Abstract
Background
Extracts of Echinacea have been used traditionally for the treatment of diverse types of infections and wounds. They have become very familiar immunostimulant herbal medicine. However, the specific immunomodulatory effect of Echinacea remains to be elucidated.
Aim
In our study, the effect of Echinacea purpurea extract on the generation of immature DCs from monocytes was described, as well as its effect on DC differentiation. In addition, an in vivo experiment was conducted to investigate whether treatment of mice with extracts derived from E. purpurea has immunomodulatory effect on murine splenic DCs.
Methods
Immature DCs were generated by incubating peripheral blood monocytes with cytokine cocktail (GM-CSF + IL-4) and matured by tumor necrosis factor-α (TNF-α). The cells were randomized to 5 groups to investigate E. purpurea effect in different stages. Phenotypic analysis of cell marker CD83-expressed on DCs was performed by flow cytometry. Mice were randomly divided into 3 groups; control, E. purpurea treated and E. purpurea-TNF-α treated group. The murine splenic DCs were isolated and phenotyped for CD83 and CD11c by flow cytometry.
Results
Treatment of monocytes with E. purpurea prior to addition of the maturation factor TNF-α resulted in a significant decrease in the yield of DC expressing CD83. On the other hand, immature DCs generated in the culture in the presence of GM-CSF and IL-4, when treated simultaneously with E. purpurea and TNF-α, exhibited an insignificant change in the yield of CD83-expressing DCs compared with untreated control. The in vivo experiments showed that splenic DCs obtained from mice treated with E. purpurea with or without TNF-α did not exhibit significant changes in CD83 or CD11c compared with those obtained from control mice.
Conclusion
Our findings suggest that the immunomodulatory mechanisms of E. purpurea impact generation fate of DCs rather than differentiation stages. The results obtained in the in vivo study utilizing murine splenic DCs supported those observed in vitro.
Keywords: Dendritic cells, Echinacea purpurea, CD83, CD11c, Murine splenic cells
1. Introduction
Immunotherapy is the treatment of a disease by producing, improving or overcoming an immune response. Immunotherapies, produced to obtain or augment an immune response, are classified as immunostimulants. On the other hand, immunotherapies prepared to reduce or suppress, are assorted as immunosuppressants [14].
Cell based immunotherapies are shown to be useful for some cancers. Immune effector cells such as lymphocytes, macrophages, dendritic cells, natural killer cells (NKs) and cytotoxic T lymphocytes (CTL), operate concurrently to guard the body toward cancer by marking unusual antigens represented on the surface of the malignant cells due to mutation [8].
The immunomodulating characteristics of plants are being examined widely to achieve the desirable effects on disease prevention. Consequently, herbal remedies have been utilized for centuries for safety, effectiveness, minor side effect and cultural acceptability. Therefore plant and its products are harmless and so, there is continuous application of plant product as an optional way to cure the patients and this approach is in practice from the ancient times [29].
Echinacea is considered one of the most popular herbal supplements used to attenuate colds, sore throats, coughs and other respiratory infections [25]. Three of the nine species of Echinacea possess medical uses: Echinacea angustifolia, Echinacea pallida and Echinacea purpurea, which are the most commonly consumed species in the United States [4]. Echinacea includes a diversity of medically essential materials that perform a role in its therapeutic effects. They involve alkylamides, caffeic acid derivatives, glycoproteins, polysaccharides, polyacetylenes, phenolic mixtures, cinnamic acids, essential oils and flavonoids [25].
E. purpurea has numerous advantageous characteristics, especially activation of the immune system, by triggering the alternating complement pathway as well as raising the number of distributing white blood cells, stimulating phagocytosis, T-cell production, lymphocytic activity, cytokine production, cellular respiration and enzyme secretion [11].
Dendritic cells (DCs) are the common effective to the most potent and professional antigen-presenting cells of the immune system required in both enhancing immune responses and maintaining tolerance [33]. In response to infections, DCs advance the production of effector CD4+ T helper 1 (Th1) and CD8+ T cell-dominated immune replies. Furthermore to these effector responses, DCs can be directed to become tolerogenic and increase regulatory T cells (Tregs), that control effector T cell responses, a process that is important for preservation of immune homeostasis and control of autoimmune diseases and hypersensitivities [6].
DCs can exist in two main states, steady state immature dendritic cells and fully mature DCs. The difference between immature and mature DCs is notably based on changes occurring on a phenotypic level and functional level [7]. Immature dendritic cells exhibited characteristics of primary cells, defined by expression of classical dendritic cell surface markers CD11c, CD11b and major histocompatibility complex class II (MHC-II). Phenotypic maturation is achieved when DCs up-regulate surface maturation markers such as CD80, CD83, and CD86 [16].
