Abstract
Background
Human Immunodeficiency Virus (HIV) and Tuberculosis (TB) are the double burden diseases of the world. The African continent takes a great share of TB-HIV cases worldwide. This study was aimed to determine the prevalence of TB-HIV co-infection in Ethiopia, using a meta-analysis based on a systematic review of published articles.
Methods
An electronic search was conducted in databases including PubMed, HINARI, EMBASE, Cochrane library and Google Scholar to extract the articles. Articles published between 1995 and November 2017 had been searched for using different keywords. The analysis was performed using MetaXL software and R statistical software (version 3.2.3).
Result
Our searches returned a total of (n = 26,746) records from 30 articles of which 21 were cross-sectional, 7 were retrospectives and 2 were prospective studies. The range of prevalence of TB-HIV co-infection was found to be from 6 to 52.1% with random effects pooled prevalence of 22% (95% CI 19–24%) and with substantial heterogeneity chi-square (X2) = 746.0, p < 0.001, (I2 = 95.84%).
Conclusion
Our analysis indicated that the prevalence of TB-HIV co-infection is high in Ethiopia with substantial regional variation. An integrated, facility-based and community-based effort towards the prevention, early detection and management of cases should be further strengthened throughout the country to mitigate the double burden disease.
Keywords: Tuberculosis, Human immunodeficiency virus, Co-infection, Prevalence, Meta-analysis
Background
Human Immunodeficiency Virus (HIV) remained as the leading cause of mortality and morbidity; in 2016 for instance, 1.8 million people were newly infected and 1 million AIDS-related deaths were registered [1]. HIV/AIDS and TB (Tuberculosis) are considered as the double burden diseases of the world. According to the World health organization (WHO) report, there were 1.5 million deaths attributed to TB out of which 26% were due to HIV-associated TB [2]. In developing countries, particularly in sub-Saharan Africa, TB is increasing due to the burden of HIV [3]. Hence, the African continent takes the greater share (74%) of the 1.2 million TB-HIV cases worldwide [2]. In Ethiopia, 4 in 100 people die due to TB-HIV co-infection and the incidence of Multidrug-resistant Tuberculosis (MDR-TB) was estimated to be 5.8 per 1000 people [4].
The burden of TB is still alarming due to the emergences of drug-resistant strains. Recent studies indicated that the prevalence of MDR-TB in Ethiopia is particularly noticeable (15%) in previously treated TB cases [5]. Among laboratory-confirmed TB cases in the Oromo region, for instance, 33% of the cases were found to be MDR-TB, majorly among the adult population [6]. This increase in MDR-TB, especially among people living with HIV is a great burden to the healthcare system; challenging the management efforts of the disease through drug interaction and immune reaction [7–9]. The associated morbidity and mortality related to noticeable economic loss of the productive society and the socioeconomic status of the country at large. In developing countries, it is estimated that around 30% of the annual household income is taken by TB and HIV associated disease [10].
In general, people living with HIV are 20 times riskier to be infected with TB as compared to HIV negative people [11], with case fatality rate of 16–35%, which is almost 4 times higher than HIV free individuals [12]. The incidence of co-infection is determined by many factors including smoking, family size, the clinical stage of HIV, use of antiretroviral therapy (ART), injectable drug use and anemia [13–15]. In addition, it is evident that HIV positivity, alcohol intake, farming occupation and previous TB contact are contributing factors for MDR-TB [6].TB and HIV co-infection has various health outcomes limiting the life expectancy of the infected host; accelerated by malnutrition, rapid progression of the disease, atypical TB presentations, delayed diagnosis and a low treatment response [16]. Active tuberculosis facilitates the transcription of HIV genes resulting in increased diversity and replication of HIV [17]. On the other hand, as the clinical stage of HIV progresses, there is an increased dissemination and extra-pulmonary TB manifestations [18]. Finally, patients suffer due to frequent pyrexia, diarrhea, severe weight loss and wasting [19, 20].
In high burden and resource-limited countries like Ethiopia, integrating health services on TB and HIV has paramount importance in order to increase the effectiveness of the diagnosis and to improve the management approaches [21]. Considering this, the WHO introduced collaborative strategies to control HIV associated TB and Ethiopia is one of the countries exercising this program as part of the health care system [22]. The Centers for Disease Control (CDC) program in Ethiopia (opened in 2001) is providing guidance and technical support towards TB-HIV co-infection and MDR-TB services. As a result, large improvements have been achieved in HIV testing, ART provision and diagnostic potential. Studying the prevalence of TB-HIV co-infection is important for programmatic changes or further improvements on the existing programs. Therefore, this study was aimed to determine the prevalence of TB-HIV co-infection in Ethiopia, using a meta-analysis based on a systematic review of published articles.
Methods
Search strategy and inclusion criteria
An electronic search was conducted to retrieve and recruit studies. Published articles of cross-sectional, prospective and retrospective cohort studies were included, which focused on the “Prevalence of TB-HIV co-infection in Ethiopia”. Databases PubMed, HINARI, EMBASE, Cochrane library, and Google Scholar search engine were used to extract journals. Articles published between 1995 and November 2017 had been searched for and their full-text was retrieved by two independent reviewers.
Inclusion criteria
The predetermined inclusion criteria for this analysis were: 1) studies conducted in Ethiopia, 2) either longitudinal/cohort or cross-sectional studies which assessed the prevalence of TB/HIV co-infection in Ethiopia and has a sample size of greater than 100 (n > 100). 3) Studies written in English. 4) Articles which were published and available online.
