Abstract
Background:
Oral human papillomavirus (HPV) infection and related oropharyngeal cancer are uncommon in lower-income countries, particularly compared to HPV-associated cervical cancer. However, little is known about the natural history of oral HPV in less-developed settings and how it compares to the natural history of cervical HPV.
Methods:
Three hundred fifty women aged 22 to 33 years from the Costa Rica Vaccine Trial provided exfoliated cells from the cervical and oral regions at 2 visits 2 years apart. Samples from both visits were tested for 25 characterized α HPV types by the SPF10 PCR-DNA enzyme immunoassay-LiPA25 version 1 system. Risk factors for oral HPV persistence were calculated utilizing generalized estimating equations with a logistic link.
Results:
Among the 82 women with characterized α oral HPV DNA detected at baseline, 14 persisted and were detected 2 years later (17.6%; 95% confidence interval [CI], 10.9–28.5%) and was similar to the persistence of α cervical HPV (40/223; 17.7%; 95% CI, 13.1–23.9%; P = 0.86). Acquisition of new α oral HPV type was low; incident infection (1.7%; 95% CI, 0.6–3.7%).
Conclusions:
Oral HPV DNA is uncommon in young women in Latin America, and often appears to clear within a few years at similar rates to cervical HPV.
Oral human papillomavirus (HPV) infections have been detected in a subset of oropharyngeal cancers.1–3 These HPV-associated cancers are etiologically distinct from other head and neck cancers and have been increasing in incidence in many developed countries.4,5 Approximately half or more of oropharyngeal cancers are now associated with HPV infection in North America, South America, and Europe.5,6
However, studies have suggested strong geographic heterogeneity in the prevalence of HPV-associated oropharyngeal cancers.6,7 Recent estimates suggest less than a quarter of oropharyngeal cancers in Central America and other less developed areas are associated with HPV infection,6 and the estimated number of HPV-associated oropharyngeal cancers in Central and South America is considerably lower than North America.8 This is in stark contrast to HPV-associated cervical cancers, which have a high burden in Central America and are currently found in less developed settings due to lack of adequate screening.
Compared with cervical HPV infection, our current understanding of the epidemiology of oral HPV infection is limited, especially in less developed settings, where HPV-associated oropharyngeal cancer appears to be less prevalent.8 In line with observed geographic trends in HPV-associated oropharyngeal cancer, a recent cross-sectional study from our group reported a low prevalence of oral HPV DNA among Costa Rican women in their 20s (2%),9 which is below the US national estimate of ∼4% in US women in their 20s.10 Additionally, when comparing oral and cervical HPV DNA within this same population of Costa Rican women, cervical HPV DNA was roughly 15 times more common than oral HPV DNA (∼30% vs ∼2%),9 suggesting that the epidemiology of HPV infection varies greatly by anatomic site.
Few longitudinal studies of oral HPV have been conducted to date11–15 and again, mostly in US populations. These studies suggest that the majority of oral HPV infections clear within 1 to 2 years,11–14 but a subset appear to persist for several years.11 However, little is known about the natural history of oral HPV in Central America and, to our knowledge, no long-term study to date has directly compared the natural history of cervical and oral HPV within the same population. Therefore, we conducted a 2-year longitudinal study of oral and cervical HPV nested within the Costa Rica Vaccine trial (CVT),16 which is conducted within a Latin American country with no reported data on oral HPV natural history. The aim of this study was to examine natural history of oral HPV and to compare it to the natural history of cervical HPV within a population of healthy Costa Rican young women.
MATERIALS AND METHODS
Study Population and Design
This study was nested within the CVT, a 4-year phase III double-blind, randomized controlled trial evaluating the efficacy of the prophylactic bivalent HPV16/18 virus-like particle vaccine.16 Women eligible for inclusion within this trial were aged 18 to 25 years, generally healthy, not pregnant or breastfeeding, willing to use contraception during the vaccination period, and willing to provide informed consent. The CVT was originally designed to evaluate vaccine efficacy against persistent cervical HPV16/18 infection and associated precancerous lesions, as previously described.16 The trial was reviewed and approved by human subjects review committees of INCIENSA (Instituto Costarricense de Investigation y Enseñanza en Nutritión y Salud) in Costa Rica and the National Cancer Institute in the United States. The trial is registered at clinicaltrials.gov, identifier NCT00128661.
