Abstract
Potato (Solanum tuberosum L.) is widely used for many industrial and food applications. Nine potato cultivars were planted and collected from a private farm in new Salihiyyah city, Sharkia governorate, Egypt to compare between them at morphological, molecular, biochemical and anatomical levels. Our results indicated that the Inova cultivar was better, however the Bafana cultivar was worse in relation to yield parameters. Inter simple sequence repeat (ISSR) molecular marker has been used to determine the genetic diversity between these nine cultivars. Through using ten primers we obtained 98 bands, 85 of which were polymorphic by 87%. The highest similarity value (0.827) was found between Caruso and Alliance as the closest but the lowest value (0.418) was found between Charlotte and Bafana as the most distant. Everest tuber contained great amounts of total phenolic and peroxidase activity, while the Bafana tuber contained small amounts of it compared to other cultivars. The phellem layer of the Everest tuber had more thickness than others and the number of phellem rows was the highest. However, the Bafana cultivar listed the lowest value compared to other cultivars. Lower values from both of total bacterial and total fungi were recorded on the tuber of the Everest cultivar. However, Bafana cultivar was recorded to have a higher value of both compared to other cultivars. We suggest that the ISSR marker is a suitable procedure to examine the potato’s genetic diversity at the DNA level. The Everest cultivar is considering the best cultivar to planting and breeding in Egypt.
Keywords: Potato cultivars, Morphological characters-ISSR-PCR, Phenol contents, Peroxidase, Phellem layer, Microbial loading
1. Introduction
Potato was first introduced outside the Andes region four centuries ago and has become an integral part of much of the world’s cuisine. It is the world’s fourth-largest food crop, following rice, wheat and maize. In Egypt the crop was introduced on a small scale during the nineteenth century. It is nowadays the second most important vegetable crop after tomato and Egypt is one of the largest producers and exporters of potatoes in Africa [1]. Currently, there are more than 3200 different potato cultivars that are cultivated in over 100 countries worldwide [2]. Its cultivation was spread and the area increased in Egypt especially in the new lands under the new irrigation systems, added the organic fertilizers and pesticides to the irrigation water. The cultivated area of potatoes in Egypt was about 183,990 feddan with an average production of 10.61 ton/feddan [3]. The nutrients and moisture content of the soil influence the number of tubers reaching maturity [4].
The correct identification, characterization and evaluation of conserved genotypes are fundamentally important for genetic improvement programs and for detecting duplicates in germplasm banks [5], [6], [7]. Genetic divergence can be evaluated based on agronomic, morphological, biochemical, physiological, molecular, and other characteristics. Studies with molecular markers have made significant contributions for understanding the genetic diversity; when compared with other types of markers, they present a greater number of polymorphic loci, which allows distinguishing between accessions that may have similar morphological and agronomical traits [5]. Molecular markers in general can be used as potential techniques for cultivar identification. These techniques are a powerful tool for determining genetic distinctness and enable characterization of particular genotypes. The recent DNA marker systems are based on PCR technology and for this reason are more suitable for routine cultivar identification, due to the small amount of DNA required, and generally fast and simple tests. Several methods were recommended for potato cultivar identification. These methods include Random Amplified Polymorphic DNA (RAPD) [8], Amplified Fragment Length Polymorphism (AFLP) [9], microsatellites – analyses of Simple Sequence Repeats (SSR) [10] or Inter-simple Sequence Repeats (ISSRs) [11], [12]. Inter-simple Sequence Repeats (ISSR) are often chosen to perform these studies considering the advantages of this molecular technique compared to other DNA markers.
Plant elaborates a vast array of natural products; many of them have evolved to confer selective advantage against microbial attack. Colored potatoes provide a natural source of phytochemicals such as carotenoids, phenolic compounds; flavonoids and anthocyanins that help to reduce the risk of chronic diseases [13], [14]. Potatoes contain a variety of phytonutrients that have antioxidant activity. Among these important health-promoting compounds are carotenoids, flavonoids, and caffeic acid, as well as unique tuber storage proteins, such as patatin, which exhibit activity against free radicals. Several Asian vegetables were classified according to total phenolic content and placed potato in the medium category where phenolic content was between 100 and 200 mg catechol/100 g [15]. Although the phenolic content of potato relative to other vegetables is low, high consumption of potato in our diet could increase the dietary intake of these bioactive compounds effectively. A variety of phytochemicals, e.g. phenolic, carotenoids and flavonoids, have been shown to possess functional properties such as antimicrobial and free radical scavenging activity [16].
Potato native periderm forms an effective barrier around the tuber that protects it from infection and dehydration. An immature periderm can make the tuber susceptible to skinning (excoriation of the skin) during harvest, which renders the tuber vulnerable to dehydration and disease as in storage [17], [18]. The potato periderm is made up of three tissues: phellem, phellogen and phelloderm [19]. The phellem (or cork) forms a series of layers at the outermost level of the periderm, and is derived from the phellogen layer (or cork cambium) underneath it. As phellem cells develop, they become suberized and then die to form a protective layer. The phelloderm cells form the innermost tier of the periderm and are similarly derived from the phellogen layer which is located directly above them. The phellogen is a single layer of meristematic cells derived from the hypoderm early during development of the tuber [20]. An immature periderm has a phellogen layer made up of cells with thin radial walls which fracture easily, allowing the phellem (skin) to scuff off [21].
The objective of this study was collecting and characterizing some of potato cultivars which are growing extensively in large areas in Egypt at morphological, molecular, biochemical and anatomical levels.
2. Materials and methods
2.1. Plant materials
We used a set of nine potato cultivars (Table 1) cultivated in sandy soil that are sown on the 15th January in two successive growing summer seasons of 2012 and 2013, under field conditions in the private farm in new Salihiyyah city, Sharkia governorate, Egypt. Both of irrigation and fertilizers systems are applied according to instructions of the ministry of agriculture.
Table 1.
List of nine potato cultivars with their pedigree and their origin.
| Name of cultivar | Pedigree of cultivar | Origin of cultivar |
|---|---|---|
| Nicola | (Clivia × 6430/1011) | Netherland |
| Everest | (Spunta × Maradonna) | Netherland |
| Charlotte | (Hansa × Danaé) | Scotland |
| Inova | (Nicola × Impala) | Netherland |
| Caruso | N.d. | Germany |
| Alliance | 185/88/359 × E 87/66 | Germany |
| Horaizon | (Russet Burbank × Sante) | Scotland |
| Slaney | (Maris Page × Cara) | England |
| Bafana | (Victoria × Felsina) | England |
N.d. = Not determined.
The field trial is (nine cultivars) designed in a complete randomized block with three replicates. The area of each replicate was 9 m2 (3 × 3 m) and had four lines of 3 m in length and 75 cm in width. Samples were collected from two summer seasons of 2012 and 2013, taking into consideration that molecular, biochemical, anatomical and microbiological analyses have been carried out on potato tubers after harvesting in the summer season of 2013. However, plant growth parameters; yield and its components have been measured in two summer seasons of 2012 and 2013.
