Abstract
This feature article for the thematic series on congestive heart failure (CHF) readmissions aims to outline important gaps in guidelines for patients with multiple comorbidities and the elderly. Congestive heart failure diagnosis manifests as a 3-phase journey between the hospital and community, during acute, chronic stable, and end-of-life (palliative) phases. This journey requires in variable intensities a combination of multidisciplinary care within tertiary hospital or ambulatory care from hospital outpatients or primary health services, within the general community. Management goals are uniform, ie, to achieve the lowest New York Heart Association class possible, with improvement in ejection fraction, by delivering gold standard therapies within a CHF program. Comorbidities are an important common denominator that influences outcomes. Comorbidities include diabetes mellitus, chronic obstructive airways disease, chronic renal impairment, hypertension, obesity, sleep apnea, and advancing age. Geriatric care includes the latter as well as syndromes such as frailty, falls, incontinence, and confusion. Many systems still fail to comprehensively achieve all aspects of such programs. This review explores these factors.
Keywords: elderly, geriatric, comorbidity, readmissions, translating guidelines, translational research
Introduction
Improvement in ejection fraction (RF) and congestive heart failure (CHF) class is the primary goal of management, and are associated with better prognosis. Doing so will also reduce readmissions.1,2 In the United States, prevalence of CHF is >5.7 million, with 670 000 new cases yearly. In Europe and globally, prevalence is >15 million and 37.7 million, respectively.3 Congestive heart failure hospitalization rates are high, and it is the leading cause in patients >65 years of age, with more than 1 million primary presentations or 1% to 2% of all hospitalizations yearly. Annual Medicare expenditure in the United States exceeds US $17 billion.3–6 Following a CHF admission, 1 in 4 are readmitted within the first month and half within 6 months, where 80% of emergency room presentations are admitted.7–13 Matching funding to readmission strategies such as pay for performance or fee-for-service, which are either health system or client focused, has not generated the desired outcomes within traditional models of care.13 In addition, presentations, readmissions, and costs for CHF are projected to increase by 50% by 2035.14–17
To achieve New York Heart Association (NYHA) functional class 1 with an improved EF, the early focus of pharmacologic therapies is now interwoven into a complex program with a range of services and therapies requiring multidisciplinary input. The OPTIMIZE-HF study showed that when such programs are used to deliver care, outcomes could be improved.18 Studies also tell us that the gap between obtaining the highest class of evidence (Class 1A), the grade of recommendation, and the need to continually fine-tune evidence at the community level remains an understudied area. Thus, the future must in some fashion envision broader clarification in recommendations taking into account an aging population with multiple comorbidities.19,20 Could this be an important consideration when addressing readmissions?
More than half of CHF patients will suffer a concomitant comorbidity such as diabetes, chronic renal impairment (CRI), smoking-induced lung diseases, obesity, sleep apnea, hypertension, and atrial fibrillation (AF).21 The elderly are also challenged by having greater comorbidities, psychological, and social vulnerability as well as geriatric syndromes such as falls, confusion, and frailty.22–24 It is not yet clear whether merely translating guidelines from a homogeneous randomized clinical trial to heterogonous population translates to improved outcomes and reduced hospital admissions. In this review, we explore CHF guidelines in the context of comorbid disease and advancing age focusing on opportunities to close the guideline gaps.
Understanding Registry and Trial Data in Translating Guidelines
Congestive heart failure still carriers a grave prognosis at all-time intervals, 30 days, 1, 5, and 10 years.25 These differences can be greater across racial subgroups and global health systems. The etiology of CHF and associated risk factors also contributes to this. Although age-adjusted incidence, prevalence, and mortality are decreasing, the absolute number of patients are increasing as patients live longer. Postdischarge event rates remain high, whereas length of hospital stay is shorter for an increasingly complex patient cohort. Congestive heart failure is thus an epidemic, where rates are projected to increase, and uses resources on many fronts the most costly being hospitalizations.25,26 Table 1 summarizes the publications that have covered this in greater detail.
Table 1.
Summary of findings from key heart failure hospitalization registries.
| Characteristics reported | Key findings |
|---|---|
| Epidemiology | • Incidence: Global 100-900/100 000; Framingham (1950-1999): F ↓ M (~420-327 vs 564 cases/100 000 pyr. Olmstead Country (2000-2010) M and F ↓ 43% and 29%. Greater in African Americans and developing nations. • Prevalence: 1% to 2% developed nations. Global 37.7 million; range <1% 40 years old; 2× ↑ each decade, peak 10% >80 years old. Lifetime risk 40 to 80 years old is 40%. • Prognosis: Framingham M 62% and F 42% 5 y; over each decade ↓ by 10% to 11%. In 1990-1999, 33% improvement. Current 5-y mortality 50%; medial survival 4.2 y (developing nations 2.61-3.72); >65 years old 30 d and 12 mo 27.5%. Inpatient mortality declining 38%, 16.4% for 30 d and 12 mo. • Admissions: 1979-2004: ↑ primary CHF diagnosis 219 to 390/100 000 pyr, and 3× ↑ admissions27,28 • Olmstead County: 1.34 admissions ppy, 63% noncardiac29 • 1999 and 2011 (Medicare patients) ↓ 1390 to 925; 100 000 pyr LOS3.1 to 1.9 d26,30 • Ethnicity: 20% and 50% ↑ Hispanics and African Americans, 50% ↓ Asians and whites31; median 30-d readmission rate 21.9% (17%-28.2%), ↓ 1.5% 2010 to 2013, 1-y rate ~67%.26,32–34 • Mismatch between per capita decline in HHF rates and static or increasing early postdischarge mortality and readmission rates in developed nations.35–40 • Medication adherence: highest rates in North America (except MRA), Western Europe, and Japan; lowest in Eastern Europe and Asia (excluding Japan) |
| Patient demographics | • Mean age: 70 to 75 y (SD 15 y). Social factors affect severity and age at first MACE. • Sex: Women have better prognosis • Ethnicity: Very minimal data. Observations suggest earlier presentation and greater severity in some (eg, African American, Hispanics). GWTG-HF intervention study, no racial disparity, and improved in-hospital mortality in African Americans and Hispanics |
| Clinical characteristics | • Ischemic CHF universal lead cause. Uncontrolled hypertension, valvular heart disease, congenital heart disease in developing nations • Cardiac comorbidities >40%-70%, eg, IHD, HT, AF • Noncardiac >33%, eg, CKD, COPD, DM; some underreported, eg, OSA, depression • Aggravators to CHF treatments, eg, in DM, COPD/asthma underreported |
| Initial clinical presentation | • BP: >50% hypertensive; ≈2% <90 mm Hg • Dyspnea—NYHA class IV >34%; class II to III, orthopnea >90% • Rales >70%; systemic congestion (JVP; peripheral edema) >66% • CXR >75% pulmonary congestion |
| Diagnostics | • ↓ Hb—50% mild, 25% moderate; ↓ Na >20%; eGFR 10% >90, 20% <30 mL/min/m2 • ECG: baseline 50%, new onset HHF 20%; 33% wide QRS • Echo: 66% EF<45% |
| In-hospital and postdischarge outcomes | • IH: mLOS: 4 to 20 d; mortality 4% to 30% • Discharge: readmission 60/90 d to 1 y—30% and 32%; mortality 60/90 d to 1 y—5.4% to 14% and 17.4%. • >50% readmissions noncardiovascular cause |
| Inpatient management | • Diuretic regimes poorly recorded; geographical variation in inotropes and vasodilators • <10% undergo procedural intervention, eg, coronary angiography |
| Morbidity and mortality predictors | • Framingham cohort • Age, weight, cardiac, and noncardiac comorbidities, systolic blood pressure • Biochemistry (renal function [BUN and SCr], serum Na, Hb, BNP, Tn), QRS duration, and evidence-based medication utilization • Admission renal function, systolic BP, elevated B-type NP, and positive Tn suggest particularly high risk for short-term morbidity and mortality • OPTIMIZE-HF—IH mortality—pneumonia (OR 1.60), worsening renal function (1.48), and ischemia (OR 1.20); postdischarge mortality ischemia (OR 1.52) and worsening renal function (OR 1.46) |
| Quality improvement initiatives | • Participation in observational registry using benchmark data reports improves outcomes. ADHERE—BB use ↑ 29% (IH) and 30% (discharge); mLOS and IH mortality ↓ 6.3 to 5.5 d and 4.5% to 3.2%. • Participation in interventional registry, eg, OPTIMIZE-HF—BB use ↑ 76% to 86%; ↓ mLOS (P < .05); ↓ trends IH/discharge/other mortality. GWTG-HF and IMPROVE-HF corroborated above study • Readmission prevention (231): (1) transitional care programs, (2) evidence-based interventions that reduce readmissions (neurohormonal blockade, AICD, CRT, cardiac rehabilitation, and exercise training), (3) emergency room discharge, (4) observation units, (5) outpatient infusion centers, (6) detecting preclinical HF deterioration (eg, technology) |
| Readmission | • OPTIMIZE-HF—Mean age 73.1 y, 48% men, mean EF 39.0%. About 61.3% of 48 612 patients had ⩾1 precipitating factors: pneumonia/respiratory process (15.3%), ischemia (14.7%), and arrhythmia (13.5%) were most frequent. • Readmissions within 30 d often relate to HF. Causes include (231) the following: 1. Patient factors: illness severity, social status determinants (race, income, education) 2. Community factors: hospital resources, community social support institutions 3. Modifiable factors: regional variations, quality of care (in-patient, discharge instructions, medication dispensing process, ambulatory care access, communication across health providers) |
| Comorbidities | • Common: HT, CRI, DM, chol., AF; OSA • Mortality ↑ |
Abbreviations: 30-dR, 30-day readmission; AF, atrial fibrillation; AICD, automated implantable cardiac defibrillator; BB, β-blocker; BP, blood pressure; BUN, blood urea nitrogen; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapies; d, days; DM, diabetes mellitus; echo, echocardiography; F, female; Hb, hemoglobin; HHF, hospitalized heart failure; HT, hypertension; IH, in-hospital; IHD, ischemic heart disease; JVP, jugular venous pressure; M, male; MACE, major adverse cardiovascular event; mLOS, median length of stay; Na, sodium <135 mEq/L; NP, natriuretic peptide; OR, odds ratio; OSA, obstructive sleep apnea; SCr, serum creatinine; SOA, state of the art; Tn, troponin.
