Abstract
Renal cell carcinoma (RCC) is not a single entity but includes various tumor subtypes that have been identified on the basis of either characteristic pathologic features or distinctive molecular changes. Clear cell RCC is the most common type of RCC and is characterized by dysregulation of the von Hippel Lindau/hypoxia-inducible factor pathway. Non–clear cell RCC represents a more heterogeneous group of tumors with diverse histopathologic and molecular features. In the past two decades, the improved understanding of the molecular landscape of RCC has led to the development of more effective therapies for metastatic RCC, which include both targeted agents and immune checkpoint inhibitors. Because only subsets of patients with metastatic RCC respond to a given treatment, predictive biomarkers are needed to guide treatment selection and sequence. In this review, we describe the key histologic features and molecular alterations of RCC subtypes and discuss emerging tissue-based biomarkers of response to currently available therapies for metastatic disease.
PATHOLOGY OF RENAL CELL CARCINOMA
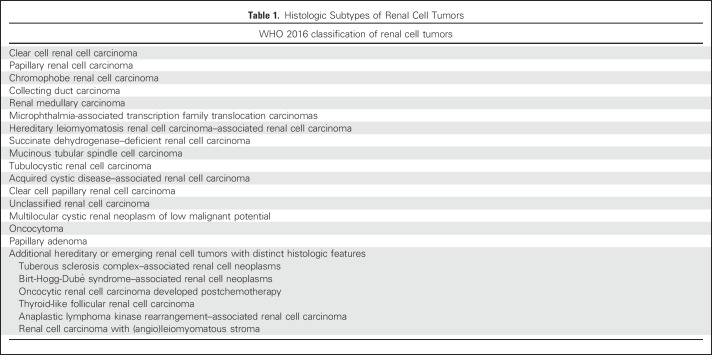
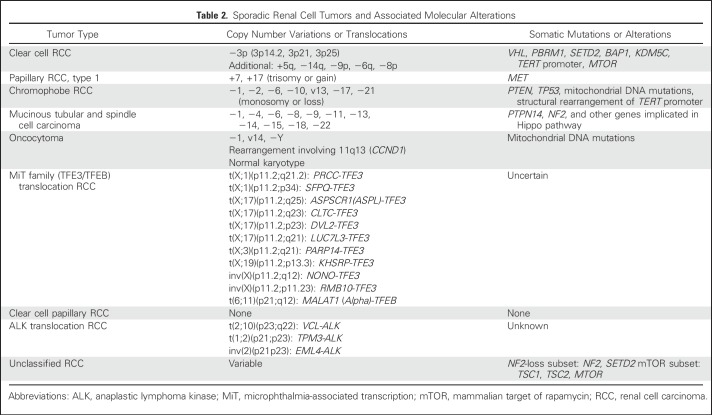
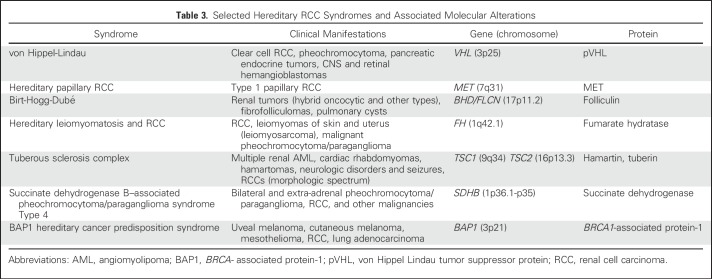
The histologic classification of renal cell carcinoma (RCC) has changed dramatically during the last few decades. Compared with the categories of clear cell carcinoma and granular cell carcinoma of the 1970s, contemporary classification has evolved from the 1997 Heidelberg classification, which first attempted to incorporate molecular genetics into the classification of RCC.1-3 In recent years, a number of new entities were added on the basis of either characteristic pathologic features or distinctive molecular alterations3 (Table 1). In general, the histologic subtyping of RCC not only guides management and conveys prognostic information but also may possess predictive value for treatment. Here we discuss the key pathologic features and molecular alterations of sporadic (Table 2) and familial (Table 3) RCC subtypes.
Table 1.
Histologic Subtypes of Renal Cell Tumors
Table 2.
Sporadic Renal Cell Tumors and Associated Molecular Alterations
Table 3.