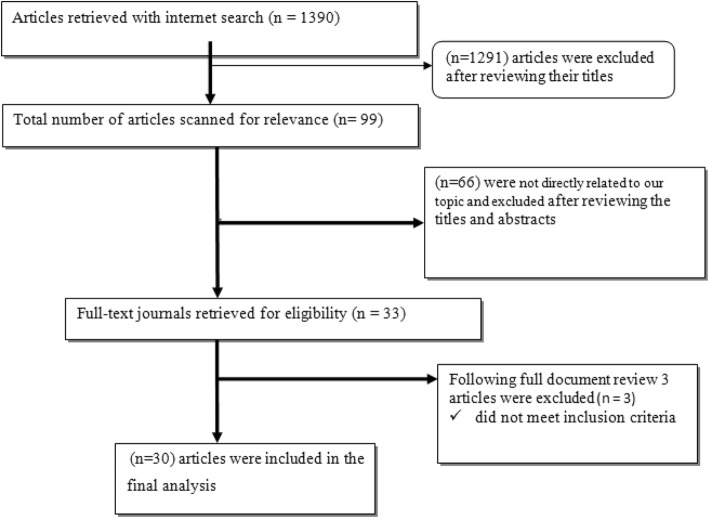
Based on these inclusion criteria, 30 articles were selected and analyzed (Fig. 1).
Fig. 1.
Flow diagram of articles selection process
Data extraction and synthesis
We developed a data collection tool to collect the findings from the published articles. The data collection tool was pretested on 5 articles and an amendment was done accordingly. After the preliminary data extraction, a careful analysis was made to record the type of the study, study setting/region, author and year of publication, number of patients/participants and the main outcomes of the study on the data collection tool.
Description of studies
As summarized in Fig. 1, the literature search with the chosen search terms firstly identified 1390 citations. We repossess 99 articles after reviewing the proximity of the title to the predetermined objectives, and after further reading the abstracts of each article, only 33 of the articles were subjected to document review. Lastly, 30 articles met the inclusion criteria for this systematic review and the meta-analysis (Fig. 1).
Statistical analysis
We performed all analyses in Meta-XL software and R statistical software (version 3.2.3). When working on binary data with low prevalence, the inverse variance weight in fixed-effects meta-analyses is sub-optimum. Hence, the estimated prevalence was determined using the variance stabilizing double arcsine transformation [23]. Wilson method was used to calculate the 95% confidence intervals; the Cochran’s Q and I2 statistic were used to determine heterogeneity [24–26]. The I2 does not essentially depend on the number of studies included. Therefore, a high, moderate and low degree of heterogeneity indicates an I2 value of 75, 50, and 25% respectively. In this study, the I2 was greater than 75% and hence the summary statistics were made using the random-effects models in which the individual study weight is the sum of the weight used in the fixed effects model and the between-study variability [27]. To analyze the basis of heterogeneity, we performed a sensitivity analysis by excluding one largest study [28] and by analyzing the groups according to the geographic area.
Results
Study characteristics
Our searches returned a total of (n = 26,746) records from 30 articles of which 21 were cross-sectional, 7 were retrospectives and 2 were prospective studies. Furthermore, 17 studies had more than 500 participants; 12 studies were conducted in the Amhara region, 6 in southern Ethiopia, 2 in Tigray, 3 in Oromia, 3 in Addis Ababa, 2 in Afar and 2 across Ethiopia.
Nineteen [19] studies determined HIV infection among TB patients (n = 21,376) and 11 studies determined TB among HIV/AIDS patients (5370) (see Table 1).
Table 1.
Summary of the studies in the review
| Author, Year, (Ref.) | Study setting/region | Study design | Study population/participants | Prev. of TB-HIV co-infection |
|---|---|---|---|---|
| Alemayehu et al., 2014 [29] | Amhara Region, Gondar University; Hospital based | Cross-sectional | HIV patients, aged 18 years and above, n = 250 | 6% |
| Alemie & Gebreselassie, 2014 [30] | Amhara Region; selected private health institutions | Cross-sectional | TB patients, n = 1153 | 20% |
| Alemu, etal,2016 [31] | Amhara Region, West Gojjam and South Gondar, Population based | Retrospective | HIV patients, children aged less than 15, n = 645 | 12% |
| Ali et al.,2016 [32] | Across Ethiopia, Population based | Cross- sectional | HIV patients, aged 15–90, n = 575 | 29.4% |
| Balcha et al.,2014 [33] | Oromia Region, Adama; Population based | Prospective | HIV-patients, aged 18 years and above, n = 812 | 17.9% |