This table summarizes selected reviews and registries on HHF, predominately from developed countries worldwide. Data on admission demographics, treatment, and outcomes are presented. Data from developing nations and some racial backgrounds are limited.
Adapted from review and meta-analysis: Previous studies6,7,10,11,13,17,23,24,28,41–46 and Appendix 1; stand-alone and trial registries: ADHERE, Acute Decompensated Heart Failure National Registry47–54; ADHERE-AP, Acute Decompensated Heart Failure National Registry International, Asia Pacific55; AHEAD, Acute Heart Failure Database56; ALARM-HF, Acute Heart Failure Global Registry of Standard Treatment57; ARIC, Atherosclerosis Risk in Communities Study58; ATTEND, Acute Decompensated Heart Failure Syndromes59,60; EFICA, Epidémiologie Francaise de l’Insuffisance Cardiaque Aigue61; EHFS II, European Heart Failure Survey II62,63; ESC-HF, European Society of Cardiology, Heart Failure64,65; IN-HF, Italian Registry on Heart Failure66; RO-AHFS, Romanian Acute Heart Failure Syndromes.67 Intervention: EVEREST, Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan23,24,68; GWTG-HF, Get With The Guidelines—Heart Failure69–74; IMPROVE-HF, Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting75; OPTIMIZE-HF, Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure.11,76–82
Natural history of heart failure and the burden on health system
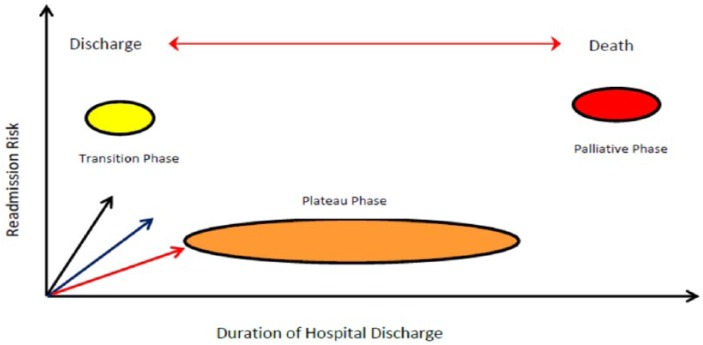
Progression of CHF or left ventricular (LV) recovery for any individual varies with the factors that influence the degree of ventricular remodeling and the causes.4,83 The greatest responders are seen with abnormalities in energetics, followed by toxins, and inflammatory cardiomyopathies. Readmissions are high when the remodeling process negatively affects systolic function.25,26,84,85 All cases will follow a 3-phase terrain of lifetime readmission risk with the highest risk “transition phase” and “palliative phase” and lowest in “plateau phase” (Figure 1).86 The cost of HF care accumulates largely from repeated hospitalization, because ambulatory care for complex CHF, with comorbidities and for elderly patients are yet to find the most cost-efficient model.5,87
Figure 1.
Natural history of heart failure (HF). Diagram demonstrates a 3-phase process once HF is diagnosed. The natural history of HF is chronological progression of left ventricular remodeling, manifesting with symptoms, physical morbidity, and early death. HF readmissions, presenting as acute decompensation, have greatest risks in the transition and palliative phases. The transition forward to more advanced phases is influenced by rate of recovery and normalization of LV function in correlation to the starting point of prior screening (black arrow), early treatment (blue arrow), and through its natural history (read arrow), and the type of cardiomyopathy, energetic defects > toxins > inflammatory causes. The slope of the arrows highlights the trajectory and prolongations toward death. Terminology: (1) Normalization of LV function, defined as an EF ⩾50%; (2) recovery of LV function, defined as an improvement in LF ejection fraction from 5% to 15 %; normalization occurs less frequently than recovery of LV function.
What have we learned from registries?
Most CHF cases are diagnosed in hospital and all patients will be admitted at some point. The past several decades have produced large, high-quality, multicenter registries, some with interventions that provided epidemiologic data as well as insight into the process of care (Table 1). The American Heart Association (AHA) has also published benchmarks for CHF taxonomy and clinical performance standards.88,89
Registries based on benchmarked data paired with interventions that optimize the delivery of proven strategies, alone, can deliver improvement in all performance measures such as readmissions and outcomes, largely through improved adherence to guidelines. Intervention tools include: evidence-based practice algorithms, standardized order sets, and discharge checklist; and access to specialist, with only 52% of Medicare patients receiving inpatient review when readmitted. Rewards-based system such as fee-for-service or award centers appear less significant. Gaps in authoritative data matching sociodemographics, comorbidities and older age support need for more heterogenous studies.13,90 Risk scoring tools are yet to deliver meaningful roles in clinical assessments.91–97
Heart Failure and Comorbidities
Early calls for recognizing multiple comorbidities98 are increasingly factored in both American and European CHF management guidelines.99–102 Positions are also increasingly provided. There remain large gaps due to homogeneity of randomized clinical trials. Among Medicare beneficiaries, 68% have ⩾2 and 14% have ⩾6 chronic conditions,20,103–105 among beneficiaries with CHF specifically >40% to 55% have ⩾5 noncardiac comorbidities, which increase preventable and all hospitalizations proportionally.29,105–108 These points have consequences for management and guidelines. We focus on diabetes mellitus (DM), CRI, chronic obstructive pulmonary disease (COPD), sleep-disordered breathing (SDB), and obesity, where guidelines are yet to recommend (advocate) a specific as opposed to generic advice.
Significance of comorbidities, trial evidence, and impacts on readmissions
First, the Global Burden of Disease Study listed 17 primary causes, with two-thirds secondary to ischemic, hypertensive, rheumatic heart diseases, and COPD. Other causes include valvular, primary genetic/hereditary and acquired, secondary (systemic diseases) cardiomyopathies, congenital heart disease, and pericardial diseases. Causes are variably associated with greater comorbidities and association with socioeconomic status and developed nation status.5,90
Second, among 4 366 489 Medicare beneficiaries with CHF, the top ranked comorbidities are hypertension (85.6%), ischemic heart disease (72.1%), hyperlipidemia (62.6%), anemia (51.2%), DM (47.1%), arthritis (45.6%), CRI (44.8%), chronic airways disease (30.9%), and AF (28.8%). Many noncardiac conditions are excluded during the run in period of randomized controlled trials (RCTs).5,21,25,26
Third, in the clinical domain, a wider racial demography, female sex, and older population are managed, whereas data remain limited or or in some cases unreliable due to variations in case definition, sampling strategies, and enrollment. Observational evidence does support greater incidence in developing nations with greater comorbidities and disease burden, and socioeconomic disadvantage in some racial groups, eg, African Americans or even variations in disease patterns e.g women and East Asians.56,109
It is thus no surprise that patient and homogenous system delivery factors are both equally risk factors for readmission. How resources are allocated within the 8 categories and 34 subdomains of a disease management taxonomy could influence readmissions.11,89 A summary of some of these factors include: firstly there are varying complexities in CHF cohort including hospitalization, complexities of comorbidities or risk factors including male sex, advanced age, or disease (eg, low systolic blood pressure), cardiac comorbidity (myocardial ischemia, AF), noncardiac comorbidity burden (CRI, DM, anemia, COPD, hyponatremia), psychosocial well-being (depression, social support, literacy), noncardiac illnesses (respiratory tract infection, falls, and fractures), history and frequency of prior hospitalization, prescription of prognostic medications, patient-related/compliance factors (nonadherence, dietary indiscretion with salt and water with weight gain, and drug and alcohol abuse), iatrogenic factors (eg, use of nonsteroidal anti-inflammatory drugs), and system-related factors (insufficient access to follow-up care and rehabilitation, poor transitions of care);27,28,30,41–46,91,110–123 secondly poor correlation between existing readmission risk scores and translation in the clinical domain;92–97 thirdly, the finding that phenotypic variables other than EF, such as comorbidity or female sex, can determine outcomes, raising questions to broaden HF classification beyond left ventricular EF (LVEF).17,31,124
Diabetes mellitus
CHF contributes to DM morbidity and mortality through arterial diseases (especially coronary) and independently, ie, “diabetic cardiomyopathies,” an evolving term.32–34 Several points are however indisputable: (1) Epidemiology observations—greater chronology and severity of DM with all stages from prediabetes, metabolic syndromes, and established diabetes, risk of CHF ([hazard ratio 1.2 to 1.7] and [12.4 vs 30.9 per 1000 person-years]). 1 in 3 admitted patients shows new-onset impaired glucose tolerance and prevalence in registries range from 25% to 40%; (2) Prognosis—higher rates of mortality and hospitalizations; (3) Pathophysiology—alterations occur with structural changes in myocardium and vasculature, unfavorable imbalance in myocardial energetics, CRI, and other organ damage.34,125
Treatment guidelines highlight the need for excellent DM control but having glaring deficiencies beyond that. Optimal treatment options and dosing are based on limited evidence, often without accounting for potential interactions contributing to suboptimal regimes. Novel pharmacotherapies are also wanting, however, SGLT2 (sodium-glucose cotransporter-2) inhibitors, is one novel agent with potential revolutionary impact on CHF prevention in DM Strong public policy for education of cardiologists and general practitioners is required to help clinical translation. With existing drugs, vasodilator β-blockers, with distinct benefits, are not presently factored.35,36 Greater evidence is needed for benefits with metformin and sulfonylureas, safe insulin dosing, safety of thiazolidinediones, translation of DPP-4 inhibitors, and GLP-1 receptor antagonist (RAs).34,37,38 Finally, lifestyle modifications are addressed through cardiac rehabilitation or diabetic education and maintained through self-care are underutilized from poor funding; for example the Australian public health fund (Medicare) rebate is for limited duration and restricted to hospital specialist, disadvantaging community specialists and ambulatory patients. This lack of resourcing has not translated as a priority health policy issue.35
Chronic renal impairment
The interaction between the failing heart and kidney is always bidirectional. This cardiorenal interaction now labeled “cardiorenal syndrome (CRS)” has been synthesized most comprehensively by Ronco et al. Short-term fluid and electrolyte imbalance, with long-term dysregulation of endocrine, sympathetic, immune (inflammatory) functions of the primary organ (heart or kidney), and secondarily every organ throughout the body contribute to a patient’s clinical presentation.39 The Acute Decompensated Heart Failure National Registry (ADHERE) database of 175 000 admissions across the Unites States consolidated on the depth of a problem already suspected highlighted that, all grades of CRS contribute to pathology in the other. The risk increasing with baseline severity, where CRI is the single strongest predictor of CHF outcome even beyond LVEF. CRS is also under-detected, and prognostic CHF treatment is often suboptimally prescribed.40
Guidelines have been cautious in taking strong positions on pharmacotherapies as advances in diagnostics have been slow. The estimated glomerular filtration rate (eGFR) is the most accurate estimate of CRI but lags by 2 to 7 days in predicting worsening CRI and cannot be used acutely to monitor renal function. Thus, using renin-aldosterone-angiotensin blockers when eGFR is between 20 and 45 mL/m becomes problematic. Novel renal function and injury biomarkers such as cystatin-C and neutrophil gelatinase–associated lipocalin have ongoing translational and cost-efficiency issues.126 As optimizing conventional CHF therapies at lower eGFRs is difficult, a novel approach to prescribing may be required including agents with extracardiac benefits and targeting systemic factors such as autonomous sympathetic overactivity, nitric oxide deficiency, and endothelial dysfunction.127 For acute heart failure, vasodilators (nesiritide; currently only selected patients), vasopressin antagonist (volume overload and resistant hyponatremia)102 and novel agents (serelaxin and ularitide) offer alternative permutations for combination therapies in nephroprotection with CHF.47,128 We anticipate stonger positions in newer guidelines.
Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease is present in a third of CHF cases, has an equal sex distribution despite a greater rate of smoking in men (suggesting greater susceptibility in women), and predicts mortality.32,48,49 Chronic obstructive pulmonary disease poses some unique challenges. First, it can be underdiagnosed due to underutilization of pulmonary function tests. Second, diagnostic challenges as the symptom of dyspnea is similar for both conditions. As pulmonary function tests are unreliable in acute settings, novel biomarkers such as B-type natriuretic peptide have been used, but more work is needed as up to 40% of respiratory distress are incorrectly admitted between cardiac and general medical units.50,51 Third, therapeutic issues such as underprescription of β-blockers in CHF while use of steroids and β-adrenergic agonist can enhance fluid retention and increase heart rates. The optimal cardioselective β-blockers is another area requiring attention. Finally, enrollment in cardiopulmonary, rehabilitation remains unclear.48,52–54
Obesity, Sleep-disordered breathing, and other comorbidities
Sleep-disordered breathing (SDB) which occurs in more than 1 in 3 HF patients, is part of a spectrum of metabolic-related conditions that are often overlooked. The common denominator is weight, where reduction targets are uniformly underachieved. Both forms of sleep apnoea are more prevalent in men and older age. Obstructive sleep apnea has greater correlations with weight and heart failure with preserved EF (HFpEF) and central sleep apnea with more severe CHF and AF. SDB is associated with greater readmissions and probably worse outcomes. The main treatment, continuous positive airway pressure effectively improves EF, physical function, and quality of life (QOL), but has issues with tolerability, and is yet to prove survival benefit.32,55,62–65,102
Obesity is a societal epidemic that is directly associated with CHF and indirectly through most CHF-associated comorbidity. It should be identified and treated with the same vigor as CHF and other comorbidities. However the resourcing involved prevents this, and so patients are at risk of accumulating additional comorbidities. Ideal body mass indices (BMIs) indexed for race are being defined. In one study, reference indices 26.5 to 30.9 kg/m2 had 27% improvement in mortality or hospitalization than those between <23.5 and >35 kg/m2. Cardiac cachexia, a contributor to mortality in lower weights, highlights the need for professional support in achieving weight reduction targets. With moderate degree of obesity (BMI <35 kg/m2), weight loss as a CHF treatment goal is not recommended. With greater obesity (BMI 35-45 kg/m2), tackling this issue with more advanced options such as bariatric surgery is showing benefits.61,66,67 Atrial, fibrillation, anemia, myopathy and deconditioning, depression, liver disease, frailty, and arthritis are other noncardiac comorbidities requiring considerations.32,48,102
Heart Failure in the Elderly
Defining elderly with a cutoff age of 70 to 80 years, registries have shown that >50% of acute HF admission (elderly - mean age 75 years or octogenarian ⩾80 years) range between 21% and 38%. Studies also demonstrate age differences in demography, clinical profiles and outcomes, comorbidities, and prognostic factors. Elderly presentations are >60% women, where 45% are new-acute HF, more likely associated with hypertension and AF, and less likely obese and diabetic. Respiratory distress is more common than peripheral edema, and atypical symptoms of sepsis, fever, confusion, fatigue, and loss of appetite are associated and added to the diagnostic difficulties (Table 2).13,22,23,56-60,68–82
Table 2.
Clinical characteristics of young versus elderly with acute heart failure (AHF).
| Young | Elderly | |
|---|---|---|
| Clinical profile | Men, obese, diabetic, coronary artery disease, less non-CV comorbidities | Women, hypertensive, nonobese, nondiabetic, atrial fibrillation, non-CV comorbidities (stroke, peripheral vascular disease, anemia, frailty) |
| Clinical presentation | Cardiac-type HF Lower SBP, higher peripheral edema |
Vascular-type HF Rales, high SBP, increased JVP, low |
| HF history | Less rales | Arterial oxygen saturation, infection |
| Laboratory findings | Acutely decompensated chronic HF Prior HF hospitalization |
New-onset HF No recent HF hospitalization |
| Echocardiography | Higher eGFR, lower levels of NPs | Lower eGFR, higher SUN, higher levels of NPs, lower Hb |
| Treatment | Reduced LV systolic function | Preserved LV systolic function, diastolic dysfunction, LA dilatation |
| Highest risk | Higher diuretic doses, more inotropes More BB, ACEi/ARBs/MRAs |
Lower diuretic doses, less inotropes Less BB, ACEi/ARBs/MRAs |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers; BB, β-blocker; CS, cardiogenic shock; CV, cardiovascular; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; JVP, jugular venous pressure; LA, left atrium; LV, left ventricle; MRA, mineralocorticoid receptor antagonists; NP, natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SUN, serum urea nitrogen.
Table data from Brouwers et al.22
Registries comparing prognostic medications with age such as IN-CHF (>70 years), OPTIMIZE-HF (>75 years), and EuroHeart Failure Survey II (EHFS II; >80 years) support significantly lower prescribing rates.13,23,68,73,74 Physiological differences with HFpEF, signs of organ impairment (lower eGFR), and perhaps uncertainty as to diagnosis and functional status influence practices. In EHFS II, and confirmed in other registries, various grades of frailty among the elderly were demonstrated by decline of independent function, self-care, and QOL with increased dependency for supported care. Data show >70% HF patients >80 years of age may be vulnerable, and this is assessable by “frailty scores.” As relatives and support services assume more care, communication pathways can become more complex even resulting in prescribing that is suboptimal or too complex.13,22,102 Gaps in organized clinical pathways undoubtedly contribute to high readmissions.
Cognitive decline could point to a range of pathologies. There is, however, no direct evidence that HF medication contributes to dementia. Mood disorder or depression is an important consideration for pseudodementia. Decompensated HF could manifest as acute delirium with prolonged hospital stay and higher mortality. Palliative and end-of-life care are usually provided after consultation with families, specialist, general practitioners, and allied health teams. In the Australian health system, providing this service with predictable intensity and duration in hospices are not a huge contributor to readmissions. In the absence of structured care, the need for regular communication and monitoring from patients or their supports, significantly contributes to high readmission rates within 3 to 6 months from discharge between 27% and 47%, where 50% relates to medication, disability, or an associated comorbid condition.22,70,71
Specific therapeutic considerations
Three major uncertainties are noted. First, the altered physiology with aging; altered pharmacodynamics, with increasing hepatic and renal impairment in the main excretory organs for drugs; and pharmacokinetics with reduced total body water content, body mass, and fat tissue. The resulting lower volume of distribution with either low or high plasma concentrations of lipophilic or hydrophilic drugs alters drug effects. Second, two thirds of elderly have >2 noncardiac comobidities and 25% have 6 or more noncardiac comorbidities. When associated with cognitive decline and polypharmacy (average of 10 medications) influences compliance and lowers safety for adverse events. Third, available social supports and ability to achieve high levels of self-efficacy can influence optimal prescribing.70
Proven prognostic therapies include RAAS inhibitors and β-blockers. Digoxin and diuretics are mainstay for symptoms. All drug classes require close monitoring of weight, electrolytes, renal functionand cardiac haemodynamics. Serum digoxin levels >0.9 nmol/L have cognitive and mortality consequences. Angiotensin-converting enzyme inhibitor long-term benefits above 75 years decline, and angiotensin receptor blockers could be more beneficial.72,73 Eplerenone could have greater tolerability as 10% of men have gynecomastia as testosterone declines with age. β-blocker data are limited; however, tolerability appears lower than younger HF patients (84% vs 76%), In an older HF population, nebivolol improved readmissions and mortality for both systolic and diastolic HF.22,70,74 Finally, with devices, morbidity and mortality are higher in patients >80 years without clear outcome benefits.68
Guideline gaps
The differences seen in the elderly excluded from RCT are co-morbidities and physiological changes of aging including an inherent increase by age alone. It thus remains unclear how much prognosis is genuinely extended or by a lead-time bias effect. Thus, factoring guidelines for octogenarians remains difficult. However, some principles must guide therapy:
Improved representation in trials, clinical trials, starting with post-marketing trials.