Selected Hereditary RCC Syndromes and Associated Molecular Alterations
Clear Cell RCC
Clear cell RCC (ccRCC) is the most common type of RCC and comprises approximately 60% of all renal cell tumors and 75% to 80% of RCCs that metastasize. The characteristic histologic findings include clear cell cytology, acinar growth patterns, and a rich vascular network. However, it is not uncommon to find tumor cells displaying granular and eosinophilic cytoplasm or areas of alveolar, cystic, solid, or pseudopapillary architecture. Rhabdoid cytologic features and sarcomatoid differentiation are associated with a worse prognosis. The presence of focal areas with the classic appearance of ccRCC in an otherwise poorly differentiated rhabdoid or sarcomatoid tumor would strongly suggest this diagnosis. Diffuse membranous staining pattern of carbonic anhydrase IX in the nonnecrotic area is a helpful adjunctive marker with high specificity when used in combination with other ancillary markers.4
Common molecular alterations identified in ccRCC are 3p loss and inactivation of the von Hippel Lindau (VHL) gene, a tumor suppressor residing at the 3p25 locus. Patients with germ line VHL mutation (ie, VHL syndrome) are predisposed to develop multiple bilateral ccRCCs in a background of renal cystic lesions. In sporadic ccRCC, VHL inactivation has been reported in 60% to 90% of cases.5-8 The inactivation of its protein product (pVHL) results in aberrant stabilization of hypoxia-inducible factor (HIF), which drives the transcription of numerous genes involved in tumor formation.9 Large-scale genomic efforts in recent years have identified other prevalent somatic mutations in ccRCC, which most frequently involve genes such as PBRM1, SETD2, BAP1, and KDM5C.5,6,10,11 Interestingly, a significant portion of these mutated genes are chromatin modifiers, although their exact roles in tumorigenesis are still under investigation. Furthermore, PBRM1, SETD2, and BAP1 are all located at 3p21 and near the VHL gene, indicating an important role for 3p loss in the oncogenesis of ccRCC. At the chromosomal level, besides 3p loss (> 90%), 5q gain (67%) and loss of 14q (45%) are relatively frequent in sporadic tumors.5 BAP1 and SETD2 mutations or protein/function loss have been associated with worse outcomes in ccRCC.12-16
The molecular characterization of ccRCC also coincidentally revealed a subtype of RCC with wild-type VHL gene and intact 3p that was not previously recognized. TCEB1 (ELOC)-mutated RCC harbors recurrent hotspot mutations of TCEB1 (8q21), a component of the VHL E3 ligase complex, and chromosome 8 loss of heterozygosity, providing another mechanism of activating the HIF pathway.6,17
Non–Clear Cell RCC
Non–clear cell RCC (nccRCC) constitutes a heterogeneous group of tumors. The current diagnostic criteria for some of these tumors rely more on histologic features, such as those for papillary, chromophobe, mucinous tubular, spindle cell, and tubulocystic carcinoma, whereas others emphasize anatomic locations (eg, collecting duct carcinoma [CDC]) or clinical associations (eg, renal medullary carcinoma in patients with sickle cell trait or other hemoglobinopathy). However, a few newly recognized subtypes are distinguished by their specific molecular alterations, such as microphthalmia-associated transcription (MiT) family translocation RCC and hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome that associates with FH germ line mutations, and succinate dehydrogenase–deficient RCC.18-22 When a tumor does not fit into one of the established histologic subtypes, it is categorized as unclassified RCC (uRCC).
Papillary RCCs (pRCCs) represent about 10% to 15% of all renal cell tumors and are the second most common renal neoplasms. Histologically, they have been divided into two types: type 1 has papillae covered by tumor cells arranged in a single layer and typically low-grade nuclei; type 2 is characterized by pseudostratified and often large tumor cells with higher nuclear grade. However, the histologically defined type 2 pRCC exhibits a rather diverse morphologic spectrum, whereas other subtypes of nccRCCs may show prominent papillary architecture and mimic type 2 pRCC, including HLRCC, MiT translocation RCC, CDC, and newly described entities such as acquired cystic disease–associated RCC, often leading to inconsistent diagnosis among pathologists. Recent genomic characterization also supports that type 2 pRCC is a heterogeneous group and can be further divided into at least three subtypes.23 In comparison, sporadic type 1 pRCC has nearly universal gains or trisomy of chromosomes 7 and 17 and less frequent gain of chromosomes 2, 3, 12, 16, and 20.23 Mutations of the MET gene (7q31), identified from hereditary pRCC patients, has been reported in 10% to 13% of sporadic type 1 cases.23,24
Chromophobe RCC (chRCC) makes up 6% to 11% of renal cell neoplasms, and stage-by-stage, it has a significantly better prognosis than ccRCC. The eosinophilic variant of chRCC consists of cells with densely eosinophilic cytoplasm and may closely mimic other oncocytic renal tumors, including benign oncocytoma. Cytogenetically, most chRCCs are characterized by combined losses involving most or all of chromosomes 1, 2, 6, 10, 13, 17, and 21.25-27 Recent comprehensive genomic analysis identified mutations in TP53 (32%) and PTEN (6%), along with defects in mitochondrial DNA and structural rearrangement involving the TERT promoter region in subsets of cases.28
MiT family translocation RCC, or TFE3 (Xp11.2) and TFEB [t(6;11)] translocation RCCs, accounts for less than 5% of all renal neoplasms in children, but it is the most common type of RCC in this age group. Approximately 10% to 15% of cases in children have a history of prior exposure to chemotherapy.29 An increasing number of cases are being recognized in adults.30 The TFE3 or TFEB gene rearrangement in these tumors (Table 2) results in an overexpression of the corresponding protein that can be detected by immunohistochemistry, a relatively specific assay that is difficult to standardize in a routine laboratory setting.31 Fluorescent in situ hybridization assays for TFE3 and TFEB translocations have also been developed.32,33 With the increased use of these ancillary tools in daily pathologic diagnosis, we are recognizing an expanding morphologic spectrum for this entity.18
