Abstract
The microalga biomass production from confectionary effluent is a possible solution for the urgent need for a live food in aquaculture. Arthrospira (Spirulina) platensis was the dominant alga in effluent of “Biscomisr a confectionary factory”, in Alexandria–Egypt. Therefore, it was isolated from the effluent samples and used throughout the study. The cyanobacterium, A. platensis was grown on the effluent using 22 Central Composite Design (22 CCD). This work addresses the best effluent dilution (WC, %) as well as sodium bicarbonate concentration (SBC) on the alga growth and biochemical composition. Total protein, carbohydrate, lipid contents and fatty acid profiles of the produced algal biomass were highly improved. The statistical analyses suggested that the main effect of (WC, %) is significant negative influences on the algal contents of proteins, lipids and carbohydrates (p > 0.01). Although it had a significant positive influence on chlorophyll (p > 0.01), no significant effect on algal β carotenes (p > 0.05) had been reported. The inter action effect of SBC together with WC, % exerted a significant negative influence on the algal proteins (p > 0.01) and no significant effect on the other responses (p > 0.05). The produced alga biomass was used for feeding the rotifer, Brachionus plicatilis for further application in aquaculture. Growth rate, reproductive rate and fecundity attributes, fatty acid content of B. plicatilis were amended. The Pearson correlation test indicated that β carotenes displayed a highly positive significant correlation with the growth rate of B. plicatilis (r = 0.733, p < 0.01) and the carbohydrates showed significant positive correlations with Egg % (r = 0.657, p < 0.05).
Keywords: Arthrospira platensis, Brachionus plicatilis, Feeding, Wastewater
1. Introduction
Arthrospira (Spirulina) platensis is a commercially important filamentous cyanobacterium that is grown in large scale and processed industrially [23]. It is now produced by several companies and sold in many health food stores around the world because it serves as a rich source of protein, vitamins, minerals, essential fatty acids, essential amino acids and pigments such as carotenoids for food, chemical and pharmaceutical industry [58], [1], [5]. It is the major known source of vitamin B12, having high protein content [28]. Due to lack of cellulose in its cell wall, 85–95% is assimilated by the organism [9]. It is also used in aquaculture as provides food for zooplankton, fish, crustacean, shellfish and bivalve cultures [41]. Also, it is applied in wastewater treatment and agriculture [58]. Biotechnological processes based on cyanobacteria have been receiving increasing interest due to their potential to produce a diverse range of chemicals and biologically active compounds [49], [61].
The rotifer, Brachionus plicatilis, is an important food organism for the first feeding stages of larval marine animals around the world. It is a cosmopolitan euryhaline species, thus it is very versatile in marine and fresh cultures. The rotifer varies in size depending on strain and culture conditions, with adult size ranging from 123 to 315 μm in length. This allows strains to be cultured for a specific size [77], [69]. Rotifers themselves have little nutritional value and only act as nutrient carriers. Therefore, rotifers are only as good as the food you feed them. It is most important that they be fed foods rich in the essential highly unsaturated fatty acids (HUFAs) before being fed to marine larvae. Several microalgae species provide these fatty acids as well as other needed nutrients [19].
Live feeds make up an essential part of larval rearing [20] and also provide an effective vehicle for the administration of probiotic cells [57]. Rotifers play an essential role as a preliminary food component to produce fish and crustacean fry for aquaculture [76], as they have a high enzymatic content which aids in the development of larval digestive systems [21]. Fish larvae can be fed Spirulina either through gut-loaded rotifers and Artemia, or through formulated microparticulate diets [51].
The confectionary industry is a major enterprise in Egypt, occupying a significant place in food supply (Egyptian Pollution Abatement Project (EPAP) [25], [27]). Generally, the raw effluent produced by confectionery industry is highly qualified by its organic content, that is composed of readily biodegradable compounds such as sugars, sweeteners, casein, vegetable oils, condensed milk, food coloring and flavoring agents, etc. [67].
There are many studies in the literature on the cultivation of Arthrospira on different wastes like kitchen wastewater [59], swine wastewater [50], agro industrial wastes and wastewaters [48] and crude oil [26]. However, there are no studies on the cultivation of A. platensis in effluent originating from the confectionary industry.
Therefore, this work aimed at attaining a double benefit of A. platensis through growing it on the confectionary effluent to be treated and to use the growing alga with the best biochemical composition for feeding the rotifer, B. plicatilis for further application in aquaculture. The collected samples were the final effluent of “Biscomisr” a confectionary factory collected from the region of discharge at the water body just before it reaches Abu Qir bay. Then the microalga flora was identified and the dominant species was isolated from the samples. The study was extended to determine the ideal effluents dilution as well as sodium bicarbonate concentration for maximum biomass production, in addition to proteins, carbohydrates and lipids production using 22 Central Composite Design (22 CCD). Fatty acid profile of the control cyanobacteruim A. platensis was compared with that giving the best growth on the diluted collected sample. Finally, the alga grown during the different experimental runs of the 22 CCD was used for feeding the rotifer, B. plicatilis for further application in aquaculture. The rotifer growth was monitored through the rotifer population density, the percentage of eggs and the daily Specific Growth Rate (SGR). In addition, fatty acid profile of the best growing B. plicatilis was also characterized to be compared with that of the control rotifer.
2. Materials and methods
2.1. The samples characterization
The samples were collected and stored under cooling to 4 °C. Physio-chemical analyses of the sample were carried out following the methods described by APHA [6]. The phytoplankton samples were immediately fixed with 4% formaldehyde for laboratory analysis. Phytoplankton samples were counted and identified using 2-mL settling chambers with a Nikon TS 100 inverted microscope at 400× magnification using [72] method, the dominant algal strain, A. platensis was used throughout the study work.
2.2. The alga strain and growth conditions
The A. platensis strain was isolated and purified in axenic cultures and used throughout this study. The preparation and maintenance of the inoculum were accomplished using Zarrouk medium according to [60], [62], standard for the cultivation of this microalga. Microalgal cultures were examined under a microscope (Olympus HB) and identified. After algal isolation, the culture medium for the runs was the collected sample itself, which was used after dilution according to the 22 CCD. The alga A. platensis cells were inoculated at a concentration of 20% (V inoculation/V media) in 500 mL Erlenmeyer flasks incubated at a thermo-statically controlled environmental chamber at 25 ± 1 °C with a continuous lightening for 15 days. Samples were taken at 3 days intervals for assessment of the cyanobacteria growth as well as estimation of biochemical composition and the pigments content of A. platensis. The experiment was carried out in triplicate, and average values were recorded.
2.2.1. Assessment of A. platensis growth
Due to varying lengths of the A. platensis filaments, they could not be counted to represent its biomass. The algal growth was assisted by measuring the optical density of the algal suspension at 680 nm [16] using spectrophotometer Spekol 1300, Analytika Jena, Spain for each experimental run of the 22 CCD.
2.2.2. Biochemical composition of A. platensis
Analyses of the A. platensis biomass produced by the end of each experimental run were carried out. Total protein was determined by the Folin-phenol method of Lowery et al. [47]. Total carbohydrate content was estimated according to the methods of Dubois et al. [24]. Lipid contents were analyzed gravimetrically after extraction with chloroform–methanol (2:1) using the Folch method as modified by Bligh and Dyer [12]. The fatty acid profiles of the tested alga and that of B. plicatilis were analyzed using gas liquid chromatography (GC system Hp, Germany, serial No. 6890 D 1530 A serial DE 00000348) equipped with a flame ionization detector; the packing column material was SP-2340.
