Abstract
Two hydrophilic spiroalkenes, azaspiro[2.3]hex-1-ene and azaspiro[2.4]hept-1-ene, were designed and synthesized. Compared to the previously reported spiro[2.3]hex-1-ene, the azaspiroalkenes exhibited greater water solubility and reactivity as dipolarophiles in the photoindued tetrazale-alkene cycloaddition reaction. In addition, an azaspiro[2.3]hex-1-ene-containing amino acid, AsphK, was found to be charged by an engineered pyrrolysyl-tRNA synthetase into proteins via amber codon suppression in E. coli as well as in mammalian cells.
Graphical Abstract
Highly strained hydrophilic bioorthogonal reporters, azaspiro[2.3]hex-1-ene and azaspiro[2.4]hept-1-ene, were synthesized and characterized.
Bioorthogonal chemistry offers a powerful tool to manipulate the biomolecule structure and function in living systems. In this approach, bioorthogonal reporters with a balanced stability and reactivity are introduced into in the biomolecule followed by the bioorthogonal ligation reaction with a biophysical probe.1 In the design of reactive, yet stable, bioorthogonal reporters, the strained ring structures have attracted most attention over the last decade because of their robust reactivity in the catalyst-free bioorthogonal cycloaddition reactions.2 These include numerous strained alkenes and alkynes such as norbornene,3 cyclooctyne,4 trans-cyclooctene (TCO),5 cyclopropene6 and their derivatives. While significant rate enhancement was observed with these strained molecules, the majority of the strained bioorthogonal reporters are highly lipophilic because of their large hydrocarbon skeleton, which may cause them to interact non-specifically with hydrophobic proteins and as a result, reduce accessibility to their cognate reaction partners.7 Indeed, several efforts were reported to introduce heteroatoms into the bioorthogonal reporters to reduce overall hydrophobicity with varying degrees of success.8 Therefore, it is highly desirable to design the hydrophilic strained chemical reporters for robust bioorthogonal cycloaddition reactions.
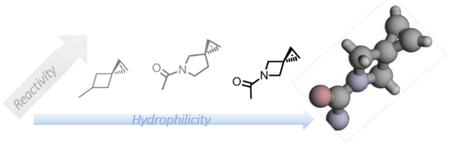
Previously, we reported the design of spiro[2.3]hex-1-ene (Sph), a highly strained spirocyclic alkene for the photoinduced tetrazole-alkene cycloaddition with k2 values approaching 104 M−1 s−1 (Fig. 1).9 An Sph-modified lysine, SphK, was genetically encoded into the class B G protein-coupled receptors (GPCRs) and subsequently labelled with a fluorophore via the tetrazole-alkene cycloaddition reaction.10 To enhance the utility of Sph as a bioorthogonal reporter, herein we report the design and synthesis of the hydrophilic analogues of Sph by introducing nitrogen into the spirocyclic ring system, structural and kinetic characterizations of the azaspiroalkenes in the photoinduced tetrazole-alkene cycloaddition reaction, and genetic encoding of an azaspirohexene-containing amino acid in bacteria as well as in mammalian cells.
Fig. 1.
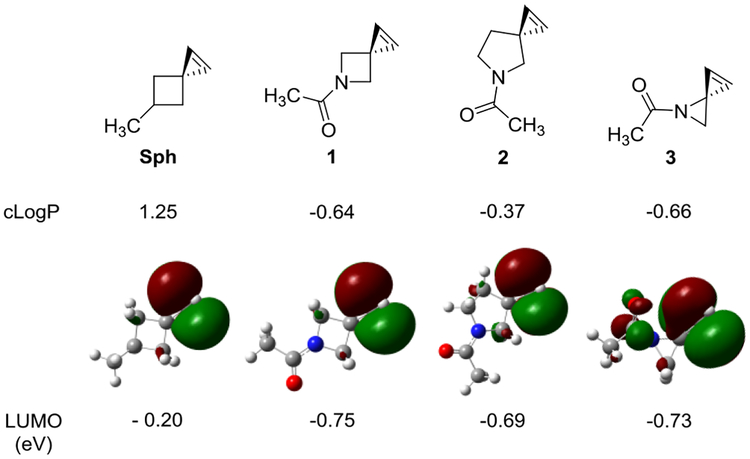
Structures of spiro[2.3]hex-1-ene and its aza derivatives with their cLogP values, optimized geometries, and LUMO energies shown below the structure. The LUMO energies were calculated using the B3LYP method at 6–31++G** level in vacuum at 298 K.
