Abstract
Poly-γ-glutamic acid (γ-PGA) is a naturally occurring biopolymer made up of repeating units of glutamic acid and can be potentially used for multiple applications. This study compared the production of γ-PGA by Bacillus subtilis and Bacillus licheniformis in GS and E media. The highest γ-PGA production was achieved using initial glycerol concentration of 40 and 80 g/l, ammonium chloride as the nitrogen source, 20 g/l glutamic acid at pH 6.5 for 72 h using E medium. On characterization, it was observed that glutamic acid was the sole component of the purified material. It contained a mixture of Na-γ-PGA and H+-γ-PGA. The survival of probiotics during freeze drying was improved by combining them with γ-PGA polymer. For Lactobacilli, 10% γ-PGA protected the cells significantly than 10% sucrose during freeze drying. γ-PGA protection was shown to improve the viability of probiotic bacteria in orange juice for 40 days. No considerable change was observed in the concentrations of citric acid, malic acid and ascorbic acid when probiotic bacteria and γ-PGA were introduced into orange juice and hence, it could be used as a non-dairy delivery platform for these bacteria.
Keywords: Poly-γ-glutamic acid, Probiotics, Cryoprotectant, Orange juice
1. Introduction
Poly-γ-glutamic acid (γ-PGA) is a natural polymer, consisting of d- and l-glutamic acids linked with amide bonding between α-amino and γ-carboxylic acids [1]. γ-PGA has been produced extensively using bacteria, especially those of Bacillus sp. [30]. The γ-PGA is biodegradable, edible, water-soluble and non-toxic to humans and environment. Therefore, it has been suggested for use as biodegradable plastics, flocculants [2], biological adhesive and food additives [17].
Probiotic food products which contain microbial strains with beneficial characters are becoming more and more popular. Consumers are attracted to these products mainly due to high publicity given by the manufacturers on their health benefits. However, these health benefits are depending on the viability of probiotic microbes and maintenance of their probiotic properties in commercial stock cultures and probiotic food products during storage. Although freeze drying has been used to preserve probiotic cultures, there are some records on loss of culture viability due to freeze-thaw process [11]. To reduce the loss of viability of probiotic cultures due to freeze drying, cryoprotectants are commonly used [19]. Although the antifreeze activity of γ-PGA is well known, it has not been used for maintaining the viability of probiotic bacteria during freeze drying.
The aim of this study was to optimize, characterize and identify γ-PGA produced by Bacillus subtilis and Bacillus licheniformis. This research was also extended for testing the viability of three probiotic strains during freeze drying and when stored in orange juice. Moreover, change in concentration of organic acids of orange juice when probiotic bacteria and γ-PGA are introduced was also investigated.
2. Materials and methods
2.1. Bacterial strains
Bacterial strains (Bacillus subtilis NCTC10400 and Bacillus licheniformis ATCC 99457) were obtained from Fermentation Biotechnology and Applied Microbiology (FERM-BAM) Centre, Al-Azhar University, Cairo, Egypt. Three probiotic bacteria, Lactobacillus rhamnosus, Lactobacillus paracasei and Lactobacillus plantarum were isolated from Egyptian dairy products collected from the Cairo markets as described by Rushdy and Gomaa [21]. The stock cultures were freeze-dried and stored at −80 °C.
2.2. Production of γ-PGA in shake flask cultures
Bacillus subtilis and B. licheniformis, the γ-PGA producers, was cultured in LB medium containing (g/l): yeast extract, 5; peptone, 10; NaCl, 10 and incubated at 37 °C for 24 h (optical density at 600 nm = 1). 5% of this culture was inoculated into 250 ml of γ-PGA production media. To determine whether the bacteria under study could produce γ-PGA in the absence of precursors, GS and E media were tested. GS medium contained (g/l): Sucrose, 50; KH2PO4, 2.7; Na2HPO4, 4.2; NaCl, 50; MgSO4·7H2O, 5; pH 7.2, whereas medium E contained (g/l): Citric acid, 12; glycerol, 80; NH4Cl, 7; MgSO4·7H2O, 0.5, FeCl3·6H2O, 0.2; K2HPO4, 0.5; CaCl2·2H2O, 0.15; MnSO4·H2O, 0.2, pH 6.5, filter sterilized l-glutamic acid was added to both media at a concentration of 2% [6], [29]. All flasks were incubated at 37 °C on a rotary shaker at 150 rpm. Samples were withdrawn at different time intervals (0, 24, 48, 72, 96 and 120 h) for analysis of cell growth and γ-PGA production. Bacterial growth was determined by measuring the absorbance at 600 nm. Cell biomass concentration was determined by the standard calibration curve between OD600 and cell dry weight.
2.3. Optimization of γ-PGA production
The effect of different glycerol concentrations in the range of 0, 20, 40, 60, 80, 100 g/l on the growth and production of γ-PGA was investigated. To study the effect of different nitrogen sources on γ-PGA production, ammonium chloride was replaced at equivalent nitrogen concentration by different nitrogen sources organic nitrogen sources like yeast extract, peptone, malt extract and soybean meal; inorganic nitrogen sources like ammonium chloride, ammonium sulfate and ammonium nitrate, sodium nitrate and potassium nitrate. The effect of initial l-glutamic acid concentrations in the range of 0, 10, 20, 30 and 40 g/l was investigated. In order to monitor the effect of pH on γ-PGA production, fermentation runs were carried out at initial pH varying from 5.0 to 8.0.
2.4. Purification of γ-PGA
The cell cultures were separated from the culture broth by centrifugation for 20 min at 12,000 rpm to remove cells. The supernatant was poured into 4 volumes ice-cold ethanol with gentle stirring and kept at 4 °C overnight to precipitate the γ-PGA. The resulting precipitate containing crude γ-PGA was collected by refrigerated centrifuge (12,000 rpm, 10 °C, for 30 min). The crude γ-PGA was then dissolved in distilled water at a concentration of 10 g/l, and any insoluble contaminants were removed by centrifugation (14,000 rpm, 10 °C, for 20 min). The γ-PGA was further purified by dialysis (membrane with the molecular weight cut off of 12,400) against 1 L of distilled water three times for 12 h to remove salts from aqueous γ-PGA solution. Finally, the γ-PGA slurry was lyophilized to prepare as γ-PGA powder. An amount of γ-PGA was determined using ninhydrin with glutamic acid as a standard [13].
