Abstract
Neuroinflammation is one of the key components contributing to the devastating outcome of ischemic stroke. Starting with stroke onset, inflammatory processes contribute both to cell damage and tissue remodeling. The early release of alarmins triggers the upregulation of multiple proinflammatory cytokines, resulting in the compromised integrity of the blood–brain barrier. From this moment on, the infiltration of peripheral immune cells, reactive gliosis and extracellular matrix (ECM) alterations become intricately intertwined and act as one unit during the tissue remodeling. While the mechanisms of leukocyte and glia activation are amply reviewed, the field of ECM modification remains as yet under explored. In this review, we focus on the interplay between neuroinflammatory cascades and ECM in the ischemic brain. By summarizing the currently available evidence obtained by in vitro research, animal experimentation and human studies, we aim to propose a new direction for the future investigation of stroke recovery.
Keywords: ischemic stroke, extracellular matrix, neuroinflammation, neuroplasticity
Introduction
Ischemic stroke remains a leading cause of death and disability in the adult population. Despite plenty of efforts devoted to understanding and treating the disease, most novel approaches have only a discouragingly limited impact on patients’ wellbeing.1 We suggest that to improve the translation of scientific advances from bench to bedside, the pathophysiology of ischemic stroke should be investigated from complementary points of view. Today, most studies of stroke put the major focus on neuronal plasticity and repair,2–4 blood–brain barrier (BBB) dysfunction,5 and neuroinflammation.6 In this review, we will address the relationship between the immune response and the reorganization of the extracellular matrix (ECM) during the acute and chronic phases of ischemic stroke. Although both aspects have been studied individually, their interaction is rarely considered in both experimental and clinical settings. We propose that the brain’s immune response and ECM regulation should be considered as a functional unit, as first proposed by Schönherr and Hausser,7 opening new perspectives in stroke treatment.
The ECM is a congregation of multiple adhesion molecules, polysaccharides, proteins and proteoglycans arranged three-dimensionally in the extracellular space. During development and adulthood, this complex fulfils various functions, such as regulating cell migration, proliferation, adhesion, differentiation,8 synaptic plasticity,9 maintenance of the BBB10 and tissue architecture, integrity and homeostasis.11 In the central nervous system (CNS), ECM can be divided into two compartments, the interstitial matrices and basement membranes (BMs).12 The interstitial matrix is based on diffuse meshworks of hyaluronic acid (HA), which incorporate mainly collagens and proteoglycans.13 The BMs are associated with the basal portion of cerebral endothelial cells and consist mainly of laminins, collagen IV, nidogens and heparan sulfate proteoglycans.14
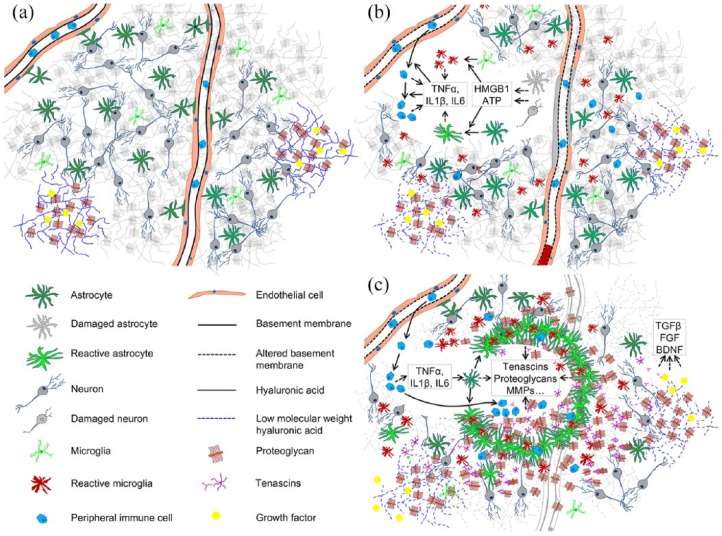
The interplay between the immune activation and the ECM is involved in different tissue compartments during the three overlapping phases of ischemic stroke: cell death and inflammation, tissue remodeling and neural plasticity and rewiring.15 Within this review, we will focus on the three main aspects of this interplay: (a) interaction between infiltrating leukocytes and endothelial BMs during the initial neuroinflammatory response; (b) reactive gliosis and ECM remodeling; (c) potential of ECM modifications for restorative therapy. For convenience, the key highlights of initial immune cell infiltration and reactive gliosis following ischemic stroke and subsequent tissue replacement are schematically summarized in Figure 1.
Figure 1.
ECM and cellular organization in healthy tissue (a), during initial neuroinflammation after stroke (b) and during subsequent tissue remodeling and matrix deposition (c).
ECM, extracellular matrix.
