Abstract
Gold nanoparticles have many applications in biomedical field. Improving delivery of anticancer agents to tumors using nanoparticles is one of the most promising research arenas in the field of nanotechnology. Eco-friendly gold nanoparticles synthesis was studied using marine bacteria Enterococcus sp. The nanoparticle synthesis started at 2 h of incubation time was identified by the formation of ruby red in the reaction mixture and SPR band centered at 545 nm. XRD shows that the strong four intense peaks indicate crystalline nature of nanoparticles. Morphology of nanoparticles analyzed by TEM shows that they are mostly spherical in shape with size ranging from 6 to 13 nm. EDX supports the presence of gold in the synthesized nanoparticles. FTIR reveals the active functional groups in the culture supernatant interaction with gold nanoparticles. As a result synthesized stable gold nanoparticles show more significant anticancer activity against HepG2 and A549 cells at 100 μg concentration of nanoparticles. This synthesis approach is simple, large scaled up a new door for development of targeted anticancer activity using gold nanoparticles and is novel in biomedical applications.
Keywords: Biosynthesis, Gold nanoparticles, Characterization, Anticancer, Sequencing
1. Introduction
Noble metal nanoparticles are having distinct properties compared to other metallic nanoparticles due to their optical, electronic and molecular recognition properties. Noble nanomaterials have large optical field enhancements due to the resonant oscillation of their free electrons in the presence of light. Because of these properties noble metal nanoparticles are greatly utilized in optical, imaging, sensors, cosmetics cancer therapy, and drug delivery [40], [28]. Among these noble metals, gold and gold nanoparticles are precious, inert and not easily oxidized when exposing to oxygen or highly acid environments [3]. Gold nanoparticles exhibit different colors such as red, blue or other colors depending their size, shape and amount of aggregation [3], [4]. These visible colors reflect the oscillations of conduction band electrons at appropriate wavelengths [29]. Gold nanoparticles are highly stable, sensitive and have higher levels of consistency. Due to these properties they are greatly admired and utilized for biomedical applications such as drug delivery, imaging, photothermal therapy and immunochromatographic detection of pathogen in food and clinical specimen [17]. Recently they are used in applications such as detection of heavy metal ions [47] and target delivery of therapeutic agent [13]. Various methods are adopted for synthesis and assembly of gold nanoparticles such as physical, chemical and biological methods. Among these methods, using biological systems is an eco-benign, less toxic, clean, and less time consuming called as green chemistry compared with other synthesis methods [8]. The biological systems for nanoparticle synthesis, microorganisms play an important role and act as living nano factory for the production of functional biomolecules [19], [25]. Microorganisms have been successfully implemented for the synthesis, nucleation, and assembly of nanomaterials. Many microorganisms, both unicellular and multicellular can produce nanoparticles either intracellular or extracellular routes. In extracellular synthesis, the cell filtrate could be used to achieving better control over size and polydispersity of nanoparticles. This synthesis method of nanoparticles using cell filtrate was more beneficial than the intracellular synthesis method [27]. Gold nanoparticles synthesized by several bacteria are Pseudomonas aeruginosa [18], Cyanobacteria [21], [22], Rhodopseudomonas capsulata [15], Escherichia coli DH5α [8], Bacillus subtilis [41], and Stenotrophomonas maltophilia [31].
Cancer is an abnormal growth of tissue or cells exhibiting uncontrolled division autonomously resulting in a progressive increase in the number of cell divisions [20]. It causes significant morbidity and mortality and is a major health problem worldwide [5] and there are increasing demands for anticancer therapy [44]. The fight against cancer is difficult particularly in the development of therapies for severely multiplying tumors. Chemotherapy is available for treatment of cancer but still it exhibits low specificity and is restricted by dose limiting toxicity. It is a challenge to find the therapy and drugs for the treatments of various types of cancer. So, conventional methods require the combination of controlled released technology and targeted drug delivery which is more effective and less harmful. Nanomaterials are expected hopefully to revolutionize cancer diagnosis and therapy [37]. Recently silver nanoparticles used in anticancer therapy for several types of cancer are Hep2 cell line [37], [7], HT-29 cell lines [6], Vero cell line [35] and breast cancer line MCF-7 [43]. In the present study we investigate gold nanoparticle synthesis using bacteria by extracellular route and their anticancer activity against liver cancer cell lines (HepG2) and lung cancer cell lines (A549).
2. Materials and methods
Chemicals such as Chloroauric acid (HAuCl4), 3-(4,5-di-methyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), and Dimethyl sulfoxide (DMSO) were purchased from Sigma Aldrich.
2.1. Isolation and identification of marine bacteria
The marine water sample was collected from Kanyakumari coastal area, Kanyakumari district, South Tamilnadu, India in a clean sample container. Isolation of marine bacteria was carried using the “Serial Dilution Method”. The isolated bacterium is stored in nutrient agar plates. The morphological and physiological characterization of the marine bacterial isolate was performed according to the methods described in Bergey’s manual of determinative bacteriology. The 16S rRNA sequencing was performed for the identification of the bacteria.
