Abstract
A new method of transgenic development called “In-planta” transformation method, where Agrobacterium is used to infect the plantlets but the steps of in vitro regeneration of plants is totally avoided. In this study, we have reported a simple In-planta method for efficient transformation of diploid cotton Gossypium hirsutum cv LRK-516 Anjali using Agrobacterium tumefaciens EHA-105 harbouring recombinant binary vector plasmid pBinAR with Arabidopsis At-NPR1 gene. Four day old plantlets were used for transformation. A vertical cut was made at the junction of cotyledonary leaves, moderately bisecting the shoot tip and exposing meristem cells at apical meristem. This site was infected with Agrobacterium inoculum. The transgenic events obtained were tested positive for the presence of At-NPR1 gene with promoter nptII gene. They are also tested negative for vector backbone integration and Agrobacterium contamination in T0 events. With this method a transformation frequency of 6.89% was reported for the cv LRK-516.
Keywords: In-planta transformation; Diploid cotton; Gossypium hirsutum cv LRK-516 Anjali; pBinAR; At-NPR1 gene, Cotyledonary leaves; Meristem cells; Transformation frequency
1. Introduction
Transgenic crop development with desired traits like biotic and abiotic stress resistance has become a reality due to the development of genetically modified (GM) crop technology [18]. Gene transfer in desirable plants was achieved by a number of methods like Agrobacterium mediated transformation, direct gene transfer by imbibition and biolistic transformation (Gene gun), chemical method, microinjection and pollen tube pathway, liposome method, shoot apex method of transformation, infiltration, and silicon carbide mediated transformation (SCMT) [58].
Cotton is one of the most economically important crops in the world. It is cultivated mainly for its fibre (lint), seed oil for human consumption and also for high protein cotton seed as a feed for cattle [64]. Cotton belongs to genus Gossypium which includes more than 50 species. Among those four are cultivable, which are Gossypium barbadense, Gossypium herbaceum, Gossypium arboreum and Gossypium hirsutum. G. hirsutum occupies 90% area of cultivation [66]. Textile industry requires a good quality fibre. Nevertheless, there are several biotic and abiotic factors which adversely affect quality and quantity of cotton. Hence, improvement of cotton by modern biotechnological approaches is required to obtain cotton plants with improved characters.
Classical breeding is one way of doing the task. But this technique is extremely time consuming and laborious [61]. Also, we need source plant possessing a desirable character that is to be incorporated in the variety of interest. But it takes a long time to achieve the goal. Genetic engineering can help to improve plant characters by transferring desirable traits using various methods of gene transfer [33], [61]. Tissue culture regeneration protocol is very much essential for development of transgenic plants. But, cotton is recalcitrant to in vitro regeneration. So far, success has been achieved through somatic embryogenesis and shoot tip organogenesis which are all genotype dependant methods [29], [63], [67]. Gossypium hirsutum cv Coker-312 and its closely related genotypes have yielded good response to gene transformation and in vitro regeneration through somatic embryogenesis and shoot tip organogenesis [15], [33], [67], [68].
Agrobacterium mediated transformation is widely used for gene transfer in plants, owning to its high transformation and relatively stable insertion, reduced copy number of transgenes, reduced co-suppression, stable expression of transgene and frequent recovery of plants with normal phenotypes [13], [23], [50]. All over the world for transgenic cotton development, Agrobacterium mediated transformation is predominantly used with somatic embryogenesis and shoot organogenesis [4], [11], [15], [20], [31], [42], [44], [51], [56], [69].
Firoozabady [15] and Umbeck [69] were the first to develop transgenic cotton plant. This was followed by a number of reports incorporating special traits. Transgenic cotton with insect resistance properties has been the most important and widely researched. Researchers who worked on this aspect include Perlak et al. [51], Cousin et al. [11], Xie et al. [73], Thomas et al. [64], Jenkins et al. [28], Li et al. [40], Li et al. [41], and Ni et al. [45]. This was followed by herbicide resistance [4], [10], [32], [39], [43], [55] and enhanced disease resistance [48], [49]. Beside these, important traits like gossypol free seeds have been also accomplished using RNA interference technology [64]. All these transgenic events were developed by Agrobacterium mediated gene transformation. Gene gun method was also used to incorporate genes in cotton [14], [43], [56]. Pollen tube pathway method was used for transgenic cotton development to overcome the problem of regeneration owing to recalcitrant nature of cotton [26], [80].
Most of these reports were related to callus based somatic embryogenesis or shoot tip organogenesis. Callus based somatic embryogenesis is of rare occurrence and very difficult in cotton. It has been achieved only in Coker 312 cultivar of G. hirsutum and its closely related genotypes [8], [19], [27], [68], [78], [79]. Even in case of Coker, the low efficiency of somatic embryogenesis, elaborate culture procedures, relatively long time period required for regeneration and high level of somaclonal variation and deviation in chromosomal numbers pose serious technical difficulties and restrict the growth of cotton biotechnology [40], [41], [72], [76], [77]. Shoot tip based method of gene transfer in diploid and tetraploid cotton varieties other than Coker has been reported [20], [21], [31], [44], [59], [75] but with low transformation efficiency.
Recently tissue culture independent transformation protocol has been reported [33], [61], [62], [63] in which the apical meristem or axillary bud or simply meristematic tissues of embryo is injured at seed germination stage and this injured part of explant is infected by Agrobacterium and this part was allowed to grow into the plant. This method totally evades the use of in vitro regeneration step by shoot tip organogenesis or callus based somatic embryogenesis. In 1986 Graves and Goldman [22] had given concept of this tissue culture independent plant transformation technique. This concept is very much significant in GM crop development and transformation studies on recalcitrant crops like cotton. This method is called “In-planta transformation”. This method of transformation has also been used in several crops and found promising, like, Bell pepper [1], Grapevine [17], Kalanchoe [30], Mulberry [34], Buckwheat [35], Peanut [52], [53], Safflower [54], Sunflower [57] and Rice [65].
Basic work on In-planta transformation was started in Arabidopsis thaliana [2], [5], [9], [37]. Pollen tube pathway had also been used in tetraploid cotton to transfer vector DNA directly to the zygotic embryo [26], [80]. Agrobacterium infection by directly microinjecting into the embryonic shoot apical meristem of germinated cotton seeds [61] and application of Agrobacterium on meristematic cells exposed by bisecting cotyledonary leaves of germinated seedling, have generated transgenic cotton [33], [63].
