Abstract
The adrenal cortex accumulates lipofuscin granules with age. Lipofuscin accumulation is also seen in adrenocortical tumors associated with Cushing syndrome (CS), particularly those with PRKAR1A mutations, such as in primary pigmented nodular adrenocortical disease (PPNAD). We investigated the presence of lipofuscin in cortisol-producing adenomas (CPAs) responsible for CS with and without the PRKACA (pLeu206Arg) somatic mutation. Ten paraffin-embedded sections of CPAs from cases with overt CS with (n=4) and without (n=6) a PRKACA mutation were microscopically examined through three detection methods, the hematoxylin-Eosin (H & E) staining, the Fontana Masson (FM) staining using light microscopy, and lipofuscin autofluorescence, using confocal laser scanning microscopy (CLSM). Sections were examined quantitatively according to the intensity of the pigmentation, as well as qualitatively based on the total number of granular pigments at all visual fields per tissue slide. Tissues from CPAs were compared to peritumoral adjacent tissues (n=5), to Conn adenomas (n=4), and PPNAD (n=3). CPAs had significantly higher number of lipofuscin-pigment granules compared to peritumoral adrenal tissue and Conn adenomas (46.9±9.5 vs. 3.8±4.8, p=0.0001). The presence of the PRKACA mutation did not increase the chances of pigmentation in the form of lipofuscin granules within CPAs associated with CS. Thus, all CPAs leading to CS accumulate lipofuscin, which presents like pigmentation sometimes seen macroscopically but always detected microscopically. PPNAD caused by PRKAR1A mutations is the best known adrenal lesion leading to CS associated with intense lipofuscin pigmentation and this was confirmed here; CPAs harboring PRKACA mutations did not have statistically significantly more pigmentation than CPAs without mutation, but a larger study might have shown a difference.
Keywords: lipofuscin, Cushing syndrome, adrenal adenoma, cortisol, Conn adenoma, PPNAD
Introduction
Age-related deposition of pigment in cells was described initially by Hanover in 1842 and subsequently named lipofuscin by Hueck in 1912 and Borst in 1922 [1]. Subcellular deposition of lipofuscin granules is a marker of aging [2]. Lipofuscin is the end product of lysosomal degradation with intracellular accumulation, since it is not degradable and cannot be removed via exocytosis [3]. The ultrastructural appearance of lipofuscin granules resembles that of secondary lysosomes [2]. The storage of lipofuscin is based on lipoid peroxidation: the lysosomes absorb the remaining intracellular products including all other products of lipoid peroxidation, but because it is not digestible by lysosomes, lipofuscin is accumulated as the residual product [3].
Generally, lipofuscin is known to accumulate in cardiac myocytes, hepatocytes, brain neurons, and the adrenal cortex as part of the aging process [1] [4] [5]. In the adrenal cortex, the intracellular volume of lipofuscin gradually increases from the outer zona glomerulosa to the inner zona reticularis [4]. Histologically, adrenocortical lipofuscin granules contain cholesterol and cholesterol esters and the autofluorescent properties are due principally to retinyl stearate molecules [5].
Lipofuscin accumulation is primarily responsible for the pigmentation seen within adrenal nodules in primary pigmented nodular adrenocortical disease (PPNAD) and, rarely, in single “black adenomas” [1] [3] [5] [6]. Cushing syndrome (CS) is defined by the presence of endogenous hypercortisolism due to various adrenal etiologies [7]. Mutations in genes encoding the cAMP-dependent protein kinase A (PKA) have been identified in patients with CS due to PPNAD and cortisol-producing adenomas (CPAs) [8]: PRKAR1A mutations cause PPNAD, whereas PRKACA genetic defects cause a wide array of abnormalities from hyperplasias that are PPNAD-like to CPAs; the latter harbor unique somatic PRKACA mutations, such as the p.Leu206Arg one that (like the PRKAR1A-inactivating mutations) lead to constitutive PKA activation and clinical CS [8] [9] [10].
We hypothesized that lipofuscin is a unique pathologic signature of adrenocortical lesions due to PKA defects and/or cortisol-producing lesions. Thus, we investigated the presence of lipofuscin in paraffin-embedded tissues from CPAs excised from patients with clinical CS that were sequenced for PRKACA/PRKAR1A defects. The data were compared with other adrenocortical lesions, such as those known to accumulate lipofuscin (i. e., PPNAD) and those that do not have any pigment (Conn’s adenomas). The presence of lipofuscin was analyzed by different staining techniques using light microscopy as well as with confocal microscopy independently of their macroscopic coloration.