To decrease the hydrophobicity, we considered bioisosteric replacement of carbon with nitrogen in the Sph skeleton. Since amines show pH-dependent charge states, we further designed the amide linkage so that the reporter structure is hydrophilic whose polarity is not pH dependent. Accordingly, three Sph derivatives were designed with the difference being the second spirocyclic ring size (Fig. 1). The calculated cLogP values for the three spiroalkenes 1–3 are −0.64, −0.37, and −0.66, respectively, all of which are significantly lower than that of Sph (Fig. 1). On their reactivity, the calculated LUMO energies of the azaspiroalkenes were lower than that of SphK, leading to the reduced HOMO−LUMO gap and likely higher cycloaddition rate with the in situ generated nitrile imines.11
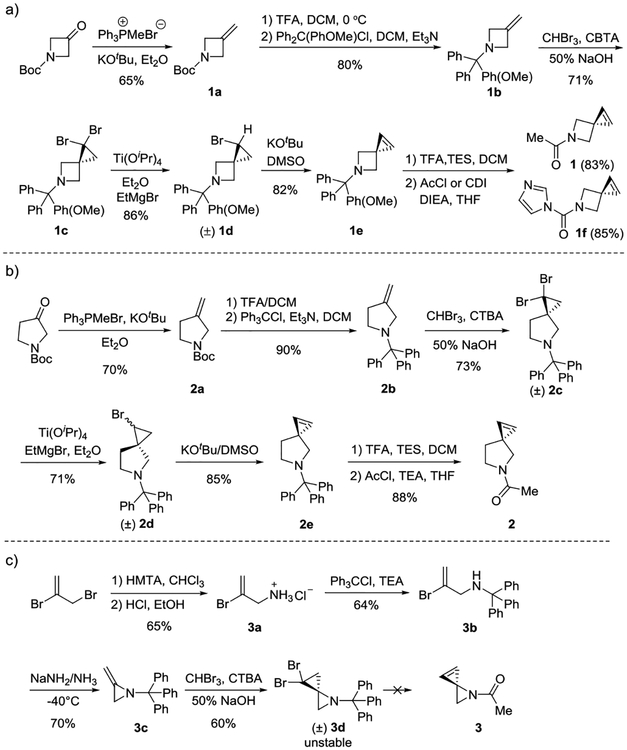
For the synthesis of azaspiroalkenes 1-3, synthetic routes analogous to Sph were adopted (Scheme 1). For azaspirohexene (1), the commercially available starting materials, N-Boc-3-oxoazetidine, was transformed into the exocyclic alkene 1a in 65% yield via the Wittig reaction. After switching the protecting group from Boc to methoxy-trityl group, the spirocycle 1c was obtained in 71% yield through dibromo carbene insertion in the presence of 50% NaOH solution. Subsequent Ti(OiPr)4 catalysed mono-debromination by EtMgBr followed by KOtBu mediated elimination of HBr produced the methoxy-trityl protected azaspirohexene 1e. Removal of the trityl group followed by acetylation by acetyl chloride afforded azaspirohexene 1 in 83% yield (Scheme 1a). For synthesis of azaspiroheptene 2, the commercially available N-Noc-3-oxopyrrolidine was used as the starting materials. An identical route was taken except that the trityl protecting group was used during the cyclopropanation, debromonation, and elimination reactions (Scheme 1b). For the synthesis of azaspiro[2.2]hep-1-ene, the key intermediate, dibromo-azaspiropentane 3d, was obtained in 60% yield after carbene insertion into the reported structure 3c12 (Scheme 1c). However, repeated attempts to convert 3d to spiropentene 3 were not successful, presumably due to the high ring strain present in such a small spirocyclic system. Moreover, spirocycle 3d decomposed gradually in solution at room temperature. Nevertheless, TLC analysis indicated that azaspirohexene 1 is indeed much more polar than the corresponding spirohexene (Table S1 in ESI). In addition, LC-MS-based measurements gave LogP values of −0.95 and −0.12 for azaspiroalkenes 1 and 2, respectively, lower than that of an acetal-substituted Sph (LogP = 0.13; Table S2 in ESI), indicating that the azaspiroalkenes are indeed more hydrophilic than spirohexene.