2.2. Morphological and yield parameters
Random samples of three plants were taken from each replicate at 90 days after sowing for vegetative growth parameters [plant height (cm) and the number of aerial stems/plant]. Yield measurements [number of tubers/plant, tuber average weight/plant and total yield/hectare (ton)] were taken after harvesting from random samples of three plants for each replicate.
2.3. Molecular analysis
Mature tubers were collected in the early morning from plants because plants maintained in the dark have lower concentrations of polyphenols that interfere with DNA extraction [22], [23]. Tubers of each cultivar were wrapped in aluminum foil, labeled and immediately immersed in liquid nitrogen to avoid DNA degradation. Approximately 100 mg of macerated tissue was transferred to 2.0 ml tubes and immersed in liquid nitrogen for DNA extraction, using the protocol of [22] with modifications. DNA quantification was performed in 1.0% agarose gels; the concentrations of the markers were measured using a 100 bp marker as a standard for comparison. A set of twenty primers were tested for ISSR. Based on the accurate amplified bands profiles and the produced polymorphic patterns of DNA fingerprinting selected ten different primers were chosen (Table 2).
Table 2.
List of ISSR primers sequences used for the analysis of nine potato cultivars with primer annealing temperatures. (R = purines: G or A; Y = pyrimidines: C or T)
| Primer code | Primer sequence | Annealing temperature (°C) |
|---|---|---|
| ISSR-3 | 5′-(TCC)5TTT-3′ | 56 |
| ISSR-10 | 5′-(TCC)5CAC-3′ | 60 |
| ISSR-16 | 5′-(AG)8YC-3′ | 56 |
| ISSR-18 | 5′-(AC)8YT-3′ | 54 |
| ISSR-21 | 5′-CAT(CA)7T-3′ | 52 |
| ISSR-24 | 5′-CGC(GATA)4-3′ | 52 |
| ISSR-26 | 5′-GAC(GATA)4-3′ | 50 |
| ISSR-27 | 5′-(AGAC)4GC-3′ | 56 |
| ISSR-28 | 5′-(GATA)4GC-3′ | 48 |
| ISSR-29 | 5′-(GACA)4AT-3′ | 52 |
ISSR amplification reactions were carried out on a Perkin-Elmer Gene Amp PCR system (model 2400) and each reaction was repeated twice. PCR amplification reactions were performed according to the protocol in [24] with some modifications. The ISSR amplification reactions were carried out in 25 μl per tube, containing 2 μl DNA (20 ng), 1 unit of Taq DNA polymerase enzyme (Promega), 2 μl 10× buffer, 2 μl MgCl2 (25 mM), 2 μl dNTPs (2.5 mM of each), 2 μl primer (10 pmol) (Operon) and 14.8 μl H2O. The following conditions were used for ISSR amplifications: an initial denaturation step of 94 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 30 s, a primer annealing step for 45 s, and an extension at 72 °C for 2 min; then a final extension was carried out at 72 °C for 5 min. The annealing temperature varied according to the melting temperature of each primer.
2.3.1. Band analysis
The reaction products were analyzed by electrophoresis on 1.4% agarose gels, stained with ethidium bromide and photographed under UV transilluminator by a digital camera with a UV filter adaptor. The synthetic DNA, ladder 100 bp (Pharmacia) was employed as molecular markers for bands of molecular size. Each amplified band profile was defined by the presence or absence of bands at particular positions on the gel. Profiles were considered different when at least one polymorphic band was identified. Fragments were scored as ‘1’ if it is present or ‘0’ if it is absent based on standard marker using GelAnalyzer 3 (Egygene) software.
2.4. Biochemical analysis
Phenolic compounds are mostly distributed between the potato cortex and skin (peel) tissues [16]. About 50% of the phenolic compounds are located in the potato peel and adjoining tissues, but the rest decrease in concentration from the outside toward the center of potato tubers [25]. Phenols were determined in an unpeeled potato tuber. Samples were taken from the skin and the upper part of the cortex (about 0.2 cm thickness) from the potato tubers for all samples. Free, bound and total phenols were determined using the colorimetric method as described by [26].
The increased peroxidase staining found in the phellem cell walls may be due to suberization of these walls, which is reported to be dependent on an anionic peroxidase [27]. Peroxidase was determined in an unpeeled potato tuber; the samples were taken from the skin and the upper part of the cortex (about 0.2 cm thickness) from the potato tubers for all samples. Peroxidase activity was carried out as the method described by Purr [28].
2.5. Anatomical analysis
Tissue blocks (1.0 × 0.5 × 0.2 cm) which included the periderm and the upper part of the cortex were cut from tubers, blocks were fixed for at least 24 h in FAA (formalin acetic alcohol) represented by the following formula: 50 ml ethyl alcohol (95%), 5 ml glacial acetic acid, 10 ml formaldehyde (37–40%), 35 ml distilled water. Then the specimens were washed and dehydrated in ascending concentrations of ethyl alcohol series, then cleared in transferring concentrations of xylene and absolute alcohol. Specimens were embedded in pure paraffin wax of melting point 52–54 °C. Sections were prepared using EPMA a rotary microtome at 14 microns. Paraffin ribbons were mounted on slides and sections were stained in safranin and light green. Sections were mounted in Canada balsam [29]. Selected sections were examined to detect histological manifestations of the chosen treatments using a light microscope (Olympus) with a digital camera (Canon power shot S80) connected to the computer; the photographs were taken by the Zoom Browser Ex Program. Dimensions of sections were measured by using Corel Draw program ver. 11.
2.6. Microbiological analysis
Samples (25 g) from each cultivar were placed in 225 ml of 0.1% sterile peptone water (w/v) in sterile stomacher bags. Samples were then homogenized using a stomacher for 6 min and diluted with 0.1% sterile peptone water to determine the microbial count associated with the samples. Serial dilutions were performed in three replicates. Aliquots (1 ml) of the diluted samples were plated into appropriate count agar plates by the pour plate technique [30]. Total aerobic bacteria counts were determined by plating the diluted samples onto plate count agar (PCA, Merck, 1.05463, Germany) and incubating the plates at 30 ± 2 °C for 2 days. Yeasts and molds were determined onto Rose Bengal Chloramphenicol Agar (RBCA, Lab M, 36, supplemented with chloramphenicol, X009) and the plates were incubated at 28 °C for 5 days. Total Actinomyses were counted on plate count agar (PCA, Merck, 1.05463, Germany) supplemented with 0.1% starch. Each microbial count was the mean of triplicates and was expressed as log CFU/g (colony forming unit/g).
2.7. Statistical analysis
Data were subjected to statistical analysis according to [31]. Mean values were compared at P < 0.05 using the least significant different test (LSD). Two-way ANOVA has been carried out to find the variance among genotypes and the variance caused by the interaction between the genotypes and the two seasons at P < 0.001. Pairwise combinations and genetic similarity were estimated following [32], [33]. The computer package SPSS was used to construct a dendrogram based on the matrix of distance using the Unweighted Pair Group Method with Arithmetic averages (UPGMA) [34].