CDC is a rare and aggressive subtype of RCC that likely originates from cells of the collecting ducts of renal medulla.34 The histologic spectrum and diagnostic features of this entity are not entirely specific, which also leads to difficulty in molecular characterization. It is noteworthy that HLRCC-associated RCCs not infrequently show the histology that fulfills the morphologic criteria of CDC.20,35
Renal medullary carcinoma is a rare and distinctive entity occurring almost exclusively in young patients with sickle cell trait or rarely other hemoglobinopathies.36 The loss of nuclear expression of SMARCB1 (INI1/BAF47) protein is a consistent finding in renal medullary carcinoma.37-39 Translocations disrupting SMARCB1 gene and homozygous deletion have recently been reported.40,41
Mucinous tubular spindle cell carcinoma, tubulocystic carcinoma, and acquired cystic disease–associated RCC are rare entities with distinctive morphologic features. Among these, the molecular features of mucinous tubular spindle cell carcinoma have been better elucidated.42,43
Diagnostic Challenges
There are some challenging areas for practicing pathologists to apply the current histologic classification of RCC. One frequently encountered issue is the pink/oncocytic tumor conundrum. Among the established common entities, benign oncocytoma and eosinophilic variants of chRCC are the two main differential diagnoses to be considered. However, a significant portion of oncocytic tumors seen in surgical specimens do not meet the standard morphologic criteria of either. Even with the recognition of patients with Birt-Hogg-Dubé syndrome,44,45 renal oncocytosis,46 clinical suspicion of tuberous sclerosis complex,47,48 and succinate dehydrogenase–deficient RCC or TFEB tumors resembling oncocytoma, we still routinely face renal oncocytic tumors with relatively low-grade nuclei that do not fit into any of the established classifications. Although the term hybrid tumor has been used by some, its meaning in a sporadic setting remains unclear. Because of these cases, many pathologists refrain from giving a definitive diagnosis, particularly when biopsy material is limited. Efforts to identify characteristic morphologic and molecular features, establish their clinical behavior, and develop ancillary tests are urgently needed for these tumors.
For nccRCCs with high-grade histologic features and aggressive clinical behaviors, we have made some progress in recent years, such as the development of reliable diagnostic markers for MiT family translocation RCC and HLRCC-associated RCC and the recognition of their wide morphologic spectrum.18,20,49-51 However, the distinction of and definition of high-grade pRCC and CDC remain controversial and frequently lead to confusion and inconsistent reporting between institutions. Given the poor outcomes and lack of standard therapy for these patients, identifying important oncogenic pathways and predictive markers to stratify patients for management or identifying those that may lead to novel treatment options should be the priority. Because of these concerns, we favor assigning high-grade nccRCCs that do not show the typical clinicopathologic presentations of the establish subtypes to the uRCC category instead of forcing them into an existing subtype. A recent molecular analysis of 62 high-grade uRCC primary tumors identified recurrent somatic mutations in 29 genes including NF2 (18%), SETD2 (18%), BAP1 (13%), KMT2C (10%), and MTOR (8%), revealing distinct molecular subsets with diagnostic and therapeutic implications.52
PREDICTIVE BIOMARKERS FOR RCC: A PRESSING NEED
Our improved understanding of the molecular pathology of RCC over the past few decades has led to the discovery of new treatments for advanced kidney cancer. The identification of the central role played by the dysregulation of the VHL/HIF pathway and the activation of mammalian target of rapamycin (mTOR) signaling in ccRCC5,9,53-55 provided a scientific rationale for developing vascular endothelial growth factor (VEGF)–targeted therapies (bevacizumab, sorafenib, sunitinib, pazopanib, axitinib, and cabozantinib) and mTOR inhibitors (temsirolimus and everolimus) in this tumor type. Recently, the demonstration that tumors can exploit immune-inhibitory signals (immune checkpoints) to evade the immune system56 has led to the development of novel immunotherapeutic agents targeting the cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed cell death protein 1 (PD-1) pathways.57,58 In patients with previously treated metastatic ccRCC (mccRCC), the anti-PD-1 antibody nivolumab produced improved overall survival (OS) and fewer serious adverse events than everolimus, leading to its approval by the US Food and Drug Administration.59 Moreover, the combination of nivolumab plus ipilimumab (anti-CTLA-4) recently showed improved clinical benefit compared with sunitinib in treatment-naïve patients with mccRCC and is now approved by Food and Drug Administration for the treatment of intermediate or poor risk previously untreated patients.60
Despite these important advances, only a minority of patients with metastatic RCC (mRCC) who receive either targeted agents or immune checkpoint inhibitors respond to a given treatment. The availability of multiple therapeutic approaches that target different molecular pathways and possibly benefit different subgroups of patients requires the identification of robust predictive biomarkers that can help clinicians select optimal treatment for individual patients. Here, we discuss potential tissue-based biomarkers of response to VEGF-targeted therapies and checkpoint inhibitors in RCC. It should be noted that, to date, most biomarkers discovery efforts have focused on ccRCC, and nccRCC subtypes remain largely understudied.
