Abstract
Alkaline serine protease was purified to homogeneity from culture supernatant of a thermophilic, alkaliphilic Bacillus sp. by 80% ammonium sulphate precipitation followed by CM-cellulose and DEAE-cellulose ion exchange column chromatography. The enzyme was purified up to 16.5-fold with 6900 U/mg activity. The protease exhibited maximum activity towards casein at pH 8.0 and at 80 °C. The enzyme was stable at pH 8.0 and 80 °C temperature up to 2 h. The Ca2+ and Mn2+ enhanced the proteolytic activity up to 44% and 36% as compared to control, respectively. However, Zn2+, K+, Ba2+, Co2+, Hg2+ and Cu2+ significantly reduced the enzyme activity. PMSF (phenyl methyl sulphonyl fluoride) completely inhibited the protease activity, whereas the activity of protease was stimulated up to two folds in the presence of 5 mM 2-mercaptoethanol. The enzyme was also stable in surfactant (Tween-80) and other commercial detergents (SDS, Triton X-100).
Keywords: Thermophilic serine protease, Oxidation-stable, Thiol-dependent, Purification, Characterization
1. Introduction
Protease catalyses the degradation of protein molecules and has been used in various industries such as food, pharmaceutical and detergent industries [29], [16]. Protease is one of the major groups of commercially available biocatalysts and covers 60% of total enzyme market [26], [9]. Microbial proteases with desirable characteristics are most suitable for biotechnological processes so they have a major share of the industrial enzyme market. Moreover, detergent formulations showed biggest worldwide enzyme consumption [5]. Currently more than 3000 enzymes are being used in various industrial processes. However, the current toolbox of enzymes is still not fulfilling the entire demands of industrial conditions and the extreme conditions of industrial reaction could not be tolerated by most available enzymes. The extremophilic microorganisms brought advancement in the field of enzymology [19], [24]. The demand of heat stable proteases with novel characteristics is high in the industries because high processing temperatures enhance the rate of reaction, increase the solubility of non-gaseous reactants and reduce microbial contamination by mesophilic organism. In the past few years, the properties of enzyme were enhanced by using the protein and genetic engineering techniques for commercialization [13]. Additionally, the use of recombinant microbes also improved the yield of extracellular protease in the culture medium by the over expressed protease gene [13]. The main industrial applications of thermostable alkaline protease are in food industry, leather industry, textile industry, pharmaceutical industry, bioremediation process, and house hold waste management, photographic industry, peptide synthesis and silk degumming processing [3].
In the present research, various bacterial strains have been isolated and screened for extracellular protease production. A thermophilic Bacillus sp. has been used for protease production. The protease was purified and biochemicals were characterized such as optimum temperature, optimum pH, and thermal stability. The effect of metal ion detergents and surfactant on the activity of protease was determined for commercialization.
2. Materials and methods
2.1. Isolation and identification of protease producing bacterial strain
Various bacterial strains were isolated from water samples of a thermal spring. 10 ml of water samples was mixed with 90 ml normal saline. Then it was serially diluted from 10−1 to 10−6 ratio with normal saline, and 100 μl of each diluted samples was inoculated on Luria–Bertani’s (LB) agar medium. The plates were incubated at 37 °C for 24 h. The isolated colonies were selected to obtain pure bacterial strains using pure culture study. Pure bacterial isolates were grown at 37 °C for 24 h onto the LB agar medium containing 1.0% casein for the screening of proteolytic activity. The clear zone of casein hydrolysis around the colony confirmed the protease production. The maximum protease producing bacterial strain was selected and identified on the basis of morphological and biochemical characteristics.
2.2. Protease assay
The activity of protease was determined through the Anson method with some modifications [2]. The enzyme solution (0.5 ml) was incubated with 5 ml of 1% (w/v) casein solution prepared in Tris–HCl buffer (pH 8.0) for 20 min at 80 °C. The reaction was stopped by addition of 1.5 ml of 0.3 M TCA (Tricarboxylic acid). The contents were centrifuged at 10,000 rpm for 10 min at 4 °C and the amino acids produced by the degradation of casein were measured as tyrosine through the method of Folin and Ciocalteu [12].
