Abstract
Aim: Arsenic trioxide (As2O3), known as pi-shuang and the most toxic compound in traditional Chinese medicine, has been used as an antitumor agent for thousands of years. Resveratrol (3,5,4’-trihydroxy-trans-stilbene) is a natural phenol that has significant anti-bacterial, anti-fungaI and antiaging activities. Our study aimed to examine the combined anticancer effects of As2O3 and resveratrol against human neuroblastoma SK-N-SH cells, and elucidate the underlying intracellular signaling. Materials and Methods: SK-N-SH cells were treated with an extremely low-dose (2-4 μM) of As2O3 alone or combined with 75 μg/ml resveratrol for further comparisons. Cell viability, apoptotic signaling as well as synergistic cytotoxic effects were estimated using the MTT assay, microscopy observation, flow cytometric analysis for loss of mitochondrial membrane potential (MMP) and reactive oxygen species (ROS), and typical quantitative western blotting analysis. Student’s t-test, and one- and two-way analysis of variance (ANOVA) were used for examination of significant differences. Results: The combined treatment was more effective than single treatment of As2O3 or resveratrol alone in suppressing cell viability, which correlated with the elevation of ROS levels. The intracellular mechanisms of cytotoxicity of As2O3 plus resveratrol were revealed as ROS accumulation and relative decrease of MMP, leading to activation of caspase-3 and -9, but not of caspase-1, -7 and-8. Combination treatment reduced the expression of B-cell lymphoma 2 (BCL2), BH3 interacting domain death agonist (BID), and BCL-x/L. Conclusion: Combined treatment at extremely low concentration of two agents from natural products, As2O3 and resveratrol, has high potential as a cocktail of anticancer drugs for neuroblastoma.
Keywords: Apoptosis, arsenic trioxide, cytotoxicity, neuroblastoma, resveratrol
Neuroblastoma is listed as the most fatal cancer among children worldwide, and exhibits a very complex biological and tremendous clinical heterogeneity (1). Remarkably, neuroblastoma is renowned for having no obvious environmental or genetic risk factors, no effective drugs, and low 5-year survival, with parents and relatives of the pediatric patient consequently suffering from much emotional and psychological pain. Clinically, the age and gender of the patient, and the stage and molecular defects are key determinants for the efficacy of treatments, prognosis and modalities of therapy. At the time of diagnosis, about 70% of patients present with distant metastasis and infants are more likely to have better prognosis than are young children (2). Standard treatment for neuroblastoma involves radiation and chemotherapy, which are continuously and rapidly being updated; however, their overall efficacy is far from satisfying. Unlike lung cancer, chemotherapeutic agents for neuroblastoma still rely on traditional pan-anticancer drugs, such as cisplatin, doxorubicin, etoposide and cyclophosphamide, without any novel drug development. In addition, the side-effects of these drug treatments for children with neuroblastoma can be extremely serious due to both acute damage and toxicity and increased occurrence of secondary tumors (3). Furthermore, even with aggressive treatment, the overall mortality remains very high in more advanced stages of neuroblastoma, with less than 50% survival rate (3). Unfortunately, even with perfect care of intensive therapeutic methodology including surgery, irradiation and chemotherapy at modern hospitals, the majority of the pediatric patients over one year of age with neuroblastoma are likely to have aggressively metastatic neuroblastoma with poor clinical outcomes, some may even die within 5 years (4). Therefore, the search for new non-toxic or low-toxic drugs for neuroblastoma therapy is especially urgent.
For thousands of years, arsenic trioxide (As2O3) has been used as a toxin in Chinese tradition and as an antitumor agent in treatment of cancer (5). In the early 2000s, As2O3 was approved by the US Food and Drug Administration for application in relapsed/refractory cancer, such as acute promyelocytic leukemia, head and neck cancer, and neuroblastoma (6-8). Its mechanisms of action including elevating the intracellular level of reactive oxygen species (ROS), causing an irreversible loss of mitochondrial membrane potential (MMP), down-regulation of B-cell lymphoma 2 (BCL2) protein expression, and activation of specific caspases, together with the induction of DNA adducts and cell-cycle arrest (9,10).
Resveratrol (3,5,4’-trihydroxy-trans-stilbene) is a natural polyphenol stilbene found in grapes and certain medicinal plants. The plant-derived polyphenolic compound, which can be extracted from grape skin, some berries and peanuts, was reported to exhibit antitumor effects both in vitro (11,12) and in vivo (13). Resveratrol produces several beneficial effects, anti-aging, anti-carcinogenic, cardioprotective, and neuroprotective effects, which have been attributed to its antioxidant, anti-inflammatory, and gene-modulating properties (14). The chemopreventive and therapeutic potential of resveratrol has been demonstrated in various kinds of cancers including lung, breast, prostate and skin cancer as well as neuroblastoma (13,15-17). Resveratrol has emerged as a potential anti-tumor agent because it has the ability to modulate the cascade signaling network involved in the proliferation and survival of cancer cells (18). In neuroblastoma, resveratrol has been shown to arrest cell-cycle progression, promote mitochondrial dysfunction, activate caspases and induce programmed cell death (19).