There are a few major advantages of In-planta transformation strategy over regular tissue culture based transformation methods. First, tissue culture regeneration protocol is not required and hence chances of somaclonal variation are least and second is, time needed to produce T0 transformants is very less compared to time taken by tissue culture protocol [26], [33], [61], [62], [63], [80]. There is one disadvantage of using this method, which is same as when we use shoot tip regeneration protocol for infected tissue, i.e. production of chimeric transgenic plants. Here, we have reported a simple In-planta transformation method for transgenic cotton development, where we have targeted the meristematic cells of bisected shoot tips from germinating seedlings.
2. Materials and methods
2.1. Gene, binary vector and bacterial strain
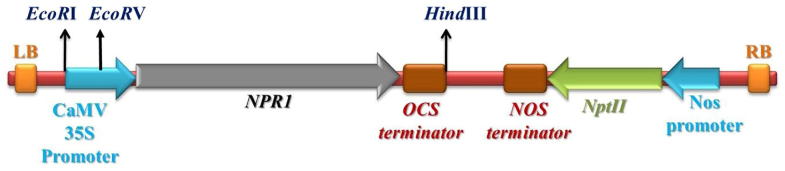
Agrobacterium tumefaciens strain EHA 105 was used for transformation. This strain carries recombinant binary vector plasmid pBinAR in which At-NPR1 gene (KF564649, non-expressor of PR gene was amplified from Arabidopsis thaliana ecotype Col-0) was cloned under CaMV-35s promoter and Octopine synthase (OCS) terminator. pBinAR is based on pBin-19 which carries the Neomycin phosphotransferase (nptII) gene, which is a Kanamycin resistance gene acting as a selectable marker and governed by the Nopaline synthase (NOS) promoter and the same NOS terminator [6] (Fig. 1).
Figure 1.
Plasmid map of recombinant binary vector pBinAR-At-NPR1. LB, left border; RB, right border. The Arabidopsis thaliana ecotype Col-0 NPR1 gene (KF564649) was amplified by PCR and cloned under Cauliflower mosaic virus (CaMV) 35S promoter and the Octopine synthase (OS) terminator. The neomycin phosphotransferase (nptII) gene was operationally fused with the Nopaline synthase (NOS) promoter and the NOS terminator.
Agrobacterium strain of EHA105 harbouring recombinant binary vector pBinAR-At-NPR1 plasmid was maintained on YEM (1 g/l Yeast Extract, 10 g/l Mannitol, K2HPO4 0.5 g/l, NaCl 0.1 g/l, MgSO4 0.2 g/l, Bactoagar 20 g/l, pH-7) agar medium [70] supplemented with 50 mg/l Kanamycin and 25 mg/l Rifampicin at 28 °C. A single colony from fresh plate of Agrobacterium was inoculated in YEM broth supplemented with 50 mg/l Kanamycin and 25 mg/l Rifampicin and incubated overnight at 28 °C in an incubator shaker at 90 rpm/min before using in transformation.
2.2. Plant material
Seeds of Gossypium hirsutum cv LRK-516 Anjali was procured from the Seema Cotton Development and Research Association, Tamil Nadu, India. The variety is vulnerable to the fungal diseases of cotton and hence selected for transformation with disease resistance gene.
2.3. Pre-treatment of seeds
Acid-delinted seeds were first rinsed with running tap water followed by shaking in liquid dishwashing detergent solution for 15 min. The seeds were then washed by agitation with double distilled water on an orbital shaker at 90 rpm for 5 to 7 min to eliminate all traces of liquid detergent. Washed seeds were then treated with the fungicide Bavistin (50% Carbendazim) solution in water (1% w/v) with agitation for 40 min followed by 3 to 4 washes with autoclaved distilled water. Treatment after this was pursued in laminar air flow. Seeds were transferred to a sterile flask containing 30 ml aqueous mercuric chloride solution (0.1% w/v) for one minute only and thoroughly washed with autoclaved distilled water 3–4 times to eliminate all traces of mercuric chloride.
2.4. Agrobacterium mediated transformation of bisected shoots
In this method of transformation, surface sterilized seeds were pre-soaked for 24 h in sterile water and sown directly in plastic cups containing autoclaved mixture of soil right and vermicompost (1:1 proportion). Cups were sterilised by wiping inside-out by 70% ethanol and then by exposing them to UV rays for two and a half hours. Only one seed was sown per pot. Total 60 seeds were sown, in replicate of three (Table 1) for germination. Four days after radicle emergence, a vertical cut was made at the junction of cotyledonary leaves, superficially along the length of the shoot apex, partially bisecting the shoot tip and exposing meristem cells, but without damaging the apical meristem (Fig. 2). Both the cotyledonary leaves were left as such attached to the seedling as they are vital for development and differentiation of meristematic tissue. Total number of seeds germinated was equal to the number of plantlets processed for Agrobacterium treatment (Table 1).
Table 1.
Percentage germination, survival rate, transformation frequency and molecular characterization of transgenic cotton G. hirsutum cv LRK-516 (Anjali) developed by leaf bisection method of In-planta Agrobacterium mediated transformation.
| Sr. No. | Number of seeds inoculated for germination | Number of seeds germinated | % of germination | Number of plantlets survived after treatment | % of survival | Number of PCR positive plantlets | % of transformation efficiency |
|---|---|---|---|---|---|---|---|
| 1 | 30 | 28 | 93.3 | 08 | 32 | 0 | 00.00 |
| 2 | 30 | 29 | 96.6 | 11 | 34 | 1 | 09.09 |
| 3 | 30 | 25 | 83.3 | 10 | 40 | 1 | 10.00 |
| Total | 90 | 82 | 91.11 | 29 | 35.36 | 2 | 06.89 |
Figure 2.
Vertical cut was made at the junction of cotyledonary leaves, superficially along the length of the shoot apex, partially bisecting the shoot tip and exposing meristem cells, but without damaging the apical meristem.
Agrobacterium culture was grown overnight from a fresh plate in YEM broth supplemented with 50 mg/l Kanamycin and 25 mg/l Rifampicin. One ml of this overnight grown culture (OD 600 = 0.6) of Agrobacterium tumefaciens was pelleted at 4000 rpm for 5 min. Supernatant was carefully discarded and the remaining concentrated culture was resuspended in 1 ml of modified vir induction medium (75 mM MES, pH 5.4, 2% glucose and 100 μM acetosyringone), based on Gould and Megallanes [20]. A 20 μl of this Agrobacterium culture was applied to the exposed meristematic cells at the site of injury. To avoid excessive drainage of applied Agrobacterium culture at the site of injury, a ring of absorbent cotton was wrapped around the wounded site, encircling the seedling, just below the cotyledonary node (Fig. 3). The plastic tape was wrapped around the cotton, which not only keeps cotyledonary leaves together but also hold leaves in their position (Fig. 4).
Figure 3.