One unit of protease was defined as “the amount of enzyme that hydrolysed casein to produce colour equivalent to 1.0 μM of tyrosine per minute under optimized reaction conditions”.
2.3. Determination of total protein
The total protein was determined through Lowry’s method using BSA (Bovine serum albumin) as a standard [20].
2.4. Purification of protease
2.4.1. Preparation of cell free supernatant
The cells of bacterial strain were separated from fermentation medium after 24 h of incubation at 37° by centrifugation at 10,000 rpm for 15 min. The cell free supernatant was used for further studies.
2.4.2. Ammonium sulphate precipitation
The supernatant was precipitated by a gradual addition of (NH4)2SO4 up to 80% saturation at 0 °C with slow stirring. The precipitates were obtained through centrifugation at 10,000 rpm for 15 min and dissolved in a minimum amount of 0.05 M Tris–HCl buffer of pH 8.0. Then enzyme solution was dialysed against the same buffer.
2.4.3. Gel permeation chromatography
Gel permeation chromatography was used for the complete purification of protease. The concentrated enzyme solution was applied on CM-Cellulose Column (20 ml bed volume, 1.6 × 50 cm). The column was pre-equilibrated with 0.05 M Tris–HCl buffer containing 5 mM CaCl2 of pH 8.0. The protein sample was eluted with 100 ml linear gradient of 0.0–1.0 M NaCl in same buffer. The fractions were collected using a fraction collector with a flow rate of 20 ml/h. The collected fractions were monitored by measuring the absorbance at 280 nm. The enzyme activity and total protein of all fractions were determined. The fractions which showed protease activity were pooled and precipitated.
The concentrated enzyme solution was dialysed overnight against 50 mM Tris–HCl containing 5 mM CaCl2 buffer of pH 8.0. This dialysate was then loaded on to a column of DEAE-cellulose (50 ml bed volume, 1.5 × 30 cm) equilibrated with 100 ml of same buffer. The sample was initially eluted with 100 ml of equilibrium buffer and then with 100 ml linear gradient of 0.0–0.5 M NaCl in the same buffer at a flow rate of 20 ml/h. Final washing was carried out with 100 ml linear gradient of 0.5–1.0 M NaCl in the same buffer. The fractions which showed enzyme activity were pooled together.
2.5. Characterization of protease
2.5.1. Effect of temperature on protease activity
In order to determine the optimum temperature of protease for maximum catalytic activity, the enzyme assay was performed in different incubation temperatures ranging from 37 °C to 90 °C at constant substrate concentration and pH for 20 min.
2.5.2. Thermal stability of protease
The thermal stability of protease was measured by pre-incubation of enzyme solution at 40 °C, 60 °C and 80 °C for different time intervals and after definite time interval the aliquots were taken for determination of residual activity.
2.5.3. Effect of pH on protease activity
The optimum pH for maximum enzymatic activity of protease was determined by measuring enzyme activity at various pH levels (pH 4–10). Various buffers such as sodium-acetate (50 mM, pH 4–5), sodium phosphate buffer (50 mM, pH 6), disodium hydrogen phosphate buffer (50 mM, pH 7), Tris–HCl (pH 8) and Glycine–NaOH (50 mM, pH 9–10) were used.
2.5.4. Effect of various inhibitors on protease activity
The effect of EDTA, PMSF and 2-mercaptoethanol on protease activity was determined by measuring the enzymatic activity of protease in the presence of various concentrations (5 mM and 10 mM) of these compounds.
2.5.5. Effect of metal ions on protease activity
The effect of different metal ions such as Mg2+, Ca2+, Cu2+, Mn2+, Zn2+, Ba2+, K+, Co2+ and Hg2+ on the activity of protease was determined by pre-incubation of enzyme solution with 5 mM of these metals in a 1:1 ratio at 37 °C for 30 min. After that aliquots were taken for the determination of enzyme activity under standard assay conditions.