Multicellular release of alarmins and early neuroinflammatory response
In cerebral ischemia, the cessation of blood flow induces severe metabolic stress causing cell death in the hypoperfused territories. Within the early phase, initial cell death causes the disruption of the BBB16 and activates the innate immune system.17 The compromised supply of oxygen and nutrients triggers the release of household molecules from the damaged cells. Heat shock protein 70 (HSP70), adenosine triphosphate (ATP) and high mobility group box 1 protein (HMGB1) are among these. In ischemic tissue, these molecules are recognized as danger signals, or alarmins. The term ‘alarmins’ was proposed to differentiate these molecular signals from the exogenous stimuli associated with pathogens.18 One of the most studied alarmins in the context of brain injury is HMGB1. In healthy tissue, this nuclear protein organizes the DNA and regulates transcription.19 Starting in the first hour after ischemia, HMGB1 translocates into the cytoplasm, undergoes posttranslational modification, and its acetylated form is released into the extracellular space.17,20 Several studies demonstrated that the released HMGB1 binds to the receptor for advanced glycation end products (RAGE) and Toll-like receptors 2 and 4 (TLR2 and TLR4) on leukocytes, neurons, astrocytes and microglia,17,21–23 inducing the multicellular release of the proinflammatory cytokines. On microglia, HMGB1 can also bind with macrophage antigen complex 1 (MAC1), driving the proinflammatory phenotype of these cells.24 Also, the early HMGB1 release can directly induce astrocyte reactivity25 and stimulate astrocytic glutamate exocytosis.25 The reactive gliosis profile is further reinforced by extracellular ATP. In fact, ATP itself acts as an important alarmin in ischemic tissue and can be secreted by multiple cell types. In the CNS, extracellular ATP binds to purinergic P2X and P2Y receptors on the plasma membrane of endothelial cells, leukocytes, neurons and glial cells, allowing the nonselective diffusion of small monovalent and bivalent cations.26 On one hand, the abundant release of ATP from reactive microglia is suggested to induce astrocyte reactivity and excitatory neurotransmission.27 But on the other hand, the reactive astrocytes may trigger the microglial inflammatory response via the release of astrocytic ATP.28,29 Apparently, extracellular ATP acts as a feed-forward element during the early stage of the induction of reactive gliosis. Although the exact interplay between the glia activation cascades remains to be clarified, current data suggest that the multicellular production of alarmins converges on the elevated extracellular levels of proinflammatory cytokines. Under both experimental and clinical conditions, these initial responses can be further amplified by infiltrating peripheral immune cells following the BBB disruption.
In addition to alarmins of intracellular origin, similar signaling properties are attributed to several ECM components, including low molecular weight HA,30,31 fibronectin and heparan sulfate.32 Similar to pathogen-associated molecular patterns (PAMPs), these molecules bind to pattern recognition receptors, leading to the activation of the immune response. It is hypothesized that the cleavage of ECM alarmins from the intact matrix can be mediated by the overproduction of reactive oxygen species.33 Under normal circumstances, high molecular weight HA serves as an ECM backbone, being essential to prevent neuronal overexcitation and epileptiform activity.34 As a result of injury, high molecular weight HA is broken down to smaller fragments, which amplify inflammatory responses by binding to TLR2 and TLR4 receptors on leukocytes.35 Interestingly, the CD44 glycoprotein, which is widely expressed by multiple brain cells (including microglia, astrocytes, lymphocytes and vascular epithelium), can sequester the low molecular weight HA, thereby limiting its inflammatory effect.36,37
Early neuroinflammatory response and peripheral immune cell infiltration
In the healthy brain, the BBB is a semipermeable barrier formed by endothelial cells, astrocytes and pericytes that allows the selective entry of secreted regulatory molecules to the brain parenchyma.38 In ischemic stroke, the loss of tight junctions between endothelial cells allows the entry of plasma proteins and peripheral immune cells.39 The BBB breakdown is triggered within the first few hours40 by the initial upregulation of proinflammatory cytokines, including tumor necrosis factor (TNF)-α and interleukin (IL)-1β.41–43 This early inflammatory response prepares the vascular surface to interact with the first leukocytes infiltrating the CNS, namely with neutrophils and monocytes. The released TNF-α and IL1β interact with p55-p75 and IL1ra receptors on the surface of endothelial cells,42,44 inducing the upregulation of endothelial adhesion molecules such as P- and E-selectins, PCAM1, ICAM1 and integrins.45,46 In turn, the enhanced adhesion slows down rolling leukocytes, leading to their immobilization on the luminal surface of capillaries and venules and facilitating migration of neutrophils and monocytes into the brain parenchyma.47 Although these mechanisms have widely been explored, more recent evidence suggests that extravasation of peripheral immune cells does not solely depend on endothelial adhesion molecules, but that ECM mediated interactions are crucially involved in this process. In fact, leukocyte infiltration is largely regulated by laminin-8 and -10 of the endothelial BMs. Following the intravital application of TNF-α in mice lacking laminin-10, BBB integrity is compromised via the destabilization of junctional cadherin,48 resulting in facilitated transmigration of leukocytes. On the other hand, laminin-8 stimulates leukocyte migration, and knockout mice lacking this BM molecule exhibit reduced recruitment of immune cells to inflammatory loci.49 Intriguingly, the composition of capillary and venular basement membranes is heterogeneous, and peripheral neutrophils tend to associate with low expression regions which contain less