Abstract
Purpose of review
Abdominal obesity, especially the increase of visceral adipose tissue (VAT), is closely associated with increased mortality related to cardiovascular disease, diabetes, and fatty liver disease. This review provides an overview of the recent advances for abdominal obesity measurement.
Recent findings
Compared to simple waist circumference, emerging three-dimensional (3D) body-scanning techniques also measure abdominal volume and shape. Abdominal dimension measures have been implemented in bioelectrical impedance analysis to improve accuracy when estimating VAT. Geometrical models have been applied in ultrasound to convert depth measurement into VAT area. Only computed tomography (CT) and MRI can provide direct measures of VAT. Recent advances in imaging allow for evaluating functional aspects of abdominal fat such as brown adipose tissue and fatty acid composition.
Summary
Waist circumference is a simple, inexpensive method to measure abdominal obesity. CT and MRI are reference methods for measuring VAT. Further studies are needed to establish the accuracy for dual-energy X-ray absorptiometry in estimating longitudinal changes of VAT. Further studies are needed to establish whether bioelectrical impedance analysis, ultrasound, or 3D body scanning is consistently superior to waist circumference in estimating VAT in different populations.
Keywords: computed tomography, MRI, three-dimensional body scanning, visceral adipose tissue
INTRODUCTION
Obesity has grown into a global health issue [1]. Obesity, especially abdominal obesity, is associated with metabolic syndrome and cardiovascular disease and also an independent risk factor of all-cause mortality [2–4]. In the third National Health and Nutrition Examination Survey study, normal-weight central obesity, as defined by high waist-to-hip ratio, was associated with higher cardiovascular mortality than BMI-defined obesity [4]. In the Dallas Heart Study of 1200 obese participants undergoing MRI, amount of visceral adipose tissue (VAT) was associated with a more severe metabolic, dyslipidemic, and atherogenic obesity phenotype compared to amount of subcutaneous adipose tissue [5]. Quantitative analysis of abdominal fat distribution, specifically VAT, is integral to understanding obesity-related comorbidities and treatment of obesity. This review provides an overview of the most popular methods to measure abdominal obesity and describes the advantages and limitations of each method.
ANTHROPOMETRY
Anthropometry has been widely used in large-scale epidemiology studies and clinical settings because of its low cost, favorable safety profile, ease of use, and applicability to all body sizes. Anthropometric measures of abdominal obesity include waist circumference, waist-to-hip ratio, and waist-to-height ratio. In a study of 168 159 participants from countries, waist circumference showed higher odds ratio with cardiovascular disease and type 2 diabetes than BMI in participants from most regions of the world [6]. Waist circumference trended for a higher correlation with MRI measured VAT than BMI (n = 1192; r = 0.80 vs. r ¼ 0.75) [7].
Waist circumference is an index of central obesity recommended by the National Institutes of Health, WHO, the American Heart Association, and the International Diabetes Foundation for screening for risk of metabolic and cardiovascular disease. However, there are limitations to this assessment mode. Cutoff points of waist circumference vary with sex and ethnic groups. There is no consensus on the best anatomic location to measure waist circumference; WHO recommends the midpoint between the last palpable rib and the iliac crest and the National Institutes of Health recommends the level of the umbilicus.
THREE-DIMENSIONAL BODY SCANNING TECHNOLOGY
Three-dimensional (3D) body scanning is a fast-growing technology that projects laser and other forms of light on the body surface and captures the reflected contour of the body with camera systems [8]. These systems can rapidly acquire hundreds of linear, circumferential, and volumetric body dimensions. Low-cost devices even for home use are now being introduced. The accuracy of laser-based 3D body scanning is higher than optical camera based, whereas the latter is much less expensive. Previous adult studies reported that the correlation between tape measured and 3D laser scanner measured waist circumference is higher than 0.99, with excellent intraobserver agreement. In a study of 473 children and adolescents, the concordance correlation coefficients were higher than 0.9 between 3D laser scanner and waist circumference and hip circumference, with intraobserver concordance correlation coefficients higher than 0.9 [9]. Soileau et al. [8] found a (mean ± standard deviation) 2.1 ± 1.8% difference between the waist circumference measured by structured light vs. laser light 3D scanning. 3D scanners that utilized a higher number of cameras produced more consistent waist circumference measurements (i.e., 16 stationary cameras vs. one oscillating camera or stationary camera) [10]. The authors suggested that the limitations including low resolution of the optical-based systems were mostly correctable when building next-generation devices. Popular media have reported smart phone apps that can perform 3D scans of objects; however, there are no peer-reviewed publications describing the efficacy of these consumer-based products. Using 3D scan apps may be a future direction for abdominal obesity evaluation when technology improvements make accurate quantification feasible.