| Belay et al.,2015 [34] | Afar and Dessie Town; in 5 health facilities. | Cross-sectional | TB patients, aged above 18 n = 110 | 40% |
| Datiko et al.,2008 [35] | Southern Ethiopia, heath facilities | Cross-sectional | TB patients, n = 1261, | 18% |
| Dengetu & dolamo, 2014 [36] | Addis Ababa; governmental health facilities | Facility-based Cross-sectional | TB patients, children aged 15–17 years, n = 579 | 35% |
| Deribew, et al.,2011 [37] | Addis Ababa; primary health care units | Facility based cross-sectional | TB patients, n = 298 | 27.2% |
| Fanosie et al.,2016 [38] | Amhara Region, University of Gondar Hospital | Institution based cross-sectional | TB patients, n = 141 | 14.1% |
| Fekadu et al., 2015 [39] | South Ethiopia | Retrospective | HIV/AIDS patients, n = 499 | 18.2%) |
| Belay et al., 2014 [40] | Afar region | Cross sectional | TB patients, n = 287 | 28.6% |
| Taddesse et al., 2013 [41] | Amhara region | Cross sectional | TB patients, n = 301 | 18.3% |
| Gebrecherkos et al., 2016 [42] | Amhara Region, North Gondar zone | Cross-sectional | TB patients, n = 282 | 27% |
| Gebreegziabiher et al., 2017 [28] | Tigray Region, Mekelle, Public health institutions | Cross-sectional | TB pregnant mother, n = 201 | 21.8% |
| Gebremariam, et al., 2016 [43] | Southern Rgion, Arsi Negele Health Center | Retrospective | TB patients, n = 1562 | 10% |
| Kassu et al.,2007 [44] | Amhara, Gondar University | Cross-sectional | TB patients, n = 257, | 52.1% |
| Kebede et al., 2014 [45] | Oromiya, Este welega | Cross- sectional | TB patients, n = 406 | 33.7% |
| Kifle et al., 2014 [46] | Amhara, Northwest Shewa | Cross- sectional | TB patients, n = 335 | 28.7% |
| Ligidi et al.,2011 [47] | Oremiya, Adama Hospital based | Cross- sectional | TB patients, n = 258 | 26.4% |
| Mekonnen et al.,2015 [48] | Tigray Rgion,Southern zone | Retrospective | TB patients, n = 973 | 24.3% |
| Mihret et al., 2014 [49] | Addis Ababa Ethiopia | Cross-sectional | TB patients, n = 418 | 23.2% |
| Mitku et al. 2016 [50] | Amhara Region, Hospital based | Retrospective | HIV positive, n = 571 | 27.7% |
| Skogmar et al.,2014 [51] | Across Ethiopia | Cross-sectional | TB patients, n = 1116 | 27.5% |
| Tarekegne et al.,2016 [52] | Amhara Rgion, Metema Hospital | Retrospective | TB patients, n = 2005 | 20.1% |
| Teklu et al., 2012 [53] | Sothern Ethiopia, Sedo, institution based | Retrospective | HIV patients, n = 459 | 7.8% |
| Wondimeneh et al., 2012 [54] | Amhara Region, Gondar university, Hospital based | Cross sectional | HIV patients, n = 400, | 7.5% |
| Yassin et al., 2004 [55] | Southern region of Ethiopia., health facilities | Prospective epidemiological | HIV patients, n = 261 | 19% |
| Degu J. et al. [56] | Southern Ethiopia, Arba Minch Hospital | Cross- sectional | TB patients, n = 190 | 20.1% |
| Alemie G.A. et al., 2014 [30] | Amhara Region, private health institutions | Cross-sectional | TB patient, n = 808 | 20% |
| EHNRI, 2014 [57] | Across Ethiopia | Survey in public health facilities | TB patients, n = 13,684 | 20% |
| EPHI, 2015 [58] | Across Ethiopia | Survey in public health facilities | TB patients, n = 7987 | 17.5% |
EHNRI Ethiopian Health and Nutrition Research Institite, EPHI Ethiopian Public Health Institute.
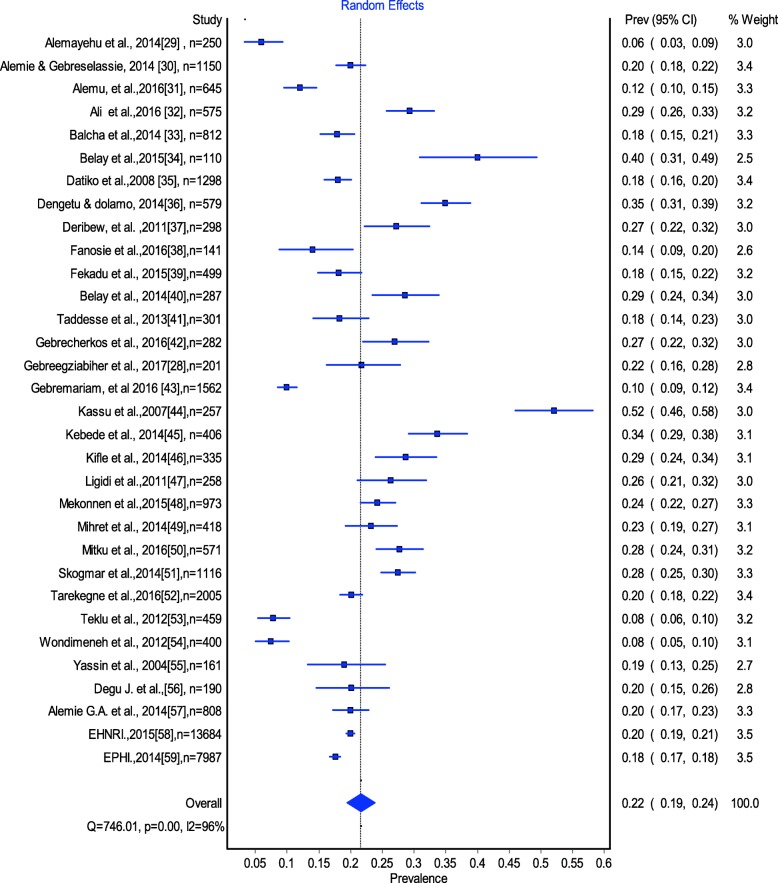
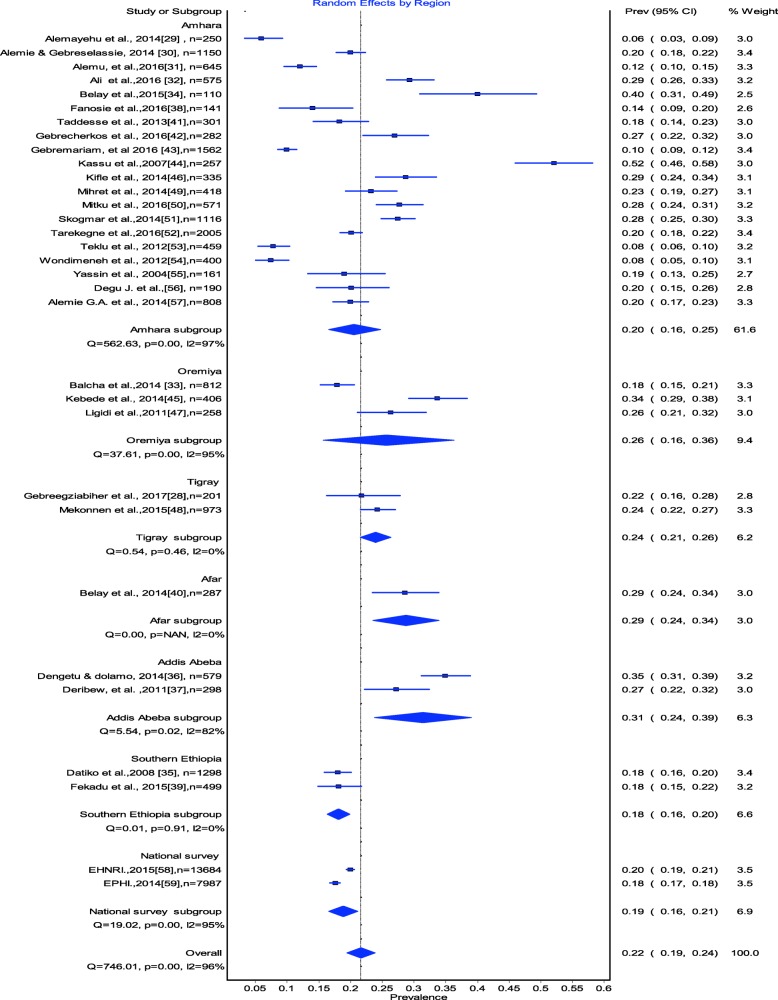
