Abstract
Background and Aims:
Though, many practitioners prefer conscious sedation (CS), it is unclear which factors most influence neurological outcome following mechanical thrombectomy under CS. The aim of this retrospective study is to identify these factors.
Methods:
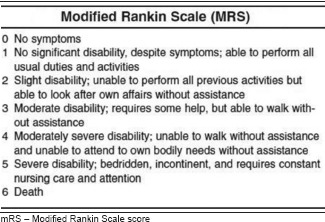
After institutional review board approval, data were collected for the patients >18 years of age who underwent endovascular treatment of AIS under CS at our comprehensive stroke centre between January 2009 and June 2015. The primary outcome measure was the modified Rankin Scale (mRS) at discharge. A good outcome was defined as mRS 0–3 and poor outcome as mRS 4–6. Univariate and logistic regression analysis were performed to identify the independent predictors of poor outcomes at discharge. A P < 0.05 was considered statistically significant.
Results:
One hundred two patients, aged 67 ± 16 years were included. The anterior cerebral circulation was affected in 88 patients (86%), and the median National Institute of Health Stroke Scale (NIHSS) score at presentation was 17.5 (range: 1–36). Overall, 21 (21%) patients had good outcome and 81 (79%) had poor outcome. Logistic regression identified the modified treatment in cerebral ischaemia (mTICI) score [odds ratio (OR): 0.443, confidence interval (CI): 0.244–0.805], NIHSS score (OR: 1.290, CI: 1.125–1.481) and previous transient ischaemic attack (TIA) (OR: 6.988, CI: 1.342–36.380) as significant independent predictors of poor outcome at discharge.
Conclusion:
The outcome of patients who underwent endovascular treatment of AIS under CS depends on the mTICI score, NIHSS score and history of previous TIA.
Key words: Acute ischaemic stroke, conscious sedation, endovascular treatment, outcomes
INTRODUCTION
Acute ischaemic stroke (AIS) is one of the leading causes of mortality and long-term disability worldwide. Intravenous tissue plasminogen activator (IV rtPA) is an approved treatment for AIS, though many patients will not qualify for the treatment because of the narrow time window for administration of rtPA. Recently, endovascular treatment with mechanical thrombectomy has been shown to yield superior outcomes than pharmacological management alone for AIS affecting the anterior circulation.[1,2,3,4,5]
The anaesthetic management of patients undergoing mechanical thrombectomy varies between general anaesthesia (GA) with or without intubation, conscious sedation (CS), to completely awake patients under local anaesthesia. Multiple studies have compared GA and CS for the endovascular treatment of AIS and have reported an association of GA with poor outcomes.[6,7,8,9,10,11,12,13,14] In most of these studies, patients who received GA had a higher pre-procedural NIHSS score and required intubation for airway protection. In these studies, GA was found to be an independent predictor for poor outcomes even after adjusting for stroke severity, though the reasons are not clear. It is speculated that the variation in the haemodynamic changes occurring during GA, mainly hypotension and hypocapnia, might lead to poorer outcomes.[14,15] Based on these studies, CS has become a popular anaesthetic choice for mechanical thrombectomy. It is unclear, however, which factors under CS anaesthesia influence clinical outcome. The aim of this retrospective study is to identify these factors.
METHODS
The institutional review board (IRB) approval was obtained to conduct this retrospective chart review of patients treated at a single hospital between January 2009 and June 2015. Patients 18 years old or older who underwent endovascular treatment for AIS under CS were included. Patients treated under GA were excluded. CS with midazolam and fentanyl titrated to the Richmond Agitation-Sedation Scale 0 to −3 was provided by trained nursing staff in neurointerventional radiology. Mechanical thrombectomies were done by one of the three fellowship-trained interventional neuroradiologists. Anaesthetic details and the vital signs during the procedure were collected from the paper chart. Comorbid diseases, medication history, laboratory parameters and social history were collected from the electronic medical record. The National Institute of Health Stroke Scale (NIHSS) score and the modified Rankin Score (mRS) at discharge were obtained from a stroke neurology database. Modified treatment in cerebral ischaemia (mTICI) scores, stroke territory, devices used for the procedure and post-procedure haemorrhagic complications were collected from a radiology database. NIHSS score was evaluated by vascular neurology residents and fellows, mTICI score was evaluated by interventional neuroradiologist and mRS score was evaluated by stroke nurses certified in the use of the mRS instrument. The primary outcome measure is the mRS at discharge. Good outcome is defined as mRS 0–3 and poor outcome as mRS 4–6.
