Abstract
New developments in cross-sectional imaging, including contrast-enhanced ultrasound, dual-energy computed tomography, multiparametric magnetic resonance imaging, single-photon emission computed tomography, and positron emission tomography, together with novel application of existing and novel radiotracers, have changed the landscape of renal mass characterization (ie, virtual biopsy) as well as the detection of metastatic disease, prognostication, and response assessment in patients with advanced kidney cancer. A host of imaging response criteria have been developed to characterize the response to targeted and immune therapies and correlate with patient outcomes, each with strengths and limitations. Recent efforts to advance the field are aimed at increasing objectivity with quantitative techniques and the use of banks of imaging data to match the vast genomic data that are becoming available. The emerging field of radiogenomics has the potential to transform further the role of imaging in kidney cancer management through eventual noninvasive characterization of the tumor histology and genetic microenvironment in single renal masses and/or metastatic disease. We review of the effect of currently available imaging techniques in the management of patients with kidney cancer, including localized, locally advanced, and metastatic disease.
INTRODUCTION
The revolution in medical imaging during the past decades has had an enormous effect on the management of kidney cancer. Routine use of imaging has led to increased detection of small renal tumors that pose diagnostic and management dilemmas. Conversely, active surveillance protocols with serial imaging and systematic evaluation of radiopathologic correlations in renal masses have enhanced our understanding of the natural history of renal cancer and potential implications of the imaging phenotype. Advances in cross-sectional imaging techniques have improved staging. However, novel treatments demand implementation of new imaging strategies to assess response beyond traditional morphologic changes. We present a comprehensive review of the current role of imaging in the management of kidney cancer, including challenges and opportunities for further development.
THE INCIDENTAL SOLID RENAL MASS: AN IMAGING DILEMMA
Kidney cancer, once considered a clinical diagnosis, is now primarily a radiologic diagnosis. Widespread use of cross-sectional imaging has resulted in a steady increase in the prevalence of kidney cancer.1 Approximately 70% of renal cell carcinomas (RCCs) are diagnosed incidentally on cross-sectional imaging.2 Increased prevalence has been greatest for lower-stage tumors, which suggests an effect of early detection.1 However, despite an increase in surgical resections, a clear effect on cancer-specific mortality has not been seen.3 Moreover, the likelihood of benign histology increases inversely with tumor size, with a prevalence approaching 20% for small renal masses (SRMs; T1a < 4 cm).4 Consequently, better presurgical characterization of SRMs may optimize patient selection for active surveillance and avoid unnecessary biopsies and/or surgery.
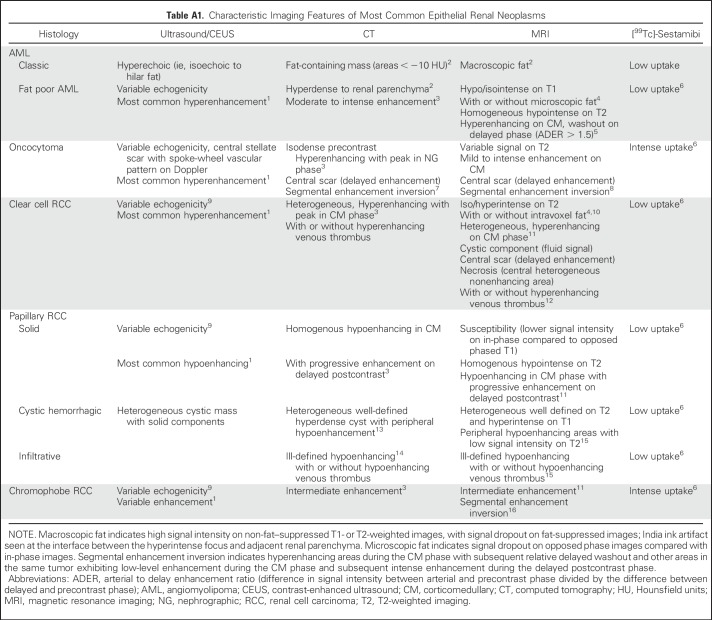
Renal angiomyolipoma (AML) and oncocytoma represent the most common benign histologies in surgical series of SRMs (44% and 35%, respectively).5 Classic AMLs that contain fatty tissue (ie, adipocytes) are easily recognized on computed tomography (CT) and magnetic resonance imaging (MRI). However, AMLs with minimal or no visible fat on imaging (also called fat poor AMLs) can be mistaken for RCC, and the patient can undergo unnecessary resection. Although a central scar may be visible in up to one third of oncocytomas,6 it is also frequently present in RCC or confused with tumor necrosis. Various CT and MRI features can assist in differentiating AML and oncocytoma from RCC (Appendix Table A1, online only). Intense uptake on [99mTc]-sestamibi single-photon emission tomography (SPECT)/CT is sensitive (87.5%) and specific (95.2%) for differentiating oncocytoma and hybrid oncocytic/chromophobe tumors from other renal masses, which demonstrate minimal or no uptake.7
CT/MRI differentiation of RCC subtypes relies primarily on analyses of postcontrast time-attenuation curves and lesion homogeneity, whereas other signal-based MRI features are also helpful. Contrast enhancement and heterogeneity of clear cell RCC (ccRCC) is significantly higher than in papillary RCC (pRCC) and chromophobe RCC.8 The relative enhancement ratio between the renal mass and the aorta on contrast-enhanced CT (CECT) is significantly lower for pRCC than for nonpapillary histology, with sensitivity and specificity of 86% and 85%, respectively (cutoff, 0.25).9 Similarly, Sun et al10 confirmed higher MRI enhancement levels during corticomedullary and nephrographic phases in ccRCC (205.6% and 247.1%, respectively) compared with pRCC (32.1% and 96.6%), whereas chromophobe RCC exhibited intermediate enhancement (109.9% and 192.5%).
Although percutaneous biopsies are highly accurate for RCC diagnosis, a recent meta-analysis reported a 14% nondiagnostic rate, 4% false-positive rate, and 3.1% false-negative rate.11 Furthermore, harms associated with biopsies are infrequent but not negligible.11 A composite MRI phenotype can predict RCC histology12 (Appendix Table A1; Fig 1) and help with management decisions. By using a predefined algorithm that was based on multiparametric MRI, Kay et al13 reported a sensitivity and specificity of 85% and 76%, respectively, for ccRCC and 80% and 94%, respectively, for pRCC. With the same algorithm, Canvasser et al14 reported a multiparametric MRI likelihood score of clear cell histology (ccLS). Sensitivity, specificity, and the positive predictive value for ccRCC using ccLS ≥ 4 was 78%, 80%, and 80%, respectively. On the basis of these results and the high specificity (95%) and positive predictive value (93%) for non-ccRCC in ccLS 1 or 2, a recommendation of surgical resection for patients with ccLS 4 or 5 lesions, active surveillance for patients with ccLS 1 to 2 lesions, and biopsy for patients with ccLS 3 lesions (particularly if renal mass < 3 cm) would result in a 20% biopsy rate; unnecessary treatment of oncocytoma and AML in 4.5% and 1.7%, respectively; and placement of 4.4% of patients with ccRCC on active surveillance.14 SRMs on active surveillance with homogeneous signal intensity on T2-weighted images exhibit a slower growth rate (ie, tumor doubling time > 2 years) and thus, may be better candidates for active surveillance.15
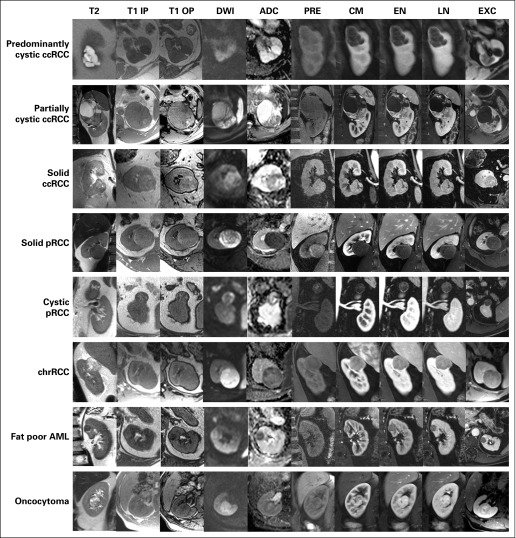
Fig 1.
