Abstract
Background and Aims:
Post-dural puncture headache (PDPH) is a consequence of spinal and epidural anaesthesia in approximately 1% of obstetric patients. The gold standard for its treatment is epidural blood patch. Sphenopalatine ganglion block (SPGB) has been proposed as a non-invasive intervention with minimal adverse effect. The primary objective of this study was to assess the efficacy of SPGB for treatment of PDPH. Secondary objectives were to assess onset of analgesia, duration of block and adverse effects.
Methods:
Twenty parturients diagnosed to have PDPH, resistant to standard treatment modalities such as intravenous fluids, abdominal binder, bed rest and caffeine, were recruited into this prospective observational study. Patients were allocated to either of the two groups. Group A patients received paracetamol 1 g 8 hourly intravenously for a day. If adequate pain relief was not achieved, diclofenac 75 mg 12 hourly was added. Patients in group B received SPGB with 2% lignocaine. Fisher's exact test, Mann–Whitney test and independent sample t-test were used for statistical analysis.
Results:
About 88.89% patients in group B had adequate pain relief within 5 min of block (P < 0.001). Pain was significantly lower in Group B for up to 8 h, with no adverse effects.
Conclusion:
SPGB is an effective initial modality for managing severe headache in patients with PDPH.
Key words: Analgesia, obstetric, post-dural puncture headache, sphenopalatine ganglion block, spinal
INTRODUCTION
Post-dural puncture headache (PDPH) is a consequence of spinal and epidural anaesthesia.[1] The gold standard for its treatment is epidural blood patch (EBP).[2] Therapeutic EBP has a success rate ranging from 68% to 90%.[3] But it is associated with sequelae such as subdural haematoma,[4] infection, meningitis and delayed radicular pain.[5] Sphenopalatine ganglion block (SPGB), a non-invasive intervention with minimal adverse effects and high efficacy, had been tried as a treatment modality of PDPH.[1,6,7,8] SPGB efficacy has been proved in the management of migraine[9] and facial pain.[10] There are a number of case reports and case series reporting the success of SPGB for the management of spinal headache in obstetric patients.[8,11] We hypothesised that administration of SPGB in patients with PDPH would reduce the severity of pain and might prove as a useful adjunct to the traditional treatment with oral or parenteral analgesics in these subset of patients.
METHODS
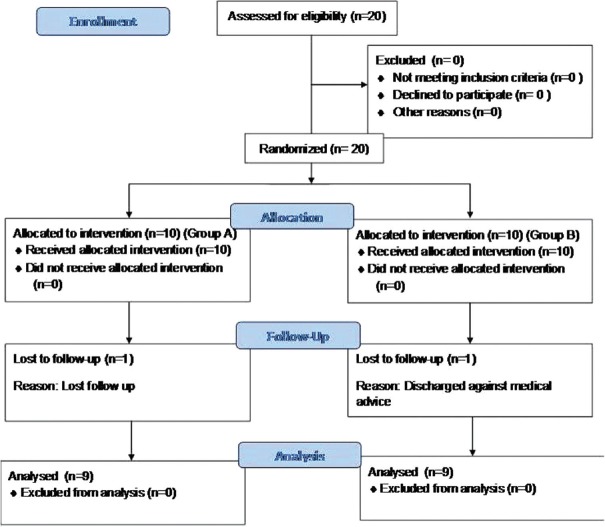
After approval from hospital ethical committee (IEC/AIMS/2016/ANES/109), this prospective unblinded observational study was conducted on 20 obstetric patients from March 2016 to September 2017. All postoperative caesarean patients received oral paracetamol 650 mg 6 hourly as per the postsurgical protocol. But when patients had PDPH, anaesthesiologists were informed. Patients with active PDPH within 7 days after subarachnoid block not relieved with standard treatment such as intravenous fluids, abdominal binder, bed rest and caffeine were recruited into the study. Patients with known coagulopathy, nasal septal deviation, polyp, history of nasal bleeding and allergy to local anaesthetics received traditional medical management only as giving sphenopalatine block (SPGB) in these patients could be technically difficult or