Abstract
Background
Acyl-coenzymeA: cholesterol acyltransferase (ACAT) 1, a key enzyme converting excess free cholesterol to cholesterol esters, has been demonstrated to be associated with the pathogenesis of Alzheimer disease (AD). However, the mechanism underlying the protective role of ACAT1 blockage in AD progression remains elusive.
Material/Methods
Human neuroblastoma SH-SY5Y cells were treated for 24 h with increasing concentrations of aggregated Aβ25–35 (5, 15, 25, and 45 μmol) with or without the ACAT1 siRNA pretreatment. Cell viability analysis was measured by CCK-8 assay. The genome-wide correlation between ACAT1 and all other probe sets was measured by the Pearson correlation coefficient (r). Western blotting was used to detect the ACAT1 protein expression in the hippocampus of APP/PSN transgenic AD mice. The mRNA level for each target was analyzed by qPCR. Western blotting was used to detect the ACAT1, cyclo-oxygenase-2 (Cox2), Calcium voltage-gated channel subunits (CACNAs), and ERK/PKC proteins in SH-SY5Y cells with or without the ACAT1 siRNA pretreatment in the presence of Aβ25–35.
Results
The expression of ACAT1 was significantly increased in the hippocampus of APP/PSN mice, and also showed an increasing trend when SH-SY5Y cells were exposed to Aβ25–35. Silencing ACAT1 significantly attenuated Aβ-induced cytotoxicity and cell apoptosis in SH-SY5Y cells. The genome-wide correlation analysis showed that Ptgs2 had the most significant correlation with Acat1 in the hippocampus of BXD RI mice. We further determined the regulatory effect of ACAT1 on COX2 expression by silencing or over-expressing ACAT1 in SH-SY5Y cells and found that silencing ACAT1 played a protective role in AD progression by regulating CACNAs and PKC/ERK signaling cascades.
Conclusions
Silencing ACAT1 attenuates Aβ25–35-induced cytotoxicity and cell apoptosis in SH-SY5Y cells, which may due to the synergistic effect of ACAT1 and COX2 through PKC/ERK pathways.
MeSH Keywords: Acyl-coenzymeA: Cholesterol Acyltransferase, Alzheimer Disease, Cyclooxygenase 2
Background
Alzheimer disease (AD) is the most common form of dementia. AD is characterized by senile plaques (SP) created by progressive accumulation of amyloid β-peptide (Aβ) and neurofibrillary tangles (NFT) composed of hyper-phosphorylated tau in brain regions. Growing evidence suggests that cellular cholesterol homeostasis is closely associated with AD pathogenesis, especially in amyloidogenesis [1,2]. In the brain, cholesterol is mainly derived from de novo synthesis via the hydroxymethyl glutaryl-CoA (HMG-CoA) reductase pathway, and is present mainly in its unesterified form in myelin sheaths and the cellular membranes of glial cells and neurons [3]. Within the cells, cholesterol is found in two forms: free cholesterol (FC) and cholesterol esters (CE). Acyl-coenzymeA: cholesterol acyltransferase (ACAT, also known as sterol O-acyltransferase, abbreviated as SOAT) catalyzes excess FC in cells into CE and mediates cellular cholesterol homeostasis [2]. There are two ACAT isoforms in mammals – ACAT1 and ACAT2 [4] – with different tissue expression patterns. ACAT1 is a resident enzyme at the endoplasmic reticulum and is ubiquitously expressed in all tissues, whereas ACAT2 is mainly expressed in the intestines and hepatocytes [5].
Although the molecular basis of the relationship between cholesterol homeostasis and AD pathogenesis is unclear, much attention also has been paid to seeking novel therapeutic targets such as statins (HMG-CoA reductase inhibitor) [6,7] and ACAT1 inhibitors [8]. Previous animal experiments showed that Aβ generation and deposition were reduced upon pharmacological inhibition of ACAT1 or by knocking out the ACAT1 gene [9,10]. Recent studies by Shibuya et al. demonstrated that ACAT1 inhibition induced an inhibitory effect against AD in neurons and microglia by stimulating autophagy. Surprisingly, the enhancing effect of ACAT1 blockage did not alter mTOR signaling or endoplasmic reticulum stress response [11,12]. The exact mechanism needs to be further explored.
ACAT1 is a key regulator in cellular cholesterol homeostasis and is associated with AD pathogenesis. However, the mechanism underlying the protective role of ACAT1 blockage in AD progression remains elusive. Previously, we have used the genetic genomics approach to dissect the genetic regulatory network for complex diseases, such as stress responses [13] and Parkinson disease [14]. The aim of the present study was to investigate the role of ACAT1 in APP/PSN double-transgenic AD mice and in human neuroblastoma SH-SY5Y cells. Genome-wide analysis was carried out in BXD RI mice to determine the regulatory gene network of Acat1 and to identify transcripts that co-vary with Acat1, which may function in the same biological network and contribute to the pathogenesis of AD. We were particularly interested in the most significant correlation between Acat1 and prostaglandin-endoperoxide synthase 2 (Ptgs2). PTGS2 encodes cyclo-oxygenase-2(COX-2), a key enzyme in prostaglandin biosynthesis, and acts as a proinflammatory mediator implicated in the pathogenesis of neurodegenerative diseases [15,16]. In addition, we attempted to validate the interaction between ACAT1 and COX2 and evaluate mechanisms underlying the protective effect of ACAT1 silencing in Aβ-induced SH-SY5Y cells.