Statistical analysis
Statistical analysis was performed with the SAS® version 9.4 (SAS Institute, Cary, North Carolina). The clinical, anaesthetic, haemodynamic and neurointerventional characteristics of patients with poor outcome were compared to those patients with good outcome using univariate statistics, specifically the Chi-square test, Fisher exact test and independent t-test. Variables which were tested to be not normally distributed were represented as median and interquartile range (IQR). All factors that were statistically significant (P < 0.05) in the univariate analysis were entered into a combination of forward and backward stepwise modelling techniques. The stepwise procedure in each specification technique was designed to construct a model that can best explain the multivariable relationships in the data. At each modelling step, a factor may be automatically added or removed based on its contribution to the overall model fit and its own significance level (P < 0.05). The final forward and backward models included slightly different subsets of significant factors. These selected factors were then combined into a final regression and rerun to reconcile between the two technical approaches and presented as the best-fitting model. The overall fit of the final model was assessed using the c-statistic, measuring the area under the receiver operating characteristic (ROC) curve and the Hosmer and Lemeshow test as a measure of goodness of fit. A model parameter with P < 0.05 was considered statistically significant.
RESULTS
A total of 102 patients with mean age 67 ± 16 years were included in the study. Eighty-eight (86%) of the patients were affected by anterior circulation stroke. The median pre-procedural NIHSS was 17.5 ranging from (1–36). Overall 21 (21%) patients had good outcome at discharge. Penumbra, Solitaire, Merci and Trevo devices were each used at least once during the study period. Fifty-five (54%) patients had mechanical thrombectomy alone without IV rtPA, 37 (36%) received intravenous tPA and mechanical thrombectomy, 5 (5%) received intra-arterial tPA, 2 (2%) received intra-arterial tPA and mechanical thrombectomy, 2 (2%) received mechanical thrombectomy and angioplasty and 1 (1%) received intravenous tPA, mechanical thrombectomy and intra-arterial tPA.
Table 1 shows the clinical and neurointerventional characteristics for both the groups at discharge. Twenty (25%) patients in the poor outcome group had post-procedure haemorrhagic complications compared with 0 (0%) in patients with good outcome (P = 0.01). About 38 (47%) patients with poor outcome had previous transient ischaemic attack (TIA) compared to 3 (14%) in patients with good outcome (P = 0.006). There was no appreciable difference in outcome based on the type of devices used during the procedure, comorbid illnesses (hypertension, diabetes mellitus, renal insufficiency, congestive heart failure, coronary artery disease, atrial fibrillation and hyperlipidaemia), smoking history or use of medications.
Table 1.
Clinical and neurointerventional characteristics by discharge outcome. Categorical variables are represented as number (percent)
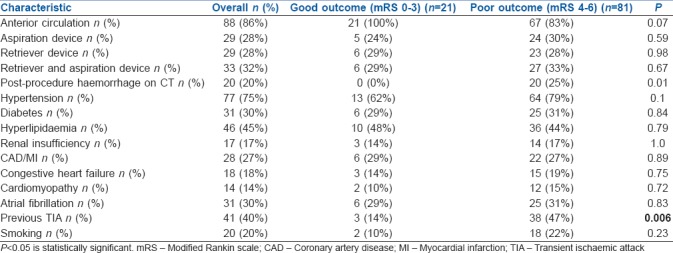
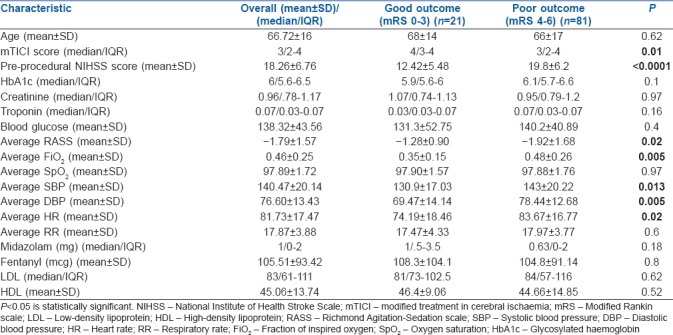
Table 2 shows the clinical, neurointerventional, laboratory and anaesthetic characteristics for both the groups at discharge. mTICI scoring after the intervention was lower (P = 0.01) and the NIHSS score was higher (P ≤ 0.0001) in the poor outcome group compared to the good outcome group. Average systolic blood pressure (SBP) and diastolic blood pressure were significantly different between both the groups, with higher values in the poor outcome group (P = 0.013, 0.005). There was no difference in outcome based on age, laboratory parameters [glucose, high-density lipoprotein (HDL), low-density lipoprotein (LDL), HbA1c and creatinine levels] or the amount of anaesthetic agent used during the procedure.