hazardous. Of the two consultants in the obstetric unit in our institute, one of them was treating PDPH with SPGB and the other was treating it with conservative measures. So, the present study was planned as an observational study to compare the efficacy of these two existing practices in the institute in relieving PDPH. After obtaining an informed consent, patients were allocated equally to either of the two groups, A and B [Figure 1]; there was no randomisation or blinding. Group A patients received paracetamol 1 g thrice daily intravenously for a day. If adequate pain relief was not achieved, intravenous diclofenac 75 mg twice daily was added. Patients in group B received spheno-palatine block, which was performed in the intensive care unit. Monitors like non-invasive blood pressure and saturation probe were attached to the patient. SPGB was performed by a transnasal approach. Few drops of lidocaine 2% was instilled into both anterior nares. Then a cotton-tipped applicator soaked in 2% lignocaine was passed through both the nares and the end of the applicator tip was positioned just superior to the middle turbinate and anterior to the pterygopalatine fossa and sphenopalatine ganglion for 5 min with the patient in supine position [Figures 2 and 3]. After 5 min, the patient was asked to sit up and presence of headache was assessed using numeric pain score (NRS), (0 – no pain to 10 – worst pain imaginable). If the pain score remains >4 in group B after 2 h, intravenous paracetamol 1 g 8 hourly was administered and diclofenac 75 mg 12 hourly was added, if required. Patients in both the groups without adequate pain relief for 3 days were considered for EBP. Pain was assessed before procedure, 30 min, 1, 2, 4, 6, 8, 12, and 24 h after the procedure. Size of spinal needle used, heart rate (HR) and mean arterial pressure (MAP) were documented at the same time points. The primary objective was to study the efficacy of SPGB for treatment of PDPH as assessed by reduction in pain score to <4. The secondary objectives were assessment of onset and duration of analgesia as well as development of any adverse effects associated with the block.
Figure 1.
Flow chart
Figure 2.
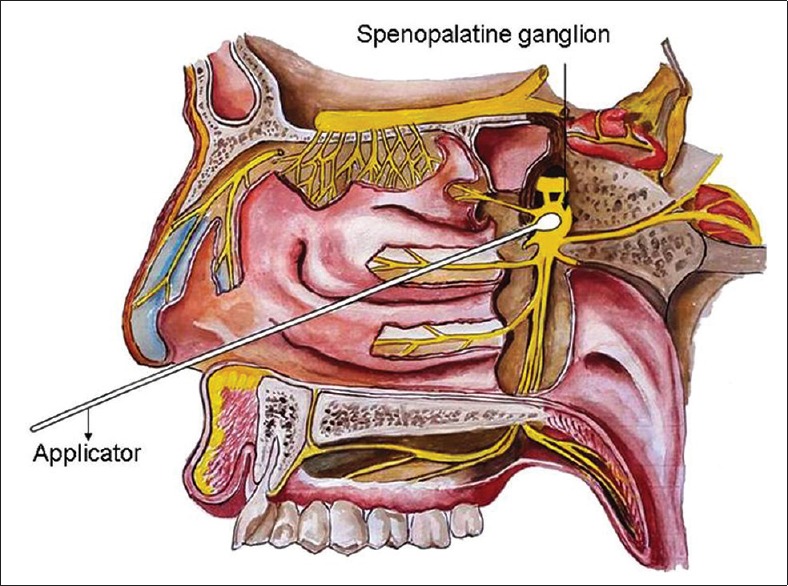
Diagrammatic representation of sphenopalatine ganglion block
Figure 3.
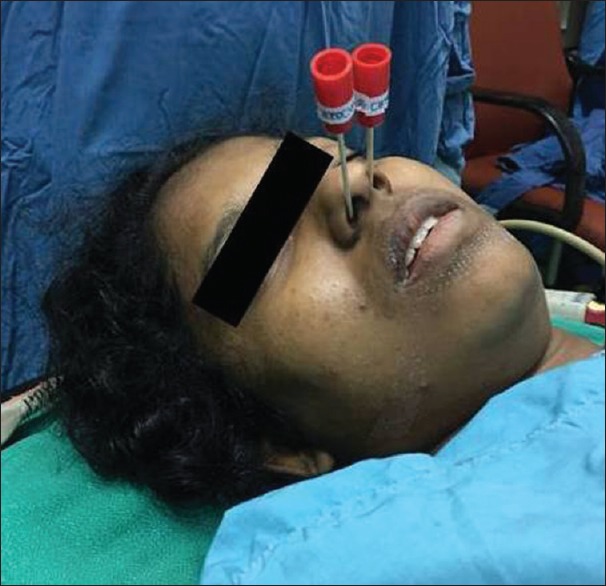
Patient receiving sphenopalatine ganglion block
As there was no similar publication available in the existing literature at the time of commencing the study, a pilot study was done. Considering time taken to attain adequate analgesia (NRS <4), as primary objective in group A versus B (240 ± 84.9 vs. 4.6 ± 1.14 min) with 95% confidence interval and 90% power, the minimum sample size required to obtain statistically significant results with a P value of 0.05 was calculated as 2 per group. However, we were able to recruit 20 cases during our study period with 10 in each group. Fisher's exact test was used to compare the pain score among groups. Mann–Whitney test was used to compare the onset of analgesia among the groups. Independent sample t-test was used to compare the size of spinal needle used, MAP, HR, height, weight and age among the groups. Statistical analyses were conducted using SPSS Version 20.0 for Windows (IBM Corporation, Armonk, NY, USA) and a P value of <0.05 was considered statistically significant.
