Abstract
The purpose of this narrative review is to summarize evidence of the epidemiology of and risk factors for kidney cancer with a focus on renal cell carcinoma in adults. The etiology of kidney cancer is largely unknown and the main epidemiologic determinants are large geographic and temporal variations in incidence rates. Established risk factors include tobacco smoking, body size, and history of hypertension and chronic kidney disease. Other suspected risk factors require additional investigation, as do the underlying biologic mechanisms that are responsible for disease occurrence. Opportunities to prevent kidney cancer include targeting modifiable risk factors—for example, smoking abstinence/cessation and body weight control—as well as interventions along the diagnostic pathway to improve early diagnosis. Molecular epidemiology, including, but not limited to, metabolomics and tumor genomics, are new areas of research that promise to play important roles in identifying some of the underlying causes of kidney cancer.
INTRODUCTION
Kidney cancer develops from the renal parenchyma. Clear cell renal cell carcinomas represent approximately 70% of kidney cancer cases in adults.1 Much of the epidemiologic knowledge pertains to kidney cancer as a whole, with a paucity of data on histologic subtypes. Tumors that arise from the renal pelvis and kidney cancer in children—Wilms tumors—are far less common than renal cell carcinomas and have different epidemiologic features that are beyond the scope of this review.
The main epidemiologic characteristics of kidney cancer are the large geographic and temporal variations in incidence rates. The list of established risk factors is limited to tobacco smoking, body size, and history of hypertension and chronic kidney diseases.
INCIDENCE AND SOCIODEMOGRAPHICS
Kidney cancer is the 13th most common cancer worldwide, accounting for 2.4% of all cancers, with more than 330,000 new cases diagnosed yearly.2 It ranks higher in Europe, North America, Australia/New Zealand, and Japan, where it is on average the 7th most common cancer. For the purpose of comparability across countries and over time, descriptive epidemiology databases often group renal cell carcinomas together with other upper urinary tract cancers.2,3
Age and Sex
Incidence rates of kidney cancer increase steadily with age, with a peak of incidence at approximately age 75 years.4,5 Worldwide, approximately one half of all cases are diagnosed before age 65 years.2
The incidence of kidney cancer is two-fold higher in men compared with women.6 This pattern has been reported repeatedly over time, across countries, and by age groups, and has so far remained unexplained. The stability of the sex ratio indicates that biologic differences between men and women, rather than lifestyle differences, such as tobacco smoking, are likely to account for much of the incidence disparities.
Geography and Ethnicity
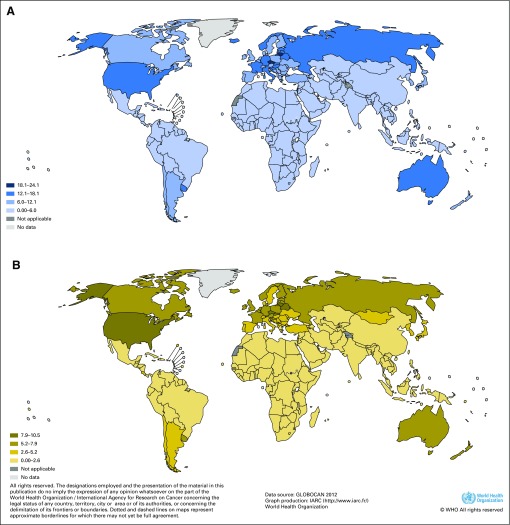
There are large variations in incidence rates around the world, with the highest incidence rates at the country level found in the Czech Republic (age-standardized rate, 21.9/100,000 in males) and Lithuania (age-standardized rate, 18.7/100,000 in males). Incidence rates are less than 2/100,000 in low-risk countries, such as China, Thailand, and African countries4 (Fig 1). In the United States, incidence rates are higher in black men (age-standardized rate, 15.6/100,000) than in white men (age-standardized rate, 14.0/100,000). Hispanics and non-Hispanics show similar rates. American Indians in the United States have intermediate rates (age-standardized rate, 10.9/100,000 in males), whereas Asians in the United States have low incidence rates (age-standardized rate, 6.4/100,000 in males). In Europe, large intracountry regional variations have been described in some countries, notably in Germany (higher incidence rates in the eastern regions of the country) and Italy (higher incidence rates in the north).7
Fig 1.