Material and Methods
Animals
All animal experiments were conducted in compliance with the Institutional Animal Care guidelines and ethics approval was granted by the Administration Committee of Jiangsu Province, China. Transgenic B6/JNju-Tg (APPswe, PSEN1dE9) (abbreviated as APP/PSN) mice were obtained from the Model Animal Research Center of Nanjing University, and age- and sex-matched (10-month-old males) wild-type C57BL/6J mice were used as controls.
Cell culture, Aβ treatment, and cell viability analysis
The human neuroblastoma cell line SH-SY5Y is commonly used in studies of neurotoxicity, oxidative stress, and neurodegenerative diseases. In the present study, SH-SY5Y cells (Shanghai Institute of Materia Medica, Chinese Academy of Sciences) were routinely grown in Dulbecco’s modified Eagle’s medium (Corning Life Sciences), supplemented with 10% fetal bovine serum. Aβ25–35 was purchased from Sigma-Aldrich. As previously described [17], Aβ25–35 was dissolved in sterilized double-distilled water at a concentration of 1 mM/ml and incubated at 37°C for 7 days to form an aggregated form. Cell viability analysis was performed using Cell Counting Kit-8 (CCK-8) assay (Beyotime). Briefly, cells were pre-incubated in 96-well plates at a density of 5×104 cells for 20 h, incubated with different concentrations of aggregated Aβ25–35 (5, 15, 25, and 45 μmol) for 24 h, and then incubated again with CCK-8 solution according to the manufacturer’s instructions.
Plasmid and siRNA transfection
Cells grown to ~70% confluence were transfected with 50~100 nM siRNAs (Biomic) to target ACAT1 or PTGS2 in SHSY-5Y cells (Table 1), and a scrambled siRNA was used as control. The ACAT1 plasmid was purchased from Genechem, and empty vector was used as the control (referred to here as vector). Transfection of siRNA and plasmids was performed using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions.
Table 1.
Primers and siRNAs used in this study.
| Primers/siRNAs | Sequence (5′-3′) |
|---|---|
| β-actin_F | CGTTGACATCCGTAAAGACC |
| β-actin_R | TAGAGCCACCAATCCACAC |
| ACAT1_F | CATCCTATTCGTCCTCAG |
| ACAT 1_R | GGGTAGTTGTCTCGGTAA |
| COX2_F | AACAGGGAGGTGCTGTACG |
| COX2_R | TGTAGCTCCTTGGTGAAGCA |
| hs-ACAT1-si-1 | GCUCUCUCUUAGAUGAACU |
| hs-ACAT1-si-2 | CCCACUCAUUUGUCAGAGA |
| hs-ACAT1-si-3 | CUCUUCAUGUUCUUUGGAA |
| hs-PTGS2-si-1 | CUUACCCACUUCAAGGGAU |
| hs-PTGS2-si-2 | CCUCAAUUCAGUCUCUCAU |
| hs-PTGS2-si-3 | GAAUCAUUCACCAGGCAAA |
RNA extraction and qPCR
Total RNA was extracted by using the RNeasy Mini kit (QIAGEN) according to the manufacturer’s instructions. cDNA was prepared by reversely transcribing total RNA (2 μg) using SuperScript III reverse transcriptase (Thermo Scientific). The mRNA level for each target was analyzed by qPCR using iTaq Universal SYBR Green Supermix (Bio-Rad) with Bio-Rad CFX manager 3.1. Primers used in this study are listed in Table 1. Relative quantification was determined using the CT method. The mRNA expression values were normalized with β-actin mRNA level.
Protein extraction and Western blotting
The tissue and cell samples were washed with ice-cold PBS and incubated for 30 min on the ice in lysis buffer. The supernatant was retained after centrifugation. Protein concentration was determined using the BCA protein assay kit (Pierce). For Western blot analysis, 100 μg total protein from each sample was separated on SDS-PAGE gel and transferred onto PVDF membranes. The membranes were blocked with 5% milk blocking buffer and incubated with the designed primary antibodies overnight at 4°C. The primary antibodies are listed in Table 2. After 3 washes with TBS, the membranes were incubated with HRP-conjugated secondary antibody at 37°C for 2 h. Bound secondary antibodies were visualized using an enhanced chemiluminescence (ECL) kit (Bio-Rad) with ChemDoc XRS and Quantity One software (Bio-Rad).
Table 2.
Primary antibodies used in the present study.
| Antibody | Dilution | Manufacturer |
|---|---|---|
| Rabbit anti-ACAT1 | 1: 500 | Abcam, Cambridge, UK |
| Goat anti-COX-2 | 1: 500 | Abcam |
| Mouse anti-PKC | 1: 1000 | Abcam |
| Mouse anti-ERK1/2 | 1: 1000 | Abcam |
| Rabbit anti-Caspase3 | 1: 1000 | Abcam |
| Rabbit anti-Cacna1b | 1: 1000 | Alomone Labs, Jerusalem, Israel |
| Rabbit anti- Cacna2d1 | 1: 1000 | CMCTAG, San Diego, USA |
| Mouse anti-β-actin | 1: 5000 |
Gene network data sets
All data sets used in this study can be publically accessed from www.genenetwork.org. The primary data set used was “Hippocampus Consortium M430v2.0 (Jun06) RMA”, which includes steady-state transcript abundance measurements collected from the hippocampus of 67 BXD RI strains, 2 reciprocal F1 hybrids, and the B6 and D2 parental strains, using the Affymetrix Mouse Genome 430 2.0 array. Detailed information for data sets can be found in the “Info” page at www.genenetwork.org.
