Abstract
Clear cell renal cell carcinoma (ccRCC) is the most common renal cell carcinoma subtype, and metastatic ccRCC is associated with 5-year survival rates of 10% to 20%. Genetically, ccRCC originates from sequential losses of multiple tumor suppressor genes. Remarkably, chromosome 3p loss occurs in more than 90% of sporadic ccRCCs. This results in concurrent one-copy loss of four tumor suppressor genes that are also mutated individually at high frequency in ccRCC (ie, VHL, 80%; PBRM1, 29% to 46%; BAP1, 6% to 19%; and SETD2, 8% to 30%). Pathogenically, 3p loss probably represents the first genetic event that occurs in sporadic ccRCC and the second genetic event in VHL-mutated hereditary ccRCC. VHL constitutes the substrate recognition module of the VCB-Cul2 E3 ligase that degrades HIF1/2α, whereas PBRM1, BAP1, and SETD2 are epigenetic modulators that regulate gene transcription. Because 3p loss and VHL inactivation are nearly universal truncal events in ccRCC, the resulting HIF1/2 signaling overdrive and accompanied tumor hypervascularization probably underlie the therapeutic benefits observed with vascular endothelial growth factor receptor inhibitors, including sorafenib, sunitinib, pazopanib, axitinib, bevacizumab, cabozantinib, and lenvatinib. Furthermore, recent marked advances in ccRCC genomics, transcriptomics, proteomics, metabolomics, molecular mechanisms, mouse models, prognostic and predictive biomarkers, and clinical trials have rendered invaluable translational insights concerning precision kidney cancer therapeutics. With an armamentarium encompassing 13 drugs that exploit seven unique therapeutic mechanisms (ie, cytokines, vascular endothelial growth factor receptor, mTORC1, cMET/AXL, fibroblast growth factor receptor, programmed cell death-1 and programmed death-ligand 1, and cytotoxic T-cell lymphocyte associated-4) to treat metastatic renal cell carcinoma, one of the imminent clinical questions concerning care of patients with metastatic ccRCC is how a personalized treatment strategy, through rationally combining and sequencing different therapeutic modalities, can be formulated to offer the best clinical outcome for individual patients. Here, we attempt to integrate recent discoveries of immediate translational impacts and discuss future translational challenges and opportunities.
INTRODUCTION
Renal cell carcinoma (RCC) encompasses more than 10 different histopathologic subtypes. Clear cell RCC (ccRCC) is most prevalent (approximately 75%) and accounts for approximately 80% of kidney cancer fatalities.1 Kidney cancer treatment has evolved dramatically over the past decade from two (around 2004) to 13 (around 2018) US Food and Drug Administration–approved drugs, representing seven distinct effective treatment mechanisms encompassing targeted and immunotherapeutic agents.1,2 At the same time, basic knowledge concerning ccRCC pathobiology has greatly expanded beyond a single mutation of the von Hippel-Lindau (VHL) tumor suppressor gene (TSG).1,3
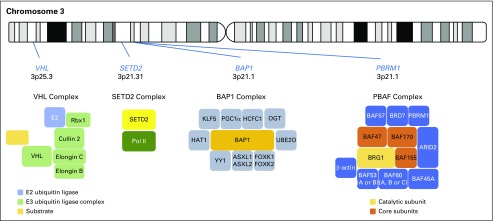
Inactivation of VHL and chromosome 3p loss were the only two known oncogenic driver events in ccRCC before international large-scale cancer genomics sequencing campaigns.4 Sequencing of primary ccRCC tumors demonstrated that VHL (approximately 80%), PBRM1/BAF180 (29% to 46%), SETD2 (8% to 30%), and BAP1 (6% to 19%) are the four most commonly mutated genes.5-8 Strikingly, all four TSGs locate at the short arm of chromosome 3 (Fig 1), and one-copy loss of 3p occurs in more than 90% of ccRCCs. Furthermore, these three newly identified 3p TSGs encode epigenetic regulators that modulate gene transcription. PBRM1 protein containing PBAF complex functions as a chromatin remodeling enzyme; the SETD2 protein functions as histone H3 lysine K36 trimethylase (H3K36TM); and the BAP1 protein functions as a ubiquitin C-terminal hydrolase (Fig 1).9 Mutations in PBRM1, SETD2, and BAP1 were also detected in non–clear cell RCC.10,11
Fig 1.
Chromosome 3p clear cell renal cell carcinoma tumor suppressor genes and their respective protein complexes.
