Highlights
-
•
Seventeen EBMT centers participated to a questionnaire on MSC manufacturing.
-
•
88% of centers manufacture MSC from bone marrow and only 2 centers from umbilical cord.
-
•
Human platelet lysate has replaced bovine serum as culture medium supplement.
-
•
Release criteria extensively differ among centers.
-
•
The results highlight the need to harmonize MSC manufacturing.
Key Words: Mesenchymal stromal cells, Graft-versus-host disease, Cellular therapy, Manufacturing, Product specification, Release criteria
Abstract
The immunosuppressive properties of mesenchymal stromal cells (MSC) have been successfully tested to control clinical severe graft-versus host disease and improve survival. However, clinical studies have not yet provided conclusive evidence of their efficacy largely because of lack of patients’ stratification criteria. The heterogeneity of MSC preparations is also a major contributing factor, as manufacturing of therapeutic MSC is performed according to different protocols among different centers. Understanding the variability of the manufacturing protocol would allow a better comparison of the results obtained in the clinical setting among different centers. In order to acquire information on MSC manufacturing we sent a questionnaire to the European Society for Blood and Marrow Transplantation centers registered as producing MSC. Data from 17 centers were obtained and analyzed by means of a 2-phase questionnaire specifically focused on product manufacturing. Gathered information included MSC tissue sources, MSC donor matching, medium additives for ex vivo expansion, and data on MSC product specification for clinical release. The majority of centers manufactured MSC from bone marrow (88%), whilst only 2 centers produced MSC from umbilical cord blood or cord tissue. One of the major changes in the manufacturing process has been the replacement of fetal bovine serum with human platelet lysate as medium supplement. 59% of centers used only third-party MSC, whilst only 1 center manufactured exclusively autologous MSC. The large majority of these facilities (71%) administered MSC exclusively from frozen batches. Aside from variations in the culture method, we found large heterogeneity also regarding product specification, particularly in the markers used for phenotypical characterization and their threshold of expression, use of potency assays to test MSC functionality, and karyotyping. The initial data collected from this survey highlight the variability in MSC manufacturing as clinical products and the need for harmonization. Until more informative potency assays become available, a more homogeneous approach to cell production may at least reduce variability in clinical trials and improve interpretation of results.
Introduction
The preparation of mesenchymal stromal cells (MSC) in experimental and clinical studies consists of a highly heterogeneous population that can be isolated from virtually all human tissues and easily expanded ex vivo. A proportion of cultured MSC, like activated fibroblasts, exhibit progenitor activity because they can differentiate in vitro into the 3 mesenchymal lineages [1], but the significance of these in vitroassays to document multipotency can be misleading and has recently been questioned [2]. However, in vivo studies have better characterized subpopulations with genuine stemness and the specific ability to form components of the osteogenic [3] and vascular [4] stem cell niche.
Also similarly to activated fibroblasts [5], MSC are endowed with unique immunomodulatory and anti-inflammatory activities on adaptive [6] and innate immune responses [7]. By reprogramming the inflammatory microenvironment [8], MSC prime tissue repair, thus making them a therapeutic tool not only to control immune-mediated ailments but also in the context of regenerative medicine 9., 10., 11.. Therefore, it is not surprising that MSC therapeutics have met a huge interest in hematopoietic stem cell transplantation (HSCT) whereby they have been exploited to treat challenging conditions such as impaired HSC engraftment 12., 13., 14. and steroid-resistant acute graft-versus-host disease (aGvHD) 15., 16., 17., 18..
The most employed and convincing clinical application of MSC in the setting of allogeneic HSCT remains the treatment of resistant aGvHD. The first report on a pediatric patient [19] paved the way to several more controlled studies or compassionate use experiences demonstrating safety and encouraging efficacy in a large portion of patients that translated into an increased survival in those showing a complete response 15., 16., 17., 18., 20., 21.. However, no randomized clinical trial has formally confirmed efficacy, with the only one completed, performed with an industrial MSC product (NCT00366145), that failed to reach the primary endpoint. An academic, European Union–funded multicenter phase III study is ongoing in Europe and will hopefully contribute to clarify the impact of MSC in GvHD. Furthermore, highly successful phase III studies are being finalized by commercial companies (NCT02336230).