Scheme 1.
Synthesis of the spiroalkenes.
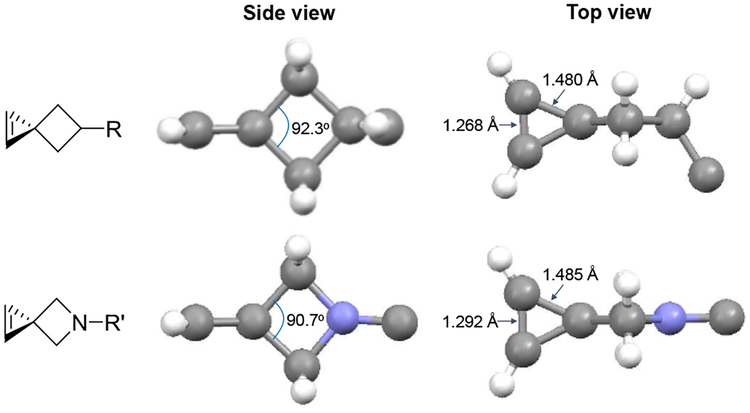
The reactivity of 3,3-disubstituted cyclopropenes, to which spiroalkenes belong, in the cycloaddition reactions are known to be critically dependent on the steric hindrance.9,13 For the nitrile imine-mediated 1,3-dipole cycloaddition, the reduced bond angle between two C3-substituents leads to lower steric hindrance and thus higher reactivity.9 To determine how the nitrogen replacement affects the bond angle, we prepared an azaspiro[2.3]hex-1-ene derivative 1f (Scheme 1a) and obtained its crystal structure (Table S2 in ESI). Compared to the Sph structure, the basic framework of azaspirohexene (Asph) stays intact with some minor changes (Fig. 2). For example, the bond angle between the two C3-substituents in Asph is 90.7o, smaller than 92.3o of Sph,9 suggesting somewhat reduced steric hindrance compared to Sph. The double-bond length in the cyclopropene ring of Asph is longer than that of Sph (1.292 Å vs. 1.268 Å; Fig. 2), indicating that the π-bond is weaker and potentially more reactive in the cycloaddition reaction.
Fig. 2.
Crystal structures of the spirohexene (top) and azaspirohexene 1f (bottom) viewed from the side and the top. The R or R’ group are omitted for clarity.