Ring of absorbent cotton was wrapped around the wound site, encircling the seedling, just below the cotyledonary node, after Agrobacterium infection.
Figure 4.
Plastic tape was wrapped around the cotton ring, to keep cotyledonary leaves together but helps to hold leaves their position. This ring was temporarily propped in pots with wooden stick for additional support to maintain balance.
The plastic cups with seedlings were covered with pre-sterilised perforated plastic cups of the same size kept inverted on the seedling cups and left undisturbed for incubation at 28 °C with 10 h of light followed by 14 h of dark period. After infection of plantlets with Agrobacterium inoculum on first day, repeat the exact same procedure of Agrobacterium infection on the same plantlets which were infected on first day by Agrobacterium, on second, third and fourth days. On the consecutive inoculum repetitions, do not remove cotton swab wrapping, keep it same right from the first day. On the fourth day of Agrobacterium inoculation, the cotton wrapping was removed carefully and wounded areas of seedlings were washed two to three times with a solution of Carbenicillin (550 mg/L). After washing, cotton ring wrapping was again done. These seedlings were allowed to grow into mature (T0) plants (Fig.5a and b). Some seedlings were physically weak and therefore support was provided. A hard paper strip or wooden stick was placed under these unbalanced cotyledons along with the cotton wrapping to keep the stem in position (Fig. 4). The growing seedlings were irrigated at regular intervals. After three to four weeks of growth in tissue culture lab, the T0 seedlings were transferred to new pots with autoclaved mixture of soil right and vermicompost (1:1) (Fig.6a and b).
Figure 5a and b.
Two week old (a) and four week (b) old T0 cotton plants after Agrobacterium inoculation.
Figure 6a and b.
Eight to ten week old T0 cotton in new pots with autoclaved mixture of soil right and vermicompost.
2.5. Molecular analysis of transgenic plants
Molecular analysis was done to confirm the integration of At-NPR1 gene in the plant genome. For this, gene specific primers of At-NPR1 gene were used. Also, to check vector backbone integration and Agrobacterium contamination, PCR testing was done. Southern blot analysis was also done to reconfirm the gene integration and to detect copy number of the gene integrated in plant genome. For all these analyses genomic DNA was isolated as follows.
2.5.1. Extraction of genomic DNA
Cotton genomic DNA was extracted by miniprep protocol using CTAB buffer. A single leaf from specific nodal portion was extracted in 800 μl of Extraction Buffer (100 mM Tris–HCl pH 8, 20 mM EDTA, 1.4 M NaCl, 2% CTAB, 2% PVP and 0.5 M glucose) in a two ml centrifuge tube. The mixture was vortexed for 45 s and incubated at 65 °C for 1 h with occasional shaking. After incubation, equal volume of chloroform: isoamyl alcohol (24:1) was added, mixed carefully and centrifuged at 12,000 rpm for 10 min at 4 °C. The supernatant was removed carefully without disturbing the interface layer and transferred to a fresh two ml centrifuge tube. 0.8 volume of ice-cold isopropanol was added to the supernatant, mixed gently by inverting the tube for 5–6 times and mixture was incubated at −20 °C for half an hour. Centrifuge the mixture at 12,000 rpm at 4 °C for 15 min. Discard supernatant, let the DNA dry for two min and dissolve the DNA pellet in 500 μl of sterile double distilled water.
RNase A was added to a final concentration of 20 μg/ml to DNA and incubated at 37 °C for half an hour. RNase A treated DNA was re-precipitated with 0.1 volume of 3 M sodium acetate (pH 5.2) and double volumes of ice cold ethanol. Incubate the mixture at −20 °C for an hour and centrifuge at 12,000 rpm at 4 °C for 10 min. Re-precipitated DNA was rinsed with 70% ethanol. Let the DNA dry for two minutes at room temperature, but not completely and re-dissolved in 200 μl of sterile double distilled water. The concentration and purity of DNA was checked by measuring the absorbance ratio A260/280. The quality of the DNA was further ascertained by resolving 1 μl DNA on 0.8% agarose gel.
2.5.2. Molecular characterization of transgenic events
Molecular characterization includes detection of At-NPR1 and nptII gene by gene specific PCR, and also examination of vector backbone sequence integration and Agrobacterium contamination by PCR testing. The PCR cocktail setup for all PCR reactions is prepared as per Table 4 and the PCR protocols were standardized as mentioned in Table 3. The presence of At-NPR1 along with nptII was verified by PCR amplification using primers NPR1-F and NPR1-R (designed to amplify 2.3 kb product, Table 2) and nptII-F and nptII-R (designed to amplify 550 bp product, Table 2). Binary vector backbone integration was checked using two pairs of primers, the first was VB-LB-F and VB-LB-R (design to amplify 925 bp region of LB and Vector Backbone, included, Table 2) and the second pair was VB-RB-F and VB-RB-R (design to amplify 1016 bp region of RB and Vector Backbone, included, Table 2). Agrobacterium contamination was checked by primer oriV-F and oriV-R (designed to amplify 246 bp of oriV gene on Ti-plasmid, Table 2). All 29 putative transformants were tested by all the primers mentioned above. Negative controls were maintained to check non-specific amplification, if any. The amplified product was checked by resolving on 1.0% agarose gel and it was documented on gel documentation system.
Table 4.
Composition of PCR cocktail prepared.
| Sr. No. | Components | Volume (μl) |
|---|---|---|
| 1 | Template DNA (25 μg/μl) | 2.0 |
| 2 | Forward primer (10 mM) | 1.0 |
| 3 | Reverse primer (10 mM) | 1.0 |
| 4 | Taq DNA polymerase (5 U/μl) | 0.3 |
| 5 | dNTPs (25 mM) | 0.5 |
| 6 | 10× PCR buffer | 5.0 |
| 7 | MgCL2 (15 mM) | 1.5 |
| 8 | Sterile double distilled water | 38.7 |
| Total | 50.0 |
All the additions and reaction setup must be done at 4 °C.
Table 3.
