Abstract
The ventricular assist device is being increasingly used as a “bridge-to-transplant” option in children with heart failure who have failed medical management. Care for this medically complex population must be optimized, including through concomitant pharmacotherapy. Pharmacokinetic/pharmacodynamic alterations affecting pharmacotherapy are increasingly discovered in children supported with extracorporeal membrane oxygenation, another form of mechanical circulatory support. Similarities between extracorporeal membrane oxygenation and ventricular assist devices supports the hypothesis that similar alterations may exist in ventricular assist device-supported patients. We conducted a literature review to assess the current data available on pharmacokinetics/pharmacodynamics in children with ventricular assist devices. We found two adult and no pediatric pharmacokinetic/pharmacodynamic studies in ventricular assist device-supported patients. While mechanisms may be partially extrapolated from children supported with extracorporeal membrane oxygenation, dedicated investigation of the pediatric ventricular assist device population is crucial given the inherent differences between the two forms of mechanical circulatory support, and pathophysiology that is unique to these patients. Commonly used drugs such as anticoagulants and antibiotics have narrow therapeutic windows with devastating consequences if under- or overdosed. Clinical studies are urgently needed to improve outcomes and maximize the potential of ventricular assist devices in this vulnerable population.
Keywords: ventricular assist device, clinical pharmacology, children, pharmacokinetics, pharmacodynamics
Heart failure and ventricular assist devices in children
An estimated 12,000–35,000 United States children <19 years of age suffer from heart failure. While representing only a small fraction of the nearly 5 million Americans affected by this syndrome, pediatric heart failure is often more severe, and children are more likely to require surgical, rather than medical intervention alone.1 When medical therapy fails, heart transplantation offers the best chance of survival.2 Nevertheless, transplantation is limited by the timeliness of an available organ from a small donor pool. This limited donor-organ availability contributes to significant mortality (16–17%) among children awaiting heart transplantation.3,4
To support children awaiting heart transplantation, ventricular assist devices are increasingly being used as a so-called bridge-to-transplant option. While initially implanted infrequently and under compassionate (or off-label) use, a significant increase in funding since 2004 has promoted ventricular assist device development as a bridge-to-transplant in children.5 In 2011, the Berlin Heart EXCOR® device was approved by the Food and Drug Administration for use in children and infants.6,7 Since that time, the use of ventricular assist devices in the pediatric population has continued to increase, although Berlin Heart EXCOR® remains the only Federal Drug Administration-approved device in children for long-term use as a bridge-to-transplant. In 2014, 33% of heart transplant recipients were bridged with some form of mechanical circulatory support, 29% of which was a ventricular assist device or total artificial heart; this represents the largest number of ventricular assist devices ever reported to the International Society for Heart and Lung Transplantation. Survival over time for children supported with ventricular assist devices or total artificial hearts is comparable to children not requiring mechanical circulatory support prior to transplant, and is significantly better than for children requiring extracorporeal membrane oxygenation (84% 5-year survival for children with ventricular assist devices or total artificial hearts, 83% for children without mechanical circulatory support, and 65% for children on extracorporeal membrane oxygenation. In 2016, the Pediatric Interagency Registry for Mechanical Circulatory Support reported an actuarial survival of 86% at 6 months in children supported with ventricular assist devices.8 In addition, during this era of increased ventricular assist device use, waitlist mortality recorded in the United Network of Organ Sharing database has declined from 16% in 1999–2004 to 8% in 2004–2012.4
Since children who are bridged to transplant with ventricular assist devices are achieving survival outcomes comparable to patients not requiring pre-operative mechanical circulatory support, the option of ventricular assist device as a bridge-to-transplant provides valuable time for patients who are dependent on a limited donor pool. As this unique patient population increases, we must continue to optimize their care in order to maximize the benefits of ventricular assist devices as a bridge towards successful heart transplantation. A major part of this care optimization is continuing to improve medical management through a better understanding of the pharmacokinetics of drugs used to support these patients. There is reason to believe that pharmacokinetics in patients supported by ventricular assist devices may be modified similarly to alterations observed with extracorporeal membrane oxygenation (which is another form of mechanical circulatory support) and due to organ dysfunction resulting from underlying heart failure, as well as exposure to cardiopulmonary bypass.9–12 This topic warrants further investigation and understanding to ensure that drugs are used safely and effectively in this population.
Pharmacotherapy in ventricular assist device-supported patients
Multiple drugs across several drug classes are routinely used in ventricular assist device-supported patients to complement the device in the management of their heart failure, to treat the side effects associated with ventricular assist device use, and to optimize overall clinical status prior to heart transplantation.13,14 A retrospective review of twenty-one children supported with ventricular assist devices at our institution between 2013–2016 found multiple drugs commonly used in this population while their device was in place. The median age of patients in the cohort was 8 years (25th, 75th percentile 0.8, 15.0). Twenty-one patients had a total of 23 ventricular device types which included Thoratec CentriMag® (52%), HeartWare® (35%), Berlin Heart EXCOR® (9%), and SynCardia Total Artificial Heart® (4%). Two patients supported with two device types underwent initial implantation of a CentriMag® and then were transitioned to a HeartWare® device. The median number of individual drugs received while a ventricular assist device was in place, including enteral and parenteral electrolyte supplements, was 70 (25th, 75th percentile 65.5, 80.5). Patients received drugs from categories including anti-infectives, anticoagulants, steroids, vasopressors/inotropes, anti-hypertensives/vasodilators, neurologic/pain/sedation drugs, and electrolytes/nutrition/gastrointestinal drugs (Table 1). The most commonly used drugs across all categories were acetaminophen, cefuroxime, dexmedetomidine, epinephrine, fentanyl, ketamine, midazolam, potassium chloride, unfractionated heparin, and vancomycin.
Table 1.