Unlike many cancers that originate from gain-of-function mutations in oncogenes such as EGFR and RAS, ccRCC manifests with prevalent loss-of-function mutations in TSGs, making the discovery of predictive biomarkers challenging.9 Nevertheless, new clinically relevant patient-derived cell line and xenograft models,12,13 novel genetically engineered mouse models,14-16 and informative outcome-based biomarker8 studies have begun to shed light on how seemingly distinct research areas are in fact highly interconnected.3 Here, we wish to update key new translational findings in ccRCC and highlight emerging biomarkers and drugs that could affect future kidney cancer care.
THE VHL-HIF-HYPOXIA-METABOLIC INNER CIRCLE
VHL: VCB-Cullin2 E3 Ligase for HIFα Degradation
The identification of mutations at the VHL gene located at 3p25 as the principal genetic event in both hereditary and somatic ccRCC cemented the quintessential role of VHL in kidney cancer pathobiology.4 VHL is a multipurpose adaptor protein that serves as the substrate recognition module of the VCB (VHL–elongin C–elongin B)-Cul2 E3 ligase that marks hypoxia-inducible factor (HIF)-1α and HIF-2α proteins for degradation.17-19 Of note, a small number of ccRCC tumors that lack VHL mutations have mutations in the TCEB1/ELOC gene that encodes elongin C.6,20
HIFs: Oxygen Sensing, Metabolic Adaptation, Mitochondria, Vascularization, and Tumorigenesis
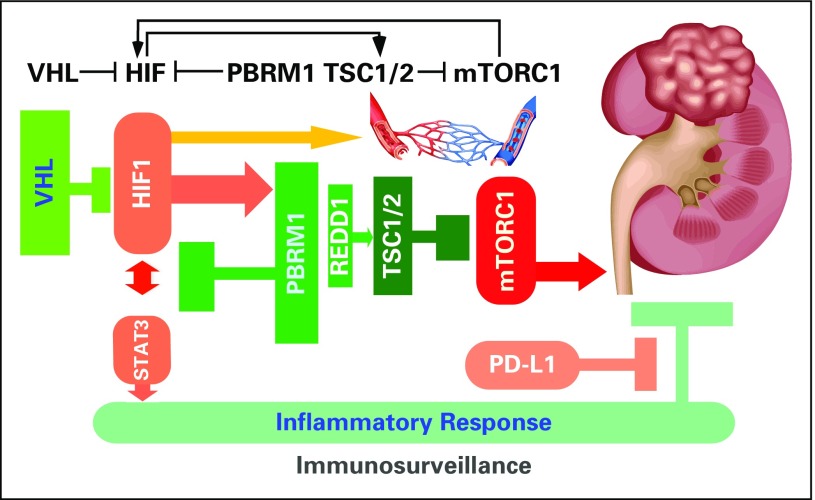
The study of oxygen sensing led to the discovery of the HIF transcription factors.17-19 Under normal oxygen tension, HIFα is prolyl hydroxylated by EGLN, ubiquitinated by VCB-Cul2, and degraded by 26S proteasome.21 Under low oxygen tension such as vessel injury, HIFα is stable and initiates a myriad of hypoxia-specific transcription programs.18 Key effects include increased glycolysis, decreased mitochondrial function, and induced neovascularization (Fig 2).17,18,21 The pathologic loss of VHL in ccRCC results in persistently elevated HIFs, which in turn incurs the classic clear cell morphology and the highly vascular nature observed with ccRCC.1,22,23 However, the long latency period (more than 30 years) between diagnosis of VHL syndrome and development of ccRCC and the insufficiency of VHL loss alone to induce ccRCC in mice argue for the necessity of cooperative events.24 Of note, the mouse model does not perfectly match the human experience because targeted loss of Vhl does not mimic the heterozygous loss of the other 3p genes seen in humans and, even if loss of chromosome 6 in mice that contains VHL was mimicked, the other genes are on different chromosomes, with Pbrm1 and Bap1 being on chromosome 14 and Setd2 on chromosome 9.
Fig 2.
The oncogenes and tumor suppressor genes operate in renal epithelium to control signaling output and prevent tumorigenesis.