Correlation analysis and gene network construction
The correlation between Acat1 (probe set 1417696_at) and all other probe sets in the Hippocampus Consortium M430v2.0 data set was analyzed by Pearson product-moment correlation analysis. Significant correlations (P Value of correlation <0.0001) with the expression level greater than 8 units were selected to construct the gene co-regulatory network for Acat1 by using Spring Model Layout Network Graphs in GeneNetwork (www.genenetwork.org) [18].
Statistical analysis
All data are expressed as means ±SEM. All statistical analyses were performed using Prism software (GraphPad). A two-tailed t test was used when 2 values were compared. For multiple comparisons, a one-way ANOVA with a Turkey’s post test was used. P values less than 0.05 were considered to be statistically significant.
Results
Elevated expression of ACAT1 in Alzheimer Disease mouse and cell models
To explore the possible role of ACAT1 in the pathogenesis of AD, the expression of ACAT1 was detected in APP/PSN and age-matched wild-type (WT) C57BL/6J mice. Compared to the WT group, the expression level of ACAT1 significantly elevated in hippocampus of APP/PSN mice (Figure 1A). ACAT1 expression was also evaluated in SH-SY5Y cells exposed to Aβ25–35. Western blot analysis showed a significant rise in ACAT1 expression after treatment with Aβ25–35 (Figure 1B). Anti-ACAT1 immunofluorescence staining in SH-SY5Y cells showed cellular morphological changes after Aβ25–35 treatment, of which the cell’s processes became shorter or disappeared (Figure 1C).
Figure 1.
Expression of ACAT1 in Alzheimer Disease mouse and cell models. (A) The expressions of ACAT1 in hippocampus of APP/PSN mice and wild-type (WT) group were detected using Western blotting (* P<0.05, N=3). (B) Representative image of immunoblots for ACAT1 (*** P<0.001, versus control group) in SH-SY5Y cells under normal condition (DMEM with FBS, Normal), control condition (DMEM without FBS, Control), and Aβ25–35 treatment (Aβ). (C) Anti-ACAT1 immunofluorescence staining in SH-SY5Y cells showed cellular morphological changes after Aβ25–35 treatment. (D) The immunoreactivity of Aβ was determined by immunohistochemistry with an anti-Aβ antibody in Cortex of APP/PSN mice (a, b) and wild-type (WT) group (c, d). The white arrows indicate Aβ plaques. These results are representative of 3 independent experiments with each condition triplicated.
Silencing ACAT1 attenuates Aβ25–35-induced cytotoxicity and cell apoptosis in SH-SY5Y neuroblastoma cells
CCK-8 assay showed that Aβ25–35 significantly decreased cell viability in a concentration-dependent manner (Figure 2A). Cell viability was reduced to 32.78% and 26.78% after exposure to 25 and 45 μmol Aβ25–35 for 24 h, respectively. In the subsequent experiments, 25 μmol/L Aβ25–35 was chosen as the optimal concentration to induce neurotoxicity. To evaluate the potential role of ACAT1 in Aβ25-35-induced SH-SY5Y cells, we inhibited the ACAT1 expression before Aβ25–35 treatment. The result showed that ACAT1_siRNA-2 had the strongest interfering effect by downregulating the ACAT1 mRNA level by 27.88%, and thus was used for subsequent RNAi experiments. CCK-8 assay showed that cell death was reduced, and cell viability was 19% in the pre-silencing ACAT1 treatment group vs. 14% in the scramble group (P<0.05) (Figure 2B), indicating that ACAT1 pre-silencing played a protective role against Aβ-induced SH-SY5Y cell death.
Figure 2.
Silencing ACAT1 inhibited Aβ25–35-induced cytotoxicity and cell apoptosis in SH-SY5Y neuroblastoma cells. (A) SH-SY5Y cells were treated with Aβ25–35 (5, 15, 25, and 45 μmol) for 24 h and cell viability was analyzed by CCK-8 assay. (B) Silencing ACAT1 significantly inhibited Aβ25–35 induced cytotoxicity. (C) Silencing ACAT1 or not before Aβ25–35 treatment, total cell lysates of SH-SY5Y cells were harvested. Expression of caspase-3 and β-actin was determined by Western blotting. Results were presented as mean ±SD of 3 independent experiments. (** P<0.01 and * P<0.05).
The protein level of caspase-3 (32 kD) was increased by 1.5-fold after Aβ25–35 (25 μmol/L) treatment (P<0.05) (Figure 2C). However, in the ACAT1_siRNA pretreated group (ACAT1_siRNA+Aβ25-35 group), the relative expression level of caspase-3 was significantly decreased (P<0.05) (Figure 2C) compared with the control group (Scramble siRNA+Aβ25–35 group), suggesting that ACAT1 pre-silencing counteracts Aβ-induced apoptosis by inhibiting caspase3 activation.