2.5. Characterization of γ-PGA
2.5.1. Amino acid analysis
The purified γ-PGA produced by B. subtilis and B. licheniformis was hydrolyzed using 6 M HCl at 110 °C for 24 h in a sealed and evacuated tube and then used for amino acid analysis. Thin layer chromatography was performed on a cellulose plate (Merck, USA) with solvent systems of butanol/acetic acid/water (3:1:1, by mass) and 96% ethanol/water (63:37, by mass). Detection of amino acids was done by spraying with 0.2% ninhydrin in acetone [24].
2.5.2. Total sugar content
The total carbohydrate content of the γ-PGA produced was determined by the phenol–sulfuric acid method [7].
2.5.3. Elemental analysis of γ-PGA
γ-PGA produced by bacteria in different media could be either in the form of a salt or free acid or a mixture of the two. To assess the percentages of different salt forms and free acid form of γ-PGA, elemental analysis was performed using Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES).
2.5.4. Measurement of FT-IR spectroscopy
A characterization of γ-PGA structure was analyzed by Fourier Transform Infrared Spectroscopy (FT-IR). The KBr mode was carried out to determine the γ-PGA produced and recorded the transmission spectra in the range of 4000–400 cm−1.
2.6. γ-PGA as a cryoprotectant
Initially, sterilization of γ-PGA is an important step before using it for a probiotic application. γ-PGA solutions (5% and 10% (w/v)) were autoclaved at 0.35 BAR & 110 °C for 30 min. Three probiotic bacteria, L. rhamnosus, L. paracasei and L. plantarum were used for these tests. Before use, the cultures were revived aseptically and grown on De Man Rogosa Sharpe (MRS) agar at 37 °C. To prepare cells for freeze drying, all microorganisms were cultured in 250 ml of MRS broth for 48 h. After incubation, viable counts were performed on MRS agar to determine the number of viable cells prior to freeze drying. The cultures were centrifuged and washed with PBS to obtain cell pellets and then resuspended in 10 ml solutions of either 10% γ-PGA, 5% γ-PGA or 10% sucrose. For cells without a cryoprotectant, 10 ml of sterile distilled water was added. The suspensions were incubated at room temperature for 1 h and then frozen at −80 °C for 24 h. The frozen cultures were then freeze dried at −40 °C for 48 h. After freeze drying, 10 ml of PBS was added to each treatment and the viability was determined. Cells were enumerated by the Miles and Misra technique which involves a 10-fold dilution series in PBS followed by aseptically plating out 20 μl of each cell suspension in triplicate on appropriate media, which were then incubated at 37 °C.
2.6.1. Protection in fruit juice
Fresh samples of orange juice were used. Bacteria were grown in MRS broth for 48 h at 37 °C. The culture was then centrifuged and washed with PBS to obtain cell pellets. Pellets were then mixed thoroughly in a 10% γ-PGA solution. This mixture was incubated at room temperature and frozen at −80 °C and freeze dried to obtain a dry powder containing cells protected with γ-PGA. 1 ml PBS was added to the dry powder and this solution was transferred to 40 ml of orange juice. The final concentration of γ-PGA in fruit juice was ∼2.5% (w/v). For tests with unprotected cells, cells were inoculated in MRS broth using the aforementioned conditions. Cell pellets were obtained after centrifugation and washing with PBS. 1 ml of PBS was added to the cells. This solution was added to orange juice. Viability was measured at days 0, 2, 4, 6, 8, 11, 13, 20, 30 and 40 for unprotected and γ-PGA-protected cells on MRS agar. After appropriate dilution, samples were plated on MRS agar and incubated at 37 °C for 48 h.
2.6.2. Organic acid concentration in fruit juice
It was important to assess the change in organic acid composition of fruit juice when unprotected and γ-PGA-protected cells were added to it to determine any alteration in its nutritional and organoleptic properties. Organic acid analysis was performed using high performance liquid chromatography (HPLC), Shimadzu Class-VPV 5.03 (Kyoto, Japan). A Prevail Organic Acid 5 μm column with a UV detector was used for citric acid, malic acid and ascorbic acid. 25 mM KH2PO4 (pH 2.5 adjusted with phosphoric acid) was used as the eluent. Before loading, the samples were filtered using 0.45 μm filters and were diluted 10-fold. Standard curves were prepared using known concentrations of chemicals to be analyzed.
2.7. Statistical analysis
All experiments were performed in two or three replicates. Analysis of variance was performed to calculate significant differences in treatment means, and the least significant difference (p < 0.05) was used to separate means, using the SPSS software.
3. Results and discussion
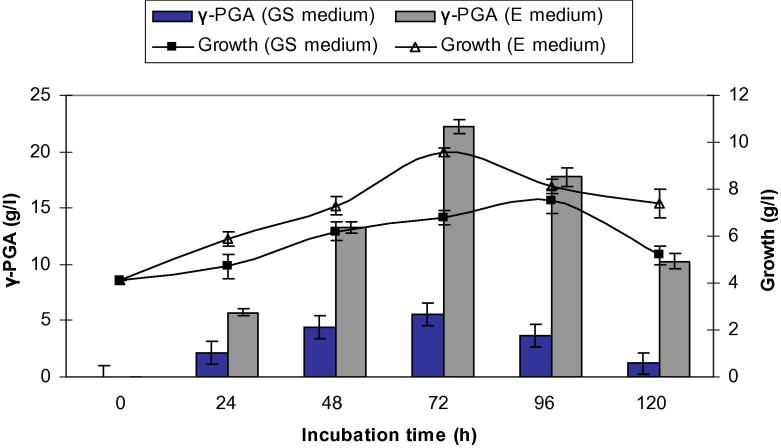
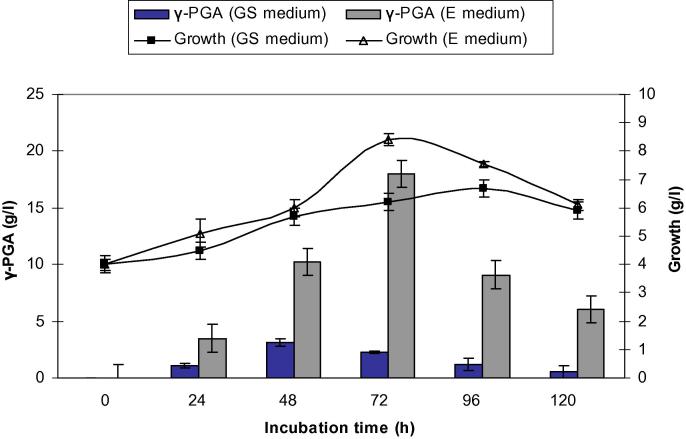
There has been a lot of attention paid to the application of poly-γ-glutamic acid for use in the field of cosmetics, biomedical and environmental industries with the feature of being less harmful to humans and the environment [16]. However, application of γ-PGA is limited due to the high cost associated with its productivity. Screening bacterial strains to find a more efficient producer is one approach to overcome this limitation [12]. Moreover, characterization of γ-PGA and identification of the factors affecting γ-PGA production have not been covered in as much detail. It is essential to have knowledge of how growth and yield of γ-PGA production changes with bacteria and medium of production. In the present study, Bacillus subtilis NCTC10400 and Bacillus licheniformis ATCC 99457 were grown in GS and E media at 37 °C and 150 rpm for 120 h in shake flasks. Samples were taken aseptically at regular intervals (24, 48, 72, 96 and 120 h) to determine growth and γ-PGA production. As shown in Figure 1, Figure 2, it was evident that both bacterial strains reached higher cell count in E medium than in GS medium. In GS medium, B. subtilis and B. licheniformis reached maximum growth (7.50 and 6.70 g/l, respectively) at 96 h, whereas in medium E, they reached the highest cell count of 9.58 and 8.40 g/l, respectively at 72 h, after which, a reduction in cell count was seen.