2.2.3. Pigments estimation
A known volume of A. platensis culture was centrifuged at 8000 rpm for 10 min and the pellet was extracted with known volume of ethyl alcohol and kept in water bath for 30 min at 60 °C, and then centrifuged again. Absorbance of the pooled extracts was measured on a spectrophotometer Spekol 1300, Analytika Jena, Spain at 650, 665, and 452 nm. Calculations were made according to the formulae described by Sengar [66] for chlorophyll and carotenoids.
2.3. Rotifer cultures
The rotifer, B. plicatilis was cultured at temperature 26 °C and salinity 24 ppt in ten plastic square tanks with 1.0 m3 capacity (one tank for each treatment food regime) at day light intensity starting with density 25 individuals/mL and collected after 3 days through 50 μm mesh size plankton net and rinsed with clear sea water, and then enriched again for short term enrichments in ten plastic containers with that of the cultures of the tested alga for each experimental run of the 22 CCD. Various A. platensis diets equivalent to (20 mg of dry powder per one cubic meter of tank capacity) produced by culturing through each of the ten experimental run of the 22 CCD were fed to the rotifer, in addition to the control rotifer. For each experimental run, three replicates were prepared to determine the assimilation of this food by the rotifers [7], [78]. Whenever, the culture water got cleared of algae, freshly harvested quantity of algae was fed to the rotifer tanks.
2.3.1. Monitoring rotifers growth
A sample of 250 μL was removed from the flasks and another from the tanks for counting rotifers under a dissecting microscope. Then we multiplied by the tank volume to determine the rotifer population density. The percentage of eggs was determined by dividing the number of females carrying eggs by the total number of rotifers found in the volume of 250 μL then multiply by 100. The daily Specific Growth Rate was calculated using the following equation described by Rombaut et al. [63]: SGR = (ln Nt − ln N0)/t; where: SGR = Specific Growth Rate; Nt = rotifer density after culture period t (individuals mL−1); N0 = initial rotifer density (individuals mL−1); t = culture period (day). In addition, fatty acid profile of the B. plicatilis growing during the 4th experimental run of 22 CCD was estimated as previously mentioned.
2.3.2. Factorial design
In this study, 22 Central Composite Design (22 CCD) was employed. Such designs are straightforward to implement, and their results can be very easily interpreted; these methods are created to measure the additive effects on a response for each of the input factors. In addition, the effects of interactions between factors can also be investigated. Statistical experimental designs have many advantages and were used in many successful degradative studies [64]. It also results in saving tremendous amount of time [8]. Table 3 shows the real and coded values of the variables used in the (22 CCD).
Table 3.
Coded levels and real values of waste effluent concentration (WC, %) and sodium bicarbonate concentration (SBC, g L−1) and results proteins content %, lipids content %, chlorophyll (mg L−1) and β carotenes (mg L−1) in the 22 CCD runs.
| Run | Factor 1 | Factor 2 | Response 1 | Response 2 | Response 3 | Response 4 | Response 5 |
|---|---|---|---|---|---|---|---|
| WC, % | SBC, g L−1 | Proteins content % | Lipids content % | Carbohydrates content % | Chlorophyll (mg L−1) | β Carotenes (mg L−1) | |
| 1 | 8.5 | 6.5 | 50 | 14.0 | 14.0 | 19.2 | 6.4 |
| 2 | 26.5 | 6.5 | 68 | 15.2 | 14.0 | 18.8 | 6.8 |
| 3 | 8.5 | 13.5 | 66 | 13.2 | 13.3 | 19.8 | 6.8 |
| 4 | 26.5 | 13.5 | 63 | 12.6 | 12.2 | 19.2 | 6.3 |
| 5 | 5.0 | 10 | 54 | 10.4 | 12.3 | 19.8 | 6.9 |
| 6 | 30.0 | 10 | 67 | 12.3 | 10.4 | 20.5 | 7.7 |
| 7 | 17.5 | 5 | 59 | 14.4 | 14.0 | 21.0 | 7.8 |
| 8 | 17.5 | 15 | 65 | 14.1 | 14.3 | 18.0 | 6.5 |
| 9 | 17.5 | 10 | 65 | 14.3 | 13.5 | 17.8 | 6.6 |
| 10 | 17.5 | 10 | 65 | 14.2 | 13.4 | 17.9 | 6.6 |
3. Results
3.1. Physicochemical characteristics of the collected sample
The results presented in Table 1 reveal that the values of pH, TS, TDS and TOC, SO4, TP, TN%, obtained for the collected sample used in this study were (6.5, 1510 mg L−1, 473 mg L−1, 254.79 mg L−1, 35.18 mg L−1, 8.64 mg L−1 and 0.02 respectively). However, COD (61.2 mg O2 L−1). Additionally, the elements’ concentrations of Na, K, Ca and Mg were 26 mg L−1, 60 mg L−1, 160.32 mg L−1 and 589.68 mg L−1, respectively.
Table 1.
Estimated values of different parameters in the confectionary waste effluent sample.
| Parameter | Unit | Sample |
|---|---|---|
| pH | – | 6.5 |
| Oil and grease | mg L−1 | 1730 |
| TS | mg L−1 | 1510 |
| TDS | mg L−1 | 473 |
| TOC | mg L−1 | 254.79 |
| SO4 | mg L−1 | 35.18 |
| TP | μg L−1 | 8.64 |
| TN % | % | 0.02 |
| COD | mg O2 L−1 | 3150 |
| BOD | mg O2 L−1 | 1340 |
| Na | mg L−1 | 26 |
| K | mg L−1 | 60 |
| Ca | mg L−1 | 160.32 |
| Mg | mg L−1 | 589.68 |
TOC: Total Organic Carbon; SO4: Sulfate; TP: Total Phosphorus; TN %: Total Nitrogen %; Na: Sodium; K: Potassium; Ca: Calcium; COD: Chemical Oxygen Demand; Ca: Calcium; Mg: Magnesium.
3.2. Microalgae flora present in the sample
Algal flora occurring in the sample was identified. The results revealed that the species belonging to 3 families were identified during January, 2014 (Table 2). The mean total phytoplankton cell abundance was 177,390 cells L−1.
Table 2.
Taxonomic composition and proportional representation of the microalgae groups at confectionary waste effluent sample.
| Group | Genus | Species | Cells L−1 | % |
|---|---|---|---|---|
| Cyanophyta | 5 | 5 | 77,760 | 43.8 |
| Chlorophyta | 6 | 8 | 75,330 | 42.5 |
| Bacillariophyta | 4 | 7 | 24,300 | 13.7 |
| Euglenophyta | 0 | 0 | 0 | 0 |
| Total | 15 | 20 | 177,390 | 100 |
The temporal pattern showed the presence of 20 taxons recorded that cyanobacteria made up the highest number (43.8%) represented by 5 genera, 5 species. Cyanophyta is represented by A. platensis, Chroococcus dispersus (Kels.), Dactylococcopsis acicularis, Merismopedia punctata Lemm., Oscillatoria limnetica Lemm followed by Chlorophyta (42.5%), 6 genera and 8 species, including Ankistrodesmus falcatus (Corda) Ralfs, A. falcatus var. mirabilis West & G.S.West, Closterium acutum, Crucigenia rectangularis (Nag.) Gay, Scenedesmus obliquus, Scenedesmus quadricauda (Turp.) Breb., Tetraedron minimum, Tetraedron trigonum. Then Bacillariophyta (13.70%); (4 genera, 7 species), Melosira granulata, Navicula gracilis Kutz., Nitzschia acicularis, Nitzschia frustulum, Nitzschia palea, Synedra tabulate, Synedra ulna. However, Euglenophyta was completely absent. A. platensis is the most dominant species of Cyanophyta. Therefore, it was isolated, identified and used throughout this experimental work.