There are two major points in designing anticancer drug investigation in cell models. The first is to find a proper control system, such as setting normal cell lines as controls, for highlighting novel findings of the efficacy of anticancer drugs. Under ideal conditions, at the dose range examined, the drug will kill cancer cells without doing any harm to normal cells. The second point is that it should be borne in mind that some toxic anticancer drugs may cause subtle, but damaging effects on normal cells, such as DNA damage to their genome. Thus, the novel combination of two natural chemotherapeutic agents at as low concentration as possible has been reported to strengthen the possible cytotoxic efficacy against cancer cells with minimal side-effects on normal cells (20-23). In this study, we aimed to examine the apoptotic effects of a novel combination of As2O3 and resveratrol on SK-N-SH cell line, and reveal the underlying signaling network in these cells.
Materials and Methods
Chemical reagents. Modified Eagle’s Minimum Essential Medium (EMEM; with 2 mM L-glutamine; Earle’s balanced salt solution containing 1.5 g/I sodium bicarbonate, 0.1 mM non-essential amino acids and 1.0 mM sodium pyruvate; 10% fetal bovine serum), trypsin, streptomycin, penicillin, and 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT) were all purchased from AllBio (Taichung, Taiwan, ROC); As2O3 and resveratrol were purchased from Sigma Co. (St. Louis, MO, USA). 2’,7’-Dichlorodihydrofluorescein diacetate (DCFH-DA), 4’,6-diamidine-2-phenylindole (DAPI) and fluorescent rhodamine123 were purchased from AllBio. All primary antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) and peroxidase-linked secondary antibody was purchased from AllBio.
Cell culture. SK-N-SH cells (American Type Culture Collection, Manassas, VA, USA) were maintained in modified EMEM. Cells were maintained in an incubator with a humidified atmosphere of 95% air and 5% CO2 at 37±1˚C. Most of the experiments were carried out 48 h after cells were seeded onto plates or dishes except the tests regarding drug treatment time.
Cell viability assay. The MTT assay was used to examine the effects of different dosages or different treatment time of specific drugs on cell proliferation or viability. Briefly, SK-N-SH cells were plated in 48-well culture plates at a density of 5×104 cells/well and allowed to adhere at 37˚C for 24 h or overnight. The following day, 0-4 μM As2O3 or 0-75 μg/ml resveratrol, alone and combination, were added to the cells and the cells were incubated for a further 48 h, after which cell growth and viability were measured by MTT assay. Wells or dishes without cells were used as blanks and were subtracted as background from each sample with drug treatment. Results were expressed as a percentage of untreated cell control group, which was set at 100% of viability or survival.
Measurement of mitochondrial membrane potential (MMP). Alterations in MMP were quantitatively followed using metric probe rhodamine123. Briefly, SK-N-SH cells pre-cultured in 6 cm tissue culture plates were treated with As2O3 alone, resveratrol alone or As2O3 plus resveratrol for 0-48 h. Twenty minutes before the cells were harvested, rhodamine123 was added directly to the culture medium to a final concentration of 30 nM. The SK-N-SH cells were then harvested by trypsinization, washed with 5 ml phosphate-buffered saline (PBS) twice at 37˚C, pelleted by centrifugation, resuspended in 500 μl fluorescence-activated cell sorting (FACS) buffer, and analyzed immediately for rhodamine123 fluorescence intensity by flow cytometry (FACS Calibur; BD Bioscience, San Jose, CA, USA). Results are expressed as a percentage of the control, which was set at MMP of 100%.
Western blotting analysis. Briefly, after treatments, the SK-N-SH cells were scraped and collected in Trident RIPA lysis buffer (GeneTex, Hsinchu, Taiwan, ROC) to an Eppendorf tube and centrifuged at 1,200 × g for 5 min at 4˚C. The proteins of each sample were quantitated and equal amounts of proteins (40 μg) were separated on 12.5% acrylamide gels by sodium dodecyl sulfate (SDS) electrophoresis and then transferred to Immobilon-P Transfer Membrane (Merck Millipore, Billerica, MA, USA). As a routine procedure, the samples non-specific binding sites were blocked with 5% dry milk in phosphate buffered saline with tween-20 (PBST), then the membranes were incubated with primary antibodies for specific proteins overnight at 4˚C. Finally, the membrane was incubated at room temperature with appropriate secondary antibody for 2 h. The membranes were treated with enhanced chemiluminescent (ECL) reagent under computer-assisted imaging analysis system (Gene Tools Match software; Syngene, CA, USA). All bands were normalized against β-actin and all the experiments were performed at least thrice while the data were quantified by Image J.
Measurement of ROS production. SK-N-SH cells were plated at a density of 2×105 cells/well into 12-well plates and incubated with As2O3 alone, resveratrol alone, or combined treatment for 0-48 h. The cells were then harvested and washed twice with PBS, re-suspended in 500 μl of DCFH-DA (10 μM), incubated at 37˚C for an extra 30 min and analyzed by flow cytometry (carried out by Dr Dai at the Instrument Center of China Medical University) to detect intracellular ROS as published elsewhere (24-26).