3. Results and discussion
3.1. Morphological and yield parameters
A number of morphological and yield parameters including plant height (cm), number of aerial stems/plant, tubers number/plant, tubers weight (kg)/plant and total yield/hectare (ton) of nine potato cultivars were investigated (Table 3) of two growing seasons (2012 and 2013), cultivars include Nicola, Everest, Charlotte, Inova, Caruso, Alliance, Horaizon, Slaney and Bafana. The mean of three independent samples from each replicate is measured and that is represented for each parameter showing that there is significant difference among some cultivars. However, in some cultivars no significant differences appeared in related to these parameters.
Table 3.
Investigation of morphological and yield parameters of nine potato cultivars growing in Egypt in two seasons of 2012 and 2013.
| Potato cultivars | Morphological parameters |
Yield parameters |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Plant height (cm) |
Number of aerial stems/plant |
Tubers number/plant |
Tubers weight/plant (kg) |
Total yield/hectare (ton) |
||||||
| 2012 | 2013 | 2012 | 2013 | 2012 | 2013 | 2012 | 2013 | 2012 | 2013 | |
| Nicola | 37.36 | 37.36 | 2.90 | 2.93 | 12.03 | 12.25 | 0.88 | 0.91 | 35.26 | 35.81 |
| Everest | 57.32 | 56.87 | 1.50 | 1.45 | 6.18 | 6.72 | 0.98 | 0.99 | 39.43 | 39.72 |
| Charlotte | 43.17 | 42.85 | 1.85 | 1.85 | 11.45 | 11.35 | 0.77 | 0.78 | 29.17 | 31.10 |
| Inova | 36.42 | 36.37 | 2.32 | 2.24 | 11.40 | 11.95 | 1.00 | 0.98 | 41.72 | 41.18 |
| Caruso | 55.61 | 54.71 | 2.77 | 2.75 | 11.20 | 11.55 | 0.66 | 0.71 | 27.00 | 27.48 |
| Alliance | 57.32 | 55.05 | 2.70 | 2.78 | 11.19 | 11.32 | 0.96 | 0.93 | 39.80 | 38.92 |
| Horaizon | 54.48 | 54.08 | 2.95 | 2.95 | 11.22 | 11.62 | 0.94 | 0.95 | 38.52 | 38.86 |
| Slaney | 36.28 | 36.55 | 2.50 | 2.52 | 10.15 | 10.75 | 0.97 | 0.94 | 40.79 | 40.52 |
| Bafana | 28.35 | 28.22 | 2.30 | 2.27 | 7.15 | 7.48 | 0.64 | 0.68 | 24.85 | 25.32 |
| L.S.D. at 0.05 | 0.99 | 0.52 | 0.20 | 0.14 | 0.21 | 0.57 | 0.03 | 0.04 | 1.33 | 1.10 |
For the morphological parameters (Table 3); in some potato cultivars, significant differences were noticed between them and others none. We observed that the highest value was obtained for both Everest and Alliance cultivars in the two seasons with a mean plant height reaching 57.32 cm for both cultivars in the first season compared to other cultivars, where these values reached to 56.87 and 55.05 cm, respectively in the second season. However, the lowest value was recorded for the Bafana and the slaney cultivars reached 28.35; 28.22 cm and 36.28; 36.55 cm, respectively in both seasons compared to the other cultivars. Variance analysis showed that the Horaizon and Nicola cultivars give the highest number of aerial stems per plant (2.95; 2.95 and 2.90; 2.93, respectively) in the two seasons compared to the other cultivars. The lowest value was recorded for the Everest cultivar and the Charlotte cultivars reached 1.50, 1.45 and 1.85, 1.85 respectively in both seasons compared to the other cultivars. The superiority of vegetative growth parameters of the potato plant grown under newly sandy soil conditions might be due to an increase in the leaves and aerial stem number per plant which led to a higher photosynthetic rate and reflect more accumulation of assimilates that caused an increase in the vegetative growth parameters.
Regarding the yield parameters (tubers number/plant, tubers weight/plant (kg) and total yield/hectare (ton); Table 3), there is a significant difference among some cultivars and other cultivars showed no significant difference. It was recorded that the Inova cultivar exhibited the highest value for the previous mentioned parameters [11.40, 11.95; 1.00, 0.98 (kg) and 41.72, 41.18 (ton)], respectively in the two seasons compared to the other cultivars. Bafana cultivar revealed the lowest value for the previous mentioned parameters [7.15, 7.48; 0.64, 0.68 (kg) and 24.85, 25.32 (ton)], respectively in the two seasons compared to the other cultivars. The superiority in potato yields as ton/hectare may be attributed to the increase in the average tuber number per plant from 6.18 to 12.25 and to the average weight of tubers per plant from 0.64 to 1.00 kg. A study showed that the average number of the aerial stems is mostly affected by cultivar characteristics [35]. On the same orientation, other study recorded that auxiliary branch number was affected by the cultivar type [36]. It was reported that potato cv. Spunta cultivar growing under Egyptian conditions produced a total yield ranging between 12 and 15 ton/feddan [37], [38]. Our results are in good accordance with that previously recorded by Morena et al. [35], Khajehpour [36] and Abou-Bakr et al. [38] on potatoes. We can interpret our results in the light that the genotype might play an important role in adaptation and response of a cultivar to both developmental and environmental cues. This equilibrium will reflect on both vegetative growth and yield parameters.
Potato genotypes with promising agronomic characteristics are those that are high yielding, stable, and with a good profile of tuber size grades across all tested environments. Different genotypes can affect vegetative growth and yield features of the potato plant as well as environmental factors. The genetic differences between cultivars of potatoes can create distinct responses to environmental cues that manifest into widely diverse responses. The interaction between the genotype and the environment and the interplay to create a large spectrum of responses can be complex. On the light of the results obtained in comparison among the nine potato cultivars, we see that both morphological and yield parameters are affected by the kind of cultivar. Both Inova and Horaizon cultivars exhibited the highest crop performance in terms of plant height (cm), number of aerial stems/plant, tubers number/plant, tubers weight/plant (kg) and total yield/hectare (ton) compared to other cultivars. This result may be interpreted in the light of the genotype of each cultivar which affects both morphological and yield parameters.
The results of a two-way analysis of variance between the two years of 2012 and 2013, cultivars and the interaction between year and cultivars in related to morphological and yield parameters of the nine potato cultivars are shown in Table 4. Variance analysis appeared that there is nostatistically significant difference between two years of 2012 and 2013 and also, there is nostatistically significant difference which resulted from the interaction between the two years and the cultivars (P > 0.001). Analysis of variance revealed significant differences among cultivars (P < 0.001). This means that the variation in both morphological and yield parameters of the potato is correlated only to the kind of genotypes of the potato cultivars.
Table 4.