3.3. Cultivation of A. platensis for improving the biochemical constituents
3.3.1. Alga growth
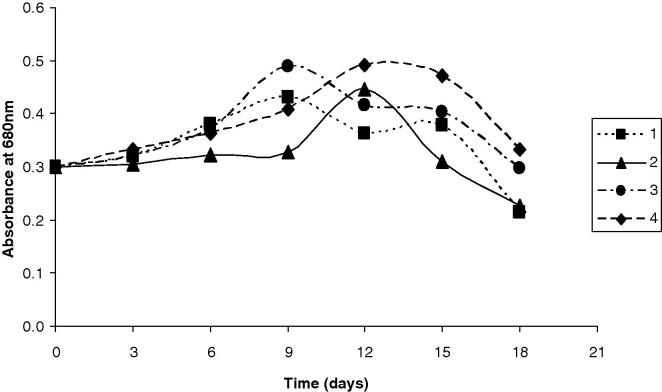
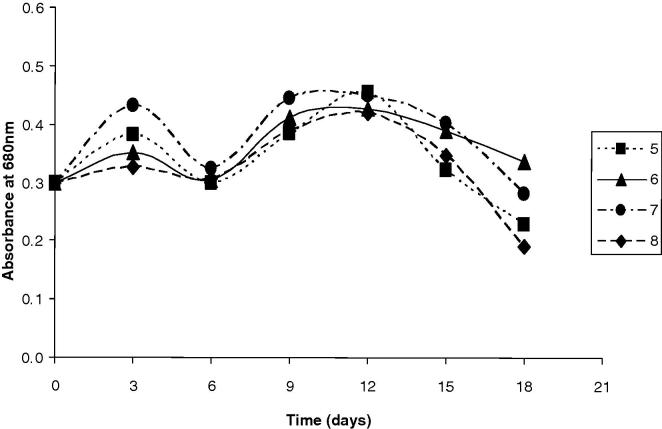
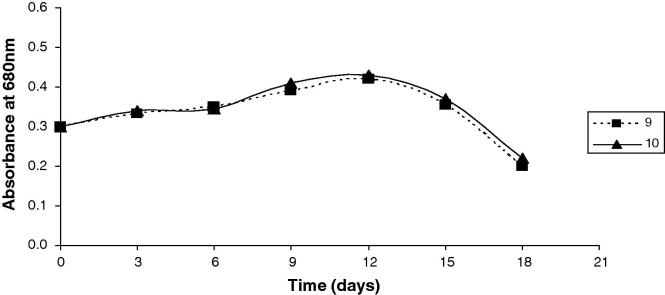
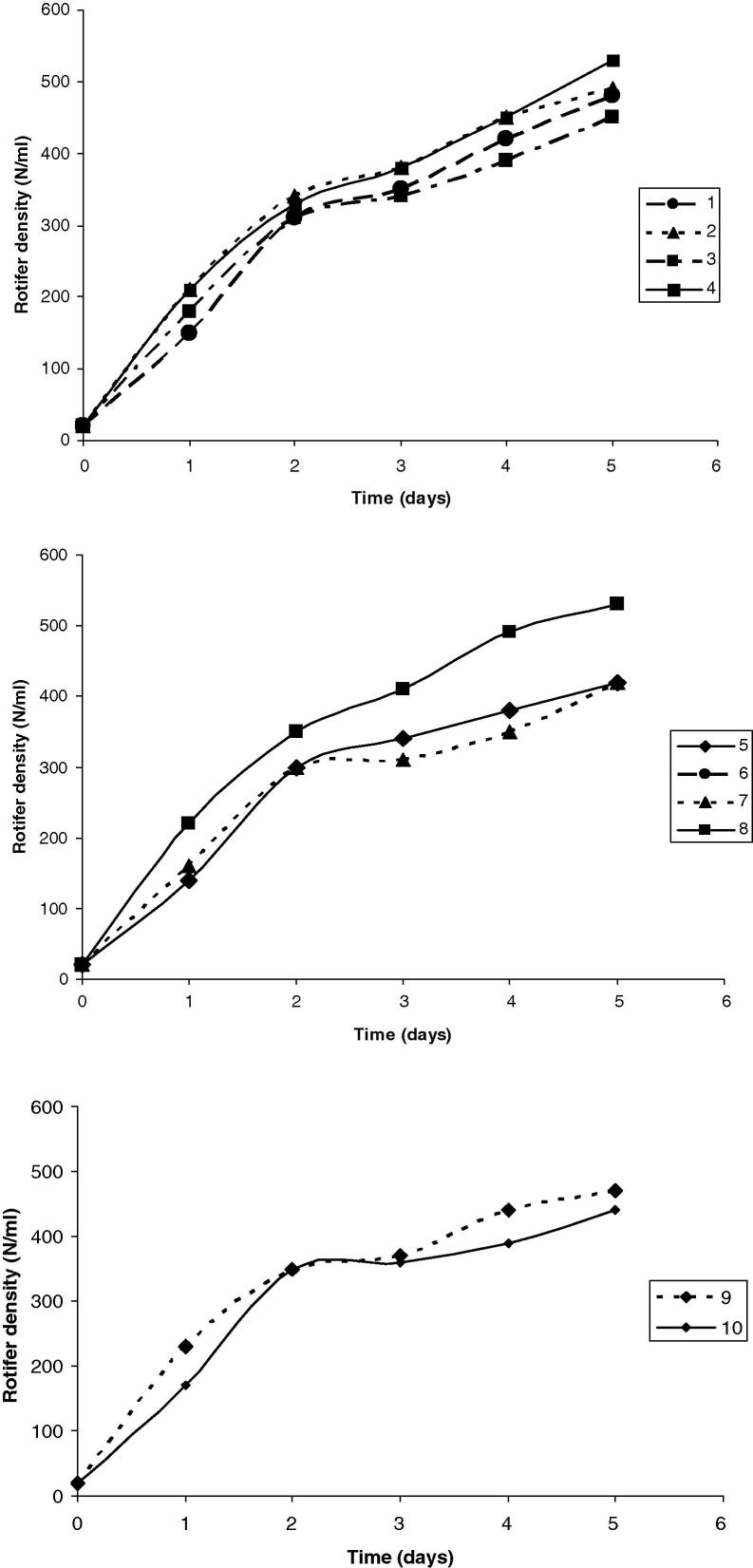
The results presented graphically in Figure 1, Figure 2, Figure 3 along with that in Table 3, show the OD680 of A. platensis obtained using different effluent loads for each run of the CCD. During the 3rd run, with the higher SBC (13.5%), compared to 1st run (6.5%), the values of the OD680 were 20% higher than the control after 12 days. The increase in the OD680 was accomplished by improvements in the biochemical composition of algal cells. Surprisingly, β carotenes (mg L−1) was spiked into the double. Proteins, lipids, and carbohydrates contents accreted 20%, 22.2% and 22.3%, respectively. However, chlorophyll decreased by 12% as the biomass became pale and lost its typical blue-green color. The 5th run, which was complemented with the smallest WC (5.0%), SBC of 10.0 g L−1, reported OD680 higher by 51.6% after 12 days. The increase in OD680 was seasoned by decrease in proteins content and lipids contents by 1.8%, 3.7%, respectively. However, carbohydrate content, chlorophyll and β carotenes augmented by 13.3%, 15.1% and 109%, respectively. The 2nd run (WC = 26.5%; SBC = 6.5 g L−1), 4th (WC = 26.5%; SBC = 13.5 g L−1) and 6th run (WC = 30.0%; SBC = 10.0 g L−1) presented cellular divisions where the OD680 unexpectedly improved by nearly 48%, 64%, 42% after 12 days for the 2nd, 4th and 6th runs, respectively. Parallel to the cellular divisions, the estimated algal biochemical constituents were highly improved, especially during the 4th run of the 22 CCD. Therefore, the algal biomass grown during the 4th run of the 22 CCD was selected for further estimation of the fatty acids. The comparison of 7th run (WC = 17.5%; SBC = 5.0 g L−1) and 8th (WC = 17.5%; SBC = 15.0 g L−1), revealed that the addition of sodium bicarbonate in the superior level resulted in the improvement of the cellular contents of proteins, lipids, carbohydrates, chlorophyll and β carotenes by 18%, 30.5%, 32.4%, 4.6% and 97%, respectively. Moreover, the result of OD680 was improved by 7%, 18% after 12 days, for 7th and 8th run, respectively. In the 9th and 10th runs, which are replicates (WC = 17.5%; SBC = 10.0 g L−1), the growth curves presented a similar behavior and log phase wherever the result of OD680 was peaked by 40%, 43% after 12 days, for 9th and 10th run, respectively. Considering the algal biochemical constituents, proteins, lipids, carbohydrates, chlorophyll and β carotenes were amended by 18%, 32%, 25%, 40% and 100%, respectively.