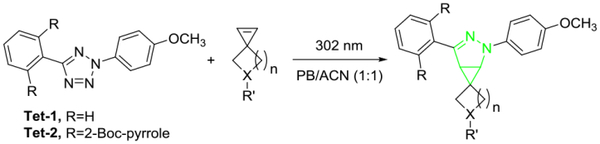
To determine the reactivity experimentally, we carried out the kinetics analysis of spiroalkenes 1, 2 and Sph in the cycloaddition reaction with two tetrazoles, Tet-1 and Tet-2,10 and the results are collected in Table 1. Because the cycloaddition produces fluorescent pyrazoline adduct, we followed the reaction progress by monitoring time-dependent fluorescence increases (Fig. S1–S6 in ESI). All reactions proceeded fast, with the second-order rate constant, k2, exceeding 104 M−1 s−1. In particular, azaspiro[2.4]hept-1-ene 2 gave the fastest reaction, with k2 value of 3.32 × 104 M−1 s−1 (Table 1). For the sterically less hindered Tet-1, azaspiroalkenes 1 and 2 reacted faster than Sph (compare entries 2 and 3 to 1 in Table 1), in agreement with their lower LUMO energies (Fig. 1). For the sterically shielded Tet-2,10 the reactivity enhancement is negligible, suggesting that the small changes in bond angle and bond length, as observed for Asph in Fig. 2, have no effect on lowering the transition state energy. The NMR studies of the cycloaddition reaction at higher mM concentrations between Tet-1 and spiroalkene 1 or 2 showed about 90~95% conversion after 2-hour photoirradiation (Fig. S7–S8 in ESI).
Table 1.
Kinetic characterization of the cycloaddition reactions between spiroalkenes and tetrazolesa
| entry | spiroalkene | tetrazole | k2 (M−1 s−1) |
|---|---|---|---|
| 1 | Sph | Tet-1 | 15,200 ± 3,500 |
| 2 | 1 | Tet-1 | 22,400 ± 7,500 |
| 3 | 2 | Tet-1 | 33,200 ± 5,500 |
| 4 | Sph | Tet-2 | 13,200 ± 1,100 |
| 5 | 1 | Tet-2 | 14,100 ± 2,200 |
| 6 | 2 | Tet-2 | 17,000 ± 3,500 |
A solution of tetrazole (1 μM) and spiroalkene (10 μM) in PB/ACN (1:1), pH = 7.4, in a quartz cuvette was photoirradiated with a 302-nm UV lamp for various times prior to fluorescence measurement. PB = phosphate buffer, ACN = acetonitrile. The formation of the pyrazoline adducts was confirmed by mass spectrometry.
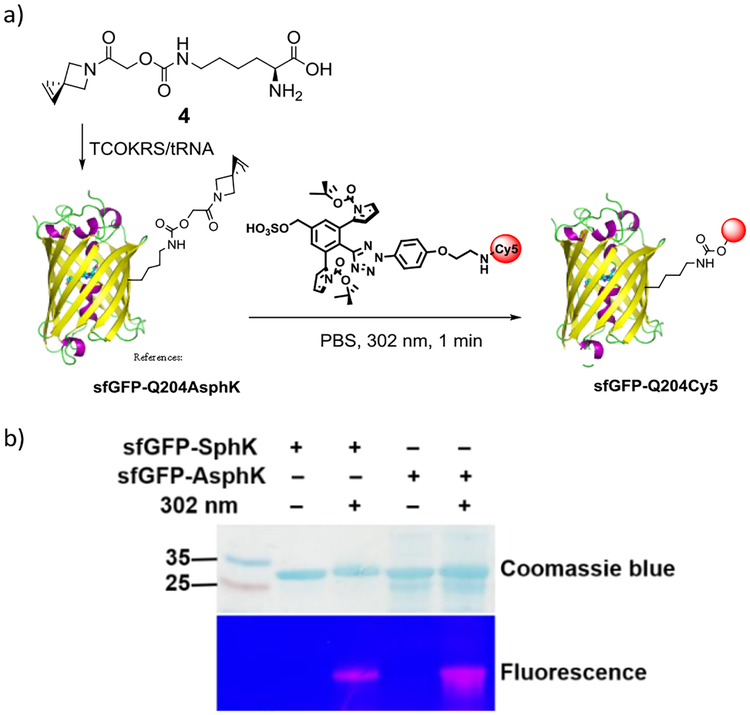
To assess the stability of azaspiroalkenes 1 and 2 in biological milieu, we incubated azaspiroalkenes with excess amount of reduced glutathione (GSH; 10 mM) for one week and found them to be stable in mixed DMSO/PBS (3:1) solvent for one week at room temperature based on 1H NMR (Fig. S9–S10 in ESI). Considering their excellent water-solubility, robust stability, and enhanced reactivity, we synthesized the azaspirohexene-conjugated lysine, AsphK (Scheme S4 in ESI), which unlike SphK turned out to be completely water-soluble. We then screened a panel of pyrrolysyl-tRNA synthetase (PylRS) variants14 for their