Results of a two-way ANOVA to find the variance among two years (2012 and 2013), the nine potato cultivars and the variance caused by the interaction between cultivars and the two years at P < 0.001.
| Source of variation | Percentage cover |
||||
|---|---|---|---|---|---|
| df | SS | MS | F-ratio | P-value | |
| Plant height (cm) | |||||
| Year | 1 | 3.001 | 3.001 | 3.481 | 0.070 |
| Cultivars∗ | 8 | 5701.804 | 712.725 | 826.716 | <0.001 |
| Year × cultivars | 8 | 6.747 | 0.843 | 0.978 | 0.468 |
| Residual | 36 | 31.036 | 0.862 | ||
| Total | 53 | 5742.588 | 108.351 | ||
| Number of aerial stems/plant | |||||
| Year | 1 | 0.000267 | 0.000267 | 0.0100 | 0.921 |
| Cultivars∗ | 8 | 12.032 | 1.504 | 56.631 | <0.001 |
| Year × cultivars | 8 | 0.0249 | 0.00312 | 0.117 | 0.998 |
| Residual | 36 | 0.956 | 0.0266 | ||
| Total | 53 | 13.013 | 0.246 | ||
| Tuber number/plant | |||||
| Year | 1 | 1.510 | 1.510 | 3.673 | 0.063 |
| Cultivars∗ | 8 | 200.873 | 25.109 | 61.078 | <0.001 |
| Year × cultivars | 8 | 0.611 | 0.0764 | 0.186 | 0.991 |
| Residual | 36 | 14.800 | 0.411 | ||
| Total | 53 | 217.794 | 4.109 | ||
| Tuber weight/plant (kg) | |||||
| Year | 1 | 0.00106 | 0.00106 | 1.397 | 0.245 |
| Cultivars∗ | 8 | 0.786 | 0.0983 | 129.777 | <0.001 |
| Year × cultivars | 8 | 0.00951 | 0.00119 | 1.570 | 0.168 |
| Residual | 36 | 0.0273 | 0.000757 | ||
| Total | 53 | 0.824 | 0.0155 | ||
| Total yield/hectare (ton) | |||||
| Year | 1 | 0.934 | 0.934 | 1.094 | 0.303 |
| Cultivars∗ | 8 | 1853.133 | 231.642 | 271.418 | <0.001 |
| Year × cultivars | 8 | 7.784 | 0.973 | 1.140 | 0.361 |
| Residual | 36 | 30.724 | 0.853 | ||
| Total | 53 | 1892.575 | 35.709 | ||
∗ indicates the presence of significant differences.
3.2. Investigation of genetic divergence of potatoes using ISSR
The results of this molecular assay in fingerprinting of the potato cultivars are presented in Table 5. Through using ten primers that were selected previously based on the number of bands that they generated and the polymorphism of these bands (Table 5), we obtained 98 bands (ranging from approximately 150 to 3000 bp), 85 of which were polymorphic (87%) and 13 monomorphic (13%). Each primer generated a mean of 8.5 polymorphic fragments. The most polymorphic primer was ISSR-26 (Table 5), which produced 15 bands, followed by the primer ISSR-24 which generated 14 polymorphic bands while the primer ISSR-3 was the less polymorphic primer which produced three polymorphic bands. A high level of polymorphism based on ISSR markers was found. The highest number of amplified ISSR fragments (68) after using all primers was detected in Nicola with an average of 6.8 per primer, while the lowest number (44) with an average of 4.4 fragments per primer was detected in Bafana (Table 5). The polymorphic patterns obtained suggested that the ISSR procedure constitutes an alternative approach that is suitable to examine the potato’s genetic diversity at the DNA level. The ISSR technique provided an efficient assessment of genetic variability in these potato cultivars, as it was also found in other studies of this crop [39], [41].
Table 5.
ISSR primers which are used for the analysis of nine potato cultivars with PCR fragment lengths [base pair (bp)], number of monomorphic, polymorphic amplified bands and polymorphism (%).
| Primer Code | PCR Fragment Length (bp) | Nicola | Everest | Charlotte | Inova | Caruso | Alliance | Horaizon | Slaney | Bafana | number of monomorphic bands | number of polymorphic bands | Polymorphism (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ISSR -3 | 150-1300 | 5 | 6 | 6 | 6 | 8 | 6 | 6 | 6 | 6 | 5 | 3 | 0.38 |
| ISSR -10 | 150-900 | 7 | 4 | 8 | 4 | 7 | 8 | 6 | 4 | 6 | 2 | 8 | 80 |
| ISSR -16 | 250-3000 | 5 | 6 | 1 | 4 | 5 | 4 | 6 | 6 | 8 | 0 | 9 | 100 |
| ISSR -18 | 250-1000 | 7 | 7 | 9 | 5 | 7 | 7 | 7 | 6 | 5 | 2 | 7 | 0.78 |
| ISSR -21 | 150-1000 | 5 | 5 | 4 | 4 | 4 | 4 | 6 | 3 | 8 | 1 | 8 | 0.89 |
| ISSR -24 | 270-2000 | 9 | 5 | 8 | 7 | 6 | 7 | 5 | 10 | 3 | 0 | 14 | 100 |
| ISSR -26 | 150-2000 | 11 | 8 | 10 | 7 | 5 | 3 | 6 | 11 | 1 | 0 | 15 | 100 |
| ISSR -27 | 150-1000 | 6 | 6 | 7 | 6 | 6 | 6 | 6 | 5 | 2 | 2 | 5 | 0.71 |
| ISSR -28 | 250-2000 | 8 | 7 | 10 | 9 | 6 | 4 | 6 | 9 | 2 | 0 | 10 | 100 |
| ISSR -29 | 350-1500 | 5 | 2 | 2 | 2 | 2 | 4 | 7 | 3 | 3 | 1 | 6 | 0.86 |
| Total | 150-3000 | 68 | 56 | 65 | 54 | 56 | 52 | 61 | 63 | 44 | 13 | 85 | 0.87 |
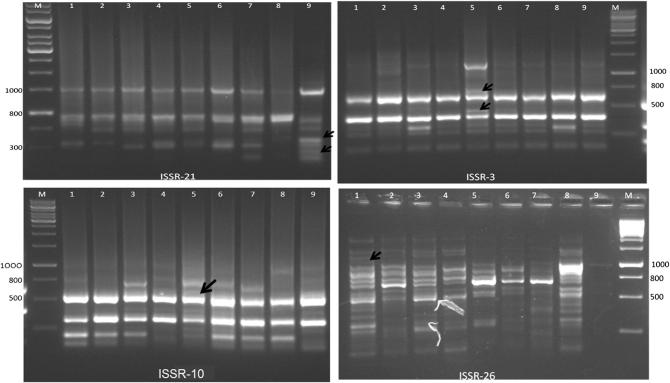
In a study, eight ISSR primers were selected and used on 34 accessions of Ipomoea (28 accessions of sweet potato and six accessions of wild species) and obtained 81 polymorphic bands, a mean of 10 bands per primer [39]. However, this mean was based on the eight most polymorphic primers, disregarding the less polymorphic primers. Another study obtained 239 polymorphic markers in 100 sweet potato accessions, using 14 ISSR primers with a mean of 17 bands per primer [40]. This high degree of polymorphism was due to the origin of these accessions, collected from China, New Guinea, and Indonesia, which are considered secondary centers of sweet potato biodiversity. Primer ISSR-24 presented one unique band (350 bp) to Nicola and two unique bands (1000 and 1600 bp) to Bafana. Primer ISSR-21 presented two unique bands (250 and 350 bp) to Bafana and primer ISSR-3 presented two unique bands (450 and 700 bp) to Caruso, but primer ISSR-10 presented one unique band (600 bp) to Caruso. Primer ISSR-29 presented one unique band (1000 bp) to Horaizon. Primer ISSR-16 presented one unique band (1100 bp) to Bafana and primer ISSR-27 presented one unique band (500 bp) to Charlotte, but primer ISSR-26 presented one unique band (1100 bp) to Nicola. Primers ISSR-18 and ISSR-28 did not present any unique band (Figue 1). Therefore; Nicola, Charlotte, Caruso, Horaizon and Bafana cultivars were distinguishable by unique ISSR markers but Everest, Inova, Alliance and Slaney cultivars were not distinguishable by unique ISSR markers.