Genes significantly correlated with Acat1 expression were collected from the BXD RI mouse hippocampus for correlation analysis
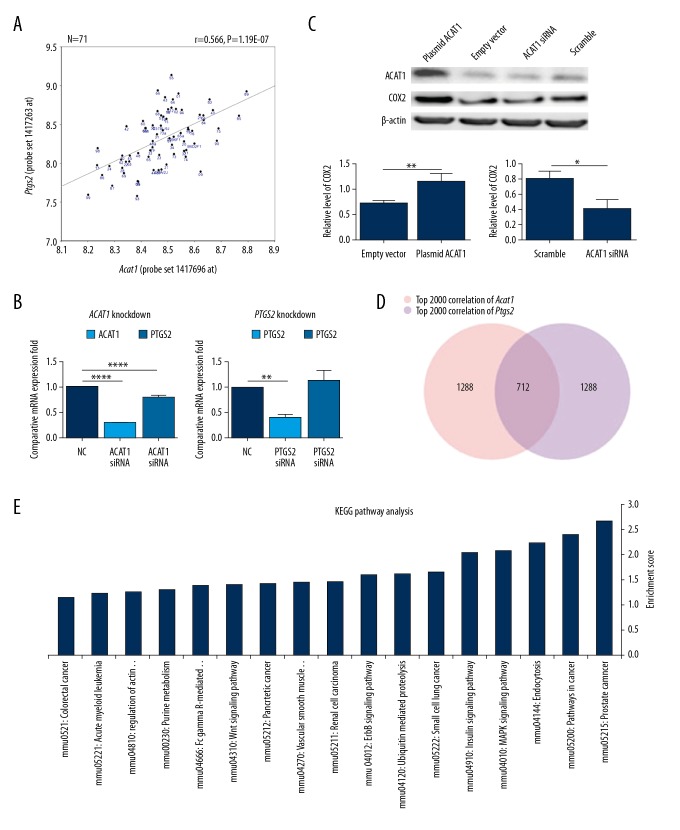
To identify potential mechanisms underlying the protective role of Acat1 silencing in AD progression, we queried the Hippocampus Consortium M430v2 (Jun 06) RMA data set to identify transcripts that co-vary with Acat1. Expression of Acat1 on the Affymetrix MOE430V2 array was interrogated by 5 probe sets. The expression level of Acat1 measured by probe set 1417696_at varied 1.5-fold across strains, with a mean expression of 8.48, and exhibited a trend of continuous change (Figure 3A). Pearson correlation analysis between Acat1 (probe set 1417696_at) and whole mouse genome transcripts in the hippocampus of the BXD RI mice revealed that 375 probe sets were significantly correlated with Acat1 (P Value of Sample correlation <0.001). To graphically evaluate the strength of the Pearson correlation coefficients, we further selected 34 genes with expression levels greater than 8 units and significantly correlated with Acat1 (P<0.0001) to generate a co-expression network (Figure 3B). The most significant correlation was Ptgs2 (probe set 1417263_at) (r=0.57, P=2.09E-07). Cyclo-oxygenase-2 (COX-2), the rate-limiting enzyme responsible for prostaglandin production, has been shown to play a key role in the regulation of inflammation, and has received attention as a therapeutic target for AD [15]. Matrix analysis showed a near-linear dependency between the expression vitiations of these 2 genes in different strains of RI BXD mice (R=0.615) (Figure 4A). These findings indicate a complex synergistic action between Acat1 and Ptgs2. Consequently, we focused on the relationship between Acat1 and Cox2 in further experiments.
Figure 3.
Expression variation and Gene network analysis of Acat1 in hippocampus of BXD RI mice. (A) The expression level of Acat1 measured by probe set 1417697_at among BXD RI strains varies 1.5-fold across strains with an average expression of 8.48. Values denote normalized relative expression levels. Strain names are indicated along the x axis. (B) Thirty-four genes were highly correlated with Acat1 (P<0.0001), with a mean expression level >8 units in the hippocampal tissue. The degree of correlation between genes was described by the Pearson correlation coefficient. The bold/thin/dashed line reflects the coefficient of 1.0–0.7, 0.7–0.5, and 0.5–0.0, respectively. The red/orange/pink color indicates a positive correlation, and the blue/green/dark color indicates a negative correlation.
Figure 4.
Correlation analysis and the expression regulation validation between Acat1 and Ptgs2. (A) Correlation Matrix analysis for Acat1 and Ptgs2 expression in BXD RI mouse hippocampus (n=71). X axis and Y axis denoted Acat1 and Ptgs2 expression, respectively. Digitally labeled dots denote the 69 strains of BXD RI mice and parental strains (C57BL/6J and DBA/2J). P=1.19E-07. (B) QPCR was performed to detect the contribution of ACAT1_siRNA to Ptgs2 expression variation (B, left) and PTGS2_siRNA treatment to ACAT1 expression variation (B, right) in human SH-SY5Y cells. (C) Western Blot analysis was performed to detect COX2 protein changes after silencing or overexpressing ACAT1. (*** P<0.001, ** P<0.01 and * P<0.05). (D) The top 2000 correlated genes for both Acat1 andPtgs2 in BXD RI mouse hippocampus were collected, and 712 genes were collected as the common co-variations with both genes through VENN analysis. Pink and purple cycles denote numbers of highly correlated genes with Acat1 or Ptgs2, respectively. (E) KEGG pathway analysis revealed 17 significant enrichment pathways; X-axis and Y-axis denotes the enriched pathway and enriched index −log (P value), respectively.
