI] [PubMed] [Google Scholar]
- 31. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869. [DOI] [PubMed] [Google Scholar]
- 32. Parving H-H, Persson F, Lewis JB, et al. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433-2446. [DOI] [PubMed] [Google Scholar]
- 33. Halbesma N, Kuiken D-S, Brantsma AH, et al. Macroalbuminuria is a better risk marker than low estimated GFR to identify individuals at risk for accelerated GFR loss in population screening. J Am Soc Nephrol. 2006;17:2582-2590. [DOI] [PubMed] [Google Scholar]
- 34. Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 1997;51:1908-1919. [DOI] [PubMed] [Google Scholar]
- 35. Huque MF. Validity of the Hochberg procedure revisited for clinical trial applications. Stat Med. 2016;35:5-20. [DOI] [PubMed] [Google Scholar]
- 36. Bland JM, Altman DG. The use of transformation when comparing two means. BMJ. 1996;312:1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Little RJ, D’Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367:1355-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nguyen T-L, Collins GS, Lamy A, et al. Simple randomization did not protect against bias in smaller trials. J Clin Epidemiol. 2017;84:105-113. [DOI] [PubMed] [Google Scholar]
- 39. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323-334. [DOI] [PubMed] [Google Scholar]
- 40. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657. [DOI] [PubMed] [Google Scholar]
- 41. Tangri N, Grams ME, Levey AS, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315:164-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gupta SC, Kim JH, Kannappan R, et al. Role of nuclear factor κB-mediated inflammatory pathways in cancer-related symptoms and their regulation by nutritional agents. Exp Biol Med (Maywood). 2011;236:658-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yamaguchi M, Moore TW, Sun A, Snyder JP, Shoji M. Novel curcumin analogue UBS109 potently stimulates osteoblastogenesis and suppresses osteoclastogenesis: involvement in Smad activation and NF-κB inhibition. Integr Biol (Camb). 2012;4:905-913. [DOI] [PubMed] [Google Scholar]
- 44. Qian J-J, Zhai X-G, Niu M-H, Zhou Q, Zhou YJ. [Curcumin inhibits iron overload-induced hepatocytic apoptosis and nuclear factor-κB activity]. Zhonghua Yi Xue Za Zhi. 2012;92:1997-2001. [PubMed] [Google Scholar]
- 45. Qiao Q, Jiang Y, Li G. Curcumin improves the antitumor effect of X-ray irradiation by blocking the NF-κB pathway: an in-vitro study of lymphoma. Anticancer Drugs. 2012;23:597-605. [DOI] [PubMed] [Google Scholar]
- 46. Liu N, Tolbert E, Pang M, et al. Suramin inhibits renal fibrosis in chronic kidney disease. J Am Soc Nephrol. 2011;22:1064-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol. 2000;15:290-301. [DOI] [PubMed] [Google Scholar]
- 48. Smith MR, Gangireddy SR, Narala VR, et al. Curcumin inhibits fibrosis-related effects in IPF fibroblasts and in mice following bleomycin-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;298:L616-L125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gaedeke J, Noble NA, Border WA. Curcumin blocks multiple sites of the TGF-beta signaling cascade in renal cells. Kidney Int. 2004;66:112-120. [DOI] [PubMed] [Google Scholar]
- 50. Song K, Peng S, Sun Z, et al. Curcumin suppresses TGF-β signaling by inhibition of TGIF degradation in scleroderma fibroblasts. Biochem Biophys Res Commun. 2011;411:821-825. [DOI] [PubMed] [Google Scholar]
- 51. Zheng S, Chen A. Disruption of transforming growth factor-beta signaling by curcumin induces gene expression of peroxisome proliferator-activated receptor-gamma in rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G113-G123. [DOI] [PubMed] [Google Scholar]
- 52. Hu Y, Liang H, Du Y, et al. Curcumin inhibits transforming growth factor-beta activity via inhibition of Smad signaling in HK-2 cells. Am J Nephrol. 2010;31:332-341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Materials_(1) for Micro-Particle Curcumin for the Treatment of Chronic Kidney Disease-1: Study Protocol for a Multicenter Clinical Trial by Matthew A. Weir, Michael Walsh, Meaghan S. Cuerden, Jessica M. Sontrop, Laura C. Chambers and Amit X. Garg in Canadian Journal of Kidney Health and Disease