Prototypical imaging phenotype of most common renal masses on multiparametric magnetic resonance imaging. Representative examples of most common histologic subtypes in renal masses. ADC, apparent diffusion coefficient; AML, angiomyolipoma; ccRCC, clear cell renal cell carcinoma; chrRCC, chromophobe renal cell carcinoma; CM, contrast-enhanced fat-saturated T1-weighted spoiled gradient echo during corticomedullary phase; DWI, diffusion-weighted imaging (b = 800); EN, contrast-enhanced fat-saturated T1-weighted spoiled gradient echo during the early nephrographic phase; EXC, contrast-enhanced fat-saturated T1-weighted spoiled gradient echo during the excretory phase; LN, contrast-enhanced fat-saturated T1-weighted spoiled gradient echo during the late nephrographic phase; pRCC, papillary renal cell carcinoma; PRE, precontrast fat-saturated T1-weighted spoiled gradient echo; T1 IP, T1-weighted in-phase gradient echo; T1 OP, T1-weighted opposed phase gradient echo; T2, T2-weighted single-shot fast spin echo.
Contrast-enhanced ultrasound (CEUS) is an alternative to CECT for renal mass characterization,16 particularly in patients with renal impairment in whom administration of iodinated contrast is undesirable. Dual-energy CT technology, introduced in the past decade, can reduce some limitations of standard CECT, such as lesion pseudoenhancement and detection of enhancement in hypoenhancing tumors (eg, pRCC).17 Diffusion-weighted MRI offers information about cellular density. A meta-analysis reported significantly lower apparent diffusion coefficients (ADCs) derived from diffusion-weighted imaging in RCC (95% CI, 1.45 to 1.77 × 10−3 mm2/s) compared with benign lesions (95% CI, 1.92 to 2.28 × 10−3 mm2/s). Oncocytomas had significantly higher ADCs than malignant lesions (1.84 to 2.17 × 10−3 mm2/s).18 Arterial spin labeled (ASL) MRI provides measures of tissue perfusion without the need of an exogenous contrast agent. Lanzman et al19 confirmed lower mean perfusion in pRCC compared with other RCC subtypes and high perfusion in oncocytomas using ASL MRI.
In general, state-of-the-art multiparametric MRI protocols are superior to CT scans for renal mass characterization in cooperative patients because of superior soft tissue contrast, characterization of fatty elements, and detection of enhancement. However, when MRI is not available, CECT is an excellent alternative to multiparametric MRI in patients with contraindication to MRI or poor breath-hold capacity. To date, positron emission tomography (PET)/CT does not play a role in the evaluation of primary tumors.
RADIOLOGIC ASSESSMENT OF CYSTIC RENAL LESIONS
A radiologic definition of cystic RCC currently is lacking. Previous reports have proposed the inclusion of neoplasms with ≥ 75% cystic components at histopathology.20 This distinction is important because increasing data indicate that cystic renal neoplasms demonstrate more indolent behavior and favorable prognosis than solid renal tumors.
Currently, patients with cystic renal lesions are managed on the basis of radiologic complexity (eg, wall thickness, internal septations, nodularity). The Bosniak classification is a well-established five-tier scale that reflects the increasing likelihood of malignancy as the lesion exhibits greater imaging complexity.21 Bosniak I (simple) and II (mildly complicated) cysts are considered benign, and Bosniak IIF (follow-up) cysts deserve longitudinal monitoring with imaging.22 The rate of malignancy in Bosniak IIF (complicated), III (indeterminate), and IV (malignant) cystic lesions is approximately 11% (range, 5% to 38%), 50% (range, 25% to 100%), and 80% (range, 67% to 100%), respectively.23 Although the Bosniak classification was developed for CECT, similar principles are applicable to ultrasound and MRI. MRI is particularly helpful to distinguish solid from cystic lesions when enhancement is equivocal on CT (ie, between 10 and 20 Hounsfield units).24 CEUS also is helpful to characterize complex cystic lesions.16
Although the Bosniak classification is an excellent tool to estimate the risk of malignancy, direct extrapolation between the Bosniak category and the need for treatment is likely not appropriate. Historically, surgical treatment was recommended for Bosniak III and IV lesions. Increasing evidence suggests that predominantly cystic renal neoplasms (ie, > 45% cystic) are more indolent than solid tumors, and have low metastatic potential.25 Moreover, the risk of metastasis may be lower than the risk of complications after surgery or ablation.26,27 Accordingly, many Bosniak category III and IV lesions, presumed to represent malignancies, may be safely followed with imaging, particularly in patients with comorbidities and/or a minimal solid component. Patients on active surveillance should undergo an initial 6-month follow-up and subsequent annual imaging follow-up.
LOCAL STAGING AND SURGICAL PLANNING
The TNM classification remains the most commonly used tool to stage kidney cancer. CT and MRI are used for local staging and accurate tumor measurements. Determination of extension beyond the kidney may be challenging. A well-defined tumor pseudocapsule is present in 66% of SRMs and better appreciated on T2-weighted imaging and contrast-enhanced MRI. This pseudocapsule is associated with low-grade histology28 and a high negative predictive value for tumor extension into perirenal and hilar fat.29 Conversely, infiltrating margins correlate with aggressive histology and behavior in pRCC and ccRCC.30-32 CT and MRI are accurate in excluding local invasion (T4 disease) when a fat plane is visualized between the renal mass and adjacent organs. Separate deposits in the perirenal fat likely indicate aggressive behavior but represent a challenge for staging (Fig 2).
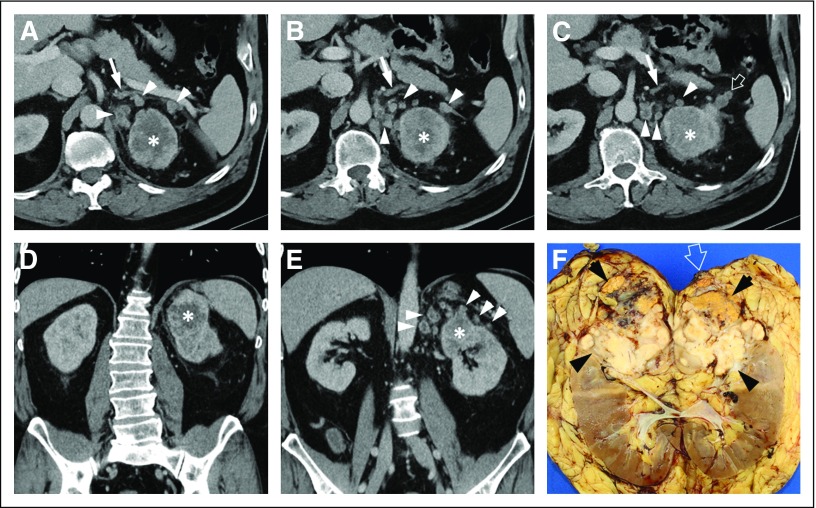
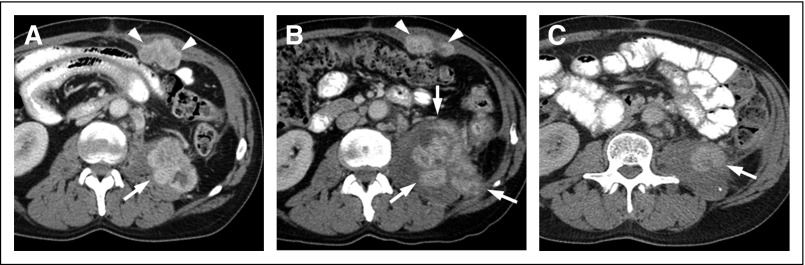
Fig 2.