An important biological function of DCs relies on the continuous sampling of their tissue environment, responding to stress, danger signals and transducing the collected molecular information to other cell types of the immune system [20]. DCs are equipped with unique sets of phylogenetically conserved Pattern Recognition Receptors (PRRs), which are specialized to recognize Microbe Associated Molecular Patterns (MAMPs) and Danger Associated Molecular Patterns (DAMPs) [28].
Activation of DCs by MAMPs and DAMPS results in the rapid, chemokine-mediated translocation of DCs to draining lymph nodes where they have the chance to contact antigen-specific T-lymphocytes to initiate adaptive immune responses [24]. This process ensures the transfer of molecular information collected in the periphery toward other cell types of both innate and adaptive immunity such as neutrophils, granulocytes, NKs, killer T cells, T- and B-lymphocytes [2].
Because of the powerful immuno-regulatory functions of DCs, there has been high attention in identifying the immunomodulating drugs that manage the various features of these cells to finally recognize means to manage the function of DCs for the intelligent pattern of DC-based immune-interventions. The aim of this research was to examine the immunomodulatory effect of ethanolic extract derived from E. purpurea on DC generation from human monocytes and to examine its effect on DCs maturation via series of in vitro experiments. We also studied whether treatment with extracts derived from E. purpurea has immunomodulatory effect on splenic murine DCs.
2. Materials and methods
2.1. Materials and reagents
Dulbecco’s modified Eagle medium (DMEM) and phosphate buffered saline-Ca2+ Mg2+ free (PBS) were obtained from Biowhittaker®, Belgium. Granulocyte macrophage-colony stimulating factor (GM-CSF), phycoerythrin (PE) anti-human CD83 monoclonal antibody (cat# 12-0839, clone: HB15e), fluorescence-activated cell sorting (FACS) buffer and FACS lyse solution were obtained from eBioscience®, Austria. Phycoerythrin hamster anti-mouse CD11c monoclonal antibody (cat# MCA1369T, clone: N418) and fluorescein isothiocyanate (FITC) rat anti-mouse CD83 monoclonal antibody (cat# MCA2747F, clone: 3D11) were obtained from AbD Serotec®, USA. IL-4 was obtained from Sigma Aldrich®, USA. TNF-α was obtained from R&D Systems®, USA. Penicillin–streptomycin solution (Pen/Strep) was obtained from Euro-lone®, Europe. Amphotericin B (Fungizone®) was obtained from Gibco®, USA. Biocoll separating solution, density 1.077 g/mL, was obtained from Biochrom®, Germany. E. purpurea dried whole plant was obtained from Haraz Co., Egypt. Heparin calcium (Cal-heparin®) 5000 U was obtained from Amoun Pharmaceutical Co., Egypt. Human albumin (Zenalb®) 20% was obtained from Bio Products Laboratory Limited, United Kingdom. All other chemicals and reagents were of high analytical grade.
2.2. Extraction of E. purpurea
Dried whole plant was powdered and soaked in absolute methanol for one day. After one day, methanolic extraction was separated by filtration and evaporated using Rota Vap instrument (Heidolph®, Germany). Then, the extract containing active constituents was divided into aliquots, weighed and stored at −20 °C [23].
2.3. In vitro experiments for culture of DCs
2.3.1. Isolation of monocytes
The present study was approved by the Research Ethics Committee of Tanta Faculty of Pharmacy. Heparinized human peripheral blood was collected and mononuclear cells were isolated by Biocoll density gradient centrifugation. Peripheral blood monocytes (PBMCs) were then placed in the incubator (Shel lab®, USA) and allowed to adhere for 2 h at 37 °C in 5% CO2. Non-adherent cells of peripheral blood lymphocytes (PBLs) were gently removed [10].
2.4. Generation of human monocyte-derived DCs
DCs were generated according to Rubinstein et al. [26]. Briefly, purified human monocytes were cultured (106 cells/mL) on day zero in DMEM medium supplemented with 100 μL amphotericin B (1 μg/mL), 100 μL Pen/Strep, IL-4 (10 ng/mL) and GM-CSF (10 ng/mL) for 9 days in order to obtain immature DCs. The cells were further fed on day 3 with fresh DMEM medium (half the original medium volume containing the same concentration of cytokine cocktail). Maturation was achieved by addition of TNF-α (10 ng/mL) dissolved in PBS on day 7 and kept for 48 h. Mature DCs were harvested and analyzed on day 9 [34].
2.5. Cell culture grouping
Regarding the above mentioned preparation of DCs, the cells were randomized to 5 groups to investigate E. purpurea effect at different stages: Group A, control immature DCs; Group B, E. purpurea added on day 7; Group C, TNF-α (10 ng/mL) added on day 7 + E. purpurea on day 8; Group D, E. purpurea added on day 7 + TNF-α (10 ng/mL) on day 8; and Group E, E. purpurea added on day 0 + TNF-α (10 ng/mL) on day 7. The prepared concentrated extract of E. purpurea was diluted in absolute ethanol to prepare 500 μg/mL solution. 0.5% of E. purpurea ethanolic solution, equivalent to 25 μg concentrated E. purpurea extract, was added to the cell culture on the time specified for each group [4]. Morphological changes of cells were examined on days 0, 7, and 9 using inverted microscope Carl Zeiss®, Germany.