3D body scanning can be used to derive new central obesity indices such as abdominal volume and body shape. Future studies are needed to compare abdominal volume and body shape measures with waist circumference in predicting VAT and obesity-related health risks.
BIOELECTRICAL IMPEDANCE ANALYSIS
Dual abdominal bioelectrical impedance analysis was developed for quantification of VAT by combining information on impedance and abdominal shape, which can be measured by built-in calipers to assess abdominal dimensions in the sagittal and coronal planes or by built-in laser to measure waist circumference. Impedance is measured by electrodes placed on the abdominal wall. Bioelectrical impedance analysis estimates were more highly correlated with total abdominal fat than VAT (r = 0.92–0.94 vs. 0.64–0.65) [11]. Dual abdominal bioelectrical impedance analysis estimates showed a higher correlation with computed tomography (CT)-measured VAT than whole-body bioelectrical impedance analysis estimates (r = 0.89, r = 0.64, respectively, P < 0.001). Some studies showed that bioelectrical impedance analysis better or equivalently estimates VAT amount compared to tape-measured waist circumference [12,13], but other studies found the opposite [11,14]. Dual abdominal bioelectrical impedance analysis seems to underestimate or has a large margin of error for VAT when VAT is high [12,14]. Large-population, multiethnic studies are needed to demonstrate whether abdominal bioelectrical impedance analysis is consistently superior to waist circumference to estimate VAT across populations.
ULTRASOUND
Ultrasound can be used to measure tissue thickness in various planes to develop novel geometric models that estimate abdominal VAT calculated from measured depths at various points around the abdominal circumference [15■]. The correlation coefficient between abdominal VAT measured by ultrasound compared with CT-measured VAT was 0.766–0.781, P < 0.001, with intrarater and inter-rater reliability of ultrasound-assessed abdominal fat thickness greater than 0.98 [15■]. There are no consistent findings on whether ultrasound estimates VAT more accurately than waist circumference [16,17]. Currently, B-mode ultrasound is more commonly used than A-mode ultrasound in obesity studies [18]. In a study of six cadavers comparing dissected tissue thickness with ultra-sound-detected thickness using A-mode and B-mode, the mean difference in subcutaneous adipose tissue thickness between A-mode and B-mode was less than 0.7 mm at the abdomen, thigh, and triceps sites [18]. No validation studies have been published examining VAT detection by A-mode ultrasound compared to CT or MRI. In summary, ultrasound can reliably estimate abdominal fat thickness, whereas the validity and reliability of ultrasound for measurement of adipose tissue areas needs further study.
DUAL-ENERGY X-RAY ABSORPTIOMETRY
Dual-energy X-ray absorptiometry (DXA) projects two beams of different energy X-rays that are collected by detectors after attenuation by the body tissues through which they have passed. The low dose of X-ray is considered safe for children and adults but most institutions prohibit its use in pregnant women.
DXA does not directly discriminate visceral from subcutaneous fat as it is a two-dimensional imaging technique, but DXA-estimated VAT volume was strongly correlated with MRI-measured VAT volume (r = 0.902, P < 0.0001) and CT-measured VAT area (r = 0.83, P < 0.0001) [19]. The Dallas Heart Study showed DXA-measured and MRI-measured VAT area correlate well, with R2 ranging from 0.82 to 0.86 (n = 2689). The inter-reader correlation was excellent (interclass correlation coefficient 0.997); however, DXA tended to underestimate VAT mass at low VAT levels and overestimate it at high VAT levels [20]. A cross-sectional study of 4950 participants showed that DXA-determined VAT mass has stronger odds ratios for type 2 diabetes and cardiovascular disease than waist circumference (i.e., 1.69–3.64 vs. 1.07–1.83) [21■].
In summary, with the high correlation between DXA and MRI to measure VAT, it is reasonable to believe that DXA is superior to waist circumference in measuring VAT in cross-sectional studies. Future studies, ideally validated by MRI or CT, are needed to establish whether DXA effectively detects longitudinal changes of VAT.