Demographics of the cohort of ventricular assist device-supported children at a single institution (2013–2016)
| Characteristic | N=21 (%) |
|---|---|
| Age at VAD implantation (years) | |
| Median (25th, 75th percentile) | 8 (0.8–15) |
| 0 – <2 | 6 (29) |
| 2 – <6 | 4 (19) |
| 6 – 18 | 11 (52) |
| Female gender | 10 (48) |
| Structural congenital heart disease | 5 (24) |
| VAD type (N=23) | |
| Berlin Heart® | 2 (9) |
| CentriMag® | 12 (52) |
| Heartware® | 8 (35) |
| Syncardia® | 1 (4) |
| Left-sided VAD only | 16 (76) |
| Median (25th, 75th percentile) duration of VAD support, days | 71 (49.5–161.5) |
VAD = ventricular assist device
Anti-infectives
Anti-infectives are commonly prescribed in the ventricular assist device population, given the high risk of post-operative infections and significant associated morbidity. According to the Pediatric Interagency Registry for Mechanical Circulatory Support registry, infection is the third most common complication during the first 3 months post-implantation (13.8 per 100 patient-months) and the most common complication after 3 months (6.1 per 100 patient-months).8 Twenty-five percent of these infections were device-related, and the remainder were systemic (blood-stream) infections. Data from a single-center pediatric cohort study show that ventricular assist device infections lead to a significantly longer duration of mechanical support and longer intensive care unit and hospital length of stay.15 Common pathogens that must be considered in both prophylactic and empiric treatment of device-related and systemic infections include Staphylococci, Enterococcus species, and gram-negative bacilli such as Pseudomonas, and Candida species.10 Consideration of methicillin-resistant Staphylococcus aureus is of particular importance. Trauma and poor wound healing at the driveline site provide potential entry for skin flora, and methicillin-resistant Staphylococcus aureus infection is particularly devastating in this population given its propensity to form biofilms, which renders clearance challenging. In a retrospective single center review of 51 children supported with ventricular assist devices, 35 (69%) experienced a total of 92 infections, 33 (36%) of which were considered ventricular assist device-specific (involving the driveline or device pocket) or ventricular assist device-related.16 Of the 10 ventricular assist device-specific infections, 2 (20%) were due to methicillin-resistant Staphylococcus aureus. A similar study reported on driveline and device pocket infections, mediastinitis, and endocarditis in 60 adults supported with ventricular assist device.17 In this cohort, 12/70 (17%) infections were due to methicillin-resistant Staphylococcus aureus.
A best evidence topic review from 2012 addressed the question of optimal antimicrobial prophylaxis surrounding ventricular assist device implantation and concluded that a beta-lactam should be used as primary prophylaxis, with the addition of vancomycin if there is an increased risk of methicillin-resistant Staphylococcus aureus infection.18 The authors also state that anti-fungal prophylaxis may benefit groups susceptible to fungal infection. In a survey of 21 centers routinely performing left ventricular assist device implantation in adults, wide variability in surgical-infection prophylaxis regimens was reported.19 The most common drug combination in use was vancomycin, a cephalosporin or quinolone, rifampin, and fluconazole. According to the 2017 consensus guidelines from the International Society for Heart and Lung Transplantation, peri-operative prophylaxis should include “coverage for Staphylococcus sp. and, in colonized patients or in centers with high methicillin-resistant Staphylococcus aureus prevalence, coverage for methicillin-resistant Staphylococcus aureus is recommended. Centers should use their local institutional epidemiology data to guide the antibiotic prophylaxis protocol for mechanical circulatory support implant procedures. Routine use of broad-spectrum Gram-negative or fungal prophylaxis is not recommended.”20 These antibiotics should be given within 1 hour of the skin incision and continued for no more than 48 hours if there are no ongoing concerns for infection.
Anticoagulants
Ventricular assist device implantation induces a hypercoagulable state by activating endothelium, hemostatic proteins, fibrinolysis, platelets, and leukocytes, leading to increased thrombin production21; this creates a persistent risk of pump thrombosis and necessitates the use of anticoagulants and antiplatelet agents throughout the duration of ventricular assist device support. Unfortunately, these drugs also inherently cause an increased risk of bleeding, which is the most common complication in the first three months post-implantation in children (15.1 per 100 patient-months).8 Given the fine balance between anticoagulation and bleeding, these drug regimens are carefully monitored, and would benefit from more individualized dosing regimens.
Recommendations provided by the 2013 International Society of Heart and Lung Transplantation Guidelines for Mechanical Circulatory Support state that anticoagulation and antiplatelet therapies should be administered with the goal of achieving “device-specific recommended international normalized ratio for warfarin and desired antiplatelet effects.”22 For adults, anticoagulation guidelines exist for the most commonly used ventricular assist devices, including the Heartmate II® and HeartWare® devices.23 In general, unfractionated heparin is the initial choice for anticoagulation once post-operative hemostasis is achieved, except in patients with a history of heparin-induced thrombocytopenia, in which case a direct thrombin inhibitor such as bivalirudin would be indicated. Warfarin is most frequently used for long-term anticoagulation and is typically started once chest tubes are removed and the patient is able to take enteral medications. An anti-platelet agent such as aspirin or dipyridamole is also added to the regimen once platelet counts have stabilized.
In pediatrics, the Edmonton protocol was developed to guide anticoagulation and antiplatelet therapy for the Berlin Heart EXCOR.24 This protocol uses thromboelastography to adjust therapy in order to reach therapeutic goals. Heparin is initiated post-operatively, followed by dipyridamole at 48 hours. Aspirin is started when chest tubes are removed. Once anticoagulation is stable, patients <1 year of age are transitioned to enoxaparin and children >1 year of age receive warfarin for long-term anticoagulation therapy with an international normalized ratio goal of 2.7–3.5. Despite the availability of this protocol, pediatric anticoagulation practices in general are much more variable between institutions compared to adults,25 which is partially because pediatric guidelines exist for only one specific device. As additional devices are increasingly being used in children, physicians typically extrapolate anticoagulation regimens from adult guidelines. In a retrospective review of data from the Pediatric Health Information System database, unfractionated heparin was the most commonly used drug across all age groups in pediatric ventricular assist device patients.25 This data also revealed trends toward more use of antiplatelet agents and oral medications over time.
In our experience, current use of antiplatelet agents in pediatrics largely mimics the use of these agents in adults; however, all of these drugs are considered off-label in children.21 Furthermore, unlike warfarin (which has an established monitoring system), antiplatelet monitoring is not well established in children. Platelet aggregometry is considered the gold standard, but there is no set target range for platelet inhibition, making it difficult to use in guiding clinical management.26 As mentioned previously, thromboelastography was used in the Edmonton protocol; while thromboelastography is the most commonly used monitoring regimen in children supported with ventricular assist devices, this regimen is fraught with limitations, including a lack of validated target ranges, as well as a lack of reproducibility and interpretability of test results.21 This is certainly an area that warrants more investigation.