Estimates of TB-HIV co-infection prevalence ranged from 6 to 52.1% (Fig. 2). The random effects pooled prevalence of TB-HIV co-infection was 22% (95% CI 179–24%) with substantial heterogeneity chi-square (X2) = 746.0, p = 0.0001, (I2 = 95.85% 95% CI: 94.9–96.6) (Fig. 2). Prevalence of TB/HIV co-infection was 20% (95% CI 16–24) in the Amhara region, 26% (95% CI 16–36) in the Oromia region, 24% (95% CI 24.21–26.0–) in the Tigray Region, 15% (95% CI 9–21) in Southern Ethiopia, 28% (95% CI 21–36) in Addis Ababa and 34% (95% CI 23–45) in the Afar region (Fig. 3).
Fig. 2.
The estimated prevalence of TB-HIV co-infection in Ethiopia: Meta-Analysis
Fig. 3.
The estimated prevalence of TB-HIV co-infection in Ethiopia by Region Meta-analysis
As part of our sensitivity analyses, we excluded one large study [28]; estimates of TB-HIV co-infection prevalence did not change.
Discussion
This systematic review and meta-analysis of TB-HIV co-infection identified 30 studies of 26,746 individuals in Ethiopia. Our pooled results confirmed that the prevalence of TB-HIV co- infection is 22% in Ethiopia. Furthermore, the high prevalence of HIV/AIDS in the general population is related to a higher prevalence of TB-HIV co-infection. This result is almost consistent with one global study [59] but higher than the finding in China (11%) [60], Nigeria (17.5%) [61] and Ghana (18.2%) [62].
According to the subgroup analysis, the highest prevalence was found in the Addis Ababa region (31%), followed by the Afar regional state (29%), whereas the lowest prevalence was found in southern Ethiopia (18%). The substantial difference between the regions might be due to the difference in the socio-cultural and demographic characteristics of the population and associated HIV prevalence [63, 64]. Supporting this, the rank of the regions based on the prevalence of HIV evidenced in the Ethiopian Demographic Health Survey (EDHS) 2016 report [64], is majorly consistent with the rank of the regions in this pooled analysis. This is logically sounding because HIV is the driving factor for TB infection as a result of the immunosuppression.
As Ethiopia is among the high burden countries for TB and HIV, it may be expected to find a high prevalence of TB-HIV co-infection. However, the pooled prevalence of TB-HIV co-infection (22%) is still higher than the WHO estimated figure (8%) in 2017 [65]. The pooled prevalence of TB-HIV co-infection in the current study is bothersome considering the feared complications and emergence of drug resistance; in Ethiopia, the estimated prevalence of MDR-TB is particularly high in previously treated cases (14%) [65]. In addition to this, HIV had been significantly associated with MDR-TB; studies indicate that there was a high rate of MDR-TB among HIV positive individuals; 14% in Addis Ababa [66], 19.4% in the Amhara region [67] and 19.5% in the Oromia region [68]. However, only 70% of those with MDR-TB are thought to be started on second-line treatment and the coverage of ART initiation was 88% in those confirmed to have TB [65]. Moreover, as part of the millennium development goals and now the health sector transformation plan, Ethiopia has been working to reduce HIV and TB associated morbidity and mortalities. Therefore, the pooled prevalence in the current study is still alarming despite the previous efforts. It implicates that efforts are still needed in reducing HIV associated TB which may include prevention, early detection and comprehensive management of cases. As part of the early detection of cases, TB contact household based screening may have a significant input to augment the facility-based screening of TB among HIV positive individuals [69]. In addition to the prevention, early diagnosis helps the early initiation of ART which in turn significantly increases the success rate of TB treatment among HIV patients [70].
