Abstract
Background
Inflammation might be a pathological mediator of cardiovascular events in patients with type 2 diabetes and high cardiovascular risk.
Methods
We investigated whether empagliflozin (EMPA) exerts anti-inflammatory effects that are reflected in decreased high-sensitivity C-reactive protein (hsCRP) values. Patients were allocated to receive a placebo (n = 51) or EMPA (n = 51) as an add-on treatment. Fasting blood samples were collected before and every 3 months after this intervention for 1 year.
Results
Empagliflozin tended to elicit reductions in BMI, HbA1c, aspartate aminotransferase, alanine aminotransferase (ALT), and gamma-glutamyl transpeptidase compared with the placebo, but the differences did not reach statistical significance. Levels of LDL-cholesterol, HDL-cholesterol, and triglycerides were unaltered, significantly increased, and decreased, respectively, by EMPA, but the differences were not statistically significant compared with the placebo. Empagliflozin for 12 months notably reduced the homeostatic model assessment of insulin resistance (HOMA-IR), remnant-like particle cholesterol (RLP-C), and hsCRP by 43%, 52% and 54%, respectively. The time courses of these reductions significantly differed from those of the placebo. Systolic and diastolic blood pressure were also significantly reduced by EMPA compared with the placebo. We applied multiple linear regression analysis to determine which factors were associated with changes in hsCRP induced by EMPA. The results revealed that alterations in hsCRP values (log [hsCRP at 12 months] minus log [hsCRP at month 0]) were significantly associated with changes in HOMA-IR, RLP-C, systolic blood pressure, HDL-C and ALT.
Conclusion
Empagliflozin decreased hs-CRP and lowered levels of remnant related lipoproteins probably via ameliorating insulin resistance. The cardiovascular benefits conferred by EMPA might be driven at least partly by anti-inflammatory effects, and this mechanism might cooperate with other EMPA-induced changes including reduced blood pressure, to achieve the degree of cardioprotection revealed by the EMPA-REG OUTCOME trial.
Trial registration UMIN Clinical Registry (UMIN000021552). Registered 21 March 2016, https://upload.umin.ac.jp/UMIN000021552
Keywords: Sodium-glucose co-transporter-2 inhibitor, High sensitivity CRP, Insulin resistance
Background
The sodium–glucose cotransporter 2 inhibitor, empagliflozin (EMPA) reduced relative risk for cardiovascular (CV) mortality, hospitalization for heart failure and death from any cause by 38%, 35% and 32%, respectively, in the EMPA-REG OUTCOME trial of patients with type 2 diabetes and high risk of CV [1]. The results of a sub-analysis of the EMPA-REG OUTCOME study of Asian patients with type 2 diabetes and high CV risk were also striking [2]. Thus, the EMPA-REG OUTCOME trial established that EMPA is cardioprotective in high-risk patients with diabetes, but the underlying mechanisms remain elusive.
Obesity-related, subacute chronic inflammation is associated with incident type 2 diabetes and atherosclerotic CV disease. Inflammation might be a pathological mediator of these commonly concurrent pathologies. Ridker et al. [3] recently showed that anti-inflammatory therapy with canakinumab, which targets the interleukin-1β innate immunity pathway, leads to a significantly lower rate of recurrent CV events than a placebo, independently of lowering LDL-cholesterol. They showed that the magnitude of the reduction in high-sensitivity C-reactive protein (hsCRP) after a single dose of canakinumab might provide a simple clinical method with which to identify individuals who are likely to benefit from continued treatment; that is, lower is better for reducing inflammation with canakinumab [4].
Empagliflozin exerts anti-inflammatory effects in experimental animal models [5, 6], but not in humans. Here, we aimed to determine whether EMPA exerts anti-inflammatory effects and whether the CV benefits of EMPA are due partly to such effects, implied by decreased hsCRP values in patients with type 2 diabetes and insulin resistance.