Figure 1.
Comparison of bacterial growth and yield of γ-PGA produced by Bacillus subtilis grown in GS and E medium. Results are means of three independent determinations. Bars correspond to standard deviation.
Figure 2.
Comparison of bacterial growth and yield of γ-PGA produced by Bacillus licheniformis grown in GS and E medium. Results are means of three independent determinations. Bars correspond to standard deviation.
Glycerol and citric acid in medium E was consumed better than sucrose in GS medium, resulting in the γ-PGA production of 22.2 and 18.0 g/l by B. subtilis and B. licheniformis, respectively at 72 h (Figure 1, Figure 2). After 72 h, the yield of γ-PGA production by both strains decreased. This could be attributed to the depletion of important medium constituents like glutamic acid, citric acid and glucose. Since γ-PGA is an extracellular, high molecular mass polymer, the culture medium becomes highly viscous with the progress of polymer production. This increased viscosity is likely to decrease the volumetric oxygen mass transfer, leading to oxygen limitation. Previous research has demonstrated that γ-PGA production in Bacillus usually takes place in the late logarithmic and stationary phases of the culture [2].
From the above mentioned results, E medium was chosen for further studies. These results were different from the findings of Kedia et al. [14] who stated that GS medium was most effective for γ-PGA production by B. subtilis natto, resulting in the production of 26–28 g/l of γ-PGA after 96 h.
3.1. Optimization of γ-PGA production
The medium composition is one of the factors that can influence yield and the properties of γ-PGA and can be used to control them. Some of the nutrient requirements and growth factors affecting γ-PGA production in different bacteria have been enumerated in the following sections.
3.1.1. Effect of glycerol concentration
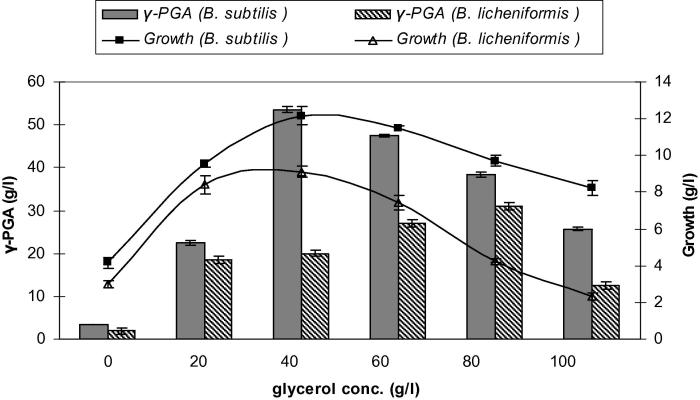
As shown in Fig. 3, glycerol as a carbon source had positive effect on γ-PGA fermentation by B. subtilis and B. licheniformis and the biomass was also comparatively higher than that without glycerol. The optimum concentration for the highest γ-PGA production reached 53.6 g/l by B. subtilis was 40 g/l, above this concentration the yield of γ-PGA started decreasing, while in the case of B. licheniformis, a higher level of glycerol was required (80 g/l). This was also the advantage of B. subtilis, as the low concentration of glycerol required would efficiently lower the industrial cost. Du et al. [6] also reported that γ-PGA production by B. licheniformis WBL-3 increased from 9.7 to 16.7 g/l as the concentration of glycerol was increased in the medium from 0 to 70 g/l.
Figure 3.
Effect of different glycerol concentrations on growth and γ-PGA production by Bacillus subtilis and Bacillus licheniformis. Results are means of three independent determinations. Bars correspond to standard deviation.
The reason for the increase in yield was found to be due to a change in the phospholipid composition of the cell membrane due to the addition of glycerol, which increased the permeability of the cell membrane for γ-PGA. Without the use of glycerol (only citric acid and glutamic acid were used in this case), the intracellular concentration of γ-PGA was quite high which hampered the synthesis of γ-PGA [6]. The addition of glycerol during production of γ-PGA was seen to decrease the molecular weight of γ-PGA, and then decreased the broth viscosity, enhanced the uptake of other nutrients, which in turn resulted in a higher γ-PGA yield [28].
3.1.2. Effect of nitrogen source
On evaluating the effect of N sources on γ-PGA production, it was found that ammonium chloride was the best nitrogen source for polymer production by both bacterial strains. As shown in Table 1, biomass production was high but γ-PGA yield was lower with organic nitrogen sources compared to inorganic nitrogen sources. Free NH4+ ions are required for γ-PGA production. The availability of free NH4+ ions from inorganic nitrogen sources is higher than that from organic nitrogen sources [8].
Table 1.