COMPUTED TOMOGRAPHY AND MAGNETIC RESONANCE IMAGING
CT and MRI directly measure VAT areas or volumes and are considered reference methods for evaluating abdominal adiposity. Compared to MRI, CT is less likely to be influenced by breathing artifact. The ionizing radiation from CT limits its use in children and in longitudinal studies. Most MRI systems have 60 cm bores, which may not accommodate individuals with severe obesity, although individuals with up to an approximate BMI of 47 have been scanned with 60 cm bore size scanners [22,23]. Larger 70 cm bore MRI facilities are becoming increasingly accessible and can accommodate patients of almost all sizes. Comprehensive discussions of the use of MRI for fat compartment measurement can be found in earlier reviews [24,25].
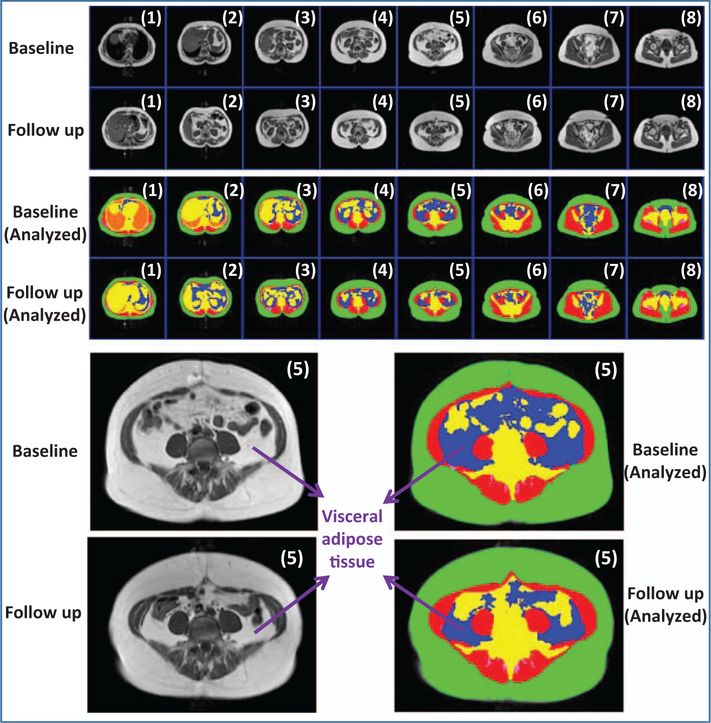
Single-slice images are often used to measure abdominal adiposity for its simplicity and to reduce radiation exposure in CT. Although single-slice imaging is a good compromise between accuracy and cost in cross-sectional studies, it may not be as accurate as total volume imaging to detect longitudinal changes in abdominal adiposity. Earlier studies used the L4–L5 intervertebral disk to localize single-slice imaging, but investigators increasingly use L2–L3 or L3–L4 disks because these sites have been found to better estimate total VAT volume. Using quantitative CT, Cheng et al. [26] confirmed that the L2–L3 location best estimates total VAT volume (r = 0.98, P < 0.001) in a healthy Chinese population, which is consistent with previous findings in western populations. Future studies of more diverse populations are needed to verify the power and generalizability of a single slice image to predict the risk for obesity-associated morbidity and mortality. Figure 1 shows that a single slice may be misleading in estimating VAT changes when breath hold is not consistent between baseline and follow up. Therefore, single slice imaging should be used cautiously in interpreting VAT changes for individual patients.
FIGURE 1.
Upper panel: Original and analyzed multislice MRI from the dome of the liver to the femoral head. Although the total VAT volume is similar between baseline and follow up, the VAT area in one MRI slice (5) is larger at baseline than at follow up. This difference is influenced by breath holding: the participant is likely inhaling during the baseline measurement but exhaling during the follow up measurement.