These clinical studies are limited not only by the intrinsic heterogeneity of GvHD patients but also by the variability in MSC preparations. According to European Regulation, MSC are classified as Advanced Therapy Medicinal Product (European Regulation EC No. 1394/2007, and complying with regulation 2004/23/EC and 2002/98/EC) and therefore require specific regulatory framework for production under Good Manufacturing Practice conditions, as well as criteria for product specification and release for clinical use [22]. Different tissue sources, expansion protocols, and product definitions are employed across European centers, thus posing the question of whether the cell product is sufficiently similar across the manufacturing units and whether results can be reasonably compared even within the same study. Moreover, the yet incomplete definition of MSC has a substantial impact on release criteria and potency assays. Mechanism-based markers for potency capable of predicting clinical efficacy have only recently been proposed [23].
All these considerations and the large use of MSC for GvHD across Europe strongly argue for the need of harmonization and standardization of the processes involved in MSC manufacturing and their release criteria. To this aim, the Cellular Therapy & Immunobiology Working Party of the European Society for Blood and Marrow Transplantation (EBMT) has conducted a survey to collect information on MSC manufacturing and product specification in approved EBMT centers registered as producing MSC.
Methods
The data reported were collected from 17 EBMT centers using a 2-phase questionnaire dedicated to several aspects of the MSC manufacturing process. The criterion used to select these centers was the participation of the center in the EBMT. The response rate was 100%, as all contacted centers replied to the first questionnaire. Centers that participated in the survey were located in Austria (Salzburg), Belgium (Leuven, Liege), Germany (Dresden, Frankfurt, Hannover), Israel (Tel-Ashomer), Italy (Bergamo, Milano, Monza, Pavia), Lithuania (Vilnius), the Netherlands (Leiden, Utrecht), Spain (Salamanca), Sweden (Stockholm), and the United Kingdom (London). They all hosted an approved facility for clinical-grade MSC manufacturing. The indication for MSC administration was treatment of steroid-resistant aGvHD developed after allogeneic HSCT or donor lymphocyte infusion.
Gathered information in the first questionnaire included tissue source (bone marrow [BM], cord blood or cord tissue, others), patient–donor matching (third-party and/or autologous MSC), medium supplements for in vitroexpansion (fetal bovine serum [FBS], human platelet lysate [hPL], antibiotics or antifungal agents), and use of fresh or frozen MSC products. Data on MSC product specification for clinical release covered expression of positive (CD90, CD105, CD73) and negative (CD45, CD31, CD3, CD19) markers by flow cytometry and their threshold for clinical use. Safety was addressed by asking practice on karyotypic analysis, sterility, mycoplasma, and endotoxin testing. Finally, centers were asked whether they performed any potency assay, including differentiation and immunosuppressive activity.
The majority of centers (71%) participating in the first phase questionnaire agreed to provide data also in the second questionnaire, which included more specific questions on technical details related to the manufacturing process. This comprised information on mononuclear cell and MSC seeding density, use of flasks and/or factories or different expansion devices (such as bioreactors), cryopreservants employed for MSC freezing, and shelf life of the product. In addition, data on the number of in vitro MSC passages and the use of pooled cell products were collected. The 2 questionnaires are included as Supplementary Material. Data were collected in 2017 and included all MSC preparations generated from the date on which the centers received approval to produce clinical-grade MSC.