HIF-1 Versus HIF-2: Friends or Foes in Tumorigenesis
HIFs are transcription factors consisting of an invariable HIF-1β and a variable, oxygen-sensing HIF-1α, HIF-2α, or HIF-3α that form heterodimeric complexes. HIF-1 and HIF-2 function as transcription activators, whereas HIF-3 functions as a repressor because of its lack of a transactivation domain. Shared and unique transcriptional targets of HIF-1 and HIF-2 have been demonstrated, explaining their common roles in hypoxia-induced gene expression and their distinct biologic outputs.17-19,22 For example, HIF-1 inhibits Myc targets, whereas HIF-2 activates these targets.25 Because cancers commonly contain hypoxic areas, the role of HIF-1 in human cancer progression has been demonstrated in many different cancer types.18 However, in ccRCC where VHL is near universally inactivated, the roles of HIF-1 and HIF-2 in tumorigenesis become perplexing.18,19 In transgenic mouse models, expression of the constitutive active, nondegradable HIF-1α but not HIF-2α mutant in the renal proximal tubule driven by the γ-glutamyl transpeptidase promoter resulted in ccRCC,26,27 whereas in human ccRCC, one-copy loss of the HIF-1α gene through the loss of chromosome 14q and the expression of HIF-2 are associated with clinical progression and poor clinical outcome.28,29 One probable scenario to reconcile these seemingly contradictory findings is that the initial expression of HIF-1 is required for ccRCC initiation and the subsequent expression of HIF-2 facilitates disease progression.
CHROMOSOME 3P PATHOBIOLOGY IN ccRCC
Chromosome 3p: The Powerful TSG Cluster
Before the cancer genomics sequencing era, it was already recognized that 3p loss is the dominating karyotypic abnormality in ccRCC.30 The biologic significance of this event in ccRCC was subsequently confirmed yet restricted to the identification of the VHL gene on 3p254 and subsequently substantiated with the discovery of three additional prevalently mutated genes in ccRCC—PBRM1 (29% to 46%), BAP1 (6% to 19%), and SETD2 (8% to 30%)—all located at 3p21.5,6,8 Hence, four TSGs that play critical roles in maintaining kidney tissue homeostasis become haploinsufficient upon one-copy loss of 3p. Interestingly, in patients with VHL syndrome, 3p loss is the second genetic event,31 whereas in somatic ccRCC, 3p loss is the first event and VHL inactivation is the second event.32 Unlike VHL, which has been studied for decades, the tumor suppressor function of PBRM1, BAP1, and SETD2 in ccRCC was largely unknown. Here, we briefly summarize recent findings and offer a glimpse into how these three epigenetic TSGs might function in suppressing the pathogenesis of ccRCC.
PBRM1
The SWI/SNF complexes are macromolecular protein complexes that use ATP to mobilize nucleosome, modulate chromatin structure, and thereby regulate central cellular, developmental, and oncogenic processes.33 There are many different complexes as a result of their interchangeable, dynamic compositions in nature.34 Notably, mutations of individual SWI/SNF subunits are detected and exhibit preferential enrichment in approximately 20% of human cancers.35 PBRM1 is the defining component of the PBAF complex and is most commonly mutated in ccRCC.36 Remarkably, the in vivo tumor suppressor role of PBRM1 in ccRCC was confirmed and reported in 2017 by three independent laboratories using three different genetically engineered mouse models where combined losses of VHL and PBRM1 led to multifocal ccRCC in mouse kidney, whereas individual losses did not.14-16
BAP1
BAP1 (BRCA1-associated protein) was first identified based on its interaction with BRCA137,38 and was recently found to be mutated in multiple cancer types such as mesothelioma, uveal melanoma, and kidney cancer.39 BAP1 is a ubiquitin C-terminal hydrolase, forms complexes (Fig 1), and regulates key cellular pathways, including the cell cycle, cellular differentiation, cell death, gluconeogenesis, and DNA damage response.39 The loss of BAP1 defines a molecular subtype of ccRCC,40 occurs near mutual exclusively with the loss of PBRM1,40 and portends poor clinical outcome.8,41,42 Genetically, the loss of BAP1 in mice results in ccRCC with aggressive morphologic features.15
SETD2
SETD2 is a histone H3 K36 methyltransferase that regulates chromatin biology and thereby modulates gene transcription and DNA repair.43,44 Intriguingly, SETD2 was recently demonstrated to methylate tubulin for cytoskeleton remodeling and STAT1 for interferon response.45,46 The prevalence of SETD2 mutations in ccRCC increases as kidney cancer progresses,7,8,47 implicating its function in preventing cancer metastasis. Besides ccRCC, SETD2 mutations were also detected in papillary RCC and unclassified RCC.48,49
KIDNEY CANCER EVOLUTION
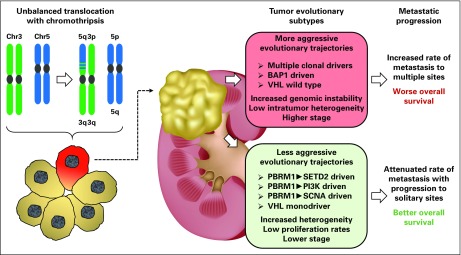
By exploiting integrated multiregional and longitudinal approaches, series of genomic studies demonstrated the presence of conspicuous intratumor heterogeneity in ccRCC through branched evolution (Fig 3).32,47,50,51 Phylogenetic study of clonal evolution of ccRCC discovered that the nearly universal loss of 3p (approximately 90%) and common gain of 5q (approximately 67%) in ccRCC5 mainly result from a single unbalanced translocation incidence involving chromosomes 3 and 5 through t(3;5) chromothripsis events that occurred decades before cancer diagnosis.32 Furthermore, deterministic evolutionary trajectories that influence primary tumor growth were revealed,51 and the hallmark genomic drivers of ccRCC metastasis are loss of 9p and 14q where CDKN2A and HIF-1α reside, respectively.50
Fig 3.