All sexually experienced women provided a cervical specimen for HPV testing at each vi sit of the CVT. At the 4-year blinded visit (hereby called the 4-year visit), all women (N = 6,351) in the both the HPV vaccine arm and the control arm were asked to provide an oral sample and were administered a questionnaire which included questions about oral sex behavior (Fig. 1).9 α oral HPV detection was rare, occurring in 1.9% of the population at this visit.9 At a subsequent visit, women in the control arm were offered the HPV vaccine. Afterward, the CVT women living in Guanacaste were followed for an additional 2 years in the “longterm follow-up study” (LTFU).17 At the LTFU follow-up visit, 2 years later (hereby called the 6-year visit), a follow-up questionnaire was administered and the oral collection was restricted to 350 women who had an α mucosal or β cutaneous oral HPV DNA detected at 4 years and attended the 6-year visit. The 66 women who attended the 6-year and had α mucosal oral HPV DNA (82 HPV types total) at 4 years were included in the 6-year visit primarily to examine oral HPV persistence. The other 284 women with only β oral HPV DNA at the 4-year visit were included primarily to expand the sample size for examining the incidence of α oral HPV (Fig. 1). Only a subset of 500 women were tested for β cutaneous HPV at the 4-year visit, and our previous study found that it was not associated with sexual behavior, but was associated with having concurrent α mucous oral HPV DNA.9 Finally, to evaluate the impact of oral hygiene and food/beverage consumption on oral specimen collection, an additional oral sample was collected the morning after the 6-year visit among a subset of these women (N = 162 of 350; the oral re-test visit).17
Figure 1.
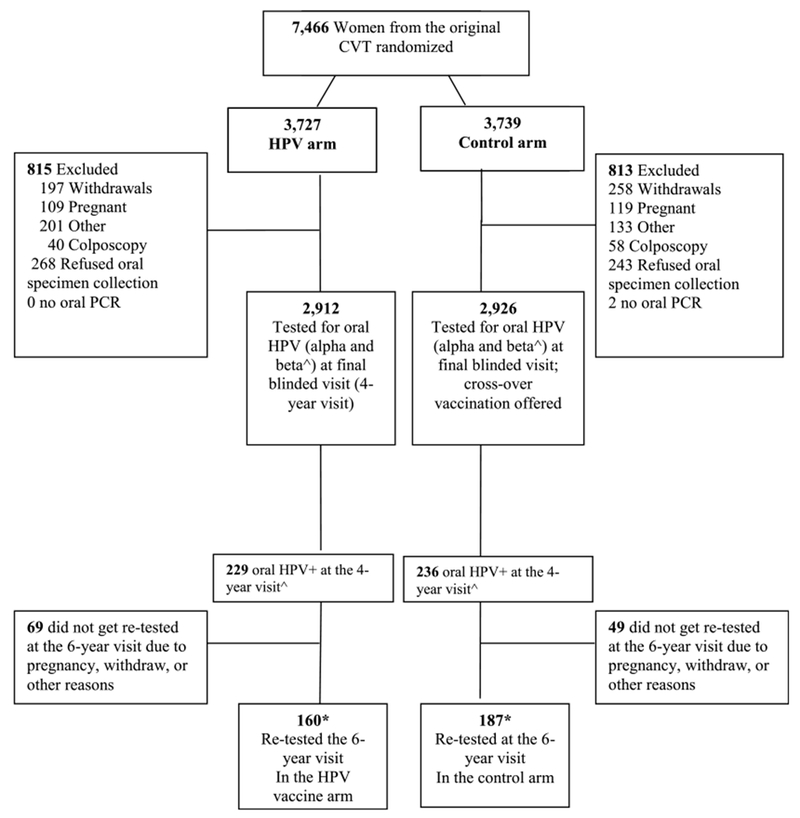
Natural history of oral HPV infection in young Costa Rican women. ^Beta oral HPV infection was only tested for in a subset of 500 women at the 4-year visit (see Lang Kuhs et al, JID2013;208(10):1643–1652 for further details). *Two women from the HPV arm and one from the control arm were oral HPV negative at the 4-year visit, but were retested at the 6-year visit. Thus in total there were 350 women tested for oral HPV at the 6 year visit, 162 from the HPV vaccine arm and 188 from the control arm.
Specimen Collection and Laboratory Analyses
Oral specimens were collected using a 30-second rinse and gargle with Scope mouthwash (Procter and Gamble Company, Cincinnati, OH) at the 4- and 6-year visits.9,18 Oral rinse samples were collected throughout the day for these participants, and had no restrictions in eating, drinking, or oral hygiene requirements before the collection. However, for the oral re-test visit (morning after the 6-year visit), participants were instructed to not eat, drink, or brush their teeth before collection of the oral rinse in the morning. Oral rinses at the re-test visit were all self-administered at participant’s homes, and participants were instructed to keep their sample in a (∼4°C) refrigerator until collection by study staff.
Specimens from each visit were kept between 2°C and 8°C until same-day processing at the local laboratory.9 Samples were centrifuged at 3000g for 10 minutes; the resulting pellet was washed with 10-mL saline solution, centrifuged, and resuspended in 1 mL of saline solution and frozen in liquid nitrogen tanks until testing. At the 4- and 6-year visits exfoliated cervical cells for liquid-based cytology and HPV DNA testing were also collected. They were collected with a Cervex brush by firmly rotating the brush 360 degrees around the cervical os five times as previously described.