Fifty-nine-year-old patient with left renal mass. (A, B, C) Axial contrast-enhanced computed tomography images and (D, E) coronal reconstructions show an ill-defined mass (asterisk) with central hypoenhancement suggestive of necrosis. The left adrenal gland is seen (arrow) surrounded by multiple heterogeneous nodules (arrowheads) in the perirenal fat. (C) A soft tissue deposit is extending beyond Gerota’s fascia (open arrow). (F) Bivalved gross specimen after radical nephrectomy that demonstrates a large infiltrative mass (arrowheads) replacing the left upper pole. A separate nodule (open arrow) is visible at the surgical margin. At histopathology, the mass was consistent with clear cell renal cell carcinoma with sarcomatoid differentiation (International Society of Urological Pathology nucleolar grade 4 [out of 4]). Invasion of the perirenal and hilar fat and lymphovascular invasion were present. Perirenal soft tissue nodules are likely intravenous tumor spread to the perirenal fat. Contrary to direct tumor spread into the perirenal fat (ie, T3 disease), noncontiguous spread to perirenal fat is not clearly typified in TNM staging. A separate nodule was present in the left adrenal gland and surgical resection of a left lung nodule identified at presentation (not shown) confirmed metastatic disease.
Tumor thrombus in the renal vein and inferior vena cava (IVC) has prognostic implications and affects the surgical approach.33 Tumor thrombus can be differentiated from bland thrombus when vascularity is present within (ie, flow on Doppler ultrasound, enhancement on CEUS, CECT, or MRI). MRI is more accurate than conventional venography and CT in delineating the extent of IVC tumor thrombus34 (Fig 3). However, determination of renal vein and IVC wall invasion remains challenging. Right-sided tumors, anteroposterior IVC diameter at the renal vein ostium of ≥ 24.0 mm, and complete occlusion of the IVC at the same level are associated with a significantly increased risk of IVC resection during nephrectomy.34
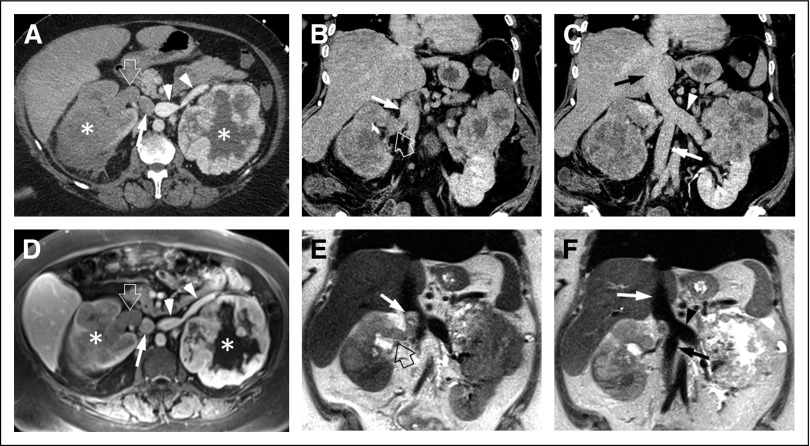
Fig 3.
Fifty-seven-year-old patient with bilateral renal masses and inferior vena cava (IVC) tumor thrombus. (A) Axial contrast-enhanced computed tomography (CT) image obtained during the corticomedullary phase shows bilateral renal masses (asterisks) and optimal opacification of the left-side renal vein (arrowheads). The right renal vein (open arrow) demonstrates lower attenuation, similar to that of the right renal mass, which is suspicious for tumor thrombus. The infrarenal IVC (arrow) is poorly opacified. (B, C) Coronal CT reconstructions from an acquisition during the venous phase shows subtle hypoenhancement of (B) the right renal vein (open arrow) and normal enhancement of the suprarenal, infrahepatic IVC (arrow); there is normal enhancement of the (C) left renal vein (arrowhead) and infrarenal IVC (white arrow). Assessment of the (C) intrahepatic IVC (black arrow) is challenging. (D) Axial contrast enhanced magnetic resonance image shows both renal masses (asterisks) and confirms the presence of hypoenhancing tumor thrombus in the right renal vein (open arrow). There is normal enhancement of the infrarenal IVC (arrow) and left renal vein (arrowheads). (E) Coronal T2-weighted images demonstrate extension of the right renal vein tumor thrombus (open arrow) into the suprarenal, infrahepatic IVC (arrow), which was not visualized on the CT examination. (F) The intrahepatic IVC (white arrow), left renal vein (arrowhead), and infrarenal IVC (black arrow) are free of tumor. Unclassified renal cell carcinoma (International Society of Urological Pathology grade 3 [out of 4]) with right renal vein and infrahepatic IVC tumor thrombus was confirmed after right radical nephrectomy. Left partial nephrectomy revealed clear cell renal cell carcinoma (International Society of Urological Pathology grade 4 [out of 4]). The left renal vein and intrahepatic and infrarenal IVC were free of tumor thrombus at surgery.
Several systems have been proposed to assess the difficulty of surgical resection of renal masses. R.E.N.A.L (radius, exophytic/endophytic, nearness to collecting system or sinus, anterior/posterior, location relative to polar lines) is a popular image-based scoring system that provides objective information on anatomic complexity of a renal mass and predicts complications and warm ischemia time during minimally invasive nephron-sparing surgery.35 The PADUA (preoperative aspects and dimensions used for anatomical score) and centrality index (C-Index) are alterative scoring systems to predict the complexity of surgery.
DIAGNOSIS OF METASTATIC DISEASE
Approximately 16% of patients with RCC have metastatic disease at diagnosis.36 Of patients with surgically excised localized tumors, 20% to 30% eventually relapse.37 The risk of metastatic disease is greatest in the 3 years after diagnosis, although late recurrences are possible, especially in younger patients with few comorbidities and larger tumors.38 Risk and/or stage-based imaging surveillance strategies have been incorporated into the National Comprehensive Cancer Network (NCCN) and American Urological Association guidelines.37,39
CECT is a mainstay imaging technique for surveillance because it is widely available and depicts lung, soft-tissue, and bone pathology. This is useful because RCC metastases involve numerous organs, including lungs, bone, nodes, liver, adrenals, and brain.40 CT limitations include radiation exposure and contrast allergies or contraindications in patients with renal impairment. According to American College of Radiology (ACR) contrast media guidelines, serum creatinine (with or without estimated glomerular filtration rate) should be available or obtained before the injection of iodinated contrast medium in all patients considered at risk for contrast-induced nephropathy; in patients older than 60 years of age; and/or in patients with a history of renal disease (including renal cancer, single kidney, renal surgery, dialysis, and renal transplant), history of hypertension that requires medical therapy, diabetes, and/or treatment with metformin or a metformin-containing regimen.41 At present, little evidence indicates that intravenous iodinated contrast material is an independent risk factor for postcontrast acute kidney injury in patients with an estimated glomerular filtration rate ≥ 30 mL/min/1.73 m2.42 However, the risks and benefits of iodinated contrast media must be considered on a case-by-case basis.