Tissue Biomarkers for VEGF-Targeted Therapies
Because VHL inactivation leads to the upregulation of several proangiogenic HIF targets (eg, VEGF, platelet-derived growth factor [PDGF]), various studies have investigated the role of members of the pVHL-HIF pathway as potential biomarkers of response to VEGF-targeted therapy in ccRCC. These included VHL gene mutations, expression of HIF-1α and HIF-2α, expression of HIF targets (eg CA-IX), and angiogenesis-related markers.7,61-65 Overall, such analyses have produced either negative or largely conflicting results, possibly because of the limited size of the analyzed patient cohorts, differences in experimental protocols, interobserver variability, or other technical issues. Thus, larger independent and more controlled studies are needed to further clarify the significance of these findings.
Although these older studies mostly relied on single-gene sequencing and single-marker immunohistochemical stains, the recent implementation of new sequencing technologies has provided a platform for large-scale biomarker discovery. Hsieh and colleagues66 evaluated the association between somatic gene mutations and treatment outcomes in a randomized trial comparing first-line sunitinib with everolimus in patients with mRCC (RECORD-3; Efficacy and Safety Comparison of RAD001 Versus Sunitinib in the First-line and Second-line Treatment of Patients With Metastatic Renal Cell Carcinoma). In the everolimus arm, tumor PBRM1 mutations were shown to be associated with a longer progression-free survival (PFS), whereas in the sunitinib arm, longer PFS correlated with KDM5C mutations. Later, a post hoc analysis from the phase III COMPARZ (Pazopanib Versus Sunitinib in the Treatment of Locally Advanced and/or Metastatic Renal Cell Carcinoma) trial in patients with treatment-naïve advanced or mccRCC found that those with PBRM1 mutant tumors had significantly improved OS and PFS compared with the PBRM1 nonmutant group.67 In line with these findings, in the phase II IMmotion 150 (A Study of Atezolizumab [an Engineered Anti-Programmed Death-Ligand 1 (PD-L1) Antibody] as Monotherapy or in Combination With Bevacizumab [Avastin] Compared to Sunitinib [Sutent] in Participants With Untreated Advanced Renal Cell Carcinoma) trial in treatment-naïve mccRCC patients, PBRM1 mutations were associated with improved PFS in the sunitinib-treated group but not in the other treatment groups.68 The potential role of PBRM1 mutations as a predictive biomarker for VEGF-targeted therapy is intriguing because results from two recent independent investigations have shown that inactivation of PBRM1, which codes for BAF180 (a subunit of the PBAF subtype of the switch-sucrose nonfermentable chromatin remodeling complex), enhances HIF signaling in human and murine VHL-deficient ccRCC cells and tumors.69,70 Because PBRM1 mutations tend to co-occur with VHL alterations in human ccRCC,5,11 it is conceivable that tumors characterized by inactivation of both genes display high dependency on HIF signaling and are therefore more sensitive to antiangiogenic therapies directed against the HIF target VEGF.
Large-scale transcriptome profiling of pretreatment ccRCC tissues has contributed to the recent identification of transcriptional signatures that might be useful in predicting clinical benefit from VEGF-targeted agents. In an exploratory analysis of the IMmotion 150 trial, the expression of six angiogenesis-associated genes was identified as a potential predictive marker of response to sunitinib.68 A different angiogenesis gene signature was found to be associated with longer PFS and OS in patients treated with first-line sunitinib or pazopanib as part of the COMPARZ trial.67 The predictive value of PBRM1 mutation and angiogenic gene signatures needs to be independently validated in the context of large controlled clinical trials.
Tissue Biomarkers for Immune Checkpoint Inhibitors
In mRCC, the PD-1 inhibitor nivolumab has been recently approved by the Food and Drug Administration for treating patients for whom previous therapy has failed. Other immune checkpoint inhibitors, including the anti-PD-1 pembrolizumab and the anti-programmed death-ligand 1 (anit-PD-L1) atezolizumab are still under development.