Statistical analyses. The data are expressed as means±standard deviation (S.D.). Statistical comparisons of different dosages for one drug and among different treatments were carried out with three or more groups using one-or two-way analysis of variance (ANOVA). In addition, Student’s t-test was used in two-group comparisons. Values of p<0.05 were considered statistically significant.
Results
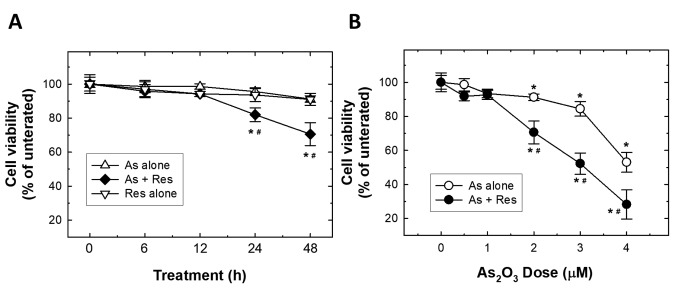
Single and combined effects of As2O3 and resveratrol on SK-N-SH cell viability. In order to evaluate the efficacy of anticancer effects of As2O3 and resveratrol, alone in and combined treatment, the cytotoxic effects of resveratrol on SK-N-SH cells were examined as a first step. Pilot experiments for resveratrol were carried out to identify a concentration of resveratrol which produced a non-significant growth inhibition in order subsequently to further evaluate its combination with As2O3. After SK-N-SH cells were treated with different doses of resveratrol for 48 h, cells were measured with the MTT assay (pilot data not shown) and from these set of data it was determined that a dose of resveratrol as low as 75 μg/ml caused no effect on cell viability for both 24 and 48 h. Very effectively, this low dose of resveratrol significantly enhanced cytotoxicity (viability of 70.6% of the control group) at 48 h when combined with an extremely low dose of 2 μM As2O3 (Figure 1A). Hence, 75 μg/ml resveratrol was selected as the dose used for the further investigation of its combination effects with different doses of As2O3 on SK-N-SH cells (Figure 1B). The results showed that 2 μM of As2O3 had cytotoxic effects on SK-N-SH cells, while 75 μg/ml resveratrol significantly enhanced the dose-dependent cytotoxic effects of As2O3 on SK-N-SH cells (Figure 1B).
Figure 1. Synergistic effects of As2O3 (As) and resveratrol (Res) on suppression of SK-N-SH cells viability. A: SK-N-SH cells were treated with 75 μg/ml resveratrol, 2 μM As2O3 or As2O3 plus resveratrol for 0-48 h and cell viability was determined applying 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay. The results represent the mean±S.D. of three independent experiments. B: Cytotoxic effect of 0, 0.5, 1,2, 3 or 4 μM of As2O3 with and without 75 μg/ml resveratrol on SK-N-SH cells at 48 h were determined by the MTT assay. Results represent the mean±S.D. of three independent experiments. Significantly different at *p<0.05 and **p<0.01.
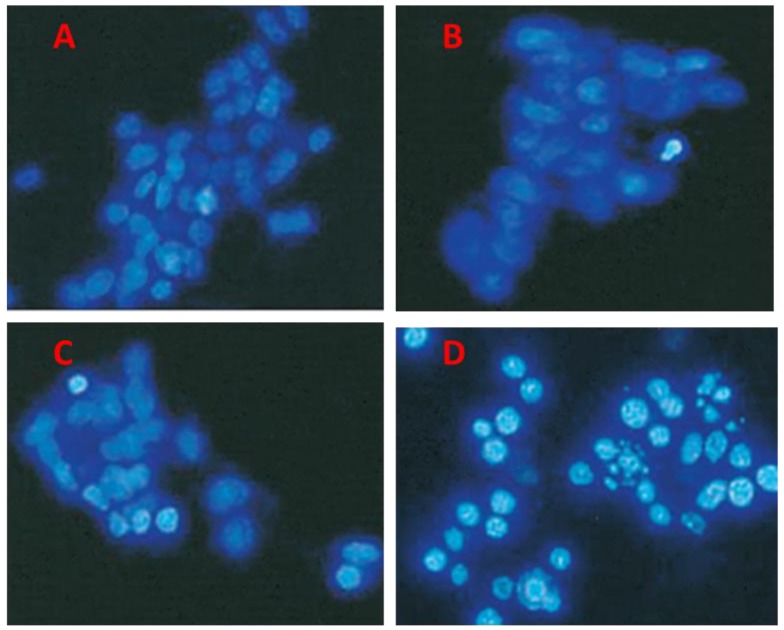
Alteration of nuclear morphology induced by combined treatment of As2O3 plus resveratrol, as observed under microscopy. After SK-N-SH cells were treated with 2 μM As2O3 alone, 75 μg/ml resveratrol alone, or their combination, nuclear morphology was examined with DAPI staining under microscopy. In contrast to the control group (Figure 2A) and single-drug-treated groups (resveratrol and As2O3 in Figure 2B and C, respectively), combined treatment led to chromatin condensation, and DNA fragmentation under fluorescent microscopy observation (Figure 2D).