Figure 1.
Results of amplification of four ISSR primers on 1.5% agarose with nine potato cultivars (M; DNA ladder marker, 1; Nicola, 2; Everest, 3; Charlotte, 4; Inova, 5; Caruso, 6; Alliance, 7; Horaizon, 8; Slaney and 9; Bafana). Black arrows indicate unique bands.
The similarity coefficient values among all cultivars based on band polymorphisms generated by ISSR after using all primers are presented in Table 6. The highest similarity value (0.827) was found between Caruso and Alliance as the closest but the lowest value (0.418) was found between Charlotte and Bafana as most distant. The differences in genetic distances which were observed in these studies were due mainly to differences in the origin of the cultivars. He et al. [40] found genetic distances of 0.17–1.48, with a mean of 0.57. Qiang et al. [41] reported genetic distances from 0.16 to 0.92, with a mean distance of 0.57. The dendrogram of genetic distances among all the tested cultivars based on band polymorphisms generated by ISSR after using the primers is shown in Figure 2. The dendrogram separated all cultivars into two clusters. First cluster formed a separate cluster with Bafana. Second cluster was further divided into two subclusters, first subcluster formed a separate subcluster with Caruso, Alliance and Horaizon and the second subcluster included Nicola, Everest, Charlotte, Inova and Slaney. Using ISSR markers, an association was found between genetic and geographic distances working with accessions from various Asian countries [41]. However, a study made with microsatellite markers, did not find correlations between geographic distances and genetic differences among sweet potato accessions in which most accessions were from the same geographic region [42].
Table 6.
Genetic similarity among the nine studied potato cultivars based on Jaccard’s coefficient.
| Potato cultivars | Everest | Charlotte | Inova | Caruso | Alliance | Horaizon | Slaney | Bafana |
|---|---|---|---|---|---|---|---|---|
| Nicola | 0.755 | 0.704 | 0.755 | 0.714 | 0.745 | 0.663 | 0.704 | 0.449 |
| Everest | 0.745 | 0.776 | 0.776 | 0.745 | 0.704 | 0.745 | 0.551 | |
| Charlotte | 0.724 | 0.745 | 0.673 | 0.612 | 0.735 | 0.418 | ||
| Inova | 0.735 | 0.745 | 0.663 | 0.724 | 0.469 | |||
| Caruso | 0.827 | 0.724 | 0.684 | 0.531 | ||||
| Alliance | 0.776 | 0.673 | 0.561 | |||||
| Horaizon | 0.694 | 0.520 | ||||||
| Slaney | 0.439 |
Figure 2.
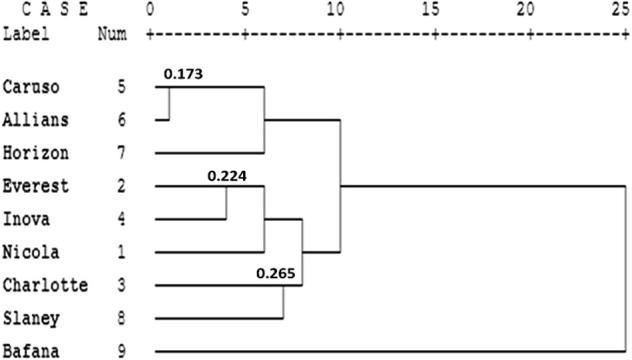
Dendrogram of genetic distances among all tested nine potato cultivars based on band polymorphisms generated by the analysis of ten ISSR primers.
A more recent study revealed that both morphological traits and the ISSR marker are highly useful for assessing genetic diversity and parental selection studies in chrysanthemum [43]. Another recent study suggested that ISSRs are very promising genetic markers for the characterization of pomegranate (Punica granatum L.) cultivars [44]. An important study suggested that by the use of ISSR and RAPD markers we were able to distinguish genetic relationships among genotypes and cultivars of hazelnut (Corylus avellana L.) which may be useful in breeding programs [45].
3.3. Detection of phenolic content and peroxidase in potato tubers
Environmental stresses or pathogen attacks have been shown to induce the generation of phenolic compounds via the phenylpropanoid pathway in plants [46]. The potato can be affected by many biotic and abiotic factors, including pathogens and environmental stresses [47], [48]. This is a serious economic problem in countries where potatoes are cultivated over large areas. Total phenolic content (TPC), free phenolics and bound phenolics were investigated in unpeeled potato tubers of nine cultivars at the harvest stage using the Folin–Ciocalteu reagent assay. Potato cultivars include Nicola, Everest, Charlotte, Inova, Caruso, Alliance, Horaizon, Slaney and Bafana; as shown in Table 7. The results revealed that, the value of total phenolic content was (6.13, 6.91, 2.79, 5.62, 4.19, 3.65, 4.58, 4.89 and 2.56 mg/g f.w.) respectively, for different cultivars. This is in accordance with previous studies reporting that the total phenolic content of potato cultivars ranged from 0.90 to 4.00 mg/g f.w. [49]. The Everest cultivar had the highest total phenolic content (6.91 mg/g f.w.), while the Bafana cultivar had the lowest phenolic content (2.56 mg/g f.w.). Phenolic compounds like pterocarpans, coumarins, flavonols, and isoflavones [47], [50] are an important group of secondary metabolites involved in resistance to pathogens due to their antimicrobial activity.
Table 7.
Measurment of phenolic compounds (total, free and bound phenols); mg/g fresh weight and peroxidase activity of unpeeled potato tubers of nine cultivars growing in Egypt.