DNA from each oral and cervical specimen was extracted via the MagNAPure LC DNA isolation procedure (Roche Diagnostics) as previously described.19 All of the extracted DNA samples were then tested for α HPV DNA by PCR amplification using the HPV SPF10 PCR-DEIA (DNA enzyme immunoassay)-LiPA25 (Line probe assay) version 1 system (Labo Biomedical Products, Rijswijk, The Netherlands), which uses SPF10 primers and LiPA25 line probe assay to provide the genotype of 25 α HPV types including 12 carcinogenic (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59) and 13 non-carcinogenic (6, 11, 34, 40, 42, 43, 44, 53, 54, 66, 68/73, 70 and 74) types.9,20,21 To increase the sensitivity of type-specific detection of HPV-16 and HPV-18 using the SPF10 DEIA system, all oral specimens that were SPF10 PCR/DEIA-positive, as well as a convenience sample of one-third of all samples (as part of quality control), were tested for the presence of HPV-16 or HPV-18 using type-specific primers detected by the TS16 and TS18 DEIA system; for cervix samples, all specimens positive for HPV DNA using SPF10 DEIA but negative for HPV-16 or HPV-18 by LiPA25 were additionally tested with type-specific primers/probes.9 At the 4-year visit only, a subset of the oral rinse specimens (n = 500) were also tested for the presence of cutaneous β, γ, μ and nu HPV types via PCR detection and a reverse-hybridization assay for typing.9
Statistical Analysis
Oral and cervical HPV types were defined as having any of the 25 characterized α HPV types by the LiPA25 line probe assay. Types were subclassifed into carcinogenic (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59); or noncarcinogenic (6, 11, 34, 40, 42, 43, 44, 53, 54, 66, 68/73, 70, and 74) HPV types. Samples that were positive by DEIA PCR, but were not positive for any of the 25 characterized α types, were considered to be “uncharacterized” and were not considered in the persistence or incidence analyses.
Study participant characteristics of the 350 female participants included in this longitudinal study were described and compared to the population of women that provided an oral rinse at the 4-year visit using chi-square, Mann-Whitney and the Kolmogorov-Smirnov tests. HPV DNA was described on a type-specific level basis, with HPV persistence defined as a type-specific DNA that was detected at the 4-year visit and subsequently detected 2 years later at the 6-year visit. Risk factors for oral HPV persistence were calculated on a type-specific level using generalized estimating equations with a logistic link using an exchangeable correlation structure to account for the correlation between multiple HPV DNA types within a single woman.22 Oral sex was defined as a woman putting her mouth on her partner’s genitals (almost all men).
Incident α oral HPV DNA types were defined as a characterized type-specific DNA type that was detected at the 6-year visit that was not observed at the 4-year visit. Given that women with an oral HPV type are still at risk of acquiring a different oral HPV type, they were included in this analysis. Type-specific concordance between the 6-year visit and the oral retest visit visits was evaluated using percent agreement and Kappa statistics with 95% confidence intervals (95% CIs). All tests were 2-sided with an α of 0.05.
RESULTS
Study Participant Characteristics
This study included 350 women who had α or cutaneous β oral HPV DNA at the 4-year visit and whom were subsequently retested for oral α HPV 2 years later (6-year visit; Fig. 1); 66 of the 350 women had at least 1 characterized α oral HPV DNA type at 4-year visit, with 12 having multiple oral HPV types. The median age at 4-year visit was 26 (interquartile range [IQR], 24–28), most never smoked (82%), were married (58%), ever used oral contraceptives (84%), had a previous pregnancy (74%), and had a history of oral sex (60%, Table 1). The majority of women who had performed oral sex only had 1 lifetime oral sex partner (63%) and had never performed oral sex until 1 or more years after the date of their first vaginal sex (88%). The median age of first vaginal sex was 17 (15–19) years, and the median age of first oral sex was 21 (IQR = 19–24). Approximately half of participants had oral sex between the 4-year and 6-year visit.
TABLE 1.
Participant Characteristics for the Subset of 350 Women Tested for α Oral HPV at the 6-y Visit Compared With the 5488 Women From the CVT Not Tested at the 6-y Visit