Effect of different nitrogen sources on growth and γ-PGA production by Bacillus subtilis and Bacillus licheniformis. Data represent the mean of 3 different readings ± standard deviation.
| Nitrogen sources | γ-PGA production (g/l) |
Bacterial growth (g/l) |
||
|---|---|---|---|---|
| B. subtilis | B. licheniformis | B. subtilis | B. licheniformis | |
| Yeast extract | 12.58 ± 1.2 | 10.22 ± 1.12 | 17.88 ± 0.99 | 14.55 ± 0.25 |
| Peptone | 11.45 ± 1.0 | 9.84 ± 2.00 | 15.24 ± 0.52 | 12.15 ± 0.12 |
| Malt extract | 20.15 ± 0.8 | 8.56 ± 1.46 | 14.52 ± 0.64 | 10.22 ± 0.14 |
| Soybean extract | 37.45 ± 0.2 | 6.47 ± 1.89 | 13.20 ± 0.22 | 9.24 ± 0.84 |
| NH4Cl | 53.68 ± 0.02 | 31.28 ± 1.25 | 12.5 ± 0.06 | 9.36 ± 0.05 |
| NH4SO4 | 42.89 ± 0.00 | 28.44 ± 1.50 | 10.0 ± 0.10 | 7.26 ± 0.11 |
| NH4NO3 | 49.11 ± 0.65 | 22.35 ± 0.05 | 8.12 ± 0.07 | 4.44 ± 0.16 |
| NaNO3 | 30.45 ± 1.5 | 20.18 ± 0.08 | 7.92 ± 0.00 | 3.12 ± 0.13 |
| KNO3 | 40.28 ± 1.0 | 19.78 ± 0.09 | 7.10 ± 0.68 | 2.51 ± 0.21 |
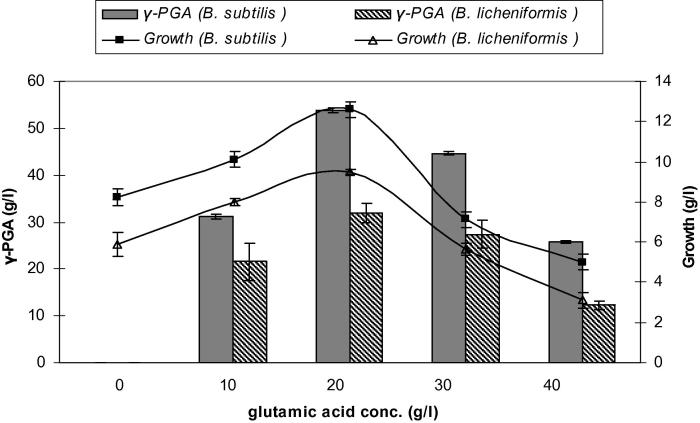
Biosynthesis of γ- PGA in bacteria is carried out in two steps. In the first step synthesis of l- and d-glutamic acid takes place, whereas in the second step these l- and d-glutamic acid units are joined together to form γ-PGA. Results obtained in the present study suggested that although B. subtilis and B. licheniformis used citric acid and glycerol as carbon sources, it is glutamic acid dependent. As shown in Fig. 4 an increase in the l-glutamic acid concentration correlated well with an increased concentration of γ-PGA polymer. The observations gave an alternative for possible process development of the production of γ-PGA. This is directly applicable in industries, as well as enhancing academic progress in bio-polymer field. The role of glutamic acid in this example is presumably an activator for the γ-PGA enzyme system but not as a carbon source.
Figure 4.
Effect of different glutamic acid concentrations on growth and γ-PGA production by Bacillus subtilis and Bacillus licheniformis. Results are means of three independent determinations. Bars correspond to standard deviation.
3.1.3. Effect of pH
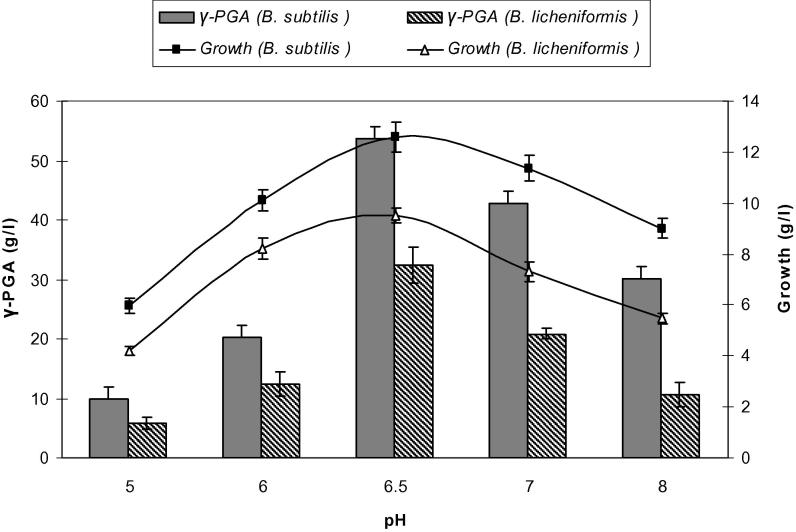
pH is a significant factor that influences the physiology of a microorganism by affecting nutrient solubility and uptake, enzyme activity, cell membrane morphology, by-product formation and oxidative-reductive reactions. The optimum pH of the medium is crucial for γ-PGA production and differs for different bacteria. It has been shown that pH 6.5 is optimal for γ-PGA produced by B. subtilis and B. licheniformis reached 53.8 and 32 g/l, respectively (Fig. 5). Most studies on fermentative production of γ-PGA were carried out at pH 6.5 [6]. During production of γ-PGA, citrate utilization occurs more rapidly and to a greater extent at pH 6.5.
Figure 5.
Effect of pH on growth and γ-PGA production by Bacillus subtilis and Bacillus licheniformis. Results are means of three independent determinations. Bars correspond to standard deviation.
3.2. γ-PGA characterization
The properties of the γ-PGA produced are crucial, since they can determine the application for which it is used.
3.2.1. Amino acid analysis
Thin layer chromatography (TLC) was used to characterize the amino acids in γ-PGA obtained from B. subtilis and B. licheniformis. Glutamic acid was the sole component of the 6 M HCl hydrolyzate of the purified material. TLC of the hydrolyzate performed on a cellulose plate and visualized with 0.2% ninhydrin indicated a single spot with Rf value of 0.33, which was identical to that of authentic glutamic acid.