Recent analysis using multiregional whole-genome and whole-exome sequencing in a cohort of 100 patients with clear cell renal cell carcinoma (ccRCC) has elucidated a model of tumor evolution for ccRCC. The earliest genetic alteration in the tumorigenic evolution of a sporadic ccRCC was shown to be loss of chromosome 3p, with the most frequent mechanism being an unbalanced translocation resulting in simultaneous loss of chromosome 3p and gain of chromosome 5q with chromothripsis at the breakpoint. This early tumor-initiating cell subsequently develops further genetic alterations to become a ccRCC, and two different groups of evolutionary trajectories were identified based on the sequential gain-of-mutation events, the degree of intratumor heterogeneity, and level of genomic instability. More aggressive evolutionary trajectories were associated with more rapid metastasis to multiple distance sites and poorer overall survival.
FUTURE DIRECTIONS
Emerging Biomarkers
The singular loss of VHL is insufficient for initiating ccRCC, which is recognized in both mouse and human VHL loss models.52 The ensuing complete loss of PBRM1 leads to the increased transcription output of HIF and STAT targets (Fig 2).14 Consequently, the dysregulated interplay between HIF and STAT upon combined losses of VHL and PBRM1 creates a feed-forward amplification loop that maximizes downstream gene expression.14 Because STATs are key transcription factors in cancer inflammation and immunity,53 the activation of the STAT3 pathway as a result of the combined loss of VHL and PBRM1 could render the resulting tumor prone to immune regulation.14 Recent approval of single-agent nivolumab (anti–programmed cell death-1 [PD-1] antibody) as second-line therapy and of the combination of ipilimumab (anti–cytotoxic T-cell lymphocyte associated-4 antibody) and nivolumab as first-line therapy for ccRCC has dramatically altered the therapeutic landscape of metastatic kidney cancer.2,54 Intriguingly, a recent publication identified PBRM1 loss as a potential genomic biomarker for the treatment response to these immune checkpoint inhibitors.55 Altogether, these mechanistic, mouse, and human ccRCC studies implicate a working hypothesis in which the disarmed STAT-PD-1 immunosurveillance can be reactivated through biologic means such as anti–PD-1 and anti–programmed death-ligand 1 (PD-L1) antibodies for therapeutic exploitation (Fig 2). Because PBRM1-mutant tumors benefited most from vascular endothelial growth factor receptor (VEGFR) inhibitor treatment, as shown in the RECORD-3 trial comparing sunitinib and everolimus,8 the lack of association between PBRM1 mutations and single-agent therapeutic benefit from the anti–PD-L1 agent atezolizumab, as reported in the IMmotion150 trial comparing atezolizumab and sunitinib, is not totally unexpected. These findings further affirm disrupted VHL-HIF biology as the first quintessential event in ccRCC pathobiology and warrant additional confirmation and refinement with large data sets.
