Abstract
A plant regeneration protocol via somatic embryogenesis was achieved in cotyledon and leaf explants of Capsicum baccatum, when cultured on MS medium supplemented with various concentrations of 2,4-dichlorophenoxy acetic acid (2,4-D, 0.5–5.0 mg l−1) in combination with Kinetin (Kn, 0.5 mg l−1) and 3% sucrose. Various stages were observed during the development of somatic embryos, including globular, heart, and torpedo-stages. Torpedo stage embryos were separated from the explants and subcultured on medium supplemented with various concentrations of different plant growth regulators for maturation. Maximum percentage (55%) of somatic embryo germination and plantlet formation was found at 1.0 mg l−1 BA. Finally, about 68% of plantlets were successfully established under field conditions. The regenerated plants were morphologically normal, fertile and able to set viable seeds.
Abbreviations: BA, 6-benzyl adenine; 2,4-D, 2,4-dichlorophenoxyacetic acid; GA3, Gibberellic acid; Kn, kinetin; MS, Murashige and Skoog (1962) medium; TDZ, thidiazuron; 2,4,5-T, 2,4,5-trichlorophenoxy acetic acid; Picloram, 4-amino-3,5,6-tri chloropicolinic acid; NAA, α-naphthalene acetic acid; IAA, indole-3-acetic acid
Keywords: Cotyledon, Leaf, Pepper, Somatic embryos
1. Introduction
Chilli pepper (Capsicum spp.) is one of the important vegetables and spice crops around the world. The genus Capsicum comprises about 30 species, of which only five species Capsicum annuum L., C. frutescens Mill., Capsicum baccatum L., C. chinense Jacq., C. pubescens Ruiz and Pavon have been domesticated and currently cultivated [1]. Capsicum species are used widely in foods, drugs, and cosmetics based on their nutritional value, flavor, aroma, texture, pungency, and color, while some are cultivated as ornamental plants due to their bright glossy fruits of various colors, shape, and sizes [2]. C. baccatum L. is a cultivated type of pepper grown primarily in Central and South America, which has been studied by several groups for its in vitro regeneration capability [3], [4]. Successful plant regeneration has been reported in C. baccatum from different explants, such as cotyledon, hypocotyl, leaf, and shoot tip explants [5], [6], [7], [8], [9], and from callus derived from both cotyledon and hypocotyl explants [10] or half seed explants [11] via multiple shoot induction.
In the case of C. baccatum, it is essential to establish a reliable and reproducible regeneration system that will allow the incorporation of desirable genes through genetic transformation. Somatic embryogenesis is the only efficient way to regenerate complete plants from individual cells, and with this system, the risk of undesirable chimerical plants is minimal. Successful plant regeneration through direct or indirect somatic embryogenesis was reported in C. annuum L. and C. chinense Jacq. using diverse explants, such as intact seedling explants [12], cotyledonary leaves [13], cotyledons, hypocotyls [13], [14], [15], stem segments and shoot tips [16], fully expanded leaf explants [17] and hypocotyl explants [18]. The development of protocols to establish embryogenic cultures using more readily available material viz. seedling and mature explants would alleviate many of the problems to improve this crop through plant biotechnology methods [4]. In the present communication, we report reproducible plant regeneration protocol via somatic embryogenesis in C. baccatum that will be useful for the genetic improvement of this species.
2. Materials and methods
2.1. Plant material
Seeds of C. baccatum PI 260434 were obtained from Regional Plant Introduction Station, Griffin, USA, through National Bureau of Plant Genetic Resources (NBPGR), New Delhi. Seeds of C. baccatum soaked for 24 h in sterile distilled water, were surface sterilized with 0.1% HgCl2 for 3–5 min, rinsed several changes of sterile distilled water, and germinated aseptically on MS [19] (Murashige and Skoog 1962) basal medium. Cotyledon, hypocotyls (3-week-old), and leaf (6-week-old) explants were obtained from axenic seedlings and cultured on various media. Single explant was placed in each tube and the explants were placed as the abaxial side of explants in contact with medium.