Details of PCR protocol optimized for transgenic event characterization.
| Primer combination | Initial denaturation |
Denaturation |
Annealing |
Extension |
Final extension |
Product size (bp) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp (°C) | Time (min) | Temp (°C) | Time (s) | Temp (°C) | Time (s) | Temp (°C) | Time (s) | Temp (°C) | Time (min) | ||
| NPR1-F NPR1-R |
94 | 5 | 94 | 40 | ⁎68–62 | 30 | 72 | 180 | 72 | 5 | 2300 |
| 01 Cycle | 45 Cycles | 01 Cycle | |||||||||
| nptII-F nptII-R |
94 | 5 | 94 | 30 | 58 | 30 | 72 | 45 | 72 | 5 | 550 |
| 01 Cycle | 35 Cycles | 01 Cycle | |||||||||
| VB-LB-F VB-LB-R |
94 | 5 | 94 | 40 | 62 | 30 | 72 | 90 | 72 | 5 | 925 |
| 01 Cycle | 35 Cycles | 01 Cycle | |||||||||
| VB-RB-F VB-RB-R |
94 | 5 | 94 | 40 | 58 | 30 | 72 | 90 | 72 | 5 | 1016 |
| 01 Cycle | 35 Cycles | 01 Cycle | |||||||||
| oriV-F oriV-R |
94 | 5 | 94 | 40 | 58 | 30 | 72 | 30 | 72 | 5 | 246 |
| 01 Cycle | 35 Cycles | 01 Cycle | |||||||||
Touchdown PCR, annealing temperature decrease from 68 °C to 62 °C.
Table 2.
Details of primers used in transgenic event characterization.
| Sr. No. | Primer ID | Primer sequence (5′-3′) | Target |
|---|---|---|---|
| 1 | NPR1-F | CTTGGCTCTGCTCGTCAATGG | Arabidopsis thaliana ecotype Col-0, NPR1 gene |
| NPR1-R | GGATGCAAAACGAAGAGCGA | ||
| 2 | nptII-F | AGATCCCGTGGGCGAAGAA | Neomycin phosphotransferase (nptII) gene from pBinAR |
| nptII-R | GTACTCGGATGGAAGCCGGT | ||
| 3 | VB-LB-F | GCTCTGCTAGGTAGCCCGATACGAT | Left border + Ti-plasmid |
| VB-LB-R | TATCCTGCCACCAGCCAGCCAACAG | ||
| 4 | VB-RB-F | TTGGCGGGTAAACCTAAGAG | Right border + Ti-plasmid |
| VB-RB-R | TCGTCGAAGGCGTCTATCG | ||
| 5 | oriV-F | ATAAGTGCCCTGCGGTATTG | oriV in Ti-plasmid |
| oriV-R | GCAGCCCTGGTTAAAAACAA | ||
2.5.3. Copy number and gene integration detection of transgene by Southern hybridization
20 μg of genomic DNA was digested with EcoRI. Following electrophoresis on 1.0% agarose gel, DNA was stained with ethidium bromide for 30 s and examined under UV light. The digested DNA fragments were transferred to positively charged nylon membranes (Pall Gelman, USA) by alkaline transfer method as described by Sambrook [81]. 500 bp PCR product of At-NPR1 gene was used to prepare probe for Southern analysis. The probe was labelled with DIG (dig-oxigenin) using a DIG-DNA labelling kit (Roche, Germany) following manufacturer’s protocol. Southern blots containing the genomic DNA were hybridized to the DIG-labelled At-NPR1 probe at 60 °C for 24 h. The hybridized probe was immuno-detected using anti-DIG–AP conjugate in the presence of the chromogenic substrate NBT/BCIP (nitro-blue tetrazolium chloride/5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt), following the manufacturer’s protocol.
2.5.4. Phenotypic characterization of cotton transgenic plants by fungal bioassay
Alternaria alternata infection assay was performed to assess activity of At-NPR1 gene in transgenic cotton. These putative transgenic cotton plants were pre-screened for fungal resistance by Detached Leaf Assay [7], [12], [48], [49], [71]. Fully developed leaves were collected from putative transgenic plants and surface sterilized with 0.1% Bavistin followed by three washes of sterile water. All these leaves were blot dried with sterile tissue paper and placed on a wet towel of filter paper and cotton, soaked with sterile distilled water in a petri plate. Freshly collected fungal spores from A. alternata were inoculated on the leaves (Fig.10a and b). The plates were sealed with parafilm and were kept in an environmental climate chamber at a 16 h/8 h day/night and photoperiod at 30 °C. Observations were taken after regular intervals.
Figure 10.
Phenotypic screening of putative transformants by fungal bioassay detached leaf method, using spore suspension of cotton pathogen Alternaria alternata. (a) Leaf form putative transgenic LRK-516 plant inoculated with Alternaria alternata spore suspension, day one. (b) Leaf form wild type non-transgenic LRK-516 plant inoculated with Alternaria alternata spore suspension, day one. (c) Leaf from putative transgenic LRK-516 plant was largely unaffected, day 14. (d) Leaf of WT non-transgenic LRK-516 plant showed infection lesions and chlorosis, day 14.
3. Results
3.1. In-planta Agrobacterium mediated transformation of bisected shoots
In the present study, G. hirsutum cv LRK-516 (Anjali) was used in transformation studies. Three replications of transformation experiment were performed (Table 1). In each experiment 30 seeds were taken for transformation. The seeds were initially sterilised and sown in sterilised plastic cup containing autoclaved mixture of soil right and vermicompost. Four day old seedlings were used to vertically bisect through cotyledonary leaves with surgical precision as described above and Agrobacterium infection was repeated three times with 24 h of interval. Altogether, out of 90 seeds used, 82 seeds were geminated (91.11% of germination). Out of these 29 seedlings were survived after transformation (35.36% of survival). It was observed that out of 29 plants two were found to be PCR positive. This gives transformation frequency of 6.89% (Table 1). From the time Agrobacterium infection to the plantlets till their proper growth and transfer in bigger pots from plastic cups, it will take at least a month and a half, and we can say this much is the time required for transgenic development by this In-planta transformation method.
3.2. Molecular analysis of transgenic plants
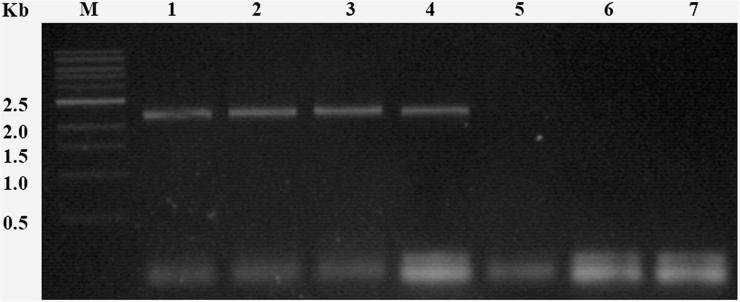
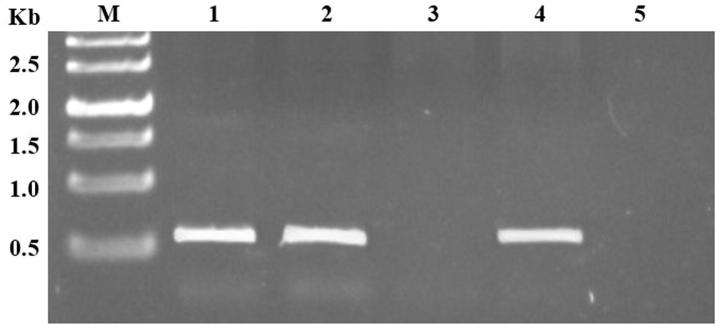
Excellent quality genomic DNA was isolated by modified CTAB method. The yield of genomic DNA was between 800 and 1400 μg/ml from 1 g of fresh leaf tissue. This DNA was used to screen plantlets by gene specific primer. PCR investigation of the putative transformants of cv LRK-516 revealed the presence of transgene with expected 2.3 kb amplicon of At-NPR1 gene (Fig. 7). These gene specific PCR positive cotton plants were also found positive for the presence of nptII gene indicated by amplification of 550 bp amplicon by nptII primers (Fig. 8). The two positive transformants were KA_LB-S.4 and KA_LB-S.5.