Alternative regimens, such as bivalirudin (a direct thrombin inhibitor) and epoprostenol (a synthetic prostacyclin analog) infusions, have been used in pediatric patients who either fail more traditional regimens due to recurrent thrombosis or develop heparin-induced thrombocytopenia.27 In a case series of 6 pediatric patients supported with a Berlin Heart EXCOR® device, 5 were successfully bridged to transplant on this new regimen while one died of multi-organ failure prior to transplant. The only major complication on the bivalirudin/epoprostenol regimen was a cerebrovascular infarct from which the patient fully recovered.
Notably, while some agents, including unfractionated heparin, warfarin, and argatroban have pediatric dosing information in their Food and Drug Administration label, none of the drugs discussed in this section have a specific indication for anticoagulation in children supported with ventricular assist devices.21
Multiple factors, including the age-related physiologic differences in hemostasis in children, require more variability in anticoagulant dosing and management.28 Clinical outcomes reflect this challenge with a recent retrospective single center review of 25 pediatric ventricular assist device patients undergoing 27 device implantations showing stroke in 22% of patients, major bleeding in 32%, and device thrombosis in 22%.29 Even patients managed with the device-specific Edmonton Protocol have demonstrated high rates of complications.19–24 Recent data from a prospective, multicenter cohort of 68 pediatric patients supported by a Berlin Heart EXCOR® ventricular assist device demonstrate major bleeding in 43% of patients (with 24% determined to be probably/definitely related to antithrombotic management), and neurologic events in 28% (with 9% determined to be probably/definitely related to antithrombotic management). Pump changes for suspected thrombosis were performed in 56% of patients. Unfortunately, there is a paucity of data with regard to the safety and timing of resuming anticoagulation in ventricular assist device patients (pediatric or adult) who have experienced a bleeding event. Data in adults with mechanical heart valves suggest that reversal, temporary cessation, and then re-initiation of oral anticoagulation therapy after intracerebral hemorrhage between 1 and 6 days (median 3 days) is safe and the risk of recurrent bleeding is low.30,31 Additional study in this area would be extremely beneficial, given the common occurrence of bleeding due to the inherent risk of thrombosis.
Miscellaneous Drugs
Additional categories of drugs frequently used in ventricular assist device patients include those supporting hemodynamics (including pulmonary vasodilators), hemostasis, and nutrition.13,14 The inflammatory state induced by ventricular assist device implantation also necessitates steroid use in some patients. Right ventricular support is of particular importance in patients after left ventricular assist device implantation since right ventricular failure is associated with increased mortality.32,33 The need for inotropic support in the immediate post-operative period is common and preference is often given to those agents that cause pulmonary vasodilation if right ventricular failure is present.10 For more long-term support, the Phosphodiesterase-5 inhibitor sildenafil is commonly used given its pulmonary vasodilation properties to help wean off inhaled nitric oxide and inotropes.34,35
When further considering the management of these patients, other potential drug categories of interest include those used for post-operative pain and sedation, as well as to treat co-morbid psychiatric conditions. While the pediatric literature is extremely limited on these topics, data identified rates of clinically significant anxiety and depression in adult patients post-left ventricular assist device implantation between 18–23%,36,37 which supports the need for further study of the pharmacologic options for patients supported by ventricular assist devices.
Pharmacokinetics in ventricular assist device-supported patients
Pharmacokinetic trials
Extensive literature shows important pharmacokinetic alterations for many forms of mechanical circulatory support (for example extracorporeal membrane oxygenation), and consequently, standard medication dosing regimens may result in therapeutic failures.38–42 Patients supported with ventricular assist devices are exposed to multiple medications that complement the device in heart failure management, treat the side effects associated with ventricular assist device use, and optimize their overall clinical status prior to heart transplantation.13,14 Commonly used medications include antibiotics and antifungals for infection prophylaxis and treatment, as well as drugs for thromboprophylaxis, sedation and analgesia, support of right heart function, nutritional supplementation, and treatment of comorbidities like renal dysfunction. Despite the prevalence of pharmacotherapy in these patients, pharmacokinetics are extremely understudied in adults with ventricular assist devices, and to date, no pharmacokinetics trials in children supported with ventricular assist devices have been reported. A single-center pharmacokinetics study of vancomycin administered per standard of care to 12 adult patients with HeartMate II continuous flow left ventricular assist devices ventricular assist device found significantly lower clearance and higher volume of distribution estimates using a 1-compartment pharmacokinetics model when compared to population-based equation estimates used in routine clinical practice.43 The authors concluded that using equations commonly used in routine clinical practice is likely to result in excessive vancomycin dosing and subsequent toxicity in patients supported with ventricular assist devices. The observed pharmacokinetics alterations are consistent with those demonstrated in non-left ventricular assist device patients with heart failure. For vancomycin specifically, decreased clearance in heart failure patients has correlated with both decreased creatinine clearance and decreased left ventricular ejection fraction.44
A second source of pharmacokinetics data in ventricular assist device patients comes from a case report of a 25-year-old woman with a history of dilated cardiomyopathy supported by a HeartWare® left ventricular assist device, a CentriMag® right ventricular assist device, and hemodialysis; this patient received extended-infusion cefepime for a multidrug resistant Pseudomonas aeruginosa ventricular assist device infection.45 The authors describe a dosing regimen of 2 grams given over 3 hours a day, as compared to the standard infusion time of 30 minutes.46 With this regimen, cefepime concentrations were maintained above the pharmacodynamic target of 4 times the minimum inhibitory concentration of the patient’s organism minimum inhibitory concentration (=8 μg/ml on hospital day 178 and 16 μg/ml on hospital day 191) for 50% of the dosing interval. The exception to this was post-dialysis, immediately prior to the next dose, when concentrations fell below the minimum inhibitory concentration, owing to the fact that hemodialysis removed 82% of the drug. Based on their experience with this patient, the authors recommended the extended-dosing regimen of 2 grams over 3 hours a day. Extended-infusion cefepime is used commonly to treat P. aeruginosa in critically ill patients to optimize time spent above the minimum inhibitory concentration. This case report did not definitively address whether or not additional pharmacokinetics alterations can be expected due to the ventricular assist device itself.47
Although not providing pharmacokinetics data, a pharmacoepidemiologic study described dosing regimens of warfarin before and after implantation of ventricular assist devices in 13 adult patients.48 This study found significant intra-individual variability in dosing before and after ventricular assist device implantation with 7/13 subjects requiring post-operative adjustment of their mean weekly dose to maintain the international normalized ratio target of 2–3. Of these 7 patients, 5 required a lower dose of warfarin post-operatively, while 2 needed a higher dose. This study excluded patients taking any additional drugs known to interact with warfarin. Based on a pharmacodynamic endpoint (target international normalized ratio), this study provides another example of the large intra- and inter-individual variability in drug exposure among subjects supported with ventricular assist devices. While variability in warfarin dosing is not specific to the ventricular assist device population, and other potential mechanisms responsible for this variability, including known mutations in the gene encoding the warfarin target VKORC1, were not addressed by the authors, the frequent dose adjustment required after ventricular assist device implantation suggests the need for more warfarin pharmacokinetic studies in this population.