Brown adipose tissue (BAT), as a metabolically active tissue, is closely related to energy regulation and obesity in humans. PET-CT, MRI, and dual energy CT can distinguish BAT from white adipose tissue [27]. A recent study of PET-CT reported that obese men with cold exposure had less activated BAT overall and less abdominal activated BAT than lean men with cold exposure (obese vs. lean, 4.9 ± 7.6 vs. 45.5 ± 43.3 ml, P = 0.02) [28■■]. Activated BAT was not detected in abdominal subcutaneous adipose tissue nor omental or mesenteric VAT. BAT-activation images included in the article show that activated BAT was found predominantly in the peri-renal and para-renal fat. Further investigation could explore whether abdominal BAT has different metabolic characteristics compared to BAT in other body regions. MRI fat fraction changes in the neck under thermal challenges correlated with hypermetabolic BAT volume (r −0.55, P = 0.04 during activation and r = 0.72, P = 0.003 during deactivation) and with¼ BAT activity (r = 0.69, P = 0.006 during deactivation) as measured by PET-CT [29]. Given that PET-CT involves exposure to ionizing radiation, MRI may serve as an alternative method to study BAT, although there are still technical challenges for quantification of BAT based on fat fraction [30].
Proton magnetic resonance spectroscopy can be used to assess polyunsaturated fatty acids in subcutaneous adipose tissue, VAT, and bone marrow adipose tissue. A significant negative correlation was observed between unsaturated fat content and VAT amount; however, there was no correlation with unsaturated fat content and other adipose tissue compartments [31]. Further studies in larger cohorts are needed to gain further insight into whether the composition of fatty acids of adipose tissue is related to metabolic health risks.
Most studies use Hounsfield Unit of about −190 to −30 for subcutaneous adipose tissue and VAT quantification in CT studies. There is no consistent threshold that can be applied for MRI adipose tissue segmentation; however, there has been tremendous growth in automation of the analysis process in recent years [32–35]. The automatic analysis of water-fat imaging methods and the conventional T1-weighted MRI has been shown to be comparable [35]. Some studies automatically separated VAT from subcutaneous adipose tissue and bone marrow adipose tissue [32–35], other studies further removed intermuscular adipose tissue in addition to using R2* mapping to remove bowel content and bone marrow fat [34]. Semiautomated segmentation is considered the reference method until fully automated segmentation methods are validated across diverse populations including infants and children. Growth in the fields of artificial intelligence and deep learning may be a future direction for fully automated, accurate 3D segmentation of adipose tissue depots [36].
ABDOMINAL ORGAN FAT QUANTIFICATION
CT and MRI have been utilized for quantifying fat contents of abdominal organs including liver, pancreas, and adrenal glands [37]. Routine CT measurement of fat content is nonspecific and may be influenced by confounding factors that alter tissue density. However, with quantitative CT technology, liver fat content measured by CT is comparable to that measured by MRI in studies validated with a postmortem biochemical fat analysis in goose [38■,39]. MRI can specifically quantify fat content and utilize a variety of pulse sequences and scan parameters to create multidimensional imaging and high resolution for soft tissues. Fat-selective MRI, chemical-shift-encoded water-fat MRI, and magnetic resonance spectroscopy are the most popular fat quantification technologies [24,25].
Substantial evidence indicates that intrahepatic fat is a major driver of metabolic complications of obesity. The fatty acid composition of liver fat can also be estimated by both magnetic resonance spectroscopy and custom-designed MRI sequences [40■], which could be applied to distinguish subtypes of nonalcoholic fatty liver disease. In a study comparing histology and MRI in 32 patients with nonalcoholic fatty liver disease, the saturated fatty acid fraction was higher in patients with nonalcoholic steatohepatitis than in patients with simple steatosis (48 ± 2% vs. 44 ± 4%; P < 0.05) [40■]. Future studies could evaluate whether fatty acid composition in different depots (i.e., liver, VAT, and subcutaneous adipose tissue) plays different roles in metabolism and development of metabolic syndrome.
Magnetic resonance spectroscopy-measured pancreatic fat has previously been reported in association with insulin resistance. Using water-fat MRI, a recent population study of 685 healthy Chinese adults reported that fatty pancreas was related to central obesity defined by waist circumference, hypertriglyceridemia, and elevated serum ferritin [41]. A recent meta-analysis reported that the presence of nonalcoholic fatty pancreas disease was associated with a significantly increased risk of arterial hypertension, type 2 diabetes, and metabolic syndrome [42].