Methods
Study design and participants
This single-center, open-label, randomized, prospective study enrolled patients without a history of medication with SGLT2 inhibitors and with HbA1c > 6.2% regardless of diet, exercise, and medical treatment other than SGLT2 inhibitors for at least 12 weeks. They were assessed at our clinic and might have had insulin resistance (BMI > 28 or homeostatic model assessment of insulin resistance [HOMA-IR] > 1.73 or fasting immunoreactive insulin [IRI] > 10 and fasting blood glucose [FBG] < 180). The patients were then allocated to receive EMPA (10 mg; n = 51) or a placebo (n = 51) as an add-on treatment. All patients continued with their administered oral hypoglycemic drugs (sulfonylureas, metformin, or an α-glucosidase inhibitor), antihypertensive agents (angiotensin II receptor blockers or calcium channel blockers), and antihyperlipidemic agents (statins or fibrates) (Table 1).
Table 1.
Baseline charasteristics and medicatoins of the participants
| Placebo (n = 51) | EMPA (n = 51) | P value | |
|---|---|---|---|
| Age (years) | 58.1 ± 9.71 | 57.4 ± 12.3 | 0.778 |
| Male/(female) | 41 (10) | 38 (13) | 0.636 |
| Baseline medication | |||
| Slfonylureas | 11 | 10 | 1 |
| Metformin | 12 | 12 | 1 |
| α-GI | 8 | 7 | 1 |
| ARB | 16 | 17 | 0.915 |
| CCB | 10 | 10 | 1 |
| Statins | 17 | 16 | 1 |
| Fibrates | 9 | 10 | 0.879 |
Data were expressed as mean ± standard deviation
α-GI α-glicosidase inhibitor, ARB angiotensin II receptor blocker, CCB calcium channel blocker
Measurements
Overnight fasting blood and urine samples were obtained at baseline and after every 3 months of EMPA or placebo administration for 1 year. All biochemical data were obtained at our laboratory, except IRI, remnant-like particle cholesterol (RLP-C), hsCRP and urinary albumin, which were assessed at LSI Medicine Corporation (Tokyo, Japan). We calculated HOMA-IR every 3 months as follows: EMPA was stopped for 72 h until urinary glucose was undetectable and then blood values of fasting glucose and IRI were measured.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). Data were statistically analyzed using EZR software version 1.21 [7]. Parameters before and 3, 6, 9, and 12 months after treatment were compared using paired t-tests. Changes in parameters over a period of 12 months between placebo and EMPA were analyzed using repeated measures ANOVA. Factors associated with changes in hsCRP were determined using multiple linear regression analysis. Continuous and categorical baseline values and medications were analyzed using t-tests and Fisher exact tests, respectively. Differences were considered statistically significant at p < 0.05.
Results
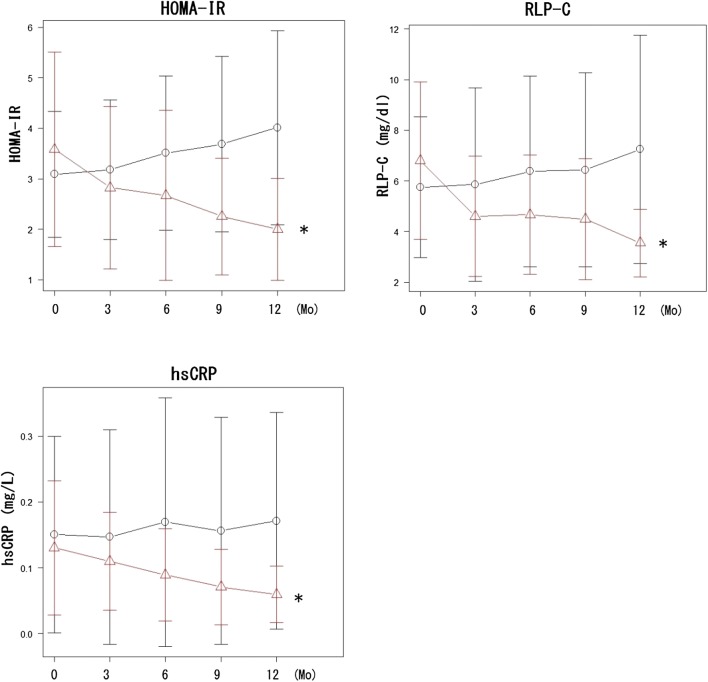
Levels of BMI, HbA1c, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma-glutamyl transpeptidase (γ-GTP) were slightly but not significantly reduced by EMPA compared with the placebo. Levels of LDL-cholesterol, HDL-cholesterol, and triglycerides were unaltered, significantly increased, and reduced, respectively, by EMPA, but the differences did not reach statistical significance compared with the placebo. Notably, HOMA-IR, RLP-C, and hsCRP were reduced by 43%, 52%, and 54%, respectively, after 12 months of EMPA. The time courses of these reductions significantly differed between EMPA and the placebo. Systolic (SBP) and diastolic blood pressure (DBP) were significantly reduced by EMPA, but not by the placebo. Estimated glomerular filtration rates (eGFR), urinary albumin excretion (measured as urinary albumin-to-creatinine ratios; ACR) did not significantly differ between the placebo and EMPA groups (Table 2, Fig. 1).