ability to charge AsphK into superfolder green fluorescent protein containing amber codon at position-204 (sfGFP-204TAG) in E. coli. To our delight, we found a PylRS variant previously reported to charge TCOK, or TCOKRS,7 supported site-specific incorporation of AsphK into sfGFP-204TAG with expression yield of 0.1 mg/L (Fig. S11 in ESI), which is significant lower than that of the SphK-encoded sfGFP mutant (4.9 mg/L) catalysed by the wild-type PylRS.9 We then compared the reactivity of AsphK to SphK in the protein context with a Cy5-conjugated tetrazole we used previously10 (Fig. 3a), and found that AsphK gave higher amount of the labelling product based on Cy5 fluorescence intensity (Fig. 3b), along with several minor, faster-migrating bands probably generated as a result of the degradation of the AsphK-encoded sfGFP protein. Because Asph showed a similar reactivity compared to Sph in the kinetic study (compare entry 5 to 4 in Table 1), we attributed this reactivity enhancement to the greater accessibility of AsphK in aqueous medium because of its hydrophilicity. Indeed, improve solvent accessibility of bioorthogonal reporters has been shown to be responsible for higher labeling yields.15
Fig. 3.
Fluorescent labelling the AsphK-encoded sfGFP with a Cy5-conjugated sterically shielded tetrazole. (a) Reaction scheme. (b) SDS-PAGE analysis of the reaction mixture after irradiating the solution of sfGFP-Q204SphK/sfGFP-Q204AsphK and Cy5-conjugated tetrazole (100 μM) in PBS with a 302-nm UV lamp for 1 min. Top panel, Coomassie blue stained gel; bottom panel, in-gel fluorescence analysis by illuminating the same gel with 365-nm UV light.
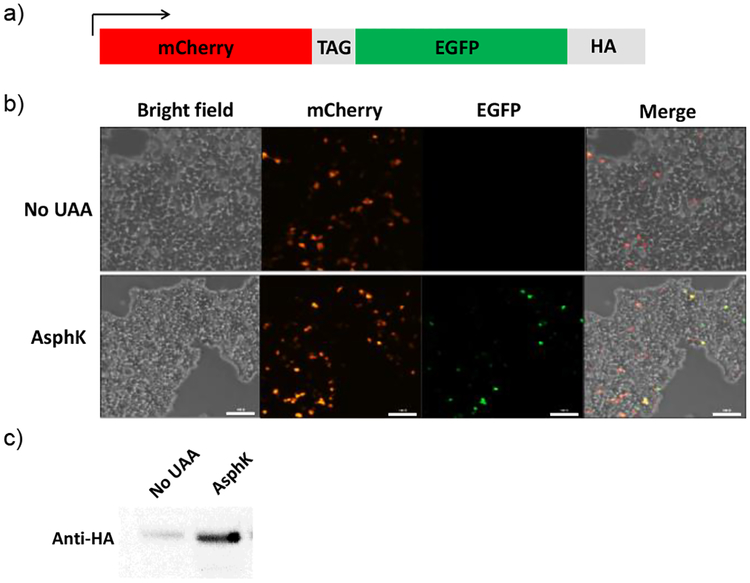
To probe whether Asph can be incorporated site-specifically into proteins in mammalian cells, we co-transfected human embryonic kidney (HEK) 293T cells (obtained from ATCC) with two plasmids, one encoding TCOKRS and tRNAPyl CUA, and the other encoding a fluorescent mCherry-EGFP reporter containing a TAG amber codon in the middle and an HA-tag at C-terminus (Fig. 4a). Cells were grown in DMEM medium supplemented with 10% FBS in the absence or presence of 1 mM AsphK for 48 h, and imaged by a fluorescent microscope. The EGFP fluorescence was detected only when AsphK was present in the medium (Fig. 3b, Fig. S12). The anti-HA western blot of the cell lysates confirmed the expression of full-length mCherry-EGFP-HA fusion protein when AsphK was present, although a low-level background suppression was also detected when AsphK was absent (Fig. 3c), which can be attributed to the promiscuity of TCOKRS.16
Fig. 4.