| Potato cultivars | Total phenols mg/g fresh weight | Free phenols mg/g fresh weight | Bound phenols mg/g fresh weight | Peroxidase μmol H2O2/mg f.w./min |
|---|---|---|---|---|
| Nicola | 6.13 | 4.29 | 1.84 | 1.31 |
| Everest | 6.91 | 4.68 | 2.23 | 4.08 |
| Charlotte | 2.79 | 1.81 | 0.98 | 0.54 |
| Inova | 5.62 | 4.15 | 1.47 | 0.77 |
| Caruso | 4.19 | 3.24 | 0.95 | 0.64 |
| Alliance | 3.65 | 2.75 | 0.90 | 0.57 |
| Horaizon | 4.58 | 3.43 | 1.15 | 0.68 |
| Slaney | 4.89 | 3.82 | 1.07 | 0.74 |
| Bafana | 2.56 | 1.79 | 0.77 | 0.45 |
| L.S.D. at 0.05 | 0.17 | 0.10 | 0.19 | 0.03 |
For the free and bound phenolics, the results were similar to those obtained in the case of total phenols. The highest values of free and bound phenols (4.68 and 2.23 mg / g fw) respectively, have been recorded with the Everest cultivar, while, the lowest values of both free and bound phenols 1.79 and 0.77 mg/g f.w. respectively, have been listed to the Bafana cultivar. The difference between the total phenolic compounds, free phenolic and bound phenolic compounds of the nine potato cultivars was statistically significant (P > 0.05). In potatoes, low temperature storage [51], light [52], wounding [53] and disease [54] can cause an increase in the phenolic content. Low temperature storage-induced activation of phenylalanine ammonia-lyase (PAL), a key regulatory enzyme in the biosynthesis of polyphenols including anthocyanins [55], and de novo synthesis of secondary metabolites [56] may be responsible for an initial increase in the phenolic content with storage. Bhatia et al. [57] recorded that secondary plant metabolites correlated with early blight resistance include phenolic compounds (tannins, flavonols, and phenols) in leaves and stems in the tomato. Moreover, the fruit of resistant tomato varieties contain a higher amount of phenolic compounds than those from susceptible varieties. The constitutive expression of phenols, which are thought to function as preformed inhibitors, is associated with nonspecific basal resistance to multiple pathogens in all plant species [58]. The correlation between resistance and defense responses such as phenol production leads to the possibility of using this as a screen for potentially resistant germplasm since we would expect that cultivars with higher basal levels of phenolics are less susceptible than those with lower basal levels.
In addition to phenolic compounds, the production of reactive oxygen species, such as hydrogen peroxide and superoxide, also has an integral role in pathogen defense [59]. Peroxidase also shows affinity to substrates involved in cellular lignification and the products of its activity have direct antimicrobial activity in the presence of hydrogen peroxide [60]. Peroxidases are known to be involved in the cross-linking of a number of cell wall polymers including suberin [27], extensin [61] and feruloylated hemicelluloses [62]. We examined the peroxidase activity in nine different potato cultivars (Nicola, Everest, Charlotte, Inova, Caruso, Alliance, Horaizon, Slaney and Bafana). In the results it appeared that, the Everest cultivar had the highest (4.080 μmol H2O2/mg f.w./min) peroxidase activity. However, The Bafana revealed the lowest (0.452 μmol H2O2/mg f.w./min) peroxidase activity. The difference between the peroxidase activities of the nine potato varieties was statistically significant (P > 0.05). It was interesting to note that potato cultivars that showed enhanced peroxidase (POD) activity also recorded a higher concentration of total phenolic contents, free phenolic compounds and bound phenolic compounds. Peroxidase is involved in the production of reactive oxygen species, which are directly toxic to the pathogen or indirectly reduce the spread of the pathogen by increasing the cross linking and lignification of the plant cell walls [63]. In addition, peroxidase enzymes are important in the production of hydrogen peroxide and have been linked to increased disease resistance in plants [64].
3.4. Examination of phellem layer in potato tubers
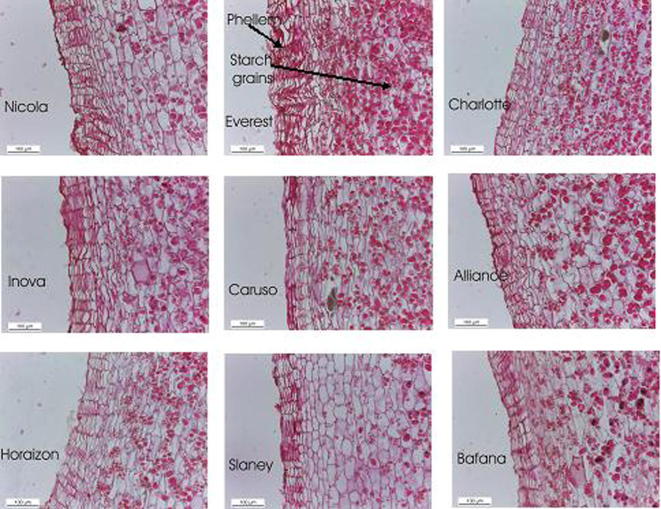
Nine cultivars of potato; Nicola, Everest, Charlotte, Inova, Caruso, Alliance, Horaizon, Slaney and Bafana; were investigated anatomically in related to the thickness of phellem (μ) and number of rows of phellem (Figue 3 and Table 8). The results showed that the thickness of the phellem and number of row phellems were (141.53 and 16, 154.34 and 18, 64.77 and 9, 138.85 and 15, 87.69 and 11, 76.30 and 10, 95.31 and 13, 129.01 and 14 as well as 63.50 and 7) respectively, the data revealed that the highest value was recorded for the Everest cultivar, while the lowest value was listed to the Bafana cultivar. The periderm of potato tuber forms an effective barrier around the tuber that protects it from infection [65]. The potato periderm is made up of three tissues; Phellem, phellogen and phelloderm [19]. The phellem or cork forms a series of layers at the outmost level of the periderm. The thickness of the phellem layer is the most reliable trait for both excoriation and microbial accumulation. The thickness of the periderm is thin and prone to fracture during harvest [65].
Figue 3.
Cross-sections through periderm of potato tubers of nine cultivars growing in Egypt illustrated both phellem thickness and number of rows of phellem (the bar for all plates = 0.1 mm).
Table 8.
Measurment of phellem thickness and the number of phellems of potato tubers of nine cultivars cultivated in Egypt. (The bar for all plates = 0.1 mm).
| Potato cultivars | Phellem thickness (μ) | Number of phellem rows |
|---|---|---|
| Nicola | 141.53 | 16 |
| Everest | 154.34 | 18 |
| Charlotte | 64.77 | 9 |
| Inova | 138.85 | 15 |
| Caruso | 87.69 | 11 |
| Alliance | 76.30 | 10 |
| Horaizon | 95.31 | 13 |
| Slaney | 129.01 | 14 |
| Bafana | 63.50 | 7 |
The thickness of the phellem layer has been measured in the tuber after their harvest in order to obtain a precise thickness or a complete thickness of the phellem layer. It was reported that the immature periderm is characterized by a meristematically active phellogen layer, while the mature periderm is characterized by a meristematically inactive phellogen layer [21]. We measured the thickness of the phellem layer for revealing the differences between potato tuber cultivars because the rest of the tuber tissue is similar in its structure, which contains parenchyma cells. These parenchyma cells are characterized with the storage of a lot of starch grains that are negatively affected in abrasion (excoriation of the skin) and microbial attack.