2.5.6. Effect of detergents and oxidizing agent on protease activity
In order to determine the influence of different detergents such as SDS, Triton X-100 and Tween 80, and oxidizing agent hydrogen peroxide on the catalytic activity of protease, 1% of these compounds were pre-incubated with enzyme solution in a 1:1 ratio for 30 min at 37 °C. Afterwards, aliquots were taken to measure the activity under defined assay conditions.
3. Results and discussion
3.1. Production of protease from bacterial strain
Various bacterial strains were isolated from water samples of a thermal spring. All these strains were screened for proteolytic activity on casein agar medium. Among these strains, Bacillus sp. showed high proteolytic activity (10–12 mm) on casein agar medium selected for further studies (Fig. 1). Then the Bacillus sp. was subjected for protease production by submerged fermentation technology.
Figure 1.

Qualitative screening of bacterial strains for proteolytic activity on casein agar medium.
3.2. Purification of protease
The crude solution containing the active extracellular protease with a specific activity of 417 U mg−1 was purified through various purification steps summarized in Table 1. Initially the enzyme was purified by ammonium sulphate precipitation and 80% ammonium sulphate precipitated maximum protease. During this step of purification, the enzyme was purified up to 3.3 fold with 66% recovery from total crude enzyme. After that, this partially purified enzyme was subjected to CM- Cellulose ion exchange column for further purification. The specific activity of protease was increased from 1392 to 5644 U mg−1 and the enzyme was purified up to 13. 5 fold with 36% yield. The concentrated protease obtained as an unbound fraction CM-Cellulose cation exchanger was loaded on anion exchanger DEAE-Cellulose column. The enzyme presented in bound fractions of DEAE-Cellulose column was finally purified up to 16.5 fold with specific activity of 6900 U mg−1. Total 16% enzyme was recovered from crude solution after performing these purification techniques. However, the autolysis due to structural unfolding might occur during purification and could be the main reason for the low enzyme yield [8], [30].
Table 1.
Purification steps of alkaline protease from Bacillus sp.
| Purification steps | Total activity (Units) | Total protein (mg) | Specific activity (U/mg) | Fold purification | Activity yield (%) |
|---|---|---|---|---|---|
| Crude enzyme | 386,460 | 925 | 417 | 1.0 | 100 |
| CM-cellulose | 141,120 | 25 | 5644 | 13.5 | 36 |
| DEAE-cellulose | 62,100 | 9.0 | 6900 | 16.5 | 16 |
3.3. Characterization of protease
3.3.1. Effect of temperature on the activity and stability of protease
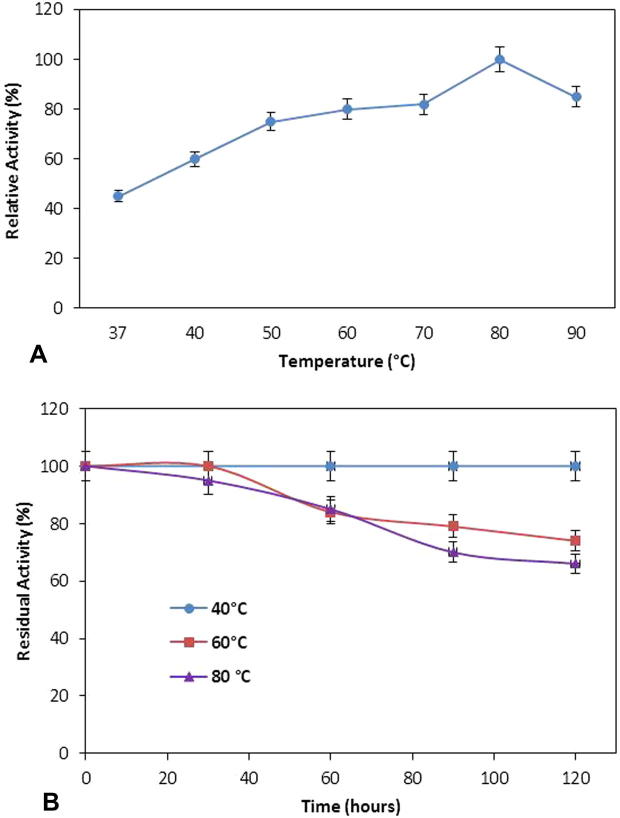
