Abstract
Context:
Blood flow–restricted training (BFRT) has been suggested to treat lower extremity muscle weakness. The efficacy of BFRT for muscle problems related to knee pathology is unclear.
Objective:
To determine whether BFRT (1) improves muscle strength and cross-sectional area (CSA) for chronic knee-related lower extremity atrophy and (2) prevents muscle atrophy after knee surgery.
Data Sources:
A systematic review of the literature from 1974 to 2017 was conducted using the PubMed and Cochrane databases.
Study Selection:
Controlled trials that used BFRT to treat chronic knee-related lower extremity muscle atrophy or to prevent muscle atrophy after knee surgery that measured the effects on quadriceps or hamstrings muscle strength or CSA were included.
Study Design:
Systematic review.
Level of Evidence:
Level 2.
Data Extraction:
Data were extracted as available from 9 studies (8 level 1, 1 level 2). Assessment of study quality was rated using the Physiotherapy Evidence Database or Methodological Index for Non-Randomized Studies instruments.
Results:
BFRT was used after anterior cruciate ligament reconstruction and routine knee arthroscopy and in patients with knee osteoarthritis or patellofemoral pain. There were a total of 165 patients and 170 controls. Vascular occlusion and exercise protocols varied; all studies except 1 incorporated exercises during occlusion, most of which focused on the quadriceps. Six of 7 studies that measured quadriceps strength reported statistically significant improvements after training. Few benefits in quadriceps CSA were reported. Hamstrings strength was only measured in 2 studies. There were no complications related to training.
Conclusion:
Published limited data show BFRT to be safe and potentially effective in improving quadriceps muscle strength in patients with weakness and atrophy related to knee pathology. The use of short-duration vascular occlusion and light-load resistance exercises appears safe after knee surgery or in arthritic knees. This treatment option requires further investigation to refine protocols related to cuff pressure and exercise dosage and duration.
Keywords: blood flow resistance training, quadriceps strengthening, resistance training
Weakness and atrophy of the quadriceps and hamstrings is a common problem in patients who have noteworthy chronic osteoarthritis and after major operations such as anterior cruciate ligament (ACL) reconstruction.6,19,44 Patients with patellofemoral or tibiofemoral arthritis may have difficulty achieving strength gains even with formal rehabilitation due to pain incurred with heavy-load resistance exercises.8,32,45 The American College of Sports Medicine recommends a minimum resistance training load of 60% to 70% of 1 repetition maximum (1 RM) to gain strength and 70% to 85% of 1 RM to achieve muscle hypertrophy.1 Training with these high loads may not be possible or may even be deleterious in painful arthritic knees. Many studies have reported lingering quadriceps weakness many months or even years after ACL reconstruction that impairs return to normal function.16,30 This occurs despite the immediate implementation of physical therapy and preventative measures such as electrical muscle stimulation, early weightbearing, and use of both closed and open kinetic chain exercises. With the focus of surgery and rehabilitation targeting return to preinjury sports activity levels in many investigations, the prevention of muscle atrophy and early recovery of muscle strength and neuromuscular function are considered paramount for athletic patients.2,3,28
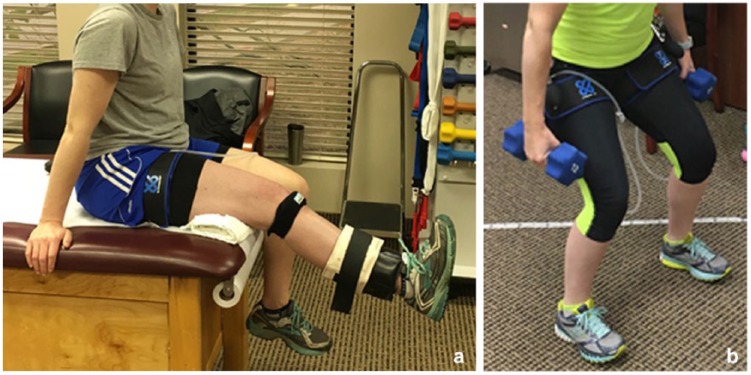
Recently, studies have begun to explore the use of blood flow–restricted training (BFRT) with low-resistance loads (such as 30% of 1 RM) in individuals who cannot tolerate high-load resistance training (Figure 1). Many investigations have shown a positive benefit of BFRT in healthy participants and athletes21,23,36,41 and in elderly individuals.7,31,33,39,46,48 Various hypotheses for the potential effectiveness of BFRT in increasing muscle strength and hypertrophy have been proposed. Hughes et al13 hypothesized that an ischemic and hypoxic muscular environment is generated during BFRT that causes high levels of metabolic stress and mechanical tension when exercise is combined with training. Metabolic stress and mechanical tension have been theorized to activate various mechanisms that induce muscle growth, such as elevated systematic hormone production, cell swelling, production of reactive oxygen species, intramuscular anabolic/anticatabolic signaling, and increased fast-twitch fiber recruitment.
Figure 1.
Examples of blood flow restriction exercise training that may be done nonweightbearing, such as (a) during knee extension, or weightbearing, such as (b) during partial squatting.
Hughes et al13 systematically reviewed 20 studies in which BFRT was used for clinical musculoskeletal rehabilitation. These authors concluded that low-load BFRT had a moderate effect on increasing strength but was less effective than heavy-load training. This review contained a wide variety of studies involving ACL reconstruction, knee osteoarthritis, older adults at risk for sarcopenia, and patients with sporadic inclusion body myositis. All types of upper and lower body BFRT were included, such as elastic band resistance training, low- to moderate-intensity walk training, body weight exercises, and low-load resistance training. Because of the heterogenic nature of this review, the effect of low-load resistance BFRT on muscle weakness and atrophy specifically related to knee pathology remains uncertain.
The purposes of this systematic review were to determine whether BFRT is effective in (1) improving quadriceps and hamstrings strength and cross-sectional area (CSA) for chronic knee-related lower extremity muscle atrophy and (2) preventing muscle atrophy after knee surgery.
Methods
Literature Search Strategy
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed in conducting this study.20 An online search was performed using PubMed for all years through 2017 and the key phrases and words blood flow restriction training, blood flow restricted exercise, occlusion resistance training, KAATSU training, and low load resistance training. The full text was accessed if the abstract suggested that this might be a clinical study in the topic of interest. In addition, reference lists from general review articles, systematic reviews, and meta-analyses obtained from the search were examined to find any other original research investigations not otherwise obtained.