Delivering all proven therapies in conjunction with geriatric teams, achieving maximal tolerable or safe doses;
Regular monitoring of treatments with comparable use of diagnostics;
Minimizing polypharmacy in all other areas, by prioritizing prognostic and QOL improving agents;
Protocols when admitted to general medical units;
Protocols for early and appropriate palliative care referrals for severely deconditioned.
Closing the Gaps
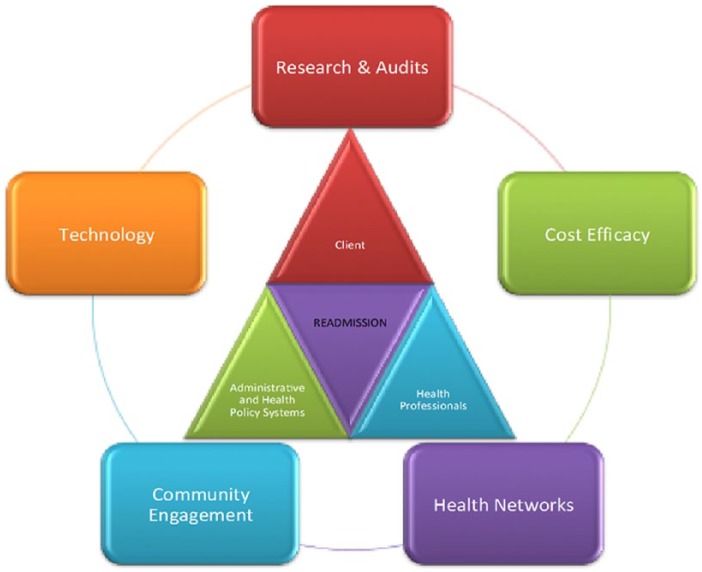
The past 2 millennia have seen the fastest advancements in public health and innovations from assessment to therapies. Universal life expectancy has increased globally when compared with any historical baseline. The evidence generating process and implementation strategies have been the biggest contributors. However, as populations develop diseases later in life or survive for longer, the variables for each patient gradually exceed the boundaries or predefined internal validity parameters of trials. We are thus starting to see plateaus in the health of subgroups, such that health budgets escalate, but cost-efficiency (or return of investment) is not readily noticeable. There is a new phase of medical practice on the horizon, one that will incorporate a greater flexibility in prescribing and management and draw in technological advances. The failure to reproduce trial-level evidence at the population level is the greatest proponent for this argument. The concept of phase 4 or postmarketing studies is well defined but not translated into clinical practice. Moving forward, several avenues to consider are discussed (Figure 2).
Figure 2.
Models for closing the gap. To address an outcome measure such as readmission requires arms of the health systems, which are often compartmentalized into silos, to overlap with common purpose. In answering, 5 key areas should be addressed: (1) defining the health jurisdiction from which most of the clients reside, (2) engagement of that community and its primary health infrastructure, (3) investing in technology to bring the gaps and address resource issues, (4) equipped for internal audits and aligning with partners to engage novel research, and (5) delivering these services at an acceptable cost.
Identification and classification of HF patients
As most of the proven evidence is derived from younger patients, health clusters must be able to develop systems that expands the efficacy of findings to all CHF patients. A surge of resources could be used at this point. While a similar prognostic target should not be denied, resources must be used to identify the support networks and readmission risks. Such scoring systems are not well developed (Table 1).13
Databases—standardizing and prioritizing implementable key performance indicators
The ability to assess real-time performance of CHF programs is vital. The AHA has published the important domains and dimensions of care, systematically going through each of this and more importantly finding a way to determine the key performance indicators, where intervention policy should be delivered will make a great impact on the measured outcome. Such information will inform risk scoring or act as a feeder for a phase 4 trials with different variables from the evidenced RCT.
Evidence generation and outcome measures—RCT or other
Defining roles
Health practitioners are defined and remunerated for clinical services, researchers for productivity in generating new knowledge, and a percentage of clinical researchers manage to function in both camps. However, it has never been entirely clear what roles health practitioners have in auditing their clinical work particularly in relation to cost-efficiency. Larger tertiary hospitals and entire systems like the British National Health System have this as a prerequisite. The responsibility to determine whether public-funded therapeutics is delivering benefits must be part of more than less systems, preferentially over-sighted by accrediting bodies. This will ensure when auditing systems are not in place, collaborations with institutes can be facilitated.
Gaps in guidelines
Gaps in guidelines can only be addressed by generating translatable evidence. This requires resourcing, infrastructure, personnel, and wide support:
Investigator-sponsored noninferiority studies: Posttranslational (phase 4) research is underutilized. Clinicians in practice will notice that with comorbidities and elderly, the external validity of RCT findings is not clearly demonstrated in real-world practice. While the scientific community does not argue against the accepted physiological basis for therapeutic benefits, the properties of one individual drug may come into question when a variant clinical scenario presents itself. Some examples here are diuretics and some renin-angiotensin blockers (e.g lisinopril, losartan) and black race and HF-class and vasodilatory β-blockers. Health systems must also achieve consensus on what constitutes translatable evidence (i.e. allows local clinicians to vary practice) and what methods to use to allow for broader question and experiments (e.g prospective audits with nested interventions or quasi-experimental studies). A noninferiority outcome for the primary and comorbid condition could be acceptable.
Novel research and institutional partnership: Strength-ening existing institutes by partnering could allow for bidirectional benefits. Most research institutes will benefit from having access to patients, and most clinical centers will benefit from the research or trial effect. Research governance requires robust support and secure infrastructure. The added personnel also imparts a culture of learning and accountability.
Improving evidence translation and cost-efficiency
Rapid translation of novel therapies: Complex patients could benefit from regular structured complex case management meetings. Incorporating relevant primary care providers is crucial; remuneration is not presently factored. At these sessions, all available proven therapies relevant to all comorbidities are discussed. Several examples are SGLT-1 inhibitors, bariatric surgery, and atrial fibrillation ablations, all conditions that persist for the patient’s life. The ability to significantly change the trajectory of a comorbid condition that alters CHF prognosis and influences prognosis must always be discussed robustly.
Engaging community and policymakers: It has never been clear what role tertiary institutions plays in prevention, yet this is often the first front to be looked at when outcomes such as readmissions are questioned. Part of this process involves delineating the community and primary care services within its primary catchment and offers regular health updates and promotes regional evidence development. Patient days where specialist units are open to the public could be factored.Strengthening understanding with governmental bodies and research governance bodies that increase the weighting of locally generated evidence and prerequisites for auditing of costly new treatments into health clusters are also considerations.
Conclusions
Readmissions in CHF are a surrogate outcome for delivery of optimal care. Although inroads have been made in improving these statistics, the growing and aging population predicts an increase in resource utilization, economic costs, and wider discrepancies in outcomes with multiple comorbidities and in the elderly. Registries have identified markers of readmission and prognostic risk; however, such information is unlikely to translate to any meaningful bedside use in the foreseeable future. Comorbidities and the elderly often have less robust evidence, as they are excluded from studies in run-in-period or outright. They may require special considerations in diagnostics, choices of therapies including dosing, and other pharmacologic considerations. As the number of questions to be asked is vast, regular quality assurance audits with prospective databases are essential. In the real world, heterogeneity in demographics and lack of relevant models of services offer few choices for a diverse problem. Several process-of-care publications on chronic disease management programs and performance markers have provided a unique opportunity to standardize CHF programs. Although the randomized clinical trial would create an artificial lens to view outcomes for CHF in the real world, it provides a solid template to conduct posttranslational or phase 4 research. The completion of cost-efficiency exercise requires a further bridge for a gap in what is considered acceptable translation research that should be funded by local authorities. Service-based research to reduce readmissions should thus primarily be focused on cost-efficiency and generating translatable research within an agreed standardized framework, for more universal acceptance. Treatments that directly improve the disease trajectory should also be explored with broad stakeholders, where again translation is the priority. Clinical outcomes including improved readmissions are the likely beneficiaries.