Material and Methods
Patients
A total of 10 female patients with CPAs, presenting with overt CS, aged between 15–49 years old (mean age at diagnosis: 39.8±10.9 years old) were operated at the National Institutes of Health (NIH) Clinical Center and included in this study in the last 2 years ([Table 1]). Age was not statistically significantly different between groups (mean age of CPA patients with the mutation: 39±10 years old, CPA patients without the mutation: 40.5±12 years old). The size of the CPAs varied from 0.6 cm to 3 cm. Adrenocortical tissue samples were collected during the operation and processed for paraffin fixation; fragments from within the tumor and the surrounding tissue were also frozen in liquid nitrogen (–80°C). Genetic analysis (Sanger sequencing) was performed in all tissues, tumor and normal, and in peripheral DNA, as described previously for mutations in the PKA pathway (PRKAR1A, PRKACA), as well for GNAS and CTNNB1 [8] [9]. Four out of these 10 patients were found positive for a single, recurrent, PRKACA somatic mutation (pLeu206Arg) [8] [9]. None of the CPAs had any PRKAR1A, GNAS or CTNNB1 mutations. All patients were investigated under a protocol for the study of adrenocortical tumors approved by the NICHD institutional review board and gave their informed consent.
Table 1.
Grading of lipofuscin pigmentation in H & E staining slides.
| Case | Nodule size(cm) | Nodule color | Number of nodules | Nodule side | H&E staining | FM staining | Confocal microscopy | PRKACA mutation | Total number of granules/Number of visual fields |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | Black-brown | Single | left | +++ | Yes | Yes | Yes | 132/6 |
| 2 | 2.4 | Gold-brown | Single | left | + | Yes | Yes | Yes | 22/6 |
| 3 | 0.6 | Dark-brown | Single | right | ++ | Yes | Yes | Yes | 16/5 |
| 4 | 3 | Dark-brown | Multiple | left | +++ | Yes | Yes | Yes | 44/5 |
| 5 | 3 | Red-brown | Single | left | ++ | Yes | NP | No | 35/6 |
| 6 | 2.5 | Black-brown | Single | left | +++ | Yes | Yes | No | 24/6 |
| 7 | 4 | Gold | Single | left | − | NP | NP | No | 10/4 |
| 8 | 6 | Brown | Single | left | ++ | Yes | Yes | No | 165/6 |
| 9 | 2 | Orange | Single | left | + | Yes | NP | No | 10/4 |
| 10 | 5 | Yellow | Single | left | + | NP | NP | No | 14/5 |
Intense (+++) ≥ 40 lipofuscin-pigment granules per slide; moderate (++)=20–40; faint (+)=10–20; negative (–) ≤ 10; CPAs: Cortisol-producing adenomas; H & E: Hematoxylin-eosin; FM: Fontanna Masson; NP: Not performed
Tissue studies
Paraffin-embedded 2 μm-thick adrenal tumor tissue samples were analyzed with three different methods for the detection and confirmation of lipofuscin pigmentation: 1) Hematoxylin-Eosin (H & E), 2) Fontana-Masson (FM) staining (Histoserv, Germantown, MD, USA), and 3) autofluorescent detection of lipofuscin by confocal laser scanning microscopy (CSLM) on unstained paraffin sections, as previously described [5] [11]. H & E was performed in all 10 tissue-samples, FM in 8 out of 10 and CSLM in 6 out of 10 tissue-samples because of insufficient material. Tissues were visualized, analyzed and images captured using the Olympus BX40 microscope fitted with a DP71 Olympus camera (Olympus). CSLM was used to visualize autofluorescent lipofuscin using the Zeiss LSM 710 confocal microscope (Zeiss, Germany), described below. All tissues analyses were performed in a blinded manner as far as the mutation status.