3.2.2. Sugar content
Polysaccharide content in the purified γ-PGA was tested by the phenol–sulfuric acid method. No sugar was detected in γ-PGA, indicating that under the optimized fermentation conditions, B. subtilis and B. licheniformis did not produce polysaccharides as a by-product.
3.2.3. Elemental analysis
Before using γ-PGA for any application, it is important to have knowledge of the form of the polymer for a better quality and a more consistent product. Moreover, assessing the form of γ-PGA is important since the percentage salt composition of the polymer would affect its solubility and would in turn affect the application for which it is used. Elemental analysis was performed using ICP-AES to identify whether the salt or the acid form of γ-PGA was produced, it was seen that most of the γ-PGA produced by bacteria was not only the sodium salt of the polymer (Na-γ-PGA), but also the acid form of γ-PGA (H+-γ-PGA) was also present. B. subtilis produced 59.90% and 37.76% of Na-γ-PGA and H+-γ-PGA, respectively, however, B. licheniformis produced 64.47% and 32.89% of Na-γ-PGA and H+-γ-PGA, respectively. The presence of Na-γ-PGA was possibly because the pH of medium E was adjusted using 3 M NaOH.
3.2.4. Identification of γ-PGA
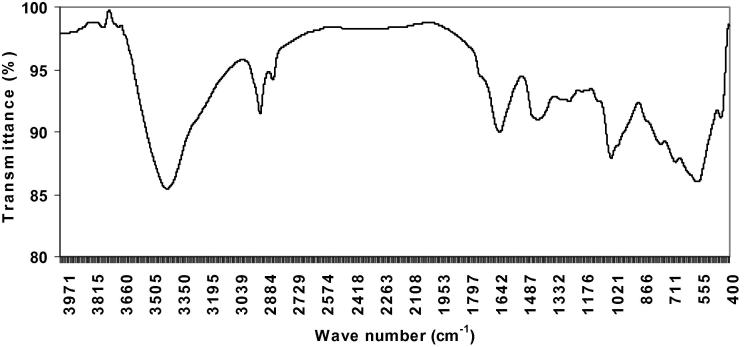
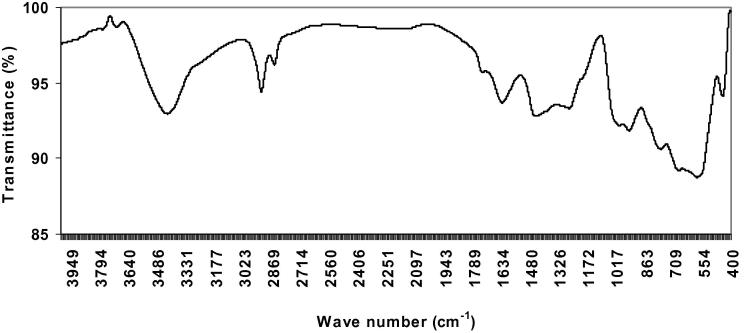
It was important to identify the produced polymer as γ-PGA, hence FT-IR spectroscopy was performed. FT-IR spectra for γ-PGA obtained by the strains under investigation were similar and they compared well to that of a commercially available γ-PGA sample. The infrared spectra of γ-PGA samples showed characteristic strong amide absorption at 1634 and 1627 cm−1, carbonyl absorption at 1432 and 1455 cm−1, and strong hydroxyl absorption at 3423 and 3427 cm−1. The absorption peaks at 1304 and 1368 cm−1 are characteristic of C O groups. The absorption peaks observed at 1194 and 1198 cm−1 are characteristic of C—N groups (Figure 6, Figure 7).
Figure 6.
FTIR spectrum of γ-PGA produced by Bacillus subtilis.
Figure 7.
FTIR spectrum of γ-PGA produced by Bacillus licheniformis.
3.3. γ-PGA as a cryoprotectant for probiotic bacteria
Probiotics can be defined as live microorganisms that when administered in adequate amounts confer a health benefit on the host. Probiotics are not only used to achieve overall well-being, but also for prevention of initiation, promotion and development of non-transmissible chronic diseases [9], [20]. Probiotics can be administered in a number of forms. They can either be in the form of capsules, health food supplements or administered in food products. Dairy foods such as milk, yoghurt and yoghurt drinks are common vehicles for delivery of probiotic bacteria [27]. The benefits of probiotic bacteria are largely dependent on their ability to survive, colonize and multiply in the host [4].
It is a common practice to freeze dry bacteria, so that they can be stored in the form of dry powders for a longer period of time. In addition, transportation of freeze dried bacteria is easier. Freeze drying is based upon sublimation and occurs in three phases – freezing, primary drying and secondary drying [18]. Probiotic cultures are frequently supplied as freeze-dried or spray-dried powders. However, the procedures used to prepare freeze dried probiotic bacteria are detrimental to cell structure and viability [22]. Freeze drying can cause protein denaturation and DNA damage that leads to a decrease in cell viability. Also, desiccated bacteria could lose viability on rehydration. The cell membrane has been reported to be the major site of damage during drying or rehydration [23].
Scientists have endeavored to use different techniques to resolve the loss of viability in probiotics. Cryoprotectants are substances that surround cells and could be used to avoid/minimize loss in viability during freeze drying, for instance, cellulose, sucrose, trehalose, skimmed milk, glycerol and DMSO [11]. These materials, although useful for certain applications, are expensive to obtain and maintain and are known to be impractical when equipment and refrigeration are not readily available [15]. In the present study, γ-PGA produced by B. subtilis and B. licheniformis was used to coat probiotic bacteria and the cryoprotectant properties were evaluated by comparison to sucrose as a cryoprotectant during freeze drying. Three commonly used probiotic bacteria (L. rhamnosus, L. paracasei and L. plantarum) were used for the tests. The effect of 10% γ-PGA, 5% γ-PGA and 10% sucrose was tested on viability of the bacteria after freeze drying.