To confirm the interaction between ACAT1 and PTGS2, qPCR was performed to detect the expression levels in SH-SY5Y cells after ACAT1_siRNA and PTGS2_siRNA treatment, respectively. QPCR was used to detect the efficiency of different sequences of siRNA, and ACAT1_siRNA-2 and PTGS2_SiRNA-2 were selected for the following experiments. Knockdown of ACAT1 mRNA reached 28.88% compared with the scrambled siRNA control group, while PTGS2 decreased to 79.54% (Figure 4B, left). However, in the condition of PTGS2 inhibition (knock down to 40.74%), the gene expression of ACAT1 did not change significantly (Figure 4B, right). Western Blot analysis revealed that COX2 was decreased significantly after ACAT1-siRNA treatment (P<0.05). To further validate the regulatory effect, SH-SY5Y cells were transfected with plasmid-over-expressed ACAT1. Western blot analysis revealed that the relative expression of COX2 was increased by 1.61-fold after ACAT1 plasmid transfection (Figure 4C). The above results suggest that COX2 expression is regulated by ACAT1 in SH-SY5Y neuroblastoma cells
Potential mechanisms responsible for the protective effect of ACAT1 silencing in Aβ-induced SH-SY5Y cells
The top 2000 correlated genes in the Hippocampus Consortium M430v2 (Jun 06) RMA dataset for both Acat1 and Ptgs2 were identified by Pearson product-moment correlation analysis, and the common co-variations in both genes were further collected through VENN analysis. We found that 712 genes with varied expression were highly correlated with both genes, meaning that these genes may be involved in Acat1 and Ptgs2 interactions (Figure 4F). KEGG pathway analysis revealed several significant enrichment pathways, including endocytosis (mmu04144), and MAPK signaling pathway (mmu04010) (Figure 4E). We were particularly interested in the MAPK signaling pathway, which is known to be involved in the transcriptional regulation of several genes encoding for proinflammatory cytokines, and their altered phosphorylation states may lead to the development of inflammatory response in AD [19,20]. Calcium voltage-gated channel subunit alpha 1 B (CACNA1b) and calcium voltage-gated channel auxiliary subunit alpha 2 delta 1 (CACNA2d1), which contributed to the Voltage-Gated Calcium Channel Subunits category, were also enriched in the MAPK pathway. Western blot results showed that the relative expression of total PKC and the ratio of phospho-ERK (p-ERK)/ERK were decreased by 87.87% (P<0.01) and 26.3% (P<0.05), respectively, after Aβ25–35 treatment as compared with the control group, suggesting that PKC and ERK are involved in Aβ25–35-induced cytotoxicity in SH-SY5Y cells. Meanwhile, the relative expression of CACNA1b and CACNA2d1 was increased by 1.63- and 1.81-fold, respectively, after Aβ25–35 treatment (Figure 5A).
Figure 5.
Effects of knockdown ACAT1 on CACNAs, PKC, and ERK phosphorylation and in Aβ-induced SH-SY5Y cells. (A) Western blot results showed that the relative expression of CACNA1b and CACNA2d1 was increased significantly, while the total PKC and the ratio of phospho-ERK (p-ERK)/ERK were decreased significantly after Aβ25-35 treatment. (B) Knockdown of ACAT1 rescued the reduction of PKC and phosphorylation level of ERK, and also retarded the upregulation of CACNA1b and CACNA2d1 induced by Aβ. (** P<0.01 and * P<0.05).
Effects of knockdown ACAT1 on PKC and ERK phosphorylation and CACNAs in Aβ-induced SH-SY5Y cells
To further explore the effect of knock down of ACAT1 on the MAPK family, the amount of PKC and ERK phosphorylation and CACNAs was assessed by Western blot. The result showed that the relative expression of total PKC and the ratio of p-ERK/ERK were increased by 1.51- and 1.45-fold, respectively, after knockdown of ACAT1 in Aβ-induced SH-SY5Y cells as compared with the control group (P<0.05) (Figure 5B). Knockdown of ACAT1 attenuated the reduction of p-ERK and PKC in Aβ-induced SH-SY5Y cells. The relative expression of CACNA1b and CACNA2d1 was decreased by 72% (P<0.01) and 52.53% (P<0.05), respectively, after knockdown of ACAT1 in Aβ-induced SH-SY5Y cells (Figure 5B). These results indicate that PKC and ERK signaling pathways might be involved in knockdown of ACAT1-mediated COX2 inhibition and neuroprotection in the AD cell model.
Discussion
ACAT1 inhibitors have recently emerged as promising drug candidates. Previously, most efforts have been focused on targeting ACAT1 to treat atherosclerosis [21,22]. Recently, the potential effect of ACAT1 inhibitors in the treatment of AD [8] and tumors [23] has also aroused much attention. Yang et al. reported that cholesterol esterification by inhibiting ACAT1 could enhance the anti-tumor activity of CD8+ T cells [23]. In vivo experiments demonstrated that ACAT1 inhibition could increase Aβ clearance and ameliorate cognitive deficits in transgenic mice [9]. Shibuya et al. reported that ACAT1 inhibition could affect the level of autophagy in neurons [12] and microglial cells [11], which could further affect Aβ clearance. However, this autophagy was not associated with the mTOR pathway [11,12]. The exact mechanism needs to be further explored. To obtain a comprehensive understanding of the possible molecular role of ACAT1 inhibition in the prevention and treatment of AD, we established an Acat1 gene regulatory network by using genome-wide association analysis in BXD RI mice.