Figure 7.
Gene specific PCR analysis for the detection of At-NPR1 cotton transgenic event from genomic DNA isolated from leaves of to putative cotton transformants of LRK-516. Lane M, 1 kb ladder; Lane 1–2, At-NPR1 cotton transgenic samples; Lane 3, positive control of Arabidopsis thaliana sample; Lane 4, positive control of pBinAR-At-NPR1 plasmid sample; Lane 5, negative control of wild type G. hirsutum cv LRK-516 (Anjali) sample; Lane 6, negative control of pBinAR plasmid; and Lane 7, PCR negative control .
Figure 8.
Screening of putative transformants of cotton for the presence of nptII gene. Lane M, 1 kb ladder; Lane 1–2, At-NPR1 cotton transgenic samples; Lane 3, negative control of wild type G. hirsutum cv LRK-516; Lane 4, positive control of pBinAR-At-NPR1 plasmid sample; and Lane 5, PCR negative control.
3.3. Southern hybridization to confirm gene integration and copy number detection
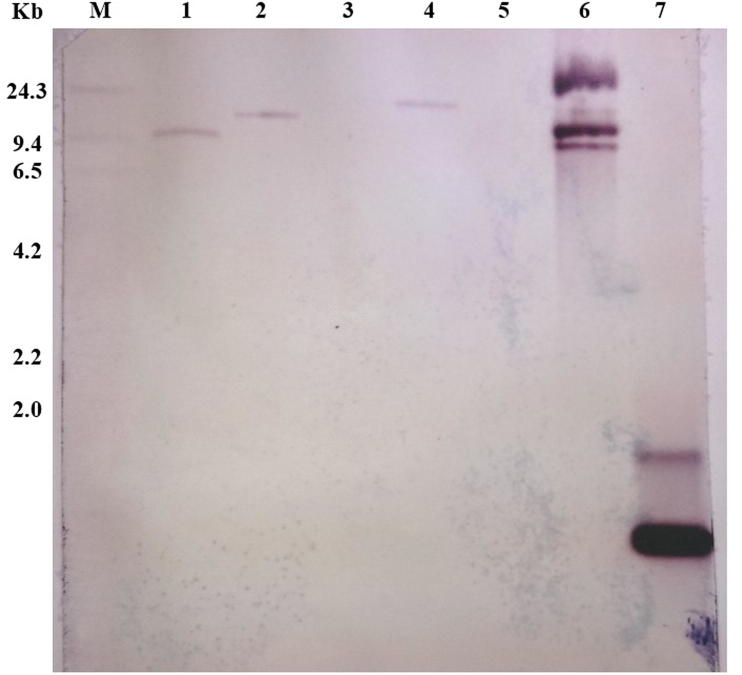
Southern blot analysis was also performed to confirm transgene integration and copy number detection (Fig. 9). Southern blot was performed with DNA samples from two positive transformants of LRK-516, wild type (i.e. non-transgenic) LRK-516, Arabidopsis thaliana ecotype Col-0, binary vector pBinAR-At-NPR1 plasmid DNA and At-NPR1 PCR product used to prepare probe for Southern blot. Single hybridization fragment was detected in each PCR positive samples along with Arabidopsis and At-NPR1 PCR product. In case of binary vector hybridization fragments showcase classic three plasmid bands. There was no hybridization in wild type LRK-516, which was expected. A single copy of the transgene was confirmed in putative transgenic cotton (Fig. 9). These results are in support of the PCR results, thus confirming the integration of At-NPR1 transgene in cotton genome and single copy integration of transgene in the plant genome.
Figure 9.
Southern blot of PCR positive At-NPR1 cotton transgenic plant samples. Genomic DNA digested with EcoRI was hybridized with DIG labelled 500 bp PCR fragment of At-NPR1 gene from Arabidopsis thaliana as DNA probe. Lane M, λ-HindIII marker; Lane 1–2, At-NPR1 cotton transgenic DNA samples. Lane 3, negative control of wild type G. hirsutum cv LRK-516 (Anjali) sample; Lane 4, positive control of Arabidopsis thaliana sample; Lane 5, negative control of pBinAR plasmid; Lane 6, positive control of pBinAR-At-NPR1 plasmid sample; and Lane 7, Probe positive 500 bp PCR fragment of At-NPR1 gene from Arabidopsis thaliana.
3.4. Checking of vector backbone integration and Agrobacterium contamination by PCR testing
It was known that the only T-DNA along with its LB and RB is integrated into the plant genome [74]. But there are reports that the vector backbone sequence also gets incorporated along with the T-DNA sequence [38]. Zhang [82] reported that transformed cotton plants had integrated backbone in their genome. Hence, our positive transgenic plants were also PCR tested by special primers (Table 2) to determine integration of vector backbone in plant genome. PCR results of two transgenic samples with VB-LB primer and VB-RB primer reveals that there was no vector backbone integration in positive samples. Also, PCR with oriV primers also confirms the absence of Agrobacterium tumefaciens itself in plant as a pathogen.
3.5. Fungal bioassay for phenotypic characterization of cotton transgenic plants
Disease resistance of the transgenic cotton was tested against the cotton fungal pathogen Alternaria alternata which causes disease Alternaria leaf blight in cotton. Disease appearance started within a week on wild type plant samples and in further weeks it gave a clear indication that transgene was working to provide resistance in transgenic plants. In this case, in the first week we found large lesions spreading beyond the inoculation site in non-transgenic WT leaves, but there was no appearance of disease on transgenic leaves. At the end of the second week the non-transgenic WT leaves showed infection lesions and chlorosis while the leaves from transgenic plants were largely unaffected (Fig.10c and d). The transgenic lines were clearly more resistant than the wild type against Alternaria alternata.