Despite active surveillance after nephrectomy, one third of patients with RCC experience disease progression.1 Although prognostic clinicopathologic models can predict survival of patients after surgery, a subset of patients (10%) characterized as low risk ultimately die of RCC.56 Epigenetic subclassifications of ccRCC offer an opportunity to improve upon clinicopathologic models. Immunohistochemistry (IHC) loss of intranuclear H3K36me3, a surrogate of SETD2 loss-of-function mutations, is associated with a two-fold increased risk of RCC-specific death in low-risk tumors.57 Similarly, loss of BAP1 expression by IHC is associated with a three-fold increased risk of RCC-specific death in low-risk tumors.58 Consistent with preclinical models showing increased enhancer of zeste homolog 2 (EZH2) activity during ccRCC invasion and metastases, increased expression of EZH2 by IHC is associated with a six-fold increased risk of RCC-specific death in low-risk tumors.59 Given the increasing incidence of small tumors (4 cm or less) as a result, in part, of the increased use of abdominal imaging, these small tumors, which are currently excluded from adjuvant trials, underscore a need to better identify patients with a low-risk prognostic score who have a higher risk of recurrence.
Emerging Therapeutics
With 13 approved drugs exploiting seven effective mechanisms in treating metastatic RCC, the field is quickly moving toward combination trials consisting of one VEGFR inhibitor and one PD-1 or PD-L1 inhibitor. The first success of such a strategy was reported in the large randomized phase II IMmotion150 trial, which revolutionized treatment of metastatic ccRCC paradigm from single-agent therapy with the VEGFR inhibitor bevacizumab or the anti–PD-1 and anti–PD-L1 agent atezolizumab to a combination of two distinct effective mechanisms.60 Because all single-agent VEGFR blockade strategies inevitably encounter resistance and the role of HIF-2 activity in ccRCC aggressiveness has been demonstrated in preclinical and clinical studies, the development of small-molecule inhibitors specifically targeting HIF-2α and HIF-β, including PT2399 and PT2385, is of clinical significance. In preclinical ccRCC studies, PT2399 exhibited on-target effects in cell-based and xenograft assays,61,62 and in a phase I clinical trial, PT2385 exhibited a favorable safety profile and activity in patients with heavily pretreated ccRCC.63 Hence, agents exploiting additional mechanisms of action such as HIF-2 could offer novel treatment modalities for effective combination therapeutic strategies in the future.
APPLIED PATHOLOGY OF CCRCC
Molecular Pathology to Incorporate Multiomics With Histopathology
Recent omic studies have presented an unprecedented, comprehensive molecular understanding of ccRCC pathobiology, which includes genomics, epigenomics, transcriptomics, proteomics, immunogenomics, and metabolomics.5,6,23,40,64 Thus far, molecular subtypes based on genomics and transcriptomics are the best studied and most applicable.3,65,66 At the chromosomal arm aberration level, commonly detected 5q gain, 9p loss, and 14q loss carry clinical significance.28,50 At the somatic mutation level, 18 genes were mutated in more than 5% of patients with in metastatic ccRCC.8
Functional Pathology to Link Molecular Pathology With Clinical Outcome
Successful interconnections between molecular pathologies and clinical outcomes are of paramount significance in translation oncology. Notably, the clinical value of individual biomarkers can be context dependent; for example, PBRM1 mutations detected in localized small ccRCC tumors could be an early indicator for disease progression whereas in advanced ccRCC tumors could be a good prognostic and predictive biomarker.7,41,42 In the targeted therapy era, the most important molecular distinction among metastatic ccRCC is probably mutation status of PBRM1 and BAP1 and chromosome 9p21 loss.8,50 By examining somatic mutations of a large ccRCC next-generation sequencing cohort and comparing first-line sunitinib versus first-line everolimus, we demonstrated that patients with PBRM1 mutation benefited significantly from first-line sunitinib and first-line everolimus with first-line progression-free survival (PFS1L) times of 11.0 and 12.8 months, respectively.8 However, patients with BAP1 mutations derived a less pronounced benefit from first-line sunitinib (PFS1L, 8.1 months) and marginal to no benefit from first-line everolimus (PFS1L, 4.9 months).8 In the metastatic ccRCC setting, PBRM1 mutation is a stronger prognostic marker associated with favorable responses to most of the currently approved treatments including immune checkpoint inhibitors. However, BAP1 mutations are probably responsive to anti-VEGFR tyrosine kinase inhibitors and irresponsive to mTORC1 inhibitors. Besides PBRM1 and BAP1, somatic mutations in SETD2, KDM5C, TSC, PTEN, MTOR, TP53, and TERT have also shown early promise in prognostication.67-70
With the improvement in histopathologic diagnosis, advances in multiomics-based molecular pathologic classification, and emergence of biomarker-centered functional pathology, the oncology community is at the early stage of implementing precision-based cancer care. Hence, we envision that an applied pathology approach integrating mechanisms, animal models, multiomics, clinical trials, and biomarkers could be of value in the future advancement of personalized, precision cancer care.