2.6. Flow cytometry analysis for CD83 expression in DCs
Approximately 106 cells were harvested and stained with anti-CD83-PE for 30 min at 4 °C. After incubation, the cells were washed with FACS buffer and finally suspended in 500 μL FACS buffer. Harvested cells were injected using a BD FACSCalibur®, USA and data were analyzed using BD CellQuest® software. Stained cells with fluorescent-labeled antibody exhibiting a higher fluorescence intensity value than exhibited by unstained control cells were considered positive [30].
2.7. In vivo experiments investigating E. purpurea effect on murine splenic DCs
2.7.1. Experimental animals
Male mice were purchased from the animal house of Geiza Institute of Ophthalmology, Cairo, Egypt. Animal care and experiments were conducted in accordance with institutional guidelines and with the approval of the Research Ethics Committee of Tanta Faculty of Pharmacy. All mice were 6–8 weeks old with an average weight between 20 and 25 g. Mice were maintained on normal balanced diet and housed in wire cages for one week under identical environmental conditions for adaptation.
2.7.2. Experimental design
Mice were randomly divided into three major groups (n = 10) according to the received treatment: Group A, B and C.
2.7.2.1. Group A
This group involved 10 mice representing control normal, which received PBS orally daily for 5 consecutive days.
2.7.2.2. Group B
This group involved 10 mice representing E. purpurea treated group. Each mouse was given E. purpurea at a dose of 30 mg/kg/day, suspended in 1.0 mL of PBS and gavaged to each animal for 5 consecutive days [1].
2.7.2.3. Group C
This group involved 10 mice representing E. purpurea + TNF-α treated group. Each mouse was given E. purpurea as in group B, and then the animal was injected with TNF-α at a dose of 5 μg/kg, i.p. one hour after the last dose of E. purpurea [12].
2.8. Isolation of spleen cells
Spleen cell suspension was prepared according to Nakano et al. [17]. Freshly acquired spleens were immediately pushed through a 40 μm sieve by mechanical squeezing using rubber rod. The cells were resuspended in PBS, then cell suspensions were centrifuged at 250 g for 10 min at 8 °C and supernatants were discarded. Then, 100 μL of cell suspension was taken and incubated for 20 min in BD FACS lysing solution for red blood cell lysis. The cells were rinsed twice in FACS buffer by centrifugation at 400 g for 10 min at room temperature.
2.9. Flow cytometry of murine splenic DCs
The spleen cells were stained with 10 μL anti-CD83-FITC and 10 μL anti-CD11c-PE. Following 30 min of incubation at 4 °C, the cells were washed twice in FACS buffer [30]. The samples were injected in flow cytometer BD FACSCalibur®, USA. Data were analyzed using BD CellQuest® software to determine the percentage of each of CD11c−CD83+ and CD11c+CD83+ expressing DCs.
2.10. Statistical analysis
All statistical analyses were accomplished by using Microsoft Office Excel 2007. Differences between groups were statistically analyzed using one way analysis of variance (ANOVA). The results were expressed as mean ± standard error (SE). P values less than 0.05 were considered statistically significant.
3. Results
3.1. Generation of DCs from PBMCs
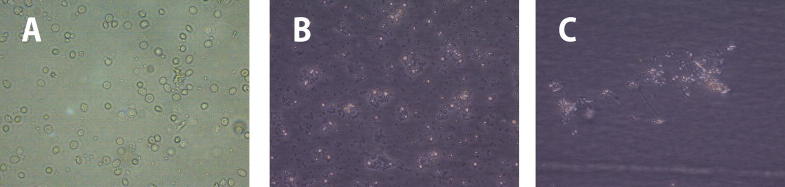
PBMCs were cultured in DMEM medium (day 0), allowed to transform to immature DCs (day 7), which were then differentiated into mature DCs in the presence of TNF-α (day 9) (Fig. 1).
Figure 1.
Morphological changes during generation and differentiation of control untreated DCs (40×). (A): Day 0: adherent monocytes. (B): Day 7: immature DCs. (C): Day 9: mature DCs.
3.2. Changes in cell morphology
Microscopically, DCs in various groups reached nearly full growth on day 7 and completely covered the culture plates on day 9, where local cell clusters were noted. On day 9, DCs appeared to be loosely adherent and have branched projections (Fig. 2A). All culture groups treated with E. purpurea showed no notable changes in the morphology of DCs compared with control untreated cultures (Fig. 2B).
Figure 2.