Table 2.
Clinical, neurointerventional, laboratory and anaesthetic characteristics by discharge outcome. Normally distributed continuous variables are represented as mean±standard deviation and others are represented as median/interquartile range
Logistic regression was performed to identify independent factors associated with poor outcome at discharge [Table 3]. All predictor variables selected using the stepwise procedure were entered into the final model as a single step rather than filtering independent variables based on their final significant P values. The final multivariable logistic regression model had a very good discrimination (c-statistic of 0.89) and a good calibration (Hosmer–Lemeshow test, Chi-square = 4.0193, P = 0.8554) with an overall area under the curve 0.89 [Figure 1]. The mTICI scoring [odds ratio (OR): 0.443, confidence interval (CI): 0.244–0.805] (P = 0.007), NIHSS score (OR: 1.290, CI: 1.125–1.481) (P = 0.0003) and TIA (OR: 6.988, CI: 1.342–36.380) (P = 0.02) were found to be the significant predictors of poor outcome at discharge.
Table 3.
Final logistic regression analysis for poor outcome at discharge
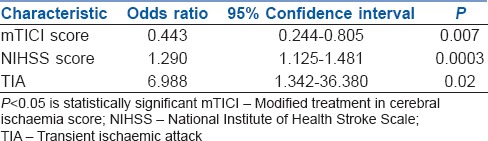
Figure 1.
Shows the receiver operating characteristic curve for the predictor variables and the entire model. Area under the curve for the overall model is 0.8903
DISCUSSION
Our study finds that the outcome of the patients at discharge who underwent endovascular treatment for AIS under CS is mainly dependent on 1) NIHSS score at presentation 2) post-treatment mTICI score and 3) a history of previous TIA. To the best of our knowledge, this is the first study which compared the outcomes under CS for the endovascular treatment of AIS. Though CS is a recommended choice when feasible for these procedures, GA cannot be avoidable in certain group of patients such as those with posterior circulation stroke or those who are highly uncooperative or agitated.
Studies which compared GA and CS during endovascular treatment of AIS have favoured CS based on superior outcomes,[6,7,8,9,10,11,12,13,14] though a recent prospective, randomised trial found no difference in outcomes between the GA and CS groups.[16] Those studies which did suggest superior outcomes with CS speculated that the haemodynamic parameters such as hypocapnia and hypotension may lead to poor outcomes under GA[14,15] and thus recommend the maintenance of normocapnia (35–45 mmHg) and SBP in the range of 140–180 mmHg.[17] In our study, we found no evidence of difficulty maintaining SBP in these patients. Indeed, most of these patients were hypertensive at presentation and several needed antihypertensive agents to lower intraprocedural SBP. It is surprising to note that higher blood pressures were associated with worse outcomes in this study. This may be related to more severe strokes or lower recanalisation rates. However, this factor was not significant in the multivariable analysis.
Excessive mechanical ventilation under GA can result in hypocapnia, which in turn may lead to cerebral vasoconstriction and exacerbate poor outcome in the setting of AIS. It is assumed that patients under CS with spontaneous ventilation maintain normocapnia, which might avoid the possibility of cerebral vasoconstriction and yield better clinical outcomes. In our study, we are not able to determine the effect of CO2 level as arterial lines were not placed for most patients. Furthermore, end-tidal CO2 (EtCO2) measurements from the nasal cannula or the non-rebreather simple oxygen mask used during CS are only indicative of adequate ventilation and do not accurately quantify CO2.