International variations in national estimates of kidney cancer age-standardized incidence rates per 100,000 for (A) men and (B) women. For men and women separately, countries were divided into four groups of fixed incidence rate intervals. Reproduced with permission from International Agency for Research on Cancer.2
Temporal Trends
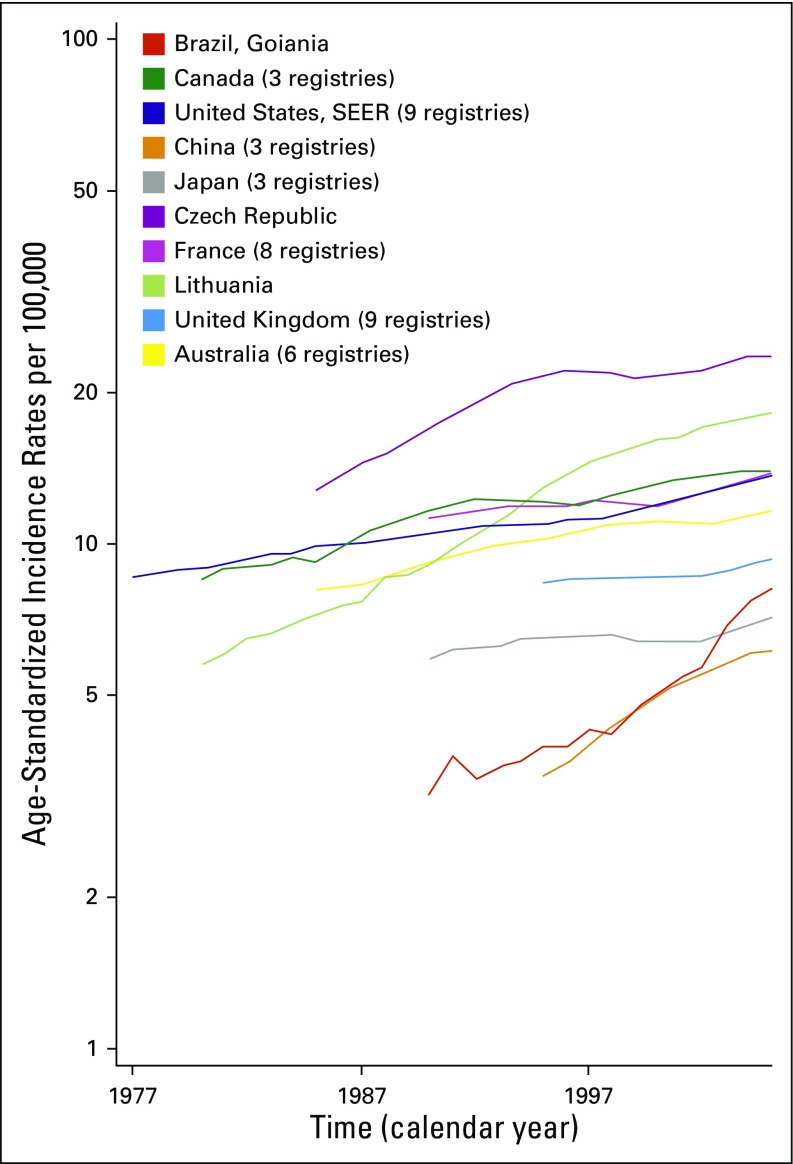
Incidence rates of kidney cancer have been increasing worldwide since the 1970s (Fig 2).3 In the United States, rates were 8.0/100,000 in males in 1975 and have increased steadily to reach 13.4/100,000 in 2012. The average annual percentage increase is approximately 2% to 3% in most countries.5 Only Austria and Poland have reported significantly decreasing rates since the early 2000s. Birth cohort and calendar period effects both contribute to the increasing rates, which indicates that changes in lifestyle and exposures to risk factors, as well as changes in tumor detection and diagnostic practices over time, are responsible for the observed temporal trends.8 In the United States, the proportion of localized kidney cancer cases increased from 45% in the 1970s to 54% in the 1990s, although trends toward increased incidence have been observed at all stages.9
Fig 2.
Temporal trends in age-standardized kidney cancer incidence rates for 10 selected registries/groups of registries (males, 1977 to 2005). Rates have been smoothed using a 5-year average. Reproduced with permission from International Agency for Research on Cancer.3
MORTALITY PATTERNS
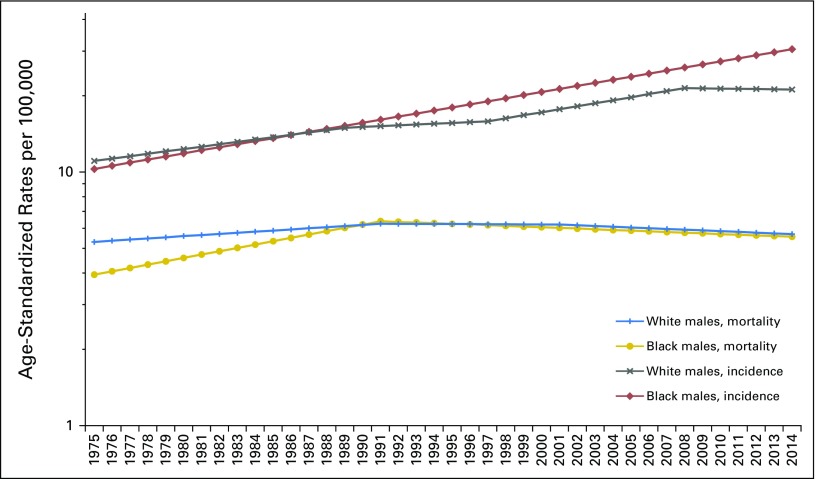
International variations in mortality follow the incidence pattern, with the highest rates observed in the Czech Republic (9.1/100,000 in males) and the Baltic countries.2 Mortality rates have been stable globally since the 1990s.5 In recent years, a decrease in mortality has been observed in most countries, with the notable exception of Brazil, Croatia, Greece, Ireland, Portugal, and Slovenia, where rates have continued to increase. In general, mortality seems to be decreasing faster in women than in men. In the United States, the decline in mortality is more pronounced for black patients, and mortality rates among black patients have remained lower than those of white patients since the 1970s4,10,11 (Fig 3). Ethnic differences in the biology and aggressiveness of kidney cancer could explain this variation, although other factors, such as competing mortality, could also play a role.12
Fig 3.