CONCLUSION
Advances in imaging technology increase the accuracy and efficiency of abdominal obesity measurement. Although tape-measured waist circumference fits the needs of large-scale epidemiological studies, 3D body scanning provides more detailed information that reflects body shape and volume. Recent improvements in dual abdominal bioelectrical impedance analysis and ultrasound are promising, particularly because ultrasound may be used to assess abdominal subcutaneous adipose tissue thickness [43] and VAT depth [44] during pregnancy. Future large-scale studies are needed to prove that bioelectrical impedance analysis or ultrasound is more accurate than waist circumference in estimating VAT in different populations. DXA is more accurate than waist circumference in estimating VAT, but future studies are needed to validate DXA’s capacity to detect VAT changes over time. CT and MRI provide multidimensional visualizations of anatomy and are reference methods for abdominal adipose tissue quantification. The versatile image acquisitions and postprocessing protocols available, especially in MRI, promote evaluating abdominal adiposity through a range of perspectives from simple morphology to functional studies.
KEY POINTS.
Waist circumference is the simplest and most economical measure of abdominal obesity.
Only CT and MRI can provide multidimensional visualizations of the anatomy and directly measure abdominal adipose tissue depots.
The emerging 3D body scanning technique measures abdominal volume and shape.
Further evidence is needed to support that bioelectrical impedance analysis, ultrasound, or 3D body scanning is consistently superior to waist circumference in evaluating abdominal obesity.
Acknowledgements
We sincerely thank Steven B. Heymsfield for his guidance and suggestions on the 3-dimensiaonal body scanning technology. We also thank Jun Chen for his suggestions on the computed tomography and magnetic resonance imaging section.
Grants support include R01 AG045761, P30 DK26687, UL1TR000040 Integrating Special Population Award on Body Composition after Bariatric Surgery, UL1TR000040 Irving Pilot Imaging Award on Quantification of Brown Adipose Tissue, and Zuckerman Mind Brain Behavior Institute Seed Grant 2018.
Financial support and sponsorship
None.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Gregg EW, Shaw JE. Global health effects of overweight and obesity. N Engl J Med 2017; 377:80–81. [DOI] [PubMed] [Google Scholar]
- 2.Kivimaki M, Kuosma E, Ferrie JE, et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health 2017; 2:e277–e285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global BMIMC. Di Angelantonio E, Bhupathiraju Sh N, Wormser D, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016; 388:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahakyan KR, Somers VK, Rodriguez-Escudero JP, et al. Normal-weight central obesity: implications for total and cardiovascular mortality. Ann Intern Med 2015; 163:827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neeland IJ, Ayers CR, Rohatgi AK, et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring) 2013; 21: E439–E447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balkau B, Deanfield JE, Despres JP, et al. International Day for the Evaluation of Abdominal Obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation 2007; 116:1942–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neamat-Allah J, Wald D, Husing A, et al. Validation of anthropometric indices of adiposity against whole-body magnetic resonance imaging: a study within the German European Prospective Investigation into Cancer and Nutrition (EPIC) cohorts. PLoS One 2014; 9:e91586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soileau L, Bautista D, Johnson C, et al. Automated anthropometric phenotyping with novel Kinect-based three-dimensional imaging method: comparison with a reference laser imaging system. Eur J Clin Nutr 2016; 70:475–481. [DOI] [PubMed] [Google Scholar]
- 9.Glock F, Vogel M, Naumann S, et al. Validity and intraobserver reliability of three-dimensional scanning compared with conventional anthropometry for children and adolescents from a population-based cohort study. Pediatr Res 2017; 81:736–744. [DOI] [PubMed] [Google Scholar]
- 10.Bourgeois B, Ng BK, Latimer D, et al. Clinically applicable optical imaging technology for body size and shape analysis: comparison of systems differing in design. Eur J Clin Nutr 2017; 71:1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Browning LM, Mugridge O, Chatfield MD, et al. Validity of a new abdominal bioelectrical impedance device to measure abdominal and visceral fat: comparison with MRI. Obesity 2010; 18:2385–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park KS, Lee DH, Lee J, et al. Comparison between two methods of bioelectrical impedance analyses for accuracy in measuring abdominal visceral fat area. J Diabetes Complicat 2016; 30:343–349. [DOI] [PubMed] [Google Scholar]
- 13.Berker D, Koparal S, Isik S, et al. Compatibility of different methods for the measurement of visceral fat in different body mass index strata. Diagn Interv Radiol 2010; 16:99–105. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Ambrosi J, Gonzalez-Crespo I, Catalan V, et al. Clinical usefulness of abdominal bioimpedance (ViScan) in the determination of visceral fat and its application in the diagnosis and management of obesity and its comorbidities. Clin Nutr 2018; 37:580–589. [DOI] [PubMed] [Google Scholar]
- 15■.Asano T, Kubota N, Koizumi N, et al. Novel and simple ultrasonographic methods for estimating the abdominal visceral fat area. Int J Endocrinol 2017; 2017:8796069. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used different geometric models to convert ultrasound measures of tissue thicknesses to VAT areas.