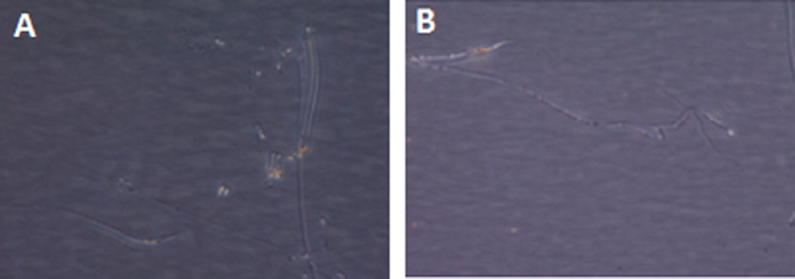
Echinacea purpurea effect on DCs morphology on day 9 (100×). (A): DCs nucleated with branched projections (control without treatment). (B): DCs in E. purpurea treated culture, there were no observable changes in all E. purpurea treated groups compared with control.
3.3. Phenotypic characterization of DCs and effect of E. purpurea
The surface expression of the important DC maturation marker CD83 was assessed by flow cytometry. The percentage of DCs that expressed CD83 was 27.25 ± 0.76% in the control culture of group A, 25.33 ± 0.88% in culture of group B, 31.66 ± 2.19% in culture of group C, 30.66 ± 2.73% in culture of group D and 17 ± 2.52% in culture of group E (Fig. 3A and B).
Figure 3.
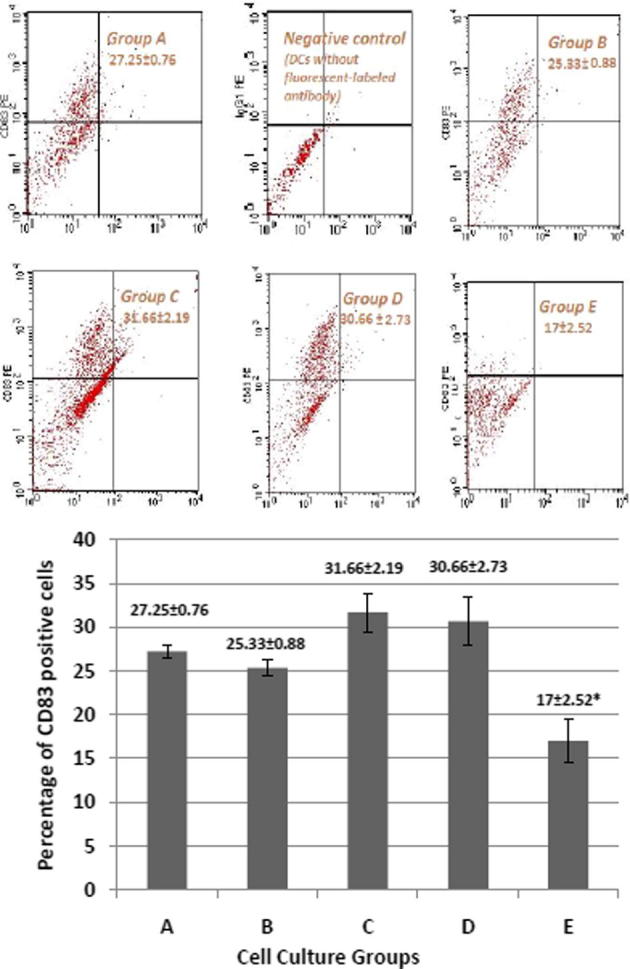
Effect of E. purpurea on CD83 expression in various cell cultures. (A): Flow cytometry showing the expression of CD83. Monocyte-derived DCs were generated in presence or absence of E. purpurea. (Group A) control group; (Group B) E. purpurea (500 μg/ml) on day 7; (Group C) TNF-α (10 ng/ml) on day 7 + E. purpurea (500 μg/ml) on day 8; (Group D) E. purpurea (500 μg/ml) on day 7 + TNF-α (10 ng/ml) on day 8; (Group E) E. purpurea (500 μg/ml) on day 0 + TNF-α (10 ng/ml) on day 7. (B): Percentage of DCs expressing CD83 in different groups. Expression of CD83 cell marker is measured by BD FACSCalibur®. Each value is the mean of 3 experiments ± SE. *P < 0.05.
E. purpurea added on day 0 (group E) significantly decreased the expression of CD83 compared to other groups (Fig. 3B). The CD83 expression was 27.25% in control group A, and decreased to 17% in E. purpurea treated group E, with a percent decreased of about 62.4%.
Group B (E. purpurea on day 7 with no TNF-α) and group D (E. purpurea on day 7 + TNF-α on day 8) showed an insignificant change in expression of CD83 compared to control group A. Group C (TNF-α on day 7 + E. purpurea on day 8) exhibited the greatest increase in CD83 (31.66%) although it was insignificant compared to control culture group A (Fig. 3B).