NCCN guidelines recommend chest radiographs or CT scans in patients with up to stage III disease,37 although chest CT is more sensitive for lung nodules. CECT or MRI can be used to monitor the abdomen. Contrast-enhanced MRI is uniquely suited to detect enhancement that represents recurrent disease after ablation. Because the risk of nephrogenic systemic fibrosis is sufficiently low or possibly nonexistent in patients imaged with standard or lower-than-standard doses of group II gadolinium-based contrast agents for contrast-enhanced MRI, renal function assessment with a questionnaire or laboratory testing is now considered optional in this setting according to ACR guidelines.41 Patients who may be administered group I or group III gadolinium-based agents still should be screened for renal impairment.
Detection of liver and pancreatic ccRCC metastases is improved with arterial phase and portal venous phase abdominal CT or MRI.43,44 Adrenal masses can present a diagnostic dilemma at initial diagnosis when no prior imaging is available. ccRCC adrenal metastases must be differentiated from lipid-poor adenomas, which can demonstrate overlapping imaging features on a dedicated adrenal CT protocol.45 Furthermore, ccRCC metastasis may exhibit lipid content that mimics lipid-rich adenomas. Increased T2-weighted imaging hyperintensity and heterogeneity on MRI recently have been shown to differentiate adrenal metastasis from adenomas; additional studies are needed to assess the role of MRI to replace tissue diagnosis.46
[99mTc]Technetium methylene diphosphate bone scans are standard of care for metastatic bone disease. However, bone scanning is of limited utility, even in patients with high pretest probability given the commonly lytic nature of this disease. In a small cohort of 36 patients with RCC with clinical suspicion for bone metastases, bone scan sensitivity ranged from 10% to 60%, depending on the applied threshold.47 Furthermore, CT is less sensitive than MRI, with a reported sensitivity of 66% for spinal metastasis.48
Although not recognized in the NCCN guidelines, [18F]fluorodeoxyglucose PET/CT or PET/MRI may be useful for the identification of metastases to peritoneum, bone, muscle, and adrenals as well as in problem solving, biopsy site selection, prognostication, and monitoring response to therapy49,50 (Appendix Fig A1, online only). Variable [18F]fluorodeoxyglucose avidity of RCC metastases, cost, availability, and radiation dose are potential limitations to use. Gerety et al51 reported a 100% sensitivity of [18F]sodium fluoride PET/CT for bone metastasis compared with 46% for CT and 29% for bone scanning. Availability, high cost, and limited reimbursement have challenged the implementation of [18F]sodium fluoride PET/CT in clinical practice.
ASSESSMENT OF TUMOR RESPONSE
Given the array of treatments available for metastatic RCC (mRCC), including therapies targeted to vascular endothelial growth factor (VEGF) receptor and other ligands; immune checkpoint inhibitors; and metastatectomy, thermal ablation, and stereotactic body radiotherapy (SBRT), imaging follow-up for mRCC has become complex. Patients with mRCC are living longer and may undergo numerous therapies; radiologists must be cognizant of past and present therapy, expected post-therapy changes, and associated toxicities during interpretation.
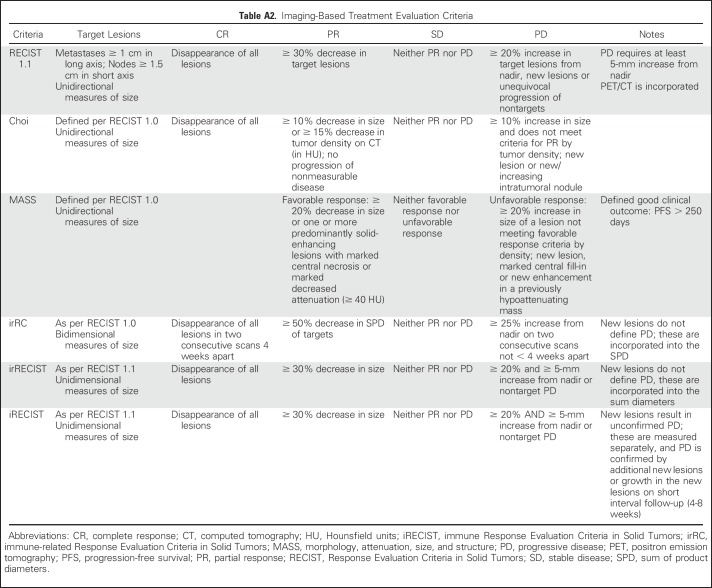
For patients with RCC treated in clinical trials, Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 remains the most commonly used response assessment method (Appendix Table A2, online only). Secondary and exploratory end points may include class-specific response assessments (eg, immune-related response criteria). In routine care, treatment change decisions may be based on the presence or absence of progressive disease and other factors. Although the utility of RECIST is evident through its longevity and range of application in a variety cancers and treatment settings, a limitation of RECIST is that a large proportion of patients with mRCC show stable disease on follow-up, especially in those treated with VEGF-targeted agents and mammalian target of rapamycin inhibitors.52 This category includes patients with 29% tumor shrinkage and 19% growth, a heterogeneous group with heterogeneous outcomes.53
It is well established that the VEGF-targeted therapies can result in modest early size changes and/or changes in tumor vascularity, which have been associated with prolonged time to progression.54,55 Several groups have sought to better define responders and nonresponders to treatment. Choi and MASS (morphology, attenuation, size, and structure) criteria have been applied to patients with mRCC treated with VEGF-targeted therapies, both of which incorporate size and density changes (Fig 4).56,57 Ten-percent tumor shrinkage also has been identified as a useful threshold indicator of response.54,55,58 Partial or favorable response according to various alternative criteria have been shown to better correlate with survival outcomes than RECIST partial response, although alternative response assessments have been less useful in early or optimally identifying progressive disease. Efforts to further improve the predictive value of response category designations have incorporated Memorial Sloan Kettering Cancer Center risk factors.59 Recently, semiautomated quantification of initial changes in CT vascular tumor burden improved differentiation of responders and nonresponders to sunitinib compared with RECIST and other alternative criteria.60
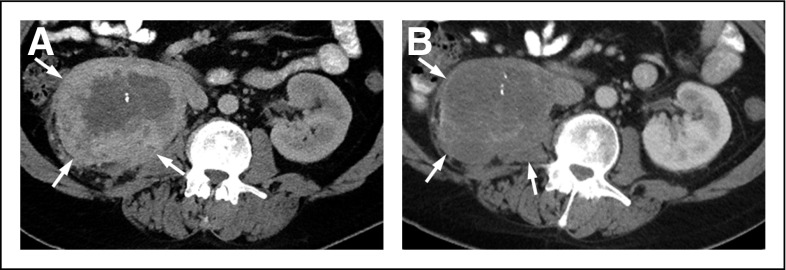
Fig 4.
Forty-five-year-old man with metastatic clear cell renal cell carcinoma. (A) Baseline contrast-enhanced axial computed tomography scan shows a large, solid, peripherally enhancing right renal mass (arrows) that represents the primary tumor. (B) Follow-up contrast-enhanced axial computed tomography scan after 3 months of sunitinib therapy demonstrates mildly decreased size but significantly decreased enhancement within the primary renal mass (arrows), which represents a partial response according to Choi or MASS (morphology, attenuation, size, and structure) criteria.