Mechanisms of altered drug disposition
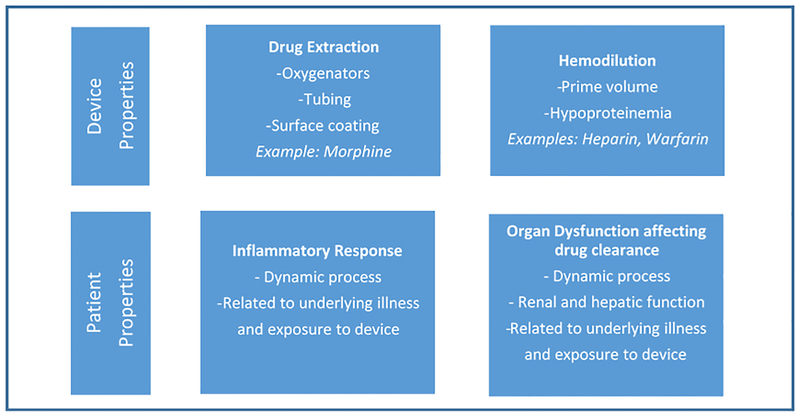
Due to the lack of pharmacokinetics trials in ventricular assist device-supported patients, population-specific dosing recommendations are limited. In contrast, pharmacokinetics data is expanding greatly for extracorporeal membrane oxygenation, which is another type of mechanical circulatory support. Multiple pharmacokinetics trials in adults and children have demonstrated altered drug disposition during extracorporeal membrane oxygenation, leading to specific dosing recommendations. While extracorporeal membrane oxygenation and ventricular assist devices are different forms of mechanical circulatory support, there are enough similarities to suggest possible mechanisms that may alter drug disposition in ventricular assist device patients, necessitating further investigation. Patients, including children, supported with extracorporeal membrane oxygenation frequently display an increased drug volume of distribution. This increase occurs via several mechanisms including: 1) drug extraction by the circuit; 2) hemodilution; and 3) physiologic changes related to critical illness/disease state (Figure 1).
Figure 1. Mechanisms of altered drug disposition during mechanical circulatory support.
This figure displays the mechanisms of altered drug disposition during mechanical circulatory support and is shown according to device and patient properties.
Contact of patient blood with the foreign surface of the extracorporeal membrane oxygenation circuit results in drug extraction, specifically altering the pharmacokinetics of highly lipophilic and protein-bound drugs.40,41,49 While some of this drug extraction is secondary to the oxygenator, which is not part of a ventricular assist device circuit, the circuit tubing itself has also been found to cause drug loss.50,51 For example, different coatings on the polyvinyl chloride tubing have been shown to affect the degree of morphine loss in the extracorporeal membrane oxygenation circuit, ranging from 41–74% after 5 minutes.51 This finding suggests that, depending on the characteristics of the drug and the type of surface coating of the device, some degree of drug loss may occur secondary to the ventricular assist device circuit itself. Ventricular assist device surface coatings usually consist of a thromboresistant heparin, and dedicated ex vivo studies would need to be performed to determine if drug loss occurs secondary to these particular surfaces. However, independent of surface coatings and the surface area of the circuit tubing, which is variable, one would expect decreased drug extraction with a ventricular assist device compared to extracorporeal membrane oxygenation due to the lack of an oxygenator.
Hemodilution has the greatest effects on drugs whose distribution is limited to the plasma compartment.41 Examples of such drugs used in patients requiring mechanical circulatory support include large molecules such as heparin, and hydrophilic drugs including aminoglycosides and warfarin. Hemodilution in extracorporeal membrane oxygenation is particularly relevant in infants, where the circuit prime volume may be more than double the native blood volume.52 However, the priming volumes of ventricular assist devices are significantly smaller than those used in extracorporeal membrane oxygenation. The smallest Berlin EXCOR® pump, for example, requires only a 10 ml prime.53 In a 3 kg infant, 10 ml would represent only a ~4% increase in circulating volume compared to ~100% increase with a standard extracorporeal membrane oxygenation circuit. For an older child with a continuous flow device such as the CentriMag pump, the prime volume of 31 ml is even less significant when compared to their circulating blood volume.54 Due to these relatively smaller prime volumes, hemodilution may play less of a role in expanding volume of distribution in ventricular assist device-supported patients compared to those requiring extracorporeal membrane oxygenation.