2.2. Extracellular biosynthesis of gold nanoparticles
The extracellular production of gold nanoparticles has wider applications in various fields. In this method, 100 ml of nutrient broth was prepared inoculated with the Enterococcus sp. and then incubated for 24–48 h at room temperature in the orbital shaker. After that the culture solution was centrifuged at 7500 rpm for 15 min. Then the supernatant was taken into a clean 250 ml conical flask and 1 mM gold chloride was added to 100 ml culture of supernatant. Then the mixture was incubated in the orbital shaker for the synthesis of gold nanoparticles. The color change was observed visually and photographs were taken. After that culture solution was centrifuged for 7500 rpm for 15 min. Then the pellet was collected and it was dried in a hot air oven the scrap and made into a powder form, it is used for the characterization of the particular nanoparticles. The powder was used for further characterization studies.
2.3. Characterization of gold nanoparticles
Extracellularly bioreduced gold ions by the culture supernatant of Enterococcus sp. were preliminarily analyzed using Double beam UV–vis spectrophotometer (Perkin Elmer, Singapore) at different wavelengths. Upon further characterization studies the synthesized gold nanoparticles were obtained by repeated centrifugation at 8000 rpm for 15 min and dried at room temperature. Crystalline structure of dried nanoparticles was characterized by XRD (Bruker, Jermany, model: D8Advance) and morphological characters such as size, shape and distribution were analyzed using the Transmission Electron Microscope (Hitachi, Model: S-3400N) with SAED. Presence of elemental gold was confirmed by EDX. The functional biomolecules associated with gold nanoparticles were characterized by FT-IR (BrukerOptik GmbH Model No – Tensor 27). The dried nanoparticle sample was ground with KBr pellets and measured at the wavelength ranging from 4000 to 400 cm−1.
2.4. Anticancer activity of gold nanoparticles against HepG2 and A549 cell lines
The HepG-2 and A549 cells were plated separately in 96 well plates at a concentration of 1 × 104 cells/well. After 24 h, cells were washed twice with 100 μl of serum-free medium and starved for an hour at 37 °C. After starvation, cells were treated with different concentrations of gold nanoparticles (1, 10, 25, 50, 100 μg/ml) for 24 h. At the end of the treatment period the medium was aspirated and serum free medium containing MTT (0.05 mg/ml) was added and incubated for 4 h at 37 °C in a CO2 incubator. The inhibitory concentration value (IC) of the gold nanoparticles was identified for normal untreated cell line. The MTT containing medium was then discarded and the cells were washed with PBS (200 μl). The crystals were then dissolved by adding 100 μl of DMSO and this was mixed properly. Spectrophotometrical absorbance of the purple blue formazan dye was measured in a microplate reader at 570 nm (Biorad 680). Cytotoxicity was determined using Graph pad prism5 software. The assay is based on the reduction of soluble yellow tetrazolium salt to insoluble purple formazan crystals by metabolically active cells. Only live cells are able to take up the tetrazolium salt. The enzyme (succinate dehydrogenase) present in the mitochondria of the live cells is able to convert internalized tetrazolium salt to formazan crystals, which are purple in color.
3. Results and discussion
Pure colonies were obtained and characterized as Enterococcus sp. based on the results described in Bergey’s manual of determinative bacteriology [16].
3.1. Phylogenetic analysis
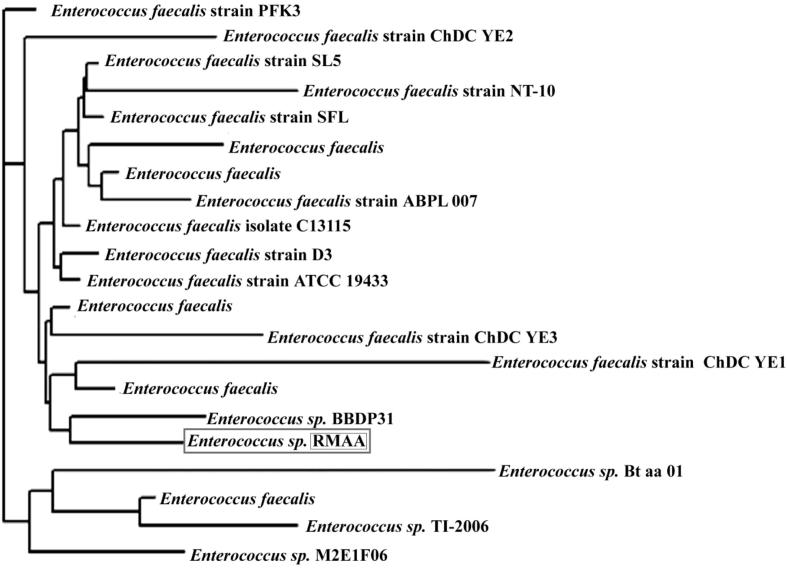
In this study, 16S rRNA genes of different species coming under Enterococcus genus (different strains of a species) were obtained from GenBank; however totally 20 species were considered as homologous sequences and selected on the basis of high sequence identity (%) for phylogenetic analysis. The cumulative results from a limited number of studies to date suggest that 16S rRNA gene sequencing provides genus identification in most cases (>90%) but less so with regard to species (65–83%), with regard to species from 1% to 14% of the isolates remaining unidentified after testing. Fukushima et al. [12] reported phylogenetic analysis using the 16S rRNA gene sequence will be able to classify some bacteria. For analyzing this hypothesis and results we have performed the phylogenetic analysis on the basis of 16S rRNA genes. Randomly, totally 20 different strains which come under the genus of Enterococcus were used for tree reconstruction. Fig. 1 shows clearly that our strain RMAA had an own branch with Enterococcus sp. with 96% bootstrap value. It is supported by the maximum sequence identity 99%. Interestingly, the strain RMAA had an own branch with Enterococcus sp. with 88% bootstrap support. It is supported by the maximum sequence identity 97%. It’s assuring that strains RMAA had a maximum identity and phylogenetically cluster with Enterococcus sp. RMAA. It confirms that RMAA belongs to Enterococcus sp.