laminin-10, collagen IV and nidogen-2.50 In ILβ stimulated tissue, neutrophils can use these regions to enter brain parenchyma. During the initial neuroinflammatory response, the infiltrating leukocytes are able to modify the BM matrix by secreting neutrophil elastase51 and macrophage metalloproteases.52 Although the exact mechanisms of leukocyte–endothelium interactions still need further investigation, they clearly result in BBB permeability and transmigration of peripheral neutrophils and macrophages during the first 24 h after stroke in rodent models.53 At later time points, BBB leakage persists, and T-cells enter the perilesional brain tissue. In different animal models of ischemic stroke, the presence of blood macrophages and T-cells in the brain parenchyma typically peaks between 7 and 30 days after stroke.53–55 Both human and mouse models show that the infiltration of regulatory T-cells has a positive effect on neurological recovery by the secretion of anti-inflammatory cytokines, IL10 and tumor growth factor (TGF)-β.56,57 In addition, regulatory T-cells can facilitate neurological recovery by producing neurotrophins such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF).58,59 Notably, elevated levels of pro- and anti-inflammatory cytokines, compromised BBB function, activated macrophages, regulatory T-cells and reactive glia can be observed several weeks after ischemia, playing an important role in tissue remodeling.15 The role of ECM in further inflammatory cascades is closely related to the modification of the interstitial matrix by reactive glia. During the recovery phase, the altered expression of glycoproteins and proteoglycans has an important effect on the behavior of immune cells in the brain parenchyma.12
Glial scar formation and extracellular matrix deposition
After initial cell death during the first hours after stroke, the inflammatory response triggers the proliferation of glial cells. Stimulated by TNF-α and IL6 cytokines, resident astrocytes become reactive [this process is usually defined by the upregulation of glial acidic protein (GFAP) expression], and form a compact glial scar around the lesion core.60,61 Glial scar astrocytes contribute to the recruitment of peripheral macrophages into the injury site by secreting chemokine (C-C motif) ligand 3 (CCL3) and chemokine (C-X-C motif) ligands 1 and 2 (CXCL1 and CXCL2).62 The enrolled macrophages remove damaged tissue components, enabling tissue remodeling. Inside the lesion core, tissue remodeling is characterized by non-neural cell proliferation and ECM deposition. Fibrocytes, fibroblasts, immune cells and surrounding glia produce massive amounts of collagens, proteoglycans and tenascins, building up the fibrotic scar. It is important to distinguish the fibrotic scar from the astrocytic scar. While the astrocytic scar surrounds the lesion site and may promote neural remodeling and rewiring,63 the fibrotic replacement of damaged tissue provides rapid wound repair at the cost of inhibiting axonal regeneration.64
The astrocytic scar limits secondary damage to brain tissue surrounding the ischemic area by restricting propagating cell death. The compromised formation of glial scars in transgenic animals leads to increased neuronal death, demyelination and worsens neurological recovery in traumatic or ischemic brain injury.65–68 These beneficial aspects of glial scar formation can partly be explained by the regulatory properties of ECM. For instance, astrocytic scar can limit the migration of reactive immune cells into the perilesional tissue by promoting chondroitin sulfate proteoglycan (CSPG)-mediated cell adhesion.69 In addition, reactive astrocytes that express tenascin C (TnC) can suppress the production of cytokines by infiltrating T-cells.70,71 The tenascin gene family comprises four genes in mammals,72 of which TnC and tenascin-R (TnR) are expressed in the CNS. TnC is a major constituent of the neural stem cell compartment73 and implicated in axon growth and guidance, synaptic plasticity and regeneration of the CNS.74,75 TnR is exclusively expressed in the CNS and associated with perineuronal nets.76
Although the glial scar is crucial for lesion demarcation, the majority of ECM molecules synthesized by reactive astrocytes limits beneficial neural plasticity in the injury recovery phase. Besides creating a compact layer in close proximity to the lesion core, reactive glia express keratan sulfate proteoglycans (KSPGs)77 and CSPGs.78,79 In a number of studies, these molecules were described to limit postinjury neuronal regeneration and axonal sprouting (Jin et al, 2018). Based on ample evidence supporting the inhibitory properties of the glial scar, several treatment strategies were proposed that counteract astrocyte proliferation and ECM accumulation (for review see Rolls and colleagues80). The suggested approaches included enzymatic ECM degradation,81,82 ECM blockade by anti-CSPG antibodies,83 inhibition of ECM formation84 and inhibition of astrocyte proliferation.85 Despite their certain relevance, these concepts are based on the perception that the glial scar is an obstacle that has to be circumvented to improve stroke recovery. However, a recent study demonstrated that after spinal cord injury, a subpopulation of scar-forming astrocytes releases a set of CSPGs that aid axon regeneration.63 Another ECM component of the glial scar, TnC,86 was shown to promote the outgrowth of retinal axons87 and sensory axon regeneration following spinal cord injury.88
In healthy nervous tissue, ECM incorporates many regulatory molecules which play pivotal roles during brain development. For example, several members of the heparan sulfate proteoglycan (HSPG) family are necessary for cell positioning, linage specification and neural wiring guidance.89,90 During development, the CSPG/HSPG expression ratio provides a bidirectional control over axon pathfinding, with HSPGs promoting and CSPGs repelling axonal outgrowth.91 Upon ischemia, the dynamics of the CSPG/HSPG ratio closely resembles the developmental profile,92 providing a potential mechanism of targeted neural rewiring.