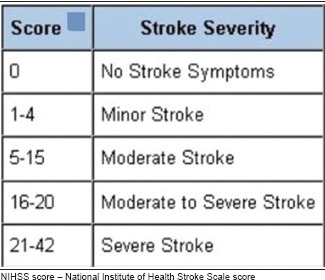
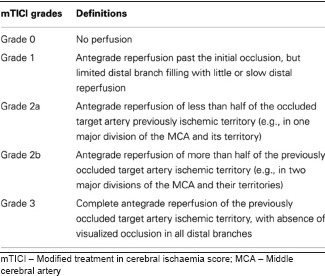
Our finding of the association of higher NIHSS score and the poor outcomes in these patients is not surprising. NIHSS score is known to be a strong predictor of functional outcome after AIS [Appendix 1].[18,19] TICI score is the most widely accepted scale to assess the extent of reperfusion after the mechanical thrombectomy and has a proven value for predicting clinical outcome after AIS [Appendix 2].[20,21] As expected, we found an association between poor outcome at discharge and lower mTICI score. Another significant finding in our study is that previous TIA is independently associated with poor outcome. The existing literature on this association is mixed. Some authors have suggested that a history of TIA confers neuroprotection due to ischaemic preconditioning, resulting in better outcomes following AIS in patients with a history of previous TIA.[22,23,24] Others, like us, have suggested that previous TIA leads to poor outcome.[25] Additional factors such as the subtype of stroke or the duration, location and timing of previous TIA might influence the association between these factors.
The overall outcomes in this cohort were worse than those reported in the recent thrombectomy trials.[26,27] This is likely related to several factors. Many of these patients were treated with older generation devices with lower recanalisation rates. In addition, patient selection was generally based on the presence of a large vessel occlusion, onset within 6 h and the absence of extensive early ischaemic changes on CT. These inclusion criteria are similar to those used in MR CLEAN[7] and these outcomes are similar.
Our study has several limitations. First, this is a single centre retrospective study with associated risk of bias. Second, sample size is relatively small due to the lack of widespread adoption of mechanical thrombectomy prior to the publication of positive trials, and therefore this study is likely underpowered to eliminate all clinically important associations between the anaesthetic factors and the neurologic outcomes. Third, some data are not universally available or of desired quality. For example, onset-to-reperfusion time and initial SBP are generally unavailable in patients transferred from outside hospitals, while EtCO2 PaCO2 were not measured in all patients, and intra-procedural blood pressures were recorded only every 10 min. Fourth, CS is generally administered by the neurointerventional team rather than by anaesthesiologists. Fifth, variations in treatment methods, including specific device used, may have influenced outcomes. Sixth, our classification and analysis of the mRS (0–2) as good outcomes resulted in a questionable validity of our model because of smaller sample size. Most clinicians agree that a mRS 0–2 reflects better the reality of a good outcome. However, there are other articles which have taken mRS (0–3) as good outcomes based on the functional independence [Appendix 3].[28,29] Finally, the method of patient selection in the early part of the review period may not reflect current practices. At our hospital, for instance, thrombectomy was considered experimental prior to publication of positive thrombectomy trials and was generally performed only for profoundly affected patients that did not qualify for other treatments, which likely skews our data towards poorer outcomes.
With all the limitations mentioned above, we conclude that no specific factor regarding CS could be identified as having a significant effect on the patient's outcome. It may be that CS has minimal physiological effects on the body. Larger multicentre prospective studies should be conducted in this area to enlighten the impact of anaesthetics on these patients.
CONCLUSION
In our study, good outcome in patients undergoing endovascular treatment for AIS under CS is associated with higher post-treatment mTICI score, lower NIHSS score at presentation and a lack of previous TIA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We would like to thank Dr. Adams Wallace for providing the list of patients, Dr. Javon Edgecombe, Dr. Thomas Madaelil for collecting the data and Dr. Colin Derdeyn for his valuable comments on the manuscript.