Genetic encoding of AsphK in mammalian cells. (a) Structural diagram of the mCherry-TAG-EGFP-HA reporter. (b) Bright field and fluorescent micrographs of HEK 293T cells transfected with plasmids encoding mCherry-TAG-EGFP-HA and TCOKRS/tRNAPyl CUA and cultured in DMEM medium supplemented with 10% FBS in the absence or presence of 1 mM AsphK. Scale bar = 1000 μm. (c) Western blot analysis of HEK 293T cell lysates probed with anti-HA antibody.
In conclusion, we have designed and synthesized two novel spiroalkene bioorthogonal reporters, azaspiro[2.3]hex-1-ene and azaspiro[2.4]hept-1-ene. Compared to the carbocyclic Sph, the azaspiroalkenes are water-soluble and exhibit improved kinetics with k2 values approaching 3.32 × 104 M−1 s−1 in the photoinduced tetrazole-alkene cycloaddition reaction. Moreover, a lysine derivative containing the azaspiro[2.3]hex-1-ene reporter (AsphK) was incorporated into sfGFP site-specifically via amber codon suppression. The AsphK-encoded sfGFP was labelled at a higher efficiency than the SphK-encoded counterpart presumably due to its greater accessibility in aqueous medium. Finally, the incorporation of AsphK into a reporter protein in mammalian cells was demonstrated. Because of its hydrophilicity and robust reactivity, Asph could find wide use in bioorthogonal studies of biomolecular structure, dynamics and function in living systems.
Supplementary Material
Acknowledgements
We gratefully acknowledge NIH (GM085092) for financial support. We thank Jordan Cox at SUNY Buffalo for solving X-ray structure for compound 1f with Cambridge Structural Database accession number: CCDC 1864586.
Footnotes
Conflicts of interest
There are no conflicts to declare.
Notes and references
- 1.Sletten EM and Bertozzi CR, Angew. Chem., Int. Ed, 2009, 48, 6974; [DOI] [PMC free article] [PubMed] [Google Scholar]; Lim RKV and Lin Q, Chem. Commun, 2010, 46, 1589; [DOI] [PMC free article] [PubMed] [Google Scholar]; Ramil CP, Q. Lin, Chem. Commun 2013, 49, 11007; [DOI] [PMC free article] [PubMed] [Google Scholar]; Lang K and Chin JW, Chem. Rev 2014, 114, 4764; [DOI] [PubMed] [Google Scholar]; Patterson DM, Nazarova LA and Prescher JA, ACS Chem. Biol 2014, 9, 592–605; [DOI] [PubMed] [Google Scholar]; Oliveira BL, Guo Z and Bernardes GJL, Chem. Soc. Rev 2017, 46, 4895. [DOI] [PubMed] [Google Scholar]
- 2.Debets MF, Berkel SSV, Dommerholt J, Dirks AJ, Rutjes FPJT, and Delft FLV, Acc. Chem. Res, 2011, 44, 805; [DOI] [PubMed] [Google Scholar]; Liu F, Liang Y and Houk KN, Acc. Chem. Res, 2017, 50, 2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han H-S, Devaraj NK, Lee J, Hilderbrand SA, Weissleder R and Bawendi MG, J. Am. Chem. Soc 2010, 132, 7838; [DOI] [PMC free article] [PubMed] [Google Scholar]; van Berkel SS, Dirks AJ, Debets MF, van Delft FL, Cornelissen JJLM, Nolte RJM and Rutjes FPJT, ChemBioChem 2007, 8, 1504; [DOI] [PubMed] [Google Scholar]; Devaraj NK, Weissleder R, Hilderbrand SA, Bioconjugate Chem 2008, 19, 2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agard NJ, Prescher JA and Bertozzi CR, J. Am. Chem. Soc 2004, 126, 15046. [DOI] [PubMed] [Google Scholar]