2.2. Culture media and conditions
All media used in the present study were MS basal medium supplemented with Indole 3-acetic acid (IAA), α-naphthalene acetic acid (NAA), 4-amino-3,5,6-trichloropicolinic acid. (Picloram), 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxy acetic acid (2,4,5-T) (0.5–5.0 mg l−1) alone or in combination with either 6-benzyl adenine (BA) or Kinetin (Kn) (0.5 or 1.0 mg l−1) and various concentrations of sucrose (1.0–10.0%). The pH of the media were adjusted to 5.8, gelled with 0.8% Difco-bacto agar before autoclaving at 121 °C and 103.4 kPa for 15–20 min. All the cultures were incubated under 16 h photoperiod with cool white fluorescent lights (40–60 μmol m−2 s−2) at 25 ± 1 °C.
2.3. Somatic embryo induction
The MS basal media supplemented with various concentrations and combinations of 2,4-D (0.5–5.0 mg l−1) and Kinetin (0.5 mg l−1) were used for the induction of somatic embryogenesis for cotyledon (3 week-old) and leaf (6 week-old) explants.
2.4. Maturation
Torpedo stage (3–5 mm in length) embryos were separated from the explants and transferred to MS medium supplemented with Abscisic acid (ABA) (0.1, 0.5, 1.0 and 2.0 mg l−1), Gibberellic acid (GA3) (0.1, 0.5, 1.0 and 2.0 mg l−1), Kn (0.1, 0.5, 1.0 and 2.0 mg l−1), and BA (0.1, 0.5, 1.0 and 2.0 mg l−1) individually for maturation (cotyledonary stage). Somatic embryos (Torpedo stage) were transferred to growth regulator-free MS basal medium to evaluate the effect of the above growth regulators on maturation of somatic embryos from torpedo-stage stage to cotyledonary stage.
2.5. Conversion into plantlets
Cotyledonary stage embryos were separated and transferred to MS medium supplemented with Abscisic acid (ABA) (0.1, 0.5, 1.0 and 2.0 mg l−1), Gibberellic acid (GA3) (0.1, 0.5, 1.0 and 2.0 mg l−1) Kn (0.1, 0.5, 1.0 and 2.0 mg l−1), and BA (0.1, 0.5, 1.0 and 2.0 mg l−1) individually for conversion into plantlets (germination). Cotyledonary stage somatic embryos were transferred to growth regulator-free MS basal medium to evaluate the effect of the above growth regulators on germination and conversion into plantlets.
2.6. Acclimatization and transfer of plantlets to soil
The embryo-derived plantlets measuring ∼5 cm height were removed from agar medium and transferred to liquid medium containing MS basal salts for 8–10 d to harden the root system. Plants were subsequently transferred to autoclaved soil mixture containing coarse sand: soil: manure (1:2:1) and covered with polythene bags. The plantlets were irrigated with tap water as and when required. These plantlets were gradually acclimatized with an increase in the temperature from 25 to 28 °C and decrease in relative humidity from 80% to 30% for 7–10 d in a control growth chamber. After 4 weeks, the plants were transferred to earthen pots containing garden soil mixed with organic manure. When the plantlets showed signs of establishment in pots with the appearance of new leaves, the polythene bags were removed gradually for acclimatization to field conditions.
2.7. Cytological procedure
Chromosome counts were carried out for randomly selected plants regenerated from somatic embryos. Root tips of 5 plants were pretreated with 0.0002 M 8-hydroxyquinoline at 18–20 °C for 3 h, then fixed in ethanol:glacial acetic acid (3:1). They were dipped in 1 N HCl and 2% aceto-orcein (9:1) for about 2 h and then squashed with a drop of 45% acetic acid. Chromosomes were counted in 2–5 well-spread cells of each plant [20].
2.8. Data analysis
A minimum of 20 explants were cultured for each treatment. All the experiments were repeated thrice and the standard deviation and standard error were calculated. Data pertaining to the percentage of embryogenesis and mean number of embryos per culture were statically analyzed using a completely randomized block design and means were evaluated the level of significance using Duncan’s new multiple range test. Among the treatments, the average figures followed by similar letter are not significantly different at the P < 0.05% level.