Table 2.
Clinical parameters of patients treated with placebo or EMPA
| Month of study | Placebo (n=51) | EMPA (n=51) | Placebo vs. EMPA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 0 | 3 | 6 | 9 | 12 | p value | |
| FBG (mg/dL) | 130.3 (25.7) | 123.5 (22.9) | 126.8 (24.8) | 124.8 (32.9) | 130.7 (26.2) | 139.1 (36.1) | 123.3 (21.9)* | 122.9 (22.4)* | 121.4 (22.5)* | 122.1 (21.3)* | 0.93 |
| IRI (mU/mL) | 9.1 (3.8) | 8.7 (3.8) | 9.2 (4.4) | 9.5 (4.5) | 10.3 (4.6)* | 10.9 (6.2) | 9.2 (4.4)* | 8.4 (4.4)* | 7.6 (3.3)* | 6.5 (2.4)* | 0.507 |
| HOMA-IR | 2.61 (1.26) | 2.70 (1.38) | 2.94 (1.54)* | 3.06 (1.67)* | 3.43 (1.92)* | 3.56 (2.01) | 2.87 (1.95)* | 2.60 (1.60)* | 2.27 (1.12)* | 2.03 (1.01)* | 0.0163† |
| HbA1c (%) | 6.84 (0.85) | 6.77 (0.81) | 6.92 (0.88) | 6.86 (0.93) | 6.88 (0.76) | 7.01 (1.1) | 6.86 (0.82)* | 6.88 (0.91)* | 6.94 (0.96)* | 6.90 (0.96)* | 0.119 |
| BMI | 30.0 (4.4) | 30.1 (4.3) | 30.2 (4.4) | 30.2 (4.6) | 30.0 (3.7) | 31.0 (4.8) | 30.0 (4.9)* | 29.5 (4.6)* | 29.4 (4.5)* | 29.4 (4.9)* | 0.385 |
| hsCRP (mg/L) | 1.46 (1.4) | 1.43 (1.56) | 1.74 (1.87) | 1.47 (1.66) | 1.71 (1.64) | 1.33 (1.0) | 1.13 (0.73) | 0.92 (0.68)* | 0.73 (0.56)* | 0.59 (0.42)* | 0.00706† |
| LDL-C (mg/dL) | 118.6 (25.1) | 121.1 (25.6) | 121.1 (23.8) | 121.9 (24.4) | 123.8 (25.8) | 112.6 (27.9) | 115.6 (27.3) | 115.1 (23.4) | 118.6 (20.0) | 114.0 (19.7) | 0.188 |
| HDL-C (mg/dL) | 55.1 (12.3) | 54.7 (10.5) | 55.4 (12.6) | 54.5 (10.6) | 53.3 (11.4) | 55.3 (12.0) | 57.8 (14.1)* | 57.3 (12.9)* | 58.8 (13.6)* | 61.2 (15.2)* | 0.222 |
| TG (mg/dL) | 129.4 (61.3) | 139.8 (81.7) | 142.6 (64.1) | 139.9 (58.4) | 153.5 (75.0) | 157.8 (80.5) | 130.4 (53.7)* | 134.3 (54.7)* | 127.6 (43.0)* | 113.4 (54.6)* | 0.553 |
| RLP-C (mg/dL) | 6.27 (3.96) | 6.74 (5.65) | 6.79 (4.28) | 6.61 (4.13) | 7.91 (5.57) | 8.13 (5.02) | 5.25 (3.17)* | 5.26 (2.90) | 4.87 (2.75)* | 3.94 (2.10)* | 0.029† |