- 5.Blackman ML, Royzen M and Fox JM, J. Am. Chem. Soc 2008, 130, 13518; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J, Šečkutė J, Cole CM and Devaraj NK, Angew. Chem., Int. Ed 2012, 51, 7476; [DOI] [PMC free article] [PubMed] [Google Scholar]; Patterson DM, Nazarova LA, Xie B, Kamber DN and Prescher JA, J. Am. Chem. Soc 2012, 134, 18638; [DOI] [PubMed] [Google Scholar]; Yu Z, Pan Y, Wang Z, Wang J and Lin Q Angew. Chem., Int. Ed 2012, 51, 10600. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ramil CP, Dong M, An P, Lewandowski TM, Yu Z, Miller LJ and Lin QJ Am. Chem. Soc 2017, 139, 13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang K, Davis L, Wallace S, Mahesh M, Cox DJ, Blackman ML, Fox JM and Chin JW, J. Am. Chem. Soc 2012, 134, 10317; [DOI] [PMC free article] [PubMed] [Google Scholar]; Nikic I, Plass T, Schraidt O, Szyman ski J, Briggs JAG, Schultz C and Lemke EA, Angew. Chem. Int. Ed 2014, 53, 2245; [DOI] [PubMed] [Google Scholar]; Uttamapinant C, Howe JD, Lang K, Beránek V, Davis L, Mahesh M, Barry NP and Chin JW, J. Am. Chem. Soc 2015, 137, 4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engelsma SB, Willems LI, van Passchen CE, van Kasteren SI, van der Marel GA, Overkleeft HS and Filippov DV, Org. Lett 2014, 16, 2744; [DOI] [PubMed] [Google Scholar]; Kozma E, Nikić I, Varga BR, Aramburu IV, Kang JH, Fackler OT, Lemke EA and Kele P, ChemBioChem 2016, 17, 1518; [DOI] [PubMed] [Google Scholar]; Siegl SJ, Vázquez A, Dzijak R, Dračínský M, Galeta J, Rampmaier R, Klepetáĭová B and Vrabel M, Chem. Eur. J 2018, 24, 2426. [DOI] [PubMed] [Google Scholar]
- 9.Yu Z and Lin Q, J. Am. Chem. Soc, 2014, 136, 4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An P, Lewandowski TM, Erbay TG, Liu P and Lin Q, J. Am. Chem. Soc, 2018, 140, 4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Song W, Hu WL and Lin Q, Angew. Chem., Int. Ed, 2009, 48, 5330; [DOI] [PMC free article] [PubMed] [Google Scholar]; An P and Lin Q, Org. Biomol. Chem, 2018, 16, 5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ince J, Ross TM, Shipman M and Slawin AMZ, Tetrahedron, 1996, 52, 7037. [Google Scholar]
- 13.Kamber DN, Nazarova LA, Liang Y, Lopez SA, Patterson DM, Shih H-W, Houk KN, Prescher JA, J. Am. Chem. Soc 2013, 135, 13680. [DOI] [PubMed] [Google Scholar]
- 14.Wan W, Tharp JM and Liu WR, Biochim. Biophys. Acta, 2014, 1844, 1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahim MK, Kota R, and Haun JB, Bioconjugate Chem 2015, 26, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang X, Song X, Faller C, Lai R, Li H, Cerny R, Niu W and Guo J, Chem. Sci 2017, 8, 1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.