Taken together, the altered expression of ECM components in reactive astrocytes can shape the extracellular space in a polarized way, preventing axonal outgrowth into the lesion core, but enabling neural rewiring under certain conditions in perilesional areas.93 Considering that reactive glia and leukocytes persist in the brain parenchyma during long periods after stroke onset, one could predict that both infiltrating immune cells and glia are central for long term ECM remodeling. Unfortunately, our current understanding of how ECM changes can influence neurological recovery is very limited. Also, there are no therapeutic approaches to manipulate these alterations, and thus further research is critically needed. Since the crude depletion of ECM components induces explicit dysregulation of neural networks,94–96 we propose that a beneficial stroke recovery requires a reversible and delicate modulation of ECM composition and structure. With this scope, matrix proteases may be promising targets for future investigation, as they comprise an intrinsic regulatory system within ECM.97
ECM remodeling and neurological recovery
Albeit the main scaffold of interstitial brain matrix is composed by HA and proteoglycans, many regulatory functions of the ECM rely on associated signaling molecules.76 Originating from different cell types, the vast majority of short range signaling factors become embedded in the extracellular meshwork. Unlike the constitutive ECM elements, the expression of these components may dynamically change, promptly responding to the augmenting cellular environment. Growth factors, ILs and molecular guidance signals can either directly bind the core protein of proteoglycans or interact with polysaccharide side chains.98 Importantly, both regulatory molecules like BDNF, fibroblast growth factor (FGF), TNF-α and TGF-β can be immersed in the same mesh as their cleavage factors. Within ECM, the two major families of neural endogenous proteases are matrix metalloproteases (MMPs) and A disintegrin and metalloproteinases with thrombospondin motifs (ADAMTSs). Both MMPs and ADAMTSs can specifically degrade proteoglycans and glycoproteins, locally modifying the ECM structure and composition.99 However, they also modulate neural plasticity by cleaving growth factors such as BDNF.100 ADAMTSs and MMPs are upregulated in perilesional areas following ischemic stroke and brain trauma, where they can promote neural recovery via ECM remodeling.101 In particular, proteoglycan digestion by ADAMTS4 was shown to enhance axonal regeneration and sprouting after spinal cord injury similar to chondroitin sulfate degradation.102 Upon ischemia, MMP2 and MMP9 are suggested to promote leukocyte infiltration, and to remove ECM barriers for neural plasticity.103 In stroke, the modulation of MMP9 activity using synthetic compounds, endogenous inhibitors and hypothermia, was recently proposed as a part of restorative therapy.104 It was also reported that in the subacute phase of ischemia MMP9 is expressed by proliferating neural progenitors in the subventricular zone, proposing a new role of MMPs in restorative neurogenesis.105
ECM remodeling as new target for stroke therapy
Taken in view of the coherent activation of brain innate immunity, peripheral immune cell infiltration and brain ECM changes, it is tempting to suggest that the available immunotherapies can support neurological recovery after stroke. However, to the best of our knowledge, there are currently no reports describing a successful usage of cytokine-directed or lymphocyte-targeted therapy in stroke, in contrast with cancer and autoimmune diseases.106 Recently, low doses of colchicine was proposed as a novel treatment to mitigate atherosclerotic plaque inflammation, which reduces recurrent stroke rates in patients.107 The beneficial effect of colchicine is presumed to be mediated by inhibition of neutrophil activation and chemotaxis.108 In rheumatoid arthritis, anti-TNF-α treatments reduced plasma levels of sulfated glycosa-minoglycans (GAGs),109 suggesting a possibility to modulate ECM metabolism by cytokine targeting. But despite these perspectives, immunotherapy has to be evaluated with great care. Firstly, the rough depletion of inflammatory cascades may interfere with glial scar formation, deteriorating stroke outcome. Secondly, the nonlocalized inhibition of proinflammatory cytokines has significant bystander effects and may result in adverse events, such as an increased susceptibility to infections.110
From our point of view, the most promising strategy to improve neurological recovery after stroke is to combine the general supportive care with a spatially confined modulation of brain ECM. In our recent work,111 we proposed that the remodeling of perineuronal ECM may promote neural rewiring in the stroke brain. To our knowledge, there are currently no treatments which enable to alter ECM ultrastructure and composition in a controlled and localized way. However, the existing evidence (Table 1), although admittedly fragmented, allows proposing two innovative directions in the search for ECM-modifying agents: (a) a delicate modification of proteoglycan complexes and (b) a subtle modulation of matrix protease activity. Based on these strategies, we believe that in the next years the combined efforts of neuroscientists and translational neurologists may allow developing new encouraging therapies that improve stroke recovery by a mode of action that is spatially confined to ECM.
Table 1.
Summary of the most important findings discussed in this review.
| HMGB1 protein is released early after the onset of brain ischemia from the degenerating neurons. HMGB1 promotes the upregulation of matrix proteases and stimulates proinflammatory cytokine synthesis. | Andersson and colleagues22; Qiu and colleagues20; Qiu and colleagues21 |
| Low molecular weight hyaluronan acts as an endogenous danger signal. | Scheibner and colleagues30 |
| Microglia activation triggers an astrocyte-driven upregulation of excitatory neurotransmission. | Pascual and colleagues27 |
| Astrocytes trigger rapid microglial response to brain injury via ATP release. | Bianco and colleagues29; Davalos and colleagues28 |
| Endothelial basement membrane matrix regulates immune cell recruitment to inflammatory loci. | Kenne and colleagues49; Song and colleagues48; Wang and colleagues51; Wang and colleagues50 |
| Astrocyte scar formation is central for axon regeneration after spinal cord injury. | Anderson and colleagues63 |
| The disturbed formation of astrocytic scar is detrimental for the recovery after traumatic brain injury. | Bush and colleagues65 |
| Chondroitin sulfate proteoglycans inhibit neurite outgrowth. | Jin and colleagues 2018112 |
| The depletion of inhibitory chondroitin sulfate proteoglycans improves neuronal regeneration and functional recovery after spinal cord injury. | Bradbury and colleagues82; Monnier and colleagues84; Moon and colleagues81; Tan and colleagues83 |
| The integrity of brain extracellular matrix is essential for retaining synaptic connectivity. | Bikbaev and colleagues94 |
| During development, the bidirectional control over axon pathfinding is determined by CSPG/HSPG expression ratio, with HSPGs promoting and CSPGs repelling axonal outgrowth. Upon ischemia, the dynamics of the CSPG/HSPG expression closely resembles the developmental profile. | Coles and colleagues91 |
| Ultrastructural modifications of perineuronal ECM are proposed as a mechanism to support neural plasticity after stroke. | Dzyubenko and colleagues111 |
ATP, adenosine triphosphate; CSPG, chondroitin sulfate proteoglycan; ECM, extracellular matrix; HMGB1, high mobility group box 1; HSPG, heparan sulfate proteoglycan.