Compared with everolimus, nivolumab improves OS in mccRCC, but only a subset of patients (20% to 25%) respond to this agent. The expression of PD-L1 in tumor cells (TCs) assessed by immunohistochemistry increases the likelihood of benefit with nivolumab in nonsquamous non–small-cell lung cancer71 but fails to identify responders reliably enough to guide clinical decision making in other cancers, including RCC.59,72,73 In the phase II dose-finding study of nivolumab in patients with mccRCC (CA209-010; BMS-936558 [MDX-1106] in Subjects With Advanced/Metastatic Clear-Cell Renal Cell Carcinoma), improvements in median PFS and overall response rate were observed in patients with TC surface PD-L1 expression but they failed to reach statistical significance.74 This result was promising, especially because TC PD-L1 expression is a well-established marker of poor clinical outcome in ccRCC.75 Nonetheless, in the phase III trial comparing nivolumab to everolimus (CheckMate 025; Study of Nivolumab [BMS-936558] vs. Everolimus in Pre-Treated Advanced or Metastatic Clear-cell Renal Cell Carcinoma), no association between PD-L1 expression and improved OS on nivolumab was found after a minimum follow-up of 14 months.59
There are several possible biologic explanations for the lack of a strong association between TC PD-L1 expression and benefit from single-agent nivolumab in ccRCC. Some ccRCCs with no detectable PD-L1 expression in TCs could respond to PD-1 blockade because in these tumors, PD-1–mediated inhibitory signals might be driven by the expression of PD-L1 in immune cells or by the expression of the second PD-1 ligand, PD-L2, rather than PD-L1. Alternatively, some PD-L1–positive ccRCCs could fail to respond to PD-1 inhibitors because their microenvironment might contain highly exhausted tumor infiltrating lymphocytes that express multiple immune checkpoint receptors (eg, PD-1, TIM-3, LAG-3) that are resistant to single checkpoint blockade. Such hypotheses are being tested in preclinical experiments and by analyzing pretreatment patient samples.76
The limited predictive value of TC PD-L1 for nivolumab in ccRCC might also be partly explained by the high level of intratumoral heterogeneity that characterizes this tumor type.77 Callea and colleagues78 recently studied a set of paired primary ccRCCs and metastases and found that in a substantial percentage of cases (approximately 20%), there was discordant TC PD-L1 expression between the primary tumor and the corresponding metastatic lesion(s). Because in RCC PD-L1 levels are commonly assessed in primary tumors, the results of these analyses might not accurately estimate PD-L1 expression in metastases, which are the target of systemic therapy. Consequently, a more accurate assessment of TC PD-L1 as a predictive biomarker for PD-1 blockade in mccRCC may require the study of metastatic lesions.
In the recent phase III trial comparing nivolumab plus ipilimumab with sunitinib (CheckMate 214; Nivolumab Combined With Ipilimumab Versus Sunitinib in Previously Untreated Advanced or Metastatic Renal Cell Carcinoma), longer PFS was observed with nivolumab plus ipilimumab than with sunitinib among patients with positive TC PD-L1 expression but not among those with negative TC PD-L1 expression.60 Validation studies are needed to confirm that TC PD-L1 expression represents a valuable predictive biomarker for the nivolumab-ipilimumab combination in RCC.
To identify potential genomic determinants of response to nivolumab, Miao et al79 recently analyzed whole exome sequencing of pretreatment tumors from patients with ccRCC treated with anti-PD-1/PD-L1 therapy alone or in combination with anti-CTLA-4. Loss-of-function somatic mutations in PBRM1 were found to be significantly more common in patients who experienced clinical benefit from checkpoint inhibitors compared with patients who had no clinical benefit. The investigators also showed that PBAF-deficient ccRCC cell lines had distinct immune-related gene expression profiles compared with PBAF-proficient ones, suggesting that PBRM1 loss in ccRCC may alter global TC expression in a way that affects tumor immune interactions and response to immunotherapy.
In conclusion, the histologic classification of RCC will likely evolve further as our understanding of the underlying molecular mechanisms continues to improve. Recent investigations have identified several emerging tissue-based predictive biomarkers in mccRCC. The clinical utility of these candidate biomarkers now needs to be validated either within prospective biomarker-driven clinical trials or through the analysis of banked specimens from large randomized efficacy trials. The collection of high-quality tumor specimens that are representative of the disease treated in patients will be key to the success of the validation process.
Future efforts should be directed toward discovering potential biomarkers of response for metastatic nccRCC. The main challenge is represented by the highly heterogeneous nature of these tumors. Correlative studies performed within ongoing clinical trials for patients with nccRCC represent a unique opportunity to advance our knowledge in this challenging area.
Footnotes
Supported by the National Institutes of Health (NIH)/National Cancer Institute (NCI) Dana-Farber/Harvard Cancer Center Kidney Cancer Specialized Programs of Research Excellence Grant No. P50 CA101942 (S.S.), the NIH/NCI Memorial Sloan Kettering Cancer Center Support Grant No. P30 CA008748 (Y.B.C. and V.E.R.), and the Society of Memorial Sloan Kettering (Y.B.C.).