TB-HIV co-infection has a great impact on the health outcome of the victims due to accelerated progression. Co-infected individuals have significantly lower years of survival as compared to those HIV positive only [60]. Witnessing this, though the case fatality ratio of TB in Ethiopia is 0.17, the case fatality ratio of TB-HIV together is estimated to be 0.28 [65]. The simultaneous occurrence of the two infections results in different immunological changes, such as the polyclonal activation of HIV harboring lymphocytes and an increased survival of the bacilli inside macrophages [71]. In addition, there is a clear impact on treatment success rate; as compared to TB alone or HIV alone, co-infected patients are more vulnerable to treatment failure and there is a low rate of treatment success [61]. Poor treatment outcome is 2 times higher among TB-HIV patients [48]. Therefore, this TB treatment failure, poor adherence to ART, the progression of the two diseases and the presence of other co-morbidities may significantly facilitate the death of TB-HIV patients [72]. As a limitation, this study did not explain the heterogeneity in terms of differences in the study setting, population or year. In addition, due to differences in the design and population of the studies, this study did not present the analysis of the trends of prevalence over the years.
Conclusion
Our analysis indicated that the prevalence of TB-HIV co-infection is high in Ethiopia with substantial regional variation. Therefore, an integrated and consistent facility-based as well as community-based effort addressing the prevention, early detection and management of TB and HIV infection should be further strengthened throughout the country to mitigate the double burden disease.
Acknowledgements
We would like to acknowledge Aksum University College of Health Sciences for providing the internet access.
Funding
This work was not funded by any external funding agent.
Availability of data and materials
Not applicable.
Abbreviations
- AIDS
Acquired Immunodeficiency Syndrome
- ART
Antiretroviral Therapy
- CDC
Centers for Disease Control
- HIV
Human Immunodeficiency Virus
- MDR-TB
Multidrug-Resistant Tuberculosis
- TB
Tuberculosis
- WHO
World Health Organization
Authors’ contributions
MT&NA: Conceived and designed the review, made the literature search, did the statistical analysis and wrote the manuscript; SW &HG: participated in statistical analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mebrahtu Teweldemedhin, Email: mebrie1216@gmail.com.
Negasi Asres, Email: negeastat@gmail.com.
Hailay Gebreyesus, Email: ghailay2015@gmail.com.
Solomon Weldegebreal Asgedom, Email: s.weldegebreal@gmail.com.
References
- 1.The Joint United nation Program on HIV/AIDS (UNAIDS) Global summary of the AIDS epidemic. Geneva: UNAIDS; 2017. [Google Scholar]
- 2.World Health Organization. Global TB report, 2016. Geneva, Switzerland:WHO; 2016.
- 3.Nachega JB, RE C. drug resistance: a global threat. Clin Infect Dis. 2003;36(1):524–530. doi: 10.1086/344657. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . Global TB report, 2017. Geneva: WHO; 2017. [Google Scholar]
- 5.Eshetie S, Gizachew M, Dagnew M, Kumera G, Woldie H, Ambaw F, et al. Multidrug resistant tuberculosis in Ethiopian settings and its association with previous history of anti-tuberculosis treatment: a systematic review and meta-analysis. BMC Infectious Diseases. 2017;17:219. doi: 10.1186/s12879-017-2323-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulisa G, Workneh T, Hordof N, Suaudi M, Abebe G, Jarso G. Multidrug-resistant Mycobacterium tuberculosis and associated risk factors in Oromia Region of Ethiopia. Int J Infect Dis. 2015;39:57–56. doi: 10.1016/j.ijid.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Towards universal access to diagnosis and treatment of multidrug-resistant and extensively drug-resistant tuberculosis by 2015. In: WHO Progress Report 2011. Geneva: WHO. p. 2011.