Temporal trends in age-standardized (2000 US population) incidence and mortality rates of kidney cancer by race in men in the United States, 1975 to 2014. Modeled rates derived from SEER data originally published by the National Cancer Institute.11
LIFESTYLE RISK FACTORS
Tobacco Smoking
Tobacco smoking has been classified as carcinogenic for the kidney by the International Agency for Research on Cancer and the United States Department of Health and Human Services.13,14 The effect size on kidney cancer risk is modest, with an approximate 30% increased risk in current smokers and a 15% increased risk in former smokers compared with never smokers.15 Epidemiologic evidence for a causal role for tobacco smoking includes a dose-response relationship between risk and the quantity of tobacco smoked per day, as well as decreased risk with increasing years of smoking cessation.16 In developed countries, it is estimated that 6% of kidney cancer deaths are a result of tobacco smoking.17
Excess Body Weight
The association between excess body weight and risk of kidney cancer has been extensively reported in large, prospective cohorts.18,19 Excess body weight has been overwhelmingly assessed via an elevation of body mass index (BMI; in kilograms per square meter). Compared with the reference BMI category (18.5 ≤ 25 kg/m2), overweight (BMI, 25 ≤ 30 kg/m2) and obese (BMI, ≥ 30 kg/m2) individuals have an estimated 28% and 77% increased risk, respectively.18 The association was demonstrated to be linear in several studies, with a 4% increase in risk for each 1-kg/m2 increment in BMI18 or a 25% increase in risk for each 5-kg/m2 increment.19 A prospective cohort study of male teenagers with BMI measured at age 16 to 19 years reported that excess body weight earlier in life is associated with an excess risk of kidney cancer later in life.20 High BMI is estimated to be responsible for 26% of incident kidney cancer cases worldwide.21
Body fatness has also been assessed using waist circumference and waist-to-hip ratio. Results have been consistent across studies, demonstrating a significant increase in risk with increasing waist circumference and waist-to-hip ratio.22 Weight cycling during adulthood has not been shown to significantly modify kidney cancer risk after accounting for baseline BMI.23 There are also no data available on the benefit of weight loss and/or long-term stabilization of lower BMI in association with the risk of kidney cancer.
Mechanisms involved have not yet been demonstrated, and research is ongoing on the role of inflammatory status, sex and growth hormone levels, metabolic state (insulin resistance and insulin-like growth factor-1 levels), and adipokine levels.24,25 It will be important to understand the mechanisms in play, particularly in the context of the obesity paradox phenomenon, whereby overweight and obese patients were shown in multiple series to have a prognostic advantage compared with patients with normal BMI.26 FASN gene expression has been implicated as a possible underlying biologic pathway that may influence the obesity paradox phenomenon,27 but replication of this finding is still needed.
Alcohol Consumption
Several meta-analyses and large prospective cohort studies were conducted on the association between alcohol consumption and risk of kidney cancer.28-32 All studies reported reduced risk in drinkers compared with nondrinkers or light drinkers. Drinkers typically have a 20% reduction in risk compared with nondrinkers and light drinkers.32 As there is no association between nonalcoholic beverage intake, nor total fluid intake and kidney cancer risk,33-36 and because the effect of alcohol consumption does not differ by type of alcoholic beverage,32 ethanol exposure likely has a mechanistic role. Hyperinsulinemia and insulin resistance in general have been associated with kidney cancer risk.37 Alcohol consumption has been shown to increase insulin sensitivity and could be associated with a reduction of kidney cancer risk via this indirect route.32,38,39
Physical Activity
Physical activity has been associated with a modest reduction in risk in a large meta-analysis of prospective cohorts.40 When considering all types of physical activities together, the highest activity category had a 13% decreased risk compared with the lowest category. Similarly, a large pooled analysis of cohort studies reported a reduced risk associated with higher levels of leisure-time physical activity.41 Sedentary behavior as measured by time spent sitting does not seem to increase the risk of kidney cancer.42 How physical activity might influence the risk of kidney cancer is unclear, and physical activity as a risk factor that is independent of excess body weight and hypertension has not been demonstrated. In its 2015 report, the World Cancer Research Fund and American Institute for Cancer Research concluded that there was limited to no evidence of a link between physical activity and kidney cancer risk.22
Diet
Conclusive epidemiologic evidence on diet and kidney cancer risk is lacking in the literature.22 Results on fruit and vegetable intake from prospective cohorts demonstrated mostly null or nonsignificant associations, or a modest reduction in risk in the highest fruit and vegetable intake categories. Data on nutrient-specific associations are lacking. Furthermore, studies that investigated the intake of fat and protein in association with the risk of kidney cancer mostly reported null associations.