RESULTS
A total of 20 patients were recruited into this study. The patients in both the groups were comparable with respect to the distribution of age, height, weight and American Society of Anesthesiologists' physical status. Preprocedural pain scores were comparable between the groups with a P value of 0.528. In group A, no patients had adequate pain relief (NRS <4) in 30 min after initiation of the study, whereas in group B, eight of nine patients (89.99%) had adequate pain relief during that time. In group A, the median pain score was ≥4 up to 2 h and from 4–24 h the median pain score remained <4. In group B after the block was performed, the median pain score was <4 up to 4 h and then rose to 4 at 6 h and subsequently it was maintained at <4 throughout the study period. While comparing the median pain score, it was seen that from 30 min to 4 h, group A had significantly higher pain score, whereas from 6 to 8 h, group A patients had significantly lower pain score than group B (P < 0.05). Though the trend remained the same from 8 to 12 h, the difference was not statistically significant (P > 0.05). Median was also used to analyse pain score, other than mean, as most of patients in Group B had a pain score of zero.
On comparing the mean pain scores between the two groups, the mean pain score in group A dropped gradually and reached a value <4 after 4 h and thereafter was maintained at that level, whereas in group B after the block was performed, the median pain score was ≤4 throughout the study period [Figure 4]. Onset of analgesia was significantly quicker in group B as compared to group A [4.1 ± 1.1 vs. 206 ± 90.6 min, P < 0.001, Table 1].
Figure 4.
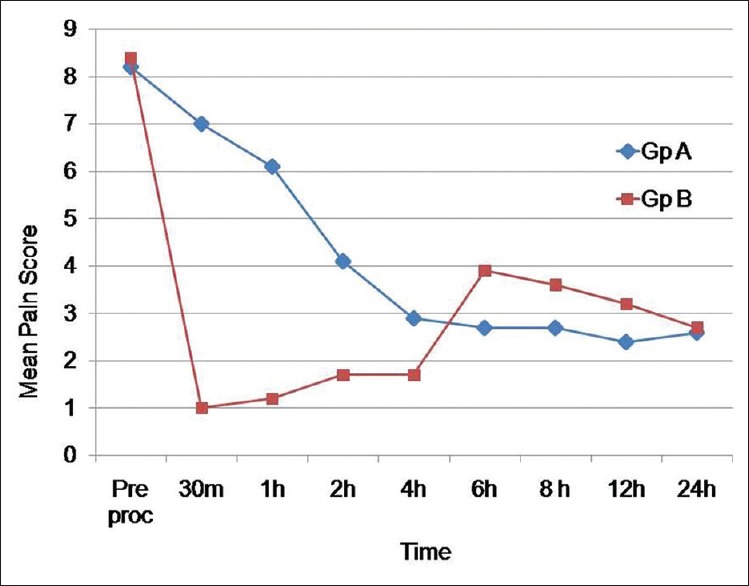
Mean pain score
Table 1.