Acknowledgments
Egor Dzyubenko and Daniel Manrique-Castano contributed equally to this work.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Dirk M Hermann  https://orcid.org/0000-0003-0198-3152
https://orcid.org/0000-0003-0198-3152
Contributor Information
Egor Dzyubenko, Department of Neurology, University Hospital Essen, Essen, Germany.
Daniel Manrique-Castano, Department of Neurology, University Hospital Essen, Essen, Germany.
Christoph Kleinschnitz, Department of Neurology, University Hospital Essen, Essen, Germany.
Andreas Faissner, Department of Cell Morphology and Molecular Neurobiology, Faculty of Biology and Biotechnology, Ruhr University Bochum, Bochum, Germany.
Dirk M. Hermann, Department of Neurology, University Hospital Essen, Hufelandstraße 55, D-45122 Essen, Germany.
References
- 1. Patel RAG, McMullen PW. Neuroprotection in the treatment of acute ischemic stroke. Prog Cardiovasc Dis 2017; 59: 542–548. [DOI] [PubMed] [Google Scholar]
- 2. Cramer SC. Treatments to promote neural repair after stroke. J Stroke 2018; 20: 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moretti A, Ferrari F, Villa RF. Neuroprotection for ischaemic stroke: current status and challenges. Pharmacol Ther 2015; 146: 23–34. [DOI] [PubMed] [Google Scholar]
- 4. Hermann DM, Chopp M. Promoting brain remodelling and plasticity for stroke recovery: therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol 2012; 11: 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lv J, Hu W, Yang Z, et al. Focusing on claudin-5: a promising candidate in the regulation of BBB to treat ischemic stroke. ProgNeurobiol 2017; 161: 79–96. [DOI] [PubMed] [Google Scholar]
- 6. Kawabori M, Yenari M. Inflammatory responses in brain ischemia. Curr Med Chem 2017; 22: 1258–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schönherr E, Hausser HJ. Extracellular matrix and cytokines: a functional unit. Dev Immunol 2000; 7: 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Faissner A, Reinhard J. The extracellular matrix compartment of neural stem and glial progenitor cells. Glia 2015; 63: 1330–1349. [DOI] [PubMed] [Google Scholar]
- 9. Dityatev A, Schachner M, Sonderegger P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci 2010; 11: 735–746. [DOI] [PubMed] [Google Scholar]
- 10. Kangwantas K, Pinteaux E, Penny J. The extracellular matrix protein laminin-10 promotes blood-brain barrier repair after hypoxia and inflammation in vitro. J Neuroinflammation 2016; 13: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skeath JB, Wilson BA, Romero SE, et al. The extracellular metalloprotease AdamTS-A anchors neural lineages in place within and preserves the architecture of the central nervous system. Development 2017; 144: 3102–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hallmann R, Zhang X, Di Russo J, et al. The regulation of immune cell trafficking by the extracellular matrix. Curr Opin Cell Biol 2015; 36: 54–61. [DOI] [PubMed] [Google Scholar]
- 13. Miyata S, Kitagawa H. Formation and remodeling of the brain extracellular matrix in neural plasticity: roles of chondroitin sulfate and hyaluronan. Biochim Biophys Acta Gen Subj 2017; 1861: 2420–2434. [DOI] [PubMed] [Google Scholar]
- 14. Pozzi A, Yurchenco PD, Iozzo RV. The nature and biology of basement membranes. Matrix Biol 2017; 57–58: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014; 81: 229–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krueger M, Bechmann I, Immig K, et al. Blood-brain barrier breakdown involves four distinct stages of vascular damage in various models of experimental focal cerebral ischemia. J Cereb Blood Flow Metab 2015; 35: 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liesz A, Hu X, Kleinschnitz C, et al. Functional role of regulatory lymphocytes in stroke: facts and controversies. Stroke 2015; 46: 1422–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 2007; 81: 1–5. [DOI] [PubMed] [Google Scholar]
- 19. Klune JR, Dhupar R, Cardinal J, et al. HMGB1: endogenous danger signaling. Mol Med 2008; 14: 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qiu J, Nishimura M, Wang Y, et al. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab 2008; 28: 927–938. [DOI] [PubMed] [Google Scholar]
- 21. Qiu J, Xu J, Zheng Y, et al. High-mobility group box 1 promotes metalloproteinase-9 upregulation through Toll-like receptor 4 after cerebral ischemia. Stroke 2010; 41: 2077–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 2000; 192: 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kokkola R, Andersson A, Mullins G, et al. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol 2005; 61: 1–9. [DOI] [PubMed] [Google Scholar]
- 24. Gao HM, Zhou H, Zhang F, et al. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci 2011; 31: 1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bonanno G, Raiteri L, Milanese M, et al. The high-mobility group box 1 cytokine induces transporter-mediated release of glutamate from glial subcellular particles (gliosomes) prepared from in situ-matured astrocytes. Int Rev Neurobiol 2007; 82: 73–93. [DOI] [PubMed] [Google Scholar]