- 8.Breen R, Smith C, Bettinson H. Paradoxical Reactions during Tuberculosis Treatment in Patients with and without HIV Co-Infection. Thorax. 2004;59:704–707. doi: 10.1136/thx.2003.019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Havlir D, Kendall M, Ive P, Kumwenda J, Swindells S, Qasba S. Timing of Antiretroviral Therapy for HIV-1 Infection and Tuberculosis. N Engl J Meds. 2011;365:482–491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell S. The economic burden of illness for households in developing countries: a review of studies focusing on malaria, tuberculosis, and human immunodeficiency virus/acquired immunodeficiency syndrome. Am J Trop Med Hyg. 2004;71(Suppl 2):147–155. doi: 10.4269/ajtmh.2004.71.147. [DOI] [PubMed] [Google Scholar]
- 11.Melkamu H, Seyoum B, Dessie Y. Determinants of Tuberculosis Infection among Adult HIV Positives Attending Clinical Care in Western Ethiopia: A Case-Control Study. AIDS Res Treat. 2013;2013:1–7. [DOI] [PMC free article] [PubMed]
- 12.Mukadi YD, Maher D, Harries A. Tuberculosis case fatality rates in high HIV prevalence populations in sub-Saharan Africa. AIDS. 2001;15:143–152. doi: 10.1097/00002030-200101260-00002. [DOI] [PubMed] [Google Scholar]
- 13.Mulugeta Dalbo TA. Incidence and Predictors of Tuberculosis among HIV/AIDS Infected Patients: A Five-Year Retrospective Follow-Up Study. Adv Infect Dis. 2016;6:70–81. doi: 10.4236/aid.2016.62010. [DOI] [Google Scholar]
- 14.Sterling TR, et al. Risk Factors for Tuberculosis after Highly Active Antiretroviral Therapy Initiation in the United States and Canada: Implications for Tuberculosis Screening. J Infect Dis. 2011;204:893–901. doi: 10.1093/infdis/jir421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seyler C, et al. Risk Factors for Active Tuberculosis after Antiretroviral Treatment Initiation in Abidjan. Amer-ican. J Respir Crit Care Med. 2005;17:123–127. doi: 10.1164/rccm.200410-1342OC. [DOI] [PubMed] [Google Scholar]
- 16.Sharma SK, Mohan A, Kadhiravan T. HIV-TB co-infection: Epidemiology, diagnosis & management. Indian J Med Res. 2005;121:550–567. [PubMed] [Google Scholar]
- 17.Toossi Z. Virological and immunological impact of tuberculosis on human immunodeficiency virus type 1 disease. J Infect Dis. 2003;188:1146–1155. doi: 10.1086/378676. [DOI] [PubMed] [Google Scholar]
- 18.Sharma SK, Kadhiravan T, Banga A, Goyal T, Bhatia I, Saha PK. Spectrum of clinical disease in a cohort of 135 hospitalised HIV-infected patients from north India. BMC Infect Dis. 2004;4:52. doi: 10.1186/1471-2334-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zumla A, Malon P, Henderson J, Grange JM. Impact of HIV infection on tuberculosis. Postgrad Med J. 2000;76:259–268. doi: 10.1136/pmj.76.895.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rupali P, Abraham OC, Zachariah A, Subramanian S, Mathai D. Aetiology of prolonged fever in antiretroviralnaive human immunodeficiency virus-infected adults. Natl Med J India. 2003;16:193–199. [PubMed] [Google Scholar]
- 21.Howard AA, El-Sadr WM. Integration of Tuberculosis and HIV Services in Sub-Saharan Africa: Lessons Learned. CID. 2010;50(S3):S238–S244. doi: 10.1086/651497. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization . Strategic framework to decrease the burden of TB/HIV. Geneva: World Health Organization; 2002. [Google Scholar]
- 23.Freeman MF, Tukey JW. Transformations Related to the Angular and the Square Root. Ann Math Stats. 1950;21:607–611. doi: 10.1214/aoms/1177729756. [DOI] [Google Scholar]
- 24.Wilson EB. Probable Inference, the Law of Succession, and Statistical Inference. J Am Stat Assoc. 1927;22:209–212. doi: 10.1080/01621459.1927.10502953. [DOI] [Google Scholar]
- 25.Newcombe RG. Two-Sided Confidence Intervals for The Single Proportion: Comparison of Seven Methods. Stat Med. 1998;17:857–872. doi: 10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(1539–1558). [DOI] [PubMed]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gebreegziabiher D, Adane K. Abebe M. A survey on undiagnosed active pulmonary tuberculosis among pregnant mothers in mekelle and surrounding Districts in Tigray, Ethiopia. Int J Mycobacteriol. 2017;6(1):43–46. doi: 10.4103/2212-5531.201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alemayehu M, Gelaw B, Abate E, Wassie L, Belyhun Y, Bekele S, et al. Active tuberculosis case finding and detection of drug resistance among HIV-infected patients: A cross-sectional study in a TB endemic area, Gondar, Northwest Ethiopia. Int J Mycobacteriol. 2014;3(2):132–138. doi: 10.1016/j.ijmyco.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alemie GA, Gebreselassie F. Common types of tuberculosis and co-infection with HIV at private health institutions in Ethiopia: a cross sectional study. BMC public health. 2014;14:319. doi: 10.1186/1471-2458-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alemu YM, Andargie G, Gebeye E. High Incidence of Tuberculosis in the Absence of Isoniazid and Cotrimoxazole Preventive Therapy in Children Living with HIV in Northern Ethiopia: A Retrospective Follow-Up Study. PloS One. 2016;11(4):e0152941. doi: 10.1371/journal.pone.0152941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali SA, Mavundla TR, Fantu R, Awoke T. Outcomes of TB treatment in HIV co-infected TB patients in Ethiopia: a cross-sectional analytic study. BMC Infect Dis. 2016;16(1):640. doi: 10.1186/s12879-016-1967-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balcha TT, Sturegard E, Winqvist N, Skogmar S, Reepalu A, Jemal ZH, et al. Intensified tuberculosis case-finding in HIV-positive adults managed at Ethiopian health centers: diagnostic yield of Xpert MTB/RIF compared with smear microscopy and liquid culture. PloS one. 2014;9(1):e85478. doi: 10.1371/journal.pone.0085478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belay M, Bjune G, Abebe F. Prevalence of tuberculosis, HIV, and TB-HIV co-infection among pulmonary tuberculosis suspects in a predominantly pastoralist area, northeast Ethiopia. Global Health Action. 