3.5. Microorganisms associated with potato tubers
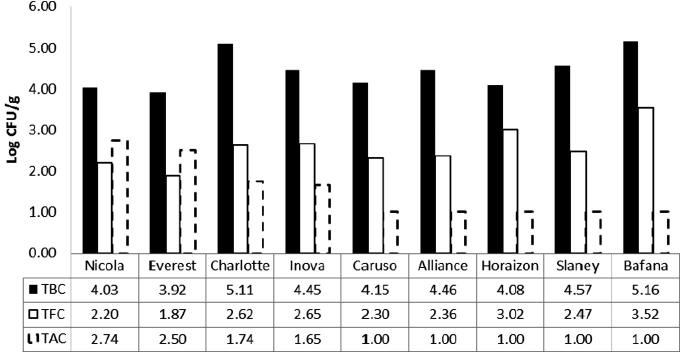
The results of microbials associated with potato tubers after harvest (total bacterial count (TBC), total fungi count (TFC), and total actinomycetes count (TAC)) are presented in Figue 4. In general, the mean log counts of TBC, TFC and TAC ranged from 5.16 to 3.92 log CFU/g for Bafana and Everest; from 3.52 to 1.87 log CFU/g for Bafana and Everest; and from 2.7 to 1.0 log CFU/g for Nicola and Bafana, respectively. In Everest, TBC was (3.92 log CFU/g) lower than all the cultivars of potato. The numbers of TFC in the Bafana samples were higher (3.52 log CFU/g) than other cultivars. The natural resistance of plants to diseases is based not only on preformed defenses, but also on induced mechanisms. The induced mechanisms are associated with local changes at the site of pathogen infection, such as the hypersensitive response (HR), which is one of the most efficient forms of plant defenses [66]. In addition to causing accumulation of antimicrobial compounds, such as phenolic compounds and phytoalexins [67], the HR also leads to an increase in the activity of peroxidases [66] and polyphenol oxidase enzymes [68] involved in defense responses [69].
Figure 4.
Estimation of total bacterial count (TBC), total fungi count (TFC) and total Actinomyces count (TAC) associated with potato tubers after their harvest.
In this study, it is obvious that in addition to the synthesis of high phenolic compounds, the thickness of the phellem and number of row phellems were variable among different potato cultivars depending on the genotype of each cultivar. These results are consistent with other results that reported that in potato plant synthesis the phenolic compounds act as a protection against bruising and injury from bacteria, fungi, viruses and insects. The Everest cultivar had the highest total phenolic content (691 mg/100 g f.w.), while the Bafana cultivar had a low value (256 mg/100 g f.w.). The thickness of the phellem and number of row phellems have the highest values for the Everest cultivar, while the lowest value was recorded to the Bafana cultivar. These cultivars exhibited changeable average to tolerance to microbial attack.
It appears that the resistant cultivars produce more secondary metabolites involved in plant defense mechanisms than the other cultivars tested. These compounds act as barriers against pathogen invasion and hence construct part of host resistance mechanisms. The increase in peroxidase (POD) activity is involved in the oxidative polymerization of hydroxyl cinnamyl alcohols to yield lignin [70] and crosslinking isodityrosine bridges in cell walls [71]. In general, the resistant cultivars have thick layers of the phellem and produce more secondary metabolites and peroxidase (POD) activity involved in plant defense mechanisms than the other cultivars tested as we noted for the number of microbials associated with potato tubers.
Acknowledgement
The authors are thankful to Dr. Samir Ahmad Marghany, Assistant professor of Food Microbiology, Agricultural Microbiology Department, Faculty of Agricultural, Zagazig University, Egypt, for helping us in part of microbial loads associated with potato cultivars.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.M.S. Ramadan, Report on Potato Production in Egypt. International Potato Course: Production, Storage, and Seed Technology. Report of Participants. International Agricultural Center, Wageningen, The Netherlands, 1981.
- 2.Hamester W., Hils U., editors. World Catalogue of Potato Varieties. Que Pub; Indianapolis: 2003. [Google Scholar]
- 3.Anonymous . Ministry of Agriculture; Cairo, Egypt: 2011. Economic and Statical Research Institute. p. 104. [Google Scholar]
- 4.M. Gaurav, College of health and human sciences, department of food science and human nutrition (Ph.D. thesis), Colorado State University, Colorado, 2012.
- 5.Goncalves L., Rodrigues R., Amaral A., Jr., Karasawa M., Sudre C. Genet. Mol. Res. 2008;7:1289–1297. doi: 10.4238/vol7-4gmr526. [DOI] [PubMed] [Google Scholar]
- 6.Laurentin H. Genet. Resour. Crop Evol. 2009;56:277–292. [Google Scholar]
- 7.Sudre C., Goncalves L., Rodrigues R., Amaral A., Jr., Riva-Souza E., Bento C. Genet. Mol. Res. 2010;9:283–294. doi: 10.4238/vol9-1gmr698. [DOI] [PubMed] [Google Scholar]
- 8.McGregor C., Lambert C., Greyling M., Louw J., Warnich L. Euphytica. 2000;113:135–144. [Google Scholar]
- 9.Van-Treuren R., Magda A., Hoekstra R., Van-Hintum J. Genet. Resour. Crop Evol. 2004;51:277–290. [Google Scholar]
- 10.Ghislain M., Spooner D., Rodríguez F., Villamón F., Núñez J., Vásquez C., Waugh R., Bonierbale M. Theor. Appl. Genet. 2004;108:881–890. doi: 10.1007/s00122-003-1494-7. [DOI] [PubMed] [Google Scholar]
- 11.Albani M., Wilkinson M. Plant Breeding. 1998;117:573–575. [Google Scholar]
- 12.Prevost A., Wilkinson M. Theor. Appl. Genet. 1999;98:107–112. [Google Scholar]
- 13.Tamimi R., Lagiou P., Adami H., Trichopoulos D. J. Intern. Med. 2002;251:286–300. doi: 10.1046/j.1365-2796.2002.00969.x. [DOI] [PubMed] [Google Scholar]
- 14.Reyes L., Miller J., Cisneros-Zevallos L. Am. J. Potato Res. 2004;81:187–193. [Google Scholar]
- 15.Kaur C., Kapoor H. Int. J. Food Sci. Technol. 2002;37:153–161. [Google Scholar]
- 16.Friedman M. J. Agric. Food Chem. 1997;45:1523–1540. [Google Scholar]
- 17.Lulai E., Orr P. Am. Potato J. 1995;72:225–241. [Google Scholar]
- 18.Lulai E., Corsini D. Physiol. Mol. Plant Pathol. 1998;53:209–222. [Google Scholar]
- 19.Reeve R., Hautala E., Weaver M. Am. Potato J. 1969;46:361–373. [Google Scholar]
- 20.Peterson R., Barker W. Bot. Gazette. 1979;140:398–406. [Google Scholar]
- 21.Lulai E., Freeman T. Ann. Bot. 2001;88:555–561. [Google Scholar]
- 22.Sharma K., Mishra A., Misra R. Afr. J. Biotechnol. 2008;7:1018–1022. [Google Scholar]
- 23.Borges A., Rosa M., Recchia G., Queiroz-Silva J., Bressan E., Veasey E.A. Sci. Agric. 2009;64:529–534. [Google Scholar]
- 24.Williams J., Kubelik A., Livak K., Rafalski J., Tingey S. Nucleic Acids Res. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasegawa D., Johnson R., Gould W. J. Agric. Food Chem. 1966;14:165–169. [Google Scholar]
- 26.Snell R., Snell G. 3rd ed. vol. III. D. van Nostrand Company Inc.; New York: 1953. Colorimetric Method of Analysis, Including Some Turbidimetric and Nephelometric Methods; pp. 225–233. [Google Scholar]
- 27.Espelie K., Franceschi V., Kolattukudy P. Plant Physiol. 1986;81:487–492. doi: 10.1104/pp.81.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purr A. Biochem. Zieitschrift. 1950;321:1–18. [PubMed] [Google Scholar]
- 29.Nassar M., El-Sahhar K. Academic Bookshop; Dokki, Giza, Egypt: 1998. Botanical Preparations and Microscopy (Microtechnique) p. 219. (in Arabic) [Google Scholar]
- 30.Goldman E., Green L.H. Practical Handbook of Microbiology. 2nd ed. CRC Press; New York: 2009. [Google Scholar]
- 31.Snedecor G.W., Cochran W.G. 7th ed. Iowa State University Press; Ames, Iowa, USA: 1980. Statistical Methods. [Google Scholar]
- 32.Lynch M. Mol. Biol. Evol. 1990;7:478–484. doi: 10.1093/oxfordjournals.molbev.a040620. [DOI] [PubMed] [Google Scholar]
- 33.Lynch M. In: DNA Fingerprinting Approaches and Applications. Burke T., Dolf G., Jefferys A.J., Wolf R., editors. Springer Verlag; Basel: 1991. pp. 113–126. [Google Scholar]
- 34.Sneath P., Sokal R. W.H. Freeman; San Francisco, USA: 1973. Numerical Taxonomy. [Google Scholar]
- 35.Morena I.D., Guillen A., Moral L.F.G. Am. Potato J. 1994;71:165. [Google Scholar]
- 36.M. Khajehpour, Production of industrial plants, 2nd ed., Jehad-e-Daneshgahi Isfahan Press, Isfahan, Iran, 2006, p. 580. ISBN: 961-6122-63-9.