4. Discussion
Cotton is one of the top GM crops occupying the largest area of agricultural land in the world [83]. Major limitations in producing GM Cotton are genotype dependant transformation of cotton, regeneration through somatic embryogenesis and shoot tip organogenesis, and large time required for its regeneration to T0 plant because cotton is recalcitrance to regeneration in tissue culture [36], [46], [47], [49].
The definitive goal of transformation method is to acquire fertile transgenic plant harbouring anticipated foreign gene(s). But, inconsistency in transformation efficiency is the major drawback with the available transformation methods. Currently, a large number of various transformation techniques are in use which are based on in vivo and in vitro strategies. In contrast, to in vitro techniques, in vivo techniques of transformation are cost effective, time saving and mostly importantly genotype independent and tissue culture independent. In this method we have attempted in vivo genotype-independent approaches through cotyledonary leaf bisection method. Our main objective was to develop disease resistance transgenic cotton using binary vector pBinAR harbouring Arabidopsis thaliana ecotype Col-0 NPR1 gene. We have successfully developed transgenic Cotton through tissue culture independent In-planta transformation protocol.
Transformation efficiency in different methods of gene transfer was quite low [33]. Since first report of first transgenic cotton [15], [69] with very low transformation efficiency, till date the frequency of transformation in cotton have markedly improved. Traditional protocols with somatic embryogenesis and shoot tip organogenesis provide transformation efficiency in the range of 0.2–5% [16], [20], [31], [44]. We have reported 6.89% of transformation frequency (Table 1) which is comparatively good. In cotyledonary leaf bisection method transformation efficiency was 8.3% [33], 0.83% [63] and in microinjection method it was 1.16% [63] and 20% [61].
Putative transformants obtained either through shoot tip organogenesis [20], [21], [31], [44], [59], [75], particle bombardment [14], [44], [57], pollen tube pathway [26], [80] or in-planta transformation [33], [61], [62], [63] all have reported chimeric plants at T0 stage. But eventually at next stage i.e. T1 level, stable transformation was achieved. In our studies we have also observed chimeric condition. When branches were PCR tested independently for the presence of At-NPR1 gene, not all the branches have shown positive results in gene specific PCR. Some branches were positive for At-NPR1, while some are negative though, gene stabilization in next generation is expected.
Addition of acetosyringone with Agrobacterium culture enhances expression of vir genes and acts as an indispensable element needed for initiation of Agrobacterium virulence [20], [24], [25], [60]. Yet, acetosyringone is not an absolute necessity for the purpose. There are other factors like, monosaccharide, plant hormone, vacuum and other strains of Agrobacterium that can intensify the effectiveness of In-planta transformation methods [3].
800–1400 μg of DNA is a good amount to harvest for molecular analysis. The two positive transformants were KA_LB-S.4 and KA_LB-S.5 characterized by PCR with gene specific primers for At-NPR1 (Fig. 7) and nptII (Fig. 8) genes. Single copy number for the transgene integrated was confirmed by Southern analysis (Fig. 9). The transgenics were also negative for vector backbone and Agrobacterium infection. Phenotypic screening against the cotton fungal pathogen Alternaria alternata shows improved resistance in transgenic cotton as compared with wild type (Fig.10a–d). These results confirm our earlier observations showing that At-NPR1 transgenic cotton lines exhibit significant resistance [48], [49].
Easy and quick production of transgenic plants is the core rewards of In-planta transformation methods. The aim to target meristematic tissues and cells based on the fact that these cells are briskly dividing and the foreign gene of importance can be easily accepted in genome of these naive cells of cotton. Moreover, there are accounts of very low occurrences of somaclonal variations and genetic mutations in the plants regenerated from meristematic tissues of shoots [20]. We are able to produce transgenic cotton cultivars which are KA_LB-S.4 and KA_LB-S.5. Successful incorporation of At-NPR1 gene in LRK-516 genome was confirmed by PCR and Southern hybridization.
Acknowledgement
Authors wish to acknowledge with thanks to the Head, Department of Biotechnology, S.G.B. Amravati University, Amravati, Maharashtra, 444602, India for providing workbench and his constant encouragement.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Arthikala M.K., Reddy K.N., Manjulatha M., Arellano E.S., Sreevathsa R., Ganeshan G. Sci. Hortic. Amsterdam. 2011;29:898–903. [Google Scholar]
- 2.Azpiroz-Leehan R., Feldmann K.A. Trends Genet. 1997;13:152–156. doi: 10.1016/s0168-9525(97)01094-9. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett J.G., Alves S.C., Smedley M., Snape J.W., Harwood W.A. Plant Methods. 2008;4:1–12. doi: 10.1186/1746-4811-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayley C., Trolinder N.L., Ray C., Morgan M.M., Quisenberry J.E., Ow D.W. Theor. Appl. Genet. 1992;83:645–649. doi: 10.1007/BF00226910. [DOI] [PubMed] [Google Scholar]