| Characteristics | CVT Women Tested at the 6-y Visit (n = 350) | CVT Women Not Tested at the 6-y Visit (n = 5488) | P |
|---|---|---|---|
| n (%) | n (%) | ||
| Age, y | |||
| Median (IQR) | 26 (24–28) | 26 (24–28) | 0.19 |
| 22–23 | 68 (19.43%) | 1257 (22.90%) | 0.07 |
| 24–25 | 85 (24.29%) | 1472 (26.82%) | |
| 26–27 | 101 (28.86%) | 1276 (23.25%) | |
| ≥ 28 | 96 (27.43%) | 1483 (27.02%) | |
| Marital status | |||
| Married or living as married | 204 (58.29%) | 3393 (61.83%) | 0.42 |
| Not married | 144 (41.14%) | 2068 (37.68%) | |
| Unknown | 2 (0.57%) | 27 (0.49%) | |
| Smoking status | |||
| Never | 287 (82.00%) | 4415 (80.45%) | 0.84 |
| Former | 39 (11.14%) | 697 (12.70%) | |
| Current | 23 (6.57%) | 365 (6.65%) | |
| Unknown | 1 (0.29%) | 11 (0.20%) | |
| Age at first vaginal intercourse, y | |||
| Median (IQR) | 17 (15–19) | 17 (15–19) | 1.00 |
| Virgin | 10 (2.86%) | 312 (5.69%) | 0.18 |
| ≥ 19 | 88 (25.14%) | 1406 (25.62%) | |
| 17–18 | 104 (29.71%) | 1598 (29.12%) | |
| ≤ 16 | 147 (42.00%) | 2166 (39.47%) | |
| Yes (age unknown) | 1 (0.29%) | 6 (0.11%) | |
| Lifetime no. sexual partners | |||
| Median (IQR) | 3 (1–5) | 2 (1–4) | 0.51 |
| 0–1 | 102 (29.14%) | 1709 (31.14%) | 0.22 |
| 2–3 | 112 (32.00%) | 1895 (34.53%) | |
| ≥ 4 | 136 (38.86%) | 1884 (34.33%) | |
| Age at first oral sex, y | |||
| Median (IQR) | 21 (19–24) | 20 (18–23) | 0.14 |
| Never had oral sex | 139 (39.71%) | 2058 (37.50%) | 0.18 |
| ≥ 21 | 106 (30.29%) | 1510 (27.51%) | |
| ≤ 20 | 105 (30.00%) | 1895 (34.53%) | |
| Unknown | 0 (0.00%) | 25 (0.46%) | |
| Lifetime no. oral sex partners | |||
| Median (IQR) | 1 (0–1) | 1 (0–2) | 0.67 |
| No oral sex/1 partner | 272 (77.71%) | 4029 (73.41%) | 0.13 |
| ≥ 2 | 78 (22.29%) | 1437 (26.18%) | |
| Unknown | 0 (0.00%) | 22 (0.40%) | |
| No. recent oral sex partners (since visit before the 6-y visit) | |||
| No oral sex | 175 (50.00%) | 2022 (47.05%) | 0.31 |
| 1 partner | 157 (44.86%) | 2043 (47.53%) | |
| ≥ 2 partners | 16 (4.57%) | 225 (5.23%) | |
| Unknown | 2 (0.57%) | 8 (0.19%) | |
| Timing of oral sex initiation | |||
| Never had oral sex | 139 (39.71%) | 2058 (37.50%) | 0.06 |
| Same age as vaginal sex initiation | 16 (4.57%) | 499 (9.09%) | |
| Before first vaginal sex | 8 (2.29%) | 152 (2.77%) | |
| After first vaginal sex | 186 (53.14%) | 2753 (50.16%) | |
| 1 to 3 y after | 89 (25.43%) | 1432 (26.09%) | |
| 4+ y after | 97 (27.71%) | 1321 (24.07%) | |
| Unknown | 1 (0.29%) | 26 (0.47%) | |
| Use of oral contraceptives | |||
| Never | 42 (12.00%) | 641 (11.68%) | 0.20 |
| Virgin | 10 (2.86%) | 312 (5.69%) | |
| Former | 159 (45.43%) | 2418 (44.06%) | |
| Current (past month) | 136 (38.86%) | 2037 (37.12%) | |
| Unknown | 3 (0.86%) | 80 (1.46%) | |
| Ever pregnant | |||
| Yes | 259 (74.00%) | 3912 (71.28%) | 0.28 |
| No | 91 (26.00%) | 1576 (28.72%) | |
| Chronic sinusitis | |||
| No | 318 (90.86%) | 5151 (93.86%) | 0.03 |
| Yes | 32 (9.14%) | 337 (6.14%) | |
| Cervical HPV status at the 4-y visit | |||
| Negative | 205 (58.57%) | 3674 (66.95%) | <0.01 |
| Positive | 144 (41.14%) | 1809 (32.96%) | |
| Not tested | 1 (0.29%) | 5 (0.09%) | |
The 350 women included in this study were similar to the other 5488 women who were not resampled at 6-year visit with respect to age, marital status, smoking status, lifetime number of sexual partners, and oral contraceptive use (all P values >0.10, Table 1). They were also similar in the number of recent and life-time oral sex partners (P values > 0.10). However, the 350 women in this study were significantly more likely to be cervical HPV positive at the 4-year visit, and more likely to have chronic sinusitis (P values < 0.05, Table 1).
Oral HPV Persistence
Among the 82 characterized α oral HPV DNA detected at the 4-year visit, 14 persisted and were detected 2 years later (17.6%; 95% CI, 10.9–28.5%; Table 2). Persistence was similar across arm, 23.6% (95% CI, 11.7–47.5%) of oral HPV types persisted in the HPV vaccinated arm and 14.8% (95% CI, 7.8–28.2%) persisted in the control arm (P = 0.41). In both arms combined, persistence was similar for the 47 carcinogenic oral HPV types (15.4%; 95% CI, 7.9–30.1%) and the 35 noncarcinogenic oral HPV types (20.5%; 10.8–39.0%), P = 0.54. The most commonly detected types were oral HPV 51, 16, 66, and 52. Ten of the 82 infections were oral HPV16 (all detected in the control arm), with only one (10%) persisting.
TABLE 2.