Interestingly, Ptgs2 and Acat1 showed a very high correlation in the gene regulatory network established in our study. To the best our knowledge, such a high correlation has not been reported in the literature before. PTGS2-encoded COX-2 is an important neuroinflammatory factor and is extensively involved in neuronal damage, inflammatory response, and neurodegenerative diseases [10,15]. An Alzheimer Disease Anti-inflammatory Prevention Trial (ADAPT) was initiated to test whether naproxen (a nonselective COX inhibitor) or celecoxib (a selective COX-2 inhibitor) could delay the onset of dementia in cognitively intact older adults with a family history of AD. However, the results showed both naproxen and celecoxib were unable to prevent the deterioration of cognitive function, suggesting that COX-2 is not suitable for use as the target for the prevention of AD [24]. Our study first demonstrated that SOAT1-encoding ACAT1 and PTGS2-encoding COX-2 had a very high correlation; ACAT1 knockdown in SH-SY5Y cells reduced the expression of COX2 significantly and ACAT1 over-expression upregulated the expression of COX2, while COX2 knockout did not have a significant effect on the expression of ACAT1 (Figure 4). These findings suggest that ACAT1 may be an upstream regulatory site of COX2. We postulate that the increased expression of COX2 upon Aβ stimulation in the present study may reflect a change in the overall environment and therefore should not be selected as the target of AD prevention, although this needs to be verified by more in vivo studies. Although COX-2 inhibitors alone cannot prevent AD, their role in the occurrence of AD should not be ignored. COX2 expression in the brain of AD patients was found to be upregulated. In addition, COX2 mediated neuroinflammation, neurodegeneration, and cognitive impairment in a rat model of AD [15]. COX2 may work together with other AD-related factors such as ACAT1 to participate in the initiation of AD. This may be the reason why COX-2 inhibitors alone do not work effectively against AD. Synergistic genes of COX2 such as ACAT1 could be considered as the action target in future research on the prevention and treatment of AD. However, it is worth noting that COX1, another member of the COX family, has also been shown to play an important role in AD pathogenesis [25–29]. In the AD brain, clusters of COX-1 expressing microglia around amyloid plaques were identified in certain cortical layers [25]. Genetic deletion of COX-1 significantly attenuates the oxidative stress and neuronal damage in response to Aβ1–42 [26]. The COX-1-selective inhibitor SC-560 improved spatial learning and memory, and reduced amyloid deposits and tau hyperphosphorylation [29]. In future work, we will further focus on whether COX1 participates in the synergistic effect of ACAT1 and COX2. It was found in this study that knockdown of ACAT1 exerted a protective effect against Aβ25–35 induced cytotoxicity in SH-SY5Y cells by inhibiting cell apoptosis and elevation of COX2 expression. In addition, we conducted a genetic correlation analysis on Acat1 and Cox2 and retrieved their common highly related genes, and found that this part of genes was enriched to 17 pathways, of which CACNA1b and CACNA2d1 were enriched to the MAPK pathway. The expression of CACNA1b and CACNA2d1 was elevated in Aβ-induced SH-SY5Y cells, and inhibition of ACAT1 decreased the expression of CACNA1b and CACNA 2d1, which was accompanied with decreased level of COX-2 and the increased level of p-ERK/ERK and PKC. These changes to some extent protected the Aβ25–35-induced AD cell model. It was reported that the level of Aβ25–35-induced PKC was decreased in PC12 cells, and activation of PKC reduced cell apoptosis [30]. The results of our study also confirmed that the level of PKC in SH-SY5Y cells was decreased 24 h after Aβ induction. However, PKC was increased after addition of Aβ25–35 to SH-SY5Y cells with previous knockout of ACAT1, suggesting that ACAT1 knockout could increase the level of PKC, which may prevent cell apoptosis. It was found in our study that the level p-ERK/ERK was decreased 24 h after addition of Aβ25–35 to SH-SY5Y cells. Some other studies used Aβ25–35 to induce PC12 cells [31], primary cortical neurons [32], and hippocampal neurons [33], and found that the level of ERK was decreased upon Aβ stimulation, resulting in cell death. Prostaglandin E (PGE)2, the chief prostanoid resulting from COX-2 activity, binds to 4 G-protein-coupled PGE receptor, EP1-4, having differential signaling abilities [34,35]. It has been demonstrated that stimulation of EP4 receptors leads to phosphorylation of ERKs through a PI3K-dependent mechanism [34], and the activation of ERK is necessary to support EP4-mediated CT26 cell proliferation [36]. Taking these results together, we indicated that the increased level of p-ERK/ERK after ACAT1 inhibition was due to the synergistic effect of ACAT1 and COX2.
It is noteworthy that some studies demonstrated that statins protect cells against apoptosis by increasing the level of p-ERK and play a role in increasing the cognitive level and attenuating the progression of AD [7,37]. As statins can reduce the synthesis of cholesterol, they have been widely used in the treatment of atherosclerosis and other cardiovascular diseases [22]. Studies in recent years demonstrated that statins decrease the level of Aβ in animal models. Some retrospective case-control studies suggested that statins reduced the risk of AD, but clinical trials and prospective cohort studies have produced inconsistent and mixed results [38,39]. A more recent study suggested that statins reduce the risk of AD, pointing out a correlation between the use of statins and the decreased risk of AD, but this decreased risk depends on sex, race/ethnicity, and the type of statins used [40]. However, there are controversies over the preventive effect of high-dose statins on AD, knowing that the statins can inhibit HMG-CoA reductase and limit the early steps of cholesterol synthesis, thus reducing the formation of many intermediate products such as anti-oxidants, which may aggravate the pathological change of AD. Unlike statins, genetic ablation or pharmacological inhibition of ACAT1 did not affect total cholesterol, but may decrease the level of Aβ in animal models [8].