2015;8(1):27949. doi: 10.3402/gha.v8.27949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Datiko DG, Yassin MA, Chekol LT, Kabeto LE, Lindtjorn B. The rate of TB-HIV co-infection depends on the prevalence of HIV infection in a community. BMC public health. 2008;8:266. doi: 10.1186/1471-2458-8-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denegetu AW. Dolamo BL. HIV screening among TB patients and co-trimoxazole preventive therapy for TB/HIV patients in Addis Ababa: facility based descriptive study. PloS One. 2014;9(2):e86614. doi: 10.1371/journal.pone.0086614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deribew A, Negussu N, Melaku Z, Deribe K. Investigation outcomes of tuberculosis suspects in the health centers of Addis Ababa, Ethiopia. PloS One. 2011;6(4):e18614. doi: 10.1371/journal.pone.0018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fanosie A, Gelaw B, Tessema B, Tesfay W, Admasu A, Yitayew G. Mycobacterium tuberculosis Complex and HIV Co-Infection among Extrapulmonary Tuberculosis Suspected Cases at the University of Gondar Hospital, Northwestern Ethiopia. PloS One. 2016;11(3):e0150646. doi: 10.1371/journal.pone.0150646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fekadu S, Teshome W, Alemu G. Prevalence and determinants of Tuberculosis among HIV infected patients in south Ethiopia. J Infect Dev Ctries. 2015;9(8):898–904. doi: 10.3855/jidc.5667. [DOI] [PubMed] [Google Scholar]
- 40.Belay M, Ameni G, Bjune G, Couvin D. Strain diversity of Mycobacterium tuberculosis isolates from pulmonary tuberculosis patients in Afar pastoral region of Ethiopia. Biomed Res Int. 2014;2014:238532. [DOI] [PMC free article] [PubMed]
- 41.Tadesse S, Tadesse T. HIV co-infection among tuberculosis patients in Dabat, northwest Ethiopia. J Infect Dis Immun. 2013;5(3):29–32. doi: 10.5897/JIDI2013.0117. [DOI] [Google Scholar]
- 42.Gebrecherkos T, Gelaw B, Tessema B. Smear positive pulmonary tuberculosis and HIV co-infection in prison settings of North Gondar Zone, Northwest Ethiopia. BMC Infect Dis. 2016;16(1):1091. doi: 10.1186/s12889-016-3761-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gebremariam G, Asmamaw G, Hussen M, Hailemariam MZ, Asegu D, Astatkie A, et al. Impact of HIV Status on Treatment Outcome of Tuberculosis Patients Registered at Arsi Negele Health Center, Southern Ethiopia: A Six Year Retrospective Study. PloS One. 2016;11(4):e0153239. doi: 10.1371/journal.pone.0153239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kassu A, Mengistu G, Ayele B, Diro E, Mekonnen F, Ketema D, et al. Coinfection and clinical manifestations of tuberculosis in human immunodeficiency virus-infected and -uninfected adults at a teaching hospital, northwest Ethiopia. J Microbiol Immunol Infect. 2007;40(2):116–122. [PubMed] [Google Scholar]
- 45.Kebede W, Keno F, Ewunetu T, Mamo G. Acceptance of Provider Initiated HIV Testing and Counseling among Tuberculosis Patients in East Wollega Administrative Zone, Oromia Regional State, Western Ethiopia. Tuberc Res Treat. 2014;2014:935713. doi: 10.1155/2014/935713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keflie TS, Ameni G. Microscopic examination and smear negative pulmonary tuberculosis in Ethiopia. Pan Afr Med J. 2014;19:162. doi: 10.11604/pamj.2014.19.162.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ligidi T, Gebre-Selassie S, Tsegaye A. The immunological status of newly diagnosed tuberculosis patients co-infected with human immunodeficiency virus-1 in Adama Hospital, Ethiopia. Ethiop Med J. 2011;49(2):75–83. [PubMed] [Google Scholar]
- 48.Mekonnen D, Derbie A, Desalegn E. TB/HIV co-infections and associated factors among patients on directly observed treatment short course in Northeastern Ethiopia: a 4 years retrospective study. BMC Res Notes. 2015;8(1):666. doi: 10.1186/s13104-015-1664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mihret A, Bekele Y, Aytenew M, Assefa Y, Wassie L, Abebe M, et al. Human immunodeficiency virus infection among new smear positive pulmonary tuberculosis patients in Addis Ababa, Ethiopia. Ethiopian Med J. 2014;2014(Suppl 1):1–6. [PubMed]
- 50.Mitku AA, Dessie ZG, Muluneh EK, Workie DL. Prevalence and associated factors of TB/HIV co-infection among HIV Infected patients in Amhara region, Ethiopia. African health sciences. 2016;16(2):588–595. doi: 10.4314/ahs.v16i2.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skogmar S, Balcha TT, Jemal ZH, Bjork J, Deressa W, Schon T, et al. Development of a clinical scoring system for assessment of immunosuppression in patients with tuberculosis and HIV infection without access to CD4 cell testing--results from a cross-sectional study in Ethiopia. Global Health Action. 2014;7:23105. doi: 10.3402/gha.v7.23105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tarekegne D, Jemal M, Atanaw T, Ebabu A, Endris M, Moges F, et al. Prevalence of human immunodeficiency virus infection in a cohort of tuberculosis patients at Metema Hospital, Northwest Ethiopia: a 3 years retrospective study. BMC Res Notes. 2016;9:192. doi: 10.1186/s13104-016-2004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teklu T, Belyhun Y, Tesfaye S, Medhin G. Trends of tuberculosis and HIV infections between 2004 and 2008 in Wolaita Sodo, southern Ethiopia. Ethiopian Med J. 2012;50(1):1–11. [PubMed] [Google Scholar]
- 54.Wondimeneh Y, Muluye D, Belyhun Y. Prevalence of pulmonary tuberculosis and immunological profile of HIV co-infected patients in Northwest Ethiopia. BMC Res Notes. 2012;5:331. doi: 10.1186/1756-0500-5-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yassin MA, Takele L, Gebresenbet S, Girma E, Lera M, Lendebo E, et al. HIV and tuberculosis coinfection in the southern region of Ethiopia: a prospective epidemiological study. Scand J Infect Dis. 2004;36(9):670–673. doi: 10.1080/00365540410020848. [DOI] [PubMed] [Google Scholar]
- 56.Degu J, Aschalew E, Bernt L. Acceptability of HIV counselling and testing among tuberculosis patients in south Ethiopia. BMC Int Health Hum Rights. 2007;7(4). [DOI] [PMC free article] [PubMed]
- 57.Ethiopian Health and Nutrition Research Institute (EHNRI)/ Ministry of Health (MOH). National TB/HIV Sentinel surveillance one year report (July 2011–June 2012). Addis Ababa, Ethiopia, 2013. .