Study Selection and Quality Assessment
To be included in the review, studies were required to be a controlled trial (randomized or nonrandomized), be published in English, use BFRT either to treat chronic lower extremity muscle atrophy or to prevent muscle atrophy after a knee operation, and report a measured effect of BFRT on quadriceps and/or hamstrings muscle strength or CSA.
Exclusion criteria included studies that (1) were off-topic; (2) investigated the acute effects of BFRT (ie, after 1 training session); (3) included trained healthy participants; (4) concerned trained patients with cardiovascular disease, obesity, or polymyositis; (5) included trained elderly patients; (6) included trained patients after voluntary immobilization; (7) included trained upper body muscles; and (8) did not provide measurement of quadriceps or hamstrings strength or CSA. General or systematic reviews, meta-analyses, editorials, case series, case reports, and laboratory animal studies were also excluded.
Study quality was evaluated using the Physiotherapy Evidence Database (PEDro) scale27 for randomized investigations or the Methological Index for Non-Randomized Studies (MINORS) criteria for nonrandomized controlled trials.40 The MINORS score is reported as a percentage of the total available points, as recommended by Wylie et al.47
Data Extraction
The following data were extracted from each article when available: study design, sex, age, diagnosis, number of training sessions, cuff pressure, occlusion protocol, exercise protocol, quadriceps and hamstrings strength measurements (isokinetic, isometric, or maximum leg press), CSA measurements for quadriceps and hamstrings using magnetic resonance imaging (MRI) or ultrasound, results of muscle biopsy, data from clinical outcome instruments, and information related to pain or discomfort during training. Effect sizes (ESs) were calculated when the data were available according to Cohen5 and were interpreted as small effects (≤0.2), moderate effects (0.5), and large effects (≥0.8).
Results
The search identified 534 articles, of which 525 were excluded (Table 1), leaving 9 studies for our review. The average PEDro score in the 8 level 1 randomized studies4,8,11,17,29,37,38,42,43 was 8 (range, 6-10), and the MINORS score in the 1 nonrandomized level 2 study42 was 79%.
Table 1.
Reasons for 525 articles excluded
| Exclusion Criteria | Articles, n |
|---|---|
| Off-topic | 205 |
| Acute effects training | 92 |
| Training in healthy participants or athletes | 55 |
| Physiology-based study | 38 |
| General review | 28 |
| Upper body training | 22 |
| Training in elderly patients | 22 |
| Case report or series | 12 |
| Study effect of cuff pressure | 11 |
| Editorial | 11 |
| Systematic review/meta-analysis | 8 |
| Laboratory (animal) study | 7 |
| Effects training after immobilization | 5 |
| Study protocol only (no results) | 4 |
| Study diseases (cardiovascular, obesity, polymyositis) | 4 |
| Survey | 1 |
The effect of BFRT in preventing muscle atrophy after knee operations was assessed in 3 studies after ACL reconstruction17,29,42 and in 1 study after routine knee arthroscopy43 (Table 2). BFRT was used to treat chronic muscle weakness due to knee osteoarthritis in 4 studies4,8,37,38 and patellofemoral pain in 1 study.11 BFRT was used in 165 patients (93 women, 72 men) whose mean age was 42.2 years. There were 170 control participants (100 women, 70 men) whose mean age was 40.6 years.
Table 2.
Study protocols
| Study Group | Protocol | |||||
|---|---|---|---|---|---|---|
| Study (Level of Evidence), Diagnosis | BFRT | Control | Treatment Sessions (Total Possible) | Cuff Material, Size, Pressure | Occlusion | Exercise During Occlusion |
| Ohta et al29 (1), ACL reconstruction | 19 males 9 females Mean age, 30.0 ± 9.7 y |
12 males 10 females Mean age, 28.0 ± 9.7 y |
2×/day weeks 2-16 p.o. (210), at home |
Air tourniquet, 180 mm Hg | Inflate duration of training, maximum 15 min. Remove 15-20 min, resume if required | Straight-leg raises, hip abduction and adduction isometrics, quadriceps isometrics, half-squats, step-ups, walk in deep flexion, elastic tube squat resistance. 20 reps 2×/day |
| Takarada et al42 (2), ACL reconstruction | 4 males 4 females Mean age, 22 ± 0.9 y |
4 males 4 females Mean age, 23 ± 1.0 y |
2×/daydays 1-15 p.o. (30), in hospital |
Pneumatic cuff width 9 cm, length 70 cm. 180 mm Hg, gradually elevated 10 mm Hg depending on patient tolerance (range, 200-260 mm Hg). Placebo cuff control group | Inflate 5 min, deflate 3 min, repeat 5× | None |
| Iversen et al17 (1), ACL reconstruction | 7 males 5 females Mean age, 24.9 ± 7.4 y |
7 males 5 females Mean age, 29.8 ± 9.3 y |
2×/day days 2-16 p.o. (30), at home |
Delphi low-pressure cuff, 14-cm wide. 130 mm Hg, increased 10 mm Hg, maximum 180 mm Hg | Inflate 5 min during exercise, deflate 3 min, repeat 5× | Quadriceps isometrics, leg extensions over knee roll, straight-leg raises. 20 reps each × 5 per session |
| Tennent et al43 (1), routine knee arthroscopy | 7 males 3 females Mean age, 37.0 y |
5 males 2 females Mean age, 27.0 y |
12 sessions weeks 3-9 p.o. (12), in clinic |
Easy-Fit Tourniquet cuff, size varied based on thigh size. Set to 70% of total arterial occlusal pressure | Inflate during exercise, maximum 5 min | 30% 1 RM BFRT: leg press, knee extension, reverse press, 1 set 30 reps, 3 sets 15 reps Exercises for controls not detailed |