Response assessment in clinical trials that use immune checkpoint inhibitors has been based in RECIST. In the phase III study of nivolumab versus everolimus in advanced RCC, response assessment was according to RECIST 1.1, although patients could continue therapy after initial progression if the investigator perceived clinical benefit.61 A spectrum of changes in immune checkpoint inhibitor treatment of RCC has been described, including early complete response, pseudoprogression, disease stability before response, mixed response with new lesions, and early progression, although the incidence of each is not well understood.62 With the use of RECIST, patients with pseudoprogression or mixed response and new lesions may be characterized as having progressive disease and discontinued from potentially effective therapy (Fig 5). Several immune-related response assessment methods have been developed to capture these patterns (immune-related response criteria, immune-related RECIST, and immune RECIST), although they are not yet widely applied in RCC.63,64 Although some differences exist among these criteria, all advise confirmation of progressive disease on a short-interval follow-up scan.
Fig 5.
Fifty-four-year-old man with metastatic renal cell carcinoma and heterogeneous initial response to immunotherapy. (A) Baseline axial contrast-enhanced computed tomography (CT) scan demonstrates metastases to the left-side psoas (arrow) and left-side anterior abdominal wall (arrowheads). (B) First follow-up abdominal CT scan after 8 weeks of immune checkpoint inhibitor treatment demonstrates marked increased size of the left-side psoas mass (arrows) suggestive of progressive disease. Conversely, some separation and decreased size of the conglomerate of anterior abdominal wall nodules (arrowheads) are seen. (C) Second follow-up CT scan after 6 additional weeks of treatment shows a significant decrease in the left-side psoas metastasis (arrow) and resolution of the abdominal wall nodules. The pattern of response in the left-side psoas metastasis (initial increase in size after treatment followed by decrease in tumor burden) has been termed pseudoprogression.
Historically, RCC has been considered radioresistant, although SBRT is being effectively used for local control in selected scenarios, including the treatment of SRM, oligometastatic disease, and brain metastasis.65,66 Imaging interpretation of lesions treated with SBRT can be challenging because of the presence of persistent/delayed enhancement after treatment67 and the lack of consensus in bone lesion response assessment on the basis of RECIST 1.1 and/or MD Anderson bone criteria.68 A potential application of SBRT in advanced RCC/mRCC is in alteration of the immune landscape to promote efficacy of immunotherapies (ie, abscopal effect) wherein imaging response assessment may be complicated further.
In conclusion, the landscape of imaging in the management of kidney cancer is evolving rapidly with new acquisition techniques and postprocessing algorithms (Appendix, Fig 6). Each imaging modality has strengths and limitations. Noninvasive histologic diagnosis in SRMs (virtual biopsy) is now feasible. Rapid implementation of new treatment modalities with different effects in primary tumors and metastatic sites has led to the investigation of alternative response criteria for improved prediction of outcomes. Standardization of these remains a challenge in clinical practice and trials.
Fig 6.
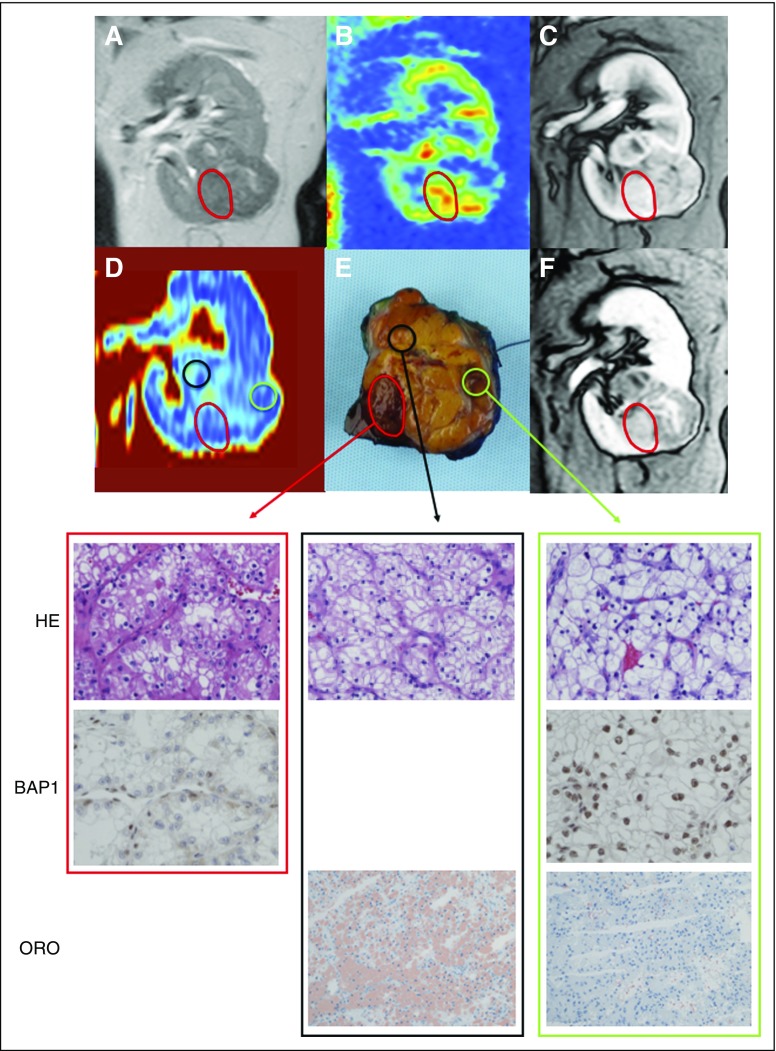
Use of multiparametric magnetic resonance imaging (MRI) as a radiomics platform for assessment of intratumor heterogeneity in clear cell renal cell carcinoma (ccRCC). (A) Coronal T2-weighted image, (B) arterial spin labeled (ASL) perfusion map, (C, F) corticomedullary and delayed venous phase from dynamic contrast-enhanced MRI, (D) Dixon fat fraction (FF) map, and (E) gross pathologic specimen. Histologic and immunohistochemical analysis revealed ccRCC grade 2 (out of 4) and BAP1 retained nuclear staining in the majority of the tumor. However, a distinct tumor nodule (red circle) demonstrates features consistent with ccRCC grade 3 and negative BAP1 nuclear staining (ie, BAP1 mutation). Multiparametric MRI in this nodule revealed (B) higher ASL perfusion (ie, 264 mL/100 g/min in nodule v 157 mL/100 g/min in the rest of the tumor), (C) increased enhancement during the corticomedullary phase, (F) retention of contrast during the delayed venous phase, and (D) decreased FF compared with the rest of the tumor. Oil red O staining (ORO) confirms (D) high-fat content in areas with high FF (black circle; FF, 8%) compared with areas with low FF (green circle; FF, 2%) on Dixon MRI. ORO staining in distinct tumor nodule (red circle) and BAP1 immunohistochemistry in superior tumor area (black circle) were not performed. HE, hematoxylin and eosin.
Appendix
Future Renal Cell Carcinoma Imaging and Radiomics
An important advantage of imaging is that it provides an analysis of the whole tumor burden in each patient. Notwithstanding the promising role of liquid biopsies, imaging is currently the only approach to assessing tumor heterogeneity in vivo. Contemporary efforts have focused on more-objective assessment of renal cell carcinoma (RCC) using quantitative techniques and/or extraction and subsequent analysis of high-dimensional data from images (ie, radiomics). Hudson et al17 compared a variety of dynamic contrast-enhanced parameters obtained from magnetic resonance imaging (MRI), computed tomography (CT), and ultrasound in patients with metastatic RCC (mRCC) who receive sunitinib. Only a change in blood volume measured by dynamic contrast-enhanced ultrasound was predictive of progression-free and overall survival.