3. Results and discussion
3.1. Somatic embryo induction
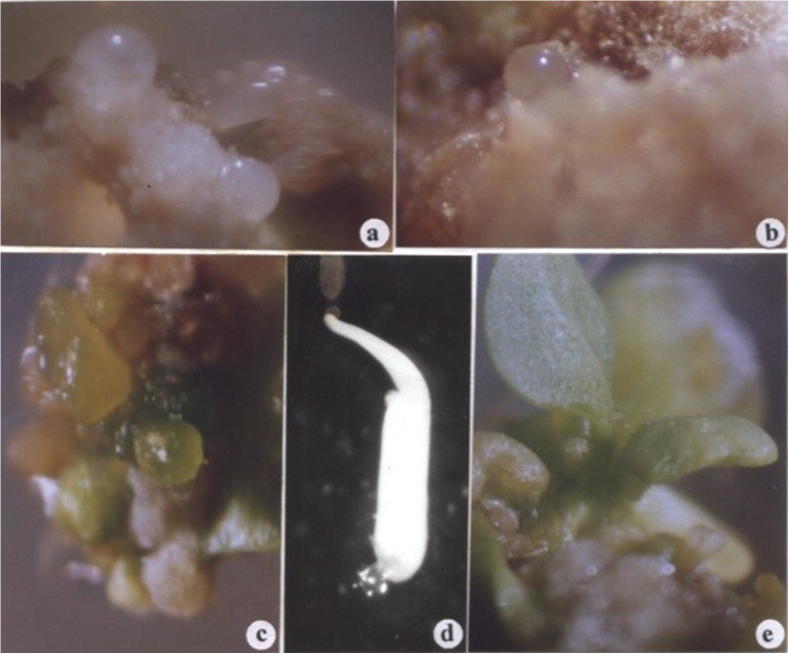
The present investigation demonstrates the induction of somatic embryogenesis from cotyledon and leaf explants cultured on various concentrations of 2,4-D in combination with Kinetin. Different explants such as, cotyledon, hypocotyl and leaf explants of C. baccatum were placed on medium containing broad concentrations of auxins (IAA, NAA, Picloram, 2,4,5-T and 2,4-D) and cytokinins (BA or Kn) alone and in combinations were examined separately in order to detect those mediating somatic embryogenesis. Callus formation at the cut ends of all explant types was observed on media containing either IAA, NAA, Picloram and 2,3,5-T in combination with Kn or Kn alone. Adventitious shoot bud formation was observed in cut ends of explants when cultured on medium containing BA alone or in combination with IAA, and NAA, whereas only callus formation was observed on medium containing BA in combination with Picloram, 2,4-D and 2,4,5-T. Somatic embryogenesis was observed from cotyledon and leaf explants, provided they were cultured on media supplemented with various concentrations of 2,4-D in combination with Kn. Small embryonic (globular) structures were observed at 15–20 d from the explants cultured on semi-solid medium containing different concentrations of 2,4-D with combination of Kinetin. The highest response in the induction of C. baccatum somatic embryos was obtained when the explants were cultured on MS medium supplemented with 2.0 mg l−1 2,4-D and 0.5 mg l−1 Kinetin for the first 15 d. Globular, glossy, white embryos started appearing from the surface of the cotyledon and leaf explants within 15–20 d after culturing, which further developed into dark green colored embryos. These structures were formed from the explants that initially were almost transparent, which then turned green color and finally dark brown. The formation of somatic embryos was asynchronous, so there were embryos in different stages of development on the same explants. Embryonic structures in the globular and heart-shaped stages were observed between 20 and 45 days. Development of somatic embryos appeared to progress through globular (Fig.1a, b), heart (Fig.1c), and torpedo stages (Fig.1c), of embryo development.
Figure 1.