Persistence of Oral and Cervical HPV Alpha Mucosal Types Among the Women With Detectable Oral HPV DNA at the 4-y Visit, Stratified by Vaccine Arm
| Overall (n = 350) |
HPV Vaccine Arm (n = 162) |
Control Arm (n = 188)* |
||||
|---|---|---|---|---|---|---|
| Test Result | No. HPVs Detected at the 4-y Visit | % (95% CI) Persisted (Detected Again at the 6-y Visit) | No. HPVs Detected at the 4-y Visit | % (95% CI) Persisted (Detected Again at 6-y Visit) | No. HPVs Detected at the 4-y Visit | (% 95% CI) Persisted (Detected Again at 6-y Visit) |
| Any oral HPV | 82 | 17.6% (10.9–28.5%) | 27 | 23.6% (11.7–47.5%) | 55 | 14.8% (7.8–28.2%) |
| Carcinogenic oral HPV | 47 | 15.4% (7.9–30.1%) | 11 | 27.7% (10.8–70.9%) | 36 | 11.3% (4.6–28.1%) |
| Noncarcinogenic oral HPV | 35 | 20.5% (10.8–39.0%) | 16 | 19.6% (7.2–53.2%) | 19 | 21.3% (9.2–49.3%) |
| Uncharacterized oral HPV | 156 | n/a | 79 | n/a | 77 | n/a |
| Any cervical HPV† | 223 | 17.7% (13.1–23.9%) | 84 | 19.3% (13.3–27.9%) | 139 | 16.5% (10.8–25.2%) |
| Carcinogenic cervical HPV† | 131 | 19.7% (13.6–28.6%) | 42 | 20.5% (11.4–37.2%) | 89 | 18.4% (11.3–29.9%) |
| Noncarcinogenic cervical HPV† | 92 | 14.9% (9.1–24.6%) | 42 | 17.9% (10.4–30.7%) | 50 | 13.4% (6.6–27.4%) |
| Uncharacterized cervical HPV† | 22 | n/a | 12 | n/a | 10 | n/a |
The majority of the women from the control arm were also HPV16/18 vaccinated after the 4 year visit.
Excludes 1 of the 350 woman who did not have cervical testing at the 4-year visit.
n/a indicates not applicable.
We next examined factors associated with oral HPV persistence among the 82 characterized α oral HPV. Older age was significantly associated with an increased odds of persistence (OR, 1.53 per each increased year of age; 95% CI, 1.12–2.10%; Table 3), because almost all of the persistent oral HPV were restricted to individuals who were 28 years of age or older when their infection was first detected. Never (OR, 13.49; 95% CI, 1.96–92.93%) and former use (OR, 6.10; 95% CI, 1.19–31.38%) of oral contraceptive was also associated with increased oral HPV persistence compared with current use.
TABLE 3.
Univariate Analyses of Risk Factors for Oral HPV Persistence Among Young Adult Women From Costa Rica (Both Arms Combined)
| Overall (N = 82) |
|||
|---|---|---|---|
| Characteristics | No. Infections | HPV Persistence, No. (%) Infection | OR (95% CI) |
| Age at the 6-y visit, y | |||
| 27 or less | 30 | 1 (3.3%) | 0.10 (0.01–0.79) |
| ≥ 28 | 52 | 13 (25.3%) | Referent |
| Continuous (per year) | 82 | 14 (17.1%) | 1.53 (1.12–2.10) |
| Continuous p-trend | 0.008 | ||
| Marital status | |||
| Married or living as married | 46 | 10 (22.5%) | Referent |
| Not married | 36 | 4 (11.4%) | 0.44 (0.12–1.56) |
| Smoking status | |||
| Never | 57 | 9 (16.5%) | Referent |
| Former | 15 | 3 (20.3%) | 1.29 (0.31–5.47) |
| Current | 10 | 2 (20.2%) | 1.28 (0.25–6.71) |
| Lifetime no. sexual partners | |||
| Continuous | 82 | 14 (17.6%) | 1.04 (0.86–1.26) |
| 0–1 | 8 | 1 (12.8%) | 0.80 (0.08–7.61) |
| 2–3 | 21 | 5 (24.4%) | 1.75 (0.49–6.32) |
| ≥ 4 | 53 | 8 (15.6%) | Referent |
| Ever had oral sex | |||
| No | 16 | 6 (37.5%) | 4.28 (1.27–14.41) |
| Yes | 66 | 8 (12.3%) | Referent |
| No. recent oral sex partners since the 4-year visit | |||
| No oral sex | 37 | 8 (21.8%) | 1.73 (0.53–5.61) |
| 1+ partners | 45 | 6 (13.9%) | Referent |
| Continuous p-trend | 0.27 | ||
| Use of oral contraceptives | |||
| Never | 7 | 3 (42.8%) | 13.49 (1.96–92.93) |
| Former | 36 | 9 (25.3%) | 6.10 (1.19–31.38) |
| Current (past month) | 39 | 2 (5.2%) | Referent |
| Ever pregnant | |||
| Y | 63 | 13 (21.2%) | Referent |
| N | 19 | 1 (5.1%) | 0.20 (0.03–1.55) |
| Chronic sinusitis | |||
| No | 70 | 13 (18.8%) | Referent |
| Yes | 12 | 1 (9.0%) | 0.43 (0.05–3.95) |
| Cervical HPV status at 4-y study visit | |||
| Negative | 35 | 11 (31.9%) | Referent |
| Positive (any HPV type) | 47 | 3 (6.6%) | 0.15 (0.04–0.61) |
| Concordant cervical HPV status at 4-y study visit | |||
| Negative | 51 | 14 (27.4%) | Referent |
| Positive* | 31 | 0 (0.0%) | N/a |
| Vaccine arm | |||
| Vaccine Arm | 27 | 6 (22.4%) | 1.50 (0.44–5.11) |
| Control arm—crossed over | 44 | 7 (16.2%) | Referent |
| Control arm—not crossed over | 11 | 1 (9.7%) | 0.56 (0.06–5.57) |
Restricted to those with same HPV type as oral at the 4-year visit.