The effect of temperature on protease activity was determined by performing the enzyme assay at various temperatures ranging from 37 °C to 90 °C. It was observed that the catalytic activity of protease was increased by increasing the temperature from 37 °C to 70 °C and reached at maximum at 80 °C (Fig. 2A). Beyond 80 °C, protease lost more than 20% activity. The optimum temperature has been reported previously between 30 °C and 70 °C for Bacillus sp. protease [14], [22]. The temperature increases the kinetic energy of substrate and enzyme molecules, and both molecules are more likely to collide with one another at higher temperatures but at certain level the temperature started to denature the enzyme molecules; therefore the enzyme activity was declined at 90 °C due to the temperature denaturation effect.
Figure 2.
Effect of different temperatures on protease activity (A) and stability (B) from Bacillus sp. (means ± S.E., n = 6).
The reversible and irreversible effect of temperature on the enzyme can be determined by comparison of the activity and stability profile curve of enzyme against various temperatures. So the thermal stability of protease was determined by pre-incubation of enzyme at 40 °C, 60 °C and 80 °C for different time intervals. The thermal stability of enzyme represents the capability of an enzyme to resist thermal denaturation and it is one of the major factors for the commercialization of enzymes in industries. The maximum stability of protease from Bacillus sp. was observed at 40 °C with retention of 100% residual activity after incubation of 120 h (Fig. 2B). However, the stability of protease declined after a further increase in temperature and at 60 °C, protease lost more than 20% of its initial activity after 120 h whereas, at 80 °C the protease lost more than 40% activity. Previously it was reported that the purified protease enzyme showed maximum heat resistant at 55 °C [1]. The thermal stability of enzyme towards high temperature for a long period makes it more applicable in industrial processes. The thermal stabilization of enzyme is promoted by the three dimensional structure of enzyme that is highly dependent on the interactions of amino acids in its primary structure through hydrogen bonding, salt bridges and ring-ring interactions. The incubation of enzyme at higher temperature for a long period of time increases the kinetic energy of molecules which destroy the three dimensional structure of enzyme at a certain level by breaking the different internal interactions between amino acids that are responsible for maintaining the structure of enzyme and the enzyme ultimately loses its activity.
3.3.2. Effect of pH on the activity and stability of protease
The pH is one of most important physical factors that has a significant impact on the activity of any enzyme. The effect of pH on the catalytic activity of protease was analysed by measuring the enzyme assay in various levels of pH medium. Bacillus sp. protease showed a broad range activity at a wide range of pH and maintained more than 80% of its relative activity at pH range of 7.0–10 (Fig. 3). The activity of protease was increased by increasing the pH from 4.0 to 8.0 and reached its maximum at pH 8. The activity started to decline when the pH of the reaction medium further increased beyond 8.0 and more than 20% relative activity was lost at pH 10. The microbial proteases were mostly reported to have optimum pH in alkaline range [10], [11], [18].
Figure 3.