Results
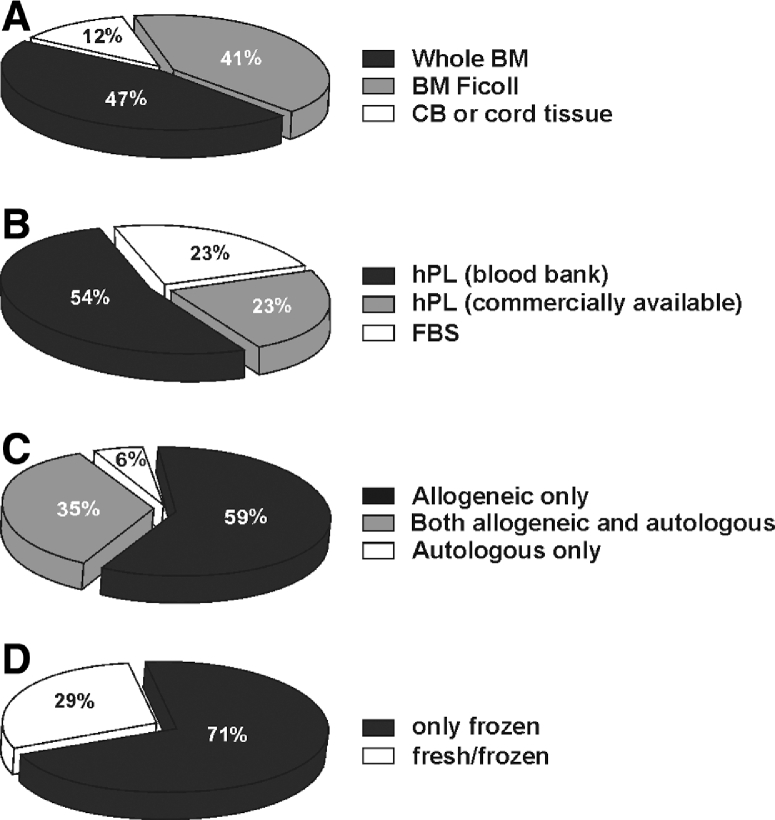
In total, more than 1500 MSC treatments have been performed in the participating facilities in over a thousand of patients. The majority of centers (88%) manufactured MSC from BM as tissue source (Ficoll or whole BM), whereas only 2 centers produced MSC from umbilical cord blood or cord tissue (Figure 1A). One of the major changes we observed in the manufacturing process has been the replacement of FBS with hPL as a medium supplement; 77% of centers currently use hPL, either commercially available or from pools of expired platelets obtained from the blood bank (Figure 1B). Further information about the specific composition of the isolation and expansion culturing media was collected through answers to the second part of the questionnaire. Analyzing the available data from 12 centers, we found that 75% of the centers employing hPL used a concentration equal to 5% in both isolation and expansion media, whereas the remaining employed 10%. Only 1 center used 5% hPL for isolation medium and 10% hPL for expansion medium. None of the centers used any growth factor for MSC manufacture. In 38% of the centers antibiotics were added to the MSC expansion medium (i.e. penicillin/streptomycin 1%), whereas no center used antifungal agents in culture. Fifty-nine percent of centers prepared only third-party allogeneic MSC, whilst 1 center manufactured only patient-derived autologous MSC (Figure 1C). For all centers, donor eligibility requirements and donor age for allogeneic products were in line with Directive 2004/23/EC and Commission Directive 2006/17/EC. 71% of facilities administered MSC exclusively from frozen batches, whereas the remaining infused both fresh and frozen products (Figure 1D).
Figure 1.
Clinical MSC production in 17 Good Manufacturing Practice facilities in EBMT centers. (A) Percentage of centers producing MSC from umbilical cord blood or cord tissue, or from BM, either using Ficoll method or adherence from whole BM. (B) Percentage of centers using FBS or hPL from commercially available manufacturers or from pooled expired platelets obtained from blood banks. (C) Distribution of centers using allogeneic or autologous MSC. (D) Percentage of centers delivering fresh or frozen MSC.