Appendix 1
Additional references for Table 1
- 1. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. [DOI] [PubMed] [Google Scholar]
- 2. Ho K, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. [DOI] [PubMed] [Google Scholar]
- 3. Ho KKL, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22:A6–A13. [DOI] [PubMed] [Google Scholar]
- 4. Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. [DOI] [PubMed] [Google Scholar]
- 5. Mahmood SS, Wang TJ. The epidemiology of congestive heart failure: the Framingham Heart Study perspective. Glob Heart. 2013;8:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kubanek M, Sramko M, Maluskova J, et al. Novel predictors of left ventricular reverse remodeling in individuals with recent-onset dilated cardiomyopathy. J Am Coll Cardiol. 2013;61:54–63. [DOI] [PubMed] [Google Scholar]
- 7. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maggioni AP. Epidemiology of heart failure in Europe. Heart Fail Clin. 2015;11:625–635. [DOI] [PubMed] [Google Scholar]
- 9. Ciapponi A, Alcaraz A, Calderón M, et al. Burden of heart failure in Latin America: a systematic review and meta-analysis. Rev Esp Cardiol. 2016;69:1051–1060. [DOI] [PubMed] [Google Scholar]
- 10. Sato N. Epidemiology of heart failure in Asia. Heart Fail Clin. 2015;11:573–579. [DOI] [PubMed] [Google Scholar]
- 11. Verdejo HE, Ferreccio C, Castro PF. Heart failure in rural communities. Heart Fail Clin. 2015;11:515–522. [DOI] [PubMed] [Google Scholar]
- 12. Sahle BW, Owen AJ, Mutowo MP, Krum H, Reid CM. Prevalence of heart failure in Australia: a systematic review. BMC Cardiovasc Disord. 2016;16:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garcia M, Mulvagh SL, Merz CN, Buring JE, Manson JE. Cardiovascular disease in women: clinical perspectives. Circ Res. 2016;118:1273–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Díaz-Toro F, Verdejo HE, Castro PF. Socioeconomic inequalities in heart failure. Heart Fail Clin. 2015;11:507–513. [DOI] [PubMed] [Google Scholar]
- 15. Cahill TJ, Kharbanda RK. Heart failure after myocardial infarction in the era of primary percutaneous coronary intervention: mechanisms, incidence and identification of patients at risk. World J Cardiol. 2017;9:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kenchaiah S, Vasan RS. Heart failure in women—insights from the Framingham Heart Study. Cardiovasc Drugs Ther. 2015;29:377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moe G. Heart failure with multiple comorbidities. Current Opin Cardiol. 2016;31:209–216. [DOI] [PubMed] [Google Scholar]
- 18. Ling LH, Kistler PM, Kalman JM, Schilling RJ, Hunter RJ. Comorbidity of atrial fibrillation and heart failure. Nat Rev Cardiol. 2016;13:131–147. [DOI] [PubMed] [Google Scholar]
- 19. Lavie CJ, Sharma A, Alpert MA, et al. Update on obesity and obesity paradox in heart failure. Prog Cardiovasc Dis. 2016;58:393–400. [DOI] [PubMed] [Google Scholar]
- 20. Gerber Y, Weston SA, Redfield MM, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meyer S, Brouwers FP, Voors AA, et al. Sex differences in new-onset heart failure. Clin Res Cardiol. 2015;104:342–350. [DOI] [PubMed] [Google Scholar]
- 22. Brouwers FP, de Boer RA, van der Harst P, et al. Incidence and epidemiology of new onset heart failure with preserved versus reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34:1424–1431. [DOI] [PubMed] [Google Scholar]
- 23. Zarrinkoub R, Wettermark B, Wändell P, et al. The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail. 2013;15:995–1002. [DOI] [PubMed] [Google Scholar]
- 24. Djoussé L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA 2009;302:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 2002;106:3068–3072. [DOI] [PubMed] [Google Scholar]
- 26. Cotter G, Cotter-Davison B, Ogah OS. The burden of heart failure in Africa. Eur J Heart Fail. 2013;15:829–831. [DOI] [PubMed] [Google Scholar]
- 27. Bocchi EA. Heart failure in South America. Curr Cardiol Rev. 2013;9:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pillai HS, Ganapathi S. Heart failure in South Asia. Curr Cardiol Rev. 2013;9:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Al-Shamiri MQ. Heart failure in the Middle East. Curr Cardiol Rev. 2013;9:174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guo Y, Lip GYH, Banerjee A. Heart failure in East Asia. Curr Cardiol Rev. 2013;9:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bloomfield GS, Barasa FA, Doll JA, Velazquez EJ. Heart failure in sub-Saharan Africa. Curr Cardiol Rev. 2013;9:157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bennett DA, Eliasz TK, Forbes A, et al. Study protocol: systematic review of the burden of heart failure in low- and middle-income countries. Syst Rev. 2012;1:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail. 2015;17:884–892. [DOI] [PubMed] [Google Scholar]
- 34. Banerjee A, Mendis S. Heart failure: the need for global health perspective. Curr Cardiol Rev. 2013;9:97–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. MacIntyre K, Capewell S, Stewart S, et al. Evidence of improving prognosis in heart failure: trends in case fatality in 66 547 patients hospitalized between 1986 and 1995. Circulation. 2000;102:1126–1131. [DOI] [PubMed] [Google Scholar]
- 36. Mosterd A, Cost B, Hoes AW, et al. The prognosis of heart failure in the general population: the Rotterdam study. Eur Heart J. 2001;22:1318–1327. [DOI] [PubMed] [Google Scholar]
- 37. Cowie MR, Wood DA, Coats AJ, et al. Survival of patients with a new diagnosis of heart failure: a population based study. Heart. 2000;83:505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McCullough PA, Philbin EF, Spertus JA, Kaatz S, Sandberg KR, Weaver WD. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol. 2002;39:60–69. [DOI] [PubMed] [Google Scholar]
- 39. Curtis LH, Whellan DJ, Hammill BG, et al. Incidence and prevalence of heart failure in elderly persons, 1994-2003. Arch Intern Med. 2008;168:418–424. [DOI] [PubMed] [Google Scholar]
- 40. Yusuf S, Rangarajan S, Teo K, et al. Cardiovascular risk and events in 17 low, middle, and high-income countries. N Engl J Med. 2014;371:818–827. [DOI] [PubMed] [Google Scholar]
- 41. Thomas KL, Hernandez AF, Dai D, et al. Association of race/ethnicity with clinical risk factors, quality of care, and acute outcomes in patients hospitalized with heart failure. Am Heart J. 2011;161:746–754. [DOI] [PubMed] [Google Scholar]
- 42. Krumholz HM, Normand ST, Wang Y. Trends in hospitalizations and outcomes for acute cardiovascular disease and stroke, 1999-2011. Circulation. 2014;130:966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yeung DF, Boom NK, Guo H, Lee DS, Schultz SE, Tu JV. Trends in the incidence and outcomes of heart failure in Ontario, Canada: 1997 to 2007. CMAJ. 2012;184:E765–E773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fonarow GC, Albert NM, Curtis AB, et al. Associations between outpatient heart failure process-of-care measures and mortality. Circulation. 2011;123:1601–1610. [DOI] [PubMed] [Google Scholar]
- 45. Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008;52:428–434. [DOI] [PubMed] [Google Scholar]
- 46. Brown DW, Haldeman GA, Croft JB, Giles WH, Mensah GA. Racial or ethnic differences in hospitalization for heart failure among elderly adults: Medicare, 1990 to 2000. Am Heart J. 2005;150:448–454. [DOI] [PubMed] [Google Scholar]
- 47. Schwartz J, Strait KM, Keshawarz A, et al. Medicare hospital quality chartbook performance report on outcome measures. Centers for Medicare & Medicaid Services, 2014. https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/hospitalqualityinits/downloads/medicare-hospital-quality-chartbook-2014.pdf.
- 48. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309: 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Krumholz HM, Hsieh A, Dreyer RP, Welsh J, Desai NR, Dharmarajan K. Trajectories of risk for specific readmission diagnoses after hospitalization for heart failure, acute myocardial infarction, or pneumonia. PLoS ONE. 2016;11:e0160492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011;306:1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schaufelberger M, Swedberg K, Koster M, Rosen M, Rosengren A. Decreasing one-year mortality and hospitalization rates for heart failure in Sweden; data from the Swedish Hospital Discharge Registry 1988 to 2000. Eur Heart J. 2004;25:300–307. [DOI] [PubMed] [Google Scholar]
- 52. Stewart S, MacIntyre K, MacLeod MM, Bailey AE, Capewell S, McMurray JJ. Trends in hospitalization for heart failure in Scotland, 1990-1996. An epidemic that has reached its peak? Eur Heart J. 2001; 22:209–217. [DOI] [PubMed] [Google Scholar]
- 53. Mosterd A, Reitsma JB, Grobbee DE. Angiotensin converting enzyme inhibition and hospitalisation rates for heart failure in the Netherlands, 1980 to 1999: the end of an epidemic? Heart. 2002;87:75–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ferreira JP, Girerd N, Rossignol P, Zannad F. Geographic differences in heart failure trials. Eur J Heart Fail. 2015;17:893–905. [DOI] [PubMed] [Google Scholar]