In critically ill patients supported with extracorporeal membrane oxygenation, a profound inflammatory response results in capillary leak and edema that can further increase volume of distribution.55–57 A similar inflammatory response is seen in patients immediately after ventricular assist device implantation. A single-center study of pro-inflammatory biomarkers in 6 adult patients before and after left ventricular assist device insertion demonstrated increased peak levels of interleukin-6 and interleukin-8, as well as prolonged time to normalization after left ventricular assist device, compared to patients who underwent cardiopulmonary bypass surgery alone.58 These findings are likely due to the ongoing inflammatory stimulus of the ventricular assist device after implantation, whereas the cardiopulmonary bypass circuit is disconnected at the conclusion of surgery. However, patients with ongoing heart failure may also suffer from a heightened inflammatory state at baseline, prior to ventricular assist device implantation.59 As a result, a transient decline in inflammatory response following ventricular assist device implantation has been described in a study of 48 adult patients with New York Heart Association class IV heart failure, who demonstrated a temporary decrease in interleukin-6, tumor necrosis factor alpha, and other inflammatory cytokines following ventricular assist device placement.60 Nevertheless, despite this initial decrease in inflammation, cytokine levels rose again and approached pre-implantation levels by 90 days, suggesting that inflammation is a recurrent process after ventricular assist device implantation. In another study comparing patients with stable end-stage heart failure to ventricular assist device recipients, oxidative inflammatory markers and total sequential organ failure assessment scores were initially elevated after ventricular assist device implantation, but both improved to pre-implant levels by 1 month.61 Based on the available data, the inflammatory response in patients after ventricular assist device implantation appears to be a highly dynamic process that varies over time and that is likely modulated by the pre-operative disease state.59 Regardless, the inflammatory response is likely to be present in the early post-operative period and its potential effect on volume of distribution or clearance should be considered when evaluating pharmacokinetics in these patients.62
Critically ill patients, including those receiving ventricular assist device support, frequently display impaired drug clearance, which is due to dysfunction of drug-eliminating organs such as the kidney and liver. In many cases, this dysfunction may be present pre-operatively, secondary to heart failure. Both renal and hepatic function tend to improve after ventricular assist device implantation, due to better end-organ perfusion and decreased hepatic and central venous congestion that results from increased cardiac output. Nevertheless, this improvement may be preceded by a brief period of worsened function, possibly because of the insult associated with surgery. Studies in sheep have shown initial increases in blood urea nitrogen, serum creatinine, total bilirubin, total protein, and liver enzymes after ventricular assist device implantation, which returned to baseline by post-implantation day 7.63 In humans, similar findings have been observed, with initial increases in blood urea nitrogen, creatinine, and bilirubin followed by normalization within 1–2 months post-ventricular assist device implantation, even for patients with abnormal pre-operative renal and hepatic function.58,64 Similarly, in a study of 15 adult ventricular assist device patients requiring pre-operative renal replacement therapy, 10 showed significant improvement in all markers of renal function after ventricular assist device implantation and were ultimately weaned off renal support.65 Of the remaining 5 who were not weaned from renal replacement therapy, 4 died of sepsis with progressive multi-organ failure and 1 remained critically ill in the hospital at the time of publication.
Hepatic function is similarly affected by ventricular assist device implantation. Twenty-three adult patients with advanced hepatic dysfunction defined as alanine aminotransferase or aspartate transaminase levels five times normal, serum total bilirubin levels three times normal, and/or necessity for a liver biopsy before or during device implantation were followed after left ventricular assist device implantation.65 Of the 20 patients who survived for more than one month post-operatively, all demonstrated significant improvement in aspartate transaminase, alanine aminotransferase, and bilirubin levels compared to pre-operative values, yet how these changes affect hepatic function, including drug metabolism and excretion, following ventricular assist device implantation remains unclear.
In an effort to determine which patients are more likely to experience organ recovery after left ventricular assist device implantation, a scoring system has been developed using pre-operative total bilirubin or creatinine adjusted for patient age (0.15 × age + 1.1 × [preoperative total bilirubin] or 0.2 × age + 3.6 × [preoperative creatinine]).66 Patients with a total bilirubin score >11 or a creatinine score >14.1 were significantly more likely to experience persistently elevated total bilirubin and creatinine levels post-operatively. Furthermore, when combined, the two scores correctly predicted 6-month mortality from multi-organ failure, stratifying patients into low-, intermediate-, and high-risk groups.
The same group developed another scoring system called the TODAI ventricular assist device score after evaluating 59 patients undergoing left ventricular assist device implantation.67 Using serum albumin, left-ventricular end-diastolic diameter, and central venous pressure, the score better predicted 1-year mortality in ventricular assist device patients compared to other scoring systems used to assess the risk of ventricular assist device implantation, including Leitz-Miller, Columbia, Seattle, Heart Failure Model, and Acute Physiology and Chronic Health Evaluation II. Whether either scoring system could be used to optimize drug dosing in ventricular assist device patients is not known, but stratification of a ventricular assist device clinical trial population by these scores is likely to greatly facilitate interpretation of the study findings and should be considered. Nonetheless, these scoring systems have been created and validated only for the adult population, so additional caution must be taken into consideration for these scoring systems in children supported with ventricular assist devices.
As a result of the dynamic changes in renal and hepatic function, drug clearance after ventricular assist device implantation may evolve from an immediate post-operative decline to a longer-term period of recovery and potential normalization. Yet patients with more severe organ dysfunction pre-ventricular assist device are at risk for permanent organ dysfunction or failure; therefore, these patients continued alteration of drug metabolism and elimination. Significantly, this body of data is largely comprised of information on adults; until more pediatric data is published, this data must be interpreted with caution when extrapolating to children.
Ventricular assist devices may also alter drug disposition through an increased volume of distribution, secondary to hemodilution, drug extraction by the circuit, and the post-operative inflammatory response. Implantable devices are unlikely to be as affected by factors related to volume of distribution and exposure to circuit surfaces given their relatively smaller size, but this should remain under consideration for the Berlin Heart EXCOR® and Centrimag® devices. Consequently, further investigation into altered drug distribution in ventricular assist device patients is needed.
Conclusions and future directions
Optimized medical and surgical interventions are needed to improve outcomes of children with severe heart failure. Pharmacokinetic and pharmacodynamics methods offer the opportunity to study the significant effects surgical interventions may have on drug exposure, efficacy, and safety. This is particularly important for children supported with ventricular assist devices, who are exposed to multiple drugs, including some with a narrow therapeutic index. Children with heart failure who are supported by ventricular assist devices are a unique patient population that combines the pathophysiology of heart failure, critical illness, and mechanical circulatory support, thereby making predictions about pharmacokinetics alterations challenging. As a result, conducting population-specific pharmacokinetics trials to identify optimal drug doses is essential.