Appendix 1
Appendix 2
Appendix 3
REFERENCES
- 1.Hussain M, Moussavi M, Korya D, Mehta S, Brar J, Chahal H, et al. Systematic review and pooled analyses of recent neurointerventional randomized controlled trials: Setting a new standard of care for acute ischaemic stroke treatment after 20 Years. Interv Neurol. 2016;5:39–50. doi: 10.1159/000442355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischaemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischaemic stroke. N Engl J Med. 2015;372:1019–30. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–95. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 5.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischaemic stroke. N Engl J Med. 2015;372:2296–306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 6.Bekelis K, Missios S, MacKenzie TA, Tjoumakaris S, Jabbour P. Anaesthesia technique and outcomes of mechanical thrombectomy in patients with acute ischaemic stroke. Stroke. 2017;48:361–6. doi: 10.1161/STROKEAHA.116.015343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berkhemer OA, van den Berg LA, Fransen PS, Beumer D, Yoo AJ, Lingsma HF, et al. MR CLEAN investigators. The effect of anesthetic management during intra-arterial therapy for acute stroke in MR CLEAN. Neurology. 2016;87:656–64. doi: 10.1212/WNL.0000000000002976. [DOI] [PubMed] [Google Scholar]
- 8.Just C, Rizek P, Tryphonopoulos P, Pelz D, Arango M. Outcomes of general anaesthesia and conscious sedation in endovascular treatment for stroke. Can J Neurol Sci. 2016;43:655–8. doi: 10.1017/cjn.2016.256. [DOI] [PubMed] [Google Scholar]
- 9.Ouyang F, Chen Y, Zhao Y, Dang G, Liang J, Zeng J. Selection of patients and anesthetic types for endovascular treatment in acute ischaemic stroke: A meta-analysis of randomized controlled trials. PLoS One. 2016;11:e0151210. doi: 10.1371/journal.pone.0151210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abou-Chebl A, Yeatts SD, Yan B, Cockroft K, Goyal M, Jovin T, et al. Impact of general anaesthesia on safety and outcomes in the endovascular arm of Interventional Management of Stroke (IMS) III trial. Stroke. 2015;46:2142–8. doi: 10.1161/STROKEAHA.115.008761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van den Berg LA, Koelman DL, Berkhemer OA, Rozeman AD, Fransen PS, Beumer D, et al. Type of Anaesthesia and differences in clinical outcome after intra-arterial treatment for ischaemic stroke. Stroke. 2015;46:1257–62. doi: 10.1161/STROKEAHA.115.008699. [DOI] [PubMed] [Google Scholar]
- 12.Brinjikji W, Murad MH, Rabinstein AA, Cloft HJ, Lanzino G, Kallmes DF. Conscious sedation versus general Anaesthesia during endovascular acute ischaemic stroke treatment: A systematic review and meta-analysis. AJNR Am J Neuroradiol. 2015;36:525–9. doi: 10.3174/ajnr.A4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li F, Deshaies EM, Singla A, Villwock MR, Melnyk V, Gorji R, et al. Impact of Anaesthesia on mortality during endovascular clot removal for acute ischaemic stroke. J Neurosurg Anesthesiol. 2014;26:286–90. doi: 10.1097/ANA.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 14.John S, Thebo U, Gomes J, Saqqur M, Farag E, Xu J, et al. Intra-arterial therapy for acute ischaemic stroke under general Anaesthesia versus monitored Anaesthesia care. Cerebrovasc Dis. 2014;38:262–7. doi: 10.1159/000368216. [DOI] [PubMed] [Google Scholar]
- 15.Löwhagen Hendén P, Rentzos A, Karlsson JE, Rosengren L, Sundeman H, Reinsfelt B, et al. Hypotension during endovascular treatment of ischaemic stroke is a risk factor for poor neurological outcome. Stroke. 2015;46:2678–80. doi: 10.1161/STROKEAHA.115.009808. [DOI] [PubMed] [Google Scholar]
- 16.Schönenberger S, Uhlmann L, Hacke W, Schieber S, Mundiyanapurath S, Purrucker JC, et al. Effect of conscious sedation vs general anaesthesia on early neurological improvement among patients with ischaemic stroke undergoing endovascular thrombectomy: A randomized clinical trial. JAMA. 2016;16:1986–96. doi: 10.1001/jama.2016.16623. [DOI] [PubMed] [Google Scholar]