- 26. Khakh BS, North RA. Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron 2012; 76: 51–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pascual O, Ben Achour S, Rostaing P, et al. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A 2012; 109: E197–E205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 2005; 8: 752–758. [DOI] [PubMed] [Google Scholar]
- 29. Bianco F, Pravettoni E, Colombo A, et al. Astrocyte-derived ATP induces vesicle shedding and IL-1 release from microglia. J Immunol 2005; 174: 7268–7277. [DOI] [PubMed] [Google Scholar]
- 30. Scheibner KA, Lutz MA, Boodoo S, et al. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol 2006; 177: 1272–1281. [DOI] [PubMed] [Google Scholar]
- 31. Gaudet AD, Popovich PG. Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Exp Neurol 2014; 258: 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol 2004; 76: 514–519. [DOI] [PubMed] [Google Scholar]
- 33. Arumugam TV, Okun E, Tang SC, et al. Toll-like receptors in ischemia-reperfusion injury. Shock 2009; 32: 4–16. [DOI] [PubMed] [Google Scholar]
- 34. Perkins KL, Arranz AM, Yamaguchi Y, et al. Brain extracellular space, hyaluronan, and the prevention of epileptic seizures. Rev Neurosci 2017; 28: 869–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang D, Liang J, Fan J, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 2005; 11: 1173–1179. [DOI] [PubMed] [Google Scholar]
- 36. Kawana H, Karaki H, Higashi M, et al. CD44 suppresses TLR-mediated inflammation. J Immunol 2008; 180: 4235–4245. [DOI] [PubMed] [Google Scholar]
- 37. Liang J, Jiang D, Griffith J, et al. CD44 is a negative regulator of acute pulmonary inflammation and lipopolysaccharide-TLR signaling in mouse macrophages. J Immunol 2007; 178: 2469–2475. [DOI] [PubMed] [Google Scholar]
- 38. Giannoni P, Badaut J, Dargazanli C, et al. The pericyte-glia interface at the blood-brain barrier. Clin Sci (Lond) 2018; 132: 361–374. [DOI] [PubMed] [Google Scholar]
- 39. Jiang X, Andjelkovic AV, Zhu L, et al. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol 2018; 163–164: 144–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abo-Ramadan U, Durukan A, Pitkonen M, et al. Post-ischemic leakiness of the blood-brain barrier: a quantitative and systematic assessment by Patlak plots. Exp Neurol 2009; 219: 328–333. [DOI] [PubMed] [Google Scholar]
- 41. Haddad M, Rhinn H, Bloquel C, et al. Anti-inflammatory effects of PJ34, a poly(ADP-ribose) polymerase inhibitor, in transient focal cerebral ischemia in mice. Br J Pharmacol 2006; 149: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Murray KN, Parry-Jones AR, Allan SM. Interleukin-1 and acute brain injury. Front Cell Neurosci 2015; 9: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clausen BH, Lambertsen KL, Babcock AA, et al. Interleukin-1beta and tumor necrosis factor-alpha are expressed by different subsets of microglia and macrophages after ischemic stroke in mice. J Neuroinflammation 2008; 5: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Botchkina GI, Meistrell ME, 3rd, Botchkina IL, et al. Expression of TNF and TNF receptors (p55 and p75) in the rat brain after focal cerebral ischemia. Mol Med 1997; 3: 765–781. [PMC free article] [PubMed] [Google Scholar]
- 45. Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol 2006; 66: 232–245. [DOI] [PubMed] [Google Scholar]
- 46. O’Carroll SJ, Kho DT, Wiltshire R, et al. Pro-inflammatory TNFα and IL-1β differentially regulate the inflammatory phenotype of brain microvascular endothelial cells. J Neuroinflammation 2015; 12: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Frijns CJM, Kappelle LJ. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke 2002; 33: 2115–2122. [DOI] [PubMed] [Google Scholar]
- 48. Song J, Zhang X, Buscher K, et al. Endothelial basement membrane laminin 511 contributes to endothelial junctional tightness and thereby inhibits leukocyte transmigration. Cell Rep 2017; 18: 1256–1269. [DOI] [PubMed] [Google Scholar]
- 49. Kenne E, Soehnlein O, Genove G, et al. Immune cell recruitment to inflammatory loci is impaired in mice deficient in basement membrane protein laminin 4. J Leukoc Biol 2010; 88: 523–528. [DOI] [PubMed] [Google Scholar]
- 50. Wang S, Voisin MB, Larbi KY, et al. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med 2006; 203: 1519–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang S, Dangerfield JP, Young RE, et al. PECAM-1, alpha6 integrins and neutrophil elastase cooperate in mediating neutrophil transmigration. J Cell Sci 2005; 118: 2067–2076. [DOI] [PubMed] [Google Scholar]
- 52. Agrawal S, Anderson P, Durbeej M, et al. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med 2006; 173: 1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grønberg NV, Johansen FF, Kristiansen U, et al. Leukocyte infiltration in experimental stroke. J Neuroinflammation 2013; 10: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vindegaard N, Munoz-Briones C, El Ali HH, et al. T-cells and macrophages peak weeks after experimental stroke: spatial and temporal characteristics. Neuropathology 2017; 37: 407–414. [DOI] [PubMed] [Google Scholar]