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Renal Cell Carcinoma in the Era of Precision Medicine: From Molecular Pathology to Tissue-Based Biomarkers
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Sabina Signoretti
Consulting or Advisory Role: AstraZeneca/MedImmune, Merck, American Association for Cancer Research, National Cancer Institute
Research Funding: AstraZeneca (Inst), Exelixis (Inst), Bristol-Myers Squibb (Inst)
Patents, Royalties, Other Intellectual Property: Royalties for CDX2 antibody from Biogenex Laboratories
Abdallah Flaifel
No relationship to disclose
Ying-Bei Chen
No relationship to disclose
Victor E. Reuter
No relationship to disclose
REFERENCES
- 1.Kovacs G, Akhtar M, Beckwith BJ, et al. : The Heidelberg classification of renal cell tumours. J Pathol 183:131-133, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Eble JN, Sauter G, Epstein JI, et al. WHO Classification of Tumours of the Urinary System and Male Genital Organs (ed3). Lyon, France, IARC Press, 2004 [Google Scholar]
- 3.Moch H, Humphrey PA, Ulbright TM, et al. WHO Classification of Tumours of the Urinary System and Male Genital Organs (ed 4). Lyon, France, IARC, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Reuter VE, Tickoo SK: Differential diagnosis of renal tumours with clear cell histology. Pathology 42:374-383, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network : Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499:43-49, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato Y, Yoshizato T, Shiraishi Y, et al. : Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet 45:860-867, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Choueiri TK, Fay AP, Gagnon R, et al. : The role of aberrant VHL/HIF pathway elements in predicting clinical outcome to pazopanib therapy in patients with metastatic clear-cell renal cell carcinoma. Clin Cancer Res 19:5218-5226, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaelin WG, Jr: Treatment of kidney cancer: Insights provided by the VHL tumor-suppressor protein. Cancer 115:2262-2272, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Kaelin WG, Jr: The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer 8:865-873, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Dalgliesh GL, Furge K, Greenman C, et al. : Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature 463:360-363, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varela I, Tarpey P, Raine K, et al. : Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 469:539-542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A, et al. : BAP1 loss defines a new class of renal cell carcinoma. Nat Genet 44:751-759, 2012. [Erratum: Nat Genet 44:1072, 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hakimi AA, Chen YB, Wren J, et al. : Clinical and pathologic impact of select chromatin-modulating tumor suppressors in clear cell renal cell carcinoma. Eur Urol 63:848-854, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hakimi AA, Ostrovnaya I, Reva B, et al. : Adverse outcomes in clear cell renal cell carcinoma with mutations of 3p21 epigenetic regulators BAP1 and SETD2: A report by MSKCC and the KIRC TCGA research network. Clin Cancer Res 19:3259-3267, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapur P, Peña-Llopis S, Christie A, et al. : Effects on survival of BAP1 and PBRM1 mutations in sporadic clear-cell renal-cell carcinoma: A retrospective analysis with independent validation. Lancet Oncol 14:159-167, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho TH, Kapur P, Joseph RW, et al. : Loss of histone H3 lysine 36 trimethylation is associated with an increased risk of renal cell carcinoma-specific death. Mod Pathol 29:34-42, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hakimi AA, Tickoo SK, Jacobsen A, et al. : TCEB1-mutated renal cell carcinoma: A distinct genomic and morphological subtype. Mod Pathol 28:845-853, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Argani P: MiT family translocation renal cell carcinoma. Semin Diagn Pathol 32:103-113, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Merino MJ, Torres-Cabala C, Pinto P, et al. : The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol 31:1578-1585, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Chen YB, Brannon AR, Toubaji A, et al. : Hereditary leiomyomatosis and renal cell carcinoma syndrome-associated renal cancer: Recognition of the syndrome by pathologic features and the utility of detecting aberrant succination by immunohistochemistry. Am J Surg Pathol 38:627-637, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill AJ, Pachter NS, Chou A, et al. : Renal tumors associated with germline SDHB mutation show distinctive morphology. Am J Surg Pathol 35:1578-1585, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Gill AJ, Hes O, Papathomas T, et al. : Succinate dehydrogenase (SDH)-deficient renal carcinoma: A morphologically distinct entity—A clinicopathologic series of 36 tumors from 27 patients. Am J Surg Pathol 38:1588-1602, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cancer Genome Atlas Research Network. Linehan WM, Spellman PT, et al. : Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med 374:135-145, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt L, Junker K, Nakaigawa N, et al. : Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene 18:2343-2350, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Kovacs A, Kovacs G: Low chromosome number in chromophobe renal cell carcinomas. Genes Chromosomes Cancer 4:267-268, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Speicher MR, Schoell B, du Manoir S, et al. : Specific loss of chromosomes 1, 2, 6, 10, 13, 17, and 21 in chromophobe renal cell carcinomas revealed by comparative genomic hybridization. Am J Pathol 145:356-364, 1994 [PMC free article] [PubMed] [Google Scholar]