3.4. In vivo study
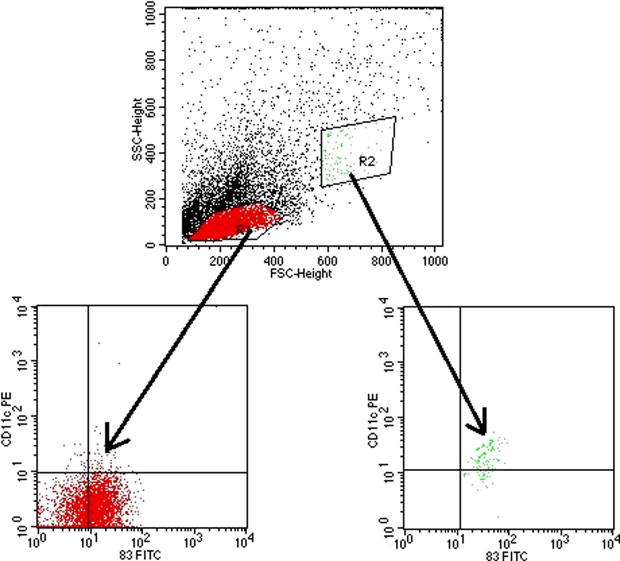
In vivo experiment was conducted to investigate whether treatment of mice with E. purpurea extract has immunomodulatory effect on murine splenic DCs. We analyzed CD11c-CD83+ and CD11c+CD83+ splenic DCs in E. purpurea-treated mice by flow cytometry (Fig. 4).
Figure 4.
Flow cytometry of cellular markers CD83 and CD11c of murine splenic DCs obtained from all animal groups. Mice of each group (n = 10) were treated with E. purpurea with or without TNF-α, then splenocytes were analyzed by flow cytometry. T cells and B cells (CD83−) were gated out. Major CD11c−CD83+ and CD11c+CD83+ populations are circled. Total CD11c−CD83+ cells are gated by R1, whereas CD11c+CD83+ population is gated by R2. SSC-Height is referred to side scattered pulse height. FSC-Height is referred to forward scattered light height.
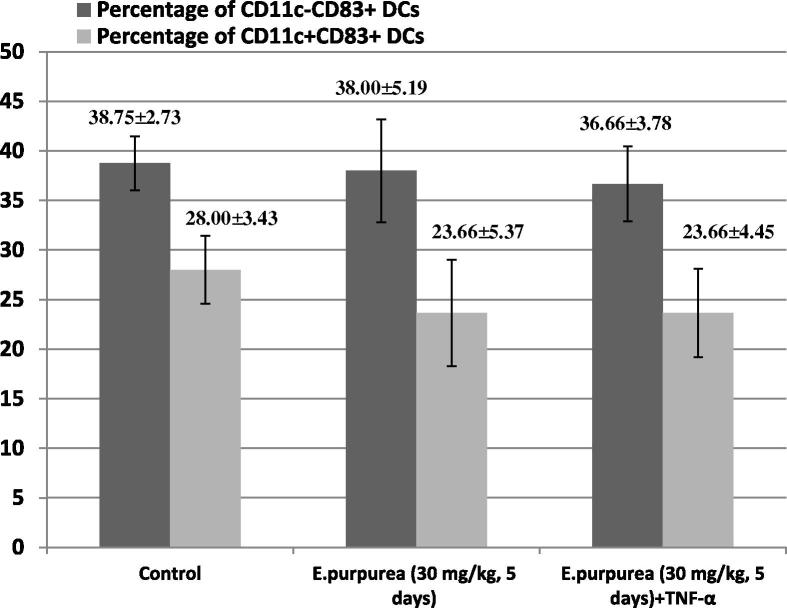
The percentage of each of CD11c−CD83+ and CD11c+CD83+ expressing DCs was determined by flow cytometry. The percentage of CD11c−CD83+ DCs was 38.75 ± 2.73% in the control group A, 38.00 ± 5.19% in group B and 36.66 ± 3.78% in group C. The percentage of CD11c+CD83+ DCs was 28.00 ± 3.43% in the control group A, 23.66 ± 5.37% in group B and 23.66 ± 4.45% in group C (Fig. 5).
Figure 5.
Effect of E. purpurea on the percentage of CD11c−CD83+ and CD11c+CD83+ murine splenic DCs. E. purpurea was administered orally, whereas TNF-α was given i.p. n = 10 for each group. Expression of CD11c and CD83 cell marker was measured by BD FACSCalibur®. Each value is the mean ± SE.
No changes in expression of the accessory molecules were observed in splenic cells obtained from different animal groups. E. purpurea-treated mice did not produce any significant change in phenotypic analysis of splenic DCs (group B and group C). Furthermore, the injection of stimulation agent TNF-α into mice of group C had no effect on the above-mentioned markers (Fig. 5).
4. Discussion
Dendritic cells (DCs) are professional antigen presenting cells (APCs) that act at the interface of the innate and adaptive branches of the immune system. DCs provoke and control immunity against pathogens and tolerance against self-antigens and commensal microorganisms [27].