Footnotes
Supported by National Institutes of Health (NIH) Grant No. R01 CA223231 (J.J.H., V.H.L., T.O., and E.H.C.), a National Cancer Institute intramural grant (C.J.R.), and NIH Grant No. R01 CA224917 (T.H.H.).
AUTHOR CONTRIBUTIONS
Conception and design: James J. Hsieh, Emily H. Cheng
Collection and assembly of data: James J. Hsieh, Valerie H. Le, Toshinao Oyama
Data analysis and interpretation: James J. Hsieh, Christopher J. Ricketts, Thai Huu Ho
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Chromosome 3p Loss–Orchestrated VHL, HIF, and Epigenetic Deregulation in Clear Cell Renal Cell Carcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
James J. Hsieh
Honoraria: Eisai, OncLive Specialty Group
Consulting or Advisory Role: Eisai
Travel, Accommodations, Expenses: Eisai
Valerie H. Le
No relationship to disclose
Toshinao Oyama
No relationship to disclose
Christopher J. Ricketts
No relationship to disclose
Thai Huu Ho
Consulting or Advisory Role: Exelixis, Genentech, Pfizer, Ipsen
Research Funding: Novartis
Emily H. Cheng
Honoraria: Eisai (I), OncLive Specialty Group (I)
Consulting or Advisory Role: Eisai (I)
Travel, Accommodations, Expenses: Eisai (I)
REFERENCES
- 1.Hsieh JJ, Purdue MP, Signoretti S, et al. : Renal cell carcinoma. Nat Rev Dis Primers 3:17009, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Tannir NM, McDermott DF, et al. : Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277-1290, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsieh JJ, Le V, Cao D, et al. : Genomic classifications of renal cell carcinoma: A critical step towards the future application of personalized kidney cancer care with pan-omics precision. J Pathol 244:525-537, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Linehan WM, Lerman MI, Zbar B: Identification of the von Hippel-Lindau (VHL) gene: Its role in renal cancer. JAMA 273:564-570, 1995 [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network : Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499:43-49, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato Y, Yoshizato T, Shiraishi Y, et al. : Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet 45:860-867, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Hakimi AA, Chen YB, Wren J, et al. : Clinical and pathologic impact of select chromatin-modulating tumor suppressors in clear cell renal cell carcinoma. Eur Urol 63:848-854, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh JJ, Chen D, Wang PI, et al. : Genomic biomarkers of a randomized trial comparing first-line everolimus and sunitinib in patients with metastatic renal cell carcinoma. Eur Urol 71:405-414, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casuscelli J, Vano Y-A, Fridman WH, et al. : Molecular classification of renal cell carcinoma and its implication in future clinical practice. Kidney Cancer 1:3-13, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen F, Zhang Y, Şenbabaoğlu Y, et al. : Multilevel genomics-based taxonomy of renal cell carcinoma. Cell Rep 14:2476-2489, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricketts CJ, De Cubas AA, Fan H, et al. : The Cancer Genome Atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep 23:313-326.e5, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sivanand S, Peña-Llopis S, Zhao H, et al. : A validated tumor graft model reveals activity of dovitinib against renal cell carcinoma. Sci Transl Med 4:137ra75, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong Y, Manley BJ, Becerra MF, et al. : Tumor xenografts of human clear cell renal cell carcinoma but not corresponding cell lines recapitulate clinical response to sunitinib: Feasibility of using biopsy samples. Eur Urol Focus 3:590-598, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nargund AM, Pham CG, Dong Y, et al. : The SWI/SNF protein PBRM1 restrains VHL-loss-driven clear cell renal cell carcinoma. Cell Rep 18:2893-2906, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu YF, Cohn S, Christie A, et al. : Modeling renal cell carcinoma in mice: Bap1 and Pbrm1 inactivation drive tumor grade. Cancer Discov 7:900-917, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espana-Agusti J, Warren A, Chew SK, et al. : Loss of PBRM1 rescues VHL dependent replication stress to promote renal carcinogenesis. Nat Commun 8:2026, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masson N, Ratcliffe PJ: Hypoxia signaling pathways in cancer metabolism: The importance of co-selecting interconnected physiological pathways. Cancer Metab 2:3, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semenza GL: HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest 123:3664-3671, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keith B, Johnson RS, Simon MC: HIF1α and HIF2α: Sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer 12:9-22, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hakimi AA, Tickoo SK, Jacobsen A, et al. : TCEB1-mutated renal cell carcinoma: A distinct genomic and morphological subtype. Mod Pathol 28:845-853, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivan M, Kaelin WG, Jr: The EGLN-HIF O2-sensing system: Multiple inputs and feedbacks. Mol Cell 66:772-779, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schito L, Semenza GL: Hypoxia-inducible factors: Master regulators of cancer progression. Trends Cancer 2:758-770, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Hakimi AA, Reznik E, Lee CH, et al. : An integrated metabolic atlas of clear cell renal cell carcinoma. Cancer Cell 29:104-116, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapitsinou PP, Haase VH: The VHL tumor suppressor and HIF: Insights from genetic studies in mice. Cell Death Differ 15:650-659, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordan JD, Thompson CB, Simon MC: HIF and c-Myc: Sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell 12:108-113, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu L, Wang G, Shevchuk MM, et al. : Generation of a mouse model of Von Hippel-Lindau kidney disease leading to renal cancers by expression of a constitutively active mutant of HIF1α. Cancer Res 71:6848-6856, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu L, Wang G, Shevchuk MM, et al. : Activation of HIF2α in kidney proximal tubule cells causes abnormal glycogen deposition but not tumorigenesis. Cancer Res 73:2916-2925, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen C, Beroukhim R, Schumacher SE, et al. : Genetic and functional studies implicate HIF1α as a 14q kidney cancer suppressor gene. Cancer Discov 1:222-235, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordan JD, Lal P, Dondeti VR, et al. : HIF-alpha effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell 14:435-446, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zbar B, Brauch H, Talmadge C, et al. : Loss of alleles of loci on the short arm of chromosome 3 in renal cell carcinoma. Nature 327:721-724, 1987 [DOI] [PubMed] [Google Scholar]
- 31.Fisher R, Horswell S, Rowan A, et al. : Development of synchronous VHL syndrome tumors reveals contingencies and constraints to tumor evolution. Genome Biol 15:433, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell TJ, Turajlic S, Rowan A, et al. : Timing the landmark events in the evolution of clear cell renal cell cancer: TRACERx Renal. Cell 173:611-623.e17, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clapier CR, Cairns BR: The biology of chromatin remodeling complexes. Annu Rev Biochem 78:273-304, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Hodges C, Kirkland JG, Crabtree GR: The many roles of BAF (mSWI/SNF) and PBAF complexes in cancer. Cold Spring Harb Perspect Med 6:a026930, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadoch C, Hargreaves DC, Hodges C, et al. : Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat Genet 45:592-601, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varela I, Tarpey P, Raine K, et al. : Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 469:539-542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu H, Mashtalir N, Daou S, et al. : The ubiquitin carboxyl hydrolase BAP1 forms a ternary complex with YY1 and HCF-1 and is a critical regulator of gene expression. Mol Cell Biol 30:5071-5085, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sowa ME, Bennett EJ, Gygi SP, et al. : Defining the human deubiquitinating enzyme interaction landscape. Cell 138:389-403, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carbone M, Yang H, Pass HI, et al. : BAP1 and cancer. Nat Rev Cancer 13:153-159, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A, et al. : BAP1 loss defines a new class of renal cell carcinoma. Nat Genet 44:751-759, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kapur P, Peña-Llopis S, Christie A, et al. : Effects on survival of BAP1 and PBRM1 mutations in sporadic clear-cell renal-cell carcinoma: A retrospective analysis with independent validation. Lancet Oncol 14:159-167, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hakimi AA, Ostrovnaya I, Reva B, et al. : Adverse outcomes in clear cell renal cell carcinoma with mutations of 3p21 epigenetic regulators BAP1 and SETD2: A report by MSKCC and the KIRC TCGA research network. Clin Cancer Res 19:3259-3267, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mar BG, Chu SH, Kahn JD, et al. : SETD2 alterations impair DNA damage recognition and lead to resistance to chemotherapy in leukemia. Blood 130:2631-2641, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanu N, Grönroos E, Martinez P, et al. : SETD2 loss-of-function promotes renal cancer branched evolution through replication stress and impaired DNA repair. Oncogene 34:5699-5708, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park IY, Powell RT, Tripathi DN, et al. : Dual chromatin and cytoskeletal remodeling by SETD2. Cell 166:950-962, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen K, Liu J, Liu S, et al. : Methyltransferase SETD2-mediated methylation of STAT1 is critical for interferon antiviral activity. Cell 170:492-506.e14, 2017 [DOI] [PubMed] [Google Scholar]