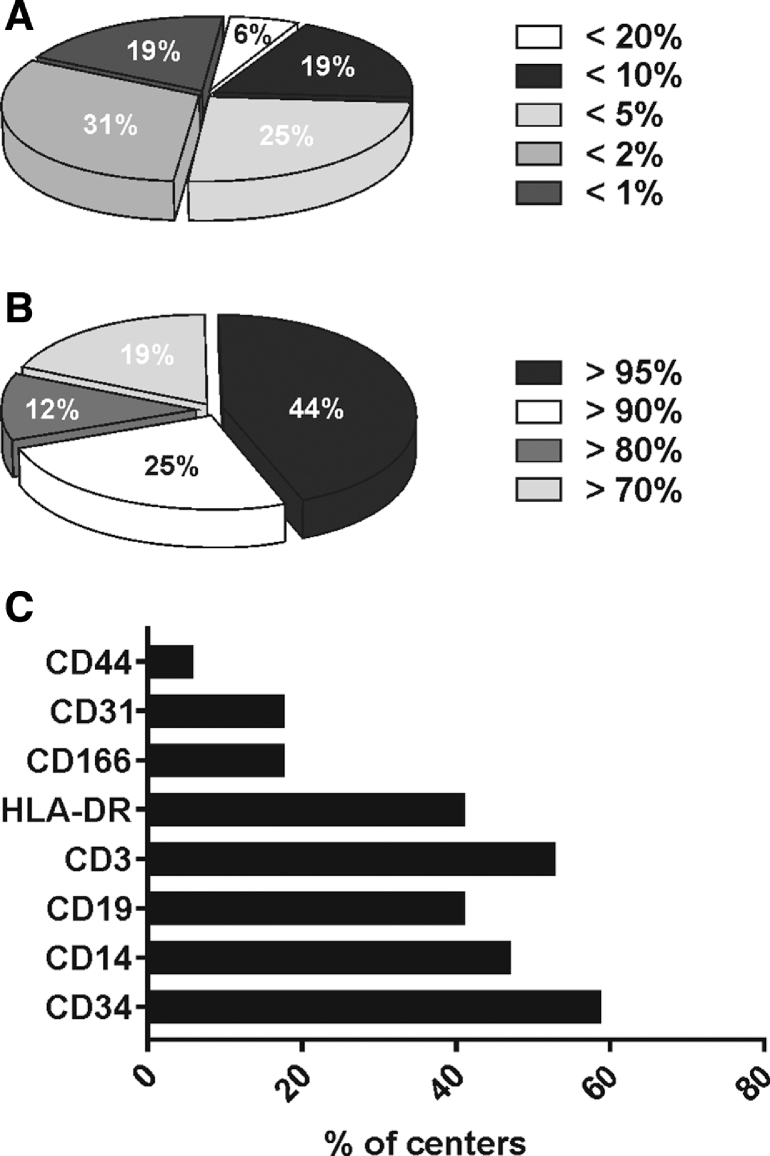
Apart from variations in the culture method, we observed a large heterogeneity in product specification. Phenotypical characterization represented a fundamental release criterion for all centers, and all surveyed facilities conducted analysis for the expression of CD73, CD90 and CD105, and CD45 through flow cytometry. However, the threshold of marker expression that is accepted to be clinically graded was highly variable, with, for example, CD45 expression varying between 1% and 20% (Figure 2A). Similarly, the threshold of positive marker expression varied from 70% to a very stringent 95%, the latter employed by nearly half of the centers (44%) (Figure 2B). Furthermore, the addition of other markers to the standard panel was unsystematic, with a high variability in the release threshold levels and in the type of markers analyzed. Most centers added CD19, CD34, CD14, HLA-DR, and CD3 negativity to the panel, while fewer centers checked expression of CD166, CD31, and CD44 (Figure 2C).
Figure 2.
Distribution charts on product specification for phenotype on clinical MSC. (A) Distribution chart on the percentage of centers using different acceptance criteria for CD45 expression. (B) Distribution chart on the percentage of centers using different acceptance criteria for expression of markers CD73, CD90, and CD105. (C) Distribution graph on the percentage of centers using other markers.