Lipofuscin quantification using light microscopy
Routine H & E staining was performed on unstained sections. Briefly, tissues sections underwent deparaffinization by xylene, followed by rehydration in graded ethanol (100%, 90%, 70%, 50%), followed by staining, as per routine H & E pathology staining procedures, previously described [12]. Following staining, coverslips were mounted and slides analyzed. For every tumor, sequential sections were cut onto individual slides whereby each slide was processed for H & E staining, then FM staining, and one remained unstained for analysis of the confocal images of autofluorescent lipofuscin. HE stained slides were observed using light microscopy (40× magnification; Olympus BX40 with a DP71 Olympus camera, Olympus). We analyzed the intensity of lipofuscin staining by quantifying the total number of cytoplasmic lipofuscin-pigment granules of all visual fields per slide for each tissue. The number of lipofuscin-pigment granules per slide of CPAs tissues was then compared with 5 peritumoral adjacent adrenal paraffin-embedded tissues (normal tissues), 4 Conn adenomas paraffin-embedded tissues (negative controls), as well 3 paraffin-embedded tissues from patients with PPNAD and PRKAR1A mutations that have been described previously (positive controls).
Lipofuscin detection using confocal scanning laser microscopy
Confocal images from unstained paraffin sections were acquired with a Zeiss LSM 710 confocal laser scanning microscope (CLSM). Lipofuscin autofluorescence was detected at the following excitation(ex)/emission(em) wavelength settings, as previously described (11): channel 1 (Ch1) - ex488nm/em505–530nm; channel 2 (Ch2) - ex561nm/em between 570–625 nm; and channel 3 (Ch3) - ex633nm/em650–700nm. Unstained sections were imaged by sequential excitation, with each laser separately, to avoid cross-talk. Individual images were superimposed to give a combined three-color image.
Statistical analysis
Statistical analysis was performed using SPSS software Version 20.0 (SPSS, Inc., Chicago, IL). Normal distribution was examined with Shapiro Wilk test; Mann-Whitney non-parametric test were performed when distribution was not normal. Statistical significance was accepted at p<0.05.
RESULTS
Patients with CPAs
A total of 10 adrenal CPAs from patients with CS were examined for pigmentation. The biochemical profile of these patients confirmed overt CS with elevated midnight cortisol levels (mean value: 393.7±249.7 nmol/l), elevated 24-hour urinary free cortisol levels (mean value: 489.5±410 nmol/24 h), and suppressed ACTH (in all cases ACTH levels were less than 1.1 pmol/l). Serum cortisol levels showed no suppression in response to the standard dexamethasone suppression test (2 mg×2 days) (mean value of serum cortisol level: 452.5±259.3 nmol/l). During the Liddle’s test, free urinary cortisol levels/24 h showed no suppression (mean value: 470.7±662.4 nmol/24 h) in all cases. Serum cortisol level showed a paradoxical increase compared to cortisol levels before the Liddle’s test in 4 cases varying from 5.2 to 34.7%, a decrease of 32% in one case and no change in 2 other cases.