Conclusions
It may be more important to explore the role of ACAT1 inhibitors in the prevention and treatment of AD. In the present study, we used siRNA interference to specifically inhibit the expression of ACAT1, and found that, compared with the Aβ stimulation group, pre-reduction of ACAT1 attenuated the cytotoxicity induced by Aβ, suggesting that ACAT1 may prove to be a meaningful target for the prevention and treatment of AD. Of course, more detailed verification is needed from animal studies.
Footnotes
Source of support: This work was supported by the grants from the National Natural Science Foundation of China (Grant No. 31571238, 31570983, and 81200828) and the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (Grant No. 16KJB310011)
Conflict of interests
None.
References
- 1.Chang TY, Yamauchi Y, Hasan M, et al. Cellular cholesterol homeostasis in Alzheimer’s disease. J Lipid Res. 2017;58:2239–54. doi: 10.1194/jlr.R075630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Paolo G, Kim TW. Linking lipids to Alzheimer’s disease: Cholesterol and beyond. Nat Rev Neurosci. 2011;12:284–96. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Liu Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell. 2015;6:254–64. doi: 10.1007/s13238-014-0131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cases S, Novak S, Zheng YW, et al. ACAT-2, a second mammalian acyl-CoA: Cholesterol acyltransferase. Its cloning, expression, and characterization. J Biol Chem. 1998;273:26755–64. doi: 10.1074/jbc.273.41.26755. [DOI] [PubMed] [Google Scholar]
- 5.Chang TY, Chang CC, Lin S, et al. Roles of acyl-coenzyme A: cholesterol acyltransferase-1 and -2. Curr Opin Lipidol. 2001;12:289–96. doi: 10.1097/00041433-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Huang W, Li Z, Zhao L, et al. Simvastatin ameliorate memory deficits and inflammation in clinical and mouse model of Alzheimer’s disease via modulating the expression of miR-106b. Biomed Pharmacother. 2017;92:46–57. doi: 10.1016/j.biopha.2017.05.060. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto N, Fujii Y, Kasahara R, et al. Simvastatin and atorvastatin facilitates amyloid beta-protein degradation in extracellular spaces by increasing neprilysin secretion from astrocytes through activation of MAPK/Erk1/2 pathways. Glia. 2016;64:952–62. doi: 10.1002/glia.22974. [DOI] [PubMed] [Google Scholar]
- 8.Shibuya Y, Chang CC, Chang TY. ACAT1/SOAT1 as a therapeutic target for Alzheimer’s disease. Future Med Chem. 2015;7:2451–67. doi: 10.4155/fmc.15.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryleva EY, Rogers MA, Chang CC, et al. ACAT1 gene ablation increases 24(S)-hydroxycholesterol content in the brain and ameliorates amyloid pathology in mice with AD. Proc Natl Acad Sci USA. 2010;107:3081–86. doi: 10.1073/pnas.0913828107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutter-Paier B, Huttunen HJ, Puglielli L, et al. The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer’s disease. Neuron. 2004;44:227–38. doi: 10.1016/j.neuron.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 11.Shibuya Y, Chang CC, Huang LH, et al. Inhibiting ACAT1/SOAT1 in microglia stimulates autophagy-mediated lysosomal proteolysis and increases Abeta1–42 clearance. J Neurosci. 2014;34:14484–501. doi: 10.1523/JNEUROSCI.2567-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibuya Y, Niu Z, Bryleva EY, et al. Acyl-coenzyme A: Cholesterol acyltransferase 1 blockage enhances autophagy in the neurons of triple transgenic Alzheimer’s disease mouse and reduces human P301L-tau content at the presymptomatic stage. Neurobiol Aging. 2015;36:2248–59. doi: 10.1016/j.neurobiolaging.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, Cai R, Lu L, et al. Genetic regulatory network analysis reveals that low density lipoprotein receptor-related protein 11 is involved in stress responses in mice. Psychiatry Res. 2014;220:1131–37. doi: 10.1016/j.psychres.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Shen Q, Wang X, Chen Y, et al. Expression QTL and regulatory network analysis of microtubule-associated protein tau gene. Parkinsonism Relat Disord. 2009;15:525–31. doi: 10.1016/j.parkreldis.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Sil S, Ghosh T. Role of cox-2 mediated neuroinflammation on the neurodegeneration and cognitive impairments in colchicine induced rat model of Alzheimer’s Disease. J Neuroimmunol. 2016;291:115–24. doi: 10.1016/j.jneuroim.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Shi S, Liang D, Chen Y, et al. Gx-50 reduces beta-amyloid-induced TNF-alpha, IL-1beta, NO, and PGE2 expression and inhibits NF-kappaB signaling in a mouse model of Alzheimer’s disease. Eur J Immunol. 2016;46:665–76. doi: 10.1002/eji.201545855. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Xu K, Yan M, et al. Protective effects of galantamine against Abeta-induced PC12 cell apoptosis by preventing mitochondrial dysfunction and endoplasmic reticulum stress. Neurochem Int. 2010;57:588–99. doi: 10.1016/j.neuint.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Lu H, Li L, Watson ER, et al. Complex interactions of Tyrp1 in the eye. Mol Vis. 2011;17:2455–68. [PMC free article] [PubMed] [Google Scholar]