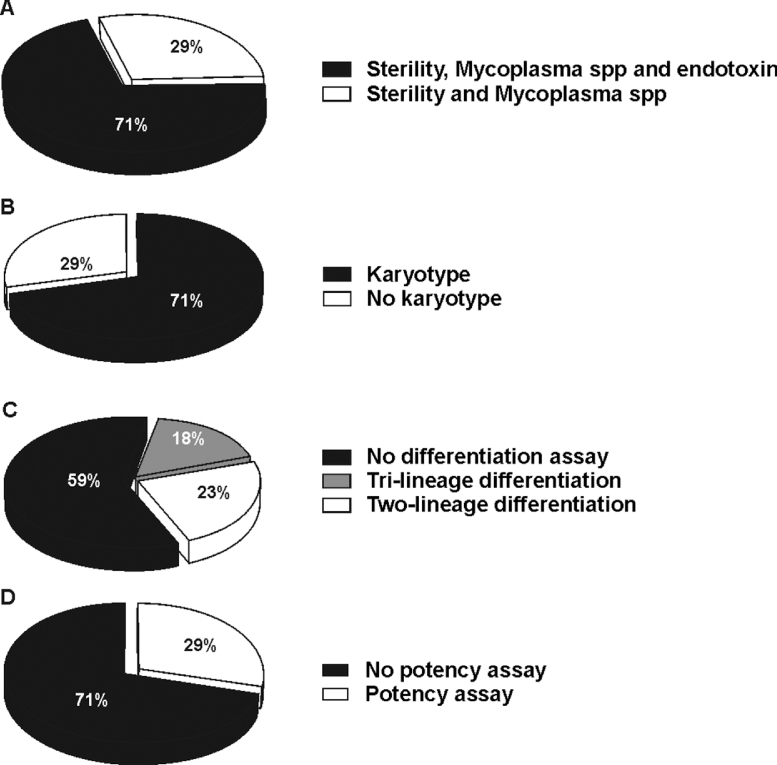
Alongside product specification, whilst all centers met the criteria of releasing sterile and mycoplasma-free product, not all of them (71%) evaluated the presence of endotoxin in the final formulation (Figure 3A). However, none of the centers ever had an out of specification result for sterility or endotoxin positivity, apart from 1 center that experienced incidental mycoplasma positivity in a product.
Figure 3.
Distribution chart on release criteria for clinical MSC. (A). Percentage of centers using sterility and absence of mycoplasma species or sterility, absence of mycoplasma species, and endotoxin levels as release criteria for clinical MSCs. (B) Percentage of centers performing analysis of karyotype. (C) Percentage of centers analyzing differentiation ability of MSC: tri-lineage differentiation into adipocytes, osteoblasts, and chondrocytes; two-lineage differentiation into adipocytes and osteoblasts; no differentiation assay carried out. (D) Percentage of centers determining immunosuppressive activity of MSC (potency assay).
When dealing with infusion of allogeneic BM MSC, only 31% of the centers responding to the second questionnaire produced pooled MSC products derived from different donors, generally obtained from 2 to 3 subjects. Noteworthy, only 1 center used MSC products generated from pooled mononuclear cells of multiple BM donors [24]. All the facilities used passage 2 or passage 3 as the limit of in vitropassage, after which cells were not released as clinical products. However, only 1 of 12 centers participating in the second phase questionnaire routinely calculated population doubling to assess MSC expansion before release.
The level of seeding densities for MSC isolation from mononuclear cells and for MSC expansion did not show any overlay at all among centers, with single facilities having identified different concentrations of cells to be plated for the manufacturing process. The majority of centers continued to perform MSC expansion in cell culture flasks or cell factories, while only 1 facility was developing a bioreactor-based expansion system. The cryopreservation medium was based on dimethyl sulphoxide, which was employed at 10% concentration in all centers except for one which adopted 5% concentration. The majority of centers measured post-thawing viability by Trypan Blue staining, with only 3 centers adopting the method of staining with 7-Aminoactinomycin D or Annexin V and Propidium Iodide followed by flow cytometry of the stained sample. The shelf life for MSC before administration displayed a vast variability, ranging from 8 months to 23 years, with 1 case in which no time limit was defined. Administration of the cells was performed intravenously or intra-arterially. Before infusion, cells were diluted with saline solution, human albumin solution, or directly administered in their cryopreservant.
The majority of centers (71%) performed karyotyping that was evaluated by means of G-banding and by analyzing a minimum number of 20 metaphases (Figure 3B). 23% of the centers determined the differentiation ability of MSC into adipocytes and osteoblasts, and only 3 facilities tested also chondrocytic differentiation (Figure 3C). 29% of the centers performed a potency assay to validate MSC immunosuppressive activity and employed it as a release criterion (Figure 3D). This test varied among centers. Whilst 1 center analyzed the immunosuppressive activity of MSC against phytohemagglutinin-activated T cells, another center did so using anti-CD3/CD28 antibody stimulation, and 2 facilities tested MSC immunosuppression on a mixed lymphocyte reaction. Moreover, these more complex assays were not always performed together, as only 2 centers tested both immunosuppression and karyotyping, 1 center analyzed differentiation capacity and karyotype, and 1 center examined differentiation alongside immunosuppressive activity.