Tissue analysis; lipofuscin localization and quantification
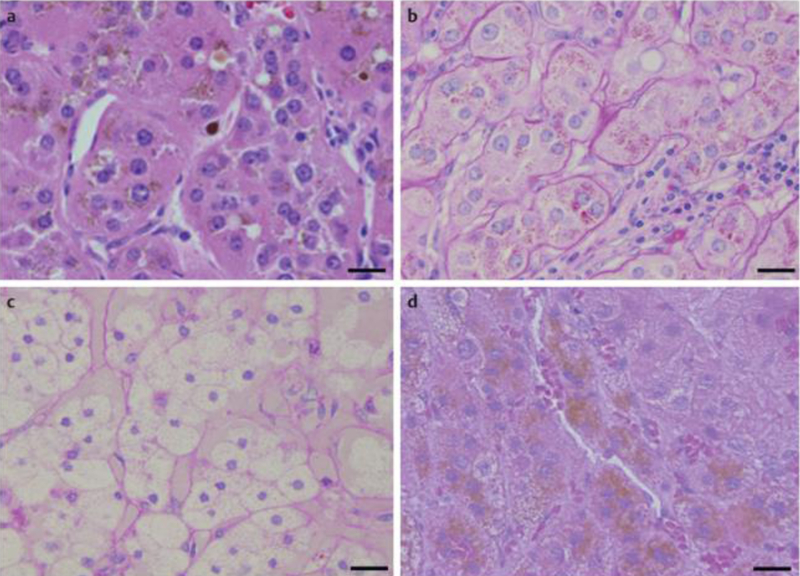
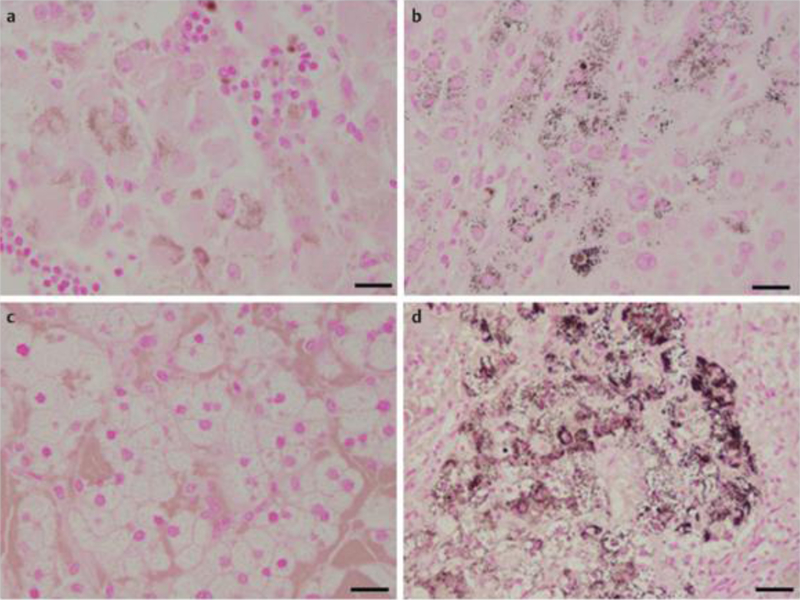
The data for each of the 10 patients are presented in [Table 1]. Analysis of lipofuscin by light microscopy of H & E stained slides showed the presence of brown-gold granules forming conglomerates of multiple granules gathered in smaller or larger groups, located in the cytoplasm in a perinuclear distribution ([Fig. 1]). Intense (+++) to moderate (++) H & E staining for lipofuscin pigmentation was observed in 3 out of 6 adrenal tissues from the CPAs without the p.Leu206Arg PRKACA mutation (1 with intense and 2 with moderate staining) ([Fig. 1a]) and in 3 out of 4 adrenal tissues from the CPAs with the pLeu206Arg mutation (2 with intense and 1 with moderate staining) ([Fig. 1b]). In comparison to these results, all 4 Conn adenomas ([Fig. 1c]) showed negative H & E staining for lipofuscin pigmentation (<10 lipofuscin-pigments granules), whereas the 3 PPNAD tissues showed intense H & E positive staining (brown pigmentation) (+++) ([Fig. 1d]). These results were also confirmed using FM staining where granules appeared also as brown-gold pigments in the cell cytoplasm ([Fig. 2]). FM staining was intensely positive (+++) in all 8 CPAs tissues in which it was performed either without ([Fig. 2a]), or with the mutation ([Fig. 2b]), negative in Conn adenomas tissues ([Fig. 2c]) and intensely positive in all samples with PPNAD (+++) ([Fig. 2d]). Quantitative analysis of HE stained slides from CPAs cases found significantly higher average number of cells with lipofuscin compared to the peritumoral normal tissue from the same patients, and Conn adenomas from patients with hyperaldosteronism (46.9±9.5 vs. 3.8±4.8, p=0.0001) ([Table 2]). Although the average number of cells with lipofuscin granules in CPAs was reduced compared to PPNAD (46.9±9.5 vs. 73.3±109.76, p=0.89), the difference was not significant. Likewise, no statistical difference in lipofuscin accumulation was observed between CPAs with the PRKACA somatic mutation (p.Leu206Arg), compared to those without PRKACA defects (53.5±55.8 vs. 28.2±56.8, respectively p=0.145) ([Table 2]). Again, the number of cells was higher but the difference was not significant, probably due to the small number of tissues studied.
Fig. 1.
Microscopic appearance of CPA without a PRKACA mutation a, CPA with a mutation (somatic) b, Conn adenoma (c, negative control), and PPNAD with a PRKAR1A mutation (germline) (d, positive control) as detected by H & E staining. All tumor cells consisted of compact cells arranged in sheets and cords with uniform nuclei: Cells of tissues-samples a, b, d are filled with numerous brown pigments. H & E staining is negative in c, intensely positive in d and positive in both CPA samples a and b. Scale bar: 20 μm. CPA: Cortisol-producing adenoma; PPNAD: Primary pigmented nodular adrenocortical disease; H & E: Hematoxylin-Eosin.