- 27.Schwerdtle RF, Störkel S, Neuhaus C, et al. : Allelic losses at chromosomes 1p, 2p, 6p, 10p, 13q, 17p, and 21q significantly correlate with the chromophobe subtype of renal cell carcinoma. Cancer Res 56:2927-2930, 1996. [Retraction: Cancer Res 1999]https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8674042&dopt=Abstract [PubMed] [Google Scholar]
- 28.Davis CF, Ricketts CJ, Wang M, et al. : The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell 26:319-330, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Argani P, Laé M, Ballard ET, et al. : Translocation carcinomas of the kidney after chemotherapy in childhood. J Clin Oncol 24:1529-1534, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Argani P, Olgac S, Tickoo SK, et al. : Xp11 translocation renal cell carcinoma in adults: Expanded clinical, pathologic, and genetic spectrum. Am J Surg Pathol 31:1149-1160, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Argani P, Lal P, Hutchinson B, et al. : Aberrant nuclear immunoreactivity for TFE3 in neoplasms with TFE3 gene fusions: A sensitive and specific immunohistochemical assay. Am J Surg Pathol 27:750-761, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Zhong M, De Angelo P, Osborne L, et al. : Dual-color, break-apart FISH assay on paraffin-embedded tissues as an adjunct to diagnosis of Xp11 translocation renal cell carcinoma and alveolar soft part sarcoma. Am J Surg Pathol 34:757-766, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pecciarini L, Cangi MG, Lo Cunsolo C, et al. : Characterization of t(6;11)(p21;q12) in a renal-cell carcinoma of an adult patient. Genes Chromosomes Cancer 46:419-426, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Srigley JR, Eble JN: Collecting duct carcinoma of kidney. Semin Diagn Pathol 15:54-67, 1998 [PubMed] [Google Scholar]
- 35.Ohe C, Smith SC, Sirohi D, et al. : Reappraisal of morphologic differences between renal medullary carcinoma, collecting duct carcinoma, and fumarate hydratase-deficient renal cell carcinoma. Am J Surg Pathol 42:279-292, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis CJ, Jr, Mostofi FK, Sesterhenn IA: Renal medullary carcinoma: The seventh sickle cell nephropathy. Am J Surg Pathol 19:1-11, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Cheng JX, Tretiakova M, Gong C, et al. : Renal medullary carcinoma: Rhabdoid features and the absence of INI1 expression as markers of aggressive behavior. Mod Pathol 21:647-652, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Calderaro J, Moroch J, Pierron G, et al. : SMARCB1/INI1 inactivation in renal medullary carcinoma. Histopathology 61:428-435, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Liu Q, Galli S, Srinivasan R, et al. : Renal medullary carcinoma: Molecular, immunohistochemistry, and morphologic correlation. Am J Surg Pathol 37:368-374, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calderaro J, Masliah-Planchon J, Richer W, et al. : Balanced translocations disrupting SMARCB1 are hallmark recurrent genetic alterations in renal medullary carcinomas. Eur Urol 69:1055-1061, 2016 [DOI] [PubMed] [Google Scholar]
- 41.Carlo MI, Chaim J, Patil S, et al. : Genomic characterization of renal medullary carcinoma and treatment outcomes. Clin Genitourin Cancer 15:e987-e994, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehra R, Vats P, Cieslik M, et al. : Biallelic alteration and dysregulation of the hippo pathway in mucinous tubular and spindle cell carcinoma of the kidney. Cancer Discov 6:1258-1266, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren Q, Wang L, Al-Ahmadie HA, et al. : Distinct genomic copy number alterations distinguish mucinous tubular and spindle cell carcinoma of the kidney from papillary renal cell carcinoma with overlapping histologic features. Am J Surg Pathol 42:767-777, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pavlovich CP, Walther MM, Eyler RA, et al. : Renal tumors in the Birt-Hogg-Dubé syndrome. Am J Surg Pathol 26:1542-1552, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Furuya M, Yao M, Tanaka R, et al. : Genetic, epidemiologic and clinicopathologic studies of Japanese Asian patients with Birt-Hogg-Dubé syndrome. Clin Genet 90:403-412, 2016 [DOI] [PubMed] [Google Scholar]
- 46.Tickoo SK, Reuter VE, Amin MB, et al. : Renal oncocytosis: A morphologic study of fourteen cases. Am J Surg Pathol 23:1094-1101, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Guo J, Tretiakova MS, Troxell ML, et al. : Tuberous sclerosis-associated renal cell carcinoma: A clinicopathologic study of 57 separate carcinomas in 18 patients. Am J Surg Pathol 38:1457-1467, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Yang P, Cornejo KM, Sadow PM, et al. : Renal cell carcinoma in tuberous sclerosis complex. Am J Surg Pathol 38:895-909, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bardella C, El-Bahrawy M, Frizzell N, et al. : Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status. J Pathol 225:4-11, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Trpkov K, Hes O, Agaimy A, et al. : Fumarate hydratase-deficient renal cell carcinoma is strongly correlated with fumarate hydratase mutation and hereditary leiomyomatosis and renal cell carcinoma syndrome. Am J Surg Pathol 40:865-875, 2016 [DOI] [PubMed] [Google Scholar]