- 47.Gerlinger M, Rowan AJ, Horswell S, et al. : Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366:883-892, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.The Cancer Genome Atlas Network : Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med 374:135-145, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen YB, Xu J, Skanderup AJ, et al. : Molecular analysis of aggressive renal cell carcinoma with unclassified histology reveals distinct subsets. Nat Commun 7:13131, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turajlic S, Xu H, Litchfield K, et al. : Tracking cancer evolution reveals constrained routes to metastases: TRACERx renal. Cell 173:581-594.e12, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turajlic S, Xu H, Litchfield K, et al. : Deterministic evolutionary trajectories influence primary tumor growth: TRACERx renal. Cell 173:595-610.e11, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nargund AM, Osmanbeyoglu HU, Cheng EH, et al. : SWI/SNF tumor suppressor gene PBRM1/BAF180 in human clear cell kidney cancer. Mol Cell Oncol 4:e1342747, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu H, Pardoll D, Jove R: STATs in cancer inflammation and immunity: A leading role for STAT3. Nat Rev Cancer 9:798-809, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Motzer RJ, Escudier B, McDermott DF, et al. : Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803-1813, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miao D, Margolis CA, Gao W, et al. : Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 359:801-806, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parker WP, Cheville JC, Frank I, et al. : Application of the stage, size, grade, and necrosis (SSIGN) score for clear cell renal cell carcinoma in contemporary patients. Eur Urol 71:665-673, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ho TH, Kapur P, Joseph RW, et al. : Loss of histone H3 lysine 36 trimethylation is associated with an increased risk of renal cell carcinoma-specific death. Mod Pathol 29:34-42, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joseph RW, Kapur P, Serie DJ, et al. : Loss of BAP1 protein expression is an independent marker of poor prognosis in patients with low-risk clear cell renal cell carcinoma. Cancer 120:1059-1067, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ho TH, Kapur P, Eckel-Passow JE, et al. : Multicenter validation of enhancer of zeste homolog 2 expression as an independent prognostic marker in localized clear cell renal cell carcinoma. J Clin Oncol 35:3706-3713, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McDermott DF, Huseni MA, Atkins MB, et al. : Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 24:749-757, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen W, Hill H, Christie A, et al. : Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539:112-117, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cho H, Du X, Rizzi JP, et al. : On-target efficacy of a HIF2alpha antagonist in preclinical kidney cancer models. Nature 539:107-111, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Courtney KD, Infante JR, Lam ET, et al. : Phase I dose-escalation trial of PT2385, a first-in-class hypoxia-inducible factor-2α antagonist in patients with previously treated advanced clear cell renal cell carcinoma. J Clin Oncol 36:867-874, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Şenbabaoğlu Y, Gejman RS, Winer AG, et al. : Tumor immune microenvironment characterization in clear cell renal cell carcinoma identifies prognostic and immunotherapeutically relevant messenger RNA signatures. Genome Biol 17:231, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rini B, Goddard A, Knezevic D, et al. : A 16-gene assay to predict recurrence after surgery in localised renal cell carcinoma: Development and validation studies. Lancet Oncol 16:676-685, 2015 [DOI] [PubMed] [Google Scholar]
- 66.Brooks SA, Brannon AR, Parker JS, et al. : ClearCode34: A prognostic risk predictor for localized clear cell renal cell carcinoma. Eur Urol 66:77-84, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manley BJ, Zabor EC, Casuscelli J, et al. : Integration of recurrent somatic mutations with clinical outcomes: A pooled analysis of 1049 patients with clear cell renal cell carcinoma. Eur Urol Focus 3:421-427, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Casuscelli J, Becerra MF, Manley BJ, et al. : Characterization and impact of TERT promoter region mutations on clinical outcome in renal cell carcinoma. Eur Urol Focus pii:S2405-4569(17)30212-2, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kwiatkowski DJ, Choueiri TK, Fay AP, et al. : Mutations in TSC1, TSC2, and MTOR are associated with response to rapalogs in patients with metastatic renal cell carcinoma. Clin Cancer Res 22:2445-2452, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Voss MH, Hakimi AA, Pham CG, et al. : Tumor genetic analyses of patients with metastatic renal cell carcinoma and extended benefit from mTOR inhibitor therapy. Clin Cancer Res 20:1955-1964, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]