| Segal et al37 (1), knee osteoarthritis | 19 males Mean age, 58.4 ± 8.7 y |
22 males Mean age, 56.1 ± 7.7 y |
3×/wk × 4 wk (12), in clinic |
Kaatsu Master BFR device, width 6.5 cm, length 65 cm. 160-200 mm Hg, gradually increased during training sessions | Inflate 5 min during exercise, deflate 1.5 min rest between sets | 30% 1 RM both groups: leg press, 1 set 30 reps, 3 sets 15 reps |
| Segal et al38 (1), knee osteoarthritis | 19 females Mean age, 56.1 ± 5.9 y |
21 females Mean age, 54.6 ± 6.9 y |
3×/wk × 4 wk (12), in clinic |
Kaatsu Master BFR device, width 6.5 cm, length 65 cm. 160-200 mm Hg, gradually increased during training sessions | Inflate 5 min during exercise, deflate 1.5 min, rest between sets | 30% 1 RM both groups: leg press, knee extension, reverse press, 1 set 30 reps, 3 sets 15 reps |
| Bryk et al4 (1), knee osteoarthritis | 17 females Mean age, 62.3 ± 7.0 y |
17 females Mean age, 60.4 ± 6.7 y |
3×/wk × 6 wk(18), in clinic | Cuff details NA. 200 mm Hg | Inflate during quadriceps exercises (time NA) | 30% 1 RM BFRT, 70% 1 RM controls: knee extension machine. BFRT 3 sets 30 reps, controls 3 sets 10 reps |
| Ferraz et al8 (1), knee osteoarthritis | 12 females Mean age, 60.3 ± 3.0 y |
HR group: 10 females Mean age, 59.9 ± 4.0 y LR group: 12 females Mean age, 60.7 ± 4.0 y |
2×/wk × 12 wk (24), in clinic | Cuff details NA. Set to 70% of total arterial occlusion pressure. Mean, 97.4 mm Hg |
Inflate during exercises and rest periods | 30% 1 RM BFRT and LR controls, 80% 1 RM HR controls. 4-5 sets × 10 reps |
| Giles et al11 (1), patellofemoral pain | 16 males 24 females Mean age, 28.5 ± 5.2 y |
20 males 19 females Mean age, 26.7 ± 5.5 y |
3×/wk × 8 wk (24), in clinic |
Cuff details NA. Set to 60% of total arterial occlusion pressure. Placebo cuff control group |
Inflate during exercise (time NA), deflate 30 s, rest between sets | 30% 1 RM BFRT, 70% 1 RM controls: leg press, knee extension. BFRT 1 set 30 reps, 3 sets 15 reps. Controls 3 sets 7-10 reps |
ACL, anterior cruciate ligament; BFRT, blood flow–restricted training; HR, high resistance; LR, low resistance; NA, not available; p.o., postoperative; reps, repetitions; RM, repetition maximum.
Four studies4,8,37,38 used power analyses to determine sample sizes, and 2 investigations8,11 calculated ESs in addition to P values. Data provided in the investigations allowed the calculation of ES in 2 additional studies.4,29
Occlusion Protocols
The vascular occlusion protocols varied in all studies except 2 from the same investigators in which the cuff pressure was gradually increased (from 160-200 mm Hg) during individual training sessions.37,38 Three studies8,11,43 set cuff pressures to a percentage of the total arterial occlusion pressure (60%-70%), 2 studies4,29 set the pressure to 1 value for all patients throughout the duration of the investigation (180-200 mm Hg), and 2 studies17,42 gradually increased the pressure throughout the duration of the investigation. Typically, occlusion was maintained during exercise sets and deflated during rest between sets. Placebo cuffs were used in the control group in 2 studies.11,42
Exercise Protocols
All studies except 142 incorporated exercises that were done with vascular occlusion. In 6 investigations,4,8,11,37,38,43 a percentage of 1 RM was selected for lower extremity exercises, and in 2 studies,17,29 low-load training was done, but not according to a percentage of 1 RM (Table 2). While some investigations focused solely on quadriceps strengthening, others included hamstrings exercises. Although not evaluated as part of the BFRT protocol, hip (abduction-adduction side-lying leg raises and Theraband resistance) and gastrocnemius-soleus exercises were also included in 2 studies.4,29
Muscle Strength
Quadriceps muscle strength was measured isokinetically in 4 studies (Table 3).29,37,38,43 The BFRT group had significantly greater improvements (measured at 60 deg/s) compared with controls in 3 investigations, with the largest magnitude of improvement noted in the study by Tennent et al.43 Two other studies4,11 measured quadriceps strength isometrically, and although neither of these studies reported a significant between-group difference, both the BFRT and control group had significant within-group improvements.
Table 3.
Changes in quadriceps strength and muscle cross-sectional area after training a
| Quadriceps Strength | Muscle CSA, Biopsy | |||||
|---|---|---|---|---|---|---|
| Study | Data | Within-Group | Between-Groups | Data | Within-Group | Between-Groups |
| Ohta et al29
ACL reconstruction 16 wk postop |
Involved-uninvolved ratios | MRI CSA I/N ratios (preop: 16 wk postop) | ||||
| 60 deg/s: BFRT, 76% ± 16%; control, 55% ± 17% | NA | P < 0.001, ES = 1.4 | Quadriceps: BFRT, 101% ± 11%; control, 92% ± 12% | NA | P = 0.04, ES = 0.78 | |
| 180 deg/s: BFRT, 77% ± 13%; control, 65% ± 13% | NA | P = 0.004, ES = 0.92 | Hamstrings: BFRT, 105% ± 19%; control, 102% ± 23% | NA | P = NS | |
| Isometric: BFRT, 84% ± 19%; control, 63% ± 19% | NA | P < 0.001, ES = 1.10 | Diameters type 1, 2 fibers medial vastus lateralis | NA | P = NS | |
| Takarada et al42
ACL reconstruction |
Not measured | MRI CSA % decrease from 3rd to 14th postop day | ||||
| Quadriceps: BFRT, 9.4% ± 1.6%; control, 20.7% ± 2.2% | P < 0.05 |
P < 0.05, ES = −5.87 |
||||
| Hamstrings: BFRT, 9.2% ± 2.6%; control, 11.3% ± 2.6% | P < 0.05 | P = NS | ||||
| Iversen et al17
ACL reconstruction |