Although the majority of oral HPV DNA were detected among those with a history of oral sex, oral HPV types detected among those reporting never having oral sex were more likely to persist (OR, 4.28; 95% CI, 1.27–14.41%). Smoking status, marital status, lifetime number of sex partners, chronic sinusitis, and vaccine status were not associated with oral HPV persistence (all P values > 0.10; Table 3).
Oral Versus Cervical HPV Persistence
Among the 350 women in this study, 223 had a detectable cervical HPV infection at the 4-year visit. Women who had a cervical HPV infection and an oral HPV infection at the 4-year visit were significantly more likely to clear their oral HPV infection (OR, 0.15; 95% CI, 0.04–0.61%; Table 3) by the 2-year visit compared to women who did not have a cervical HPV infection. None of the 31 women who had the same type-specific infection at the cervical and oral sites had their oral HPV infection persist to the 6-year visit.
Among cervical HPV detected at the 4-year visit, 40 of the 223 (17.7%; 95% CI, 13.1–23.9%; Table 2) were detected at the 6-year visit, which was similar to the oral HPV persistence in this population (17.6%; P = 0.86). Cervical and oral HPV persistence were also similar when restricting to carcinogenic (19.7% vs 15.4%, P = 0.48) and noncarcinogenic types (14.9% vs 20.5%; P = 0.54, Table 2). The percentage of persistent cervical HPV in our study was also similar to the cervical HPV persistence in the overall source population of 5832 women from the overall CVT (17.7% vs 16.5%; P = 0.62).
Oral HPV Incidence and Oral Rinse Agreement in the Retest Visit
Among the 350 women in this study, there were 6 incident α oral HPV DNA types detected among 6 women (1.71%; 95% CI, 0.63–3.69%). This included 3 carcinogenic (HPV16, 2 HPV51) and 3 noncarcinogenic types (HPV 53, 70, 68/73), with 2 in the vaccinated arm and 4 in the control arm (including one with oral HPV16 DNA).
Among the 162 women who provided an oral rinse in the morning before any oral hygiene/eating/drinking (retest visit), 145 had the same oral HPV status as the previous day (either consistently positive or negative). This amounted to a percent agreement of 89.5%, and a κ of 0.32 (95% CI, 0.07–0.56%). There were 22 women with a detectable oral HPV DNA at the 6-year visit or the retest visit, 5 had the same oral HPV type at both visits, 3 had different oral HPV types at the 2 visits, 3 were only detected at the 6-year visit, and 11 were only detected at only the re-test visit (Table 4). In total, there were twice as many individuals with oral HPV DNA detected in the retest visit versus the 6-year visit (22 vs 11 infections, P = 0.01).
TABLE 4.
Women Level Analysis Among the 162 Women Tested For Oral HPV at 6-y Visit and the Retest Visit*
| Retest Visit (Morning Rinse) | |||
|---|---|---|---|
| Oral HPV+ | Oral HPV− | ||
| 6-y visit | Oral HPV+ | 5 | 6† |
| Oral HPV− | 11 | 140 | |
| Agreement = 89.5% | |||
| κ = 0.32 (95% CI, 0.07–0.56) | |||
| HPV+ agreement = 45.5%; HPV− agreement = 92.7% | |||
There were 22 infections detected among the 19 oral HPV+ women in the LOR visit, and 11 infections detected among the 11 oral HPV+ women at the 6-year visit (P = 0.01).
All 6 women who are classified as oral HPV+ at the 6-year visit and oral HPV− at the re-test visit did not have the same type-specific oral HPV DNA type present at the 6-year visit. However, 3 of these 6 women did have a different oral HPV type detected at the retest visit that was not present at the 6-year visit.
DISCUSSION
In this study of young women from Costa Rica, oral HPV detection was rare, and when detected, the oral HPV types did not usually persist for 2 years. In addition, we found that oral HPV commonly clears at a similar rate as cervical HPV. This suggests that the higher cervical cancer burden in Costa Rica, and other countries may be more driven by the considerably higher risk of acquisition of cervical HPV compared with oral HPV rather than differences in clearance.