| AST (IU/L) | 27.2 (13.8) | 27.8 (11.6) | 25.8 (9.4) | 26.9 (9.2) | 27.1 (9.9) | 28.7 (14.2) | 25.6 (10.8)* | 26.2 (10.1) | 24.7 (8.6) | 23.9 (9.4)* | 0.583 |
| ALT (IU/L) | 33.2 (22.4) | 35.3 (24.6) | 34.4 (20.1) | 34.3 (21.2) | 35.7 (24.6) | 37.0 (21.0) | 32.0 (20.3)* | 32.0 (19.6)* | 31.0 (20.2)* | 28.6 (20.1)* | 0.6 |
| γGTP (IU/L) | 50.1 (36.1) | 49.5 (33.2) | 50.7 (34.3) | 48.5 (28.7) | 51.3 (30.5) | 46.5 (32.4) | 39.2 (23.8)* | 41.0 (25.7)* | 39.0 (24.6)* | 35.1 (22.3)* | 0.0558 |
| eGFR (mL/min/1.73m2) | 71.9 (18.6) | 71.7 (19.5) | 71.9 (19.0) | 72.3 (17.5) | 71.6 (19.1) | 74.3 (16.3) | 70.9 (16.4)* | 71.2 (15.4)* | 72.9 (17.4) | 72.1 (16.6) | 0.956 |
| ACR (mg/gCr) | 69.7 (289) | 106.1 (425) | 104.0 (467) | 111.0 (514) | 61.8 (149) | 94.9 (244) | 45.3 (78.3) | 40.2 (68.7) | 39.6 (65.1) | 36.0 (62.4) | 0.806 |
| SBP (mmHg) | 128.0 (15.6) | 127.0 (17.4) | 128.6 (16.6) | 129.9 (15.5) | 128.7 (15.6) | 130.5 (21.2) | 125.1 (14.8)* | 126.3 (14.7)* | 122.4 (13.7)* | 121.0 (13.3)* | 0.022† |
| DBP (mmHg) | 77.5 (10.3) | 75.8 (9.3) | 77.1 (9.7) | 77.2 (10.7) | 75.5 (14.2) | 78.1 (9.8) | 71.9 (16.4)* | 73.3 (11.0)* | 72.2 (10.3)* | 72.8 (10.5)* | 0.0187† |
Values are shown as means ± SD in parentheses
BW body weight, BMI body mass index, FBG fasting blood glucose, HbA1c hemoglobin A1c, IRI immuno reactive insulin, HOMA-IR homeostatic model assessment of insulin resistance, LDL-C LDL cholesterol, HDL-C HDL choresterol, TG triglyceride, RLP-C remnant-like particle cholesterol, AST aspartate aminotransferase, ALT alanine amino transferase, γGTP gamma-glutamyl transpeptidase, eGFR estimated glomerular filtration rate, ACR albumin-to-creatinine ratio, hsCRP highly sensitivity C-reactive protein, SBP systolic blood pressure, DBP diastolic blood pressure
*Intragroup comparison: p < 0 .05 (paired t test)
†Intergroup comparison for 12 months between placebo and EMPA: p < 0 .05 (repeated measures ANOVA)
Fig. 1.