Not measured | MRI CSA % decrease from 2nd to 16th postop day | ||||
| Quadriceps: BFRT, 13.8% ± 1.1%; control, 13.1% ± 1.0% | NA | P = NS | ||||
| Tennent et al43
Knee arthroscopy 9 wk postop |
60 deg/s N·m/kg (% improvement b ) | P = 0.03 | Not done | |||
| BFRT pre: 99.83; post: 211.92 (75%) | P = 0.002 | |||||
| Control pre: 126.7; post: 171.5 (33.5%) | P = 0.2 | |||||
| Segal et al37
Knee osteoarthritis males 4 wk posttraining |
60 deg/s N·m mean increase (% improvement) | P = NS | Not done | |||
| BFRT: –0.1 ± 3.3 N·m (0.4%) | P = NS | |||||
| Control: 7.0 ± 3.0 N·m (6.7%) | P < 0.05 | |||||
| Leg press 1 RM kg mean increase (% improvement) | P = NS | |||||
| BFRT: 11.3 ± 14.0 (3.1%) | P = 0.003 | |||||
| Control: 13.5 ± 16.8 (4.7%) | P < 0.002 | |||||
| Segal et al38
Knee osteoarthritis females 4 wk posttraining |
60 deg/s N·m/kg mean increase | P < 0.01 | MRI volume % increase | P = NS | ||
| BFRT: 0.07 ± 0.03 | P = 0.02 | Quadriceps: BFRT, 1.3% ±0.80%; control, 0.01% ± 0.73% | ||||
| Control: −0.05 ± 0.03 | P = 0.05 | |||||
| Leg press 1 RM kg mean increase | P < 0.05 | |||||
| BFRT: 28.3 ± 4.8 | P < 0.001 | |||||
| Control: 15.6 ± 4.5 | P = 0.005 | |||||
| Bryk et al4
Knee osteoarthritis 6 wk posttraining |
Isometric kg force/kg body weight (% improvement) | P = NS | Not done | |||
| BFRT pre: 23.2 ± 8.4; post: 40.0 ± 0.2 (17%) | P = 0.001, ES = 2.83 | |||||
| Control pre: 24.1 ± 10.1; post: 33.5 ± 12.9 (9%) | P = 0.001, ES = 0.81 | |||||
| Ferraz et al8 Knee osteoarthritis 12 wk posttraining |
Leg press 1 RM % improvement | CT CSA % increase quadriceps | ||||
| BFRT: 26% |
P < 0.0001 ES = 1.01 |
P = 0.0004 vs LR P = NS vs HR |
||||
| High-resistance group: 33% |
P < 0.0001 ES = 0.82 |
P < 0.0001 vs LR | BFRT: 7% |
P < 0.0001 ES = 0.39 |
P = 0.02 vs LR P = NS vs HR |
|
| Low-resistance group: 8% |
P = NS ES = 0.23 |
|||||
| Knee extension 1 RM % improvement | High-resistance group: 8% |
P < 0.0001 ES = 0.54 |
P = 0.007 vs LR | |||
| BFRT: 23% |
P < 0.0001 ES = 0.86 |
P = 0.0005 vs LR P = NS vs HR |
||||
| High-resistance group: 22% |
P < 0.0001 ES = 0.83 |
P = 0.0004 vs LR | Low-resistance group: 2% |
P = NS ES = 0.12 |
||
| LR control: 7% |
P = NS ES = 0.21 |
|||||
| Giles et al11
Patellofemoral pain 8 wk posttraining |
Isometric N·m (% improvement) | P = NS | Ultrasound quadriceps size (cm) | P = NS | ||
| BFRT pre: 131.2 ± 61.9; post: 166.4 ± 59.4 (27%) | P < 0.001, ES = 0.58 | BFTR pre: 7.9 ± 1.3; post: 8.0 ± 1.1 | NS | |||
| Control pre: 135.1 ± 55; post: 158.7 ± 57.4 (17%) | P < 0.001, ES = 0.42 | Control pre: 7.7 ± 1.4; post: 7.9 ± 1.2 | NS | |||
ACL, anterior cruciate ligament; BFRT, blood flow–restricted training; CSA, cross-sectional area; CT, computed tomography; ES, effect size; HR, high resistance; I/N, involved/noninvolved; LR, low resistance; MRI, magnetic resonance imaging; NA, not available; NS, not significant; RM, repetition maximum.
Data are shown as means ± SDs (when available). Percentage improvement data provided when available. ESs calculated according to Cohen when possible.
When outliers (1 from each group) removed.
Segal et al37,38 determined changes in 1 RM by comparing the pretraining leg press value with that obtained on completion of the investigation. While there was no between-group difference in this value in men with knee osteoarthritis, women with osteoarthritis in the BFRT group had a greater increase compared with controls (mean improvement, 28.3 kg and 15.6 kg, respectively; P < 0.05). Ferraz et al8 determined changes in 1 RM in leg press and knee extension values between the BFRT group and 2 control groups. Patients in the high-resistance control group had similar significant improvements in these values to those in the BRFT group. However, patients in the low-resistance control group failed to significantly improve these strength indices.
Hamstrings strength was measured in only 2 studies.29,43 Ohta et al29 reported that ratios of the involved limb/uninvolved limb showed significant differences between the BFRT and control groups 16 weeks after ACL reconstruction at 60 deg/s (81% ± 14% and 72% ± 15%, respectively; P = 0.05; ES, 0.62) and at 180 deg/s (84% ± 18% and 74% ± 12%, respectively; P = 0.04; ES, 0.65). Tennent et al43 reported significant improvements in isokinetic hamstrings strength in both the BFRT (99.83-141.68 N·m/kg; P = 0.002) and control groups (105.51-132.71 N·m/kg; P < 0.05); however, there was no between-group significant difference. Of note, the BFRT group had a larger deficit in hamstring strength at the onset of the study (approximately 3 weeks postoperatively) compared with the control group (31.09 and 7.77 N·m/kg, respectively).
Muscle CSA
The 3 studies on ACL reconstruction all measured CSA using MRI on completion of BFRT, which varied from 16 days to 16 weeks postoperatively.17,29,42 Ohta et al29 reported a significant between-group effect for the quadriceps involved/uninvolved ratio but not for the hamstrings ratio. The BFRT group in the study by Takarada et al42 had a significantly smaller percentage decrease in quadriceps CSA compared with the control group (9.4% and 20.7%, respectively; P < 0.05; ES, –5.87) 15 days postoperatively; however, there was no between-group difference in the percentage decrease in hamstrings CSA. Iversen et al17 reported no between-group difference in the percentage decrease in quadriceps CSA measured 16 days postoperatively.