Figure 1.
Phylogenetic tree is based on the 16S rRNA gene sequence of bacteria.
3.2. Biosynthesis of gold nanoparticles using Enterococcus sp.
3.2.1. Visual inspection
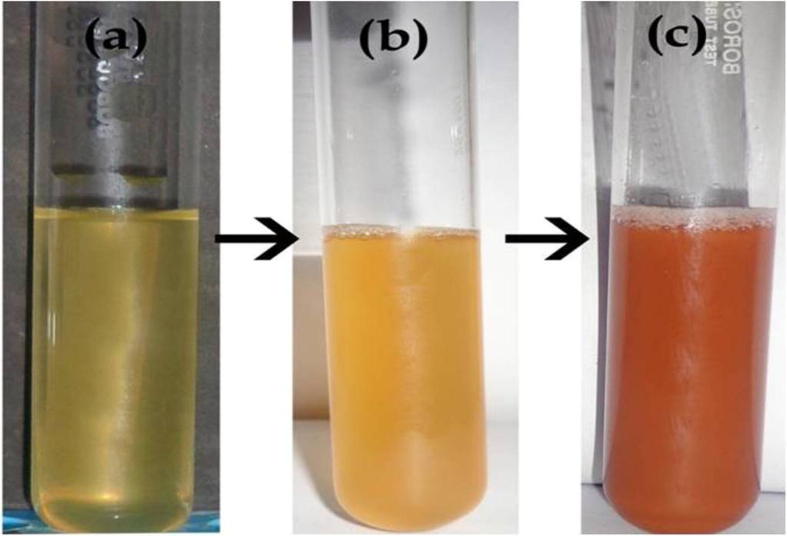
The visual inspection is the preliminary analysis process to confirm the synthesis of gold nanoparticles using the Enterococcus sp. Fig. 2a shows the culture supernatant of Enterococcus sp. Fig. 2b shows the 1 h incubation of gold chloride with Enterococcus sp. Fig. 2c exhibits the 24 h incubation of gold chloride with Enterococcus sp. The formation of ruby red color observed after 1 h reveals the synthesis of gold nanoparticles using the Enterococcus sp. The intensity of ruby red color was increased with increased incubation time from 2 to 12 h. After that the appearance of dark ruby red color indicates the gold nanoparticle synthesis process is completed. The color of the solution is changed from yellow to ruby red for gold due to the excitation of surface plasmon vibrations in the gold nanoparticles [33]. Several hydroquinones with excellent redox properties could act as electron shuttle in metal reductions [9]. Thus, it was evident that electron shuttles or other reducing agents released by the bacterial strains were capable to reduce the ions to nanoparticles [1].
Figure 2.
Biosynthesis of gold nanoparticles using Enterococcus sp. (a) Bacterial culture (b) HAuCl4 with bacterial culture initial at 1 h incubation (c) final color 24 h incubation.