Fig. 2.
Microscopic appearance of the same tissues samples as detected by FM staining. Similarly FM staining is negative in c, intensely positive in d and positive in both CPA samples a and b. Scale bar: 20 μm. CPA: Cortisol-producing adenoma; PPNAD: Primary pigmented nodular adrenocortical disease; FM: Fontanna Masson.
Table 2.
Lipofuscin scoring in CPA tissuses (with and without the PRKACA mutation) compared to positive (PPNAD) and negative controls (Conn adenoma and peritumoral tissues).
| Tissues | Sample numbers | Number of lipofuscin granules per slide (mean±SD) | Presence of lipofuscin† | Statistcal significance (p) |
|---|---|---|---|---|
| CPAs | 10 | 46.9±9.5 | +++ | p*=0.0001 |
| PRKACA somatic mutation | 4 | 53.5±55.8 | +++ | p**=0.145 |
| No PPKACA mutation | 6 | 28.2±56.8 | ++ | |
| PPNADs | 3 | 73.3±109.76 | +++ | p***=0.89 |
| Peritumoral tissues | 5 | 1.6±1.8 | − | |
| Conn adenoma | 4 | 3.8±4.8 | − |
Grading of lipofuscin pigmentation in H & E staining slides: intense (+++) ≥40 lipofuscin-pigment granules per slide; moderate (++)=20–40; faint (+)=10–20; negative (–) ≤10
Comparison of the number of lipofuscin granules per slide of CPAs (n=10 tissues) tissues vs. Conn adenomas (n=4) and peritumoral tissues (n=5)
Comparison of the number of lipofuscin granules per slide of PPNAD (n=3) tissues vs. CPAs tissues (n=10); CPAs: Cortisol producing adenomas; PPNAD: Primary pigmented nodular adrenocortical disease
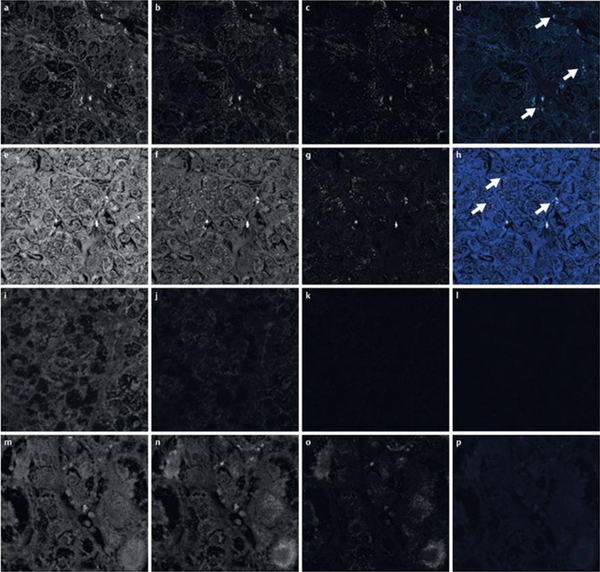
CLSM showed autofluorescent material corresponding to lipofuscin-pigment granules according to its localization and quantity in unstained paraffin sections in both groups of CPAs with and without PRKACA mutations ([Fig. 3]). Lipofuscin-pigmented granules demonstrated autofluorescence in CPAs, when excitation was with the 488 nm and 633 nm laser, but not the 561 nm laser.
Fig. 3.
Autofluorescence detection of lipofuscin in unstained paraffin section from CPA without the PRKACA mutation (a–d), CPA with the PRKACA mutation (e–h), Conn adenoma (i–l), and PPNAD (m–p), with the following CLSM excitation/emission wavelengths, where images are shown black and white: channel 1 (Ch1, blue) - 488 nm/505–530 nm (a, e, i, m); channel 2 (Ch2, green) – 543 nm/560–615 nm (b, f, j, n); and channel 3 (Ch3, red) – 633 nm/over 650 nm (c, g, k, o). Merged images are shown in color whereby small quantities of lipofuscin are detectable (d, h, l, p). Compared to control groups, larger quantities of lipofuscin, detectable by autofluorescence, are detectable in CPAs in Ch1 and Ch3, corresponding to lipofuscin and represented in merge channels (d, h, l, p; white arrows). CPA: Cortisol-producing adenoma; PPNAD: Primary pigmented nodular adrenocortical disease; CLSM: Confocal laser scanning microscopy.