- 58.Ethiopian Public Health Institute (EPHI) Ministry of Health (MOH). National TB/HIV Sentinel Surveillance annual report (July–June 2015).
- 59.Gao J, Zheng P. Fu H. Prevalence of TB/HIV co-infection in countries except China: a systematic review and meta-analysis. PloS One. 2013;8(5):e64915. doi: 10.1371/journal.pone.0064915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maimaiti R, Zhang Y, Pan K, Mijiti P, Wubili M, Musa M, Andersson R. High prevalence and low cure rate of tuberculosis among patients with HIV in Xinjiang,China. BMC Infect Dis. 2017;17(1):15. doi: 10.1186/s12879-016-2152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Babatunde OI, Christiandolus EO, Bismarck EC, Emmanuel OI, Chike AC, Gabriel EI. Five years retrospective cohort analysis of treatment outcomes of TB-HIV patients at a PEPFAR/DOTS Centre in South Eastern Nigeria. African Health Sci. 2016;16(3):655–662. doi: 10.4314/ahs.v16i3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osei E, Der J, Owusu R, Kofie P, Axame WK. The burden of HIV on Tuberculosis patients in the Volta region of Ghana from 2012 to 2015: implication for Tuberculosis control. BMC Infect Dis. 2017;17(1):504. doi: 10.1186/s12879-017-2598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lakew Y, Benedict S, Haile D. Social determinants of HIV infection, hotspot areas and subpopulation groups in Ethiopia: evidence from the National Demographic and Health Survey in 2011. BMJ open. 2015;5(11):e008669. doi: 10.1136/bmjopen-2015-008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Central Statistical Agency (CSA) [Ethiopia] and ICF. Ethiopia Demographic and Health Survey 2016: HIV Report. Addis Ababa, Ethiopia, and Rockville, Maryland: CSA and ICF. p. 2018.
- 65.World Health Organization. Global TB report, 2017.
- 66.Bruchfeld J, Aderaye G, Palme IB, Bjorvatn B, Ghebremichael S, Hoffner S, Lindquist L. Molecular epidemiology and drug resistance of Mycobacterium tuberculosis isolates from Ethiopian pulmonary tuberculosis patients with and without human immunodeficiency virus infection. J Clin Microbiol. 2002;40(5):1636–1643. doi: 10.1128/JCM.40.5.1636-1643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nigus DM, Lingerew W, Beyene B, Tamiru A, Lemma M, Melaku MY. Prevalence of multi drug resistant tuberculosis among presumptive multi drug resistant tuberculosis cases in Amhara National Regional State, Ethiopia. J Mycobac Dis. 2014;4(152):2161–1068. [Google Scholar]
- 68.Mulisa G, Workneh T, Hordofa N, Suaudi M, Abebe G, Jarso G. Multidrug-resistant Mycobacterium tuberculosis and associated risk factors in Oromia Region of Ethiopia. Int J Infect Dis. 2015;39:57–61. doi: 10.1016/j.ijid.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 69.Shapiro AE, Variava E, Rakgokong MH, Moodley N, Luke B, Salimi S, Chaisson RE, Golub JE, Martinson NA. Community-based targeted case finding for tuberculosis and HIV in household contacts of patients with tuberculosis in South Africa. Am J Respir Crit Care Med. 2012;185(10):1110–1116. doi: 10.1164/rccm.201111-1941OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shastri S, Naik B, Shet A, Rewari B, De Costa A. TB treatment outcomes among TB-HIV co-infections in Karnataka, India: how do these compare with non-HIV tuberculosis outcomes in the province? BMC Public Health. 2013;13(1):838. doi: 10.1186/1471-2458-13-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rossouw T, Tucker JD, Zyl GU, Sikwesi K, Godfrey C. Barriers to HIV remission research in low-and middle-income countries. J Int AIDS Soc. 2017;20(1):21521. [DOI] [PMC free article] [PubMed]
- 72.Agbor AA, Bigna JJ, Billong SC, Tejiokem MC, Ekali GL, Plottel CS, Noubiap JJ, Abessolo H, Toby R, Koulla-Shiro S. Factors associated with death during tuberculosis treatment of patients co-infected with HIV at the Yaoundé Central Hospital, Cameroon: an 8-year hospital-based retrospective cohort study (2006–2013) PloS One. 2014;15(9):12–e115211. doi: 10.1371/journal.pone.0115211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.