3.2.2. UV–vis spectroscopy
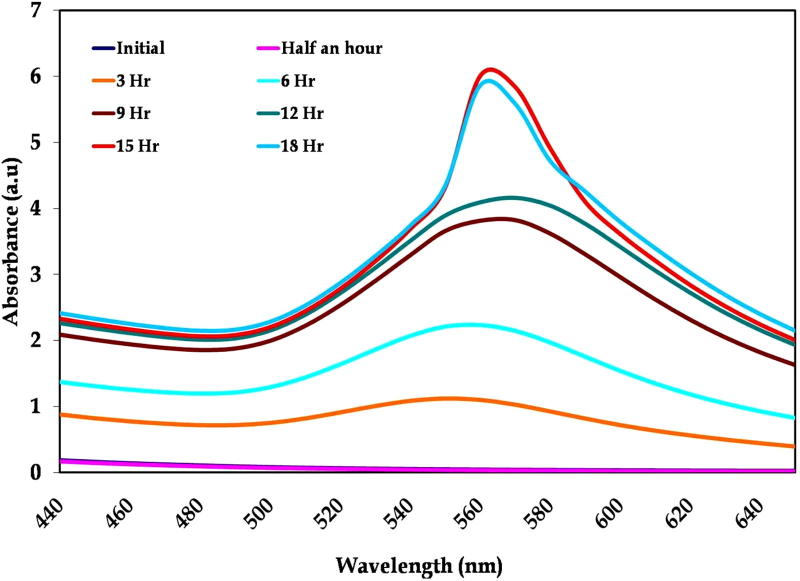
The collection of excitation present in the surface of the nanoparticles known as surface plasmon resonance was used for the confirmation of synthesis, size and shape of the nanoparticles [10]. The UV–vis spectra recorded from the Enterococcus sp. with gold ion reaction mixture at different reaction times are plotted in Fig. 3. The spectra show a well-defined surface plasmon band centered at around 545 nm for gold nanoparticles, which is the characteristic absorbance of gold nanoparticles [14]. The color intensity of gold is raised while increasing the incubation time and it reveals the formation of an increased number of nanoparticles. Observation of this peak, assigned to a surface plasmon is well-documented for various metal nanoparticles with sizes ranging from 2 nm to 100 nm [38], [39]. The stability of nanoparticles is mainly based on the capping agents like enzymes and proteins present in the bacteria [11].
Figure 3.
Absorbance spectra of biosynthesis of gold nanoparticles using marine bacteria Enterococcus sp.
3.2.3. X-ray diffraction
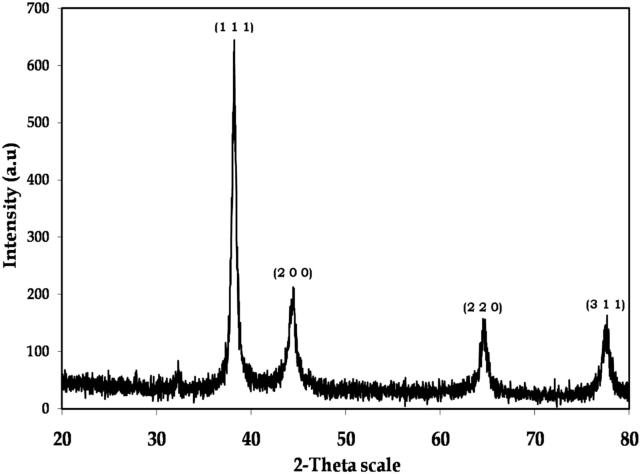
The shape, size, stability (high and low), visible aggregation and precipitation of the nanoparticles synthesized from bacterial isolate were identified through XRD analysis [39]. The XRD pattern of gold nanoparticles is shown in Fig. 4. The XRD pattern shows intense peaks in the whole spectrum of 2θ values, ranging from 20° to 80°. It is important to know the exact nature of the formed gold nanoparticles can be deduced from the XRD spectrum of the sample. XRD spectra of pure crystalline gold structures have been published by the Joint Committee on Powder Diffraction Standards (file nos. 04-0784). A comparison of our XRD spectrum with the standard sample confirmed that the gold nanoparticles had been formed in the form of crystalline. The strong peaks at 2θ values of 38.21°, 44.44°, 64.61° and 77.63° correspond to (1 1 1), (2 0 0), (2 2 0) and (3 1 1) set of planes for fcc gold nanoparticles. Thus, the XRD pattern clearly demonstrates that the gold nanoparticles synthesized by the bio-method are crystalline in nature.
Figure 4.
Representative XRD pattern of gold nanoparticles formed after reaction of Enterococcus sp. with HAuCl4.
3.2.4. Transmission Electron Microscope
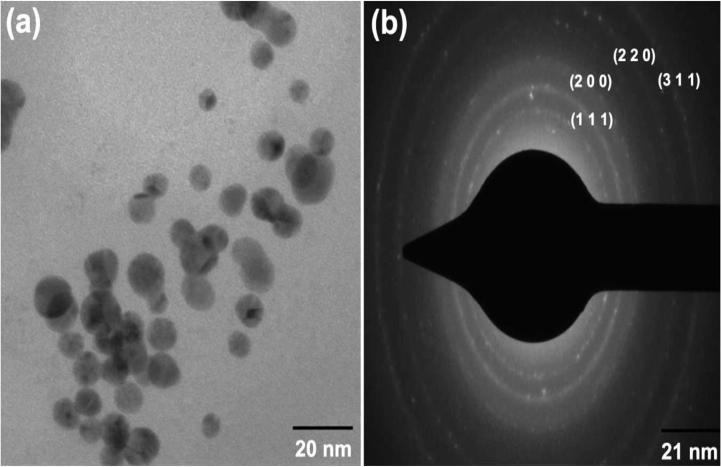
The structure and size distribution of gold nanoparticles were analyzed using TEM (Fig. 5a). In general, the gold nanoparticles were mostly spherical in shape with size ranging from 6–13 nm. Most of the nanoparticles were aggregates with only a few of them scattered, as observed under this microscope. The gold nanoparticles are uniformly distributed with spherical in shape and smaller nanoparticles were found in the average size of 10 nm. This falls nearer to the range of gold nanoparticles produced using other microbes like Shewanella algae, Bacillus sp., Aspergillus flavus [46], [32], [45]. The difference in size and shape of nanoparticles synthesized by bio-method is due to the different growth phases of particles. SAED pattern shows (Fig. 5b) four circular rings corresponding to (1 1 1), (2 0 0), (2 2 0) and (3 1 1) were documented to confirm the synthesized gold NPs are crystalline in nature.