- 55. Stubbe T, Ebner F, Richter D, et al. Regulatory T cells accumulate and proliferate in the ischemic hemisphere for up to 30 days after MCAO. J Cereb Blood Flow Metab 2013; 33: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lobo-Silva D, Carriche GM, Castro AG, et al. Balancing the immune response in the brain: IL-10 and its regulation. J Neuroinflammation 2016; 13: 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol 2010; 10: 170–181. [DOI] [PubMed] [Google Scholar]
- 58. Brait VH, Arumugam TV, Drummond GR, et al. Importance of T lymphocytes in brain injury, immunodeficiency, and recovery after cerebral ischemia. J Cereb Blood Flow Metab 2012; 32: 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chan A, Yan J, Csurhes P, et al. Circulating brain derived neurotrophic factor (BDNF) and frequency of BDNF positive T cells in peripheral blood in human ischemic stroke: effect on outcome. J Neuroimmunol 2015; 286: 42–47. [DOI] [PubMed] [Google Scholar]
- 60. Yuan YM, He C. The glial scar in spinal cord injury and repair. Neurosci Bull 2013; 29: 421–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sofroniew MV. Astrogliosis. Cold Spring Harb Perspect Biol 2014; 7: a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chamorro A, Meisel A, Planas AM, et al. The immunology of acute stroke. Nat Rev Neurol 2012; 8: 401–410. [DOI] [PubMed] [Google Scholar]
- 63. Anderson MA, Burda JE, Ren Y, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016; 532: 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dias DO, Goritz C. Fibrotic scarring following lesions to the central nervous system. Matrix Biol 2018; 68–69: 561–570. [DOI] [PubMed] [Google Scholar]
- 65. Bush TG, Puvanachandra N, Horner CH, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 1999; 23: 297–308. [DOI] [PubMed] [Google Scholar]
- 66. Faulkner JR, Herrmann JE, Woo MJ, et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 2004; 24: 2143–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li L, Lundkvist A, Andersson D, et al. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab 2008; 28: 468–481. [DOI] [PubMed] [Google Scholar]
- 68. Wanner IB, Anderson MA, Song B, et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci 2013; 33: 12870–12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Miura R, Aspberg A, Ethell IM, et al. The proteoglycan lectin domain binds sulfated cell surface glycolipids and promotes cell adhesion. J Biol Chem 1999; 274: 11431–11438. [DOI] [PubMed] [Google Scholar]
- 70. Puente Navazo MD, Valmori D, Ruegg C. The alternatively spliced domain TnFnIII A1A2 of the extracellular matrix protein tenascin-C suppresses activation-induced T lymphocyte proliferation and cytokine production. J Immunol 2001; 167: 6431–6440. [DOI] [PubMed] [Google Scholar]
- 71. Jachetti E, Caputo S, Mazzoleni S, et al. Tenascin-C protects cancer stem-like cells from immune surveillance by arresting T-cell activation. Cancer Res 2015; 75: 2095–2108. [DOI] [PubMed] [Google Scholar]
- 72. Chiquet-Ehrismann R, Tucker RP. Tenascins and the importance of adhesion modulation. Cold Spring Harb Perspect Biol 2011; 3. pii: a004960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Faissner A, Roll L, Theocharidis U. Tenascin-C in the matrisome of neural stem and progenitor cells. Mol Cell Neurosci 2017; 81: 22–31. [DOI] [PubMed] [Google Scholar]
- 74. Joester A, Faissner A. The structure and function of tenascins in the nervous system. Matrix Biol 2001; 20: 13–22. [DOI] [PubMed] [Google Scholar]
- 75. Faissner A, Pyka M, Geissler M, et al. Contributions of astrocytes to synapse formation and maturation - potential functions of the perisynaptic extracellular matrix. Brain Res Rev 2010; 63: 26–38. [DOI] [PubMed] [Google Scholar]
- 76. Dzyubenko E, Gottschling C, Faissner A. Neuron-Glia interactions in neural plasticity: contributions of neural extracellular matrix and perineuronal nets. Neural Plast 2016; 2016: 5214961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang H, Uchimura K, Kadomatsu K. Brain keratan sulfate and glial scar formation. Ann N Y Acad Sci 2006; 1086: 81–90. [DOI] [PubMed] [Google Scholar]
- 78. Soleman S, Filippov MA, Dityatev A, et al. Targeting the neural extracellular matrix in neurological disorders. Neuroscience 2013; 253: 194–213. [DOI] [PubMed] [Google Scholar]
- 79. Wiese S, Karus M, Faissner A. Astrocytes as a source for extracellular matrix molecules and cytokines. Front Pharmacol 2012; 3: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci 2009; 10: 235–241. [DOI] [PubMed] [Google Scholar]
- 81. Moon LD, Asher RA, Rhodes KE, et al. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat Neurosci 2001; 4: 465–466. [DOI] [PubMed] [Google Scholar]
- 82. Bradbury EJ, Moon LD, Popat RJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002; 416: 636–640. [DOI] [PubMed] [Google Scholar]
- 83. Tan AM, Colletti M, Rorai AT, et al. Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. J Neurosci 2006; 26: 4729–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Monnier PP, Sierra A, Schwab JM, et al. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci 2003; 22: 319–330. [DOI] [PubMed] [Google Scholar]
- 85. Tian DS, Dong Q, Pan DJ, et al. Attenuation of astrogliosis by suppressing of microglial proliferation with the cell cycle inhibitor olomoucine in rat spinal cord injury model. Brain Res 2007; 1154: 206–214. [DOI] [PubMed] [Google Scholar]