Comparison of pain score, onset of analgesia and mean arterial pressure between groups
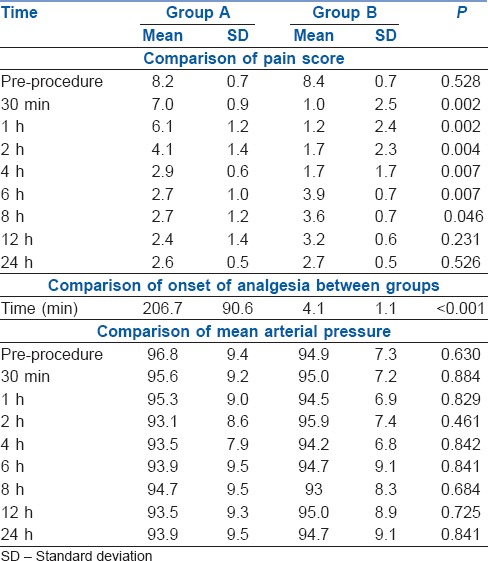
The baseline HR and MAP did not show any significant difference between the groups. HR and mean arterial pressure were compared between the two groups but the difference was not found to be statistically significant at any time point with a P value of >0.005 [Table 1 and Figure 5]. The sizes of the spinal needle used in both groups were comparable (P > 0.05).
Figure 5.
Changes in heart rate
DISCUSSION
The results of our study suggest that SPGB could be effectively used as an initial modality in the treatment of PDPH for rapid control of severe pain. Though statistical analysis revealed lower pain scores in group A after 4 h, SPGB was found to provide adequate pain relief with NRS <4 throughout the study period. Majority of patients in group B did not require rescue analgesic up to 6 h. But the intensity of pain that developed later was less and managed well by intravenous medications showing that SPGB is an effective initial modality for managing PDPH. None of the patients in our study group had any adverse effect associated with the block. PDPH is a complication that arises after accidental dural puncture while locating epidural space, following spinal anaesthetic, especially with use of large gauge cutting needles or with multiple attempts.[8,12,13] Obstetric patients are at greater risk because of gender predisposition, younger age and greater exposure to neuraxial techniques.[14,15,16,17] It usually resolves spontaneously but may interfere with the mother's ability to take care of herself and baby. It may also extend the length of hospital stay. In some patients, the headache lasts for months or even years[18] and if not properly managed could evolve into chronic headache.[14] A rare life-threatening complication of an untreated PDPH, caused by traction on bridging cerebral veins, is the development of intracranial subdural haematoma.[15,16] The distress the parturient experiences when symptoms are severe justifies active management of the pain.[15]
SPGB has been widely used in the treatment of migraine headaches, cluster headache, trigeminal neuralgia and orofacial pain.[17] Though EBP is considered the gold standard for the treatment of PDPH[19] with a success rate of around 75%,[12,15] it is associated with complications such as bleeding, infection and neurological sequelae. Neurological complications include motor and sensory deficits, meningitis, hearing loss, Horner's syndrome and subdural haematoma. EBP could itself cause another accidental dural puncture. Some patients may require a second EBP if the first one fails. SPGB may be a safer alternative in the treatment of PDPH.
Sphenopalatine ganglion is an extracranial parasympathetic ganglion about 5 mm in size located in the pterigopalatine fossa, posterior to the middle nasal turbinate and anterior to the pterigoid canal.[11] It has sympathetic, parasympathetic and somatic sensory roots. Only the preganglionic parasympathetic fibres synapse within the ganglion. Post-ganglionic sympathetic neurons along with somatic sensory fibres from the maxillary division of trigeminal nerve pass through the ganglion. All these fibres are blocked by SPGB.[17]
According to Monro–Kellie hypothesis, the sum of the volumes of the brain, cerebrospinal fluid and blood in the intracranial compartment remains constant. If the volume of one constituent reduces, the volume of another constituent must increase to maintain the equilibrium. After dural puncture, there is a continuous loss of CSF through the dural tear. So in order to maintain intracranial volume, there is compensatory vasodilation resulting in headache.[20] SPGB produces symptomatic relief by blocking the parasympathetic induced vasodilation.
The SPGB is minimally invasive, with minimal side effects, and produces good and rapid analgesia. When used as first-line treatment in the management of PDPH, it produces analgesia quicker than that produced by conservative measures. Its use can avoid the requirement for an EBP, an invasive procedure associated with complications. SPGB can be performed by transnasal, transoral, sub-zygomatic and lateral infratemporal approaches. Transnasal is the easiest, least invasive approach which can be done at bedside. Hence, we opted for this route in our study. The efficacy of SPGB in relieving pain secondary to PDPH has been well proven[1,6,7,8] and it is considered as a safe procedure as the contraindications are local nasal infections and base of skull fracture only.[7]
For performing SPGB, the patient has to lie in supine position with the neck in extension. A long applicator with a cotton swab at the tip, soaked with local anaesthetic, is inserted parallel to the floor of the nose until resistance is encountered. At this point the swab will be at the posterior pharyngeal wall superior to the middle turbinate. The applicator has to be retained for 5 min and then removed [Figures 2 and 3]. The procedure has to be repeated in the other nostril.[10] The complications associated with transnasal approach include mild discomfort during procedure, bleeding and numbness of the throat.