Figure 5.
HR-TEM images of gold nanoparticles formed by bacteria (a) 50 nm scale and (b) selected area diffraction pattern.
3.2.5. Energy dispersive X-ray analysis
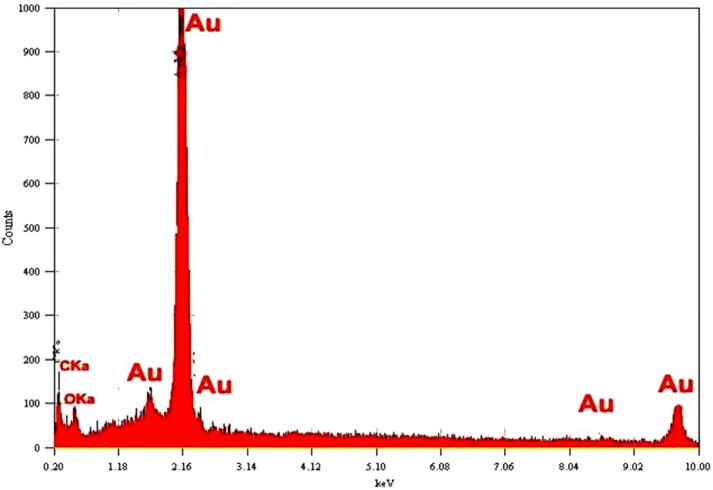
The Fig. 6 shows the energy dispersive X-ray analysis (EDX) of Enterococcus sp. derived gold NPs reveal the strongest signal in the gold region and confirm the formation of Au nanoparticles. Metallic gold nanocrystals generally show typical strong optical absorption peak was observed due to surface plasmon resonance [24].
Figure 6.
EDX spectrum for gold nanoparticles synthesized from marine bacteria Enterococcus sp.
3.2.6. Fourier Transmittance Infrared Spectroscopy
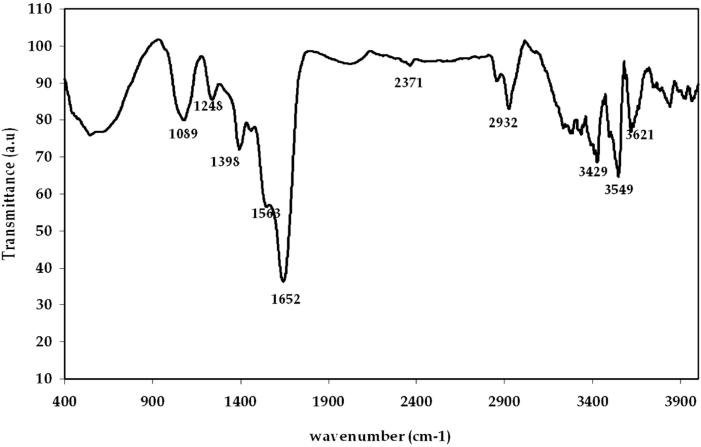
FTIR spectrum of gold nanoparticles suggests that the biological molecules like proteins and enzymes could possibly execute the function for the formation and stabilization of the gold nanoparticles using the Enterococcus sp. [11]. The gold nanoparticles synthesized from Enterococcus sp. (Fig. 7) having the peak at 3621 cm−1 and 3429 cm−1 correspond to O—H stretching group of phenols and alcohol. The band at 3549 cm−1 shows O—H stretching of carboxylic groups, the band at 2932 cm−1 indicates C—H stretching of alkanes. The strong peak at 1652 cm−1 shows C O stretching of amides. The intense peak at 1398 cm−1 corresponds to C—H in plane bend of alkenes. The weak band at 1248 cm−1 and 1089 cm−1 shows that C—N stretching of aliphatic amines and C—O stretching of carboxylic, amines respectively. Lin et al. previously reported that the hydroxyl groups (O—H) have a stronger ability to bind with gold ions [23].
Figure 7.
FT-IR spectra of biologically synthesized gold nanoparticles using the bacteria Enterococcus sp.
The exact mechanism leading to the reduction of metal ions is yet to be elucidated for marine bacteria Enterococcus sp. The previous reports suggest that the variations in the peak positions indicate that the proteins responsible for synthesis of gold nanoparticles and they can bind to gold nanoparticles through free amine groups in the proteins [33]. That the amine group of protein can bind to nano gold reveals that the amine group might be involved in reduction and stabilization of synthesized nanoparticles.