Figure 2. Single and combined treatment of As2O3 and resveratrol induced alteration of nuclear morphology. SK-N-SH cells were untreated (A), or treated with 75 μg/ml resveratrol alone (B), 2 μM As2O3 alone (C) or As2O3 plus resveratrol (D) for 48 h. Chromatin condensation and nuclear fragmentation of SK-N-SH cells were observed using staining with 4’,6-diamidine-2-phenylindole and imaged under fluorescent microscopy. Representative images from triplicate experiments are shown.
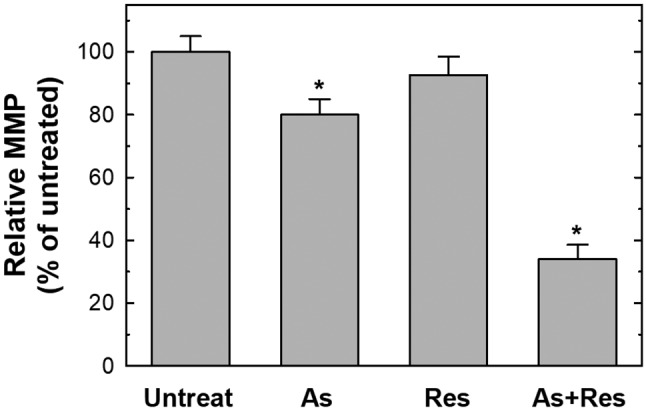
Combined treatment with As2O3 plus resveratrol induced apoptosis via the loss of MMP and alteration of the levels of apoptosis-related proteins. The loss of MMP is reported as an early event marker in activation of the mitochondrial pathway in programmed cell death, which is regulated by the balance among BCL2 family proteins. Since the loss of MMP is a critical marker in cells undergoing apoptosis, we evaluated whether the treatment of As2O3 plus resveratrol had any effect on the MMP of SK-N-SH cells by using rhodamine123, the florescent indicator for mitochondrial membrane integrity. As presented in Figure 3, there was no substantial change of the MMP after treatment with 75 μg/ml resveratrol alone. However, there was a significant loss of MMP in SK-N-SH cells after treatment with 2 μM As2O3, indicating disruption of the MMP in these cells. Interestingly, when the cells were treated with As2O3 plus resveratrol for 48 h, an obvious decrease in MMP was observed and the effects were synergistic (Figure 3).
Figure 3. Single and combined treatment with As2O3 (As) and resveratrol (Res) reduced the mitochondrial membrane potential (MMP). SK-N-SH cells were untreated, or treated with 75 μg/ml resveratrol alone, 2 μM As2O3 alone or As2O3 plus resveratrol for 48 h. After harvesting, 50 nM rhodamine123 was added to each sample, incubated for 30 min at 37˚C and MMP was analyzed with flow cytometry. The average MMP of the control group was set as 100% for statistical comparison. Results represent the mean±S.D. of three independent experiments. *Significantly different at p<0.05.
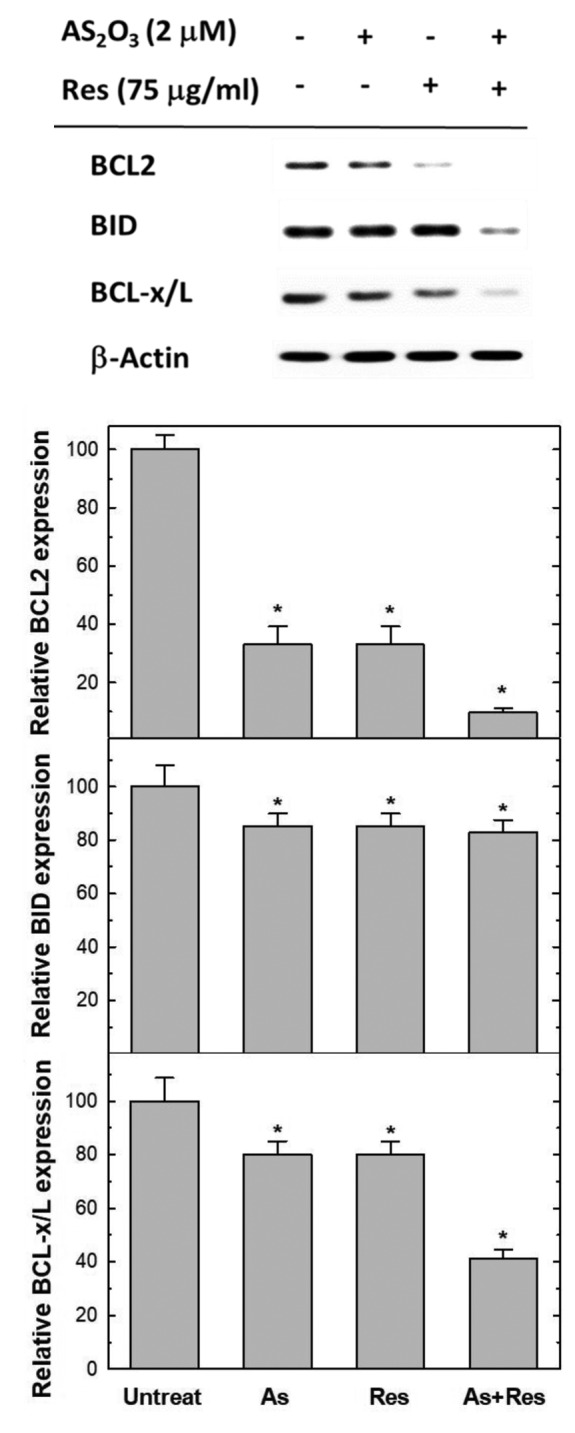
BCL2, BID and BCL-x/L have been recognized to behave as inhibitors of programmed cell death. Therefore, we determined the levels of BCL2, BID and BCL-x/L proteins to evaluate the effect of As2O3 alone, resveratrol alone and their combination on the expression of these proteins in SK-N-SH cells. The results revealed that the combined treatment with As2O3 and resveratrol resulted in a marked reduction in the levels of BCL2, BID and BCL-x/L protein, in addition to the slight effects of As2O3 alone and resveratrol alone on them. The effect of As2O3 and resveratrol was again of a synergistic type (Figure 4).