Figure 1.
Time course of Arthrospira platensis for 1–4 runs of the CCD. The 1st run (WC = 8.5%; SBC = 6.5 g L−1); the 2nd run (WC = 26.5%; SBC = 6.5 g L−1); the 3rd run (WC = 8.5%; SBC = 13.5 g L−1) and 4th run (WC = 26.5%; SBC = 13.5 g L−1). WC: wastewater concentration; SBC: sodium bicarbonate concentration.
Figure 2.
Time course of Arthrospira platensis for 5–8 runs of the CCD. The 5th run (WC = 5.0%; SBC = 10.0 g L−1); the 6th run (WC = 30.0%; SBC = 10.0 g L−1); the 7th (WC = 17.5%; SBC = 5.0 g L−1) and the 8th run (WC = 17.5%; SBC = 15.0 g L−1).
Figure 3.
Time course of Arthrospira platensis for the 9th and 10th runs of the CCD. The 9th run (WC = 17.5%; SBC = 10.0 g L−1) and 10th run (WC = 17.5%; SBC = 10.0 g L−1).
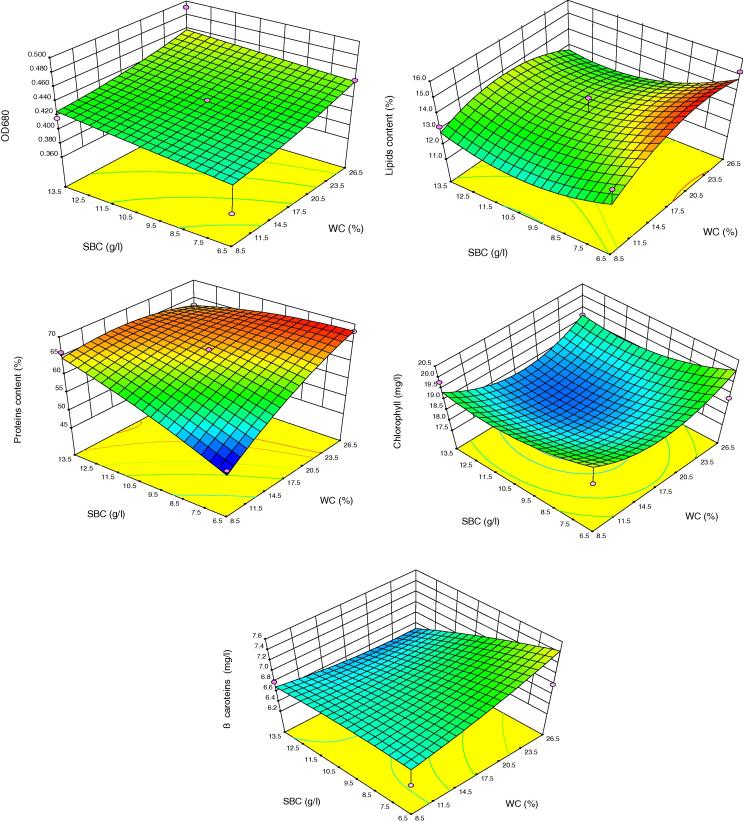
The results presented graphically in Fig. 4 show the surface responses of alga contents of proteins, lipids, chlorophyll and β carotenes due to waste concentration (WC, %) and sodium bicarbonate concentration (SBC, g L−1). Considering the main effects for SBC, it has a significant positive influence on the alga content of proteins and chlorophyll (p > 0.01). However, it has no significant influences on the other responses. WE exerted highly significant negative influences in the answers of proteins content, lipids, carbohydrates (p > 0.01). But it has highly positive significant influence on chlorophyll (p > 0.01). However, no significant effects on the response of algal β carotenes (p > 0.05) have been reported. The inter action effect of SBC together with WC, % exerted a significant negative influence on the responses of algal proteins (p > 0.01) and no significant influence on the other responses (p > 0.05).
Figure 4.
Interaction effect between waste effluent concentration (WC, %) and sodium bicarbonate concentration [SBC (g/L)] on OD680, proteins content %, lipids content % as well as chlorophyll (mg L−1), and β carotenes (mg Ll−1) of Arthrospira platensis.
3.3.2. The fatty acid methyl ester profile of the A. platensis
The fatty acid content of the A. platensis biomass cultivated on the 4th run of the 22 CCD (Table 4) revealed differences concerning the polyunsaturated fraction, where the control biomass had lower levels of C18:2c, and lower levels of monounsaturated fatty acids. The biomass produced from the cultures grown on the 4th run presented fatty acid content three times higher than that of the control biomass (7.22 g/100 g of sample). Unlike what was observed with A. platensis, the saturated fraction of fatty acids of the alga biomass was dominant when compared to the mono- and polyunsaturated fractions. The main components of the saturated fraction were Myristic Acid (C14:0) and Pentadecanoic Acid (C15:0). Heneicosylic Acid (C21:0) which was absent in the control alga, was represented in that grown in the 4th run of the CCD. The most important fatty acids in the monounsaturated fraction were cis-10-Pentadecenoic Acid (C15:1). The ω-7 unsaturated fatty acids were represented by Palmitoleic Acid (C16:1) and Oleic Acid (C18:1c) which appeared as the most important fatty acids in the polyunsaturated fraction. The ω-9 unsaturated were represented by Erucic Acid (C22:1).
Table 4.
Fatty acids methyl ester profile of Arthrospira platensis (control) and that grown on the 4th run of the 22 CCD for 12 days.