Fortunately, conducting pharmacokinetics studies in this population is feasible. First, the drugs of interest are administered per standard of care. Second, ventricular assist device patients frequently require laboratory monitoring, making them very amenable to opportunistic pharmacokinetics sample collection. This strategy has been successfully used to develop population pharmacokinetics models.68 This type of modeling approach would allow for the inclusion of specific ventricular assist device characteristics (ventricular assist device type, flow rate, etc.), as well as individual patient physiologic alterations as covariates in the modeling, and may help predict inter-individual variability in drug exposure. A complementary strategy could also leverage the use of physiologically-based pharmacokinetics modeling, an alternate modeling strategy that could accommodate the physiologic alterations associated with ventricular assist device implantation. Following an opportunistic pharmacokinetics trial in ventricular assist device patients, the collected data could be combined with adult trial information to develop a pediatric physiologically-based pharmacokinetics model.69 Either model type could ultimately be applied to identify optimal dosing associated with different types of ventricular assist devices and different levels of physiologic alterations. Overall, this approach would maximally leverage pediatric opportunistic, adult ventricular assist device, and ex-vivo ventricular assist device data to minimize the number of ventricular assist device-supported children enrolled in clinical trials.
Acknowledgements
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
K.D.H. receives salary support for research from the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR001117, U01TR001803-01) and from the Gilead Cardiovascular Scholars Program. KMW receives support from the Pediatric Critical Care and Trauma Scientist Development Program (5K12HD047349), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (1K23HD075891), and the United States government for his work in pediatric and neonatal clinical pharmacology (Government Contract HHSN267200700051C, PI: Benjamin under the Best Pharmaceuticals for Children Act). D.G. receives support for research from NICHD (K23HD083465) and the nonprofit organization Thrasher Research Fund (www.thrasherresearch.org). C.P.H receives support for research from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (1K23HD090239) and the United States government for his work in pediatric and neonatal clinical pharmacology (Government Contract HHSN267200700051C, PI: Benjamin under the Best Pharmaceuticals for Children Act).
Footnotes
Ethical standards
This research did not involve human and/or animal experimentation.
References
- 1.Hsu DT, Pearson GD. Heart failure in children: part I: history, etiology, and pathophysiology. Circ Heart Fail 2009; 2: 63–70. [DOI] [PubMed] [Google Scholar]
- 2.Rossano JW, Dipchand AI, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Nineteenth Pediatric Heart Transplantation Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant 2016; 35: 1185–1195. [DOI] [PubMed] [Google Scholar]
- 3.Almond CS, Thiagarajan RR, Piercey GE, et al. Waiting list mortality among children listed for heart transplantation in the United States. Circulation 2009; 119: 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zafar F, Castleberry C, Khan MS, et al. Pediatric heart transplant waiting list mortality in the era of ventricular assist devices. J Heart Lung Transplant 2015; 34: 82–88. [DOI] [PubMed] [Google Scholar]
- 5.Section on Cardiology and Cardiac Surgery; Section on Orthopaedics. Off-label use of medical devices in children. Pediatrics 2017; 139 pii: e20163439. doi: 10.1542/peds.2016-3439. [DOI] [PubMed] [Google Scholar]
- 6.Fraser CD Jr, Jaquiss RD, Rosenthal DN, et al. Prospective trial of a pediatric ventricular assist device. N Engl J Med 2012; 367: 532–541. [DOI] [PubMed] [Google Scholar]
- 7.Almond CS, Morales DL, Blackstone EH, et al. Berlin Heart EXCOR pediatric ventricular assist device for bridge to heart transplantation in US children. Circulation 2013; 127: 1702–1711. [DOI] [PubMed] [Google Scholar]
- 8.Blume ED, Rosenthal DN, Rossano JW, et al. Outcomes of children implanted with ventricular assist devices in the United States: first analysis of the Pediatric Interagency Registry for Mechanical Circulatory Support (Pediatric Interagency Registry for Mechanical Circulatory Support). J Heart Lung Transplant 2016; 35: 578–584. [DOI] [PubMed] [Google Scholar]
- 9.Upperman JS, Lacroix J, Curley MA, et al. Specific etiologies associated with multiple organ dysfunction syndrome in children: part 1. Pediatr Crit Care Med 2017;18:S50–S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarral OA, Saso S, Harling L, et al. Organ dysfunction in patients with left ventricular impairment: what is the effect of cardiopulmonary bypass? Heart Lung Circ 2014;23:852–862. [DOI] [PubMed] [Google Scholar]
- 11.Sasse M, Dziuba F, Jack T, et al. In-line filtration decreases systematic inflammatory response syndrome, renal and hematologic dysfunction in pediatric cardiac intensive care patients. Pediatr Cardiol 2015;36:1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apostolakis E, Filos KS, Koletsis E, Dougenis D. Lung dysfunction following cardiopulmonary bypass. J Card Surg 2010;25:47–55. [DOI] [PubMed] [Google Scholar]
- 13.Dang NC, Naka Y. Perioperative pharmacotherapy in patients with left ventricular assist devices. Drugs Aging 2004; 21: 993–1012. [DOI] [PubMed] [Google Scholar]
- 14.Ensor CR, Paciullo CA, Cahoon WD Jr, Nolan PE Jr. Pharmacotherapy for mechanical circulatory support: a comprehensive review. Ann Pharmacother 2011; 45: 60–77. [DOI] [PubMed] [Google Scholar]
- 15.Fragasso T, Ricci Z, Grutter G, et al. Incidence of healthcare-associated infections in a pediatric population with an extracorporeal ventricular assist device. Artif Organs 2011; 35: 1110–1114. [DOI] [PubMed] [Google Scholar]
- 16.Cabrera AG, Khan MS, Morales DL, et al. Infectious complications and outcomes in children supported with left ventricular assist devices. J Heart Lung Transplant 2013; 32: 518–524. [DOI] [PubMed] [Google Scholar]