Figure 4. Single and combined treatment with As2O3 and resveratrol reduced the expression of B-cell lymphoma 2 (BCL2) family proteins. SK-N-SH cells were untreated, or treated with 75 μg/ml resveratrol alone, 2 μM As2O3 alone or As2O3 plus resveratrol for 48 h. Equal amounts of protein lysates from each sample were subjected to 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto polyvinylidene fluoride membranes, and subsequently immunoblotted with anti-BCL2, anti-BH3 interacting domain death agonist (BID), anti-B-cell lymphoma (BCL)-x/L, and anti-β-actin. The signals were visualized via enhanced chemiluminescence and quantitated with the Image J software. Results represent the mean±S.D. of three independent experiments. *Significantly different at p<0.05.
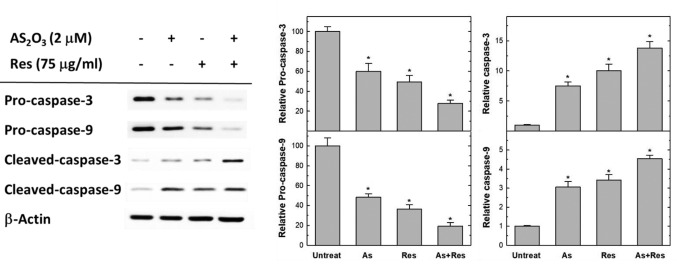
Apoptosis-related caspases were altered by combined treatment with As2O3 plus resveratrol. In order to address the role of caspase in apoptosis induced by resveratrol and As2O3, we investigated activities of caspase-3 and-9. The western blot analysis of pro-caspase-3 and pro-caspase-9 protein is illustrated in Figure 5. Combined treatment with resveratrol and As2O3 elevated the expression of pro-and cleaved forms of caspase-3 and caspase-9 proteins in SK-N-SH cells markedly. There were obviously reverse correlations for the elevation of cleaved forms of caspase-3 and caspase-9, while the ones of pro-caspase-3 and pro-caspase-9 decreased.
Figure 5. Single and combined treatment with As2O3 (As) and resveratrol (Res) altered the expression of apoptosis-related caspases. SK-N-SH cells were treated with 75 μg/ml resveratrol, 2 μM of As2O3 or drugs combined for 48 h. Equal amounts of protein lysates from each sample were subjected to 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) together with the protein markers, transferred onto polyvinylidene fluoride membranes, and subsequently blotted with specific anti-procaspase-3, anti-procaspase-9, anti-cleaved caspase-3, anticleaved caspase-9 and anti-β-actin antibodies for 2 h. The final signals for each specific protein on the same membranes were visualized by enhanced chemiluminescence kit and quantitated with the Image J software. Results represent the mean±S.D. of three independent experiments. *Significantly different at p<0.05.
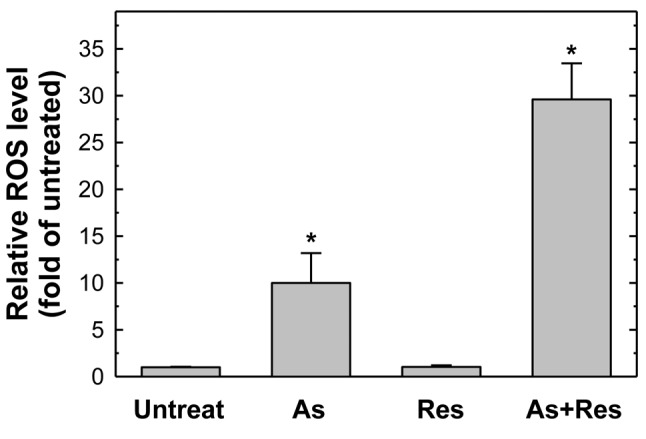
Combined treatment with As2O3 plus resveratrol resulted in elevated ROS production. The elevation of ROS in the cells may play an important role in apoptotic cells. Using the ROS-sensitive probe DCFH-DA detected by flow cytometry, it was found that As2O3 alone, but not resveratrol, elevated the production of ROS. The highlight finding is that 75 μg/ml resveratrol, which is generally considered an antioxidant, can synergistically enhance the production of ROS by 2 μM As2O3 in SK-N-SH cells (Figure 6).