MEDICAL HISTORY
Hypertension
Hypertension predisposes to kidney cancer.43,44 In the United States, a history of hypertension has been estimated to double the risk of kidney cancer in white patients and triple the risk in black patients.45 Prospective cohort studies consistently report dose-response associations between blood pressure at baseline and kidney cancer risk,46-49 even when restricting the risk analysis to more than 5 years after blood pressure measurement when reverse causation is less likely.50
Antihypertensive medication has also been associated with an increased risk of kidney cancer, but it is difficult to disentangle the effect of the condition from the effect of treatment.51 In a study with repeated measures of blood pressure over time, a decreased risk was observed with the reduction of blood pressure.50 This indicates that the hypertensive condition, rather than the treatment, is more likely to be the risk factor. Furthermore, controlling the condition through the use of hypertensive medication may be an effective therapeutic intervention in the prevention of kidney cancer. Hypertension also seems to be biologically independent from obesity in increasing the risk of kidney cancer, with a cumulative effect among individuals who present with both conditions.46,50 Although underlying mechanisms are not yet well described, renal injury, hypoxia, or inflammation caused by hypertension may play a role.52,53
Chronic Kidney Disease and Kidney Stones
Chronic kidney disease increases the risk of kidney cancer two-fold to three-fold.54-57 Evidence suggests that the increase in risk is more pronounced in black than in white Americans, which might contribute to the higher incidence rates in black patients considering that chronic kidney disease is also more prevalent in black patients.57-59
An association between a history of kidney stones and a subsequent risk of kidney cancer was reported in several case-control studies, but prospective cohort studies have been inconclusive, which indicates that surveillance and reporting biases may have artificially increased risk estimates in case-control studies.60-62
Diabetes Mellitus
The association between diabetes mellitus and kidney cancer risk has been assessed in several prospective cohort studies, with some suggestive evidence of an independent biologic effect from diabetes comorbidities, such as obesity and hypertension.63-65 A history of diabetes would be associated with a 40% excess risk of kidney cancer.63
ENVIRONMENTAL AND OCCUPATIONAL EXPOSURES
Trichloroethylene
Trichloroethylene is mostly known for its use as a metal cleaner and degreaser.66 The International Agency for Research on Cancer classified the occupational exposure to trichloroethylene as carcinogenic to humans, relying on a body of sufficient evidence that this chemical causes kidney cancer.66 The latest meta-analysis on the topic estimated that occupational exposure to trichloroethylene confers a 30% to 40% excess risk of kidney cancer.67 Levels lower than those found in an occupational setting have not been reported to be associated with kidney cancer.
Aristolochic Acid
Chronic and acute exposures to aristolochic acid have historically been linked to Balkan endemic nephropathy and carcinomas of the upper urinary tract.68 Exposure comes from the ingestion of Aristolochia plants, either unintentionally through contamination of food (chronic exposure) or intentionally as herbal traditional remedies (acute exposure). It was more recently hypothesized that this renal toxicant could also increase the risk of renal cell carcinoma, as typical mutational signatures and specific DNA adducts were found in patients who were diagnosed in Romania.69,70
Others
Other environmental exposures, such as outdoor air pollution, pesticides, arsenic in drinking water, and lead, were examined in several studies, which demonstrated suggestive but not conclusive evidence of an association with kidney—renal parenchyma—cancer risk.71-74
GENETIC RISK FACTORS
The majority of kidney cancer cases are sporadic, with only 3% to 5% occurring within a familial context.75 Von Hippel-Lindau syndrome is the most common genetic syndrome associated with an increase of kidney cancer risk and accounts for approximately 1% of renal cell carcinomas.76 Common genetic variants associated with kidney cancer risk were discovered through genome-wide association studies, and 13 loci have so far been implicated.77
OTHER RISK FACTORS
Height has been consistently associated with kidney cancer risk—independently of weight—with an approximate 30% increased risk for every 10-cm increase in height.78,79 Mechanisms involved are not clear but could involve growth hormones levels, genetic background, and childhood exposures.
Several studies have investigated the link between reproductive factors in women and kidney cancer risk. In a large meta-analysis, a significant increased risk was reported in women who underwent hysterectomy, but surveillance bias cannot be ruled out in this observation.80 The roles of other reproductive related–factors, such as parity and age at menarche/menopause, have remained inconclusive.44
Association between vitamin D levels and kidney cancer risk cancer has been systematically reviewed by the World Cancer Research Fund and American Institute for Cancer Research.81 Whereas the existing evidence does not rule out a possible protective effect of adequate vitamin D levels against kidney cancer development, results were inconsistent across studies.