RCC genome sequencing and identification of multiple mutations with prognostic significance have brought about attempts to associate imaging features with mutations. The ability to noninvasively characterize the genetic milieu of tumors is of special interest in RCC given intratumoral and intrapatient heterogeneity (Fig 6). Early radiogenomic studies in clear cell RCC have found associations between KDM5C and BAP1 mutations and renal vein invasion and BAP1 and ill-defined tumor margins and calcification, respectively.18,19 A challenge of such approaches is that, beyond Von Hippel-Lindau mutations, other mutations have a relatively low frequency and thus require larger data sets.
Jamshidi et al20 evaluated a 28-trait image array from preoperative CT images of clear cell RCC and identified four features (ie, pattern of necrosis, sharp or infiltrating transition zone between tumor and normal renal parenchyma, discrete enhancing rim or hypoattenuating rim that circumscribes the tumor) that correlated with a previously validated prognostic gene signature. These four traits, when combined into a radiogenomic risk score, predicted outcomes in patients with surgically resected clear cell RCCs. The radiogenomic risk score was trained and validated in independent data sets and was predictive of disease-specific survival independent of stage, grade, and performance status.
Take-Home Points
Multiparametric MRI and contrast-enhanced CT are useful tools to predict histologic diagnosis in renal masses. Noninvasive renal mass characterization with imaging aids in management by avoiding unnecessary surgeries in benign masses (angiomyolipoma, oncocytoma) and improving candidate selection for active surveillance versus definitive treatment.
Imaging features of malignant renal masses and aggressive behavior have also been defined. CT and MRI of malignant renal masses aid in staging and surgical planning, and scoring systems have been developed to predict surgical complexity and the risk of complications.
In general, contrast-enhanced CT is the mainstay imaging technique for metastatic surveillance in patients without contraindications to contrast administration (patients with history of allergy to iodinated contrast and/or elevated estimated glomerular filtration rate). Contrast-enhanced MRI can be performed for surveillance in patients with renal impairment when group II gadolinium-based contrast agents are administered because of the sufficiently low or potentially nonexistent risks of nephrogenic systemic fibrosis associated with these agents.
Response assessment in patients with metastatic disease is conventionally according to Response Evaluation Criteria in Solid Tumors (RECIST) guidelines, although adjunct methods have been developed to capture the spectrum of response and progression in patients treated with molecularly targeted therapies and immune checkpoint inhibitors.
Contemporary RCC imaging research efforts aim to develop quantitative techniques to noninvasively characterize tumors’ genetic features and predict outcomes.
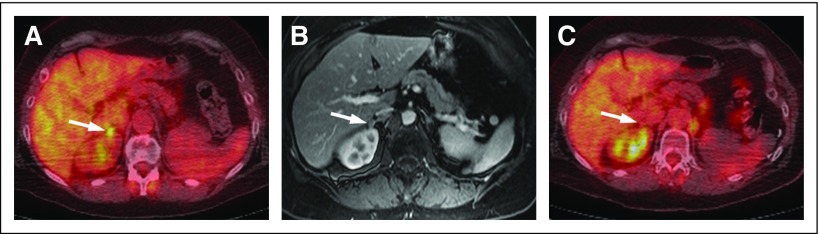
Fig A1.
Fifty-seven-year-old woman with metastatic renal cell carcinoma to the right adrenal gland. (A) Axial fused [18F]fluorodeoxyglucose (FDG) positron emission tomography/computed tomography image demonstrates a new (ie, compared with prior imaging [not shown]), small, FDG-avid right-side adrenal nodule (arrow) that represents a new site of oligometastatic disease. (B) Axial fat-suppressed T1-weighted contrast-enhanced magnetic resonance image confirms the presence of a small, right-sided adrenal nodule (arrow). (C) Follow-up axial fused FDG positron emission tomography/computed tomography image after microwave ablation demonstrates resolution of the focal-intense FDG uptake (arrow), which suggests adequate coverage of the lesion. No new sites of disease were present elsewhere.
Table A1.
Characteristic Imaging Features of Most Common Epithelial Renal Neoplasms
Table A2.
Imaging-Based Treatment Evaluation Criteria
Supplemental References
Atri M, Tabatabaeifar L, Jang HJ, et al: Accuracy of Contrast-enhanced US for Differentiating Benign from Malignant Solid Small Renal Masses. Radiology 276:900-8, 2015
Jinzaki M, Silverman SG, Akita H, et al: Renal angiomyolipoma: a radiological classification and update on recent developments in diagnosis and management. Abdom Imaging 39:588-604, 2014
Pierorazio PM, Hyams ES, Tsai S, et al: Multiphasic enhancement patterns of small renal masses (</=4 cm) on preoperative computed tomography: utility for distinguishing subtypes of renal cell carcinoma, angiomyolipoma, and oncocytoma. Urology 81:1265-71, 2013
Hindman N, Ngo L, Genega EM, et al: Angiomyolipoma with minimal fat: can it be differentiated from clear cell renal cell carcinoma by using standard MR techniques? Radiology 265:468-77, 2012
Sasiwimonphan K, Takahashi N, Leibovich BC, et al: Small (<4 cm) renal mass: differentiation of angiomyolipoma without visible fat from renal cell carcinoma utilizing MR imaging. Radiology 263:160-8, 2012
Gorin MA, Rowe SP, Baras AS, et al: Prospective Evaluation of (99m)Tc-sestamibi SPECT/CT for the Diagnosis of Renal Oncocytomas and Hybrid Oncocytic/Chromophobe Tumors. Eur Urol 69:413-6, 2016
Kim JI, Cho JY, Moon KC, et al: Segmental enhancement inversion at biphasic multidetector CT: characteristic finding of small renal oncocytoma. Radiology 252:441-8, 2009
Kay FU, Canvasser NE, Xi Y, et al: Diagnostic Performance and Interreader Agreement of a Standardized MR Imaging Approach in the Prediction of Small Renal Mass Histology. Radiology 287:543-553, 2018
Sidhar K, McGahan JP, Early HM, et al: Renal Cell Carcinomas: Sonographic Appearance Depending on Size and Histologic Type. J Ultrasound Med 35:311-20, 2016
Zhang Y, Udayakumar D, Cai L, et al: Addressing metabolic heterogeneity in clear cell renal cell carcinoma with quantitative Dixon MRI. JCI Insight 2, 2017
Sun MR, Ngo L, Genega EM, et al: Renal cell carcinoma: dynamic contrast-enhanced MR imaging for differentiation of tumor subtypes--correlation with pathologic findings. Radiology 250:793-802, 2009
Pedrosa I, Sun MR, Spencer M, et al: MR imaging of renal masses: correlation with findings at surgery and pathologic analysis. Radiographics 28:985-1003, 2008
Rosenkrantz AB, Matza BW, Portnoy E, et al: Impact of size of region-of-interest on differentiation of renal cell carcinoma and renal cysts on multi-phase CT: preliminary findings. Eur J Radiol 83:239-44, 2014
Egbert ND, Caoili EM, Cohan RH, et al: Differentiation of Papillary Renal Cell Carcinoma Subtypes on CT and MRI. AJR Am J Roentgenol 201:347-55, 2013
Rosenkrantz AB, Sekhar A, Genega EM, et al: Prognostic implications of the magnetic resonance imaging appearance in papillary renal cell carcinoma. Eur Radiol 23:579-87, 2013
Schieda N, Al-Subhi M, Flood TA, et al: Diagnostic accuracy of segmental enhancement inversion for the diagnosis of renal oncocytoma using biphasic computed tomography (CT) and multiphase contrast-enhanced magnetic resonance imaging (MRI). Eur Radiol 24:2787-94, 2014
Hudson JM, Bailey C, Atri M, et al: The prognostic and predictive value of vascular response parameters measured by dynamic contrast-enhanced-CT, -MRI and -US in patients with metastatic renal cell carcinoma receiving sunitinib. Eur Radiol, 2018
Karlo CA, Di Paolo PL, Chaim J, et al: Radiogenomics of clear cell renal cell carcinoma: associations between CT imaging features and mutations. Radiology 270:464-71, 2014
Shinagare AB, Vikram R, Jaffe C, et al: Radiogenomics of clear cell renal cell carcinoma: preliminary findings of The Cancer Genome Atlas-Renal Cell Carcinoma (TCGA-RCC) Imaging Research Group. Abdom Imaging 40:1684-92, 2015
Jamshidi N, Jonasch E, Zapala M, et al: The Radiogenomic Risk Score: Construction of a Prognostic Quantitative, Noninvasive Image-based Molecular Assay for Renal Cell Carcinoma. Radiology 277:114-23, 2015
Footnotes
Supported by National Cancer Institute grants U01 CA207091 (to I.P.), P50CA196516 (to I.P.), and R01CA154475 (to I.P.).