Segal et al38 detected negligible increases of quadriceps volume after 4 weeks of training women with knee osteoarthritis in both the BFRT and the control groups (1.3% and 0.01%, respectively). Giles et al11 used ultrasound to measure quadriceps muscle thickness in patients with patellofemoral pain and reported minimal change in size in either group after 8 weeks of training. Ferraz et al8 reported significant increases in CSA measured with computed tomography in both the BRFT group (7% increase; P < 0.0001; ES, 0.39) and the high-resistance control group (8% increase; P < 0.0001; ES, 0.54), but no change in the low-resistance control group.
Outcome Scales
Outcome instruments were used in 6 studies (Table 4).4,8,11,37,38,43 Overall, significant improvements were reported in symptoms and function in both the BFRT and the control groups at the conclusion of these investigations, but few between-group differences were found. For instance, Bryk et al4 reported significant improvements in the Lequesne scale after 6 weeks of training in both BFRT and control groups; however, the BFRT group had less anterior knee pain during training sessions. Giles et al11 reported no differences between BFRT and control patients in the Kujala patellofemoral score after training, but the BFRT group reported a greater reduction in pain with daily activities (ES, 0.53).
Table 4.
Effect of training on outcome scales
| BFRT Group | Control Group | |||||||
|---|---|---|---|---|---|---|---|---|
| Study | Outcome Scale | Pre-training | Post-training | P | Pre-training | Post-training | P | Between-Group Comparison, P |
| Tennent et al43
Knee arthroscopy |
KOOS | |||||||
| Pain | 52.8 | 75.0 | 0.0001 | 69.4 | 77.8 | 0.04 | NS | |
| Symptoms | 47.1 | 76.8 | 0.003 | 67.9 | 71.4 | NS | NS | |
| ADL | 58.1 | 88.2 | 0.0009 | 73.5 | 75.0 | NS | NS | |
| QOL | 31.3 | 59.3 | 0.003 | 43.8 | 62.5 | NS | NS | |
| Sport | 10.0 | 47.5 | 0.0009 | 35.0 | 70.0 | 0.04 | NS | |
| VR-12 | ||||||||
| PCS | 0.86 | 46.3 | 0.001 | 36.5 | 47.7 | 0.04 | NS | |
| MCS | 51.20 | 60.2 | 0.04 | 57.6 | 56.2 | NS | 0.01 | |
| Segal et al37
Knee osteoarthritis, males |
KOOS | |||||||
| Pain | ~83 | ~86 | NS | ~76 | ~81 | NS | NS | |
| Segal et al38
Knee osteoarthritis, females |
KOOS | |||||||
| Pain | ~80 | ~82 | NS | ~76 | ~78 | NS | NS | |
| Bryk et al4
Knee osteoarthritis |
Lequesne scale | 11.5 | 6.5 | 0.001 | 13.0 | 7.0 | 0.001 | NS |
| VAS knee pain | 6.5 | 3.2 | 0.001 | 6.0 | 3.5 | 0.001 | NS | |
| VAS knee pain with training | — | 2.5 | — | — | 6.2 | — | 0.01 | |
| Ferraz et al8
Knee osteoarthritis |
WOMAC | HR | HR | |||||
| Pain | 6.9 | 4.0 | 0.02 | 7.2 | 4.0 | NS | NP | |
| Stiffness | 3.6 | 2.1 | 0.01 | 3.5 | 2.0 | NS | NP | |
| Physical function | 21.0 | 10.3 | 0.02 | 25.9 | 14.6 | 0.02 | NP | |
| Total score | 31.5 | 17.1 | 0.008 | 36.6 | 21.2 | 0.02 | NP | |
| WOMAC | LR | LR | ||||||
| Pain | 7.9 | 4.0 | 0.001 | NP | ||||
| Stiffness | 2.8 | 1.8 | NS | NP | ||||
| Physical function | 24.4 | 12.7 | NS | NP | ||||
| Total score | 35.1 | 18.4 | 0.005 | NP | ||||
| Giles et al11
Patellofemoral pain |
Kujala patellofemoral score | 73.6 | 86.5 | <0.001 | 72.6 | 83.2 | <0.001 | NS, ES = 0.23 |
| VAS worst pain | 55.7 | 27.4 | <0.001 | 51.4 | 25.6 | <0.001 | NS, ES = 0.27 | |
| VAS ADL | 58.2 | 21.6 | <0.001 | 42.5 | 24.1 | < 0.001 | 0.02, ES = 0.53 | |
ADL, activities of daily living; BFRT, blood flow–restricted training; ES, effect size; HR, high resistance; KOOS, Knee injury and Osteoarthritis Outcome Scores; LR, low resistance; MCS, mental component score; NP, not provided; NS, not significant; PCS, physical component score; QOL, quality of life; VAS, visual analog scale; VR-12, Veterans RAND 12-Item Health Survey; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Recommendations for BFRT
Overall, 6 of the 8 studies concluded BFRT was effective and should be considered after ACL reconstruction,29,42 after routine knee arthroscopy,43 in arthritic knees,4,38 and in cases of patellofemoral pain (Table 5).11 Segal et al37 found no benefit in 19 men with knee arthritis, and Iversen et al17 reported unsatisfactory results in 12 patients after ACL reconstruction.
Table 5.