OPPORTUNITIES FOR PREVENTION AND FUTURE DIRECTIONS
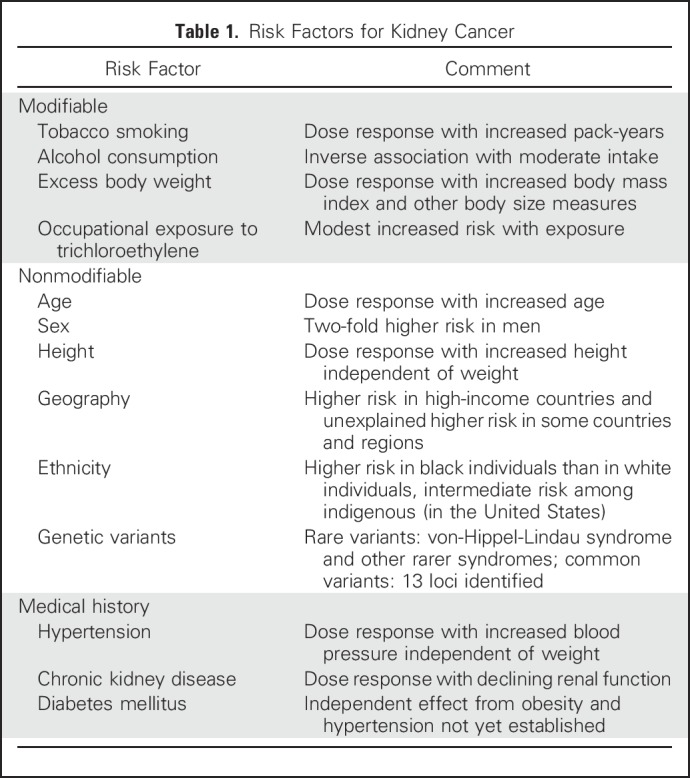
The etiology of kidney cancer is largely unknown, although causes are thought to be multifactorial (Table 1). To date, a range of risk factors has been studied, some of which are modifiable and therefore offer an opportunity for primary prevention. Tobacco smoking abstinence or cessation, avoiding overweight/obesity, and control of hypertension are likely to be major players in keeping kidney cancer risk low. To this end, primary care providers and public health agencies play a significant role in the encouragement and adoption of healthy behaviors. On a broader scale, country-level factors related to geography and environmental exposures, including trichloroethylene and others yet to be identified, would require system-level interventions to influence not only kidney cancer risk, but population health overall. At the same time, kidney cancer is not generally considered an occupational cancer. Strong, consistent evidence for an association between environmental exposures in general and kidney cancer have not been reported.
Table 1.
Risk Factors for Kidney Cancer
It is clear that more evidence is needed to gain a more complete understanding of the etiology, epidemiology, and risk factors for kidney cancer. Future research must therefore ensure our improved understanding of the underlying mechanisms that are associated with kidney cancer. For example, although excess body weight has been demonstrated to be associated with an increased risk of kidney cancer in a dose-response manner, overweight and early obese states have also been associated with improved survival among patients with cancer. This obesity paradox in cancer, kidney cancer included, requires additional investigation.26 The sex-ratio difference in patients with kidney cancer also requires additional mechanistic understanding.6 Furthermore, any independent effects from diabetes mellitus, obesity, and hypertension require delineation.
Molecular epidemiology, including but not limited to metabolomics and tumor genomics, is an exciting new area of research that has yet to be fully used for the study of kidney cancer. Metabolomics can provide insight into metabolic derangements that underlie disease and lead to the discovery of new therapeutic treatments as well as the discovery of biomarkers for early diagnosis and/or prognosis.82 Tumor genomics promises to causally identify individual tumors via mutational signatures that are specific to underlying exo- or endogenic causes.69,83
Finally, improvements along the diagnostic pathway can lead to earlier diagnosis and better prognosis. At present, evidence for blood- or urine-based biomarkers of kidney cancer is lacking. Identifying such biomarkers could have a major impact on overall survival and quality of life for patients worldwide, allowing for the detection of kidney cancer and recurrences at an earlier stage.