Different developmental stages of direct somatic embryogenesis and plant regeneration in Capsicum baccatum. (a) Globular embryos developing directly from cotyledon explants (b) isolated single globular stage embryo (c) cluster of various developmental stages somatic embryos (d) cotyledonary stage of somatic embryo (unequal cotyledons) (e) dicotyledonary stage of somatic embryo.
In the present study, in C. baccatum somatic embryos were produced in the presence of 2,4-D and Kn, but not with 2,4-D alone. This suggests that in C. baccatum both 2,4-D and Kn (cytokinin) were necessary for somatic embryogenesis. In many plants, it is evident that cytokinins can exert promotive effect on somatic embryogenesis. Auxins and cytokinin are the main plant growth regulators involved in the regulation of plant cell cycling, division and differentiation, playing key roles in somatic embryogenesis of many species [21], [22], [23], [24] including Capsicum species [13], [14], [18], [25]. In the present study, asynchronous development of somatic embryos was noticed as reported earlier in C. annuum [25], [26], [27], [28] and C. chinense [29].
3.2. Effect of sucrose concentration on somatic embryogenesis
A combination of 2.0 mg l−1 2,4-D with 0.5 mg l−1 Kn and 3% sucrose was found to be the most efficient medium composition to produce maximum number of somatic embryos from cut surfaces of cotyledon and leaf explants (Table 1). Leaf explants showed the maximum percentage of somatic embryogenesis when compared to cotyledon explants. The concentration of sucrose in the induction medium has been shown to be quite important for the induction of somatic embryogenesis in several plants including pepper [25], [26]. To evaluate the effect of sucrose on the induction and development of somatic embryogenesis, the explants were cultured on medium supplemented with different concentrations of sucrose (1.0–10.0%). The concentrations of sucrose in the medium were also found to have a profound influence on somatic embryogenesis in several plants [21], [22], [23], [24] including C. annuum [25], [26], [27], [28] and C. chinense [29]. Sucrose at 3% was found to be optimum for the induction and development of somatic embryos in cotyledon and leaf explants of C. baccatum. As sucrose concentration increased, the development of somatic embryos was inhibited. Profuse callus formation was observed on media supplemented with high concentrations of sucrose (4.0–10.0%). In the present study, in C. baccatum the induction of somatic embryogenesis was observed with 3% sucrose, our results are in agreement with other species of Capsicum; C. annuum [16] and in C. chinense [13], [14], [15], [18], while high concentrations of sucrose were found more effective in the induction of somatic embryogenesis in C. annuum [17], [25], [26], [27], [28].
Table 1.
Effect of different concentrations of 2,4-D and Kn on somatic embryogenesis from cotyledon and leaf explants of Capsicum baccatum L.
| Growth regulators concentration (mg l−1) | Cotyledon |
Leaf |
|||
|---|---|---|---|---|---|
| % cultures showing | Mean No. of embryos | % cultures showing | Mean No. of embryos | ||
| 2,4-D | Kn | Embryogenesis | Per explant | Embryogenesis | Per explant |
| 0.5 | 0.5 | 33.3 ± 1.28f | 2.4 ± 0.43gh | 53.6 ± 1.62f | 6.2 ± 0.72ef |
| 1.0 | 0.5 | 76.4 ± 1.54bc | 6.2 ± 0.73e | 77.2 ± 1.28de | 8.6 ± 0.53cd |
| 1,5 | 0.5 | 78.2 ± 1.68bc | 8.3 ± 0.57c | 92.3 ± 1.54ab | 14.8 ± 0.83bc |
| 2.0 | 0.5 | 86.6 ± 1.58a | 12.4 ± 0.38a | 96.5 ± 1.92ab | 18.3 ± 0.58a |
| 2.5 | 0.5 | 81.1 ± 1.92bc | 9.2 ± 0.84b | 89.2 ± 1.38bc | 11.4 ± 0.68bc |
| 3.0 | 0.5 | 76.2 ± 1.38bc | 7.3 ± 0.92d | 83.6 ± 1.42cd | 8.9 ± 0.43cd |
| 4.0 | 0.5 | 68.2 ± 1.46d | 4.8 ± 0.63f | 72.2 ± 1.46de | 5.4 ± 0.65ef |
| 5.0 | 0.5 | 42.8 ± 1.72e | 1.8 ± 0.78gh | 54.9 ± 1.68f | 2.8 ± 0.82g |
Each experiment had 20 replications and was repeated thrice.