- 17.Monkowski DH, Axelrod P, Fekete T, Hollander T, Furukawa S, Samuel R. Infections associated with ventricular assist devices: epidemiology and effect on prognosis after transplantation. Transpl Infect Dis 2007; 9: 114–120. [DOI] [PubMed] [Google Scholar]
- 18.Acharya MN, Som R, Tsui S. What is the optimum antibiotic prophylaxis in patients undergoing implantation of a left ventricular assist device? Interact Cardiovasc Thorac Surg 2012; 14: 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker PC, DePestel DD, Miles NA, Malani PN. Surgical infection prophylaxis for left ventricular assist device implantation. J Card Surg 2011; 26: 440–443. [DOI] [PubMed] [Google Scholar]
- 20.Kusne S, Mooney M, Danziger-Isakov L, et al. An ISHLT consensus document for prevention and management strategies for mechanical circulation support infection. J Heart Lung Transplant 2017;36:1137–1153. [DOI] [PubMed] [Google Scholar]
- 21.Massicotte MP, Bauman ME, Murray J, Almond CS. Antithrombotic therapy for ventricular assist devices in children: do we really know what to do? J Thromb Haemost 2015; 13 Suppl 1: S343–350. [DOI] [PubMed] [Google Scholar]
- 22.Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013; 32: 157–187. [DOI] [PubMed] [Google Scholar]
- 23.Baumann Kreuziger LM. Management of anticoagulation and antiplatelet therapy in patients with left ventricular assist devices. J Thromb Thrombolysis 2015; 39: 337–344. [DOI] [PubMed] [Google Scholar]
- 24.Steiner ME, Bomgaars LR, Massicotte MP, Berlin Heart EXCOR Pediatric VAD IDE study investigators. Antithrombotic therapy in a prospective trial of a pediatric ventricular assist device. ASAIO J 2016; 62: 719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moffett BS, Cabrera AG, Teruya J, Bomgaars L. Anticoagulation therapy trends in children supported by ventricular assist devices: a multi-institutional study. ASAIO J 2014; 60: 211–215. [DOI] [PubMed] [Google Scholar]
- 26.Adachi I, Kostousov V, Hensch L, Chacon-Portillo MA, Teruya J. Management of hemostasis for pediatric patients on ventricular-assist devices. Semin Thromb Hemost 2018;44:30–37. [DOI] [PubMed] [Google Scholar]
- 27.Rutledge JM, Chakravarti S, Massicotte MP, Buchholz H, Ross DB, Joashi U. Antithrombotic strategies in children receiving long-term Berlin Heart EXCOR ventricular assist device therapy. J Heart Lung Transplant 2013;32:569–573. [DOI] [PubMed] [Google Scholar]
- 28.Monagle P, Chan AKC, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141: e737S–801S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein ML, Robbins R, Sabati AA, et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS)-defined morbidity and mortality associated with pediatric ventricular assist device support at a single US center: the Stanford experience. Circ Heart Fail 2010; 3: 682–688. [DOI] [PubMed] [Google Scholar]
- 30.Butler AC, Tait RC. Restarting anticoagulation in prosthetic heart valve patients after intracranial haemorrhage: a 2-year follow-up. Br J Haematol 1998;103:1064–1066. [DOI] [PubMed] [Google Scholar]
- 31.Romualdi E, Micieli E, Ageno W, Squizzato A. Oral anticoagulant therapy in patients with mechanical heart valve and intracranial haemorrhage. A systematic review. Thromb Haemost 2009;101:290–297. [PubMed] [Google Scholar]
- 32.Kormos RL, Teuteberg JJ, Pagani FD, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg 2010; 139: 1316–1324. [DOI] [PubMed] [Google Scholar]
- 33.Lampert BC, Teuteberg JJ. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant 2015; 34: 1123–1130. [DOI] [PubMed] [Google Scholar]
- 34.Klodell CT Jr, Morey TE, Lobato EB, et al. Effect of sildenafil on pulmonary artery pressure, systemic pressure, and nitric oxide utilization in patients with left ventricular assist devices. Ann Thorac Surg 2007; 83: 68–71; discussion 71. [DOI] [PubMed] [Google Scholar]
- 35.Houston BA, Kalathiya RJ, Hsu S, et al. Right ventricular afterload sensitivity dramatically increases after left ventricular assist device implantation: a multi-center hemodynamic analysis. J Heart Lung Transplant 2016; 35: 868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Modica M, Ferratini M, Torri A, et al. Quality of life and emotional distress early after left ventricular assist device implant: a mixed-method study. Artif Organs 2015;39:220–227. [DOI] [PubMed] [Google Scholar]
- 37.Brouwers C, Denollet J, Caliskan K, et al. Psychological distress in patients with a left ventricular assist device and their partners: an exploratory study. Eur J Cardiovasc Nurs 2015;14:53–62. [DOI] [PubMed] [Google Scholar]
- 38.Wildschut ED, Ahsman MJ, Houmes RJ, et al. Pharmacotherapy in neonatal and pediatric extracorporeal membrane oxygenation (ECMO). Curr Drug Metab 2012; 13: 767–777. [DOI] [PubMed] [Google Scholar]
- 39.Wildschut ED, van Saet A, Pokorna P, Ahsman MJ, Van den Anker JN, Tibboel D. The impact of extracorporeal life support and hypothermia on drug disposition in critically ill infants and children. Pediatr Clin North Am 2012; 59: 1183–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harthan AA, Buckley KW, Heger ML, Fortuna RS, Mays K. Medication adsorption in to contemporary extracorporeal membrane oxygenator circuits. J Pediatr Pharmacol Ther 2014;19:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shekar K, Roberts JA, Mcdonald CI, et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care 2012;16:R194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watt KM, Gonzalez D, Benjamin DK Jr, et al. Fluconazole population pharmacokinetics and dosing for prevention and treatment of invasive Candidiasis in children supported with extracorporeal membrane oxygenation. Antimicrob Agents Chemother 2015;59:3935–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jennings DL, Makowski CT, Chambers RM, Lanfear DE. Dosing of vancomycin in patients with continuous-flow left ventricular assist devices: a clinical pharmacokinetic analysis. Int J Artif Organs 2014; 37: 270–274. [DOI] [PubMed] [Google Scholar]
- 44.Shimamoto Y, Fukuda T, Tominari S, et al. Decreased vancomycin clearance in patients with congestive heart failure. Eur J Clin Pharmacol 2013; 69: 449–457. [DOI] [PubMed] [Google Scholar]