- 55. Egwim C, Dixon B, Ambrosy AP, Mentz RJ. Global variations in patient populations and outcomes in heart failure clinical trials. Curr Heart Fail Rep. 2017;14:30–39. [DOI] [PubMed] [Google Scholar]
- 56. Frigerio M, Mazzali C, Paganoni AM, et al. Trends in heart failure hospitalizations, patient characteristics, in-hospital and 1-year mortality: a population study, from 2000 to 2012 in Lombardy. Int J Cardiol. 2017;236:310–314. [DOI] [PubMed] [Google Scholar]
- 57. Heywood JT, Fonarow GC, Costanzo MR, et al. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–430. [DOI] [PubMed] [Google Scholar]
- 58. Fonarow GC, Heywood JT, Heidenreich PA, Lopatin M, Yancy CW. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2007;153:1021–1028. [DOI] [PubMed] [Google Scholar]
- 59. Heywood JT. The cardiorenal syndrome: lessons from the ADHERE database and treatment options. Heart Fail Rev. 2004;9:195–201. [DOI] [PubMed] [Google Scholar]
- 60. Adams KF Fonarrow GC Emerman CL et al.;. ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–216. [DOI] [PubMed] [Google Scholar]
- 61. Fonarow GC, Yancy CW, Heywood JT, et al. Adherence to heart failure quality-of-care indicators in US hospitals: analysis of the ADHERE Registry. Arch Intern Med. 2005;165:1469–1477. [DOI] [PubMed] [Google Scholar]
- 62. Adams KF, Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–216. [DOI] [PubMed] [Google Scholar]
- 63. Abraham WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005;46:57–64. [DOI] [PubMed] [Google Scholar]
- 64. Fonarow GC; ADHERE Scientific Advisory Committee. The Acute Decompensated Heart Failure National Registry (ADHERE): opportunities to improve care of patients hospitalized with acute decompensated heart failure. Rev Cardiovasc Med. 2003;4:S21–S30. [PubMed] [Google Scholar]
- 65. Atherton JJ, Hayward CS, Wan Ahmad WA, et al. Patient characteristics from a regional multicenter database of acute decompensated heart failure in Asia Pacific (ADHERE International-Asia Pacific). J Card Fail. 2012;18:82–88. [DOI] [PubMed] [Google Scholar]
- 66. Nieminen MS, Brutsaert D, Dickstein K, et al. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–2736. [DOI] [PubMed] [Google Scholar]
- 67. Cleland JG, Swedberg K, Follath F, et al. ; Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology: the EuroHeart Failure Survey Programme—a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24:442–463. [DOI] [PubMed] [Google Scholar]
- 68. Maggioni AP, Dahlström U, Filippatos G, et al. ; Heart Failure Association of the European Society of Cardiology (HFA). EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2013;15:808–817. [DOI] [PubMed] [Google Scholar]
- 69. Maggioni AP, Dahlstrom U, Filippatos G, et al. EURObservational Research Programme: the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2010;12:1076–1084. [DOI] [PubMed] [Google Scholar]
- 70. Oliva F, Mortara A, Cacciatore G, et al. Acute heart failure patient profiles, management and in-hospital outcome: results of the Italian Registry on Heart Failure Outcome. Eur J Heart Fail. 2012;14:1208–1217. [DOI] [PubMed] [Google Scholar]
- 71. Zannad F, Mebazaa A, Juilliere Y, et al. Clinical profile, contemporary management and one-year mortality in patients with severe acute heart failure syndromes: the EFICA study. Eur J Heart Fail. 2006;8:697–705. [DOI] [PubMed] [Google Scholar]
- 72. Chioncel O, Vinereanu D, Datcu M, et al. The Romanian Acute Heart Failure Syndromes (RO-AHFS) registry. Am Heart J. 2011;162: 142–153.e1. [DOI] [PubMed] [Google Scholar]
- 73. Spinar J, Parenica J, Vitovec J, et al. Baseline characteristics and hospital mortality in the Acute Heart Failure Database (AHEAD) Main registry. Crit Care. 2011;15:R291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sato N, Kajimoto K, Keida T, et al. Clinical features and outcome in hospitalized heart failure in Japan (from the ATTEND Registry). Circ J. 2013;77:944–951. [DOI] [PubMed] [Google Scholar]
- 75. Sato N, Kajimoto K, Asai K, et al. Acute decompensated heart failure syndromes (ATTEND) registry. A prospective observational multicenter cohort study: rationale, design, and preliminary data. Am Heart J. 2010;159:949–955.e1. [DOI] [PubMed] [Google Scholar]
- 76. Follath F, Yilmaz MB, Delgado JF, et al. Clinical presentation, management and outcomes in the Acute Heart Failure Global Survey of Standard Treatment (ALARM-HF). Intensive Care Med. 2011;37:619–626. [DOI] [PubMed] [Google Scholar]
- 77. Foraker RE, Rose KM, Suchindran CM, Chang PP, McNeill AM, Rosamond WD. Socioeconomic status, Medicaid coverage, clinical comorbidity, and rehospitalization or death after an incident heart failure hospitalization: atherosclerosis risk in communities cohort (1987 to 2004). Circ Heart Fail. 2011;4:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kociol RD, Horton JR, Fonarow GC, et al. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circulation. 2011;4:628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fonarow GC, Abraham WT, Albert NM, et al. Day of admission and clinical outcomes for patients hospitalized for heart failure: findings from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Circ Heart Fail. 2008;1:50–57. [DOI] [PubMed] [Google Scholar]
- 80. Fonarow GC, Abraham WT, Albert NM, et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med. 2008;168:847–854. [DOI] [PubMed] [Google Scholar]
- 81. Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll of Cardiol. 2007;50:768–777. [DOI] [PubMed] [Google Scholar]
- 82. O’Connor CM, Abraham WT, Albert NM, et al. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J. 2008;156:662–673. [DOI] [PubMed] [Google Scholar]
- 83. Fonarow GC, Abraham WT, Albert NM, et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med. 2008;168:847–854. [DOI] [PubMed] [Google Scholar]
- 84. Gheorghiade M, Abraham WT, Albert NM, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217–2226. [DOI] [PubMed] [Google Scholar]
- 85. Fonarow GC, Albert NM, Curtis AB, et al. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation. 2010;122:585–596. [DOI] [PubMed] [Google Scholar]
- 86. Heidenreich PA, Hernandez AF, Yancy CW, Liang L, Peterson ED, Fonarow GC. Get with the guidelines program participation, process of care, and outcome for Medicare patients hospitalized with heart failure. Circ Cardiovasc Qual Outcomes. 2012;5:37–43. [DOI] [PubMed] [Google Scholar]
- 87. Kociol RD, Peterson ED, Hammill BG, et al. National survey of hospital strategies to reduce heart failure readmissions: findings from the Get With the Guidelines-Heart Failure registry. Circ Heart Fail. 2012;5:680–687. [DOI] [PubMed] [Google Scholar]
- 88. Eapen ZJ, Fonarow GC, Dai D, et al. Comparison of composite measure methodologies for rewarding quality of care: an analysis from the American Heart Association’s Get With The Guidelines program. Circ Cardiovasc Qual Outcomes. 2011;4:610–618. [DOI] [PubMed] [Google Scholar]
- 89. Hernandez AF, Fonarow GC, Liang L, Heidenreich PA, Yancy C, Peterson ED. The need for multiple measures of hospital quality: results from the Get With the Guidelines-Heart Failure Registry of the American Heart Association. Circulation. 2011;124:712–719. [DOI] [PubMed] [Google Scholar]
- 90. Dunlay SM, Gheorghiade M, Reid KJ, et al. Critical elements of clinical follow-up after hospital discharge for heart failure: insights from the EVEREST trial. Eur J Heart Fail. 2010;12:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. O’Connor CM, Miller AB, Blair JE, et al. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction: results from Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) program. Am Heart J. 2010;159:841–849. [DOI] [PubMed] [Google Scholar]
- 92. Gheorghiade M, Pang PS, Ambrosy AP, et al. A comprehensive, longitudinal description of the in-hospital and post-discharge clinical, laboratory, and neurohormonal course of patients with heart failure who die or are re-hospitalized within 90 days: analysis from the EVEREST trial. Heart Fail Rev. 2012;17:485–509. [DOI] [PubMed] [Google Scholar]
- 93. Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. [DOI] [PubMed] [Google Scholar]
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/ or publication of this article: A.D. is supported by a Heart Foundation Future Leader fellowship 100472 from the National Heart Foundation of Australia. All co-authors have won independent and governmental research funding.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: PI conceived and designed the experiments, analyzed the data and wrote the first draft of the manuscript. PI, DL, CN, AD, THM, DLH contributed to the writing of the manuscript. PI, DL, CN, AD, THM, DLH agree with manuscript results and conclusions. PI, DL, CN, AD, THM, DLH jointly developed the structure and arguments for the paper. PI, DL, CN, AD, THM, DLH made critical revisions and approved final version. All authors reviewed and approved of the final manuscript.