Overall results a
| Quadriceps Strength | Quadriceps CSA | Outcome Scales: Pain | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Total No. Training Sessions Possible | Major Muscle Groups Exercised | Improved in BFRT Group? | BFRT Significantly Greater Than Control? | Improved in BFRT Group? | BFRT Significantly Greater Than Control? | Improved in BFRT Group? | BFRT significantly Greater Than Control? | BFRT Recommended? |
| Ohta et al29
ACL reconstruction |
210 | Quadriceps, hamstrings, hip | — | Yes | — | Yes | — | — | Yes |
| Takarada et al42
ACL reconstruction |
30 | None | — | — | Yes | Yes | — | — | Yes |
| Iversen et al17
ACL reconstruction |
30 | Quadriceps | — | — | — | No | — | — | No |
| Tennent et al43
Knee arthroscopy |
12 | Quadriceps, hamstrings | Yes | Yes | — | — | Yes | No | Yes |
| Segal et al37
Knee osteoarthritis males |
12 | Quadriceps | No | No | — | — | No | No | No |
| Segal et al38
Knee osteoarthritis females |
12 | Quadriceps, hamstrings | Yes | Yes | No | No | No | No | Yes |
| Bryk et al4
Knee osteoarthritis |
18 | Quadriceps, hamstrings, hip, gastrocnemius | Yes | No | — | — | Yes | Yes | Yes |
| Ferraz et al8
Knee osteoarthritis |
24 | Quadriceps | Yes | Yes vs LR No vs HR |
Yes | Yes vs LR No vs HR |
Yes | No | Yes |
| Giles et al11
Patellofemoral pain |
24 | Quadriceps | Yes | No | No | No | Yes | No | Yes |
ACL, anterior cruciate ligament; BFRT, blood flow–restricted training; CSA, cross-sectional area; HR, high resistance; LR, low resistance.
— = factor not analyzed in study.
Adverse Events
Adverse events related to BFRT were rarely encountered. Dropouts occurred due to discomfort with training in the series of Ohta et al29 (2 of 24 [8%] enrolled) and Segal et al37 (1 of 20 [5%] enrolled). No other adverse events were reported.
Discussion
The limited published data show BFRT to be safe and potentially effective in improving quadriceps strength in patients with knee-related weakness and atrophy. There were no complications related to BFRT. Improvements in study design and refinements in protocols related to cuff pressure and exercise dosage and duration are required to further advance our knowledge of this treatment option.
The low-resistance load of 30% 1 RM used in 6 studies was effective in improving quadriceps strength without eliciting a pain response that may be incurred with high-load resistance levels. Ferraz et al8 reported a dropout rate of 25% in patients in the high-resistance training control group due to exercise-induced knee pain. Few effects of training were noted in quadriceps CSA, and future data are required to determine whether changes in training dose or duration may improve this finding. Hamstrings strength was measured after training in only 2 studies,29,43 and conclusions could not be reached regarding the efficacy of BFRT on this factor. One study that included isolated hamstrings exercises (reverse press) at 30% 1 RM reported an approximate 40% increase in hamstrings strength in the treatment group and a 17% increase in the control group.43
The study protocols varied in cuff pressures selected for training, with pressures ranging from a mean of 97.4 mm Hg8 to as high as 260 mm Hg (depending on patient tolerance42). Only 4 studies provided information regarding cuff type and size.17,37,38,42 Three studies set the pressure to a percentage of total arterial occlusal pressure. Patterson et al32 recommended this type of approach to determine the appropriate blood flow–restricted pressure to minimize any cardiovascular risk and underlying tissue compression damage. These authors recommended using a handheld Doppler to make individual patient measurements and selected a range of 40% to 80% of limb occlusion for training. Four studies in our review gradually increased cuff pressure during training sessions or over the course of the study as tolerated. Systematic reviews of healthy participants have noted large variations in cuff pressure, as well as exercise doses and durations.21,25,36,41 Slysz et al41 included 47 studies of healthy participants in their review; muscular strength changes were available for 400 participants, and muscular hypertrophy data were available for 377 participants. The authors noted that no single cuff pressure produced equal blood flow restriction between participants and noted the need for the development of a model that would produce equal occlusion for all patients. With the data available, these authors recommended a cuff pressure of >150 mm Hg, a resistance load of 30% 1 RM, and a training duration of at least 8 weeks to produce noteworthy increases in muscle strength and size. Others have recommended cuff pressures be individually adjusted based on cuff width, limb circumference, and composition of muscle and fat in the limb to produce equivalent blood flow restriction.22,35 A recent study by Hughes et al14 compared interface pressure, perceived exertion, and pain among 3 different blood flow restriction systems in 18 healthy male participants. The study concluded that a system that automatically adjusts pressure during exercise is most likely the most beneficial tool to use for patient tolerance and adherence to a BFRT program.
In our review, it appeared that a minimum of 12 sessions is required to achieve measurable strength gains. The time of cuff inflation in the other studies was typically 5 minutes, although 3 studies noted inflation of the cuff during exercise periods that were not timed. Further work is necessary to determine the appropriate cuff pressure and training dose and duration for rehabilitation of knee-related muscle atrophy and to determine whether a protocol similar to that used for healthy participants should be followed.
In healthy participants and athletes, BFRT has been shown to produce significant gains in muscle strength and hypertrophy21,25,36,41; however, the underlying mechanisms responsible for these findings remain unclear.13,34 The reduced blood flow is hypothesized to bring about an ischemic/hypoxic environment that increases levels of metabolic stress, increases recruitment of fast-twitch muscle fibers, elevates systematic hormones, induces cell swelling, and increases production of reactive oxygen species.13,34 In addition, authors34 have theorized that mechanical tension acts in a synergistic manner with metabolic stress to produce muscle hypertrophy. BFRT does not appear to induce acute skeletal muscle damage, as Loenneke et al24 noted in a review of studies that reported no prolonged decrements in muscle function or swelling and no elevation in blood biomarkers of muscle damage. Mechanisms for potential improvements in quadriceps and hamstrings strength in patients with knee-related muscle atrophy after BFRT are unknown at present.
A few small case series were found in this systematic review that assessed the effects of BFRT after total knee arthroplasty (3 patients)10 and in patients with chronic atrophy after lower extremity trauma (7 patients).15 The results were encouraging, with all patients demonstrating improvements in isokinetic strength and no complications reported. Although these series were not included in our formal review, the positive results provide further evidence of the safety and efficacy of this training to augment traditional rehabilitation protocols.