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Imaging Advances in the Management of Kidney Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Katherine M. Krajewski
Employment: Ironwood Pharmaceuticals (I)
Stock or Other Ownership: Ironwood Pharmaceuticals (I)
Honoraria: ASCO
Ivan Pedrosa
Honoraria: Dava Oncology
Research Funding: Philips Healthcare (Inst), Siemens Healthineers (Inst)
Patents, Royalties, Other Intellectual Property: Co-inventor of patents with Philips Healthcare; no royalties received
REFERENCES
- 1.Chow WH, Devesa SS, Warren JL, et al. : Rising incidence of renal cell cancer in the United States. JAMA 281:1628-1631, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Laguna MP, Algaba F, Cadeddu J, et al. : Current patterns of presentation and treatment of renal masses: A Clinical Research Office of the Endourological Society prospective study. J Endourol 28:861-870, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Mallin K, Ritchey J, et al. : Decreasing size at diagnosis of stage 1 renal cell carcinoma: Analysis from the National Cancer Data Base, 1993 to 2004. J Urol 179:2131-2135, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Johnson DC, Vukina J, Smith AB, et al. : Preoperatively misclassified, surgically removed benign renal masses: A systematic review of surgical series and United States population level burden estimate. J Urol 193:30-35, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Kutikov A, Fossett LK, Ramchandani P, et al. : Incidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imaging. Urology 68:737-740, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Perez-Ordonez B, Hamed G, Campbell S, et al. : Renal oncocytoma: A clinicopathologic study of 70 cases. Am J Surg Pathol 21:871-883, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Gorin MA, Rowe SP, Baras AS, et al. : Prospective evaluation of (99m)Tc-sestamibi SPECT/CT for the diagnosis of renal oncocytomas and hybrid oncocytic/chromophobe tumors. Eur Urol 69:413-416, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Kim JK, Kim TK, Ahn HJ, et al. : Differentiation of subtypes of renal cell carcinoma on helical CT scans. AJR Am J Roentgenol 178:1499-1506, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Herts BR, Coll DM, Novick AC, et al. : Enhancement characteristics of papillary renal neoplasms revealed on triphasic helical CT of the kidneys. AJR Am J Roentgenol 178:367-372, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Sun MR, Ngo L, Genega EM, et al. : Renal cell carcinoma: Dynamic contrast-enhanced MR imaging for differentiation of tumor subtypes--correlation with pathologic findings. Radiology 250:793-802, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Patel HD, Johnson MH, Pierorazio PM, et al. : Diagnostic accuracy and risks of biopsy in the diagnosis of a renal mass suspicious for localized renal cell carcinoma: Systematic review of the literature. J Urol 195:1340-1347, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedrosa I, Chou MT, Ngo L, et al. : MR classification of renal masses with pathologic correlation. Eur Radiol 18:365-375, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Kay FU, Canvasser NE, Xi Y, et al. : Diagnostic performance and interreader agreement of a standardized MR imaging approach in the prediction of small renal mass histology. Radiology 287:543-553, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canvasser NE, Kay FU, Xi Y, et al. : Diagnostic accuracy of multiparametric magnetic resonance imaging to identify clear cell renal cell carcinoma in cT1a renal masses. J Urol 198:780-786, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodelzon K, Mussi TC, Babb JS, et al. : Prediction of growth rate of solid renal masses: Utility of MR imaging features--preliminary experience. Radiology 262:884-893, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Barr RG, Peterson C, Hindi A: Evaluation of indeterminate renal masses with contrast-enhanced US: A diagnostic performance study. Radiology 271:133-142, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Mileto A, Allen BC, Pietryga JA, et al. : Characterization of incidental renal mass with dual-energy CT: Diagnostic accuracy of effective atomic number maps for discriminating nonenhancing cysts from enhancing masses. AJR Am J Roentgenol 209:W221-W230, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Lassel EA, Rao R, Schwenke C, et al. : Diffusion-weighted imaging of focal renal lesions: A meta-analysis. Eur Radiol 24:241-249, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Lanzman RS, Robson PM, Sun MR, et al. : Arterial spin-labeling MR imaging of renal masses: Correlation with histopathologic findings. Radiology 265:799-808, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corica FA, Iczkowski KA, Cheng L, et al. : Cystic renal cell carcinoma is cured by resection: A study of 24 cases with long-term followup. J Urol 161:408-411, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Bosniak MA: The current radiological approach to renal cysts. Radiology 158:1-10, 1986 [DOI] [PubMed] [Google Scholar]
- 22.Hindman NM, Hecht EM, Bosniak MA: Follow-up for Bosniak category 2F cystic renal lesions. Radiology 272:757-766, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Hindman NM: Imaging of cystic renal masses. Radiol Clin North Am 55:259-277, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Israel GM, Bosniak MA: How I do it: Evaluating renal masses. Radiology 236:441-450, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Park JJ, Jeong BC, Kim CK, et al. : Postoperative outcome of cystic renal cell carcinoma defined on preoperative imaging: A retrospective study. J Urol 197:991-997, 2017 [DOI] [PubMed] [Google Scholar]
- 26. doi: 10.2214/AJR.14.13149. Smith AD, Allen BC, Sanyal R, et al: Outcomes and complications related to the management of Bosniak cystic renal lesions. AJR Am J Roentgenol 204:W550-W566, 2015. [DOI] [PubMed] [Google Scholar]
- 27. doi: 10.1016/j.juro.2017.09.078. Chandrasekar T, Ahmad AE, Fadaak K, et al: Natural history of complex renal cysts: Clinical evidence supporting active surveillance. J Urol 199:633-340, 2017. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita Y, Honda S, Nishiharu T, et al. : Detection of pseudocapsule of renal cell carcinoma with MR imaging and CT. AJR Am J Roentgenol 166:1151-1155, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Pretorius ES, Siegelman ES, Ramchandani P, et al. : Renal neoplasms amenable to partial nephrectomy: MR imaging. Radiology 212:28-34, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Hill H, Christie A, et al. : Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539:112-117; 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jamshidi N, Jonasch E, Zapala M, et al. : The radiogenomic risk score stratifies outcomes in a renal cell cancer phase 2 clinical trial. Eur Radiol 26:2798-2807, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Rosenkrantz AB, Sekhar A, Genega EM, et al. : Prognostic implications of the magnetic resonance imaging appearance in papillary renal cell carcinoma. Eur Radiol 23:579-587, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gayed BA, Youssef R, Darwish O, et al. : Multi-disciplinary surgical approach to the management of patients with renal cell carcinoma with venous tumor thrombus: 15 year experience and lessons learned. BMC Urol 16:43, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Psutka SP, Boorjian SA, Thompson RH, et al. : Clinical and radiographic predictors of the need for inferior vena cava resection during nephrectomy for patients with renal cell carcinoma and caval tumour thrombus. BJU Int 116:388-396, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Kutikov A, Uzzo RG: The R.E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 182:844-853, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Stat Facts: Kidney and Renal Pelvis Cancer. https://seer.cancer.gov/statfacts/html/kidrp.html.