References
- 1. Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126:501–506. [DOI] [PubMed] [Google Scholar]
- 2. Cintron G, Johnson G, Francis G, Cobb F, Cohn JN. Prognostic significance of serial changes in left ventricular ejection fraction in patients with congestive heart failure. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87:VI17–VI23. [PubMed] [Google Scholar]
- 3. Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation. 2008;117:e25–e146. [DOI] [PubMed] [Google Scholar]
- 4. Chaudhry SP, Stewart GC. Advanced heart failure: prevalence, natural history, and prognosis. Heart Fail Clin. 2016;12:323–333. [DOI] [PubMed] [Google Scholar]
- 5. Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13:368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure associated hospitalizations in the United States. J Am Coll Cardiol. 2013;61:1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chun S, Tu JV, Wijeysundera HC, et al. Lifetime analysis of hospitalizations and survival of patients newly admitted with heart failure. Circ Heart Fail. 2012;5:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cubbon RM, Gale CP, Kearney LC, et al. Changing characteristics and mode of death associated with chronic heart failure caused by left ventricular systolic dysfunction: a study across therapeutic eras. Circulation. 2011;4:396–403. [DOI] [PubMed] [Google Scholar]
- 9. Joynt KE, Jha AK. Who has higher readmission rates for heart failure, and why? implications for efforts to improve care using financial incentives. Circ Cardiovasc Qual Outcomes. 2011;4:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ross JS, Chen J, Lin Z, et al. Recent national trends in readmission rates after heart failure hospitalization. Circulation. 2010;3:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407–413. [DOI] [PubMed] [Google Scholar]
- 12. Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med. 1994;331:1564–1575. [DOI] [PubMed] [Google Scholar]
- 13. Soundarraj D, Singh V, Satija V, Thakur RK. Containing the cost of heart failure management: a focus on reducing readmissions. Heart Fail Clin. 2017;13:21–28. [DOI] [PubMed] [Google Scholar]
- 14. Iyngkaran P. Editorial: optimizing chronic heart failure care beyond randomised controlled trials - what are the problem areas and potential solutions? Curr Cardiol Rev. 2016;12(3):164-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zannad F, Agrinier N and, Alla F. Heart failure burden and therapy. Europace. 2009;11:v1–v9. [DOI] [PubMed] [Google Scholar]
- 16. Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385:812–824. [DOI] [PubMed] [Google Scholar]
- 17. Konstam MA. Seeking therapeutic precision in heart failure: is ejection fraction really the way? deconstructing the CHARM of heart failure with mid-range ejection fraction. Eur J Heart Fail. 2018; 20:1240–1242. doi: 10.1002/ejhf.1205. [DOI] [PubMed] [Google Scholar]
- 18. Fonarow GC, Abraham WT, Albert NM, et al. Influence of a performance-improvement initiative on quality of care for patients hospitalized with heart failure: results of the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF). Arch Intern Med. 2007; 167:1493–1502. [DOI] [PubMed] [Google Scholar]
- 19. Iyngkaran P, Liew D, McDonald P, et al. Phase 4 studies in heart failure—what is done and what is needed? Curr Cardiol Rev. 2016;12:216–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferreira JP, Girerd N, Rossignol P, Zannad F. Geographic differences in heart failure trials. Eur J Heart Fail. 2015;17:893–905. [DOI] [PubMed] [Google Scholar]
- 21. Iyngkaran P, Majoni W, Cass A, et al. Northern territory perspectives on heart failure with comorbidities—understanding trial validity and exploring collaborative opportunities to broaden the evidence base. Heart Lung Circ. 2015;24:536–543. [DOI] [PubMed] [Google Scholar]
- 22. Katsanos S, Bistola V, Parissis JT. Acute heart failure syndromes in the elderly: the European perspective. Heart Fail Clin. 2015;11:637–645. [DOI] [PubMed] [Google Scholar]
- 23. Dharmarajan K, Rich MW. Epidemiology, pathophysiology, and prognosis of heart failure in older adults. Heart Fail Clin. 2017;13:417–426. [DOI] [PubMed] [Google Scholar]
- 24. Gheorghiade M, Vaduganathan M, Fonarow G, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol. 2013;61:391–403. [DOI] [PubMed] [Google Scholar]
- 25. Roger VL. Epidemiology of heart failure. Circ Res. 2013;113:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154:260–266. [DOI] [PubMed] [Google Scholar]
- 28. Gwadry-Sridhar FH, et al. A systematic review and meta-analysis of studies comparing readmission rates and mortality rates in patients with heart failure. Arch Intern Med. 2004;164:2315–2320. [DOI] [PubMed] [Google Scholar]
- 29. Centers for Medicare and Medicaid Services. Chronic Conditions Among Medicare Beneficiaries (Chartbook). Baltimore, MD: Centers for Medicare and Medicaid Services; 2012. [Google Scholar]
- 30. Hummel SL, Pauli NP, Krumholz HM, et al. Thirty-day outcomes in Medicare patients with heart failure at heart transplant centers. Circ Heart Fail. 2011;3:244–252. [DOI] [PubMed] [Google Scholar]
- 31. Ahmad T, Pencina MJ, Schulte PJ, et al. Clinical implications of chronic heart failure phenotypes defined by cluster analysis. J Am Coll Cardiol. 2014;64:1765–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ernande L, Derumeaux G. Diabetic cardiomyopathy: myth or reality? Arch Cardiovasc Dis. 2012;105:218–225. [DOI] [PubMed] [Google Scholar]
- 34. Lehrke M, Marx N. Diabetes mellitus and heart failure. Am J Cardiol. 2017;120:S37–S47. [DOI] [PubMed] [Google Scholar]
- 35. Iyngkaran P, Harris M, Ilton M, et al. Implementing guideline based heart failure care in the northern territory: challenges and solutions. Heart Lung Circ. 2014;23:391–406. [DOI] [PubMed] [Google Scholar]
- 36. Iyngkaran P, Toukhsati SR, Thomas MC, Jelinek MV, Hare DL, Horowitz JD. A review of the external validity of clinical trials with beta-blockers in heart failure. Clin Med Insights Cardiol. 2016;10:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paneni F, Luscher TF. Cardiovascular protection in the treatment of type 2 diabetes: a review of clinical trial results across drug classes. Am J Cardiol. 2017;120:S17–S27. [DOI] [PubMed] [Google Scholar]
- 38. Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME trial. Eur Heart J. 2016;37:1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardio-renal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. [DOI] [PubMed] [Google Scholar]
- 40. Iyngkaran P, Thomas M, Majoni W, Anavekar NS, Ronco C. Comorbid heart failure and renal impairment: epidemiology and management. Cardiorenal Med. 2012;2:281–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aizawa H, Imai S, Fushimi K. Factors associated with 30-day readmission of patients with heart failure from a Japanese administrative database. BMC Cardiovasc Disord. 2015;15:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kang SM, Cho MC. Prognostic factors in hospitalization for heart failure in Asia. Heart Fail Clin. 2015;11:543–550. [DOI] [PubMed] [Google Scholar]
- 43. Dunlay SM, Redfield MM, Weston SA, et al. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009;54:1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vivo RP, Krim SR, Liang L, et al. Short- and long-term rehospitalization and mortality for heart failure in 4 racial/ethnic populations. J Am Heart Assoc. 2014;3:e001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heidenreich PA, Sahay A, Kapoor JR, Pham MX, Massie B. Divergent trends in survival and readmission following a hospitalization for heart failure in the Veterans Affairs health care system 2002 to 2006. J Am Coll Cardiol. 2010;56:362–368. [DOI] [PubMed] [Google Scholar]
- 46. Aranda JM, Jr, Johnson JW, Conti JB. Current trends in heart failure readmission rates: analysis of Medicare data. Clin Cardiol. 2009;32:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anker SD, Ponikowski P, Mitrovic V, Peacock WF, Filippatos G. Ularitide for the treatment of acute decompensated heart failure: from preclinical to clinical studies. Eur Heart J. 2015;36:715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hopper I, Kotecha D, Chin KL, Mentz RJ, von Leuder TG. Comorbidities in heart failure: are there gender differences? Curr Heart Fail Rep. 2016;13:1–12. [DOI] [PubMed] [Google Scholar]
- 49. De Blois J, Simard S, Atar D, Agewall S. COPD predicts mortality in HF: the Norwegian Heart Failure Registry. J Card Fail. 2010;16:225–229. [DOI] [PubMed] [Google Scholar]
- 50. Dharmarajan K, Strait KM, Lagu T, et al. Acute decompensated heart failure is routinely treated as a cardiopulmonary syndrome. PLoS ONE. 2013;8:e78222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hawkins NM, Petrie MC, Jhund PS, Chalmers GW, Dunn FG, McMurray JJ. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail. 2009;11:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Smith BM, Prince MR, Hoffman EA, et al. Impaired left ventricular filling in COPD and emphysema: is it the heart or the lungs? the multi-ethnic study of atherosclerosis COPD study. Chest. 2013;144:1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jabbour A, Macdonald PS, Keogh AM, et al. Differences between beta-blockers in patients with chronic heart failure and chronic obstructive pulmonary disease: a randomized crossover trial. J Am Coll Cardiol. 2010;55:1780–1787. [DOI] [PubMed] [Google Scholar]
- 54. Mentz RJ, Fiuzat M, Kraft M, Lindenfeld J, O’Connor CM. Bronchodilators in heart failure patients with COPD: is it time for a clinical trial? J Card Fail. 2012;18:413–422. [DOI] [PubMed] [Google Scholar]
- 55. Sin DD, Fitzgerald F, Parker JD, et al. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–1106. [DOI] [PubMed] [Google Scholar]
- 56. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 57. Metra M, Mentz RJ, Chiswell K, et al. Acute heart failure in elderly patients: worse outcomes and differential utility of standard prognostic variables. Insights from the PROTECT trial. Eur J Heart Fail. 2015;17:109–118. [DOI] [PubMed] [Google Scholar]
- 58. Mogensen UM, Ersboll M, Andersen M, et al. Clinical characteristics and major comorbidities in heart failure patients more than 85 years of age compared with younger age groups. Eur J Heart Fail. 2011;13:1216–1223. [DOI] [PubMed] [Google Scholar]
- 59. Murad K, Goff D, Morgan T, et al. Burden of comorbidities and functional and cognitive impairments in elderly patients at the initial diagnosis of heart failure and their impact on total mortality: the cardiovascular health study. J Am Coll Cardiol. 2015;HF3:542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Metra M, Cotter G, El-Khorazaty J, et al. Acute heart failure in the elderly: differences in clinical characteristics, outcomes, and prognostic factors in the VERITAS study. J Card Fail. 2015;21:179–188. [DOI] [PubMed] [Google Scholar]
- 61. Ramani GV, McCloskey C, Ramanathan RC, et al. Safety and efficacy of bariatric surgery in morbidly obese patients with severe systolic heart failure. Clin Cardiol. 2008;31:516–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Paulino A, Damy T, Margarit L, et al. Prevalence of sleep-disordered breathing in a 316-patient French cohort of stable congestive heart failure. Arch Cardiovasc Dis. 2009;102:169–175. [DOI] [PubMed] [Google Scholar]