Sterilization of γ-PGA is important because there is a need to eradicate any residual bacteria from the fermentation that might interfere with subsequent viable cell count results for the probiotic bacteria. Different techniques were previously tested for the sterilization of γ-PGA. While γ-irradiation of γ-PGA could work on a large scale, it is too costly when smaller amounts of γ-PGA need to be sterilized. In addition, irradiation would result in cross-linking of γ-PGA, thus increasing its water absorption capability [26]. A polymer with a high water absorption capability would form hydrogels in solution. This is not ideal since it would change the palatability of the foodstuff in which it is to be used. Autoclaving at 0.35 BAR & 110 °C for 30 min, did not seem to affect the structure of γ-PGA. A previous study has reported that the decomposition temperature of Na-γ-PGA is 340 °C[10]. Therefore, heating γ-PGA at 110 °C would not disrupt its structure. Although using this technique for sterilization of γ-PGA did not affect the structural bonds within the polymer, it reduced its molecular weight. Interestingly, it has been shown that γ-PGA with a lower molecular weight has a better antifreeze activity than a higher molecular weight γ-PGA. This may explain why sterilizing γ-PGA enhanced its cryoprotectant ability. Therefore, autoclaving γ-PGA could not only sterilize the polymer, but might also improve its efficiency in protecting the cells when they are freeze dried.
It was observed that when no cryoprotectant was used to protect the cells, L. rhamnosus showed a reduction in viability of 1.5 log CFU/ml. When 10% sucrose was used to protect L. rhamnosus during freeze drying, 1.0 log CFU/ml reduction in viability was observed. However, when cells were protected with 10% γ-PGA produced by B. subtilis and B. licheniformis, loss in viability was reduced to 0.6 and 0.8 log CFU/ml, respectively (Table 2). For L. paracasei, a log reduction of 2.8 CFU/ml was observed when cells were freeze dried without any cryoprotectant. When cells were protected using 10% γ-PGA produced by B. subtilis and B. licheniformis, log reduction of 1.1 and 1.7 CFU/ml, respectively was observed. However, when 10% sucrose was used to protect L. paracasei during freeze drying, 1.9 log CFU/ml reduction in viability was observed. A more pronounced reduction in viability (2.9 log CFU/ml) was observed when L. plantarum cells were freeze dried without any cryoprotectant. When L. plantarum cells were protected with 10% γ-PGA produced by B. subtilis and B. licheniformis, only 0.8 and 1.1 log CFU/ml reduction in viability was observed (Table 2). Although 5% γ-PGA was also able to protect the cells during freeze drying, it was not as efficient as 10% γ-PGA. However, its cryoprotectant ability was comparable to 10% sucrose.
Table 2.
Effect of γ-PGA and sucrose on viability of probiotic bacteria during freeze drying. Data represent the mean of 3 different readings ± standard deviation.
| Bacterial species | Before freeze drying | After freeze drying |
|||||
|---|---|---|---|---|---|---|---|
| No cryoprotectant | Sucrose (10%) |
γ-PGA of B. subtilis (10%) |
γ-PGA of B. subtilis (5%) |
γ-PGA of B. licheniformis (10%) |
γ-PGA of B. licheniformis (5%) |
||
| L. rhamnosus | 9.2 ± 0.11 | 7.7 ± 0.13 | 8.2 ± 0.3 | 8.6 ± 0.5 | 8.0 ± 0.6 | 8.4 ± 0.16 | 7.9 ± 0.8 |
| L. paracasei | 9.5 ± 0.5 | 6.7 ± 0.6 | 7.6 ± 0.9 | 8.4 ± 0.3 | 7.4 ± 0.2 | 7.8 ± 0.3 | 7.1 ± 0.13 |
| L. plantarum | 9 ± 0.12 | 6.1 ± 0.18 | 7.7 ± 0.6 | 8.2 ± 0.8 | 7.0 ± 0.7 | 7.9 ± 0.6 | 6.7 ± 0.11 |
Although γ-PGA is known to be non-toxic, it is crucial to ascertain the toxigenic potential of its source [3]. In the present study, the results successfully confirmed that B. subtilis and B. licheniformis, the bacteria used to produce γ-PGA for the novel application, do not contain any of the genes that are usually responsible for toxin production. In addition, the absence of hemolytic or lecithinase activity was also demonstrated. Therefore, this study suggests that γ-PGA could be used as a food ingredient for the delivery of probiotic bacteria.
3.3.1. γ-PGA protection of probiotic bacteria in juice
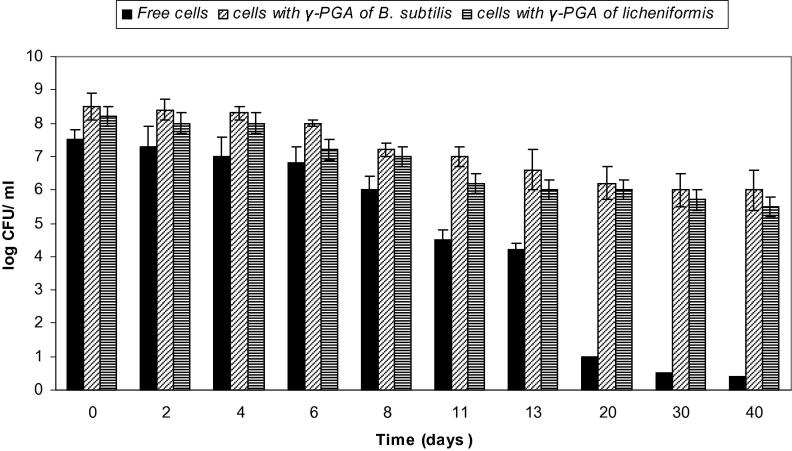
Most probiotic bacteria in foods are delivered via dairy products such as milk, yoghurts and yoghurt drinks. There is a need to develop non-dairy vehicles for administration of probiotics for individuals who are lactose intolerant or allergic to milk proteins, and are unable to consume dairy probiotic products. Fruit juices are good candidates for this purpose, since they are rich in nutrients and are palatable [5]. Also, fruit juices often have oxygen scavenging agents, such as ascorbic acid, which promote anaerobic growth conditions. In addition, fruit juices contain high amounts of sugars which could encourage growth of the added probiotic bacteria. However, probiotic viability in fruit juice is hampered due to factors such as pH, storage temperature, oxygen levels, naturally present antimicrobials and/or presence of competing microorganisms [9], [25]. Hence, it is important to protect the viability of probiotic bacteria in such environments. γ-PGA was utilized for this novel application where it was shown to protect probiotic bacteria in orange juice for 40 days at 4 °C.