Figure 6. Single and combined treatment with As2O3 and resveratrol elevated the production of intracellular reactive oxygen species (ROS). SK-N-SH cells were untreated, or treated with 75 μg/ml resveratrol alone, 2 μM As2O3 alone or As2O3 plus resveratrol for 48 h. After harvesting, cells were stained with 2’,7’-dichlorofluorescin diacetate dye and subjected to flow cytometric analysis. The average ROS level of the control group was set as 100% for statistical comparison. Results represent the mean±S.D. of three independent experiments. *Significantly different at p<0.05.
Discussion
Neuroblastoma is a childhood disease threatening the lives of almost half of the patients diagnosed (27). The elucidation of the molecular mechanisms of human neuroblastoma and potential drugs against it help us to further comprehensively understand the treatment responses and develop optimal therapeutic agents for better neuroblastoma therapy. In the late 20th century, As2O3, a traditional Chinese medicine was firstly applied for its anticancer capacity in the fight against acute promyelocytic leukemia (28). Its antitumor mechanism may lie in its activation of apoptosis-related proteins (28). In the same period, the induction of apoptosis by arsenite was observed in other types of cells including lymphocytes, fibroblasts and, most interestingly, neurons (29-31). In 2007, As2O3 was proposed for treatment of neuroblastoma, and several clinical trials have also shown that As2O3 induced cell death at clinically tolerable doses for patients with acute myeloid leukemia, myelodysplastic syndromes, multiple myeloma, chronic myelogenous leukemia and lymphoma (32). In 2012, Keim and colleagues reported that As2O3 treatment of human SH-SY5Y neuroblastoma cells led to caspase-3/7 activation and to chromatin fragmentation via the up-regulation of p53 and c-JUN proteins (33). However, the detailed mechanism is still largely unknown and whether As2O3 treatment can be effective against other neuroblastoma cells in addition to SH-SY5Y cells has not yet been examined.
There are several highlights of the current study. Firstly, we confirmed the antitumor effect of As2O3 on another human neuroblastoma cell line, SK-N-SH. Secondly, at the lowest dose of As2O3 ever published, 2 μM of As2O3, increased the ROS level and triggered the activation of apoptotic signaling pathways in SK-N-SH cells, resulting in induction of neuroblastoma cells to undergo apoptosis. Thirdly, with the novel combination with resveratrol, the overall cytotoxic effect of As2O3 was synergistically enhanced. By our group and others, As2O3 was found to induce oxidative stress in NB4 (34) and other types of cells (29,35,36). Resveratrol, an antioxidant, was not capable of inducing ROS activation in cells, but dramatically elevated the level of As2O3-induced ROS (Figure 6). Consequently, the elevation of ROS may cause the loss of MMP (Figure 4), alterations of BCL2, BID and BCL-x/L (Figure 5), and activation of caspase-3 and-9 (Figure 3). We did not find any change in the levels of caspase-6, -7, -10, and-12 by As2O3 alone nor combined with resveratrol (data not shown). In 2012, As2O3 was reported to activate the cleaved form of casepase-3 and-7. Thus, the signaling in different neuroblastoma cells by As2O3 may vary (33). The origin of such ROS is not yet known and the involvement of other signaling molecules requires greater investigation. In addition, animal experiments providing evidence for the in vivo efficacy of As2O3 plus resveratrol are urgently needed before clinical trials can be undertaken.
In summary, the application of the natural phenol resveratrol has been extended from its being a simply antioxidant, anti-aging and anti-fungal agent to adjuvant to As2O3 in neuroblastoma treatment. The effect of novel combined treatment of two natural chemotherapeutic agents, As2O3 with resveratrol, has been revealed in SK-N-SH cells and may have great potential as an anti-neuroblastoma cocktail.
Conflict of Interest
The Authors declare no conflict of interest in regard to this study.
Acknowledgements
The Authors appreciate Hsin-Ting Li, Huai-Mei Hsu, Yun-Chi Wang and Yu-Shih Wang for their excellent technical assistance. This study was supported mainly by the Taichung Veterans General Hospital to Dr. Cheng alone and Dr. Cheng and Dr. Wang (TCVGH-1074903B and TCVGH-HK-1078004, respectively) and partially by research grant from Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW107-TDU-B-212-123004).