Mean ± SE within a column followed by the same letters are not significantly different at P < 0.05 according to DNMRT.
3.3. Embryo maturation, germination and conversion into plantlets
A critical step in somatic embryogenesis is the maturation process. The efficient conversion of cotyledonary somatic embryos into plantlets is also an important step for whole plant regeneration and mass proliferation. The globular and heart-shaped were seen more frequently than torpedo-shaped stages. It was necessary to transfer the somatic embryos to the germination media within 20–30 d, otherwise dedifferentiation set in, resulting in callus formation. Torpedo embryos were separated from explants subcultured onto MS media supplemented with various concentrations of ABA or GA3 or BA or Kn for maturation and germination. Subculturing was carried out every 30 days. Among the different treatments tested, medium supplemented with BA at 1.0 mg l−1 was found to be most effective for maturation and conversion to whole plantlets (Table 2). Under these conditions, embryos continued germinating, and shoots started developing within 20 d. The torpedo shape embryos give different types of fused embryos, absent cotyledons, a single cotyledon (Fig.1d), incorrectly formed cotyledons, lack of leaf primordia and deformations in the apical meristem. The apical meristem of somatic embryos failed to elongate during germination, making somatic embryos unable to resume normal vegetative development. When torpedo-stage embryos remained attached to the explants without subculture on cytokinin (BA) containing medium they were unable to convert into cotyledonary stage and germinate to give complete plants. Maximum percentage (55%) of torpedo stage somatic embryos converted into cotyledonary stage (Fig.1e). Cotyledonary stage embryos were further transferred to MS basal supplemented with different concentrations of various plant growth regulators for germination and conversion into plantlets. The germination and conversion of somatic embryos from two different explants were significantly different (Table 2). Torpedo stage somatic embryos derived from leaf explants were efficient in conversion into cotyledonary stage and germination than the somatic embryos derived from cotyledon explants. A maximum number of plantlets were obtained after 80–90 d of initial culture of cotyledon and leaf explants. Taking these results into consideration, it may be noted that cytokinins have stronger enhancing effects on early and later stage somatic embryonic development in C. baccatum.
Table 2.
Conversion and germination of Torpedo stage somatic embryos from cotyledon and leaf explants of Capsicum baccatum L.
| Growth regulator concentration (mg l−1) | Cotyledon |
Leaf |
||||||
|---|---|---|---|---|---|---|---|---|
| BA | No. of Torpedo stage embryos | Mean No. of embryos converted into cotyledonary stage | Mean No. of embryos germinated | Mean No. of plantlets recovered | No. of Torpedo stage embryos | Mean No. of embryos converted into cotyledonary stage | Mean No. of embryos germinated | Mean No. of plantlets recovered |
| 0.1 | 106 | 14.3c | 6.6cd | 2.3bc | 103 | 18.3d | 8.8cd | 4.6c |
| 0.5 | 104 | 15.6c | 8.6b | 3.3b | 106 | 23.6c | 14.6b | 6.3b |
| 1.0 | 103 | 28.6a | 15.6a | 7.3a | 102 | 40.3a | 22.3a | 12.3a |
| 2.0 | 104 | 25.5b | 7.6bc | 3.6b | 107 | 28.6b | 9.3cd | 6.6b |
Each experiment had 20 replications and was repeated thrice.
Mean ± SE within a column followed by the same letters are not significantly different at P < 0.05 according to DNMRT.