Delivery of adjuvant to DCs may be a promising strategy to treat autoimmune disorders. But, to produce immunity rather than tolerance, it is crucial to provide the DCs with an activation signal [5]. Although a limited number of surface molecules are preferentially expressed by DCs, high-density CD83 expression is serving as an excellent marker for DC characterization [21].
In the present study, PBMCs were cultured and allowed to generate DCs, which became mature after addition of TNF-α to the culture. Mature DCs were identified by microscopical examination and flow cytometry to detect the expression of the cell specific marker CD83.
DCs undergo several changes during maturation including morphological changes, loss of endocytic and phagocytic receptors, chemokine secretion, upregulation of costimulatory molecules, translocation of MHC–II biomarkers to the cell surface and cytokine secretion [31]. Ultimately these changes prepare DCs to interact successfully with T cells in the context of antigen. Activation of the antigen-specific T cell results in clonal expansion and differentiation, leading to adaptive immunity [19].
E. purpurea has been shown to have immunostimulatory effects on monocytes, macrophages, natural killer cells and T cells in vitro [9], but few studies have attempted to elucidate the immunomodulatory effect of E. purpurea on DCs.
In our work, we set out to define morphological variations and CD83 phenotypic analysis of DCs after exposure of cultured peripheral blood monocytes to E. purpurea extract at different time intervals. In addition, we carried out phenotypic analysis of splenic DCs obtained from mice treated with E. purpurea daily for 5 days.
Our results showed that addition of 0.5% of 500 μg/mL E. purpurea ethanolic extract on day 0 of culture (group E) produced a significant reduction of the expression of immunosuppressant cell marker CD83 by 62.4% in cultured DCs when examined on day 9 compared to control cultures. These results were confirmed by the work of Wang et al. [32], who reported that treatment of cultured DCs with E. purpurea extracts at 500 μg/mL greatly decreased the expression of CD83 by 61% in comparison with negative control DCs.
Because the addition of alcoholic extract of E. purpurea on day 0 to PBMC cultures decreased the expression of CD83 analyzed on day 9, we thought to analyze the expression of this molecule at multiple time days. Our results showed that the expression of CD83 was not significantly changed following treatment of generated DCs with E. purpurea extract added on day 7 or day 8 either in the presence or absence of TNF-α (group B, C and D). We inferred that the timing of addition of E. purpurea extract is critical. We suggest that E. purpurea extract impacts on DC precursors not on differentiated DCs. This observation is important since this extract can be used as an immunomodulator, which marked with reduced expression of CD83 in group E.
Depending on phenotypic and functional states, DCs may promote immunogenic or tolerogenic responses. Although various Toll-like receptors, such as TLR3, TLR4, TLR5, TLR7 and TLR8, induce immune activation, others can silence immune responses through tolerance induction in DCs. TLR2 activation induces IL-10 production, inhibits TLR7/TLR9 signaling and prevents IFN-α and -β production [13].
Despite the immunogenic ability of DCs in raising immune responses, which has been attributed to only role in the immune system, they have also been ascribed opposite roles in tolerance induction and silencing of immune responses. DCs play a critical role in the induction of different subsets of T cells, such as Th1, Th2, Th17 and regulatory T cells (Tregs) [18].
The source material for experimental studies is usually an aqueous or ethanol extract of whole, aerial parts or roots of the dried plant of E. purpurea. The chemical compositions of the known marker compounds, such as caffeic acid, alkylamides and polysaccharides differ substantially between such preparations [3]. Polysaccharides are typically present at the highest concentration in aqueous or fresh pressed juice extracts while alkylamides are more likely to be major constituents in ethanolic extracts [22]. The present data demonstrated that the effect of E. purpurea was more apparent on monocytes rather than fully cultured DCs, whereby this effect might be contributed by the alkylamide content of E. purpurea alcoholic extract.E. purpurea drug is indicated for prevention and treatment of common cold and influenza. Also, it is prescribed as prophylactic therapy in cases of increased susceptibility to infections, inflammatory skin diseases and recurrent vaginal candidiasis. Its bioavailability study revealed that following oral consumption of 675 mg/70 kg of E. purpurea prepared from dried ethanolic extract, alkylamides appeared in plasma after 20 min and reached a maximum concentration of 336 ± 131 ng/mL in human plasma, which demonstrates that alkylamides are bioavailable [15].
In our study, in vivo experiments were performed to compare E. purpurea effect on murine splenic DCs to its effect in vitro. We examined the effect of E. purpurea on different subsets of splenic DCs in mice through phenotypic analysis. In accordance with the results obtained in vitro, the in vivo experiment indicated that oral treatment of mice with E. purpurea extract, with or without TNF-α, daily for 5 days produced no significant effect on the subsets of splenic DCs CD11c−CD83+ and CD11c+CD83+ regarding the phenotypic analysis. Because we did not study E. purpurea extract effect on DC precursors in vivo, we could not exclude the possibility of its effect on PBMCs in vivo. This possibility requires further study.