Time course of HOMA-IR, RLP-C and log[hsCRP] in patients treated with EMPA (triangles, red line) or placebo (circles, black line). Data are presented as mean ± SD. Group differences parameters between baseline and 12 months later between placebo and EMPA were analyzed *p < 0.05 (repeated measures ANOVA); p = 0.0163, p = 0.029 and p = 0.00706 for HOMA-IR, RLP-C and hsCRP, respectively
We applied multiple linear regression analysis to identify factors associated with changes in hsCRP in the EMPA group. The objective variable comprised changes in hsCRP over a period of 12 months (log [hsCRP at 12 months] minus log [hsCRP at month 0]), and changes in FBG, IRI, BMI, HbA1c, AST, ALT, γ-GTP, LDL-cholesterol, HDL-cholesterol, RLP-C, eGFR, SBP, and DBP over 12 months were included as explanatory variables. The findings of these analyses (R2 = 0.9671, p = 4.709 × 10−8) revealed that changes in hsCRP were significantly associated with changes in HOMA-IR (t = 2.541, p = 0.0274), RLP-C (t = 2.528, p = 0.0281), SBP (t = − 2.324, p = 0.0403), HDL-cholesterol (t = − 2.411, p = 0.0346), and ALT (t = − 2.372, p = 0.0370) (Table 3).
Table 3.
Association between changes in log[hsCRP] and various parameters coefficients
| Estimate | Std. error | t value | P | |
|---|---|---|---|---|
| ∆ACR (mg/gCr) | 0.0006272 | 0.0003121 | 2.01 | 0.0697 |
| ∆BMI | − 0.004537 | 0.01105 | − 0.411 | 0.6892 |
| ∆DBP (mmHg) | − 0.002671 | 0.002109 | − 1.266 | 0.2316 |
| ∆eGFR (ml/min/1.73m2) | 0.0002273 | 0.002345 | 0.097 | 0.9246 |
| ∆γGTP (IU/L) | − 0.001513 | 0.001401 | − 1.08 | 0.3031 |
| ∆AST (IU/L) | 0.003259 | 0.001935 | 1.684 | 0.1203 |
| ∆ALT (IU/L) | − 0.003582 | 0.00151 | − 2.372 | 0.037* |
| ∆HbA1c (%) | 0.04275 | 0.02592 | 1.65 | 0.1273 |
| ∆HDL-C (mg/dL) | − 0.002794 | 0.001159 | − 2.411 | 0.0346* |
| ∆HOMA-IR | 0.06453 | 0.0254 | 2.541 | 0.0274* |
| ∆LDL (mg/dL) | 0.0008015 | 0.0008732 | 0.918 | 0.3784 |
| ∆RLPC (mg/dL) | 0.06442 | 0.02548 | 2.528 | 0.0281* |
| ∆SBP (mmHg) | − 0.002882 | 0.00124 | − 2.324 | 0.0403* |
| ∆TG (mg/dL) | 0.000000518 | 0.0003293 | 0.002 | 0.9988 |
R2 = 0.9855, p-value = 4.709 × 10−8
Multiple linear regression analysis was used to test the association between change in log[hsCRP] and changes in various parameters across 12 months in EMPA group
hsCRP high sensitivity C-reactive protein, ACR albumin-to-creatinine ratio, BMI body mass index, eGFR estimated glomerular filtration rate, GTP, gamma-glutamyl transpeptidase, AST aspartate aminotransferase, ALT alanine aminotransferase, HbA1c hemoglobin A1c, HDL-C HDL cholesterol, LDL-C LDL cholesterol, TG triglyceride, RLP-C remnant-like particle cholesterol, SBP systolic blood pressure, DBP diastolic blood pressure
*p < 0.05
Discussion
This study showed that the SGLT2 inhibitor, EMPA, decreases hsCRP, lowers levels of remnant lipoproteins and ameliorates insulin resistance.
Insulin resistance is not a simple matter of deficient glucose uptake in response to insulin, but a multifaceted syndrome that significantly increases risk for CV disease [8]. How SGLT2 inhibitors improve insulin resistance has been examined using a glucose clamp procedure [9, 10]. However, the observation periods were relatively short and the patient cohorts were quite small because long-term assessment using this technique is difficult to implement. Here, we repeatedly measured HOMA-IR to evaluate changes in insulin resistance. We found that EMPA decreased HOMA-IR and significantly decreased hsCRP and RLP-C.