This study found that over three quarters of the prevalent oral HPV types are not detected 2 years later. This estimate is similar to other estimates, predominately from the US-based studies.11–15 Although these studies have suggested that oral HPV DNA can occasionally persist for several years, persistent oral HPV infections appear to more commonly occur in men, smokers, and older individuals.11,14,15 Our study similarly found oral HPV persistence to be associated with older age, which may be the results of infections in older women reflecting longer-term persistent infections (i.e., left truncation) which in turn are more likely to continue to persist, a phenomenon described in the cervix. One may also postulate that a decline in immune function with age may reduce the ability to clear infections, but our age span is relatively small, making this an unlikely explanation.11,14,15 However, this study did not observe a strong association with smoking status, which has been observed in larger studies.11 To our knowledge, we are the first to observe an association between not using oral contraceptives and oral HPV persistence. This is in contrast to the cervical HPV literature, which has suggested that current oral contraceptive use is positively associated with cervical HPV persistence.23 However, we should note that this study had a limited number of persistent oral HPV infections detected, and thus we could not conduct more robust adjusted analyses.
This study also found the persistence of oral and cervical HPV infection to be similar, despite having very large differences in acquisition. Although, this is, to our knowledge, the first longer-term comparison of oral and cervical HPV persistence, our finding does support a short-term US study that suggested that the 6-month persistence for oral and cervical HPV infection may be similar in women.24 However, further study across anatomic sites is necessary because differences in persistence are quite possible given local immunologic differences.25 Indeed, a previous study suggested a higher persistence of anal HPV infection than oral HPV infection in HIV-infected individuals from the United States.14
As expected, this study observed a low incidence of oral HPV in these young Costa Rica women. This low incidence is likely the primary driver behind the approximately 15 times lower oral HPV prevalence compared with cervical HPV prevalence in this population.9 There are likely several explanations for this low incidence. Previous studies have suggested that oral HPV incidence is lower among women potentially because the risk of oral HPVacquisition is lower when performing oral sex on a man compared with a woman.11,26 Additionally, the incidence may be particularly low in this population given that many women in this study appear to have first initiated oral sex years after vaginal sex. Thus, it is possible that these women may have developed natural HPV immunity from their cervical HPV infection, which may protect them against subsequent oral HPV acquisitions.27 Third, many of the women in this study were HPV16/18 vaccinated, likely explaining the low number of oral HPV16/18 infections detected (as oral HPV16 is often the most common type in unvaccinated populations).10
Finally, it is possible that the oral HPV prevalence and incidence may be artificially low due to modest sensitivity of the oral rinse. Previous studies have suggested that only about half of individuals with HPV-associated oropharyngeal cancer have detectable oral HPV infection via the oral rinse.28 This study suggests that procedures for oral HPV collection could have an impact on the sensitivity, as we detected twice as many oral HPV infections when requiring participants to provide their oral rinse sample in the morning before any drinking, eating, or oral hygiene. Although, we should note this is based on a small number of infections (22 vs 11). Although studies have suggested similar short-term HPV type-specific agreement for repeat sampling by the oral rinse29,30 compared with anal HPV (swab) and cervical HPV (brush) infections,31–33 it is possible that oral hygiene or other behaviors maybe limit the sensitivity of the oral rinse. Indeed, a recent study suggested a nonsignificantly higher sensitivity for their pretoothbrush abrasion oral rinse samples compared with their posttoothbrush abrasion oral rinse samples.34
There were several limitations to this study. Given the low frequency of oral HPV in this population, we had a limited numbers to examine persistence and were limited to univariate analyses for risk factors for oral HPV persistence. It is possible that some of the detections of oral HPV DNA represented deposition rather than established infection, as those reporting oral sex in this study were more likely to clear their HPV “infection” by follow-up. Additionally, we were limited to only 1 follow-up visit 2 years after the baseline visit for oral HPV collection to examine persistence and incidence. Thus, some of the infections may represent reinfections and we may have missed many short-term infections between visits and may have underestimated the persistence given the potential limited sensitivity of the oral rinse for collection. Although HPV vaccination is not known to impact HPV persistence, it is possible that there could be differences to other nonvaccinated populations. There were also a number of strengths to this study because it is one of the first long-term oral HPV natural history study in the developing world. This study used validated laboratory and statistical techniques and is also one of the first studies to compare the natural history of oral HPV and cervical HPV infection.
This study suggests that oral HPV infection is uncommon in young women in Latin America, and is likely to clear within a few years when it occurs. The rate of oral HPV clearance is highly similar to cervical HPV clearance, suggesting that the considerably higher burden of cervical cancer compared with oropharyngeal cancer in Latin America may be much more driven by a large difference in acquisition.
Footnotes
Please see Supplemental Digital Content 1, http://links.lww.com/OLQ/A180, for Investigators in the Costa Rica HPV Vaccine Trial (CVT) Group, funding, other notes, and conflict of interest.