Footnotes
Supported by Research Council of Norway Grant No. 267776/H10 (to T.L.L.), within the framework of an agreement between the Research Council of Norway and the Norwegian University of Science and Technology.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Epidemiology and Risk Factors for Kidney Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Ghislaine Scelo
No relationship to disclose
Tricia L. Larose
No relationship to disclose
REFERENCES
- 1.Gansler T, Fedewa S, Amin MB, et al. : Trends in reporting histological subtyping of renal cell carcinoma: Association with cancer center type. Hum Pathol 74:99-108, 2018 [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer : GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012 v1.0. http://publications.iarc.fr/Databases/Iarc-Cancerbases/GLOBOCAN-2012-Estimated-Cancer-Incidence-Mortality-And-Prevalence-Worldwide-In-2012-V1.0-2012
- 3.International Agency for Research on Cancer : CI5Plus: Cancer incidence in five continents time trends. http://ci5.iarc.fr/CI5plus/Default.aspx
- 4.International Agency for Research on Cancer Cancer Incidence in Five Continents, Vol. XI. http://ci5.iarc.fr/CI5-XI/Default.aspx
- 5.Znaor A, Lortet-Tieulent J, Laversanne M, et al. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67:519–530. doi: 10.1016/j.eururo.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Scelo G, Li P, Chanudet E, et al. : Variability of sex disparities in cancer incidence over 30 years: The striking case of kidney cancer. Eur Urol Focus 10.1016/j.euf.2017.01.006 [epub ahead of print on January 31, 2017] [DOI] [PubMed] [Google Scholar]
- 7.Li P, Znaor A, Holcatova I, et al. : Regional geographic variations in kidney cancer incidence rates in European countries. Eur Urol 67:1134-1141, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Znaor A, Laversanne M, Bray F: Less overdiagnosis of kidney cancer? An age-period-cohort analysis of incidence trends in 16 populations worldwide. Int J Cancer 141:925-932, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Hock LM, Lynch J, Balaji KC: Increasing incidence of all stages of kidney cancer in the last 2 decades in the United States: An analysis of surveillance, epidemiology and end results program data. J Urol 167:57-60, 2002 [PubMed] [Google Scholar]
- 10.Lipworth L, Tarone RE, McLaughlin JK: Renal cell cancer among African Americans: An epidemiologic review. BMC Cancer 11:133, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Cancer Institute : Surveillance, Epidemiology, and End Results Program: Fast stats. https://seer.cancer.gov/faststats/
- 12.Lipworth L, McLaughlin JK, Tarone RE, et al. : Renal cancer paradox: Higher incidence but not higher mortality among African-Americans. Eur J Cancer Prev 20:331-333, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans : Personal Habits and Indoor Combustions. Volume 100 E. Lyon, France, International Agency for Research on Cancer, 2012 [PMC free article] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services : The health consequences of smoking—50 years of progress: A report of the Surgeon General, 2014. https://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html
- 15.Cumberbatch MG, Rota M, Catto JW, et al. : The role of tobacco smoke in bladder and kidney carcinogenesis: A comparison of exposures and meta-analysis of incidence and mortality risks. Eur Urol 70:458-466, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Hunt JD, van der Hel OL, McMillan GP, et al. : Renal cell carcinoma in relation to cigarette smoking:Meta-analysis of 24 studies. Int J Cancer 114:101-108, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Dy GW, Gore JL, Forouzanfar MH, et al. : Global burden of urologic cancers, 1990-2013. Eur Urol 71:437-446, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Wang F, Xu Y: Body mass index and risk of renal cell cancer: A dose-response meta-analysis of published cohort studies. Int J Cancer 135:1673-1686, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Bhaskaran K, Douglas I, Forbes H, et al. : Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5.24 million UK adults. Lancet 384:755-765, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leiba A, Kark JD, Afek A, et al. : Adolescent obesity and paternal country of origin predict renal cell carcinoma: A cohort study of 1.1 million 16 to 19-year-old males. J Urol 189:25-29, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Arnold M, Pandeya N, Byrnes G, et al. : Global burden of cancer attributable to high body-mass index in 2012: A population-based study. Lancet Oncol 16:36-46, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Cancer Research Fund/American Institute for Cancer Research : Continuous Update Project report: Diet, nutrition, physical activity and kidney cancer. http://wcrf.org/kidney-cancer-2015
- 23.Stevens VL, Jacobs EJ, Patel AV, et al. : Weight cycling and cancer incidence in a large prospective US cohort. Am J Epidemiol 182:394-404, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klinghoffer Z, Yang B, Kapoor A, et al. : Obesity and renal cell carcinoma: Epidemiology, underlying mechanisms and management considerations. Expert Rev Anticancer Ther 9:975-987, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Byers T, Sedjo RL: Body fatness as a cause of cancer: Epidemiologic clues to biologic mechanisms. Endocr Relat Cancer 22:R125-R134, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Lennon H, Sperrin M, Badrick E, et al. : The obesity paradox in cancer: A review. Curr Oncol Rep 18:56, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albiges L, Hakimi AA, Xie W, et al. : Body mass index and metastatic renal cell carcinoma: Clinical and biological correlations. J Clin Oncol 