Three reports have described an association between hsCRP and insulin resistance [11–13]. The present study found a significant association between decreased hsCRP and ameliorated HOMA-IR, suggesting that improved insulin resistance results in anti-inflammatory effects.
Remnant lipoproteins are thought to be atherogenic. Remnant-like particle cholesterol (RLP-C) reflects amounts of various remnant lipoproteins in the blood. Increased levels of RLP-C are thought to comprise a significant and independent risk factor for coronary artery disease (CAD) and to predict future coronary events in patients with CAD and type 2 diabetes [14]. The present study found a significant correlation between changes with EMPA in RLP-C and in hsCRP. We recently found that EMPA decreases RLP-C levels in close association with ameliorated insulin resistance at 3 months in patients with diabetes and insulin resistance [15]. The present study found that this suppressive effect of EMPA on RLP-C continued for 12 months.
The CV benefits of EMPA might be due in part to anti-inflammatory effects as indicated by the 54% decrease in hsCRP in the present study. Arima et al. found that hsCRP levels were associated with future coronary heart disease events in a general Japanese population [16]. However, although hsCRP levels are far lower among Asians than other populations, the striking reduction in CV risk among Asians was similar to that in the overall population in the EMPA-REG OUTCOME trial [2]. Thus, a reduction in hsCRP within the lower range might confer a considerable benefit in CV risk reduction among Japanese patients with type 2 diabetes and insulin resistance.
This study has several limitations. We evaluated insulin resistance using HOMA-IR, which is not a good indicator under poor diabetes control. Another is that measuring HOMA-IR immediately after EMPA administration might not always reflect insulin resistance. This is because SGLT2 inhibitors work by inhibiting glucose reabsorption in the proximal tubules of the kidney, which results in increased urinary glucose excretion and decreased blood glucose levels. Thus, we excluded patients with poorly controlled diabetes from the present study. We also stopped EMPA administration for 72 h until SGLT2 inhibition became ineffective, then measured blood glucose and IRI in fasting blood samples to calculate HOMA-IR. Since the findings of linear regression analyses cannot define cause-and-effect between hsCRP and HOMA-IR and other parameters, further study is needed to clarify this issue.
Conclusion
This study showed that the SGLT2 inhibitor, EMPA, ameliorated insulin resistance and might consequently decrease levels of hsCRP and remnant lipoproteins. We also showed that EMPA significantly decreased SBP and DBP. These results suggest that the CV benefits of EMPA might be driven at least in part, by anti-inflammatory effects that are particularly beneficial for patients with insulin resistance who might have cardiac dysfunction and/or vascular inflammation. This mechanism should cooperate with other EMPA-induced changes such as reduced blood pressure as shown herein and enhanced diuresis [17] to achieve the degree of cardioprotection revealed by the EMPA-REG OUTCOME trial.
Authors’ contributions
This work was basically done by SH herself. The author read and approved the final manuscript.
Acknowledgements
The author thanks the members of the Tohto Clinic for helping her implement this work plan.
Competing interests
The author declares that she has no competing interests.
Availability of data and materials
The datasets analyzed during the current study are not publicly available due to some relevant ongoing studies, but may be available from the corresponding author of this article on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by the ethic committee at the Tohto Clinic, and written informed consent was obtained from all participating patients before the initiation of the study.