- 5.Bechtold N., Ellis J., Pelletier G. C. R. Acad. Sci. Paris Life Sci. 1993;316:1194–1199. [Google Scholar]
- 6.Bevan M. Nucl. Acids Res. 1984;12:8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boydom A., Dawit W., Getaneh W. J. Plant Pathol. Microbiol. 2013;4:5. [Google Scholar]
- 8.Cao J.L., Zhang X.L., Jin S.X., Yang X.Y., Zhu H.G., Fu L.L. Acta Agron. Sin. 2008;34(2):224–231. [Google Scholar]
- 9.Chang S.S., Park S.K., Kim B.C., Kang B.J., Kim D.U., Nam H.G. Plant J. 1994;5:551–558. [Google Scholar]
- 10.Chen Z.X., Llewellyn D.J., Fan Y.L., Li S.J., Guo S.D., Jiao G.L., Zhao J.X. Sci. Agric. Sin. 1994;27(2):31–37. [Google Scholar]
- 11.Cousins Y.J., Lyon B.R., Liewellyn D.J. Aust. J. Plant Physiol. 1991;18:481–494. [Google Scholar]
- 12.Emani C., Garcia J.M., Lopata-Finch E., Pozo M.J., Uribe P., Kim D.J., Sunilkumar G., Cook D.R., Kenerley C.M., Rathore K.S. Plant Biotechnol. J. 2003;1:321–333. doi: 10.1046/j.1467-7652.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 13.Enriquez-Obregon G.A., Prieto-Samsonov D.L., de la Riva G.A., Perez M.I., Selman-Housein G., Vazquz-Padron R.I. Plant Cell Tissue Organ Culture. 1999;59:159–168l. [Google Scholar]
- 14.Finer J.J., McMullen M.D. Plant Cell Rep. 1990;8:586–589. doi: 10.1007/BF00270059. [DOI] [PubMed] [Google Scholar]
- 15.Firoozabady E., Deboer D.L., Merlo D.J., Halk E.L., Amerson L.N., Rashka K.E., Murray E.E. Plant Mol. Biol. 1987;10:105–116. doi: 10.1007/BF00016148. [DOI] [PubMed] [Google Scholar]
- 16.Francisco J.L.A., Giovanni R.V., Silvia B.R., Elibio L.R. Plant Sci. 2005;168:1227–1233. [Google Scholar]
- 17.Fujita K., Matsuoka T., Suzuki S., Takayanagi T. J. Plant Biochem. Biotechnol. 2009;18:161–167. [Google Scholar]
- 18.Gasser C.S., Fraley R.T. Transgenic Crops Sci. Am. 1992;266:62–69. [Google Scholar]
- 19.Gonzalez-Benito M.E., Carvalho J.M., Perez C. Pesq. Agropec. Bras. Brasilia. 1997;32(5):485–488. [Google Scholar]
- 20.Gould J.H., Megallanes-Cedeno M. Plant Mol. Biol. Rep. 1998;16:1–10. [Google Scholar]
- 21.Gould J.H., Banister S., Hasegawa O., Fahima M., Smith R.H. Plant Cell Rep. 1991;10:35–38. doi: 10.1007/BF00233024. [DOI] [PubMed] [Google Scholar]
- 22.Graves A., Goldman S. Plant Mol. Biol. 1986;7:43–50. doi: 10.1007/BF00020130. [DOI] [PubMed] [Google Scholar]
- 23.Hansen G., Shillito R.D., Chilton M.D. Proc. Nat. Acad. Sci. USA. 1997;94:11726–11730. doi: 10.1073/pnas.94.21.11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Y., Jones H.D., Chen S., Chen X.M., Wang D.W., Li K.X., Wang D.S., Xia L.Q. J. Exp. Bot. 2010;61:1567–1581. doi: 10.1093/jxb/erq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiei Y., Koman T. Nature. 2008;3:824–834. doi: 10.1038/nprot.2008.46. [DOI] [PubMed] [Google Scholar]
- 26.Huang G.C., Dong V.M., Sun J.S. Chin. Sci. Bull. 1999;44 698- 672. [Google Scholar]
- 27.Ikram-ul-Haq Afr. J. Biotechnol. 2005;4(2):206–209. [Google Scholar]
- 28.Jenkins J.N., McCarty J.C., Buehler R.E., Kiser J., Williams R.E., Wofford T. Agron. J. 1997;89:768–780. [Google Scholar]
- 29.John M.E. Crit. Rev. Biotechnol. 1997;17:185–208. [Google Scholar]
- 30.Jung Y., Rhee Y., Auh C.K., Shim H., Choi J.J., Kwon S.T., Yang J.S., Kim D., Kwon M.H., Kim Y.S., Lee S. Plant Cell Rep. 2009;28:1593–1602. doi: 10.1007/s00299-009-0758-3. [DOI] [PubMed] [Google Scholar]
- 31.Kategari I.S., Vamadevaiah H.M., Udikeri S., Khadi B.M., Polumetla A.K. Curr. Sci. India. 2007;93:1843–1847. [Google Scholar]
- 32.Keller G., Spatola L., McCabe D., Martinelli B., Swain W., John M.E. Transgenic Res. 1997;6:385–392. [Google Scholar]
- 33.Keshamma E., Rohini S., Rao K.S., Madhusudhan B., Udayakumar M. J. Cotton Sci. 2008;12:264–272. [Google Scholar]
- 34.Kojima M., Shiori H., Nogawaa M., Nozue M., Matsumoto D., Wada A., Saiki V., Kiguchi K. J. Biosci. Bioeng. 2004;98:136–139. doi: 10.1016/S1389-1723(04)70256-X. [DOI] [PubMed] [Google Scholar]
- 35.Kojima M., Arai Y., Iwase N., Shiratori K., Shioiri H., Nozue M. Biosci. Biotechnol. Biochem. 2000;64:845–847. doi: 10.1271/bbb.64.845. [DOI] [PubMed] [Google Scholar]
- 36.Kumria R., Sunni-Chan V.G., Das D.K., Gupta S.K., Reddy V.S., Bhatnagar R.K., Leekavathi S. Plant Cell Rep. 2003;21:635–639. doi: 10.1007/s00299-002-0554-9. [DOI] [PubMed] [Google Scholar]
- 37.Labra M., Vannini C., Grassi F., Bracale M., Balsemin M., Basso B., Sala F. Theor. Appl. Genet. 2004;109:1512–1518. doi: 10.1007/s00122-004-1773-y. [DOI] [PubMed] [Google Scholar]
- 38.Lange M., Vincze E., Moller M.G., Holm P.B. Plant Cell Rep. 2006;25:815–820. doi: 10.1007/s00299-006-0140-7. [DOI] [PubMed] [Google Scholar]
- 39.Leelavathi S., Sunnicham V.G., Kumaria V., Vijaykanth G.P., Bhatnagar R.K., Reddy V.S. Plant Cell Rep. 2004;22:465–470. doi: 10.1007/s00299-003-0710-x. [DOI] [PubMed] [Google Scholar]
- 40.Li Y.E., Chen Z.X., Wu X., Li S.J., Jiao G.L., Wu J.H., Fan X.P., Meng J.H., Zhu Z., Wang W., Zhu Y., Xu H.L., Xiao G.F., Li X.H. Acta Gossypii Sin. 1998;10:237–243. [Google Scholar]