Discussion
The immunosuppressive properties of MSC have been widely exploited in the clinical setting to control aGvHD resistant to conventional treatments. Despite the encouraging experience and the positive impact on overall survival, there is still no formal proof of efficacy, and the factors predictive of clinical responses have not yet been identified. What certainly complicates the interpretation of the results is the potential heterogeneity of MSC preparations and the absence of any mechanistic potency assay. Using the information from 17 EBMT-affiliated transplant centers registered to manufacture MSC, our survey has effectively documented a high variability in culture methods and, even more importantly, in the list of release criteria for the clinical product.
Despite a decline in its use as source of HSC for transplantation, BM remains the main MSC source. Certainly, a significant change in the manufacturing process has been the substitution, in most facilities, of an animal protein-based additive (FBS) with hPL as a culture supplement [25]. Another common finding is the administration of third-party MSC products that have been stored in frozen batches. This has been made indispensable to allow acquisition of data on quality release tests. At the same time, it meets the important need of having an off-the-shelf product promptly available for the treatment of patients affected by severe GvHD. Amongst the implementation of the safety measures we observed that although there is no evidence that infused MSC engraft irrespective of whether they are obtained from the patient or a third-party donor, the majority of centers regularly performs MSC karyotyping. Most centers never had an anomalous karyotyping result, however, this might be due to the sensitivity of the sampling and of the test. Considering the practically inexistent risk of malignant transformation 26., 27., this labor-intensive procedure should be reviewed.
Our survey also highlights the vast heterogeneity in product specification. This includes the phenotypic markers as well as the functional assays to test MSC potency before their clinical use. These many discrepancies are clearly the results of an inaccurate scientific definition of the MSC product and in particular the confusion between progenitor and anti-inflammatory activities that do not necessarily overlap. Many current release criteria should be revised because they are not entirely substantiated by scientific evidence or have been defined in an era in which many of the aspects of MSC biology had not yet been identified. Furthermore, the limited knowledge of their mode of action has certainly hindered the development of informative potency assays by which to select the most efficacious cell batch. It was only very recently that new insights into the immunosuppressive activity of MSC [23] have suggested not only to investigate the cell preparation but also the cell recipient.
In conclusion, this initial report highlights the variability present in MSC manufacturing and release criteria across EBMT centers. Such variability may impact on MSC therapeutic activity and further complicate the already difficult interpretation of clinical results. Although release criteria should adapt to new scientific findings rather than opinionated consensus statements, manufacturing a single homogenous therapeutic reagent would at least eliminate one variable and allow us to concentrate on the more complex nature of MSC recipients.
Acknowledgments
The authors thank all members of the Cell Therapy & Immunobiology Working Party of the EBMT.
Financial disclosure: Supported by grants from the Bloodwise specialist programme 14019 and Bloodwise specialist programme 12006 (to F.D.); Bloodwise Clinical Research Training Fellowship 15029 (to A.G.); Italian Association for Cancer Research-AIRC and Italian Ministry of University and Research-MIUR (to C.B.); KWF grant UU 2015-7601 (to J.K.);, Ricerca Corrente no. 80380 (to M.A.A.).
Conflict of interest statement: C.B. received research support from Molmed s.p.a and Intellia Therapeutics and received the fee for the participation to an advisory Board by GSK. The other authors disclose no potential conflicts of interest.
Authorship statement: C.T. and M.E.B. contributed equally to this study.
Footnotes
Financial disclosure: See Acknowledgments on page 2369.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.bbmt.2018.07.015.