Funding
This work isn’t receiving any funding.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ACR
albumin-to-creatinine ratio
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- CAD
coronary artery disease
- CV
cardiovascular
- eGFR
estimated glomerular filtration rate
- EMPA
empagliflozin
- FBG
fasting blood glucose
- HbA1c
hemoglobin A1c
- HDL
high-density lipoprotein
- HOMA-IR
homeostatic model assessment of insulin resistance
- hsCRP
high sensitivity C-reactive protein
- IRI
immunoreactive insulin
- LDL
low-density lipoprotein
- RLP-C
remnant-like particle cholesterol
- SGLT2
sodium–glucose cotransporter 2 inhibitor
- γGTP
gamma-glutamyl transpeptidase
References
- 1.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 2.Kaku K, Lee J, Mattheus M, Kaspers S, George J, Woerle HJ, EMPA-REG OUTCOME® Investigators Empagliflozin and cardiovascular outcomes in asian patients with type 2 diabetes and established cardiovascular disease-results from EMPA-REG OUTCOME®. Circ J. 2017;81:227–234. doi: 10.1253/circj.cj-16-1148. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, CANTOS Trial Group Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391:319–328. doi: 10.1016/s0140-6736(17)32814-3. [DOI] [PubMed] [Google Scholar]
- 5.Ojima A, Matsui T, Nishino Y, Nakamura N, Yamagishi S. Empagliflozin, an inhibitor of sodium-glucose cotransporter 2 exerts anti-inflammatory and antifibrotic effects on experimental diabetic nephropathy partly by suppressing AGEs-receptor axis. Horm Metab Res. 2015;47:686–692. doi: 10.1055/s-0034-1395609. [DOI] [PubMed] [Google Scholar]
- 6.Steven S, Oelze M, Hanf A, Kröller-Schön S, Kashani F, Roohani S, Welschof P, Kopp M, Gödtel-Armbrust U, Xia N, et al. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017;13:370–385. doi: 10.1016/j.redox.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, Xiong J, Perez Z, Norton L, Abdul-Ghani MA, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509–514. doi: 10.1172/JCI70704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuba R, Matsuba I, Shimokawa M, Nagai Y, Tanaka Y. Tofogliflozin decreases body fat mass and improves peripheral insulin resistance. Diabetes Obes Metab. 2018;20:1311–1315. doi: 10.1111/dom.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uemura H, Katsuura-Kamano S, Yamaguchi M, Bahari T, Ishizu M, Fujioka M, Arisawa K. Relationships of serum high-sensitivity C-reactive protein and body size with insulin resistance in a Japanese cohort. PLoS ONE. 2017;12:e0178672. doi: 10.1371/journal.pone.0178672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Effoe VS, Correa A, Chen H, Lacy ME, Bertoni AG. High-sensitivity C-reactive protein is associated with incident type 2 diabetes among african americans: the Jackson Heart Study. Diabetes Care. 2015;38:1694–1700. doi: 10.2337/dc15-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alemzadeh R, Kichler J. Gender differences in the association of insulin resistance and high-sensitivity c-reactive protein in obese adolescents. J Diabetes Metab Disord. 2014;13:35. doi: 10.1186/2251-6581-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukushima H, Sugiyama S, Honda O, Koide S, Nakamura S, Sakamoto T, Yoshimura M, Ogawa H, Fujioka D, Kugiyama K. Prognostic value of remnant-like lipoprotein particle levels in patients with coronary artery disease and type II diabetes mellitus. J Am Coll Cardiol. 2004;43:2219–2224. doi: 10.1016/j.jacc.2003.09.074. [DOI] [PubMed] [Google Scholar]
- 15.Hattori S. Empagliflozin decreases remnant-like particle cholesterol in type 2 diabetes patients with insulin resistance. J Diabetes Investig. 2018;9:870–874. doi: 10.1111/jdi.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arima H, Kubo M, Yonemoto K, Doi Y, Ninomiya T, Tanizaki Y, Hata J, Matsumura K, Iida M, Kiyohara Y. High-sensitivity C-reactive protein and coronary heart disease in a general population of Japanese: the Hisayama study. Arterioscler Thromb Vasc Biol. 2008;28:1385–1391. doi: 10.1161/atvbaha.107.157164. [DOI] [PubMed] [Google Scholar]
- 17.McMurray J. EMPA-REG-the “diuretic hypothesis”. J Diabetes Complications. 2016;30:3–4. doi: 10.1016/j.jdiacomp.2015.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are not publicly available due to some relevant ongoing studies, but may be available from the corresponding author of this article on reasonable request.