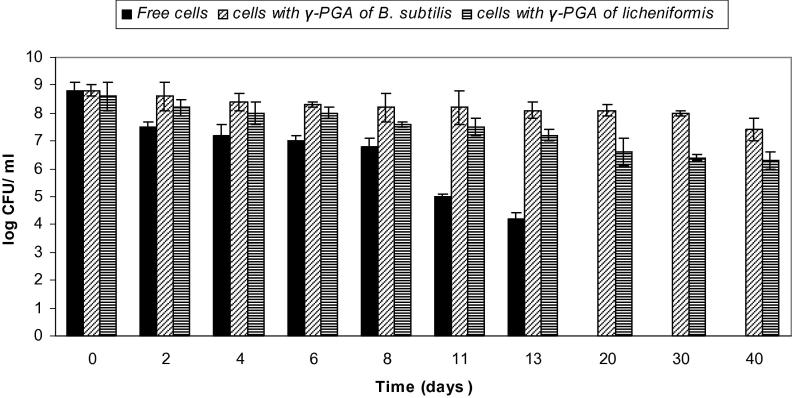
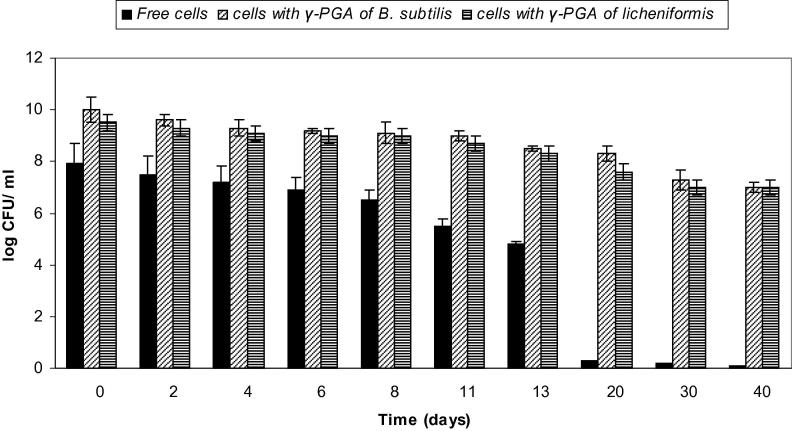
As shown in Fig. 8, the effect of 10% γ-PGA on the viability of L. rhamnosus in orange juice for 40 days was tested. A log reduction of 6.5 CFU/ml was seen in viability of L. rhamnosus cells that were not protected with γ-PGA at 20 days. In contrast, cells survived well for 40 days with a viability of 6 and 5.5 log CFU/ml when protected with γ-PGA produced by B. subtilis and B. licheniformis, when compared to their initial count of 8.5 and 8.2 log CFU/ml, respectively. On day 20, unprotected L. paracasei showed complete loss in viability, whereas a log reduction of only 0.7 and 1.4 CFU/ml was observed for γ-PGA-protected cells (Fig. 9). γ-PGA protected L. plantarum cells showed only 1.7 and 1.9 log reduction in viability after 20 days in comparison to a 7.6 log decrease in viability for unprotected cells (Fig. 10).
Figure 8.
Viability of L. rhamnosus in orange juice (free cells and cells coated with γ-PGA produced by B. subtilis and B. licheniformis). Results are means of three independent determinations. Bars correspond to standard deviation.
Figure 9.
Viability of L. paracasei in orange juice (free cells and cells coated with γ-PGA produced by B. subtilis and B. licheniformis). Results are means of three independent determinations. Bars correspond to standard deviation.
Figure 10.
Viability of L. plantarum in orange juice (free cells and cells coated with γ-PGA produced by B. subtilis and B. licheniformis). Results are means of three independent determinations. Bars correspond to standard deviation.
3.3.2. Change in organic acid concentration in orange juice
It was important to identify changes in concentration of organic acids, if any, when probiotic bacteria and γ-PGA are introduced to preserve the organoleptic properties and nutritional value of fruit juices. Organic acid analysis was performed using HPLC. It was seen that fresh orange juice contained 0.636 g/l of ascorbic acid (Table 3). This concentration dropped to 0.360 g/l for juice that was stored at 4 °C for 40 days. When L. rhamnosus was added to orange juice without γ-PGA, it was seen that the concentration of ascorbic acid in the juice reduced to 0.373 mg/l after 40 days. Likewise, when L. rhamnosus protected with γ-PGA produced by B. subtilis and B. licheniformis was added to orange juice, the concentration of ascorbic acid after 40 days was 0.393and 0.380 g/l, respectively. The concentrations of citric acid and malic acid in fresh orange juice were 13.91 g/l and 1.51 g/l respectively. After 40 days, orange juice with unprotected L. paracasei showed a slightly reduced concentration of citric acid (13.29 g/l). The citric acid concentration was further reduced slightly (12.31 and 12.00 g/l) for orange juice with γ-PGA-protected cells. The concentration of malic acid in orange juice with unprotected cells of L. plantarum did not differ from that of orange juice without cells after 40 days. Overall, it can be said that addition of γ-PGA-protected lactobacilli did not seem to change the organoleptic and nutritional properties of orange juice substantially. γ-PGA protection was shown to improve the viability of probiotic bacteria in orange juice and hence, it could be used as a non-dairy delivery platform for these bacteria.
Table 3.
Organic acids content of fresh and preserved orange juice as affected by the addition of probiotic L. rhamnosus, L. paracasei and L. plantarum protected with γ-PGA. Data represent the mean of 3 different readings ± standard deviation.