- 86. Roll L, Mittmann T, Eysel UT, et al. The laser lesion of the mouse visual cortex as a model to study neural extracellular matrix remodeling during degeneration, regeneration and plasticity of the CNS. Cell Tissue Res 2012; 349: 133–145. [DOI] [PubMed] [Google Scholar]
- 87. Siddiqui S, Horvat-Brocker A, Faissner A. The glia-derived extracellular matrix glycoprotein tenascin-C promotes embryonic and postnatal retina axon outgrowth via the alternatively spliced fibronectin type III domain TNfnD. Neuron Glia Biol 2008; 4: 271–283. [DOI] [PubMed] [Google Scholar]
- 88. Andrews MR, Czvitkovich S, Dassie E, et al. Alpha9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration. J Neurosci 2009; 29: 5546–5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yu C, Griffiths LR, Haupt LM. Exploiting heparan sulfate proteoglycans in human neurogenesis-controlling lineage specification and fate. Front Integr Neurosci 2017; 11: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Condomitti G, de Wit J. Heparan Sulfate proteoglycans as emerging players in synaptic specificity. Front Mol Neurosci 2018; 11: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Coles CH, Shen Y, Tenney AP, et al. Proteoglycan-specific molecular switch for RPTPsigma clustering and neuronal extension. Science 2011; 332: 484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Leadbeater WE, Gonzalez AM, Logaras N, et al. Intracellular trafficking in neurones and glia of fibroblast growth factor-2, fibroblast growth factor receptor 1 and heparan sulphate proteoglycans in the injured adult rat cerebral cortex. J Neurochem 2006; 96: 1189–1200. [DOI] [PubMed] [Google Scholar]
- 93. George N, Geller HM. Extracellular matrix and traumatic brain injury. J Neurosci Res 2018; 96: 573–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bikbaev A, Frischknecht R, Heine M. Brain extracellular matrix retains connectivity in neuronal networks. Sci Rep 2015; 5: 14527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bukalo O, Schachner M, Dityatev A. Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience 2001; 104: 359–369. [DOI] [PubMed] [Google Scholar]
- 96. Geissler M, Gottschling C, Aguado A, et al. Primary hippocampal neurons, which lack four crucial extracellular matrix molecules, display abnormalities of synaptic structure and function and severe deficits in perineuronal net formation J Neurosci 2013; 33: 7742–7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yong VW. Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat Rev Neurosci 2005; 6: 931–944. [DOI] [PubMed] [Google Scholar]
- 98. Maeda N. Proteoglycans and neuronal migration in the cerebral cortex during development and disease. Front Neurosci 2015; 9: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lu P, Takai K, Weaver VM, et al. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 2011; 3. pii: a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tsilibary E, Tzinia A, Radenovic L, et al. Neural ECM proteases in learning and synaptic plasticity. Prog Brain Res 2014; 214: 135–157. [DOI] [PubMed] [Google Scholar]
- 101. Song I, Dityatev A. Crosstalk between glia, extracellular matrix and neurons. Brain Res Bull 2018; 136: 101–108. [DOI] [PubMed] [Google Scholar]
- 102. Tauchi R, Imagama S, Natori T, et al. The endogenous proteoglycan-degrading enzyme ADAMTS-4 promotes functional recovery after spinal cord injury. J Neuroinflammation 2012; 9: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol 2010; 10: 712–723. [DOI] [PubMed] [Google Scholar]
- 104. Chaturvedi M, Kaczmarek L. Mmp-9 inhibition: a therapeutic strategy in ischemic stroke. Mol Neurobiol 2014; 49: 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lee SR. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci 2006; 26: 3491–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wraith DC. The future of immunotherapy: a 20-year perspective. Front Immunol 2017; 8: 1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Georgios T, Aristeidis HK, Georgios G, et al. The role of colchicine in the prevention of cerebrovascular ischemia. Curr Pharm Des 2018; 24: 668–674. [DOI] [PubMed] [Google Scholar]
- 108. Paschke S, Weidner AF, Paust T, et al. Technical advance: inhibition of neutrophil chemotaxis by colchicine is modulated through viscoelastic properties of subcellular compartments. J Leukoc Biol 2013; 94: 1091–1096. [DOI] [PubMed] [Google Scholar]
- 109. Szeremeta A, Jura-Półtorak A, Koźma EM, et al. Effects of a 15-month anti-TNF-α treatment on plasma levels of glycosaminoglycans in women with rheumatoid arthritis. Arthritis Res Ther 2018; 20: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. van der Meer JW, Popa C, Netea MG. Side effects of anticytokine strategies. Neth J Med 2005; 63: 78–80. [PubMed] [Google Scholar]
- 111. Dzyubenko E, Manrique-Castano D, Kleinschnitz C, et al. Topological remodeling of cortical perineuronal nets in focal cerebral ischemia and mild hypoperfusion. Matrix Biol 2018; 74: 121–132. [DOI] [PubMed] [Google Scholar]
- 112. Jin J, Tilve S, Huang Z, et al. Effect of chondroitin sulfate proteoglycans on neuronal cell adhesion, spreading and neurite growth in culture. Neural Regen Res 2018; 13(2): 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]