Discussion
In the present study, we tested the hypothesis that CPAs with PRKACA defects accumulate lipofuscin pigment more frequently or more intensely than other adrenocortical lesions. This hypothesis was based on the observation that PPNAD that is caused by mutations in yet another gene of the PKA pathway (PRKAR1A) has intense pigmentation due to lipofuscin accumulation.
This preliminary study confirmed something that clinical pathologists and adrenal surgeons already know: overall, CPAs had more lipofuscin than peritumoral normal adrenal tissue and samples from aldosterone-producing Conn’s adenomas. However, with regards to PRKACA, we found that a mutant PRKACA caused less intense lipofuscin accumulation than PRKAR1A-mutant PPNAD. Overall lipofuscin accumulation in CPAs did not differ statistically between adenomas with and without PRKACA mutations but there was a higher presence of lipofuscin in most PRKACA-mutant tumors, indicating that a larger study might have shown a difference.
Data concerning the role of lipofuscin accumulation in the function of adrenocortical adenomas are not available. Lipofuscin accumulation in functional so called “black” adenomas was associated with overt CS [11]; in other studies, lipofuscin accumulation in adenomas was associated with subclinical CS [5] [14]. It should be noted that autopsy series have also shown incidental pigmented adrenal nodules with an incidence from 2.2% to 10.4% of the population [15].
Pigmented or “black” adenomas of the adrenal cortex were first described by Lucksch in 1912 [16] and by Baker, in the English literature in 1938 [17]. These were initially regarded as non-functional neoplasms of unknown clinical significance, being documented mainly in autopsy studies [18]. Observations of increased lipofuscin autofluorescence in pigmented CPAs [19] [20] [21] were later described in the literature using different staining methods and by electron microscopy. Indeed, CS was the most common presentation of “black” adrenal adenomas [4] [5] [18] [22].
It should be noted that whether a tissue is pigmented due to lipofuscin accumulation is not easy to confirm. The distinction between lipofuscin and other pigments such as melanin is based on specific characteristics of the pigmented granules: the autofluorescence of lipofuscin is its most characteristic feature. Macroscopically melanin is brown to black, whereas lipofuscin pigment granules are described as yellowish-brown and fluorescent [23]. Tissue localization is also helpful as melanin is mainly detected in skin cells, hair, eyes (retina, iris, and choroid) and in the cell bodies of some neurons, notably in the substantia nigra and locus coeruleus of the brain stem, whereas lipofuscin has been mainly detected in cardiac muscle, hepatocytes, brain nerve tissue and adrenal cortex. However, melanin and lipofuscin can co-exist in adrenal tissues [24]. In animal studies, adrenal lipofuscin pigment had a maximum emission maximum at a range 640–660 nm and 330–380 nm at excitation [25]. Melanin emission ranged between 360–560 nm at excitation. In our study, lipofuscin-pigmented granules demonstrated autofluorescence in CPAs, when excitation was with the 488 nm and 633 nm laser, but not with the 561 nm laser.
Data from different staining methods showed that lipofuscin-pigment granules are positive for H & E, periodic acid–Schiff (PAS), acid-fast (long Ziehl-Neelsen), the Schmorl method and FM stainings [5]. Conversely, lipofuscin-containing granules are negative for Berlin blue staining [5]. In our study, lipofuscin granules appeared as brown pigment with cytoplasmic localization in both H&E and FM staining with a more clear appearance in the FM staining and were responsible for the variation of brown color intensity. Accordingly, two of the CPAs with the greater number of pigment granules had a more intense dark brown appearance pigmentation. These were PRKACA-mutant CPAs.
The accumulation of lipofuscin-like materials results from the progressive oxidation of unsaturated fatty acids by oxygen-derived free radicals in lysosomes [26]. Mitochondrial injury causing impaired excretion of lipid-containing pigments was hypothesized as a possible mechanism responsible for pigmentation in the “black” adrenal adenomas [27]. In particular, the comparison of “black” adenomas with non-pigmented adrenal adenomas had showed that the ultrastructure of “black” adenoma cells was similar to the compact cells of “yellow” cortical adenomas. In addition, a paradoxical cortisol rise similar to that in PPNADs in cases with “black” adenomas causing CS has been described [24]. Interestingly, in our study CPAs with moderate/intense staining for lipofuscin (>20 granules/slide) had a paradoxical increase of cortisol in response to the Liddle’s test ranging from 5.5–34.7%, whereas CPAs with faint staining for lipofuscin (<20 granules/slide) had either no change or a decrease of cortisol in response to dexamethasone. Clearly, the small number of cases does not allow statistical comparisons, but the suggestion is that there might be a correlation of lipofuscin accumulation and a paradoxical increase of cortisol secretion in response to dexamethasone, such as the one that has been reported in PPNAD [28].