References
- 1.Kamihara J, Ma C, Fuentes Alabi SL, Garrido C, Frazier AL, Rodriguez-Galindo C, Orjuela MA. Socioeconomic status and global variations in the incidence of neuroblastoma: call for support of population-based cancer registries in low-middle-income countries. Pediatr Blood Cancer. 2017;64:321–323. doi: 10.1002/pbc.26244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiegel B, Harris TM, Edwards MK, Smith RR, Azzarelli B. MR of intracranial neuroblastoma with dural sinus invasion and distant metastases. Am J Neuroradiol. 1991;12:1198–1200. [PMC free article] [PubMed] [Google Scholar]
- 3.Gutenberg A, Schulten HJ, Gunawan B, Ludwig HC, Bruck W, Larsen J, Rohde V. CNS tumor 22 years after spinal neuroblastoma IV: Diagnostic dilemma between recurrence and secondary malignancy. Pediatr Neurosurg. 2009;45:61–68. doi: 10.1159/000204906. [DOI] [PubMed] [Google Scholar]
- 4.Leclair MD, Hartmann O, Heloury Y, Fourcade L, Laprie A, Mechinaud F, Munzer C, Rubie H. Localized pelvic neuroblastoma: excellent survival and low morbidity with tailored therapy–The 10-year experience of the French Society of Pediatric Oncology. J Clin Oncol. 2004;22:1689–1695. doi: 10.1200/JCO.2004.04.069. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, Chen GQ, Shen ZX, Sun GL, Tong JH, Wang ZY, Chen SJ. Expanding the use of arsenic trioxide: Leukemias and beyond. Semin Hematol. 2002;39:22–26. doi: 10.1053/shem.2002.33611. [DOI] [PubMed] [Google Scholar]
- 6.Evens AM, Tallman MS, Gartenhaus RB. The potential of arsenic trioxide in the treatment of malignant disease: past, present, and future. Leuk Res. 2004;28:891–900. doi: 10.1016/j.leukres.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Jakubowicz-Gil J, Paduch R, Piersiak T, Glowniak K, Gawron A, Kandefer-Szerszen M. The effect of quercetin on pro-apoptotic activity of cisplatin in HeLa cells. Biochem Pharmacol. 2005;69:1343–1350. doi: 10.1016/j.bcp.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Kumagai T, Shih LY, Hughes SV, Desmond JC, O’Kelly J, Hewison M, Koeffler HP. 19-Nor-1,25(OH)2D2 (a novel, noncalcemic vitamin D analogue), combined with arsenic trioxide, has potent antitumor activity against myeloid leukemia. Cancer Res. 2005;65:2488–2497. doi: 10.1158/0008-5472.CAN-04-2800. [DOI] [PubMed] [Google Scholar]
- 9.Baumgartner M, Sturlan S, Roth E, Wessner B, Bachleitner-Hofmann T. Enhancement of arsenic trioxide-mediated apoptosis using docosahexaenoic acid in arsenic trioxide-resistant solid tumor cells. Int J Cancer. 2004;112:707–712. doi: 10.1002/ijc.20462. [DOI] [PubMed] [Google Scholar]
- 10.Scholz C, Wieder T, Starck L, Essmann F, Schulze-Osthoff K, Dorken B, Daniel PT. Arsenic trioxide triggers a regulated form of caspase-independent necrotic cell death via the mitochondrial death pathway. Oncogene. 2005;24:1904–1913. doi: 10.1038/sj.onc.1208233. [DOI] [PubMed] [Google Scholar]
- 11.Suh J, Kim DH, Surh YJ. Resveratrol suppresses migration, invasion and stemness of human breast cancer cells by interfering with tumor-stromal cross-talk. Arch Biochem Biophys. 2018;643:62–71. doi: 10.1016/j.abb.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Flores-Perez A, Elizondo G. Apoptosis induction and inhibition of HeLa cell proliferation by alpha-naphthoflavone and resveratrol are aryl hydrocarbon receptor-independent. Chem Biol Interact. 2018;281:98–105. doi: 10.1016/j.cbi.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 13.Song X, Shu XH, Wu ML, Zheng X, Jia B, Kong QY, Liu J, Li H. Postoperative resveratrol administration improves prognosis of rat orthotopic glioblastomas. BMC Cancer. 2018;18:871. doi: 10.1186/s12885-018-4771-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juhasz B, Varga B, Gesztelyi R, Kemeny-Beke A, Zsuga J, Tosaki A. Resveratrol: A multifunctional cytoprotective molecule. Curr Pharm Biotechnol. 2010;11:810–818. doi: 10.2174/138920110793262079. [DOI] [PubMed] [Google Scholar]
- 15.Rasheduzzaman M, Jeong JK, Park SY. Resveratrol sensitizes lung cancer cell to TRAIL by p53 independent and suppression of Akt/NF-kappaB signaling. Life Sci. 2018;208:208–220. doi: 10.1016/j.lfs.2018.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Rossi EL, Khatib SA, Doerstling SS, Bowers LW, Pruski M, Ford NA, Glickman RD, Niu M, Yang P, Cui Z, DiGiovanni J, Hursting SD. Resveratrol inhibits obesity-associated adipose tissue dysfunction and tumor growth in a mouse model of postmenopausal claudin-low breast cancer. Mol Carcinog. 2018;57:393–407. doi: 10.1002/mc.22763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng TM, Chin YT, Ho Y, Chen YR, Yang YN, Yang YC, Shih YJ, Lin TI, Lin HY, Davis PJ. Resveratrol induces sumoylated COX-2-dependent anti-proliferation in human prostate cancer LNCaP cells. Food Chem Toxicol. 