34:3655-3663, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JE, Hunter DJ, Spiegelman D, et al. : Alcohol intake and renal cell cancer in a pooled analysis of 12 prospective studies. J Natl Cancer Inst 99:801-810, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Song DY, Song S, Song Y, et al. : Alcohol intake and renal cell cancer risk: A meta-analysis. Br J Cancer 106:1881-1890, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bagnardi V, Rota M, Botteri E, et al. : Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br J Cancer 112:580-593, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karami S, Daugherty SE, Purdue MP: A prospective study of alcohol consumption and renal cell carcinoma risk. Int J Cancer 137:238-242, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wozniak MB, Brennan P, Brenner DR, et al. : Alcohol consumption and the risk of renal cancers in the European Prospective Investigation Into Cancer and Nutrition (EPIC). Int J Cancer 137:1953-1966, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Lee JE, Giovannucci E, Smith-Warner SA, et al. : Total fluid intake and use of individual beverages and risk of renal cell cancer in two large cohorts. Cancer Epidemiol Biomarkers Prev 15:1204-1211, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Lee JE, Hunter DJ, Spiegelman D, et al. : Intakes of coffee, tea, milk, soda and juice and renal cell cancer in a pooled analysis of 13 prospective studies. Int J Cancer 121:2246-2253, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Allen NE, Balkwill A, Beral V, et al. : Fluid intake and incidence of renal cell carcinoma in UK women. Br J Cancer 104:1487-1492, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deckers IA, van den Brandt PA, van Engeland M, et al. : Long-term dietary sodium, potassium and fluid intake: Exploring potential novel risk factors for renal cell cancer in the Netherlands Cohort Study on diet and cancer. Br J Cancer 110:797-801, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Häggström C, Rapp K, Stocks T, et al. : Metabolic factors associated with risk of renal cell carcinoma. PLoS One 8:e57475, 2013. [Erratum: PLoS One 8:10.1371/annotation/bb4481d0-a1ac-4fd9-aa57-e267f719a189, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koppes LL, Dekker JM, Hendriks HF, et al. : Moderate alcohol consumption lowers the risk of type 2 diabetes: A meta-analysis of prospective observational studies. Diabetes Care 28:719-725, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Calle EE, Kaaks R: Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4:579-591, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Behrens G, Leitzmann MF: The association between physical activity and renal cancer: Systematic review and meta-analysis. Br J Cancer 108:798-811, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore SC, Lee IM, Weiderpass E, et al. : Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med 176:816-825, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.George SM, Moore SC, Chow WH, et al. : A prospective analysis of prolonged sitting time and risk of renal cell carcinoma among 300,000 older adults. Ann Epidemiol 21:787-790, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McLaughlin JK, Lipworth L, Tarone RE: Epidemiologic aspects of renal cell carcinoma. Semin Oncol 33:527-533, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Chow WH, Dong LM, Devesa SS: Epidemiology and risk factors for kidney cancer. Nat Rev Urol 7:245-257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colt JS, Schwartz K, Graubard BI, et al. : Hypertension and risk of renal cell carcinoma among white and black Americans. Epidemiology 22:797-804, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weikert S, Boeing H, Pischon T, et al. : Blood pressure and risk of renal cell carcinoma in the European prospective investigation into cancer and nutrition. Am J Epidemiol 167:438-446, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Stocks T, Van Hemelrijck M, Manjer J, et al. : Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension 59:802-810, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Sanfilippo KM, McTigue KM, Fidler CJ, et al. : Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of US men and women. Hypertension 63:934-941, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun LM, Kuo HT, Jeng LB, et al. : Hypertension and subsequent genitourinary and gynecologic cancers risk: A population-based cohort study. Medicine (Baltimore) 94:e753, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chow WH, Gridley G, Fraumeni JF, Jr, et al. : Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med 343:1305-1311, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Yoon C, Yang HS, Jeon I, et al. : Use of angiotensin-converting-enzyme inhibitors or angiotensin-receptor blockers and cancer risk: A meta-analysis of observational studies. CMAJ 183:E1073-E1084, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palm F, Nordquist L: Renal oxidative stress, oxygenation, and hypertension. Am J Physiol Regul Integr Comp Physiol 301:R1229-R1241, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mennuni S, Rubattu S, Pierelli G, et al. : Hypertension and kidneys: Unraveling complex molecular mechanisms underlying hypertensive renal damage. J Hum Hypertens 28:74-79, 2014 [DOI] [PubMed] [Google Scholar]