The conservative measures for treatment of PDPH include adopting supine position, hydration, abdominal binders, analgesics, caffeine, sumatriptan and laxatives.[17] Our findings are consistent with recent case reports in the literature on the effectiveness of the block in treatment of spinal headache. On comparing the time taken to obtain clinical effect, SPGB provided a quicker and better relief than conservative measures. Adequate pain relief was obtained with 2% lignocaine,[21] as well as ropivacaine[18] when used to perform sphenopalatine block in obstetric patients with PDPH. These patients had pain relief for 12–24 h. The longer duration of analgesia achieved could be attributed to the use of longer acting local anaesthetic such as ropivacaine. Pain relief following SPGB for management of acute headache had shown promising results.[22,23] However, the mechanism could be mechanical stimulation of sphenopalatine ganglion as well, since saline placebo also resulted in pain relief.[22] Bilateral SPGB with cotton-tipped applicator saturated with 0.5% levobupivacaine could result in optimal pain relief within 5 min. Therefore, patients presenting with PDPH should be considered primarily for SPGB and rescue EBP be used only if needed.[19]
The median onset of adequate pain relief was 240 min (minimum 60–maximum 360 min) in group A in our study. This could be because additional IV diclofenac was added to paracetamol only after 2 h, if the pain relief was inadequate, and not all patients had received diclofenac as well. During this study, we realised that there is a high incidence of PDPH, which could be because our unit is a high volume teaching institute with large volume of caesarean sections being done mainly under regional anaesthesia. One-year audit (1120 cases) was performed following the present study and a change of practice was decided upon aiming to reduce the incidence of PDPH by mainly changing the type of spinal needle used from Quincke needle to Whitacre.
Limitations of our study were that it was nonrandomised. It was not a blinded study as patients, anaesthetist performing the block and the person collecting the data were aware of the group allocated. It was not registered in Clinical Trial Registry as it was conducted as an observational study. We could have increased the duration of analgesia by either increasing the concentration of lignocaine to 4% or by using longer acting local anaesthetic such as bupivacaine or by repeating the block. When the block had failed or on return of the pain, block could have been repeated or IV analgesics given earlier to produce better patient comfort and satisfaction. The block could have been done on the bedside to reduce discomfort to the patient.
CONCLUSION
SPGB is an effective initial modality for managing severe headache in patients with PDPH.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors acknowledge Dr. M. Gopalan for pictorial representation of sphenopalatine ganglion block.
REFERENCES
- 1.Nair AS, Rayani BK. Sphenopalatine ganglion block for relieving postdural puncture headache: Technique and mechanism of action of block with a narrative review of efficacy. Korean J Pain. 2017;30:93–7. doi: 10.3344/kjp.2017.30.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atim A, Ergin A, Yanarates O, Kuyumcu M, Kurt E. Epidural blood patch for the management of post-dural puncture headache. J Nervous Sys Surgery. 2009;2:67–71. [Google Scholar]
- 3.Oedit R, van Kooten F, Bakker S, Dippel D. Efficacy of the epidural blood patch for the treatment of post lumbar puncture headache BLOPP: A randomised, observer-blind, controlled clinical trial. BMC Neurol. 2005;5:12. doi: 10.1186/1471-2377-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies JM, Murphy A, Sullivan GO, Smith M. Subdural hematoma after dural puncture headache treated by epidural blood patch. Br J Anaesth. 2001;86:720–3. doi: 10.1093/bja/86.5.720. [DOI] [PubMed] [Google Scholar]
- 5.Desai MJ, Dave AP, Martin MB. Delayed radicular pain following two large volume epidural blood patches for post-lumbar puncture headache: A case report. Pain Physician. 2010;13:257–62. [PubMed] [Google Scholar]