3.2.7. Anticancer activity of gold nanoparticles
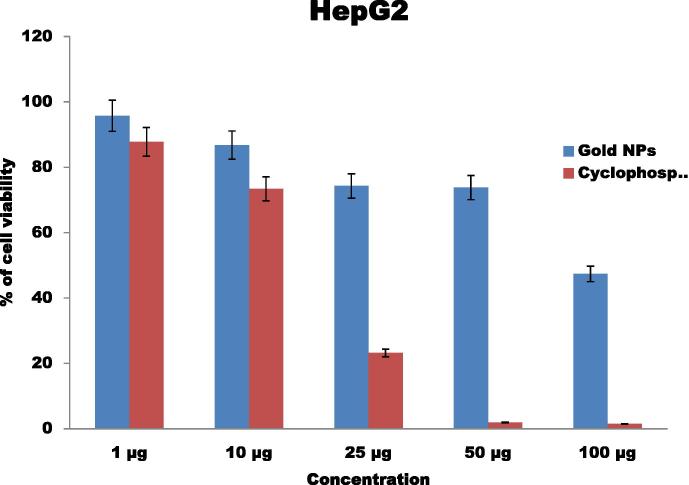
In vitro cytotoxic activity against Hep-G2 and A549 cell line at different concentrations was evaluated and compared with the standard drug cyclophosphamide. The anticancer activities of the gold nanoparticles were performed with different concentrations such as 1 μg, 10 μg, 25 μg, 50 μg and 100 μg. The anticancer activity of gold nanoparticles against HepG2 increased while in the concentration of gold nanoparticles (Fig. 8). Gold nanoparticles exhibit good results when compare with the standard cyclophosphamide. Previously, the anticancer activity of Silver and gold nanoparticles has been studied against Dalton’s lymphoma ascite (DLA) cell lines, Human laryngeal Hep-2 cell lines and human leukemic monocyte lymphoma respectively [42], [7], [36].
Figure 8.
Anticancer activities of gold nanoparticles against liver cancer (HepG2) cell lines.
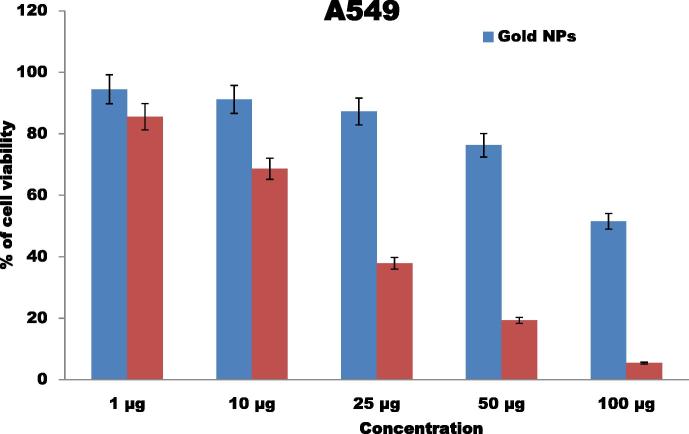
The anticancer effect of gold nanoparticles against HepG-2 and lung cancer cell (A549) lines was performed. The results show the good cytotoxic activity against the cancer cells (Fig. 9). The concentration of gold nanoparticles plays an important role in the anticancer activity. The gold nanoparticles are having the good results against A549 in that 100 μg show fine results followed by 50 μg, 25 μg and 1 μg. The lowest inhibitory action was observed form the concentration of 10 μg. Nam et al. [30] designed functionalized gold nanoparticles using dendrimers for fight cancer cells. Similarly, the silver nanoparticles synthesized by brown seaweed Ulva show good cytotoxic activity against human laryngeal cancer (Hep-2) cell line, human breast cancer (MCF 7) cell line and human colon cancer (HT 29) cell line [6] and breast cancer line MCF-7 [16]. Recently, Rajeshkumar et al. [34] synthesized the silver nanoparticles and reported their great potential to inhibit the cell viability of liver and lung cancer cell lines
Figure 9.
Anticancer activity of gold nanoparticles against lung cancer (A549) cells.
The cytotoxic effect of gold nanoparticles is the result of active physicochemical interaction of gold atoms with the functional groups of intracellular proteins, as well as with the nitrogen bases and phosphate groups in DNA [2]. In previous report Sriram et al. [42] reported that the nanoparticles acquiring anticancer properties are known for their potential ability to slow down the activities of abnormally expressed signaling proteins, such as Akt and Ras, cytokine-based therapies, DNA- or protein based vaccines against specific tumor markers, and tyrosine kinase inhibitors which exhibit a consistent antitumor effect [26].
In this report, the anticancer activity was observed and that the synthesized gold nanoparticles induce a dose dependant inhibition activity against lung and liver cells. Some of the approved chemotherapeutic agents were caused side effect and high cast. Therefore there is an important need to develop alternative medicines against this deadly disease. Synthesized gold nanoparticles to fulfill the need of new therapeutic treatment were discovered.