In conclusion, our results imply that the immunomodulatory mechanisms of E. purpurea affect generation fate of DCs rather than differentiation stages. The in vivo study employing murine splenic DCs confirmed those observed in vitro. It would be worthwhile to investigate the response of DCs to isolated E. purpurea constituents separately, such as alkylamides and polysaccharides.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
Contributor Information
N.E. El-Ashmawy, Email: nahlaelashmawy@yahoo.com.
E.A. El-Zamarany, Email: dr_enas_arafa@hotmail.com.
M.L. Salem, Email: mohamedlabibsalem@yahoo.com.
H.A. El-Bahrawy, Email: helbahrawy@yahoo.com.
G.M. Al-Ashmawy, Email: ghadaashmawy@yahoo.com.
References
- 1.Abouelella A.M.K. J. Vet. Sci. 2007;8(4):341–351. doi: 10.4142/jvs.2007.8.4.341. Available from: < http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2868149&tool=pmcentrez&rendertype=abstract> (Accessed August 17, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann M.F., Kopf M., Marsland B.J. Nat. Rev. Immunol. 2006;6(2):159–164. doi: 10.1038/nri1776. Available from: < http://www.ncbi.nlm.nih.gov/pubmed/16491140> (Accessed December 30, 2014) [DOI] [PubMed] [Google Scholar]
- 3.Barnes J. J. Pharm. Pharmacol. 2005;57(8):929–954. doi: 10.1211/0022357056127. Available from: < http://www.ncbi.nlm.nih.gov/pubmed/16102249> (Accessed November 26, 2014) [DOI] [PubMed] [Google Scholar]
- 4.Benson J.M. Food Chem. Toxicol. 2010;48(5):1170–1177. doi: 10.1016/j.fct.2010.02.007. Available from: < http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2883451&tool=pmcentrez&rendertype=abstract> (Accessed August 23, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohn L., Delamarre L. Front. Immunol. 2014;5:255. doi: 10.3389/fimmu.2014.00255. Available from: < http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4039009&tool=pmcentrez&rendertype=abstract> (Accessed July 10, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everts B., Pearce E.J. Front. Immunol. 2014;5:203. doi: 10.3389/fimmu.2014.00203. Available from: < http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4021118&tool=pmcentrez&rendertype=abstract> (Accessed July 18, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferlazzo G., Moretta L. Crit. Rev. Oncog. 2014;19(1–2):67–75. doi: 10.1615/critrevoncog.2014010827. Available from: < http://www.ncbi.nlm.nih.gov/pubmed/24941374> (Accessed October 31, 2014) [DOI] [PubMed] [Google Scholar]
- 8.Gallois A., Bhardwaj N. Front. Immunol. 2013;4:436. doi: 10.3389/fimmu.2013.00436. Available from: < http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3857536&tool=pmcentrez&rendertype=abstract> (Accessed October 31, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldrosen M.H., Straus S.E. Nat. Rev. Immunol. 2004;4(11):912–921. doi: 10.1038/nri1486. Available from: < http://www.ncbi.nlm.nih.gov/pubmed/15516970> (Accessed August 23, 2014) [DOI] [PubMed] [Google Scholar]
- 10.Hashimdeen S.S. Clin. Hemorheol. Micro. 2013;55(4):501–512. doi: 10.3233/CH-131786. Available from: < http://www.ncbi.nlm.nih.gov/pubmed/24099989> (Accessed December 20, 2014) [DOI] [PubMed] [Google Scholar]
- 11.Hudson J.B. J. Biomed. Biotechnol. 2012;2012:769896. doi: 10.1155/2012/769896. Available from: < http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3205674&tool=pmcentrez&rendertype=abstract> (Accessed August 17, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiao S.-F. J. Biomed. Sci. 2014;21:1. doi: 10.1186/1423-0127-21-1. Available from: < http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3902418&tool=pmcentrez&rendertype=abstract> (Accessed August 18, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadowaki N. J. Exp. Med. 2001;194(6):863–869. doi: 10.1084/jem.194.6.863. Available from: < http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2195968&tool=pmcentrez&rendertype=abstract> (Accessed December 30, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koido S. World J. Gastroenterol. 2013;19(46):8531–8542. doi: 10.3748/wjg.v19.i46.8531. Available from: < http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3870498&tool=pmcentrez&rendertype=abstract> (Accessed October 31, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthias A. Life Sci. 2005;77(16):2018–2029. doi: 10.1016/j.lfs.2005.04.009. Available from: < http://www.ncbi.nlm.nih.gov/pubmed/15919096> (Accessed December 17, 2014) [DOI] [PubMed] [Google Scholar]