- 19.Franco R, Martinez-Pinilla E, Navarro G, et al. Potential of GPCRs to modulate MAPK and mTOR pathways in Alzheimer’s disease. Prog Neurobiol. 2017;149–50:21–38. doi: 10.1016/j.pneurobio.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Giraldo E, Lloret A, Fuchsberger T, et al. Abeta and tau toxicities in Alzheimer’s are linked via oxidative stress-induced p38 activation: Protective role of vitamin E. Redox Biol. 2014;2:873–77. doi: 10.1016/j.redox.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomoda H, Omura S. Potential therapeutics for obesity and atherosclerosis: Inhibitors of neutral lipid metabolism from microorganisms. Pharmacol Ther. 2007;115:375–89. doi: 10.1016/j.pharmthera.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Ding L, Biswas S, Morton RE, et al. Akt3 deficiency in macrophages promotes foam cell formation and atherosclerosis in mice. Cell Metab. 2012;15:861–72. doi: 10.1016/j.cmet.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang W, Bai Y, Xiong Y, et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531:651–55. doi: 10.1038/nature17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ADAPT-FS Research Group. Follow-up evaluation of cognitive function in the randomized Alzheimer’s disease anti-inflammatory prevention trial and its follow-up study. Alzheimers Dement. 2015;11:216–25. doi: 10.1016/j.jalz.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yermakova AV, Rollins J, Callahan LM, et al. Cyclooxygenase-1 in human Alzheimer and control brain: Quantitative analysis of expression by microglia and CA3 hippocampal neurons. J Neuropathol Exp Neurol. 1999;58:1135–46. doi: 10.1097/00005072-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Choi SH, Bosetti F. Cyclooxygenase-1 null mice show reduced neuroinflammation in response to beta-amyloid. Aging (Albany, NY) 2009;1:234–44. doi: 10.18632/aging.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi SH, Aid S, Bosetti F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol Sci. 2009;30:174–81. doi: 10.1016/j.tips.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dargahi L, Nasiraei-Moghadam S, Abdi A, et al. Cyclooxygenase (COX)-1 activity precedes the COX-2 induction in Abeta-induced neuroinflammation. J Mol Neurosci. 2011;45:10–21. doi: 10.1007/s12031-010-9401-6. [DOI] [PubMed] [Google Scholar]
- 29.Choi SH, Aid S, Caracciolo L, et al. Cyclooxygenase-1 inhibition reduces amyloid pathology and improves memory deficits in a mouse model of Alzheimer’s disease. J Neurochem. 2013;124:59–68. doi: 10.1111/jnc.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Xing D, Zhu D, et al. Low-power laser irradiation inhibiting Abeta25–35-induced PC12 cell apoptosis via PKC activation. Cell Physiol Biochem. 2008;22:215–22. doi: 10.1159/000149799. [DOI] [PubMed] [Google Scholar]
- 31.Qian W, Li H, Pan N, et al. Curcumin treatment is associated with increased expression of the N-methyl-D-aspartate receptor (NMDAR) subunit, NR2A, in a rat PC12 cell line model of Alzheimer’s disease treated with the acetyl amyloid-beta peptide, Abeta(25–35) Med Sci Monit. 2018;24:2693–99. doi: 10.12659/MSM.906933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu T, Jin H, Sun QR, et al. Neuroprotective effects of emodin in rat cortical neurons against beta-amyloid-induced neurotoxicity. Brain Res. 2010;1347:149–60. doi: 10.1016/j.brainres.2010.05.079. [DOI] [PubMed] [Google Scholar]
- 33.Millucci L, Ghezzi L, Bernardini G, et al. Conformations and biological activities of amyloid beta peptide 25–35. Curr Protein Pept Sci. 2010;11:54–67. doi: 10.2174/138920310790274626. [DOI] [PubMed] [Google Scholar]
- 34.Fujino H, Xu W, Regan JW. Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. J Biol Chem. 2003;278:12151–56. doi: 10.1074/jbc.M212665200. [DOI] [PubMed] [Google Scholar]
- 35.Majumder M, Dunn L, Liu L, et al. COX-2 induces oncogenic micro RNA miR655 in human breast cancer. Sci Rep. 2018;8:327. doi: 10.1038/s41598-017-18612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pozzi A, Yan X, Macias-Perez I, et al. Colon carcinoma cell growth is associated with prostaglandin E2/EP4 receptor-evoked ERK activation. J Biol Chem. 2004;279:29797–804. doi: 10.1074/jbc.M313989200. [DOI] [PubMed] [Google Scholar]
- 37.Zhi WH, Zeng YY, Lu ZH, et al. Simvastatin exerts antiamnesic effect in Abeta25–35-injected mice. CNS Neurosci Ther. 2014;20:218–26. doi: 10.1111/cns.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGuinness B, Passmore P. Can statins prevent or help treat Alzheimer’s disease? J Alzheimers Dis. 2010;20:925–33. doi: 10.3233/JAD-2010-091570. [DOI] [PubMed] [Google Scholar]
- 39.McGuinness B, Craig D, Bullock R, et al. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2016;(1):CD003160. doi: 10.1002/14651858.CD003160.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zissimopoulos JM, Barthold D, Brinton RD, et al. Sex and race differences in the association between statin use and the incidence of Alzheimer disease. JAMA Neurol. 2017;74:225–32. doi: 10.1001/jamaneurol.2016.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]