4. Conclusion
Extracellular synthesis of gold nanoparticles was achieved using culture supernatant of Enterococcus sp. Synthesized gold nanoparticles were initially identified by a formation of ruby red color and UV–vis spectrophotometer analysis shows the surface plasmon resonance band at 545 nm. Crystalline structure of gold nanoparticles was confirmed by XRD and SAED. TEM image shows the size, shape and distribution of nanoparticles. Carboxylic and amine groups are the functional groups present in the gold nanoparticles identified by FTIR. Anticancer activity of extracellularly synthesized gold nanoparticles was carried out by MTT assay against Hep-G2 and lung cancer cell (A549) lines. In this study the increased anticancer activity was found to be at an increased concentration of gold nanoparticles. This biosynthesis approach was easy, large scaled up and eco-friendly. Thus synthesized nanoparticles were more efficient in the biomedical applications in cancer treatment for their high anticancer activity.
Acknowledgements
Authors gratefully acknowledge STIC, Cochin for providing SEM and EDX, VIT University, Vellore for XRD, IIT Bombay for TEM facility.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Ahmad R.S., Sara M., Hamid R.S., Hossein J., Ashraf-Asadat N. Proc. Biochem. 2007;42:919–923. [Google Scholar]
- 2.Blagoi Yu P., Galkin V.L., Gladchenko G.O., Kornilova S.V., Sorokin V.A., Shkorbatov A.G. Naukova Dumka; Kiev: 1991. p. 272. [Google Scholar]
- 3.Daniel M.C., Astruc D. Chem. Rev. 2004;104:293. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 4.Dash S.S., Bag B.G. Appl. Nanosci. 2012 doi: 10.1007/s13204-012-0179-4. [DOI] [Google Scholar]
- 5.Devi J.S., Bhimba B.V. Asian Pac. J. Trop. Dis. 2012:S87–S93. [Google Scholar]
- 6.Devi J.S., Bhimba B.V. Sci. Rep. 2012;1(4):242. [Google Scholar]
- 7.Devi J.S., Bhimba B.V., Ratnam K. Int. J. Pharm. Pharm. Sci. 2012;4(4):710–715. [Google Scholar]
- 8.Du L., Jiang H., Liu X., Wang E. Electrochem. Commun. 2007;9(5):1165–1170. [Google Scholar]
- 9.Duran N., Marcato P.D., Alves O.L., D’Souza G., Esposito E. J. Nanobiotechnol. 2005;3:8–14. doi: 10.1186/1477-3155-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fayaz M.A., Tiwary C.S., Kalaichelvan P.T., Venkatesan R. Colloid. Surf B Biointerf. 2009;75:175–178. doi: 10.1016/j.colsurfb.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 11.Fayaz M.A., Girilal M., Venkatesan R., Kalaichelvan P.T. Colloid. Surf B Biointerf. 2011;88(1):287–291. doi: 10.1016/j.colsurfb.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Fukushima M., Kakinuma K., Kawaguchi R. J. Clinic. Microbiol. 2002;40:2779–2785. doi: 10.1128/JCM.40.8.2779-2785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosha P., Hana G., Dea M., Kima C.K., Rotello V.M. Adv. Drug. Del. Rev. 2008;60:1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Govindaraju K., Basha S.K., Kumar V.G., Singaravelu G. J. Mater. Sci. 2008;43:5115–5122. [Google Scholar]
- 15.He S., Zhang Y., Guo Z., Gu N. Biotechnol. Program. 2008;24:476–480. doi: 10.1021/bp0703174. [DOI] [PubMed] [Google Scholar]
- 16.Holt J.G., Krieg R.N., Sneath P.H.A., Staley J.T., Williams S.T. ninth ed. Williams and Wilkins Baltimore; 1994. Bergey’s Manual of Determinative Bacteriology. [Google Scholar]
- 17.Huang X., Jain P.K., El-Sayed I.H. Photochem. Photobiol. 2006;82:412–417. doi: 10.1562/2005-12-14-RA-754. [DOI] [PubMed] [Google Scholar]
- 18.Husseiny I.M., El-Aziz A.M., Badr Y., Mahmoud A.M. Spectrochimica. Acta Part A. 2007;67:1003–1006. doi: 10.1016/j.saa.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 19.Joerger T.K., Joerger R., Olsson E., Granqvist C.G. Trends Biotechnol. 2001;19:15. doi: 10.1016/s0167-7799(00)01514-6. [DOI] [PubMed] [Google Scholar]
- 20.Kanchana A., Balakrishna M. Int. J. Pharm. Pharm. Sci. 2011;3(4):356–364. [Google Scholar]