| Fatty Acid (FA) | Structural formula | Control | Treated |
|---|---|---|---|
| % FA | % FA | ||
| Capric Acid (C10:0) | CH3(CH2)8COOH | 0.003 | 0.016 |
| Undecylic Acid (C11:0) | CH3(CH2)9COOH | 0.017 | 0.044 |
| Lauric Acid (C12:0) | CH3(CH2)10COOH | 0.068 | 0.172 |
| Tridecylic Acid (C13:0) | CH3(CH2)11COOH | 0.281 | 0.769 |
| Myristoleic Acid (C14:1) | CH3(CH2)3CH CH(CH2)7COOH | 0.117 | 0.334 |
| Myristic Acid (C14:0) | CH3(CH2)12COOH | 0.476 | 1.350 |
| cis-10-Pentadecenoic Acid (C15:1) | C16H30O2 | 0.337 | 0.912 |
| Pentadecanoic Acid (C15:0) | CH3(CH2)13COOH | 0.554 | 1.544 |
| Palmitoleic Acid (C16:1) | CH3(CH2)5CH CH(CH2)7COOH | 0.194 | 0.508 |
| Palmitic Acid (C16:0) | CH3(CH2)14COOH | 0.222 | 0.814 |
| Linoleic acid (C18:2c) | CH3(CH2)4CH CHCH2CH CH(CH2)7COOH | 0.030 | 0.109 |
| Oleic Acid (C18:1c) | CH3(CH2)7CH CH(CH2)7COOH | 0.032 | 0.089 |
| Heneicosylic Acid (C21:0) | CH3(CH2)19COOH | – | 0.461 |
| Erucic Acid (C22:1) | CH3(CH2)7CH CH(CH2)11COOH | 0.0159 | 0.094 |
| Sat. FA | 1.62 | 5.26 | |
| Mono unsat. | 0.70 | 1.84 | |
| Poly unsat. | 0.03 | 0.12 | |
| ∑ FA in g/100 g of sample | 2.35 | 7.22 |
3.4. Rotifer growth characteristics
3.4.1. Culture of B. plicatilis with A. platensis
Rotifers reared on the microalga, A. platensis during the different experimental runs of the 22 CCD are presented graphically in (Fig. 5a–c). Monitoring the microalga growth in the different runs revealed a maximum OD680 value at 12th day followed by a phase of reduction. The rotifers were introduced when the microalga was in full growth phase. The density of the rotifers exceeded the 530 individuals/mL toward 5th day of their growth on the A. platensis of the 4th, 6th and 8th runs of the 22 CCD.
Figure 5.
Variation of Brachionus plicatilis population densities (N/mL) fed with a live Arthrospira platensis grown during the different experimental runs of the 22 CCD.
3.4.2. B. plicatilis hatched eggs%, growth rate
The growth patterns of the B. plicatilis reared on the algal strain yielded different net reproduction rates (Table 5), generally eliciting the highest rate during the 4th, 6th and 8th experimental run of the 22 CCD. In general, there were no clear trends as to which algal prey elicited the highest growth potential.
Table 5.
Hatched Eggs %, growth rate of Brachionus plicatilis grown on Arthrospira platensis of the different experimental runs of the 22 CCD for 12 days.
| Run number | Factor 1 | Factor 2 |
|---|---|---|
| GR | Egg % | |
| 1 | 0.02 | 23.3 |
| 2 | 0.06 | 27.7 |
| 3 | 0.02 | 18.6 |
| 4 | 0.06 | 25.0 |
| 5 | 0.07 | 25.0 |
| 6 | 0.11 | 28.3 |
| 7 | 0.06 | 20.0 |
| 8 | 0.11 | 33.3 |
| 9 | 0.02 | 23.3 |
| 10 | 0.06 | 20.0 |
3.4.3. Fatty acids methyl ester profile of B. plicatilis biomass
The fatty acid content of the B. plicatilis biomass grown on A. platensis of the 4th run of the 22 CCD exceeds that of the control by nearly 58% (Table 6). The saturated fraction of fatty acids of B. plicatilis biomass was dominant when compared to the mono- and polyunsaturated fractions (3.49 g/100 g of sample). The main components of the saturated fraction were Undecylic Acid (C11:0), Lauric Acid (C12:0), Tridecylic Acid (C13:0), Myristic Acid (C14:0), Pentadecanoic Acid (C15:0), Palmitic Acid (C16:0), Octadecanoic Acid (C18:0) and Eicosanoic Acid (C20:0). The most important fatty acid in the saturated fraction was Pentadecanoic Acid (C15:0). Generally, lower levels of monounsaturated fatty acids were observed. The monounsaturated fractions were cis-10-Pentadecenoic Acid (C15:1). The ω-7 unsaturated fatty acids were represented by Palmitoleic Acid (C16:1) and Oleic Acid (C18:1c) which appeared as the most important fatty acids in the polyunsaturated fraction. The ω-9 unsaturated were represented by Erucic Acid (C22:1) slightly increased in the treated sample than that in the control. The polyunsaturated fatty acids were completely absent from both control and treated samples.
Table 6.
Fatty acids methyl ester profile of Brachionus plicatilis biomass grown on Arthrospira platensis of the 4th experimental run of the 22 CCD.
| Fatty Acid (FA) | Structural formula | Control | Treated |
|---|---|---|---|
| % FA | % FA | ||
| Undecylic Acid (C11:0) | CH3(CH2)9COOH | 0.01 | 0.01 |
| Lauric Acid (C12:0) | CH3(CH2)10COOH | 0.10 | 0.09 |
| Tridecylic Acid (C13:0) | CH3(CH2)11COOH | 0.35 | 0.53 |
| Myristoleic Acid (C14:1) | CH3(CH2)12COOH | 0.17 | 0.19 |
| Myristic Acid (C14:0) | CH3(CH2)12COOH | 0.51 | 1.01 |
| Pentadecanoic Acid (C15:0) | CH3(CH2)13COOH | 0.66 | 1.14 |
| Palmitoleic Acid (C16:1) | CH3(CH2)5CH CH(CH2)7COOH | 0.38 | 0.42 |
| Palmitic Acid (C16:0) | CH3(CH2)14COOH | 0.41 | 0.57 |
| Oleic Acid (C18:1c) | CH3(CH2)7CH CH(CH2)7COOH | 0.02 | 0.09 |
| Octadecanoic AcidC18:0 | CH3(CH2)16COOH | 0.06 | 0.09 |
| Paullinic Acid (C20:1) | CH3(CH2)5CH = CH(CH2)11COOH | 0.01 | 0.14 |
| Eicosanoic Acid (C20:0) | CH3(CH2)18COOH | 0.02 | 0.04 |
| Erucic Acid (C22:1) | CH3(CH2)7CH CH(CH2)11COOH | 0.01 | 0.02 |
| Sat. FA | 2.22 | 3.49 | |
| Mono unsat. | 0.58 | 0.87 | |
| Poly unsat. | – | – | |
| ∑ FA in g/100 g of sample | 2.80 | 4.36 |
3.5. Correlation analysis
The statistical relationships between the Egg %, growth rate of B. plicatilis and the nutritional composition of the A. platensis (β carotenes, chlorophyll, carbohydrates, lipids, proteins) during the different experimental runs of the 22 CCD were analyzed using Pearson correlation. Only β carotenes displayed a highly positive significant correlation with the growth rate of the rotifer (r = 0.733, p < 0.01). On the other hand, among the biochemical constituents of A. platensis, the carbohydrates showed significant positive correlations with Egg % (r = 0.657, p < 0.05). None of the other correlations between Egg %, growth rate of B. plicatilis and the other nutritional composition of the A. platensis were statistically significant (p > 0.05).
4. Discussion
The characteristics of the collected samples were highly variable but comparable to those reported by previous studies. The physicochemical characteristics of the effluent were within the range of the values recorded by Sivasubramanian [67] during their studies. However, the constituents of the confectionery effluent sample differ according to the raw materials used in this industry.
The industrial by-products with potential use in Ghanaian aquaculture were reviewed by Obirikorang et al. [55]. They stated that these by-products represent huge potentials as alternative aqua feed protein sources because of their abundance, very affordable prices and healthy nutritional profiles for fish growth.
In this study, A. platensis succeeded to grow very well on the confectionery waste effluent during the different experimental runs of the 22 CCD. The alga may utilize sugar present in the effluent. As expected, the results ensured the suitability of using A. platensis to grow on confectionery waste effluent. Moreover, the biochemical composition of the produced algal biomass was highly improved. Up to the current knowledge, this is the first report on the growth of A. platensis on confectionery waste effluent and subsequent rearing of B. plicatilis on it. Juneja et al. [40], reviewed the effects of different environmental factors and nutrient availability on the biochemical composition of algae.