The transition of globular to polar embryogenesis is a critical step in embryogenesis. Somatic embryos did not progress globular stage to other advanced stages, or many of the embryos that reach the torpedo and cotyledonary stages showed a wide range of malformations and did not convert to plantlets. A high frequency of deformed somatic embryos restricted the germination and conversion into normal plantlets in C. baccatum, similar observations were also made in C. annuum [25] and C. chinense [13], [14], [26]. Steinitz et al. [25] suggested an inhibitory role of auxins in apical meristem development of pepper somatic embryos. This phenomenon could be provoked by genetic, epigenetic, or physiological factors [14]. In C. baccatum, the presence of an exogenous cytokinin in the medium is required for maturation and germination of somatic embryos into normal plantlets. Thus, the removal of the somatic embryos from the auxin-containing medium must be crucial to normal cotyledon development [26]. The positive effect of cytokinin on somatic embryos maturation and germination as described here is consistent with the earlier observations made with C. annuum [17], [26], [27], [28] and C. chinense [14], [29]. The supplementation of cytokinins during the histo-differentiation phase can compensate for the detrimental effects of auxins on the meristem development [30]. In the present investigation, cytokinins were found to be effective plant growth regulators for the induction, maturation and germination of somatic embryos in C. baccatum. The persistence of this behavior in C. baccatum also contributes to a marked reduction in the rates of germination and conversion of somatic embryos into normal plants as observed in C. annuum [26], [27].
3.4. Plantlet hardening and acclimatization
The complete plantlets obtained after germination were found to be diploid (2n = 24) and devoid of any chromosomal aberrations. The establishment of in vitro grown plantlets were transferred to pots containing soil mixture (coarse sand: soil: manure in the 1:2:1 ratio) and maintained in the controlled environment for 10–15 d for acclimatization (Fig.2a). The regenerated plants showed 68% survival in the field after transplantation (Fig.2b). The regenerated plants did not show any detectable variation in morphology or growth characteristics as compared to the respective donor plants and they flowered normally with 100% pollen viability and able to set viable seeds. No cytological anomaly was observed in our studies, indicating a genotypic stability of somatic embryo derived plants.
Figure 2.
Acclimatization and hardening of Capsicum baccatum L. plants derived from somatic embryos. (a) Potted plantlets after 3 weeks, (b) complete plant with normal fruits.
4. Conclusions
In the present study, we have shown that C. baccatum could be successfully regenerated in vitro via somatic embryogenesis from cotyledon and leaf explants. Somatic embryos derived from leaf explants are more pronounced than the cotyledon explants in induction, maturation and conversion into complete plantlets. The protocol developed will be useful for rapid in vitro vegetative propagation and genetic transformation studies of this species. Further experiments, aimed at fine tuning the C. baccatum somatic embryogenesis system and encouraging more cells within an explant to follow the path of embryogenesis, are currently being pursued.
Acknowledgements
We thank Director, National Bureau of Plant Genetic Resources (NBPGR), New Delhi, India for providing seed material. Thanks are also due to Dr. B. Steinitz, Department of Plant Genetics, Institute of Plant Sciences, A.R.O., The Volcani Center, Bet Dagan, Israel for helpful comments on the manuscript.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Csillery G. Acta Agron. Hung. 2006;54:151–166. [Google Scholar]