In conclusion, we have confirmed that CPAs in patients with overt CS show accumulation of brown pigment in the cytoplasm with autofluorescent characteristics corresponding to lipofuscin granules. A somatic PRKACA mutation did not affect significantly the presence or absence of lipofuscin in CPAs although the intensity of its presence may be increased in patients with somatic PRKACA defects and may be associated with paradoxical increases in response to dexamethasone, both features of PPNAD that is caused by PRKAR1A mutations. A larger study is needed to examine this possibility.
Acknowledgments
Funding: This work was supported by the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH) (Dr. Stratakis) and by the IKY National Foundation, Athens, Greece (Dr. Angelousi).
Footnotes
Declaration of interest: There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported
References
- 1.Cheng B, Tserng KY, Kowal J, Buekers KS, Abraham S & Gerhart JP. Characterization and identification of an adrenal age-related nonpolar fluorescent substance. Endocrinology 1996; 137:2447–2456. [DOI] [PubMed] [Google Scholar]
- 2.Cheng B, Hornick TR, Hassan MO, Chou SC, Abraham S, Kowal J. Effects of prolonged ACTH-stimulation on adrenocortical accumulation of lipofuscin granules in aged rats. Tissue Cell 1999; 31:594–604. [DOI] [PubMed] [Google Scholar]
- 3.Terman A & Brunk UT. Lipofuscin: mechanisms of formation and increase with age. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica 1998; 106: 265–276. [DOI] [PubMed] [Google Scholar]
- 4.Kovacs K, Horvath E & Feldman PS. Pigmented adenoma of adrenal cortex associated with Cushing’s syndrome: light and electron microscopic study. Urology 1976; 7: 641–645. [DOI] [PubMed] [Google Scholar]
- 5.Odanaka M, Katabami T, Inoue M & Tadokoro M. Adrenal black adenoma associated with preclinical Cushing’s syndrome. Pathol Int 2003; 53: 796–799. [DOI] [PubMed] [Google Scholar]
- 6.Lindholm J, Juul S, Jorgensen JO. et al. Incidence and late prognosis of cushing’s syndrome: a population-based study. J Clin Endocrinol Metab 2001; 86: 117–123 [DOI] [PubMed] [Google Scholar]
- 7.Lindholm J, Juul S, Jorgensen JO, Astrup J, Bjerre P, Feldt-Rasmussen U, Hagen C, Jorgensen J, Kosteljanetz M, Kristensen L, Laurberg P, Schmidt K & Weeke J. Incidence and late prognosis of cushing’s syndrome: a population-based study. J Clin Endocrinol Metab 2001; 86: 117–123. [DOI] [PubMed] [Google Scholar]
- 8.Stratakis CA. E pluribus unum? The main protein kinase A catalytic subunit (PRKACA), a likely oncogene, and cortisol-producing tumors. J Clin Endocrinol Metab 2014; 99: 3629–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beuschlein F, Fassnacht M, Assie G, Calebiro D, Stratakis CA, Osswald A, Ronchi CL, Wieland T, Sbiera S, Faucz FR, Schaak K, Schmittfull A, Schwarzmayr T, Barreau O, Vezzosi D, Rizk-Rabin M, Zabel U, Szarek E, Salpea P, Forlino A, Vetro A, Zuffardi O, Kisker C, Diener S, Meitinger T, Lohse MJ, Reincke M, Bertherat J, Strom TM & Allolio B. Constitutive Activation of PKA Catalytic Subunit in Adrenal Cushing’s Syndrome. N Engl J Med 2014; 370:1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carney JA, Lyssikatos C, Lodish MB & Stratakis CA. Germline PRKACA amplification leads to Cushing syndrome caused by 3 adrenocortical pathologic phenotypes. Hum Pathol 2015; 46: 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erem C, Hacihasanoglu A, Cinel A, Cobanoglu U, Ersoz HO, Ahmetoglu A, Ukinc K & Kocak M. Adrenal black adenoma associated with Cushing’s syndrome. Endocrine 2004; 25: 253–257. [DOI] [PubMed] [Google Scholar]