- 45.Heil EL, Lowery AV, Thom KA, Nicolau DP. Treatment of multidrug-resistant pseudomonas aeruginosa using extended-infusion antimicrobial regimens. Pharmacotherapy 2015; 35: 54–58. [DOI] [PubMed] [Google Scholar]
- 46.Lexicomp. Cefepime drug information. Lexicomp web site; http://online.lexi.com/action/home. Accessed September 13, 2017. [Google Scholar]
- 47.Nicasio AM, Ariano RE, Zelenitsky SA, et al. Population pharmacokinetics of high-dose, prolonged-infusion cefepime in adult critically ill patients with ventilator-associated pneumonia. Antimicrob Agents Chemother 2009; 53: 1476–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jennings DL, Brewer R, Williams C. Impact of continuous flow left ventricular assist device on the pharmacodynamic response to warfarin early after implantation. Ann Pharmacother 2012; 46: 1266–1267. [DOI] [PubMed] [Google Scholar]
- 49.Shekar K, Roberts JA, McDonald CI, et al. Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: results from an ex vivo study. Crit Care 2015; 19: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Preston TJ, Hodge AB, Riley JB, Leib-Sargel C, Nicol KK. In vitro drug adsorption and plasma free hemoglobin levels associated with hollow fiber oxygenators in the extracorporeal life support (ECLS) circuit. J Extra Corpor Technol 2007; 39: 234–237. [PMC free article] [PubMed] [Google Scholar]
- 51.Preston TJ, Ratliff TM, Gomez D, et al. Modified surface coatings and their effect on drug adsorption within the extracorporeal life support circuit. J Extra Corpor Technol 2010; 42: 199–202. [PMC free article] [PubMed] [Google Scholar]
- 52.Duke University Hospital ECMO Steering Committee. ECMO Set Up and Management Policy. Duke University Sites@Duke web site; https://sites.duke.edu/micu/files/2016/02/ECMO-Set-Up-Management.pdf. Published May 2, 2015. Accessed September 13, 2017. [Google Scholar]
- 53.Food and Drug Administration (FDA) Pediatric Advisory Committee. Berlin Heart Inc. EXCOR Pediatric the Ventricular Assist Device for Children. FDA web site; https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM461241.pdf. Updated September 16, 2015. Accessed September 13, 2017. [Google Scholar]
- 54.Thoratec Corporation. CentriMag: Magnetically Levitated Circulatory Support System. Columbia University Department of Surgery web site; http://columbiasurgery.org/sites/default/files/lvad_centrimag.pdf. Accessed September 13, 2017. [Google Scholar]
- 55.Anderson HL 3rd, Coran AG, Drongowski RA, Ha HJ, Bartlett RH. Extracellular fluid and total body water changes in neonates undergoing extracorporeal membrane oxygenation. J Pediatr Surg 1992; 27: 1003–1007; discussion 1007–1008. [DOI] [PubMed] [Google Scholar]
- 56.Butler J, Pathi VL, Paton RD, et al. Acute-phase responses to cardiopulmonary bypass in children weighing less than 10 kilograms. Ann Thorac Surg 1996; 62: 538–542. [PubMed] [Google Scholar]
- 57.Seghaye MC, Grabitz RG, Duchateau J, et al. Inflammatory reaction and capillary leak syndrome related to cardiopulmonary bypass in neonates undergoing cardiac operations. J Thorac Cardiovasc Surg 1996; 112: 687–697. [DOI] [PubMed] [Google Scholar]
- 58.Corry DC, DeLucia A 3rd, Zhu H, et al. Time course of cytokine release and complement activation after implantation of the HeartMate left ventricular assist device. ASAIO J 1998; 44: M347–351. [DOI] [PubMed] [Google Scholar]
- 59.Petretta M, Condorelli GL, Spinelli L, et al. Circulating levels of cytokines and their site of production in patients with mild to severe chronic heart failure. Am Heart J 2000; 140: E28. [DOI] [PubMed] [Google Scholar]
- 60.Clark AL, Loebe M, Potapov EV, et al. Ventricular assist device in severe heart failure: effects on cytokines, complement and body weight. Eur Heart J 2001; 22: 2275–2283. [DOI] [PubMed] [Google Scholar]
- 61.Caruso R, Verde A, Campolo J, et al. Severity of oxidative stress and inflammatory activation in end-stage heart failure patients are unaltered after 1 month of left ventricular mechanical assistance. Cytokine 2012; 59: 138–144. [DOI] [PubMed] [Google Scholar]
- 62.Slaviero KA, Clarke SJ, Rivory LP. Inflammatory response: an unrecognised source of variability in the pharmacokinetics and pharmacodynamics of cancer chemotherapy. Lancet Oncol 2003; 4: 224–232. [DOI] [PubMed] [Google Scholar]
- 63.Reddy RC, Goldstein AH, Pacella JJ, Cattivera GR, Clark RE, Magovern GJ Sr. End organ function with prolonged nonpulsatile circulatory support. ASAIO J 1995; 41: M547–551. [DOI] [PubMed] [Google Scholar]
- 64.Russell SD, Rogers JG, Milano CA, et al. Renal and hepatic function improve in advanced heart failure patients during continuous-flow support with the HeartMate II left ventricular assist device. Circulation 2009; 120: 2352–2357. [DOI] [PubMed] [Google Scholar]
- 65.Demirozu ZT, Etheridge WB, Radovancevic R, Frazier OH. Results of HeartMate II left ventricular assist device implantation on renal function in patients requiring post-implant renal replacement therapy. J Heart Lung Transplant 2011; 30: 182–187. [DOI] [PubMed] [Google Scholar]
- 66.Imamura T, Kinugawa K, Shiga T, et al. Preoperative levels of bilirubin or creatinine adjusted by age can predict their reversibility after implantation of left ventricular assist device. Circ J 2013; 77: 96–104. [DOI] [PubMed] [Google Scholar]
- 67.Imamura T, Kinugawa K, Shiga T, et al. Novel risk scoring system with preoperative objective parameters gives a good prediction of 1-year mortality in patients with a left ventricular assist device. Circ J 2012; 76: 1895–1903. [DOI] [PubMed] [Google Scholar]
- 68.Gonzalez D, Melloni C, Yogev R, et al. Use of opportunistic clinical data and a population pharmacokinetic model to support dosing of clindamycin for premature infants to adolescents. Clin Pharmacol Ther 2014; 96: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hornik CP, Wu H, Edginton AN, Watt K, Cohen-Wolkowiez M, Gonzalez D. Development of a pediatric physiologically-based pharmacokinetic model of clindamycin using opportunistic pharmacokinetic data. Clin Pharmacokinet 2017; 56: 1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]