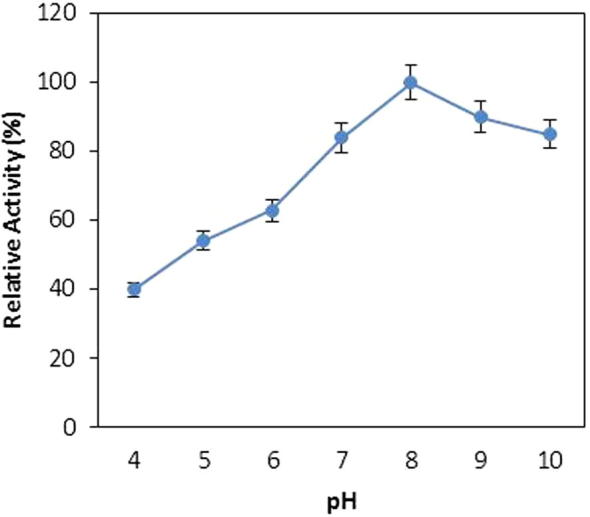
Effect of various pHs on protease activity from Bacillus sp. (means ± S.E., n = 6).
3.3.3. Effect of different inhibitors on the activity of protease
The effect of various inhibitors on the activity of Bacillus sp. protease was determined by pre-incubation of enzyme separately with various concentrations (5.0 mM and 10 mM) of EDTA, PMSF and 2-mercaptoethanol in 0.1 M Tris–HCl buffer of pH 8.0 at 37 °C for 30 min. The proteolytic activity of enzyme was partially then entirely inhibited with 5 mM and 10 mM PMSF, respectively (Fig. 4). PMSF is known as compound to inhibit both thiol as well as serine proteases [21]. This extracellular and alkaliphilic protease secreted from Bacillus sp. may possibly be categorized as serine-type protease. 2-Mercaptoethanol enhanced the proteolytic activity of protease up to 2 fold as compared to control. A similar observation has been previously documented about the Bacillus mojavensis protease activation due to 2-mercaptoethanol [25]. On the other hand the EDTA could not inhibit the activity of protease. The results indicate that this protease can be categorized into thiol-dependant-serine proteases.
Figure 4.
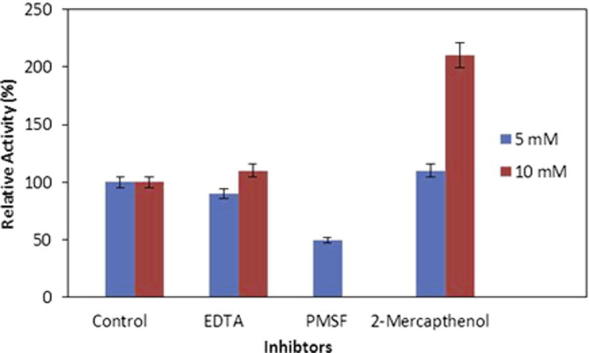
Effect of various inhibitors on protease activity from Bacillus sp. (means ± S.E., n = 6).
3.3.4. Effect of various metals on the activity of protease
The effects of various metal ions such as Ca2+, Mn2+, Mg2+, Ba2+, Zn2+, Cu2+, Co2+ and Hg2+ on the activity of protease were tested by pre-incubation of 5 mM of these metal ions for 30 min at 37 °C individually with the enzyme solution (Table 2). The activity of protease was enhanced up to 43% and 31% in the presence of Ca2+ and Mn2+ respectively as compared to control, indicating that these ions have stimulating effects on protease activity. A similar investigation has been reported previously that Mn2+ and Ca2+ stimulate the protease activity [23], [15], [10]. Metal ions are known to function as cofactor for enzyme activities and after act as ion or salt bridges among two nearby amino acid residues preserve the rigid confirmation of enzyme molecules. The activity of protease was reduced in the presence of, Ba2+, Zn2+, Cu2+, Fe2+, Mg2+, Ba2+, Zn2+ and Li2+. More than 70% activity of protease reduced by Hg2 as compared to control. Protease inhibition by Hg2+, Cu2+, Fe2+, Mg2+, Ba2+, Zn2+ and Li2+ was also reported by Bhatiya and Jadeja [6]. The protease from Brevibacillus (Bacillus brevis) was also inhibited by Hg2+, Zn2+ and Cu2+ [4]. These metal ions chemically react with the protein thiol-group in addition to tryptophan and histidine amino acid in the polypeptide chain of enzyme. Moreover, the disulphide bonds were noted to hydrolytically breakdown in the presence of silver and mercury metal ions [18].