- 16.Mellman I. Cancer Immunol. Res. 2013;1(3):145–149. doi: 10.1158/2326-6066.CIR-13-0102. Available from: < http://cancerimmunolres.aacrjournals.org/content/1/3/145.full> (Accessed July 17, 2014) [DOI] [PubMed] [Google Scholar]
- 17.Nakano H., Yanagita M., Gunn M.D. J. Exp. Med. 2001;194(8):1171–1178. doi: 10.1084/jem.194.8.1171. Available from: < http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2193516&tool=pmcentrez&rendertype=abstract> (Accessed August 18, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohnmacht C. J. Exp. Med. 2009;206(3):549–559. doi: 10.1084/jem.20082394. Available from: < http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2699126&tool=pmcentrez&rendertype=abstract> (Accessed December 5, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piqueras B. Blood. 2006;107(7):2613–2618. doi: 10.1182/blood-2005-07-2965. Available from: < http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1895384&tool=pmcentrez&rendertype=abstract> (Accessed December 19, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pisetsky D.S. Clin. Immunol. (Orlando, Fla.) 2012;144(1):32–40. doi: 10.1016/j.clim.2012.04.006. Available from: < http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3724456&tool=pmcentrez&rendertype=abstract> (Accessed December 4, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prazma C.M., Tedder T.F. Immunol. Lett. 2008;115(1):1–8. doi: 10.1016/j.imlet.2007.10.001. Available from: < http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2699889&tool=pmcentrez&rendertype=abstract> (Accessed August 17, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pugh N.D. Int. Immunopharmacol. 2008;8(7):1023–1032. doi: 10.1016/j.intimp.2008.03.007. Available from: < http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2467439&tool=pmcentrez&rendertype=abstract> (Accessed December 20, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ragupathi G. Vaccine. 2008;26(37):4860–4865. doi: 10.1016/j.vaccine.2008.06.098. Available from: < http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2565601&tool=pmcentrez&rendertype=abstract> (Accessed August 17, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randolph G.J., Angeli V., Swartz M.A. Nat. Rev. Immunol. 2005;5(8):617–628. doi: 10.1038/nri1670. Available from: < http://www.ncbi.nlm.nih.gov/pubmed/16056255> (Accessed December 19, 2014) [DOI] [PubMed] [Google Scholar]
- 25.Rezaie A. Jundishapur J. Nat. Pharmaceut. Prod. 2013;8(2):60–64. Available from: < http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3941908&tool=pmcentrez&rendertype=abstract> (Accessed August 17, 2014) [PMC free article] [PubMed] [Google Scholar]
- 26.Rubinstein M.P. J. Immunol. (Baltimore, Md.: 1950) 2002;169(9):4928–4935. doi: 10.4049/jimmunol.169.9.4928. Available from: < http://www.ncbi.nlm.nih.gov/pubmed/12391205> (Accessed December 20, 2014) [DOI] [PubMed] [Google Scholar]
- 27.Scott C.L., Aumeunier A.M., Mowat A.M. Trends Immunol. 2011;32(9):412–419. doi: 10.1016/j.it.2011.06.003. Available from: < http://www.ncbi.nlm.nih.gov/pubmed/21816673> (Accessed July 16, 2014) [DOI] [PubMed] [Google Scholar]
- 28.Shimizu K., Fujii S. Front. Biosci. 2008;13:6193–6201. doi: 10.2741/3147. Available from: < http://www.ncbi.nlm.nih.gov/pubmed/18508653> (Accessed December 30, 2014) [DOI] [PubMed] [Google Scholar]
- 29.Shrestha G., St Clair L.L., O’Neill K.L. Phytother. Res. 2014 doi: 10.1002/ptr.5251. Available from: < http://www.ncbi.nlm.nih.gov/pubmed/25339289> (Accessed October 31, 2014) [DOI] [PubMed] [Google Scholar]
- 30.Stetler-Stevenson M., Braylan R.C. Semin. Hematol. 2001;38(2):111–123. Available from: < http://www.ncbi.nlm.nih.gov/pubmed/11309693> (Accessed December 20, 2014) [PubMed] [Google Scholar]
- 31.Trombetta E.S., Mellman I. Annu. Rev. Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. Available from: < http://www.ncbi.nlm.nih.gov/pubmed/15771591> (Accessed July 9, 2014) [DOI] [PubMed] [Google Scholar]
- 32.Wang C.-Y. Genomics. 2006;88(6):801–808. doi: 10.1016/j.ygeno.2006.08.011. Available from: < http://www.ncbi.nlm.nih.gov/pubmed/17011161> (Accessed August 23, 2014) [DOI] [PubMed] [Google Scholar]
- 33.Yamada K. J. Ethnopharmacol. 2011;137(1):231–235. doi: 10.1016/j.jep.2011.05.017. Available from: < http://www.ncbi.nlm.nih.gov/pubmed/21619924> (Accessed August 8, 2014) [DOI] [PubMed] [Google Scholar]
- 34.Ye Z. Cancer Biotherap. Radiopharmaceut. 2006;21(6):613–622. doi: 10.1089/cbr.2006.21.613. Available from: < http://www.ncbi.nlm.nih.gov/pubmed/17257077> (Accessed December 20, 2014) [DOI] [PubMed] [Google Scholar]