- 6.Cohen S, Levin D, Mellender S, Zhao R, Patel P, Grubb W, et al. Topical Sphenopalatine Ganglion Block Compared With Epidural Blood Patch for Postdural Puncture Headache Management in Postpartum Patients: A Retrospective Review. Reg Anesth Pain Med. 2018;43:880–884. doi: 10.1097/AAP.0000000000000840. [DOI] [PubMed] [Google Scholar]
- 7.Channabasappa SM, Manjunath S, Bommalingappa B, Ramachandra S, Banuprakash S. Transnasal sphenopalatine ganglion block for the treatment of postdural puncture headache following spinal anesthesia. Saudi J Anaesth. 2017;11:362–3. doi: 10.4103/sja.SJA_59_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furtado I, Lima IF, Pedro S. Ropivacaine use in transnasalsphenopalatine ganglion block for post dural puncture headache in obstetric patients - case series. Rev Bras Anestesiol. 2018;68:421–4. doi: 10.1016/j.bjane.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cady R, Saper J, Dexter K, Manley HR. A double-blind, placebo-controlled study of repetitive transnasalsphenopalatine ganglion blockade with tx360® as acute treatment for chronic migraine. Headache. 2015;55:101–16. doi: 10.1111/head.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Candido KD, Massey ST, Sauer R, Darabad RR, Knezevic NN. A novel revision to the classical transnasal topical sphenopalatine ganglion block for the treatment of headache and facial pain. Pain Physician. 2013;16:E769–78. [PubMed] [Google Scholar]
- 11.Kent S, Mehaffey G. Trasnasalsphenopalatine ganglion block for the treatment of postdural puncture headache in obstetric patients. J Clin Anaesth. 2016;34:194–6. doi: 10.1016/j.jclinane.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Boonmak P, Boonmak S. Epidural blood patching for preventing and treating post-dural puncture headache. Cochrane Database Syst Rev. 2010:CD001791. doi: 10.1002/14651858.CD001791.pub2. doi: 10.1002/14651858.CD001791.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Turnbull DK, Shepherd DB. Post-dural puncture headache: Pathogenesis, prevention and treatment. Br J Anaesth. 2003;91:718–29. doi: 10.1093/bja/aeg231. [DOI] [PubMed] [Google Scholar]
- 14.Cardoso JM, Sa M, Gra ca R, Reis H, Almeida L, Pinheiro C, et al. Sphenopalatine ganglion block for postdural puncture headache in ambulatory setting. Rev Bras Anestesiol. 2017;67:311–3. doi: 10.1016/j.bjan.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Khonsary SA, Ma Q, Villablanca P, Emerson J, Malkasian D. Clinical functional anatomy of the pterygopalatine ganglion, cephalgia and related dysautonomias: A review. Surg Neurol Int. 2013;4:S422–8. doi: 10.4103/2152-7806.121628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mokri B. The Monro-Kellie hypothesis: Applications in CSF volume depletion. Neurology. 2001;56:1746–8. doi: 10.1212/wnl.56.12.1746. [DOI] [PubMed] [Google Scholar]
- 17.Windsor RE, Jahnke S. Sphenopalatine ganglion blockade: A review and proposed modification of the transnasal technique. Pain Physician. 2004;7:283–6. [PubMed] [Google Scholar]
- 18.Vallejo MC, Mandell GL, Sabo DP, Ramanathan S. Ropivacaine use in transnasalsphenopalatine ganglion block for post dural puncture headache in obstetric patients-case series. Anesth Analg. 2000;91:916–20. doi: 10.1097/00000539-200010000-00027. [DOI] [PubMed] [Google Scholar]
- 19.Thew M, Paech MJ. Management of postdural puncture headache in the obstetric patient. Curr Opin Anaesthesiol. 2008;21:288–92. doi: 10.1097/ACO.0b013e3282f8e21a. [DOI] [PubMed] [Google Scholar]
- 20.Schaffer JT, Hunter BR, Ball KM, Weaver CS. Noninvasivesphenopalatine ganglion block for acute headache in the emergency department: A randomized placebo-controlled trial. Ann Emerg Med. 2015;65:503–10. doi: 10.1016/j.annemergmed.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Kent S, Mehaffey G. Trasnasalsphenopalatine ganglion block for the treatment of postdural puncture headache in the ED. Am J Emerg Med. 2015;1714:e1–2. doi: 10.1016/j.ajem.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Lux EA, Althaus A. Is there a difference in postdural puncture headache after continuous spinal anesthesia with 28g microcatheters compared with punctures with 22g Quincke or sprotte spinal needles? Local Reg Anesth. 2014;7:63–7. doi: 10.2147/LRA.S68828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwak K-H. Postdural puncture headache. Korean J Anesthesiol. 2017;70:136–43. doi: 10.4097/kjae.2017.70.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]