2018;112:67–75. doi: 10.1016/j.fct.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486:95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahman MA, Kim NH, Kim SH, Oh SM, Huh SO. Antiproliferative and cytotoxic effects of resveratrol in mitochondria-mediated apoptosis in rat b103 neuroblastoma cells. Korean J Physiol Pharmacol. 2012;16:321–326. doi: 10.4196/kjpp.2012.16.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li XK, Motwani M, Tong W, Bornmann W, Schwartz GK. Huanglian, A chinese herbal extract, inhibits cell growth by suppressing the expression of cyclin B1 and inhibiting CDC2 kinase activity in human cancer cells. Mol Pharmacol. 2000;58:1287–1293. doi: 10.1124/mol.58.6.1287. [DOI] [PubMed] [Google Scholar]
- 21.Maeda H, Hori S, Ohizumi H, Segawa T, Kakehi Y, Ogawa O, Kakizuka A. Effective treatment of advanced solid tumors by the combination of arsenic trioxide and L-buthionine-sulfoximine. Cell Death Differ. 2004;11:737–746. doi: 10.1038/sj.cdd.4401389. [DOI] [PubMed] [Google Scholar]
- 22.Notarbartolo M, Poma P, Perri D, Dusonchet L, Cervello M, D’Alessandro N. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett. 2005;224:53–65. doi: 10.1016/j.canlet.2004.10.051. [DOI] [PubMed] [Google Scholar]
- 23.Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB, Kondo S. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65:3336–3346. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- 24.Hsiao YT, Kuo CL, Chueh FS, Liu KC, Bau DT, Chung JG. Curcuminoids induce reactive oxygen species and autophagy to enhance apoptosis in human oral cancer cells. Am J Chin Med. 2018;46:1145–1168. doi: 10.1142/S0192415X1850060X. [DOI] [PubMed] [Google Scholar]
- 25.Tsai CW, Yang MD, Hsia TC, Chang WS, Hsu CM, Hsieh YH, Chung JG, Bau DT. Dithiothreitol enhanced arsenic-trioxide-induced cell apoptosis in cultured oral cancer cells via mitochondrial dysfunction and endoplasmic reticulum stress. Environ Toxicol. 2017;32:17–27. doi: 10.1002/tox.22208. [DOI] [PubMed] [Google Scholar]
- 26.Wu SH, Bau DT, Hsiao YT, Lu KW, Hsia TC, Lien JC, Ko YC, Hsu WH, Yang ST, Huang YP, Chung JG. Bufalin induces apoptosis in vitro and has antitumor activity against human lung cancer xenografts in vivo. Environ Toxicol. 2017;32:1305–1317. doi: 10.1002/tox.22325. [DOI] [PubMed] [Google Scholar]
- 27.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 28.Shao W, Fanelli M, Ferrara FF, Riccioni R, Rosenauer A, Davison K, Lamph WW, Waxman S, Pelicci PG, Lo Coco F, Avvisati G, Testa U, Peschle C, Gambacorti-Passerini C, Nervi C, Miller WH Jr. Arsenic trioxide as an inducer of apoptosis and loss of PML/RAR alpha protein in acute promyelocytic leukemia cells. J Natl Cancer Inst. 1998;90:124–133. doi: 10.1093/jnci/90.2.124. [DOI] [PubMed] [Google Scholar]
- 29.Chen YC, Lin-Shiau SY, Lin JK. Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. J Cell Physiol. 1998;177:324–333. doi: 10.1002/(SICI)1097-4652(199811)177:2<324::AID-JCP14>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Zhu XH, Shen YL, Jing YK, Cai X, Jia PM, Huang Y, Tang W, Shi GY, Sun YP, Dai J, Wang ZY, Chen SJ, Zhang TD, Waxman S, Chen Z, Chen GQ. Apoptosis and growth inhibition in malignant lymphocytes after treatment with arsenic trioxide at clinically achievable concentrations. J Natl Cancer Inst. 1999;91:772–778. doi: 10.1093/jnci/91.9.772. [DOI] [PubMed] [Google Scholar]
- 31.Namgung U, Xia Z. Arsenite-induced apoptosis in cortical neurons is mediated by c-JUN N-terminal protein kinase 3 and p38 mitogen-activated protein kinase. J Neurosci. 2000;20:6442–6451. doi: 10.1523/JNEUROSCI.20-17-06442.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pettersson HM, Karlsson J, Pietras A, Ora I, Pahlman S. Arsenic trioxide and neuroblastoma cytotoxicity. J Bioenerg Biomembr. 2007;39:35–41. doi: 10.1007/s10863-006-9058-6. [DOI] [PubMed] [Google Scholar]
- 33.Keim A, Rossler OG, Rothhaar TL, Thiel G. Arsenite-induced apoptosis of human neuroblastoma cells requires p53 but occurs independently of c-JUN. Neuroscience. 2012;206:25–38. doi: 10.1016/j.neuroscience.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Gurr JR, Bau DT, Liu F, Lynn S, Jan KY. Dithiothreitol enhances arsenic trioxide-induced apoptosis in NB4 cells. Mol Pharmacol. 1999;56:102–109. doi: 10.1124/mol.56.1.102. [DOI] [PubMed] [Google Scholar]
- 35.Miller WH Jr., Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–3903. [PubMed] [Google Scholar]
- 36.Gupta S, Yel L, Kim D, Kim C, Chiplunkar S, Gollapudi S. Arsenic trioxide induces apoptosis in peripheral blood T-lymphocyte subsets by inducing oxidative stress: A role of BCL2. Mol Cancer Ther. 2003;2:711–719. [PubMed] [Google Scholar]