Appendix. Supplementary materials
Supplementary Data
References
- 1.Dominici M, Le Blanc K, Mueller I. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 2.Bianco P, Cao X, Frenette PS. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lv F-J, Tuan RS, Cheung KMC, Leung VYL. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 4.Crisan M, Yap S, Casteilla L. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 6.Krampera M, Glennie S, Dyson J. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 7.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Cheung TS, Dazzi F. Mesenchymal-myeloid interaction in the regulation of immunity. Semin Immunol. 2018;35:59–68. doi: 10.1016/j.smim.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Ciccocioppo R, Bernardo ME, Sgarella A. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60:788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 10.Perin EC, Sanz-Ruiz R, Sánchez PL. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: the PRECISE Trial. Am Heart J. 2014;168 doi: 10.1016/j.ahj.2014.03.022. 88-95.e2. [DOI] [PubMed] [Google Scholar]
- 11.Laroye C, Gibot S, Reppel L, Bensoussan D. Concise review: mesenchymal stromal/stem cells: a new treatment for sepsis and septic shock. Stem Cells. 2017;35:2331–2339. doi: 10.1002/stem.2695. [DOI] [PubMed] [Google Scholar]
- 12.Lazarus HM, Koc ON, Devine SM. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11:389–398. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 13.de Lima M, McNiece I, Robinson SN. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367:2305–2315. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trento C, Marigo I, Pievani A. Bone marrow mesenchymal stromal cells induce nitric oxide synthase-dependent differentiation of CD11b+ cells that expedite hematopoietic recovery. Haematologica. 2017;102:818–825. doi: 10.3324/haematol.2016.155390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Blanc K, Frassoni F, Ball L. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 16.Ball LM, Bernardo ME, Roelofs H. Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III-IV acute graft-versus-host disease. Br J Haematol. 2013;163:501–509. doi: 10.1111/bjh.12545. [DOI] [PubMed] [Google Scholar]
- 17.von Dalowski F, Kramer M, Wermke M. Mesenchymal stromal cells for treatment of acute steroid-refractory graft versus host disease: clinical responses and long-term outcome. Stem Cells. 2016;34:357–366. doi: 10.1002/stem.2224. [DOI] [PubMed] [Google Scholar]
- 18.Sánchez-Guijo F, Caballero-Velázquez T, López-Villar O. Sequential third-party mesenchymal stromal cell therapy for refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20:1580–1585. doi: 10.1016/j.bbmt.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Le Blanc K, Rasmusson I, Sundberg B. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 20.Introna M, Lucchini G, Dander E. Treatment of graft versus host disease with mesenchymal stromal cells: a phase I study on 40 adult and pediatric patients. Biol Blood Marrow Transplant. 2014;20:375–381. doi: 10.1016/j.bbmt.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 21.Galleu A, Deplano S, Szydlo R. Mesenchymal stromal cells for the treatment of steroid-resistant acute graft versus host disease: factors influencing clinical responses. Haematologica. 2017;102(suppl 2):336–337. [Google Scholar]
- 22.de Girolamo L, Lucarelli E, Alessandri G. Mesenchymal stem/stromal cells: a new “cells as drugs’’ paradigm. Efficacy and critical aspects in cell therapy. Curr Pharm Des. 2013;19:2459–2473. doi: 10.2174/1381612811319130015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galleu A, Riffo-Vasquez Y, Trento C. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aam7828. 9:eaam7828. [DOI] [PubMed] [Google Scholar]
- 24.Kuci Z, Bonig H, Kreyenberg H. Mesenchymal stromal cells from pooled mononuclear cells of multiple bone marrow donors as rescue therapy in pediatric severe steroid-refractory graft-versus-host disease: a multicenter survey. Haematologica. 2016;101:985–994. doi: 10.3324/haematol.2015.140368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avanzini MA, Bernardo ME, Cometa AM. Generation of mesenchymal stromal cells in the presence of platelet lysate: a phenotypic and functional comparison of umbilical cord blood- and bone marrow-derived progenitors. Haematologica. 2009;94:1649–1660. doi: 10.3324/haematol.2009.006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogel G. Cell biology. To scientists’ dismay, mixed-up cell lines strike again. Science. 2010;329:1004. doi: 10.1126/science.329.5995.1004. [DOI] [PubMed] [Google Scholar]
- 27.Torsvik A, Røsland GV, Svendsen A. Spontaneous malignant transformation of human mesenchymal stem cells reflects cross-contamination: putting the research field on track—letter. Cancer Res. 2010;70:6393–6396. doi: 10.1158/0008-5472.CAN-10-1305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data