One problem highlighted in this review is that only 2 studies provided ES calculations in addition to P values. ES measures the magnitude of the effects of treatment and is especially relevant in studies with small sample sizes.9 It is probable that some statistically significant findings (P < 0.05) may have limited clinical relevance. The question of what percentage of muscle strength gain from BFRT represents a minimal clinically important difference (MCID)18 remains questionable. In musculoskeletal disorders, this may be influenced by the diagnosis and magnitude of muscle weakness and atrophy. For instance, one may arbitrarily set a value of strength gain of 10% as the MCID; however, if a deficit of quadriceps peak torque between limbs at baseline is 50%, a 10% gain in strength may not be clinically meaningful to the patient. An additional problem detected in this review was the sample size selected in many studies. Only 4 studies4,8,37,38 conducted a prospective power calculation of the size required to discern a detectable difference (95% CI) between BFRT and control groups. Future studies should calculate both ESs and sample sizes to minimize the occurrence of a type II statistical error.12 Another problem noted in our review was the lack of consistency in reporting muscle strength data. One study29 provided only limb ratios (involved/uninvolved limb), and another study37 reported only the percentage change from baseline values of knee extensor strength. Future studies should report the peak torque values at baseline and follow-up and normalize all data by body weight.
A history of deep venous thrombosis was considered a contraindication for training in the investigations of Segal et al.37,38 Giles et al11 excluded patients at elevated risk of deep vein thrombosis (lower limb surgery within the previous 6 months, cardiovascular conditions, or high blood pressure). Tennent et al43 performed bilateral lower extremity duplex ultrasounds before and on completion of their investigation, to rule out vascular problems. All studies were negative, and no adverse events were reported. In the study by Bryk et al,4 a vascular surgeon assessed femoral and tibial pulses to exclude potential vascular risks before patients were entered in the trial. Hughes et al13 reviewed 20 studies of BFRT used for musculoskeletal rehabilitation and reported few to no adverse events reported. The conclusion was made that correct implementation of this training option presents no greater risk than traditional training modes.26
Conclusion
Published data show BFRT to be potentially effective in improving quadriceps strength in patients with knee-related weakness and atrophy. The use of short-duration vascular occlusion and low-load resistance exercises appears safe and not deleterious after knee surgery or in arthritic knees. This treatment option requires further investigation to refine protocols related to cuff pressure and exercise dosage and duration.
Footnotes
The authors report no potential conflicts of interest in the development and publication of this article.
References
- 1. American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41:687-708. [DOI] [PubMed] [Google Scholar]
- 2. Ardern CL, Glasgow P, Schneiders A, et al. 2016 Consensus statement on return to sport from the First World Congress in Sports Physical Therapy, Bern. Br J Sports Med. 2016;50:853-864. [DOI] [PubMed] [Google Scholar]
- 3. Ardern CL, Taylor NF, Feller JA, Webster KE. Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med. 2014;48:1543-1552. [DOI] [PubMed] [Google Scholar]
- 4. Bryk FF, Dos Reis AC, Fingerhut D, et al. Exercises with partial vascular occlusion in patients with knee osteoarthritis: a randomized clinical trial. Knee Surg Sports Traumatol Arthrosc. 2016;24:1580-1586. [DOI] [PubMed] [Google Scholar]
- 5. Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Academic Press; 1977. [Google Scholar]
- 6. de Jong SN, van Caspel DR, van Haeff MJ, Saris DB. Functional assessment and muscle strength before and after reconstruction of chronic anterior cruciate ligament lesions. Arthroscopy. 2007;23:21-28, 28.e1-e3. [DOI] [PubMed] [Google Scholar]
- 7. de Souza TMF, Libardi CA, Cavaglieri CR, et al. Concurrent training with blood flow restriction does not decrease inflammatory markers. Int J Sports Med. 2018;39:29-36. [DOI] [PubMed] [Google Scholar]
- 8. Ferraz RB, Gualano B, Rodrigues R, et al. Benefits of resistance training with blood flow restriction in knee osteoarthritis. Med Sci Sports Exerc. 2018;50:897-905. [DOI] [PubMed] [Google Scholar]
- 9. Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141:2-18. [DOI] [PubMed] [Google Scholar]
- 10. Gaunder CL, Hawkinson MP, Tennent DJ, Tubb CC. Occlusion training: pilot study for postoperative lower extremity rehabilitation following primary total knee arthroplasty. US Army Med Dep J. 2017;(2-17):39-43. [PubMed] [Google Scholar]
- 11. Giles L, Webster KE, McClelland J, Cook JL. Quadriceps strengthening with and without blood flow restriction in the treatment of patellofemoral pain: a double-blind randomised trial. Br J Sports Med. 2017;51:1688-1694. [DOI] [PubMed] [Google Scholar]
- 12. Greenfield ML, Kuhn JE, Wojtys EM. A statistics primer. Power analysis and sample size determination. Am J Sports Med. 1997;25:138-140. [DOI] [PubMed] [Google Scholar]
- 13. Hughes L, Paton B, Rosenblatt B, Gissane C, Patterson SD. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br J Sports Med. 2017;51:1003-1011. [DOI] [PubMed] [Google Scholar]
- 14. Hughes L, Rosenblatt B, Gissane C, Paton B, Patterson SD. Interface pressure, perceptual, and mean arterial pressure responses to different blood flow restriction systems. Scand J Med Sci Sports. 2018;28:1757-1765. [DOI] [PubMed] [Google Scholar]
- 15. Hylden C, Burns T, Stinner D, Owens J. Blood flow restriction rehabilitation for extremity weakness: a case series. J Spec Oper Med. 2015;15:50-56. [PubMed] [Google Scholar]
- 16. Ingersoll CD, Grindstaff TL, Pietrosimone BG, Hart JM. Neuromuscular consequences of anterior cruciate ligament injury. Clin Sports Med. 2008;27:383-404. [DOI] [PubMed] [Google Scholar]
- 17. Iversen E, Rostad V, Larmo A. Intermittent blood flow restriction does not reduce atrophy following anterior cruciate ligament reconstruction. J Sport Health Sci. 2016;5:115-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katz NP, Paillard FC, Ekman E. Determining the clinical importance of treatment benefits for interventions for painful orthopedic conditions. J Orthop Surg Res. 2015;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Konishi Y, Fukubayashi T. Relationship between muscle volume and muscle torque of the hamstrings after anterior cruciate ligament reconstruction. J Sci Med Sport. 2010;13:101-105. [DOI] [PubMed] [Google Scholar]