- 63. Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol. 2011;57:119–127. [DOI] [PubMed] [Google Scholar]
- 64. Bradley TD, Logan AG, Kimoff RJ, et al. CANPAP investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. [DOI] [PubMed] [Google Scholar]
- 65. Lavie CJ, McAuley PA, Church TS, et al. Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol. 2014;63:1345–1354. [DOI] [PubMed] [Google Scholar]
- 66. Poirier P, Cornier MA, Mazzone T, et al. Bariatric surgery and cardiovascular risk factors: a scientific statement from the American Heart Association. Circulation. 2011;123:1683–1701. [DOI] [PubMed] [Google Scholar]
- 67. Mandviwala T, Khalid U, Deswal A. Obesity and cardiovascular disease: a risk factor or a risk marker? Curr Atheroscler Rep. 2016;18:21. [DOI] [PubMed] [Google Scholar]
- 68. Iacoviello M, Antoncecchi V. Heart failure in elderly: progress in clinical evaluation and therapeutic approach. J Geriatr Cardiol. 2013;10:165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rich MW. Management of heart failure in the elderly. Heart Fail Rev. 2002;7:89–97. [DOI] [PubMed] [Google Scholar]
- 70. Lemay G, Azad N. Management of chronic heart failure in the older population. J Geriatr Cardiol. 2014;11:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wong CY, Chaudhry SI, Desai MM, et al. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med. 2011;124:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Flather MD, Yusuf S, Køber L, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. Lancet. 2000;355:1575–1581. [DOI] [PubMed] [Google Scholar]
- 73. Pfeffer MA, Swedberg K, Granger CB, et al. Effect of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-overall programme. Lancet. 2003;362:759–766. [DOI] [PubMed] [Google Scholar]
- 74. Rich MW. Pharmacotherapy of heart failure in the elderly: adverse events. Heart Fail Rev. 2012;17:589–595. [DOI] [PubMed] [Google Scholar]
- 75. Komajda M, Hanon O, Hochadel M, et al. Contemporary management of octogenarians hospitalized for heart failure in Europe: Euro Heart Failure Survey II. Eur Heart J. 2009;30:478–486. [DOI] [PubMed] [Google Scholar]
- 76. Stein GY, Kremer A, Shochat T, et al. The diversity of heart failure in a hospitalized population: the role of age. J Card Fail. 2012;18:645–653. [DOI] [PubMed] [Google Scholar]
- 77. Herrero-Puente P, Martin-Sanchez FJ, Fernandez-Fernandez M, et al. Differential clinical characteristics and outcome predictors of acute heart failure in elderly patients. Int J Cardiol. 2012;155:81–86. [DOI] [PubMed] [Google Scholar]
- 78. Tinetti ME, McAvay GJ, Chang SS, et al. Contribution of multiple chronic conditions to universal health outcomes. J Am Geriatr Soc. 2011;59:1686–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chaudhry SI, Wang Y, Gill TM, Krumholz HM. Geriatric conditions and subsequent mortality in older patients with heart failure. J Am Coll Cardiol. 2010;55:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pulignano G, Del Sindaco D, Tavazzi L, et al. Clinical features and outcomes of elderly outpatients with heart failure followed up in hospital cardiology units: data from a large nationwide cardiology database (IN-CHF registry). Am Heart J. 2002;143:45–55. [DOI] [PubMed] [Google Scholar]
- 81. Fonarow GC, Abraham WT, Albert NM, et al. Age and gender-related differences in quality of care and outcomes of patients hospitalized with heart failure (from OPTIMIZE-HF). Am J Cardiol. 2009;104:107–115. [DOI] [PubMed] [Google Scholar]
- 82. Mahjoub H, Rusinaru D, Souliere V, et al. Long-term survival in patients older than 80 years hospitalised for heart failure. A 5-year prospective study. Eur J Heart Fail. 2008;10:78–84. [DOI] [PubMed] [Google Scholar]
- 83. Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128:388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rush CJ, Campbell RT, Jhund PS, et al. Falling cardiovascular mortality in heart failure with reduced ejection fraction and implications for clinical trials. JACC Heart Fail. 2015;3:603–614. [DOI] [PubMed] [Google Scholar]
- 85. Givertz MM, Mann DL. Epidemiology and natural history of recovery of left ventricular function in recent onset dilated cardiomyopathies. Curr Heart Fail Rep. 2013;10:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Desai AS. The three-phase terrain of heart failure readmissions. Circ Heart Fail. 2012;5:397–399. [DOI] [PubMed] [Google Scholar]
- 87. Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol. 2014;171:368–376. [DOI] [PubMed] [Google Scholar]
- 88. Iyngkaran P, Tinsley J, Smith D, et al. Northern territory heart failure initiative—clinical audit (NTHFI-CA)—a prospective database on the quality of care and outcomes for acute decompensated heart failure admission in the northern territory: study design and rationale. BMJ Open. 2014;4:e004137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Krumholz HM, Currie PM, Riegel B, et al. A taxonomy for disease management: a scientific statement from the American Heart Association Disease Management Taxonomy Writing Group. Circulation. 2006;114:1432–1445. [DOI] [PubMed] [Google Scholar]
- 90. Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. [DOI] [PubMed] [Google Scholar]
- 91. Krumholz HM, Parent EM, Tu N, et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157:99–104. [PubMed] [Google Scholar]
- 92. Ferrero P, Iacovoni A, Di Elia E, Vaduganathan M, Gavazzi A, Senni M. Prognostic scores in heart failure—critical appraisal and practical use. Int J Cardiol. 2015;188:1–9. [DOI] [PubMed] [Google Scholar]
- 93. Echouffo-Tcheugui JB, Greene SJ, Papadimitriou L, et al. Population risk prediction models for incident heart failure: a systematic review. Circ Heart Fail. 2015;8:438–447. [DOI] [PubMed] [Google Scholar]
- 94. Yang H, Negishi K, Otahal P, et al. Clinical prediction of incident heart failure risk: a systematic review and meta-analysis. Open Heart. 2015;2:e000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rahimi K, Bennett D, Conrad N, et al. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail. 2014;2:440–446. [DOI] [PubMed] [Google Scholar]
- 96. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ross JS, Mulvey GK, Stauffer B, et al. Statistical models and patient predictors of readmission for heart failure: a systematic review. Arch Intern Med. 2008;168:1371–1386. [DOI] [PubMed] [Google Scholar]
- 98. Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351:2870–2874. [DOI] [PubMed] [Google Scholar]
- 99. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;136:1–75. [DOI] [PubMed] [Google Scholar]
- 100. Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2016;134:e282–e293. [DOI] [PubMed] [Google Scholar]
- 101. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 102. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 103. Arnett DK, Goodman RA, Halperin JL, et al. AHA/ACC/HHS strategies to enhance application of clinical practice guidelines in patients with cardiovascular disease and comorbid conditions: from the American Heart Association, American College of Cardiology, and US department of health and human services. Circulation. 2014;130:1662–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Iorio A, Pozzi A, Senni M. Addressing the heterogeneity of heart failure in future randomized trials. Curr Heart Fail Rep. 2017;14:197–202. [DOI] [PubMed] [Google Scholar]
- 105. Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–1233. [DOI] [PubMed] [Google Scholar]
- 106. Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Luscher TF. Heart failure and comorbidities: renal failure, diabetes, atrial fibrillation, and inflammation. Eur Heart J. 2015;36:1415–1417. [DOI] [PubMed] [Google Scholar]
- 108. Shaffer JA, Maurer MS. Multiple chronic conditions and heart failure. JACC Heart Fail. 2015;3:551–553. [DOI] [PubMed] [Google Scholar]
- 109. Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fonarow GC, Abraham WT, Albert NM, et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med. 2008;168:847–854. [DOI] [PubMed] [Google Scholar]
- 111. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366:1366–1369. [DOI] [PubMed] [Google Scholar]
- 112. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. [DOI] [PubMed] [Google Scholar]
- 113. Saito M, Negishi K, Marwick TH. Meta-analysis of risks for short-term readmission in patients with heart failure. Am J Cardiol. 2016;117:626–632. [DOI] [PubMed] [Google Scholar]
- 114. Zaya M, Phan A, Schwarz ER. Predictors of re-hospitalization in patients with chronic heart failure. World J Cardiol. 2012;4:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. McNaughton CD, Collins SP, Kripalani S, et al. Low numeracy is associated with increased odds of 30-day emergency department or hospital recidivism for patients with acute heart failure. Circ Heart Fail. 2013;6:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Collins SP, Lindsell CJ, Storrow AB, et al. Early changes in clinical characteristics after emergency department therapy for acute heart failure syndromes: identifying patients who do not respond to standard therapy. Heart Fail Rev. 2012;17:387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Au AG, McAlister FA, Bakal JA, Ezekowitz J, Kaul P, van Walraven C. Predicting the risk of unplanned readmission or death within 30 days of discharge after a heart failure hospitalization. Am Heart J. 2012;164:365–372. [DOI] [PubMed] [Google Scholar]
- 119. Metra M, Davison B, Bettari L, et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? the role of congestion and its interaction with renal function. Circulation. 2012;5:54–62. [DOI] [PubMed] [Google Scholar]
- 120. Allen LA, Gheorghiade M, Reid KJ, et al. Identifying patients hospitalized with heart failure at risk for unfavorable future quality of life. Circ Cardiovasc Qual Outcomes. 2011;4:389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Epstein AM, Jha AK, Orav EJ. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365:2287–2295. [DOI] [PubMed] [Google Scholar]
- 122. van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183:E391–E402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Setoguchi S, Stevenson LW. Hospitalizations in patients with heart failure: who and why. J Am Coll Cardiol. 2009;54:1703–1705. [DOI] [PubMed] [Google Scholar]
- 124. Kerkhof PLM, Peace RA, Macfarlane PW. Sex- and age-related reference values in cardiology, with annotations and guidelines for interpretation. Adv Exp Med Biol. 2018;1065:677–706. doi: 10.1007/978-3-319-77932-4_41. [DOI] [PubMed] [Google Scholar]
- 125. Perrone-Filardi P, Paolillo S, Costanzo P, Savarese G, Trimarco B, Bonow RO. The role of metabolic syndrome in heart failure. Eur Heart J. 2015;36:2630–2634. [DOI] [PubMed] [Google Scholar]
- 126. Iyngkaran P, Schneider H, Devarajan P, Anavekar N, Krum H, Ronco C. Cardio-renal syndrome: new perspective in diagnostics. Semin Nephrol. 2012;32:3–17. [DOI] [PubMed] [Google Scholar]
- 127. Iyngkaran P, Anavekar N, Majoni W, Thomas MC. The role and management of sympathetic overactivity in cardiovascular and renal complications of diabetes. Diabetes Metab. 2013;39:290–298. [DOI] [PubMed] [Google Scholar]
- 128. Ponikowski P, Mitrovic V, Ruda M, et al. A randomized, double-blind, placebo-controlled, multicentre study to assess haemodynamic effects of serelaxin in patients with acute heart failure. Eur Heart J. 2014;35:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]