- 41.Li Y.E., Jiao G.L., Li S.J., Fan X.P., Wu X., Chen Z.X. Sci. Agric. Sin. 1998;31:96–98. [Google Scholar]
- 42.Lyon B.R., Cousins Y.L., Llewellyn D.J., Dennis E.S. Transgenic Res. 1993;2:162–169. [Google Scholar]
- 43.McCabe D.E., Martinell B.J. Biotechnology. 1993;11:596–598. [Google Scholar]
- 44.Nandeshwar S.B., Moghe S., Chakrabarty P.K., Deshattiwar M.K., Kranthi K., Anandkumar P., Mayee C.D., Khadi B.M. Plant Mol. Biol. Rep. 2009;27:549–557. [Google Scholar]
- 45.Ni W.C., Zhang Z.L., Guo S.D. Sci. Agric. Sin. 1998;31:8–13. [Google Scholar]
- 46.Ouma J.P., Young M.M., Reichert N.A. Afr. J. Biotechnol. 2004;3(6):313–318. [Google Scholar]
- 47.Ozyigit I.I., Kahraman M.V., Ercan O. Afr. J. Biotechnol. 2007;6(1):003–008. [Google Scholar]
- 48.Parkhi V., Kumar V., Campbell L.M., Bell A.A., Rathore K.S. J. Phytopathol. 2010;158(11–12):822–825. [Google Scholar]
- 49.Parkhi V., Kumar V., Campbell L.M., Bell A.A., Shah J., Rathore K.S. Transgenic Res. 2010;19(6):959–975. doi: 10.1007/s11248-010-9374-9. [DOI] [PubMed] [Google Scholar]
- 50.Park S.H., Pinson S.R.M., Smith R.H. Plant Mol. Biol. 1996;32:1135–1148. doi: 10.1007/BF00041397. [DOI] [PubMed] [Google Scholar]
- 51.Perlak F.J., Deaton R.W., Armstrong R.L., Fuchs R.L., Sims S.R., Greenplate J.T., Fischhoff D.A. Biotechnology. 1990;8:939–943. doi: 10.1038/nbt1090-939. [DOI] [PubMed] [Google Scholar]
- 52.Rohini V.K., Rao S.K. Plant Sci. 2000;150:41–49. [Google Scholar]
- 53.Rohini V.K., Rao S.K. Plant Sci. 2001;160:883–892. doi: 10.1016/s0168-9452(00)00462-3. [DOI] [PubMed] [Google Scholar]
- 54.Rohini V.K., Rao S.K. Ann. Bot. 2006;86:1043–1049. [Google Scholar]
- 55.Rajasekaran K., Grula J.W., Hudspeth R.L., Pofelis S., Anderson D.W. Mol. Breed. 1996;2:307–319. [Google Scholar]
- 56.Rajasekaran K., Hudspeth R.L., Cary J.W., Anderson D.M., Cleveland T.E. Plant Cell Rep. 2000;19:539–545. doi: 10.1007/s002990050770. [DOI] [PubMed] [Google Scholar]
- 57.Rao S.K., Rohini V.K. Ann. Bot. 1999;83:347–354. [Google Scholar]
- 58.Rao A.Q., Bakhsh A., Kiani S., Shahzad K., Shahid A., Husnain T., Riazuddin S. Biotechnol. Adv. 2009;27:753–763. doi: 10.1016/j.biotechadv.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 59.Satyavathi V.V., Prasad V., Lakshmi G., Lakshmi S. Plant Sci. 2002;162:215–223. [Google Scholar]
- 60.Seo M.S., Bae C.H., Choi D.O., Rhim S.L., Seo S.C., Song P.S., Lee H.Y. Plant Biotechnol. 2002;29:85–92. [Google Scholar]
- 61.Shou H., Palmer R.G., Wang K. Plant Mol. Biol. Rep. 2002;20:325–334. [Google Scholar]
- 62.Sivakumar S., Premkumar G., Siva G., Kanakachari M., Vigneswaran M., Vinoth S., Kumar T.S., Jayabalan N. Int. J. Curr. Biotechnol. 2014;2:12. [Google Scholar]
- 63.Solanki V.H., Khandelwal V., Patel D.H., Mahatma M.K. Indian J. Plant Physiol. 2011;16(3–4):303–308. [Google Scholar]
- 64.Sunilkumar G., Campbell L.M., Puckhaber L., Stipanovic R.D., Rathore K.S. PNAS USA. 2006;103(48):18054–18059. doi: 10.1073/pnas.0605389103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Supartana P., Shimizu T., Shiori H., Nogawa M., Nozue M., Kojima M. J. Biosci. Bioeng. 2005;100:391–397. doi: 10.1263/jbb.100.391. [DOI] [PubMed] [Google Scholar]
- 66.Thomas J.C., Adam D.G., Keppenne V.D., Wasmann C.C., Brown J.K., Kanost M.R., Bohnert J.J. Plant Cell Rep. 1995;14:758–762. doi: 10.1007/BF00232917. [DOI] [PubMed] [Google Scholar]
- 67.Tian Z.C., Shen J.W., Jun Z., Wang Z.G., Tian Z.Z. Biotechnol. Lett. 2010;32:547–555. [Google Scholar]
- 68.Trolinder N.L., Xhixian C. Plant Cell Rep. 1989;8:133–136. doi: 10.1007/BF00716824. [DOI] [PubMed] [Google Scholar]
- 69.Umbeck P.G., Tohnson K., Barton, Swain W. Plant Biotechnol. 1987;5:263–266. [Google Scholar]
- 70.Vincent J.M. International Biological Programme. vol. 15. Blackwell Scientific Publisher; Oxford: 1970. (Handbook). [Google Scholar]
- 71.Vleeshouwers V.G.A.A., van-Dooijeweert W., Keizer L.C.P., Sijpkes L., Govers F., Colon L.T. Eur. J. Plant Pathol. 1999;5:241250. [Google Scholar]
- 72.Voo K.S., Rugh C.L., Kamalay J.C. In Vitro Cell Dev. Biol. 1991;27:117–124. [Google Scholar]
- 73.Xie D.X., Fan Y.L., Ni W.C., Huang J.Q. China Sci. (B) 1991;4:367–373. [Google Scholar]
- 74.Zambryski J., Tempe J., Schell J. Ann. Rev. Plant Physiol. Mol. Biol. 1992;43:465–490. [Google Scholar]
- 75.Zapata C., Srivalakanakul M., Park S.H., Lee B.M., Salas M.G., Smith R.H. Plant Cell Tissue Organ. 1999;12:43–50. [Google Scholar]
- 76.Zhang B.H., Li X.L., Li F.L., Li F.G. Boreali-occidentalis Sin. 1993;2(4):24–28. [Google Scholar]
- 77.Zhang B.H., Zhao B.S. China Agricultural Press; Beijing: 1997. Cotton Biotechnology and Its Application. [Google Scholar]
- 78.Zhang B.H., Liu F., Yao C.B. Plant Cell Tissue Organ. 2000;60:89–94. [Google Scholar]
- 79.Zhang B.H., Feng R., Liu F., Wang Q. Bot. Bull. Acad. Sin. 2001;42:9–16. [Google Scholar]
- 80.Zhou G.Y., Wang J., Zeng Y., Huang J., Quian S., Liu G. Methods Enzymol. 1983;101:433–481. doi: 10.1016/0076-6879(83)01032-0. [DOI] [PubMed] [Google Scholar]
- 81.Sambrook J., Fritsch E.F., Maniatis T. Cold spring Harbor Laboratory press; 1989. Molecular Cloning. Plain View. [Google Scholar]
- 82.Zhang J., Hong Y. Protocol Transgenic Cotton. 2012;958:95–107. [Google Scholar]
- 83.USDA, Cotton: World Market and Trend, USDA FAS, 2014.