Table 2.
Effect of different metal ions (5 mM), detergents and oxidizing agents (1%) on the activity of protease from Bacillus sp. after 30 min at 37 °C.
| Enzyme activity (U/mg) | Relative activity (%) | |
|---|---|---|
| Metal ions (5 mM) | ||
| Ca2+ | 9660 | 140 |
| Mn2+ | 8970 | 130 |
| Mg2+ | 5520 | 80 |
| Zn2+ | 5175 | 75 |
| Ba2+ | 5106 | 74 |
| Cu2+ | 3450 | 50 |
| Co2+ | 3105 | 45 |
| Hg2+ | 2070 | 30 |
| Control | 6900 | 100 |
| Detergents and oxidizing agents (1%) | ||
| Tween-80 | 5520 | 80 |
| SDS | 5175 | 75 |
| Triton-X-100 | 4140 | 60 |
| Hydrogen peroxide | 3105 | 45 |
| Control | 6900 | 100 |
3.3.5. Effect of different surfactants and detergents on the activity of protease
A good quality protease should also be steady and active in the presence of a range of various surfactants, bleaching agents and detergents, besides its pH and temperature stability. One of the prominent aspects of this Bacillus sp. producing protease is its stability towards Tween-80, SDS, Triton X-100 and oxidizing agents H2O2 (Table 2). This remarkable stability towards SDS, oxidizing agents and surfactant is significant because enzyme with oxidation and SDS stability from wild type microorganism are not commonly known except from few reports [28], [27], [17], [7]. Protein engineering and recombinant DNA technology have been applied to produce high quality of a bioengineered enzyme which is stabile in various industrial conditions. Purafect, Durazym and Maxapem are commercially available proteases in which bleach and oxidizing-stability has been invaded through protein engineering techniques and site directed mutagenesis [11], [13]. However, present Bacillus sp. excreted a novel thermophilic and alkaliphilic protease which has an edge over all the other available commercial proteases, this enzyme already has substantial oxidation stability and could be applied in various industrial preparations.
4. Conclusion
The protease from thermophilic Bacillus sp. showed excellent catalytic performance at various physico-chemical conditions. The protease showed activity in the presence of detergent, surfactant and oxidizing agent at elevated temperature of 80 °C. Bacillus sp. protease with its novel catalytic properties can be applied in various detergent industries. The enzyme might be a potential contribution to the existing protease family as well as emerging enzyme industry in Pakistan.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.M.S. Ammar, R.A. Bayoumi, A.M.H. El-Kasaby, A.M. Soliman, 5th Int Sci Conf, Al Azhar Univ, Fac Sci, Cairo, Egypt, 2003, pp. 54.