- 17.Talke PO, Sharma D, Heyer EJ, Bergese SD, Blackham KA, Stevens RD. Society for Neuroscience in Anaesthesiology and Critical Care Expert consensus statement: Anesthetic management of endovascular treatment for acute ischaemic stroke: Endorsed by the Society of NeuroInterventional Surgery and the Neurocritical Care Society. J Neurosurg Anesthesiol. 2014;26:95–108. doi: 10.1097/ANA.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 18.Adams HP, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, et al. Baseline NIHSS Scale score strongly predicts outcome after stroke. A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST) Neurology. 1999;53:126–31. doi: 10.1212/wnl.53.1.126. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed R, Zuberi BF, Afsar S. NIHSS scale score and early prediction of outcome after stroke. J Coll Physicians Surg Pak. 2004;14:267–9. [PubMed] [Google Scholar]
- 20.Kleine JF, Wunderlich S, Zimmer C, Kaesmacher J. Time to redefine success? mTICI 3 versus mTICI 2b recanalization in middle cerebral artery occlusion treated with thrombectomy. J Neurointerv Surg. 2017;9:117–121. doi: 10.1136/neurintsurg-2015-012218. [DOI] [PubMed] [Google Scholar]
- 21.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Cerebral Angiographic Revascularization Grading (CARG) Collaborators; STIR Revascularization working group; STIR modified treatment in cerebral ischaemia (mTICI) Task Force. Recommendations on angiographic revascularization grading standards for acute ischaemic stroke: A consensus statement. Stroke. 2013;44:2650–63. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.German Stroke Study Collaboration. Weber R, Diener HC, Weimar C. Why do acute ischaemic stroke patients with a preceding transient ischaemic attack present with less severe strokes? Insights from the German Stroke Study. Eur Neurol. 2011;66:265–70. doi: 10.1159/000331593. [DOI] [PubMed] [Google Scholar]
- 23.Arboix A, Cabeza N, García-Eroles L, Massons J, Oliveres M, Targa C, et al. Relevance of transient ischaemic attack to early neurological recovery after nonlacunar ischaemic stroke. Cerebrovasc Dis. 2004;18:304–11. doi: 10.1159/000080356. [DOI] [PubMed] [Google Scholar]
- 24.Moncayo J, de Freitas GR, Bogousslavsky J, Altieri M, van Melle G. Do transient ischaemic attacks have a neuroprotective effect? Neurology. 2000;54:2089–94. doi: 10.1212/wnl.54.11.2089. [DOI] [PubMed] [Google Scholar]
- 25.Hoshino T, Mizuno S, Shimizu S, Uchiyama S. Clinical features and functional outcome of stroke after transient ischaemic attack. J Stroke Cerebrovasc Dis. 2013;22:260–6. doi: 10.1016/j.jstrokecerebrovasdis.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Campbell BC, Hill MD, Rubiera M, Menon BK, Demchuk A, Donnan GA, et al. Safety and efficacy of solitaire stent thrombectomy: Individual patient data meta-analysis of randomized trials. Stroke. 2016;47:798–806. doi: 10.1161/STROKEAHA.115.012360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carvalho A, Cunha A, Rodrigues M, Figueiredo S, Paredes L, Gregório T, et al. Mechanical thrombectomy in acute ischaemic stroke: Initial single-center experience and comparison with randomized controlled trials. J Stroke Cerebrovasc Dis. 2017;26:589–94. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.116. [DOI] [PubMed] [Google Scholar]
- 28.Naidech AM, Beaumont JL, Berman M, Francis B, Liotta E, Maas MB, et al. Dichotomous “Good Outcome” indicates mobility more than cognitive or social quality of life. Crit Care Med. 2015;43:1654–9. doi: 10.1097/CCM.0000000000001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potter JF, Robinson TG, Ford GA, Mistri A, James M, Chernova J, et al. Controlling hypertension and hypotension immediately post-stroke (CHHIPS): A randomised, placebo-controlled, double-blind pilot trial. Lancet Neurol. 2009;8:48–56. doi: 10.1016/S1474-4422(08)70263-1. [DOI] [PubMed] [Google Scholar]