- 16.Rolfe ED, Sleigh A, Finucane FM, et al. Ultrasound measurements of visceral and subcutaneous abdominal thickness to predict abdominal adiposity among older men and women. Obesity 2010; 18:625–631. [DOI] [PubMed] [Google Scholar]
- 17.Gradmark AM, Rydh A, Renstrom F, et al. Computed tomography-based validation of abdominal adiposity measurements from ultrasonography, dual-energy X-ray absorptiometry and anthropometry. Br J Nutr 2010; 104:582–588. [DOI] [PubMed] [Google Scholar]
- 18.Wagner DR, Thompson BJ, Anderson DA, Schwartz S. A-mode and B-mode ultrasound measurement of fat thickness: a cadaver validation study. Eur J Clin Nutr 2018; 72:1–6; doi: 10.1038/s41430-018-0085-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Cheung AS, de Rooy C, Hoermann R, et al. Correlation of visceral adipose tissue measured by Lunar Prodigy dual X-ray absorptiometry with MRI and CT in older men. Int J Obes (Lond) 2016; 40:1325–1328. [DOI] [PubMed] [Google Scholar]
- 20.Neeland IJ, Grundy SM, Li X, et al. Comparison of visceral fat mass measurement by dual-X-ray absorptiometry and magnetic resonance imaging in a multiethnic cohort: the Dallas Heart Study. Nutr Diabetes 2016; 6:e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21■.Vasan SK, Osmond C, Canoy D, et al. Comparison of regional fat measurements by dual-energy X-ray absorptiometry and conventional anthropometry and their association with markers of diabetes and cardiovascular disease risk. Int J Obes (Lond) 2018; 42:850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study found that DXA-determined visceral fat mass has stronger odds ratios for type 2 diabetes and cardiovascular disease than waist circumference.
- 22.Alsina ME, Ruiz-Tovar J, Bernabeu A. Evolution of liver steatosis quantified by MR imaging and MR spectroscopy, in morbidly obese patients undergoing sleeve gastrectomy: short-term outcomes. Obes Surg 2017; 27: 1724–1728. [DOI] [PubMed] [Google Scholar]
- 23.Pourhassan M, Gluer CC, Pick P, et al. Impact of weight loss-associated changes in detailed body composition as assessed by whole-body MRI on plasma insulin levels and homeostatis model assessment index. Eur J Clin Nutr 2017; 71:212–218. [DOI] [PubMed] [Google Scholar]
- 24.Hu HH, Chen J, Shen W. Segmentation and quantification of adipose tissue by magnetic resonance imaging. MAGMA 2016; 29:259–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heymsfield SB, Hu HH, Shen W, Carmichael O. Emerging technologies and their applications in lipid compartment measurement. Trends Endocrinol Metab 2015; 26:688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng X, Zhang Y, Wang C, et al. The optimal anatomic site for a single slice to estimate the total volume of visceral adipose tissue by using the quantitative computed tomography (QCT) in Chinese population. Eur J Clin Nutr 2018; 72:1–9; doi: 10.1038/s41430-018-0122-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borga M, Virtanen KA, Romu T, et al. Brown adipose tissue in humans: detection and functional analysis using PET (positron emission tomography), MRI (magnetic resonance imaging), and DECT (dual energy computed tomography). Methods Enzymol 2014; 537:141–159. [DOI] [PubMed] [Google Scholar]
- 28■■.Leitner BP, Huang S, Brychta RJ, et al. Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci USA 2017; 114:8649–8654. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes BAT’s anatomic distribution and functional capacity in lean and obese healthy young men under tolerable cold exposure. They found that obese men had less abdominal activated BAT than lean men.