Conflict of interest and sources of funding: none declared.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
REFERENCES
- 1.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst 2008; 100: 407–420. [DOI] [PubMed] [Google Scholar]
- 2.Gillison ML, Lowy DR. A causal role for human papillomavirus in head and neck cancer. The Lancet 2004; 363:1488–1489. [DOI] [PubMed] [Google Scholar]
- 3.Agalliu I, Gapstur S, Chen Z, et al. Associations of oral α-, β-, and γ-human papillomavirus types with risk of incident head and neck cancer. JAMA Oncol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol 2013; 31:4550–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the united states. J Clin Oncol 2011;29:4294–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellsagué X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: Comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst 2016; 108:djv403. [DOI] [PubMed] [Google Scholar]
- 7.Anantharaman D, Abedi-Ardekani B, Beachler DC, et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int J Cancer 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine 2012; 30(Suppl 5):F12–F23. [DOI] [PubMed] [Google Scholar]
- 9.Lang Kuhs KA, Gonzalez P, Struijk L, et al. Prevalence of and risk factors for oral human papillomavirus among young women in costa rica. J Infect Dis 2013; 208:1643–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the united states, 2009–2010. JAMA 2012; 307:693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beachler DC, Sugar EA, Margolick JB, et al. Risk factors for acquisition and clearance of oral human papillomavirus infection among HIV-infected and HIV-uninfected adults. Am J Epidemiol 2015; 181:40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreimer AR, Pierce Campbell CM, Lin HY, et al. Incidence and clearance of oral human papillomavirus infection in men: the HIM cohort study. Lancet 2013; 382:877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelstein ZR, Schwartz SM, Hawes S, et al. Rates and determinants of oral human papillomavirus infection in young men. Sex Transm Dis 2012; 39:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beachler DC, D’Souza G, Sugar EA, et al. Natural history of anal vs oral HPV infection in HIV-infected men and women. J Infect Dis 2013; 208:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Souza G, Wentz A, Kluz N, et al. Sex differences in risk factors and natural history of oral human papillomavirus infection. J Infect Dis 2016; 213:1893–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrero R, Hildesheim A, Rodriguez AC, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in guanacaste, costa rica. Vaccine 2008; 26: 4795–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez P, Hildesheim A, Herrero R, et al. Rationale and design of a long term follow-up study of women who did and did not receive HPV 16/18 vaccination in guanacaste, costa rica. Vaccine 2015; 33: 2141–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lum A, Le Marchand L. A simple mouthwash method for obtaining genomic DNA in molecular epidemiological studies. Cancer Epidemiol Biomarkers Prev 1998; 7:719–724. [PubMed] [Google Scholar]
- 19.Herrero R, Quint W, Hildesheim A, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in costa rica. PLoS One 2013; 8:e68329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleter B, van Doorn LJ, Schrauwen L, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol 1999; 37:2508–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleter B, van Doorn LJ, ter Schegget J, et al. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol 1998; 153:1731–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue X, Gange SJ, Zhong Y, et al. Marginal and mixed-effects models in the analysis of human papillomavirus natural history data. Cancer Epidemiol Biomarkers Prev 2010; 19:159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marks M, Gravitt PE, Gupta SB, et al. Combined oral contraceptive use increases HPV persistence but not new HPV detection in a cohort of women from thailand. J Infect Dis 2011; 204: 1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Souza G, Fakhry C, Sugar EA, et al. Six-month natural history of oral versus cervical human papillomavirus infection. Int J Cancer 2007; 121:143–150. [DOI] [PubMed] [Google Scholar]
- 25.Fakhry C, Marks MA, Gilman RH, et al. Comparison of the immune microenvironment of the oral cavity and cervix in healthy women. Cytokine 2013; 64:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaturvedi AK, Graubard BI, Broutian T, et al. NHANES 2009–2012 findings: association of sexual behaviors with higher prevalence of oral oncogenic human papillomavirus infections in U.S. men. Cancer Res 2015;75:2468–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beachler DC, Jenkins G, Safaeian M, et al. Natural acquired immunity against subsequent genital human papillomavirus infection: a systematic review and meta-analysis. J Infect Dis 2015; 213: 1444–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007; 356: 1944–1956. [DOI] [PubMed] [Google Scholar]
- 29.D’Souza G, Sugar E, Ruby W, et al. Analysis of the effect of DNA purification on detection of human papillomavirus in oral rinse samples by PCR. J Clin Microbiol 2005; 43:5526–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broutian TR, He X, Gillison ML. Automated high throughput DNA isolation for detection of human papillomavirus in oral rinse samples. J Clin Virol 2011; 50:270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flores R, Abalos AT, Nielson CM, et al. Reliability of sample collection and laboratory testing for HPV detection in men. J Virol Methods 2008; 149:136–143. [DOI] [PubMed] [Google Scholar]
- 32.Daniel RW, Ahdieh L, Hayden D, et al. Intra-laboratory reproducibility of human papillomavirus identification in cervical specimens by a polymerase chain reaction-based assay. J Clin Virol 2000; 19:187–193. [DOI] [PubMed] [Google Scholar]
- 33.Gravitt PE, Lacey JV Jr, Brinton LA, et al. Evaluation of self-collected cervicovaginal cell samples for human papillomavirus testing by polymerase chain reaction. Cancer Epidemiol Biomarkers Prev 2001; 10:95–100. [PubMed] [Google Scholar]
- 34.Ong JJ, Read T, Chen M, et al. Improving oral human papillomavirus detection using toothbrush sampling in HIV-positive men who have sex with men. J Clin Microbiol 2014; 52:2206–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]