- 37.Motzer RJ, Jonasch E, Agarwal N, et al. : Kidney cancer, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 15:804-834, 2017 [DOI] [PubMed] [Google Scholar]
- 38.Stewart-Merrill SB, Thompson RH, Boorjian SA, et al. : Oncologic surveillance after surgical resection for renal cell carcinoma: A novel risk-based approach. J Clin Oncol 33:4151-4157, 2015 [DOI] [PubMed] [Google Scholar]
- 39. doi: 10.1016/j.juro.2009.07.004. Campbell SC, Novick AC, Belldegrun A, et al: Guideline for management of the clinical T1 renal mass. J Urol 182:1271-1279, 2009. [DOI] [PubMed]
- 40. Bianchi M, Sun M, Jeldres C, et al: Distribution of metastatic sites in renal cell carcinoma: A population-based analysis. Ann Oncol23:973-980, 2012. [DOI] [PubMed]
- 41. American College of Radiology: Manual on Contrast Media Version, 2017. https://www.acr.org/Clinical-Resources/Contrast-Manual.
- 42.Davenport MS, Cohan RH, Ellis JH: Contrast media controversies in 2015: Imaging patients with renal impairment or risk of contrast reaction. AJR Am J Roentgenol 204:1174-1181, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Palmowski M, Hacke N, Satzl S, et al. : Metastasis to the pancreas: Characterization by morphology and contrast enhancement features on CT and MRI. Pancreatology 8:199-203, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Raptopoulos VD, Blake SP, Weisinger K, et al. : Multiphase contrast-enhanced helical CT of liver metastases from renal cell carcinoma. Eur Radiol 11:2504-2509, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Choi YA, Kim CK, Park BK, et al. : Evaluation of adrenal metastases from renal cell carcinoma and hepatocellular carcinoma: Use of delayed contrast-enhanced CT. Radiology 266:514-520, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Schieda N, Krishna S, McInnes MDF, et al. : Utility of MRI to differentiate clear cell renal cell carcinoma adrenal metastases from adrenal adenomas. AJR Am J Roentgenol 209:W152-W159, 2017 [DOI] [PubMed] [Google Scholar]
- 47.Staudenherz A, Steiner B, Puig S, et al. : Is there a diagnostic role for bone scanning of patients with a high pretest probability for metastatic renal cell carcinoma? Cancer 85:153-155, 1999 [PubMed] [Google Scholar]
- 48.Buhmann Kirchhoff S, Becker C, Duerr HR, et al. : Detection of osseous metastases of the spine: Comparison of high resolution multi-detector-CT with MRI. Eur J Radiol 69:567-573, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Kayani I, Avril N, Bomanji J, et al. : Sequential FDG-PET/CT as a biomarker of response to sunitinib in metastatic clear cell renal cancer. Clin Cancer Res 17:6021-6028, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Nakatani K, Nakamoto Y, Saga T, et al. : The potential clinical value of FDG-PET for recurrent renal cell carcinoma. Eur J Radiol 79:29-35, 2011 [DOI] [PubMed] [Google Scholar]
- 51. Gerety EL, Lawrence EM, Wason J, et al: Prospective study evaluating the relative sensitivity of 18F-NaF PET/CT for detecting skeletal metastases from renal cell carcinoma in comparison to multidetector CT and 99mTc-MDP bone scintigraphy, using an adaptive trial design. Ann Oncol 26:2113-2118, 2015. [DOI] [PMC free article] [PubMed]
- 52.Choueiri TK, Escudier B, Powles T, et al. : Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1814-1823, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grünwald V, McKay RR, Krajewski KM, et al. : Depth of remission is a prognostic factor for survival in patients with metastatic renal cell carcinoma. Eur Urol 67:952-958, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krajewski KM, Guo M, Van den Abbeele AD, et al. : Comparison of four early posttherapy imaging changes (EPTIC; RECIST 1.0, tumor shrinkage, computed tomography tumor density, Choi criteria) in assessing outcome to vascular endothelial growth factor-targeted therapy in patients with advanced renal cell carcinoma. Eur Urol 59:856-862, 2011 [DOI] [PubMed] [Google Scholar]
- 55. Thiam R, Fournier LS, Trinquart L, et al: Optimizing the size variation threshold for the CT evaluation of response in metastatic renal cell carcinoma treated with sunitinib. Ann Oncol21:936-941, 2010. [DOI] [PubMed]
- 56.Smith AD, Shah SN, Rini BI, et al. : Morphology, attenuation, size, and structure (MASS) criteria: Assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy. AJR Am J Roentgenol 194:1470-1478, 2010 [DOI] [PubMed] [Google Scholar]
- 57.van der Veldt AA, Meijerink MR, van den Eertwegh AJ, et al. : Choi response criteria for early prediction of clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Br J Cancer 102:803-809, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krajewski KM, Franchetti Y, Nishino M, et al. : 10% tumor diameter shrinkage on the first follow-up computed tomography predicts clinical outcome in patients with advanced renal cell carcinoma treated with angiogenesis inhibitors: A follow-up validation study. Oncologist 19:507-514, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith AD, Shah SN, Rini BI, et al. : Utilizing pre-therapy clinical schema and initial CT changes to predict progression-free survival in patients with metastatic renal cell carcinoma on VEGF-targeted therapy: A preliminary analysis. Urol Oncol 31:1283-1291, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Smith AD, Zhang X, Bryan J, et al. : Vascular tumor burden as a new quantitative CT biomarker for predicting metastatic RCC response to antiangiogenic therapy. Radiology 281:484-498, 2016 [DOI] [PubMed] [Google Scholar]
- 61.Motzer RJ, Escudier B, McDermott DF, et al. : Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803-1813, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Velasco G, Krajewski KM, Albiges L, et al. : Radiologic heterogeneity in responses to anti-PD-1/PD-L1 therapy in metastatic renal cell carcinoma. Cancer Immunol Res 4:12-17, 2016 [DOI] [PubMed] [Google Scholar]
- 63.Seymour L, Bogaerts J, Perrone A, et al. : iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 18:e143-e152, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolchok JD, Hoos A, O’Day S, et al. : Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res 15:7412-7420, 2009 [DOI] [PubMed] [Google Scholar]
- 65.Hoerner-Rieber J, Duma M, Blanck O, et al. : Stereotactic body radiotherapy (SBRT) for pulmonary metastases from renal cell carcinoma-a multicenter analysis of the German working group “Stereotactic Radiotherapy.” J Thorac Dis 9:4512-4522, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang CJ, Christie A, Lin MH, et al. : Safety and efficacy of stereotactic ablative radiation therapy for renal cell carcinoma extracranial metastases. Int J Radiat Oncol Biol Phys 98:91-100, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun MR, Brook A, Powell MF, et al. : Effect of stereotactic body radiotherapy on the growth kinetics and enhancement pattern of primary renal tumors. AJR Am J Roentgenol 206:544-553, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McDonald R, Probyn L, Poon I, et al. : Tumor response after stereotactic body radiation therapy to nonspine bone metastases: An evaluation of response criteria. Int J Radiat Oncol Biol Phys 93:879-881, 2015 [DOI] [PubMed] [Google Scholar]