- 12.Fischer AH, Jacobson KA, Rose J & Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc; 2008; 1: 2008 pdb prot4986. [DOI] [PubMed] [Google Scholar]
- 13.Markelic M, Velickovic K, Golic I, Klepal W, Otasevic V, Stancic A, Jankovic A, Vucetic M, Buzadzic B, Korac B & Korac A. The origin of lipofuscin in brown adipocytes of hyperinsulinaemic rats: the role of lipid peroxidation and iron. Histol Histopathol 2013; 28: 493–503. [DOI] [PubMed] [Google Scholar]
- 14.Inomoto C, Sato H, Kanai G, Hirukawa T, Shoji S, Terachi T, Kajiwara H & Osamura RY. Black adrenal adenoma causing preclinical Cushing’s syndrome. Tokai J Exp Clin Med 2010; 35: 57–61. [PubMed] [Google Scholar]
- 15.Damron TA, Ward WG & Stewart A. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base Report. Clinical orthopaedics and related research 2007; 459: 40–47. [DOI] [PubMed] [Google Scholar]
- 16.Lucksch F. Uber pigmentierte Adenome der Nebennieren. Beitr. z. Pathol. Anat. u.z.allg.Pathol. Bd. 1916;215 [Google Scholar]
- 17.Baker MR. A pigmented adenoma of the adrenal. Arch Pathol 1938; 26: 845–852 [Google Scholar]
- 18.Robinson MJ, Pardo V & Rywlin AM. Pigmented nodules (black adenomas) of the adrenal. An autopsy study of incidence, morphology, and function. Hum Pathol 1972; 3: 317–325. [DOI] [PubMed] [Google Scholar]
- 19.Tseng CH, Chang GK, Wong QY & Lin JI. Cushing’s syndrome and functioning adrenal black adenoma. South Med J 1978; 71: 1166–1168. [DOI] [PubMed] [Google Scholar]
- 20.Zaniewski M & Sheeler LR. Cushing’s syndrome associated with functional black adenoma of the adrenal cortex. South Med J 1980; 73: 1410–1412. [DOI] [PubMed] [Google Scholar]
- 21.Ueda Y, Tanaka H, Murakami H, Ninomiya T, Yamashita Y, Ichikawa M, Kondoh T, Chiba T. A functioning black adenoma of the adrenal gland. Intern Med 1997; 36: 398–402. [DOI] [PubMed] [Google Scholar]
- 22.Caplan RH & Virata RL. Functional black adenoma of the adrenal cortex. A rare cause of primary aldosteronism. Am J Clin Pathol 1974; 62: 97–103. [DOI] [PubMed] [Google Scholar]
- 23.Shimokawa I, Higami Y, Horiuchi S, Iwasaki M, Ikeda T. Advanced glycosylation end products in adrenal lipofuscin. J Gerontol A Biol Sci Med Sci 1998; 53: 49–51. [DOI] [PubMed] [Google Scholar]
- 24.Kamalanathan S, Mahesh DM, Muruganandham K & Basu D. Black adrenal adenoma:distinction from PPNAD. BMJ Case Rep 2012; 3: 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mochizuki Y, Park MK, Mori T, Kawashima S. The difference in autofluorescence features of lipofuscin between brain and adrenal. Zoolog Sci 1995; 12: 283–288. [DOI] [PubMed] [Google Scholar]
- 26.Bahu RM, Battifora H & Shambaugh G 3rd. Functional black adenoma of the adrenal gland. Light and electron microscopical study. Arch Pathol 1974; 98: 139–142. [PubMed] [Google Scholar]
- 27.Balazs M Functioning “black adenoma” of the adrenal gland with emphasis on ultrastructural studies. Zentralbl Pathol 1991; 137: 151–156. [PubMed] [Google Scholar]
- 28.Bourdeau I, Lacroix A, Schürch W, et al. Primary pigmented nodular adrenocortical disease: paradoxical responses of cortisol secretion to dexamethasone occur in vitro and are associated with increased expression of the glucocorticoid receptor. J Clin Endocrinol Metab 2003;88:3931–7. [DOI] [PubMed] [Google Scholar]