Bicarbonate alkalinity and carbon can be provided by the addition of sodium bicarbonate to the cultivation medium. In synthetic cultivation media for the cultivation of Spirulina sp. the most frequently used medium is Zarrouk medium, which provides carbon as sodium bicarbonate in the amount of 16.8 g L−1 [73]. In the earlier studies, sodium bicarbonate was added to media containing anaerobically digested wastes in amounts in the range of 9–17 g L−1, but 2–4 g L−1 is now considered as sufficient [68]. Furthermore, [46] suggested that supplementation of sodium bicarbonate need not exceed 0.5% of the culture medium to be adequate for Spirulina growth in effluents of anaerobically digested pig and cattle manure. It has been suggested that many factors, including bicarbonate (HCO3−) furnishes the carbon skeleton needed for algal biomass particularly, proteins. It may be essential for protein synthesis in the organism, probably because sodium is necessary for the activity of certain enzymes involved in protein synthesis, formation of co-enzymes and adenosine phosphates and biosynthesis of sulfur-containing proteins, respectively [4]. Moreover, algae put up different metabolic products during photosynthesis, using only light and nutrients. The relative amounts of these products are tightly linked to environmental and nutrient conditions, including CO2 levels. Carbon is required as non-mineral nutrient for algal growth as reported by Walker [74]. Similar to the results reported from this study, the OD680 and biochemical constituents of the tested alga had greatly affected when grown under different concentrations of sodium bicarbonate as compared by control culture after 12 days. The statistical analyses have already revealed that sodium bicarbonate exerted a significant positive effect on the algal constituents of proteins and chlorophyll (p > 0.01). In accordance with the study results, previous studies have shown sodium bicarbonate can be added as a form of inorganic carbon to increase cell dry weight, FAME, and pigment production [17], [31], [75]. Even though, [56] reported that inorganic carbon of alkalinity in the form of bicarbonate was consumed rapidly, in turn causing the attenuation of cell growth of Scenedesmus sp. Moreover, it has been reported that the elevated CO2 concentrations decrease the relative concentrations of proteins and pigments in the cells but increase carbohydrate content of the A. platensis which was accompanied by reduction in the maximum biomass yield as reported by Gordillo et al. [32].
The alteration in the biomass color observed in the alga culture grown in the 3rd run could be attributed to elevated CO2 concentrations. It was reported that S. platensis grown on elevated CO2 concentrations resulted in an increase in carbohydrate content; decrease in proteins and pigments [32]. Also, [11] stated that some microalgae can undergo a carotenogenesis process, in response to various environmental and cultural stresses (e.g., light, temperature, salts, nutrients), where the alga stops growth and changes dramatically its carotenoid metabolism, accumulating secondary carotenoids as an adaptation to severe environments.
Another important factor to be considered during cultivation of microalgae on waste effluents is the dilution. Ref. [44], reported that the dilution of the digested waste is needed for the growth of Arthrospira, regarding the ammonia toxicity, which is certainly the major factor explaining the need for considerable dilution of the waste. In this study, using larger effluent concentrations, observed in 7th run and 8th runs, the control of the pH through the sodium bicarbonate buffer is necessary due to the fact that the wastewater causes a decrease of the pH in the medium, making the micro alga growth unfeasible. It can be predicted that the low effluent concentration does not cause pH variations that limit the growth of the micro alga, thus the buffer effect of the bicarbonate is necessary. The improvement in cellular growth observed in 2nd, 4th and 6th runs may be attributed to the higher effluent concentration, which contains ingredients that support the alga growth. In this respect, [67] reported that Chlorella vulgaris grows very well in the raw confectionery effluent. He added that the alga utilizes sugar present in the effluent. The statistical analyses suggested that WE exerted significant negative influences in the answers of algal contents of proteins, lipids and carbohydrates (p > 0.01). But it has a significant positive influence on chlorophyll (p > 0.01). However, no significant effect on the response of algal carotenes has been reported (p > 0.05). Likewise, [15], [65] found that the effluent from an anaerobic sludge blanket from a pig farm was a suitable medium for the growth of A. platensis, obtaining an enriched biomass with up to 57% protein and employing 20% of this effluent in the culture media. Moreover, [56] cultivated the microalga Scenedesmus sp. on swine effluent wastewater diluted 1/10 up on the addition to the culture.
Phytoplankton has a significant role in aquaculture as means of enriching zooplankton on-feeding to fish and other larvae. In recent years, mass culture of unicellular algae such as diatoms (Chaetoceros and Skeletonema) and small phytoplankters (Isochrysis, Tetraselmis and Chlorella) is becoming quite popular for feeding larvae of fishes, prawns, shrimps and molluscs in aqua hatcheries. Phytoplankton (live foods) which is eaten by zooplankton forms the basis of the food chain [19]. Moreover, [39] examined the possibility of the integrated multi-trophic aquaculture (IMTA) model consisting of Styela clava, microalgae, and Stichopus japonicus, in which microalgae could remove dissolvable nutrients produced by S. clava and S. japonicus to maintain the dissolvable nutrients at a lower level, and provide sufficient dissolved oxygen in the water body through photosynthesis at the same time. Additionally, live foods contain all the essential nutrients including protein, vitamins, and minerals together with essential PUFAs which are transferred through the food chain [53] and consequently, are commonly known as “living capsules of nutrition”. These capsules are able to tread the water column and are constantly available to fish and shellfish larvae to stimulate their feeding response [13]. Moreover, this HUFA composition results in significantly higher DHA and EPA concentrations in rotifers than for cultures grown on mixtures of algae and baker’s yeast as reported by Lé ger et al. [45].
A good selection of microalgal species is available to support the aquaculture industry. However for some particular applications or industry sectors, new species with improved nutritional quality or growth characteristics could improve hatchery efficiency [45]. Spirulina becomes one of the most commonly used microalgae in aqua feeds as it is a rich source of protein, vitamins, minerals, essential fatty acids, essential amino acids and pigments such as carotenoids that have potent antioxidant and antiinflammatory activities [1], [3], [5], [36]. Moreover, the antioxidant and/or antiinflammatory activities of Spirulina or its extracts have become an interesting point for many researchers either in vitro or in vivo suggesting its beneficial effect as a feed additive [2], [38].
Zooplankton is required as a first food for many cultured fish; for others it contributes to faster growth and higher survival. Larvae of fish and shellfish cannot feed artificial supplemented feed. They require small size live foods for their nutrition. Live foods are easily digestible protein rich diet for fish and shellfish. In nature, zooplankton is one of the primary foods of fish larvae. Two of the dominant zooplankton groups are Rotifera and Copepoda [13], [19]. For the enrichment or boosting of rotifers, several approaches can be followed including the feeding of rotifers on a complete diet or long-term enrichment (rearing of the rotifers on the enrichment diet for more than 24 h) [22]. Moreover, [30] developed an automatic continuous culture system with a filtration unit, a culture unit and a harvest unit to improve the stability in the mass production of rotifers fed on C. vulgaris.
In this study, we rigorously examined if growth and biochemical differences in A. platensis strains elicit differences in the growth attributes of B. plicatilis. The cyanobacteruim, A. platensis grown under standard conditions and that overripe under the different experimental runs of the 22 CCD were then fed to rotifers, and a number of parameters in B. plicatilis were measured, which were growth rate, reproductive rate and fecundity attributes. The rotifer B. plicatilis species complex is the essential live food for the initial stage of larval rearing of marine fishes [33], and is also studied in nutritional science for aquaculture [43] and as a culture for probiotics [35].