- 2.Bosland P.W., Votava E.J. second ed. CABI; Cambridge, MA, USA: 2012. Peppers: Vegetables and Spice Capsicums. [Google Scholar]
- 3.Ochoa-Alejo N., Ramirez-Malagon R. In Vitro Cell. Dev. Biol. Plant. 2001;37(6):701–729. [Google Scholar]
- 4.Kothari S.L., Joshi A., Kachhwaha S., Ochoa-Alejo N. Biotechnol. Adv. 2010;28:35–48. doi: 10.1016/j.biotechadv.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Sun Z., Wang M. Capsicum Newsletter. 1990;8–9:42. [Google Scholar]
- 6.Fari M., Csanyi M., Mityko J., Peredi A., Szasz A., Csillag A. Hort. Sci. (Hungary) 1995;27:9–15. [Google Scholar]
- 7.Christopher T., Rajam M.V. Plant Cell Tissue Organ Culture. 1996;46:245–250. [Google Scholar]
- 8.Venkataiah P., Christopher T., Subhash K. Capsicum Eggplant Newsletter. 2001;20:68–71. [Google Scholar]
- 9.Venkataiah P., Christopher T., Subhash K. Sci. Hort. 2006;107:117–122. [Google Scholar]
- 10.Subhash K., Sumalini K. Capsicum News Letter. 1990;8–9:40–41. [Google Scholar]
- 11.Valera-Motero L.L., Phillips G.C. In Vitro Cell. Dev. Biol. Plant. 2005;41:470–476. [Google Scholar]
- 12.Kaparakis G., Alderson P.G. J. Hort. Sci. Biotechnol. 2002;77(2):186–190. [Google Scholar]
- 13.Lopez-Puc G., Canto-Flick A., Barredo-Pool F., Zapata-Castillo P., Montalvo-Peniche M., Barahona-Perez F., Santana-Buzzy N. HortScience. 2006;41(6):1–7. [Google Scholar]
- 14.Santana-Buzzy N., Lopez-Puc G., Canto-Flick A., Barredo-Pool F., Balam-Uc E., Aviles-Vinas S., Solıs-Marroquın D., Lecona-Guzman C., Bello-Bello J., Gomez-Uc E., Mijangos-Cortes J.O. HortScience. 2009;44(1):113–118. [Google Scholar]
- 15.Aviles-Vinas S.A., Lecona-Guzman C.A., Canto-Flick A., Lopez-Erosa S., Santana-Buzzy N. Plant Biotechnol. Rep. 2013;7:277–286. [Google Scholar]
- 16.Khan H., Siddique I., Anis M. Biol. Planta. 2006;50(4):789–792. [Google Scholar]
- 17.Kintzois S., Drossopoulos J.B., Lymperopoulos Ch. Plant Cell Tissue Organ Culture. 2001;67:55–62. [Google Scholar]
- 18.Zapata-Castillo Y.P., Canto-Flick A., Lopez-Puc G., Solıs-Ruiz A., Barahona-Perez F., Santana-Buzzy N. HortScience. 2007;42(2):1–5. [Google Scholar]
- 19.Murashige T., Skoog F. Physiol. Plant. 1962;15:473–497. [Google Scholar]
- 20.Qin X., Rotino G.L. Plant Cell Tissue Organ Culture. 1995;41:145–149. [Google Scholar]
- 21.Feher A., Pasternak T.P., Dudits D. Plant Cell Tissue Organ Culture. 2003;74(3):201–228. [Google Scholar]
- 22.Jimenez V.M. Plant Growth Reg. 2005;47:91–110. [Google Scholar]
- 23.You C.R., Fan T.J., Gong X.Q., Bian F.H., Liang L.K., Qu F.N. Plant Cell Tissue Organ Culture. 2011;107:233–242. [Google Scholar]
- 24.Pinto D.L.P., de Almeida A.M.R., Rego M.M., da Silva M.L., de Oliveira E.L., Otoni W.C. Plant Cell Tissue Organ Culture. 2011;107:521–530. [Google Scholar]
- 25.Steinitz B., Kusek M., Tabib Y., Paran I., Zelcer A. In Vitro Cell. Dev. Biol. Plant. 2003;39:296–303. [Google Scholar]
- 26.Binzel M.L., Sankhala N., Joshi S., Sankhla D. Plant Cell Rep. 1996;15:536–540. doi: 10.1007/BF00232989. [DOI] [PubMed] [Google Scholar]
- 27.Kaparakis G., Alderson P.G. J. Plant Growth Reg. 2008;27:110–114. [Google Scholar]
- 28.Harini I., Sita G.L. Plant Sci. 1993;89:107–112. [Google Scholar]
- 29.Solis-Ramos L.Y., Nahuath-Dzib S., Andrade-Torres A., Barredo-Pool F., Gonzalez-Estrada T., Castano de la Serna E. Biologia. 2010;65(3):504–511. [Google Scholar]
- 30.Merkle S.A. In: Bajaj Y.P.S., editor. vol. 30. Springer Verlag; Berlin: 1995. pp. 388–403. (Somatic Embryogenesis and Synthetic Seeds I. Biotechnology in Agriculture and Forestry). [Google Scholar]