- 2.Anson M.L. J. Gen. Physiol. 1938;22:79–89. doi: 10.1085/jgp.22.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anwar A., Saleemuddin M. Bioresour. Technol. 1998;64:175–183. [Google Scholar]
- 4.Banerjee U.C., Sani R.K., Azmi W., Soni R. Proc. Biochem. 1999;35:213–216. [Google Scholar]
- 5.Beg Q.K., Sahai V., Gupta R. Proc. Biochem. 2003;39:203–206. [Google Scholar]
- 6.Bhatiya R., Jadeja G.R. EJEAF Chem. 2010;9:594–599. [Google Scholar]
- 7.Blumentals I.I., Robinson A.S., Kelly R.M. Appl. Environ. Microbiol. 1990;56:1992–1998. doi: 10.1128/aem.56.7.1992-1998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chomsri N. The Graduate School, Chiangmai University; Chiangmai: 2001. Thermostable Protease Enzymes. Master Thesis in Biotechnology. [Google Scholar]
- 9.Cowan D.A. Industrial enzymes. In: Moses V., Cape R.E., editors. Harwood Academic Publishers; Switzerland: 1994. pp. 326–328. (Biotechnology, The Science and The Business). [Google Scholar]
- 10.Durham D.R., Stewart D.B., Stellwag E.J. J. Bacteriol. 1987;169:2762–2768. doi: 10.1128/jb.169.6.2762-2768.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erikson N. 2nd ed. The Macmillan Publisher Ltd; 1987. Industrial Enzymology; pp. 1–8. [Google Scholar]
- 12.Folin O., Ciocalteu V.J. J. Biol. Chem. 1929;73:627–650. [Google Scholar]
- 13.Gupta R., Beg Q.K., Lorenz P. Appl. Microbiol. Biotechnol. 2002;59:15–21. doi: 10.1007/s00253-002-0975-y. [DOI] [PubMed] [Google Scholar]
- 14.Huang G.R., Ying T.J., Huo P., Jiang J.X. Afr. J. Biotechnol. 2006;5:2433–2438. [Google Scholar]
- 15.Jaswal R.K., Kocher G.S. Internet J. Microbiol. 2007;4:1–8. [Google Scholar]
- 16.Kalisz H.M. Springer-Verlag, Berlin Heidelberg; 1988. Microbial proteases; pp. 1–65. (Advances in Biochemical Engineering/Biotechnology). [Google Scholar]
- 17.Klingeberg M., Galinsky B., Sjoholm C., Kasche V., Antranikian G. Appl. Environ. Microbiol. 1995;61:3098–3104. doi: 10.1128/aem.61.8.3098-3104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar C.G., Tiwari M.P. Biotechnol. Tech. 1999;13:224–235. [Google Scholar]
- 19.Ladenstein R., Antranikian G. Adv. Biochem. Eng. Biotechnol. 1998;61:37–85. doi: 10.1007/BFb0102289. [DOI] [PubMed] [Google Scholar]
- 20.Lowry O.H., Rosembrough N.J., Farr A.L., Randall R.J. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 21.Mikola L., Mikola J. Occurrence and properties of different types of peptidases in higher plants. In: Dalling M.J., editor. Vol. 1. CRC Press Inc; Boca Raton, Florida: 1986. pp. 97–117. (Plant Proteolytic Enzymes). [Google Scholar]
- 22.Morya V.K., Yadav D. Internet J. Microbiol. 2010;8:1–7. [Google Scholar]
- 23.Nascimento W.C.A., Martins M.L.L. Braz. J. Microbiol. 2004;35:9–96. [Google Scholar]
- 24.Niehaus F., Bertoldo C., Kahler M., Antranikian G. Appl. Microbiol. Biotechnol. 1999;51:711–729. doi: 10.1007/s002530051456. [DOI] [PubMed] [Google Scholar]
- 25.Qasim K.B., Rani G. Enzyme Microb. Technol. 2003;32:294–304. [Google Scholar]
- 26.Rao M.A., Tankasale A., Ghatge M., Desphande V. Microbiol. Mol. Biol. Rev. 1998;62:597–634. doi: 10.1128/mmbr.62.3.597-635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saeki K., Hitomi J., Okuda M., Hatada Y., Kageyami Y., Takaiwa M., Kubota H., Hagihara H., Kobayashi T., Kawai S. Extremophiles. 2002;6:65–72. doi: 10.1007/s007920100224. [DOI] [PubMed] [Google Scholar]
- 28.Takami H., Kobayashi T., Aono R., Horikoshi K. Appl. Microbiol. Biotechnol. 1992;38:101–108. doi: 10.1007/BF00169427. [DOI] [PubMed] [Google Scholar]
- 29.Ward O.P. Applied Science Publishers; London: 1983. Proteinases; pp. 251–317. (Microbia1 Enzymes and Biotechnology). [Google Scholar]
- 30.Ward O.P. Comprehensive Biotechnol. 1985;3:789–818. [Google Scholar]