The nutritional aspects of rotifers have received major attention in larviculture and several commercial products have been launched to increase the lipid and vitamin content in rotifers [18], [34]. Herein, the density of the rotifers reached the maximum individuals/mL toward 5th day of their growth on the A. platensis of the 4th, 6th and 8th runs of the 22 CCD. There were no clear trends as to which algal prey elicited the highest growth potential. In this respect, [54], [70] studied the influence of the biochemical composition of food on that of the rotifer B. plicatilis and found that there is a positive correlation between the protein content of the food and that of the rotifers, they added that the percentage of protein values ranged between 28.8% and 61.3% with different feed types. Additionally, it is well established that in rotifers, the fatty acid profile is chiefly determined by the diet as stated by Isik et al. [37].
Generally, it is known that different algal foods of B. plicatilis can yield substantially different reproductive rates [69]. Food type had a significant effect on the maximal population density as reported by Navarro and Yufera [42], [52]. Similar to the results of this study, the algal pigment (β carotenes) displayed a highly positive significant correlation with the growth rate of the B. plicatilis (r = 0.733, p < 0.01) and the carbohydrates showed significant positive correlations with Egg % (r = 0.657, p < 0.05). Algae fed to rotifer cultures will alter the lipid and fatty acid composition of the rotifers [10]. Additionally, [14] studied the chemical composition of rotifers fed on different food at three growth phases; found that the total lipid as percentage dry matter varied between 8.5% and 19.4%. Similar to the findings of this study wherever, the fatty acid content of the B. plicatilis biomass grown on A. platensis of the 4th run of the 22 CCD was highly improved and exceeds that of the control. The saturated fraction of fatty acids of B. plicatilis biomass was the predominant fractions. In this respect, [71] found a correlation between the percentage composition of the short chain fatty acids 14:0 + 16:0 in microalgae, and larval growth rates growing on it. They reasoned that diets with higher percentages of the saturated fats were more beneficial for the rapidly growing larvae, because energy is released more efficiently from saturated fats than unsaturated fats. Similar to the findings of this study were the saturated fraction of fatty acids of the alga biomass grown on the waste effluent was dominant as compared to the mono- and polyunsaturated fractions. The main components of the saturated fraction were Myristic Acid (C14:0) and Pentadecanoic Acid (C15:0) which is the most important fatty acid in the saturated fraction. In accordance with the study results, [29] reported the digestibility of various oil seed cakes/meals in African catfish diets. This study is an economically useful message, as it will reduce the extent of rigor required to maintain production.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Abdel-Daim M.M. Small Rumin. Res. 2014;120:234–241. [Google Scholar]
- 2.Abdel-Daim M.M., Abuzead S.M., Halawa S.M. PLoS One. 2013;8:e72991. doi: 10.1371/journal.pone.0072991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdelkhalek N.K.M., Ghazy Emad W., Daim Mohamed M. Abdel. Environ. Sci. Pollut. Res. 2014;22:3023–3031. doi: 10.1007/s11356-014-3578-0. [DOI] [PubMed] [Google Scholar]
- 4.Abu G.O., Ogbonda K.H., Aminigo R.E. Afr. J. Biotechnol. 2007;6:2550–2554. [Google Scholar]
- 5.Alvarenga R.R., Rodrigues P.B., Cantarelli V.S., Zangeronimo M.G., Júnior J.W.S., Silva L.R., Santos L.M., Pereira L.J. Rev. Bras. Zootec. 2011;40:992–996. [Google Scholar]
- 6.APHA, American Public Health Association Standard Methods for Examination of Water and Wastewater, 21st ed., Washington, DC, USA, 2000.
- 7.Araujo A.B., Hagiwara A. Hydrobiologia. 2005;546:553–558. [Google Scholar]
- 8.Auden J., Gruner J., Nüesch J., Knüsel F. Pathol. Microbiol. 1967;30:856–866. doi: 10.1159/000161751. [DOI] [PubMed] [Google Scholar]
- 9.Babadzhanov A.S., Abdusamatova N., Yusupova F.M., Faizullaeva N., Mezhlumyan L.G., Malikova M.K. Chem. Nat. Compd. 1999;40:276–279. [Google Scholar]
- 10.Ben-Amotz A., Fishler R., Schneller A. Mar. Biol. 1987;95:31–36. [Google Scholar]
- 11.Bhosale P. Appl. Microbiol. Biotechnol. 2004;63:351–361. doi: 10.1007/s00253-003-1441-1. [DOI] [PubMed] [Google Scholar]
- 12.Bligh E.G., Dyer W.J. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 13.M.R. Brown, in: L.E. Cruz-Suárez, D. Ricque-Marie, M. Tapia-Salazar, M.G. Gaxiola-Cortés, N. Simoes (Eds.), Avances en Nutrición Acuícola VI. Memorias del VI Simposium Internacional de NutriciónAcuícola, 3 al 6 de Septiembre del 2002, Cancún, Quintana Roo, México.
- 14.Cariç M., Sanko-Njire J., Skaramuca B. Aquaculture. 1993;110:141–150. [Google Scholar]
- 15.Chaiklahan R., Chirasuwan N., Siangdung W., Paithoonrangsarid K., Bunnag B. J. Microbiol. Biotechnol. 2010;20(3):609–614. doi: 10.4014/jmb.0907.07026. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y., Lee M. J. Mar. Sci. Technol. 2012;20:233–236. [Google Scholar]
- 17.Costa J.A.V., Colla L.M., Filho P.D. Zeitschrift Naturforschung C, J. Biosci. 2003;58:76–80. doi: 10.1515/znc-2003-1-214. [DOI] [PubMed] [Google Scholar]
- 18.Coutteau P., Sorgeloos P. Freshw. Biol. 1997;38:501–512. [Google Scholar]
- 19.Das P., Mandal S.C., Bhagabati S.K., Akhtar M.S., Singh S.K. Front. Aquacult. 2012;5:69–86. [Google Scholar]
- 20.Delbos B., Schwarz M.H. VA Co. Ext. Publ. 2009;600:1–3. [Google Scholar]
- 21.Demir O., Diken G. Afr. J. Biotechnol. 2011;10:15065–15071. [Google Scholar]
- 22.Dhert P., Rombaut G., Suantika G., Sorgeloos P. Aquaculture. 2001;200:129–146. [Google Scholar]
- 23.Dillon J.C., Phuc A.P., Dubacq J.P. World Rev. Nutr. Diet. 1995;77:32–46. doi: 10.1159/000424464. [DOI] [PubMed] [Google Scholar]
- 24.Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Anal. Chem. 1956;28:350–356. [Google Scholar]
- 25.The Egyptian Pollution Abatement Project (EPAP), Self-Monitoring Manual: Confectionary Industry, Ministry of state for environmental Affairs, Egyptian Environmental Affairs Agency (EEAA), 2003.
- 26.El-Sheekh M.M., Hamouda R.A. Desalin. Water Treat. 2014;52:7–9. [Google Scholar]
- 27.Environmental monitoring program for the North Lakes (E.M.P.N.L.), <http://www.eeaa.gov.eg/arabic/main/env_water.asp>.
- 28.Estrada J.E.P., Bescós P.B., Fresno A.M.V. Il Farmaco. 2001;56:497–500. [Google Scholar]
- 29.Fagbenro O.A. Aquacult. Int. 1998;6:317–322. [Google Scholar]
- 30.Fu Y., Hada H., Yamashita T., Yoshida Y., Hino A. Hydrobiologia. 1997;358:145–151. [Google Scholar]
- 31.Gardner R.D., Keith E.C., Mus F., Macur R., Moll K., Eustance E., Carlson R., Gerlach R., Fields M., Peyton B. J. Appl. Phycol. 2012:1–10. doi: 10.1007/s10811-011-9782-0. [DOI] [Google Scholar]
- 32.Gordillo F.J.L., Jiménez C., Figueroa F.L., Niell F.X. J. Appl. Phycol. 1998;10:461–469. [Google Scholar]
- 33.Hagiwara A., Suga K., Akazawa A., Kotani T., Sakakura Y. Aquaculture. 2007;268:44–52. [Google Scholar]
- 34.M. Harel, D. Clayton, Feed formulation for terrestrial and aquatic animals, US Patent 20070082008 (WO/2004/080196), 2004.