- 21.Lengke M., Fleet M.E., Southam G. Langmuir. 2006;22:2780–2787. doi: 10.1021/la052652c. [DOI] [PubMed] [Google Scholar]
- 22.Lengke M., Ravel B., Fleet M.E., Wanger G., Gordon R.A., Southam G. Environ. Sci. Technol. 2006;40:6304–6309. doi: 10.1021/es061040r. [DOI] [PubMed] [Google Scholar]
- 23.Lin Z., Wu J., Xue R., Yang Y. Spectrochim. Acta A. 2005;61:761–765. doi: 10.1016/j.saa.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 24.Magudapatty P., Gangopadhyayrans B.K., Panigrahi Nair K.G.M., Dhara S. Phys. B. 2001;299:142–146. [Google Scholar]
- 25.Mandal D., Bolander M.E., Mukhopadhyay D., Sarkar G., Mukherjee P. Appl. Microbiol. Bioechnol. 2006;69:485–492. doi: 10.1007/s00253-005-0179-3. [DOI] [PubMed] [Google Scholar]
- 26.Martins D., Frungillo L., Anazzetti M.C., Melo P.S., Duran N. Int. J. Nanomed. 2010;5:77–85. [PMC free article] [PubMed] [Google Scholar]
- 27.Mohanpuria P., Rana N.K., Yadav S.K. J. Nanopart. Res. 2008;10:507–517. [Google Scholar]
- 28.Mukherjee P., Ahmad A., Mandal D., Senapati S., Sainkar S.R., Khan M.I., Ramani R., Parischa R., Kumar P.A.V., Alam M., Sastry M., Kumar R. Angew. Chem. Int. Ed. 2001;40:3585–3588. doi: 10.1002/1521-3773(20011001)40:19<3585::aid-anie3585>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 29.Murphy C.J., Gole A.M., Stone J.W., Sisco P.N., Alkilany A.M., Goldsmith E.C., Baxter S.C. Acc. Chem. Res. 2008;41:1721–1730. doi: 10.1021/ar800035u. [DOI] [PubMed] [Google Scholar]
- 30.Nam J., Won N., Jin H., Chung H., Kim S. J. Am. Chem. Soc. 2009;131:13639–13645. doi: 10.1021/ja902062j. [DOI] [PubMed] [Google Scholar]
- 31.Nangia Y., Wangoo N., Goyal N., Shekhawat G., Suri C.R. Microb. Cell Fact. 2009;8:39. doi: 10.1186/1475-2859-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Pugazhenthiran N., Anandan S., Kathiravan G., Prakash N.K.U., Crawford S., Ashokkumar M. J. Nanopart. Res. 2009;11:1811–1815. [Google Scholar]
- 33.Rajasekharreddy P., Rani P.U., Sreedhar B. J. Nanopart. Res. 2010;12:1711–1721. [Google Scholar]
- 34.Rajeshkumar S., Malarkodi C., Vanaja M., Annadurai G. J. Mol. Struct. 2016;1116:165–173. [Google Scholar]
- 35.Renugadevi K., Inbakandan D., Bavanilatha M., Poornima V. Int. J. Pharm. Bio. Sci. 2012;3(3):437–445. [Google Scholar]
- 36.Rieznichenko L.S., Dybkova S.M., Gruzina T.G., Ulberg Z.R., Todor I.N., Lukyanova N.Y., Shpyleva S.I., Chekhun V.F. Exp. Oncol. 2012;34:25–28. [PubMed] [Google Scholar]
- 37.Rosarin F.S., Arulmozhi V., Nagarajan S., Mirunalini S. Asian Pac. J. Trop. Med. 2012:1–10. doi: 10.1016/S1995-7645(12)60193-X. [DOI] [PubMed] [Google Scholar]
- 38.Sastry M., Mayya K.S., Bandyopadhyay K. Colloid. Surf A Physicochem. Eng. Aspects. 1997;127:221–228. [Google Scholar]
- 39.Sastry M., Patil V., Sainkar S.R., Phy J. Chem. B. 1998;102:1404–1410. [Google Scholar]
- 40.Shankar S.S., Rai A., Ahmad A., Sastry M. J. Colloid. Interf. Sci. 2004;275:496–502. doi: 10.1016/j.jcis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Southam G., Beveridge T.J. Geochim. Cosmochim. Acta. 1994;58:4527–4530. [Google Scholar]
- 42.Sriram M.I., ManiKanth S.B., Kalishwaralal K., Gurunathan S. Int. J. Nanomed. 2010;5:753–762. doi: 10.2147/IJN.S11727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thangaraju N., Venkatalakshmi R.P., Chinnasamy A., Pandian K. Nano Biomed. Eng. 2012;4(2):89–94. [Google Scholar]
- 44.Unno Y., Shino Y., Kondo F., Igarashi N., Wang G., Shimura R., Yamaguchi T., Asano T., Saisho H., Sekiya S., Shirasawa H. Clin. Cancer Res. 2005;11(12):4553–4560. doi: 10.1158/1078-0432.CCR-04-2610. [DOI] [PubMed] [Google Scholar]
- 45.Vigeshwaran N., Ashtaputre M., Nachane R.P., Paralikar K.M., Balasubramanya H. Mater. Lett. 2007;61:1413–1418. [Google Scholar]
- 46.Yasuhiro K., Kaori O., Norizoh S., Toshiyuki N., Shinsuke N., Hajime H., Yoshio T., Tomoya U. J. Biotechnol. 2007;128:648–653. [Google Scholar]
- 47.Yungjin K., Johnson R.C., Hupp J.T. Nano. Lett. 2001;1:165–167. [Google Scholar]