| Condition | Citric acid (g/l) | Malic acid (g/l) | Ascorbic acid (g/l) | |
|---|---|---|---|---|
| Fresh orange juice | 13.91 ± 1.20 | 1.51 ± 0.02 | 0.636 ± 0.02 | |
| Stored orange juice | 13.70 ± 1.10 | 1.75 ± 0.03 | 0.360 ± 0.03 | |
| Addition of L. rhamnosus | Unprotected | 13.20 ± 1.00 | 1.87 ± 0.00 | 0.373 ± 0.01 |
| Protected with γ-PGA of B. subtilis | 12.30 ± 0.98 | 1.23 ± 0.01 | 0.393 ± 0.00 | |
| Protected with γ-PGA of B. licheniformis | 11.60 ± 1.50 | 1.20 ± 0.01 | 0.380 ± 0.05 | |
| Addition of L. paracasei | Unprotected | 13.29 ± 1.20 | 1.92 ± 0.06 | 0.378 ± 0.04 |
| Protected with γ-PGA of B. subtilis | 12.31 ± 1.24 | 1.43 ± 0.06 | 0.398 ± 0.01 | |
| Protected with γ-PGA of B. licheniformis | 12.00 ± 1.00 | 1.50 ± 0.05 | 0.390 ± 0.03 | |
| Addition of L. plantarum | Unprotected | 13.50 ± 0.51 | 1.71 ± 0.04 | 0.378 ± 0.05 |
| Protected with γ-PGA of B. subtilis | 12.10 ± 0.54 | 1.30 ± 0.02 | 0.384 ± 0.07 | |
| Protected with γ-PGA of B. licheniformis | 11.90 ± 0.1 | 1.25 ± 0.07 | 0.381 ± 0.06 | |
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Ashiuchi M. In: Microbiology Monographs. Hamano Y., editor. Vol. 15. Verlag Berlin Heidelberg; NewYork: 2010. pp. 77–93. [Google Scholar]
- 2.Bajaj I., Singhal R. Bioresour. Technol. 2011;102:5551–5561. doi: 10.1016/j.biortech.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 3.Bhat A.R., Irorere V.U., Bartlett T., Hill D., Kedia G., Morris M.R., Charalampopoulos D., Radecka I. AMB Express. 2013;3:36–45. doi: 10.1186/2191-0855-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgain J., Gaiani C., Linder M., Scher J. J. Food Eng. 2011;104:467–483. [Google Scholar]
- 5.Ding W., Shah N. Int. Food Res. J. 2008;15:219–232. [Google Scholar]
- 6.Du G., Yang G., Qu Y., Chen J., Lun S. Process Biochem. 2005;40:2143–2147. [Google Scholar]
- 7.Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F.M. Anal. Chem. 1956;28:350–356. [Google Scholar]
- 8.Goto A., Kunioka M. Biosci. Biotechnol. Biochem. 1992;56:1031–1035. doi: 10.1271/bbb.56.1031. [DOI] [PubMed] [Google Scholar]
- 9.Granato D., Branco G., Nazzaro F., Cruz A., Faria J. Comp. Rev. Food Sci. 2010;9:292–302. doi: 10.1111/j.1541-4337.2010.00110.x. [DOI] [PubMed] [Google Scholar]
- 10.Ho G.H., Ho T.I., Hsieh K.H., Su Y.C., Line P.Y., Yang J., Yang K.H., Yang S.C. J. Chin. Chem. Soc. 2006;53:1363–1384. [Google Scholar]
- 11.Jagannath A., Raju P.S., Bawa A.S. Food Sci. Technol. 2010;43:1197–1203. [Google Scholar]
- 12.Ju W.T., Song Y.S., Jung W.J., Park R.D. Biotechnol. Lett. 2014;36:2319–2324. doi: 10.1007/s10529-014-1613-3. [DOI] [PubMed] [Google Scholar]
- 13.Kambourova M., Tangney M., Priest F.G. Appl. Environ. Microbiol. 2001;67:1004–1007. doi: 10.1128/AEM.67.2.1004-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kedia G., Hill D., Hill R., Radecka I. J. Nanosci. Nanotechnol. 2010;10:1–9. doi: 10.1166/jnn.2010.2614. [DOI] [PubMed] [Google Scholar]
- 15.Krumnow A., Sorokulova I., Olsen E., Globa L., Barbaree J., Vodyanoy V. J. Microbiol. Methods. 2009;78:189–194. doi: 10.1016/j.mimet.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Lee N.R., Go T.H., Lee S.M., Jeong S., Park G.T., Hong C.O., Son H.J. Saudi J. Biol. Sci. 2014;21:153–158. doi: 10.1016/j.sjbs.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee C.Y., Kuo M.I. Food Hydrocolloids. 2011;25:1034–1040. [Google Scholar]
- 18.Meng X., Stanton C., Fitzgerald G., Daly C., Ross Food Chem. 2008;106:1406–1416. [Google Scholar]
- 19.Otero M., Espeche M., Macias M.N. Process Biochem. 2007;42:1406–1411. [Google Scholar]
- 20.Prakash S., Tomaro-Duchesneau C., Saha S., Cantor A. J. Biomed. Biotechnol. 2011;3:1–12. doi: 10.1155/2011/981214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rushdy A.A., Gomaa E.Z. Ann. Microbiol. 2013;63:81–90. [Google Scholar]
- 22.Saarela M., Virkajärvi I., Alakomi H., Mattila-Sandholm T., Vaari A., Suomalainen T., Mättö J. J. Appl. Microbiol. 2005;99:1330–1339. doi: 10.1111/j.1365-2672.2005.02742.x. [DOI] [PubMed] [Google Scholar]
- 23.Santivarangkna C., Kulozik U., Foerst P. J. Appl. Microbiol. 2008;105:1–13. doi: 10.1111/j.1365-2672.2008.03744.x. [DOI] [PubMed] [Google Scholar]
- 24.Shih I.L., Van Y.T., Yeh L.C., Lin Y.N. Bioresour. Technol. 2001;78:267–272. doi: 10.1016/s0960-8524(01)00027-x. [DOI] [PubMed] [Google Scholar]
- 25.Shyu Y., Sung W.J. Mar. Sci. Technol. 2010;18(6):895–900. [Google Scholar]
- 26.Sung M.H., Park C., Kim C.J., Poo H., Soda K., Ashiuchi M. Chem. Rec. 2005;5:352–366. doi: 10.1002/tcr.20061. [DOI] [PubMed] [Google Scholar]
- 27.Vinderola G., Binetti A., Burns P., Reinheimer J. Front. Microbiol. 2011;2:1–6. doi: 10.3389/fmicb.2011.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Q., Xu H., Liang J., Yao J. Appl. Biochem. Biotechnol. 2010;160:386–392. doi: 10.1007/s12010-008-8320-2. [DOI] [PubMed] [Google Scholar]
- 29.Xu H., Jiang M., Li H., Lu D., Ouyang P. Process Biochem. 2005;40:519–523. [Google Scholar]
- 30.Yong X., Raza W., Yu G., Ran W., Shen Q., Yang X. Bioresour. Technol. 2011;102:7548–7554. doi: 10.1016/j.biortech.2011.05.057. [DOI] [PubMed] [Google Scholar]