- 54.Shebl FM, Warren JL, Eggers PW, et al. : Cancer risk among elderly persons with end-stage renal disease: A population-based case-control study. BMC Nephrol 13:65, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lowrance WT, Ordoñez J, Udaltsova N, et al. : CKD and the risk of incident cancer. J Am Soc Nephrol 25:2327-2334, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Butler AM, Olshan AF, Kshirsagar AV, et al. : Cancer incidence among US Medicare ESRD patients receiving hemodialysis, 1996-2009. Am J Kidney Dis 65:763-772, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hofmann JN, Corley DA, Zhao WK, et al. : Chronic kidney disease and risk of renal cell carcinoma: Differences by race. Epidemiology 26:59-67, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hofmann JN, Schwartz K, Chow WH, et al. : The association between chronic renal failure and renal cell carcinoma may differ between black and white Americans. Cancer Causes Control 24:167-174, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lipworth L, Mumma MT, Cavanaugh KL, et al. : Incidence and predictors of end stage renal disease among low-income blacks and whites. PLoS One 7:e48407, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheungpasitporn W, Thongprayoon C, O’Corragain OA, et al. : The risk of kidney cancer in patients with kidney stones: A systematic review and meta-analysis. QJM 108:205-212, 2015 [DOI] [PubMed] [Google Scholar]
- 61.Chung SD, Liu SP, Lin HC: A population-based study on the association between urinary calculi and kidney cancer. Can Urol Assoc J 7:E716-E721, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shih CJ, Chen YT, Ou SM, et al. : Urinary calculi and risk of cancer: A nationwide population-based study. Medicine (Baltimore) 93:e342, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larsson SC, Wolk A: Diabetes mellitus and incidence of kidney cancer: A meta-analysis of cohort studies. Diabetologia 54:1013-1018, 2011 [DOI] [PubMed] [Google Scholar]
- 64.Joh HK, Willett WC, Cho E: Type 2 diabetes and the risk of renal cell cancer in women. Diabetes Care 34:1552-1556, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harding JL, Shaw JE, Peeters A, et al. : Cancer risk among people with type 1 and type 2 diabetes: Disentangling true associations, detection bias, and reverse causation. Diabetes Care 38:264-270, 2015 [DOI] [PubMed] [Google Scholar]
- 66.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans : Trichloroethylene, Tetrachloroethylene, and Some Other Chlorinated Agents. Volume 100 E. Lyon, France, International Agency for Research on Cancer, 2014 [PMC free article] [PubMed] [Google Scholar]
- 67.Karami S, Lan Q, Rothman N, et al. : Occupational trichloroethylene exposure and kidney cancer risk: A meta-analysis. Occup Environ Med 69:858-867, 2012 [DOI] [PubMed] [Google Scholar]
- 68.Debelle FD, Vanherweghem JL, Nortier JL: Aristolochic acid nephropathy: A worldwide problem. Kidney Int 74:158-169, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Scelo G, Riazalhosseini Y, Greger L, et al. : Variation in genomic landscape of clear cell renal cell carcinoma across Europe. Nat Commun 5:5135, 2014 [DOI] [PubMed] [Google Scholar]
- 70.Turesky RJ, Yun BH, Brennan P, et al. : Aristolochic acid exposure in Romania and implications for renal cell carcinoma. Br J Cancer 114:76-80, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raaschou-Nielsen O, Pedersen M, Stafoggia M, et al. : Outdoor air pollution and risk for kidney parenchyma cancer in 14 European cohorts. Int J Cancer 140:1528-1537, 2017 [DOI] [PubMed] [Google Scholar]
- 72.Karami S, Boffetta P, Rothman N, et al. : Renal cell carcinoma, occupational pesticide exposure and modification by glutathione S-transferase polymorphisms. Carcinogenesis 29:1567-1571, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saint-Jacques N, Brown P, Nauta L, et al. : Estimating the risk of bladder and kidney cancer from exposure to low-levels of arsenic in drinking water, Nova Scotia, Canada. Environ Int 110:95-104, 2018 [DOI] [PubMed] [Google Scholar]
- 74.Boffetta P, Fontana L, Stewart P, et al. : Occupational exposure to arsenic, cadmium, chromium, lead and nickel, and renal cell carcinoma: A case-control study from Central and Eastern Europe. Occup Environ Med 68:723-728, 2011 [DOI] [PubMed] [Google Scholar]
- 75.Haas NB, Nathanson KL. Hereditary kidney cancer syndromes. Adv Chronic Kidney Dis. 2014;21:81–90. doi: 10.1053/j.ackd.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maher ER, Neumann HP, Richard S. von Hippel-Lindau disease: A clinical and scientific review. European Journal of Human Genetics. 2011;19:617–23. doi: 10.1038/ejhg.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scelo G, Purdue MP, Brown KM, et al. Genome-wide association study identifies multiple risk loci for renal cell carcinoma. Nat Commun. 2017;8:15724. doi: 10.1038/ncomms15724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Green J, Cairns BJ, Casabonne D, et al. Height and cancer incidence in the Million Women Study: Prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. Lancet Oncol. 2011;12:785–794. doi: 10.1016/S1470-2045(11)70154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wirén S, Häggström C, Ulmer H, et al. : Pooled cohort study on height and risk of cancer and cancer death. Cancer Causes Control 25:151-159, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karami S, Daugherty SE, Purdue MP: Hysterectomy and kidney cancer risk: A meta-analysis. Int J Cancer 134:405-410, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Darling AL, Abar L, Norat T: WCRF-AICR continuous update project: Systematic literature review of prospective studies on circulating 25-hydroxyvitamin D and kidney cancer risk. J Steroid Biochem Mol Biol 164:85-89, 2016 [DOI] [PubMed] [Google Scholar]
- 82.Clish CB: Metabolomics: An emerging but powerful tool for precision medicine. Cold Spring Harb Mol Case Stud 1:a000588, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alexandrov LB, Stratton MR: Mutational signatures: The patterns of somatic mutations hidden in cancer genomes. Curr Opin Genet Dev 24:52-60, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]