- 51.Smith SC, Trpkov K, Chen YB, et al. : Tubulocystic carcinoma of the kidney with poorly differentiated foci: A frequent morphologic pattern of fumarate hydratase-deficient renal cell carcinoma. Am J Surg Pathol 40:1457-1472, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen YB, Xu J, Skanderup AJ, et al. : Molecular analysis of aggressive renal cell carcinoma with unclassified histology reveals distinct subsets. Nat Commun 7:13131, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gnarra JR, Tory K, Weng Y, et al. : Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet 7:85-90, 1994 [DOI] [PubMed] [Google Scholar]
- 54.Xu J, Pham CG, Albanese SK, et al. : Mechanistically distinct cancer-associated mTOR activation clusters predict sensitivity to rapamycin. J Clin Invest 126:3526-3540, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsieh JJ, Purdue MP, Signoretti S, et al. : Renal cell carcinoma. Nat Rev Dis Primers 3:17009, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pardoll DM: The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252-264, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Topalian SL, Hodi FS, Brahmer JR, et al. : Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443-2454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hodi FS, O’Day SJ, McDermott DF, et al. : Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711-723, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Motzer RJ, Escudier B, McDermott DF, et al. : Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803-1813, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Motzer RJ, Tannir NM, McDermott DF, et al. : Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277-1290, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choueiri TK, Vaziri SA, Jaeger E, et al. : von Hippel-Lindau gene status and response to vascular endothelial growth factor targeted therapy for metastatic clear cell renal cell carcinoma. J Urol 180:860-865, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Peña C, Lathia C, Shan M, et al. : Biomarkers predicting outcome in patients with advanced renal cell carcinoma: Results from sorafenib phase III Treatment Approaches in Renal Cancer Global Evaluation Trial. Clin Cancer Res 16:4853-4863, 2010 [DOI] [PubMed] [Google Scholar]
- 63.Terakawa T, Miyake H, Kusuda Y, et al. : Expression level of vascular endothelial growth factor receptor-2 in radical nephrectomy specimens as a prognostic predictor in patients with metastatic renal cell carcinoma treated with sunitinib. Urol Oncol 31:493-498, 2013 [DOI] [PubMed] [Google Scholar]
- 64.Choueiri TK, Cheng S, Qu AQ, et al. : Carbonic anhydrase IX as a potential biomarker of efficacy in metastatic clear-cell renal cell carcinoma patients receiving sorafenib or placebo: Analysis from the Treatment Approaches in Renal Cancer Global Evaluation Trial (TARGET). Urol Oncol 31:1788-1793, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zucca LE, Morini Matushita MA, da Silva Oliveira RJ, et al. : Expression of tyrosine kinase receptor AXL is associated with worse outcome of metastatic renal cell carcinomas treated with sunitinib. Urol Oncol 36:11.e13-11.e21, 2018 [DOI] [PubMed] [Google Scholar]
- 66.Hsieh JJ, Chen D, Wang PI, et al. : Genomic biomarkers of a randomized trial comparing first-line everolimus and sunitinib in patients with metastatic renal cell carcinoma. Eur Urol 71:405-414, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Voss MH, Kuo F, Chen D, et al. : Integrated biomarker analysis for 412 renal cell cancer (RCC) patients (pts) treated on the phase 3 COMPARZ trial: Correlating common mutation events in PBRM1 and BAP1 with angiogenesis expression signatures and outcomes on tyrosine kinase inhibitor (TKI) therapy. J Clin Oncol 35, 2017. (suppl; abstr 4523) [Google Scholar]
- 68.McDermott DF, Huseni MA, Atkins MB, et al. : Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 24:749-757, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao W, Li W, Xiao T, et al. : Inactivation of the PBRM1 tumor suppressor gene amplifies the HIF-response in VHL-/- clear cell renal carcinoma. Proc Natl Acad Sci USA 114:1027-1032, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nargund AM, Pham CG, Dong Y, et al. : The SWI/SNF Protein PBRM1 Restrains VHL-Loss-Driven Clear Cell Renal Cell Carcinoma. Cell Reports 18:2893-2906, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Borghaei H, Paz-Ares L, Horn L, et al. : Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627-1639, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferris RL, Blumenschein G, Jr, Fayette J, et al. : Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375:1856-1867, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma P, Retz M, Siefker-Radtke A, et al. : Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol 18:312-322, 2017 [DOI] [PubMed] [Google Scholar]
- 74.Motzer RJ, Rini BI, McDermott DF, et al. : Nivolumab for metastatic renal cell carcinoma: Results of a randomized phase II trial. J Clin Oncol 33:1430-1437, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thompson RH, Dong H, Kwon ED: Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res 13:709s-715s, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Pignon JC, Jegede O, Horak C, et al. : Evaluation of predictive biomarkers for nivolumab in metastatic clear cell renal cell carcinoma (mccRCC) using RECIST and immune-related (IR) RECIST. J Clin Oncol 36, 2018. (suppl; abstr 619) [Google Scholar]
- 77.Gerlinger M, Rowan AJ, Horswell S, et al. : Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366:883-892, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Callea M, Albiges L, Gupta M, et al. : Differential expression of PD-L1 between primary and metastatic sites in clear-cell renal cell carcinoma. Cancer Immunol Res 3:1158-1164, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miao D, Margolis CA, Gao W, et al. : Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 359:801-806, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]