- 20. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:B2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lixandrão ME, Ugrinowitsch C, Berton R, et al. Magnitude of muscle strength and mass adaptations between high-load resistance training versus low-load resistance training associated with blood-flow restriction: a systematic review and meta-analysis. Sports Med. 2018;48:361-378. [DOI] [PubMed] [Google Scholar]
- 22. Loenneke JP, Fahs CA, Rossow LM, et al. Blood flow restriction pressure recommendations: a tale of two cuffs. Front Physiol. 2013;4:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loenneke JP, Kim D, Fahs CA, et al. Effects of exercise with and without different degrees of blood flow restriction on torque and muscle activation. Muscle Nerve. 2015;51:713-721. [DOI] [PubMed] [Google Scholar]
- 24. Loenneke JP, Thiebaud RS, Abe T. Does blood flow restriction result in skeletal muscle damage? A critical review of available evidence. Scand J Med Sci Sports. 2014;24:e415-e422. [DOI] [PubMed] [Google Scholar]
- 25. Loenneke JP, Wilson JM, Marin PJ, Zourdos MC, Bemben MG. Low intensity blood flow restriction training: a meta-analysis. Eur J Appl Physiol. 2012;112:1849-1859. [DOI] [PubMed] [Google Scholar]
- 26. Loenneke JP, Wilson JM, Wilson GJ, Pujol TJ, Bemben MG. Potential safety issues with blood flow restriction training. Scand J Med Sci Sports. 2011;21:510-518. [DOI] [PubMed] [Google Scholar]
- 27. Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713-721. [PubMed] [Google Scholar]
- 28. Mohtadi NG, Chan DS. Return to sport-specific performance after primary anterior cruciate ligament reconstruction: a systematic review. Am J Sports Med. 2018;46(13):3307-3316. [DOI] [PubMed] [Google Scholar]
- 29. Ohta H, Kurosawa H, Ikeda H, Iwase Y, Satou N, Nakamura S. Low-load resistance muscular training with moderate restriction of blood flow after anterior cruciate ligament reconstruction. Acta Orthop Scand. 2003;74:62-68. [DOI] [PubMed] [Google Scholar]
- 30. Palmieri-Smith RM, Thomas AC, Wojtys EM. Maximizing quadriceps strength after ACL reconstruction. Clin Sports Med. 2008;27:405-424. [DOI] [PubMed] [Google Scholar]
- 31. Patterson SD, Ferguson RA. Enhancing strength and postocclusive calf blood flow in older people with training with blood-flow restriction. J Aging Phys Act. 2011;19:201-213. [DOI] [PubMed] [Google Scholar]
- 32. Patterson SD, Hughes L, Head P, Warmington S, Brandner C. Blood flow restriction training: a novel approach to augment clinical rehabilitation: how to do it. Br J Sports Med. 2017;51:1648-1649. [DOI] [PubMed] [Google Scholar]
- 33. Patterson SD, Leggate M, Nimmo MA, Ferguson RA. Circulating hormone and cytokine response to low-load resistance training with blood flow restriction in older men. Eur J Appl Physiol. 2013;113:713-719. [DOI] [PubMed] [Google Scholar]
- 34. Pearson SJ, Hussain SR. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015;45:187-200. [DOI] [PubMed] [Google Scholar]
- 35. Scott BR, Loenneke JP, Slattery KM, Dascombe BJ. Exercise with blood flow restriction: an updated evidence-based approach for enhanced muscular development. Sports Med. 2015;45:313-325. [DOI] [PubMed] [Google Scholar]
- 36. Scott BR, Loenneke JP, Slattery KM, Dascombe BJ. Blood flow restricted exercise for athletes: a review of available evidence. J Sci Med Sport. 2016;19:360-367. [DOI] [PubMed] [Google Scholar]
- 37. Segal N, Davis MD, Mikesky AE. Efficacy of blood flow-restricted low-load resistance training for quadriceps strengthening in men at risk of symptomatic knee osteoarthritis. Geriatr Orthop Surg Rehabil. 2015;6:160-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Segal NA, Williams GN, Davis MC, Wallace RB, Mikesky AE. Efficacy of blood flow-restricted, low-load resistance training in women with risk factors for symptomatic knee osteoarthritis. PM R. 2015;7:376-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shimizu R, Hotta K, Yamamoto S, et al. Low-intensity resistance training with blood flow restriction improves vascular endothelial function and peripheral blood circulation in healthy elderly people. Eur J Appl Physiol. 2016;116:749-757. [DOI] [PubMed] [Google Scholar]
- 40. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological Index for Non-Randomized Studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73:712-716. [DOI] [PubMed] [Google Scholar]
- 41. Slysz J, Stultz J, Burr JF. The efficacy of blood flow restricted exercise: a systematic review & meta-analysis. J Sci Med Sport. 2016;19:669-675. [DOI] [PubMed] [Google Scholar]
- 42. Takarada Y, Takazawa H, Ishii N. Applications of vascular occlusion diminish disuse atrophy of knee extensor muscles. Med Sci Sports Exerc. 2000;32:2035-2039. [DOI] [PubMed] [Google Scholar]
- 43. Tennent DJ, Hylden CM, Johnson AE, Burns TC, Wilken JM, Owens JG. Blood flow restriction training after knee arthroscopy: a randomized controlled pilot study. Clin J Sport Med. 2017;27:245-252. [DOI] [PubMed] [Google Scholar]
- 44. Thomas AC, Villwock M, Wojtys EM, Palmieri-Smith RM. Lower extremity muscle strength after anterior cruciate ligament injury and reconstruction. J Athl Train. 2013;48:610-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vanwye WR, Weatherholt AM, Mikesky AE. Blood flow restriction training: implementation into clinical practice. Int J Exerc Sci. 2017;10:649-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vechin FC, Libardi CA, Conceição MS, et al. Comparisons between low-intensity resistance training with blood flow restriction and high-intensity resistance training on quadriceps muscle mass and strength in elderly. J Strength Cond Res. 2015;29:1071-1076. [DOI] [PubMed] [Google Scholar]
- 47. Wylie JD, Hartley MK, Kapron AL, Aoki SK, Maak TG. Failures and reoperations after matrix-assisted cartilage repair of the knee: a systematic review. Arthroscopy. 2016;32:386-392. [DOI] [PubMed] [Google Scholar]
- 48. Yasuda T, Fukumura K, Tomaru T, Nakajima T. Thigh muscle size and vascular function after blood flow-restricted elastic band training in older women. Oncotarget. 2016;7:33595-33607. [DOI] [PMC free article] [PubMed] [Google Scholar]