- 37.R.S.E. Anwar, Faculty of agricultural (Ph.D. thesis), Zagazig University, Egypt, 2005.
- 38.Abou-Bakr M.H.A., Hassan H.R., Nassar D.M.A. Mansoura Univ. J. Agric. Sci. 2005;30:6701–6721. [Google Scholar]
- 39.Hu J., Nakatani M., Lalusin A., Kuranouch T., Fujimura T. Breeding Sci. 2003;53:297–304. [Google Scholar]
- 40.He X., Liu Q., Ishiki K., Zhai H., Wang Y. Plant Genet. Breeding. 2007;150:35–41. [Google Scholar]
- 41.Qiang L., Qing Chang L., Hong Z., Dai-Fu M., Xin W., Xue-Qin L., Yu-Ping W. Acta Agron. Sin. 2008;34:972–977. [Google Scholar]
- 42.Veasey E., Borges A., Rosa M., Silva J., Bressan E., Peroni N. Genet. Mol. Biol. 2008;31:725–733. [Google Scholar]
- 43.Baliyana D., Sirohia A., Kumara M., Kumara V., Malika S., Sharmab S., Sharma S. Sci. Hortic. 2014;167:164–168. [Google Scholar]
- 44.Ajal E.A., Jbir R., Melgarejo P., Hernández F., Haddioui A., Hannachi A.S. Biochem. Syst. Ecol. 2014;56:24–31. [Google Scholar]
- 45.Mohammadzedeh M., Fattahi R., Zamani Z., Khadivi-Khub A. Sci. Hortic. 2014;167:17–26. [Google Scholar]
- 46.Dixon R.A., Paiva N.L. Plant Cell. 1995;7:1085–1087. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Juskiewicz J., Zdunczyk Z., Fornal J. Acta Biochim. Pol. 2005;52:725–729. [PubMed] [Google Scholar]
- 48.Rauscher G.M., Smart C.D., Simko I., Bonierbale M., Mayton H., Greenland A., Fry W.E. Theor. Appl. Genet. 2006;112:674–687. doi: 10.1007/s00122-005-0171-4. [DOI] [PubMed] [Google Scholar]
- 49.Stushnoff C., Holm D., Thompson M.D., Jiang W., Thompson H.J., Joyce N., Wilson P. Am. J. Potato Res. 2008;85:267–276. [Google Scholar]
- 50.Harborne J.B. Biochem. Syst. Ecol. 1999;27:335–367. [Google Scholar]
- 51.Rhodes M.J.C., Wooltorton L.S.C. Phytochemistry. 1978;17:1225–1229. [Google Scholar]
- 52.Percival G.C., Baird L. J. Agric. Food Chem. 2000;48:2476–2482. doi: 10.1021/jf9909095. [DOI] [PubMed] [Google Scholar]
- 53.Reyes L., Villarreal J., Cisneros-Zevallos L. Food Chem. 2007;101:1254–1262. [Google Scholar]
- 54.Smith B., Rubery P. Planta. 1981;151:535–540. doi: 10.1007/BF00387431. [DOI] [PubMed] [Google Scholar]
- 55.Jiang Y., Joyce D.C. Plant Growth Regul. 2003;39:171–174. [Google Scholar]
- 56.Lewis C.E., Walker J.R., Lancaster J.E., Sutton K.H. J. Sci. Food Agric. 1998;77:45–57. [Google Scholar]
- 57.Bhatia I., Uppal D., Bajaj K. Indian Phytopathol. 1972;25:231–235. [Google Scholar]
- 58.Nicholson R., Hammerschmidt R. Annu. Rev. Phytopathol. 1992;30:369–389. [Google Scholar]
- 59.Lamb C., Dixon R. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- 60.Ride J. Physiol. Mol. Plant Pathol. 1975;5:125–134. [Google Scholar]
- 61.Cooper J., Varner J. Biochem. Biophys. Res. Commun. 1983;112:161–167. doi: 10.1016/0006-291x(83)91811-9. [DOI] [PubMed] [Google Scholar]
- 62.Tan K., Hoson T., Masuda Y., Kamisaka S. Physiol. Plant. 1991;38:397–403. [Google Scholar]
- 63.Hammond-Kosack K., Jones J. Plant Cell. 1996;8:1773–1791. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bindschedler L., Dewdney J., Blee K., Stone J., Asai T., Plotnikov J., Denoux C., Hayes T., Gerrish C., Davies D., Ausubel F., Bolwell G. Plant J. 2006;47:851–863. doi: 10.1111/j.1365-313X.2006.02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sabba R., Lulai E. Ann. Bot. 2002;90:1–10. doi: 10.1093/aob/mcf147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kortekamp A., Zyprian E. J. Plant Physiol. 2003;160:1393–1400. doi: 10.1078/0176-1617-01021. [DOI] [PubMed] [Google Scholar]
- 67.Ortega X., Velasquez J., Perez L. Biol. Res. 2005;38:89–99. doi: 10.4067/s0716-97602005000100011. [DOI] [PubMed] [Google Scholar]
- 68.Agrios G.N. 4th ed. Academic Press; San Diego: 1997. Plant Pathology. 93–114. [Google Scholar]
- 69.Thipyapong P., Hunt M., Steffens J. Planta. 2004;220:105–117. doi: 10.1007/s00425-004-1330-6. [DOI] [PubMed] [Google Scholar]
- 70.Vance C., Kirk T., Sherwood R. Annu. Rev. Phytopathol. 1980;18:259–288. [Google Scholar]
- 71.Fry S.C. Biochem. J. 1982;204:449–455. doi: 10.1042/bj2040449. [DOI] [PMC free article] [PubMed] [Google Scholar]