- 35.Hirata H., Murata O., Yamada S., Ishitani H., Wachi M. Hydrobiologia. 2004;387/388:495–498. [Google Scholar]
- 36.Hosseini S.M., Khosravi-Darani K., Mozafari M.R. Mini Rev. Med. Chem. 2013;13:1231–1237. doi: 10.2174/1389557511313080009. [DOI] [PubMed] [Google Scholar]
- 37.Isik O., Sarihan E., Kusvuran E., Gul O., Erbatur O. Aquaculture. 1999;174:299–311. [Google Scholar]
- 38.Ismail M.F., Ali D.A., Fernando A., Abdraboh M.E., Gaur R.L., Ibrahim W.M., Raj M.H., Ouhtit A. Int. J. Biol. Sci. 2009;5:377–387. doi: 10.7150/ijbs.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ju B., Chen L., Xing R., Jiang A. Aquacult. Int. 2015;23:471–497. [Google Scholar]
- 40.Juneja A., Ceballos R.M., Murthy G.S. Energies. 2013;6:4607–4638. [Google Scholar]
- 41.Kamal A.A., Ahmad I.Z. Int. J. Innov. Appl. Stud. 2014;7:251–261. [Google Scholar]
- 42.Kelman D., Ben-Amotz A., Berman-Frank I. Environ. Microbiol. 2009;11:1897–1908. doi: 10.1111/j.1462-2920.2009.01913.x. [DOI] [PubMed] [Google Scholar]
- 43.Kotani T., Genka T., Fushimi H., Hayashi M., Dierckens K., Sorgeloos P. Fish. Sci. 2009;75:1–10. [Google Scholar]
- 44.Laliberté G., Olguin E.J., de la Noue J. Taylor & Francis; London: 1997. Mass Cultivation and Wastewater Treatment Using Spirulina. [Google Scholar]
- 45.Lé ger Ph., Grymonpre D., Van Ballaer E., Sorgeloos P. EAS Spec. Publ. No. 10. Eur Aquacult Sot; Bredene, Belgium: 1989. pp. 141–142. [Google Scholar]
- 46.Lincoln E.P., Wilkie A.C., French B.T. Biomass Bioenergy. 1996;10:63–68. [Google Scholar]
- 47.Lowery O.H., Rosembrough N.J., Farr A.L., Randall R.J. J. Biol. Chem. 1951;193:267–275. [PubMed] [Google Scholar]
- 48.Markou G., Georgakakis D. Appl. Energy. 2011;88:3389–3401. [Google Scholar]
- 49.Mata T.M., Martins A.A., Caetano N.S. Renewable Sustainable Energy Rev. 2010;14:217–232. [Google Scholar]
- 50.Mezzomo N., Saggiorato A.G., Siebert R., Tatsch P.O., Lago M.C., Hemkemeier M., Costa J.A.V., Bertolin T.E., Colla L.M. Ciênc. Tecnol. Aliment. 2010;30:173–178. [Google Scholar]
- 51.Moosavi B.J., Montajami S. J. Fish. Int. 2013;8:74–77. [Google Scholar]
- 52.Navarro N., Yufera M. Aquaculture. 1998;166:297–309. [Google Scholar]
- 53.M.B. New, in: Anans do Aquacultura Brasil I, Recife, 1998, pp. 2–6.
- 54.Nhu C.V. Asian Fish Sci. 2004;17:357–363. [Google Scholar]
- 55.Obirikorang K.A., Amisah S., Fialor S.C., Skov P.V. Aquacult. Int. 2014;23:403–425. [Google Scholar]
- 56.Park J., Jin H., Lim B., Park K., Lee K. Bioresour. Technol. 2010;101:8649–8657. doi: 10.1016/j.biortech.2010.06.142. [DOI] [PubMed] [Google Scholar]
- 57.Patra S.K., Mohamed K.S. Aquacult. Int. 2003;11:505–514. [Google Scholar]
- 58.Pelizer L.H., Carvalho J.C.M., Sato S., deOliveiraMoraes I. Electron. J. Biotechnol. 2002;5(No. 3) [Google Scholar]
- 59.Promya J., Traichaiyaporn S., Deming R.L. Int. J. Agric. Biol. 2008;10:437–441. [Google Scholar]
- 60.B. Raoof, B.D. Kaushika, R. Prasanna, Formulation of a low-cost medium for mass production of Spirulina, Division of Microbiology, Indian Agricultural Research Institute, New Delhi 110, 012, India and the Centre for Conservation and Utilization of Blue-Green Algae, Indian Agricultural Research Institute, New Delhi, 110 012, India, 2006.
- 61.Rasool M., Sabina E.P., Lavanya B. Biol. Pharm. Bull. 2006;29:2483–2487. doi: 10.1248/bpb.29.2483. [DOI] [PubMed] [Google Scholar]
- 62.Richmond A. CRC Press; Boca-Ratón, FL: 1986. Handbook of Microalgal Mass Culture. pp. 212–230. [Google Scholar]
- 63.Rombaut G., Suantika G., Boon N., Maertens S., Dhert P., Top E., Sorgeloos P., Verstraete W. Aquaculture. 2001;198:237–252. [Google Scholar]
- 64.Saravanan P., Pakshirajan K., Saha P. J. Environ. Sci. 2008;20:1508–1513. doi: 10.1016/s1001-0742(08)62557-7. [DOI] [PubMed] [Google Scholar]
- 65.Sassano C.E.N., Gioielli L.A., Almeida K.A., Sato S., Perego P., Converti A., Carvalho J.C.M. Biomass Bioenergy. 2007;31:593–598. [Google Scholar]
- 66.Sengar H. Planta. 1970;90:243–266. doi: 10.1007/BF00387177. [DOI] [PubMed] [Google Scholar]
- 67.Sivasubramanian V. ENVIS Newslett. 2011;9(4 Oct–Dec) [Google Scholar]
- 68.Slotton D.G., Goldman C.R., Frank A. Nutr. Rep. Int. 1989;40:1165–1172. [Google Scholar]
- 69.Snell T.W., Carrillo K. Aquaculture. 1984;73:359–367. [Google Scholar]
- 70.Srivastava A., Hamre K., Stoss J., Chakrabarti R., Tonheim S.K. Aquaculture. 2006;254:534–543. [Google Scholar]
- 71.Thompson J.N. Evolution. 1993;47:1585–1594. doi: 10.1111/j.1558-5646.1993.tb02177.x. [DOI] [PubMed] [Google Scholar]
- 72.Utermöhl’s H. Mitt. Int. Ver. Theor Angew. Limnol. 1958;9:1–38. [Google Scholar]
- 73.Vonshak A. Taylor & Francis; London: 1997. Spirulina platensis (Arthrospira), Physiol. Cell-Biol. Biotechnol. [Google Scholar]
- 74.Walker J.B. Arch. Biochem. Biophys. 1954;53:1–8. doi: 10.1016/0003-9861(54)90227-1. [DOI] [PubMed] [Google Scholar]
- 75.White D., Pagarette A., Rooks P., Ali S.T. J. Appl. Phycol. 2012;25:153–165. [Google Scholar]
- 76.Yoshimura K., Tanaka K., Yoshimatsu T. Aquaculture. 2003;227:165–172. [Google Scholar]
- 77.Yufera M. Aquaculture. 1982;27:55–61. [Google Scholar]
- 78.Zhang D.M., Yoshimatsu T., Furuse M. Aquaculture. 2005;248:51–57. [Google Scholar]