- 29.Deng J, Neff LM, Rubert NC, et al. MRI characterization of brown adipose tissue under thermal challenges in normal weight, overweight, and obese young men. J Magn Reson Imaging 2018; 47:936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones TA, Wayte SC, Reddy NL, et al. Identification of an optimal threshold for detecting human brown adipose tissue using receiver operating characteristic analysis of IDEAL MRI fat fraction maps. Magn Reson Imaging 2018; 51:61–68. [DOI] [PubMed] [Google Scholar]
- 31.Machann J, Stefan N, Wagner R, et al. Intra- and interindividual variability of fatty acid unsaturation in six different human adipose tissue compartments assessed by 1 H-MRS in vivo at 3 T. Nmr Biomed 2017; 30:e3744. doi: 10.1002/nbm.3744. Epub 2017 May 25. [DOI] [PubMed] [Google Scholar]
- 32.Hui SCN, Zhang T, Shi L, et al. Automated segmentation of abdominal subcutaneous adipose tissue and visceral adipose tissue in obese adolescent in MRI. Magn Reson Imaging 2018; 45:97–104. [DOI] [PubMed] [Google Scholar]
- 33.Jha S, Topol EJ. Adapting to artificial intelligence radiologists and pathologists as information specialists. JAMA 2016; 316:2353–2354. [DOI] [PubMed] [Google Scholar]
- 34.Addeman BT, Kutty S, Perkins TG, et al. Validation of volumetric and single-slice MRI adipose analysis using a novel fully automated segmentation method. J Magn Reson Imaging 2015; 41:233–241. [DOI] [PubMed] [Google Scholar]
- 35.Fallah F, Machann J, Martirosian P, et al. Comparison of T1-weighted 2D TSE, 3D SPGR, and two-point 3D Dixon MRI for automated segmentation of visceral adipose tissue at 3 Tesla. MAGMA 2017; 30:139–151. [DOI] [PubMed] [Google Scholar]
- 36.Lee H, Troschel FM, Tajmir S, et al. Pixel-level deep segmentation: artificial intelligence quantifies muscle on computed tomography for body morpho-metric analysis. J Digit Imaging 2017; 30:487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Unal E, Karaosmanoglu AD, Akata D, et al. Invisible fat on CT: making it visible by MRI. Diagn Interv Radiol 2016; 22:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38■.Xu L, Duanmu Y, Blake GM, et al. Validation of goose liver fat measurement by QCT and CSE-MRI with biochemical extraction and pathology as reference. Eur Radiol 2018; 28:2003–2012. [DOI] [PubMed] [Google Scholar]; This study used goose liver to validate quantitative CT for liver fat measurement.
- 39.Cheng X, Blake GM, Brown JK, et al. The measurement of liver fat from single-energy quantitative computed tomography scans. Quant Imaging Med Surg 2017; 7:281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40■.Leporq B, Lambert SA, Ronot M, et al. Simultaneous MR quantification of hepatic fat content, fatty acid composition, transverse relaxation time and magnetic susceptibility for the diagnosis of nonalcoholic steatohepatitis. NMR Biomed 2017; 30:e3766. doi: 10.1002/nbm.3766. Epub 2017 Jul 5. [DOI] [PubMed] [Google Scholar]; This study processed customize-designed MRI and generated maps of monounsaturated and polyunsaturated fatty acids of the liver.
- 41.Wong VW, Wong GL, Yeung DK, et al. Fatty pancreas, insulin resistance, and β-cell function: a population study using fat-water magnetic resonance imaging. Am J Gastroenterol 2014; 109:589–597. [DOI] [PubMed] [Google Scholar]
- 42.Singh RG, Yoon HD, Wu LM, et al. Ectopic fat accumulation in the pancreas and its clinical relevance: a systematic review, meta-analysis, and meta-regression. Metabolism 2017; 69:1–13. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy NJ, Peek MJ, Quinton AE, et al. Maternal abdominal subcutaneous fat thickness as a predictor for adverse pregnancy outcome: a longitudinal cohort study. BJOG 2016; 123:225–232. [DOI] [PubMed] [Google Scholar]
- 44.Ray JG, De Souza LR, Park AL, et al. Preeclampsia and preterm birth associated with visceral adiposity in early pregnancy. J Obstet Gynaecol Can 2017; 39:78–81. [DOI] [PubMed] [Google Scholar]